Abstract
MRI of the breast is the most sensitive test for breast cancer detection and outperforms conventional imaging with mammography, digital breast tomosynthesis or ultrasound. However, the long scan time and relatively high costs limit its widespread use. Hence, it is currently only routinely implemented in the screening of women at an increased risk of breast cancer. To overcome these limitations, abbreviated DCE-MRI protocols have been introduced that substantially shorten image acquisition and interpretation time while maintaining a high diagnostic accuracy. Efforts to develop abbreviated MRI protocols reflect the increasing scrutiny on the disproportionate contribution of radiology to the rising overall healthcare expenditures. Healthcare policy makers are now focusing on curbing the use of advanced imaging examinations such as MRI while continuing to promote the quality and appropriateness of imaging. An important cornerstone of value-based healthcare defines value as the patient’s outcome over costs. Therefore, the concept of fast, abbreviated MRI exam is very appealing given its high diagnostic accuracy coupled with the possibility of marked reducing the cost of a MRI examination. Given recent concerns about gadolinium-based contrast agents, unenhanced MRI techniques such as DWI are also being investigated for breast cancer diagnosis. Although further larger prospective studies, standardized imaging protocol and reproducibility studies are necessary, initial results with abbreviated MRI protocols suggest that it seems feasible to offer screening breast DCE-MRI to a broader population. This article aims to give an overview of abbreviated and fast breast MRI protocols, their utility for breast cancer detection, and their emerging role in the new value-based healthcare paradigm that has replaced the fee-for-service model.
Keywords: Breast MRI, abbreviated MRI, breast cancer, gadolinium-based contrast agents, unenhanced, diffusion-weighted Imaging
Introduction
Breast cancer is one of the leading causes of death among women in the United States, with early detection of cancer being the key to improved prognosis and survival. Randomized controlled trials have found that screening mammography has decreased the mortality for breast cancer by 30% (1). However, with a sensitivity of approximately 70%, mammography has its limitations. Particularly in women with dense breasts, cancers might be occult on mammography (2). Magnetic resonance imaging (MRI) of the breast is undisputedly the most sensitive of imaging methods to detect cancer with a higher cancer detection rate than mammography, digital breast tomosynthesis and ultrasound. Adjunct screening with DCE-MRI was first recommended for women at high (>20%) lifetime risk of breast cancer (3–5), facilitating earlier cancer detection and reducing interval cancers (6–15) in this population. This prompted a most recent similar recommendation for its use in women at intermediate (>15%) lifetime risk of breast cancer (16). Meanwhile, for women at average risk of breast cancer, there is evidence that they might also benefit from screening MRI. Kuhl et al. investigated the utility of MRI as a supplemental screening tool in 2120 women at average breast cancer risk, and found that MRI depicted 60 additional breast cancers, while 12 of 13 incident cancers were found with MRI alone (17). Despite such encouraging results, breast MRI is currently not implemented in the screening of women at average risk of breast cancer, with the limiting factor being its relatively high direct and indirect costs and wide-spread availability compared with conventional imaging (18,19).
Apart from the initial purchase price of the MRI equipment, major drivers of the high costs of breast MRI are the relatively long acquisition limiting high-volume patient through-put and interpretation times involved in a full diagnostic protocol. In efforts to overcome these limitations and facilitate increased access to screening breast MRI, Kuhl et al. initially introduced an abbreviated breast MRI protocol for screening that substantially shortened examination and reading times; at the same time, this protocol maintained a diagnostic accuracy equivalent to a full diagnostic protocol (20). Thereafter, several other studies have investigated different abbreviated and high-temporal resolution MRI protocols for breast cancer diagnosis and screening with similar results (21–42). This review will provide an overview of the current published literature on abbreviated breast MRI and its potential clinical applications and challenges. We will also discuss the emerging roles of fast temporal resolution, unenhanced MRI strategies in an abbreviated MR protocol, and workflow issues to implement abbreviated MR exams into the clinical workflow.
Dynamic Contrast-enhanced MRI of the Breast
DCE-MRI allows the assessment of high-resolution breast morphology and enhancement kinetics to depict angiogenesis as a tumor-specific feature (43). At any given field strength, DCE-MRI is the most sensitive modality for breast cancer detection with a pooled sensitivity of 93%; DCE-MRI has good pooled specificity of 71% (5,6,44–46).
In high-risk women, several studies have demonstrated that DCE-MRI is the superior screening modality compared with conventional imaging techniques. Schrading et al. (47) demonstrated that MRI had both high specificity and positive predictive value. Obdeijn et al. (48) found a sensitivity of 93.6% for MRI alone in a population of 93 BRCA1 mutation-carriers, with mammography giving no additional value to MRI screening in women below the age of 40. Riedl et al. (49) demonstrated that MRI in high-risk patients had a sensitivity of 90% and almost half of all cancers (45%) in their cohort were detected by MRI only. Similar results were found by Krammer et al. (50).
Central to breast cancer detection using DCE-MRI is the combination of morphological features and functional information derived from enhancement kinetics; this need is supported by multiple studies and noted in the revised MR BI-RADS lexicon (51,52). There is consensus that the ideal breast MRI protocol should incorporate both a high spatial and high temporal resolution MRI protocol. Such a protocol enables simultaneous and accurate assessment of lesion morphology and lesion enhancement kinetics and optimizes sensitivity and specificity. However, these are two competing demands: a high spatial resolution protocol requires thinner slices for better delineation of breast morphology, ultimately leading to longer acquisition times (51). A high temporal resolution (acquisition speed) protocol requires faster acquisition speeds for a more accurate depiction of enhancement kinetics, leading to a tradeoff in the assessment of morphologic features.
In the clinical setting, breast DCE-MRI protocols attempt to balance between the two demands, with most protocols including a three-plane localizer sequence, T2-weighted or short TI inversion recovery (STIR) sequence, T1-weighted pre-contrast sequence, or three or more T1-weighted post-contrast sequences. Sequences are performed with or without fat saturation, while post-processing usually include subtraction and maximum intensity projection (MIP) images. Currently, full diagnostic protocols are used uniformly for indications such as pre-operative staging, detection of scar versus recurrence, response assessment and therapy monitoring of neoadjuvant chemotherapy, cancer of unknown primary and assessment of equivocal findings on conventional imaging (3,53,54). Currently, there is no recommendation on protocol adjustments when breast DCE-MRI is used solely for screening purposes (5,55).
Abbreviated MRI
As we mentioned, Kuhl et al. in 2014 were the first to report on the feasibility of an abbreviated breast MRI protocol for breast cancer screening, consisting of an unenhanced T1-weighted and first contrast-enhanced T1-weighted sequence, subtraction imaging and a single MIP image (20). This abbreviated protocol was generated from a full diagnostic protocol in 606 examinations of 443 women; the two protocols were compared regarding acquisition time, cancer yield and diagnostic accuracy (Figure 1). This groundbreaking study found that image acquisition and interpretation time could be reduced without having a negative impact on diagnostic accuracy. With the abbreviated MRI protocol, the acquisition time was substantially decreased to 3 minutes, compared with 17 minutes for the full diagnostic protocol. The interpretation time of the abbreviated protocol was 28 seconds on average and 2.8 seconds when the MIP image alone was evaluated (the interpretation time for the full protocol was not measured). If compared with mammography, the average interpretation time for the abbreviated MRI protocol proved to be much faster than the interpretation of screening mammography of 2–4 minutes (56). Of 606 MRI studies, all 11 cancers were found with equivalent diagnostic accuracy for the abbreviated and full protocols, while evaluation of the MIP image alone missed one cancer.
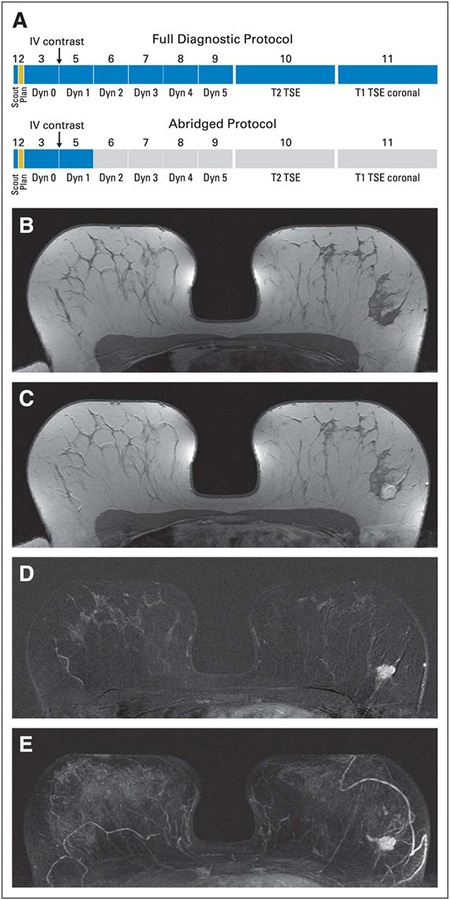
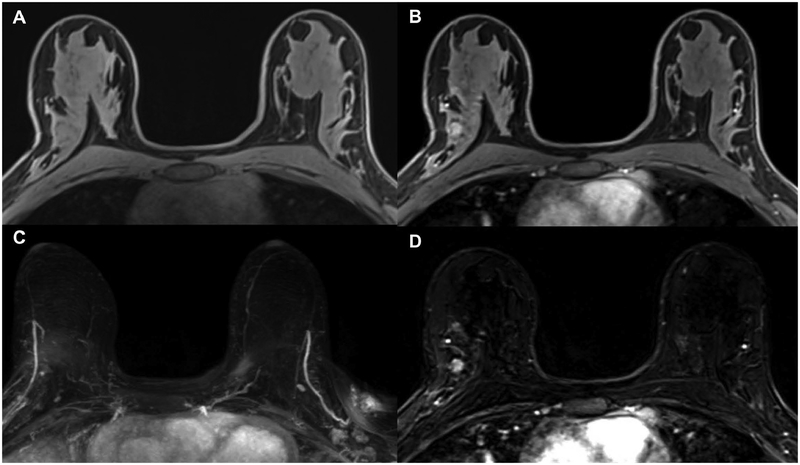
Figure 1.
(A) Schematic presentation of full diagnostic and abbreviated protocols and (B to E) clinical example of calculation of first postcontrast subtracted (FAST) and maximum intensity projection (MIP) images in patient with left-sided breast cancer. (B) Midbreast section of baseline (precontrast) dynamic acquisition (A; Dyn 0); (C) corresponding section of first postcontrast dynamic acquisition (A; Dyn 1); (D) corresponding FAST image, generated by subtracting image (B) from image (C); and (E) MIP image, generated by fusing all FAST sections into single three-dimensional–like projection image. Scout is automatic survey. Dyn refers to single dynamic acquisition consisting of image stack of 27 to 33 individual axial images; Dyn 0 is baseline dynamic acquisition, obtained before contrast agent injection, and Dyn 1 to Dyn 5 are five consecutive dynamic acquisitions obtained after contrast injection. IV, intravenous; TSE, turbo spin echo. Reprinted with permission from: Kuhl CK, Schrading S, Strobel K, et al. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–10.
The initial findings by Kuhl et al. were supported by a study by Mango et al. who in 2015 reported the feasibility of an abbreviated protocol consisting of fat-saturated T1-weighted pre-contrast, early post-contrast T1, and subtraction MIP sequences (38). All 100 cancers of known breast cancer patients were found by at least one of four readers, with a sensitivity of 96% for the first post-contrast image, 96% for the first post-contrast subtraction image, and 93% for the MIP image (Figure 2). Both the Kuhl et al. and Mango et al. studies suggest that the MIP image alone is not sufficient for the detection of breast cancer or an accurate BI-RADS assessment; however, this might be accomplished using only the first post-contrast sequence. Similar to the Kuhl et al. study, the Mango et al. study yielded substantially decreased image acquisition and interpretation times of 10–15 minutes and 44 seconds, respectively.
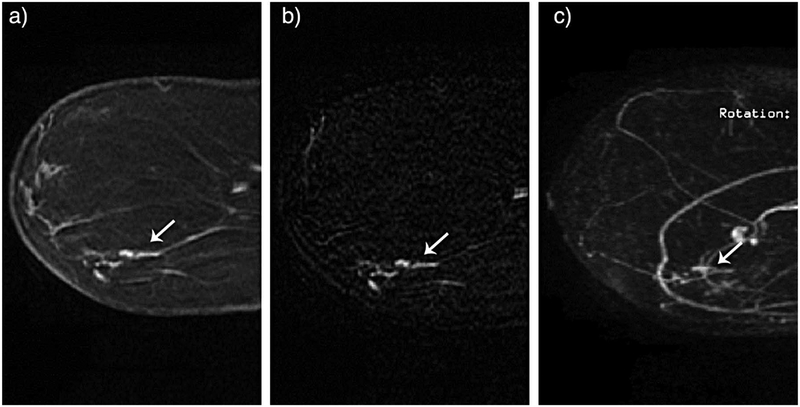
Figure 2.
54-year-old female for high risk screening. New linear nonmass enhancement in the lower outer left breast measuring 1.9 cm. MRI guided core biopsy yielded DCIS with microinvasion. Cancer recognized by all 4 radiologists on reading of the abbreviated protocol. First post-contrast sequence (A), first post-contrast subtraction sequence (B) and subtraction maximum intensity projections (MIP) sequence (C). Reprinted with permission from: Mango VL, Morris EA, Dershaw D, et al. Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur J Radiol. 2015;84(1):65–70.
Since these initial studies, numerous others have investigated the use of different abbreviated MRI protocols for breast cancer screening and the assessment of known cancer lesions. Moschetta et al. found no significant differences in diagnostic accuracy between a full and an abbreviated protocol including STIR, T2-weighted sequences, a pre-contrast and single post-contrast T1-weighted sequence (36). Dogan et al. investigated an abbreviated high-risk screening protocol consisting of T2-weighted imaging and Dixon sequence for DCE-MRI, yielding the same image quality equivalent to a separate full protocol (26). Choi et al. evaluated the usefulness of an abbreviated MRI protocol with fat-suppressed T2-weighted imaging, pre- and post-contrast T1-weighted images, and subtracted MIPs in 799 examinations for screening of 725 women with a history of breast cancer. In this study, 12 malignancies where detected with 5 lesions visible on MRI alone (25).
Whereas the abovementioned studies included T2-weighted or STIR sequences in their protocols, others solely relied on DCE-MRI. Petrillo et al. investigated an abbreviated protocol including one pre-contrast T1-weighted and the first post-contrast T1-weighted series in 508 patients, yielding similar results with comparable diagnostic accuracy of the abbreviated and the full protocols (32). Romeo at al. used a protocol that included localizer, pre-contrast, and three time-point post-contrast sequences to enable the assessment of the delayed enhancement. This abbreviated protocol yielded comparable results to a full diagnostic breast MRI protocol (AUC 0.98 vs. 0.99; p=0.76) (29).
A major advantage of breast MRI is that it is independent of breast density, which is a limiting factor of screening with mammography. In this context, Chen et al. investigated an abbreviated protocol with T1-weighted pre-, post and MIP images in 478 patients with dense breasts. There was no significant difference in cancer yield between the abbreviated and full diagnostic protocol (31). Currently, the ECOG-ACRIN 1141 Trial (57), “Comparison of Abbreviated Breast MRI and Digital Breast Tomosynthesis in Breast Cancer Screening,” is ongoing. This prospective randomized phase II trial aims to compare abbreviated breast MRI exam and digital breast tomosynthesis for detecting cancer in women with dense breasts. This trial has been designed to investigate abbreviated breast MRI for the screening of average-risk women with dense breasts and thus results are highly anticipated.
Given these different abbreviated MRI protocols, efforts have been made to identify the sequences that are necessary for a confident breast cancer diagnosis. Strahle et al. used a full diagnostic protocol in 452 lesions to prospectively identify the most important sequences for breast MRI screening (33). They concluded that T2-weighted, T1-weighted pre-contrast, first and late post-contrast images are necessary and enable a rapid breast cancer screening with a scan time of 7.5 minutes. Heacock et al. compared three different protocols in 107 cancers and found that T2-weighted imaging increased lesion conspicuity without altering the cancer detection rate (24).
Recently, other aspects of using abbreviated breast MRI protocols have been investigated. In a recent study, Panigrahi et al. assessed the impact of an abbreviated protocol on the assigned BI-RADS assessment category. Comparison of a full diagnostic protocol to an abbreviated protocol in 1052 cases demonstrated that the full protocol resulted in a change in final BI-RADS assessments in only 3.4% of the cases (30).
In the studies reviewed, the abbreviated protocols consisted of unenhanced and first contrast-enhanced T1-weighted sequences, while some of them additionally included T2-weighted, STIR, second contrast-enhanced, subtraction and MIP images. The different studies and protocols are summarized in Table 1. While the time to interpret images was also substantially reduced using the abbreviated protocol in most studies (34,36), only one study showed no difference in interpretation time between a full and an abbreviated protocol (37). In almost all the studies reviewed, the short acquisition time and fast image interpretation of varying abbreviated protocols had no negative effect on diagnostic accuracy (20,36,38) (Figures 3, 4 and 5).
Table 1. Abbreviated MRI Protocols used in 21 studies.
Note: Abbreviated protocols 2 and 3 were read by the same reader using the different additional sequences and/or available prior imaging.
| Reference | Abbreviated Protocol 1 | Abbreviated Protocol 2 | Abbreviated Protocol 3 |
|---|---|---|---|
| Platel et al. 2014 | Five standard and twenty ultrafast view-sharing contrast-enhanced T1-weighted images | ||
| Kuhl et al. 2014 | Unenhanced T1-weighted imaging without fat saturation First contrast-enhanced T1-weighted imaging without fat saturation Subtraction MIP |
||
| Mann et al. 2014 | Contrast-enhanced TWIST T1-weighted imaging | ||
| Mango et al. 2015 | Unenhanced fat-saturated T1-weighted imaging First contrast-enhanced fat-saturated T1-weighted imaging Fat-saturated T1-weighted imaging Subtraction MIP |
||
| Grimm et al. 2015 | Fat-saturated T2-weighted imaging Unenhanced fat-saturated T1-weighted imaging First contrast-enhanced fat-saturated T1-weighted imaging |
Abbreviated 1 protocol + Second contrast-enhanced fat-saturated T1-weighted imaging |
|
| Harvey et al. 2016 | Unenhanced fat-saturated T1-weighted imaging Contrast-enhanced fat-saturated T1-weighted imaging Subtraction MIP |
||
| Heacock et al. 2016 | Unenhanced fat-saturated T1-weighted imaging First contrast-enhanced fat-saturated T1-weighted Subtraction No previous imaging |
Abbreviated protocol 1 + With previous imaging |
Abbreviated protocol 1 + With previous imaging + Fat-saturated T2-weighted imaging |
| Moschetta et al. 2016 | STIR Turbo spin-echo T2-weighted imaging Unenhanced THRIVE Intermediate contrast-enhanced THRIVE (3 min after gadolinium injection) |
||
| Pineda et al. 2016 | Contrast-enhanced fat-saturated T1-weighted imaging with high spatial and temporal resolution | ||
| Abe et al. 2016 | Eight high-temporal-resolution and four standard contrast-enhanced T1-weighted images | ||
| Machida et al. 2017 | Unenhanced fat-saturated T1-weighted imaging First contrast-enhanced fat-saturated T1-weighted imaging |
||
| Chen et al. 2017 | Subtraction MIP |
||
| Strahle et al. 2017 | T2-weighted imaging Unenhanced fat-saturated T1-weighted imaging Early contrast-enhanced fat-saturated T1-weighted imaging Late contrast-enhanced fat-saturated T1-weighted imaging |
||
| Petrillo et al. 2017 | Unenhanced fat-saturated T1-weighted imaging First contrast-enhanced fat-saturated T1-weighted imaging |
||
| Panigrahi et al. 2017 | Unenhanced fat-saturated T1-weighted imaging First contrast-enhanced fat-saturated T1-weighted imaging Subtraction MIP |
||
| Romeo et al. 2017 | Unenhanced fat-saturated T1-weighted imaging Contrast-enhanced fat-saturated T1-weighted imaging subtractions at 3 time points |
||
| Oldrini et al. 2017 | MIP | Contrast-enhanced TRICKS acquisitions at 12 time points + MIP |
Contrast-enhanced TRICKS acquisitions at 12 time points + Unenhanced fat-saturated T1-weighted imaging + T2-weighted imaging + MIP |
| Choi et al. 2018 | T2-weighted imaging Unenhanced fat-saturated T1-weighted imaging Contrast-enhanced fat-saturated T1-weighted imaging MIP |
||
| Dogan et al. 2018 | T2-weighted imaging Contrast-enhanced fat-saturated T1-weighted imaging |
||
| Oldrini et al. 2018 | Unenhanced fat-saturated T1-weighted imaging First contrast-enhanced fat-saturated T1-weighted imaging Subtraction |
||
| Jimenez et al. 2018 | Contrast-enhanced T1-weighted STELLR imaging |
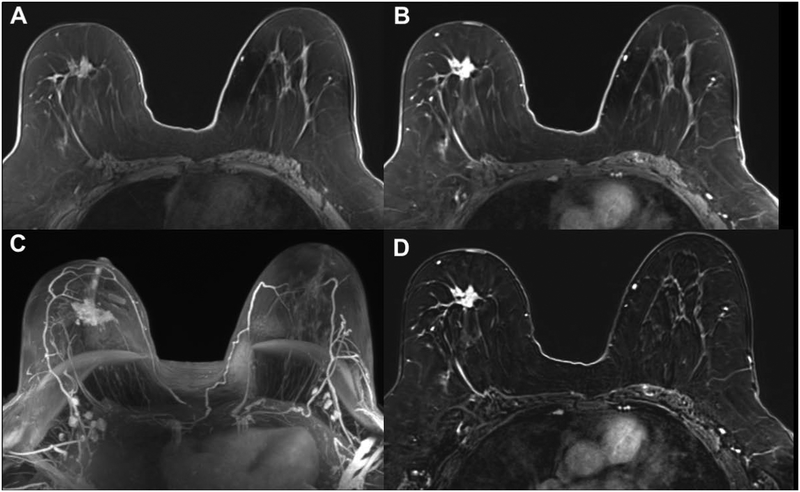
Figure 3.
Invasive lobular carcinoma (ILC) in the right breast of a 59-year-old patient. The irregular, spiculated 3.5 cm mass (A) demonstrates heterogeneous contrast enhancement on early contrast-enhanced axial T1W (B), maximum intensity projections (MIP) (C), and subtraction images axial T1W post-contrast (D) and was accurately diagnosed with an abbreviated MRI protocol.
Figure 4.
29-year-old patient with biopsy-proven breast cancer in the right breast. Abbreviated MRI of the breast including unenhanced (A), early contrast-enhanced axial T1W (B), maximum intensity projections (MIP) (C) and subtraction images axial T1W post-contrast (D) was useful for the assessment of the true extent of disease, revealing multiple contrast-enhancing satellite lesions in the right breast.
Figure 5.
Fibroadenoma in the right breast in a 26-year-old patient with a BRCA2 gene mutation. On high risk screening MRI there is a 0.9 cm mass lesion in the right breast, showing homogeneous contrast enhancement with a type I enhancement curve in the post contrast axial T1W (B) and subtraction images axial T1W post-contrast (D). This benign lesion was not depicted on maximum intensity projections (MIP) images (C).
To date, the abbreviated breast MRI exam has been performed in eight different countries and in over 4,500 women. A shared finding in almost all abbreviated MRI studies is that the cancers detected were mainly early stage invasive cancers with percentages ranging from 64% to 97%. The ductal carcinomas in situ (DCIS) detected with abbreviated MRI screening were predominately intermediate or high-grade DCIS (20,22,24,26,29,30,32,34,35,38). This is in good agreement with a recent study by Sung et al. who found that in women at high breast cancer risk who underwent screening with both mammography and MRI, invasive cancers were more likely to be detected with MRI, while most cancers found with mammography were DCIS (58). All these studies highlight that MRI using an abbreviated protocol is poised to be a supplemental screening tool designed to detect mammographically occult and biologically relevant breast cancer (59).
Kinetic Information with High Temporal Resolution Protocols
The shortened acquisition time of abbreviated MRI protocols comes at the expense of kinetic information of breast tumors. However, the consensus is that kinetic information over time in addition to tumor morphology adds valuable information and increases specificity. Benign lesions typically show an initial slow or medium/delayed persistent enhancement, while an initial fast/delayed washout is highly indicative of malignancy. A full diagnostic protocol that includes several time points after contrast agent application would allow the semi-/quantitative assessment of tumor enhancement kinetics over time; however, an abbreviated MRI protocol that typically includes only one post-contrast scan would not allow such an assessment.
To overcome this limitation of abbreviated MRI protocols, several studies have explored the utility of shortened MRI protocols performed with imaging acceleration techniques (60). Acceleration techniques decrease acquisition time while maintaining high spatial resolution (61). Hence, high temporal resolution can be used for the extraction of kinetic information without a tradeoff in high spatial resolution and diagnostic confidence.
View-sharing is an important acceleration technique, where k-space periphery is undersampled, and data from different k-spaces are used to achieve high spatial resolution while contrast is measured in the center of k-space (62). A commonly used view-sharing sequence for acquisition of both high temporal and spatial resolution imaging is time-resolved angiography with stochastic trajectory (TWIST) (63,64). Mann et al. used a TWIST-based DCE-MRI protocol, comparing the diagnostic performance of the maximum slope of enhancement with a conventionally acquired kinetic curve (41). Their protocol shortened imaging time while maintaining cancer yield and diagnostic accuracy, suggesting that delayed phase of the kinetic curve might not be needed for an accurate lesion detection and classification (Figures 6 and 7). Platel et al. found similar results when using a computer-aided diagnosis system (40).
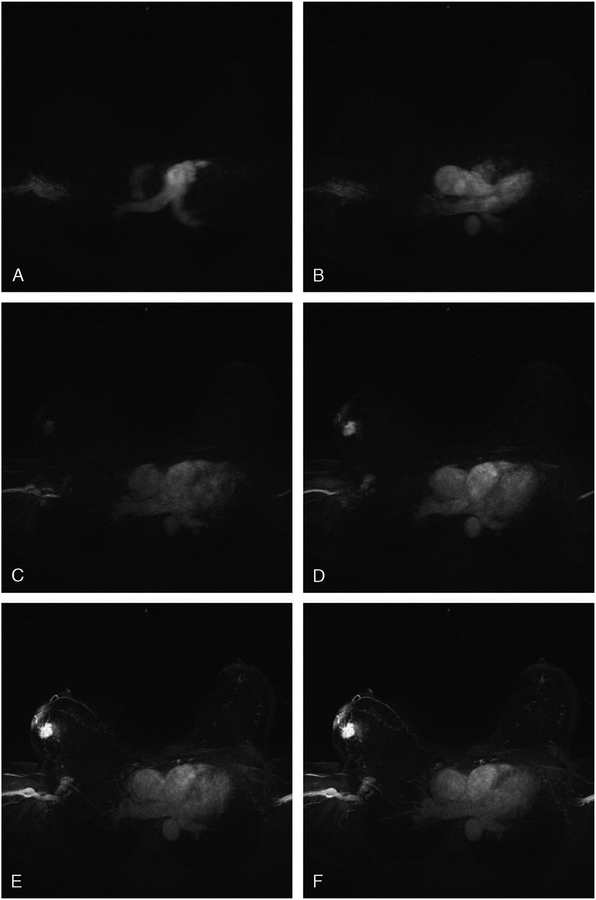
Figure 6.
Selected stills (maximum intensity projections) from a movie of contrast inflow. In A, only the pulmonary artery enhances. In B, the contrast has reached the aorta. In C, the tumor in the right breast starts to enhance, just as the overlying infiltrated skin. In D, the tumor stands out like a light bulb in a further empty breast. In E, the draining veins become visible, and in F, minimal normal glandular tissue enhancement is seen. Reprinted with permission from: Mann RM, Mus RD, van Zelst J, et al. A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol. 2014;49(9):579–85.
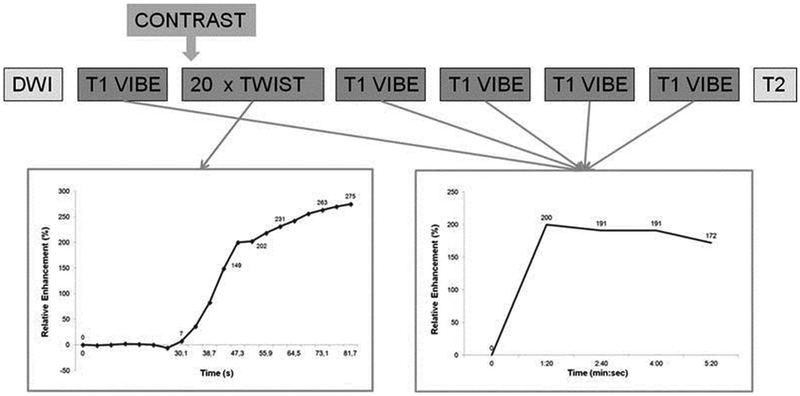
Figure 7.
Schematic drawing of the breast MRI scan protocol: The TWIST acquisitions allow evaluation of the contrast inflow in the lesion, whereas the VIBE acquisitions are used for 3 time point analysis, creating the classic contrast enhancement versus time curve. Reprinted with permission from: Mann RM, Mus RD, van Zelst J, et al. A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol. 2014;49(9):579–85.
Pineda et al. first explored a different acceleration technique using Fourier sampling and a high sensitivity-encoding (SENSE) acceleration factor (39). They demonstrated that lesion conspicuity was highest on early images in the first minute after contrast administration, with significant differences in time of arrival between malignant and benign lesions. A follow-up study by Abe et al. confirmed these initial results and demonstrated that fast MRI is at least equal in diagnostic performance compared with standard diagnostic MRI protocols (42).
Most recently, Jimenez et al. developed a volumetric imaging technique with 0.8-mm isotropic resolution and 10-s/volume rate for breast cancer using parallel imaging and spatial compressed sensing. This approach allowed the assessment of lesion morphology and early-phase perfusion with a total scan time of only 6 minutes. The authors conclude that upon further validation, this technique may translate to high-performance, rapid breast cancer screening with MRI (21).
Oldrini et al. evaluated time resolved imaging of contrast kinetics (TRICKS) acquisitions compared with an abbreviated as well as a full diagnostic protocol (23). The authors achieved an improved specificity when TRICKS acquisitions were added to the abbreviated protocol, outperforming the full protocol.
In summary, the results of these studies indicate that even with abbreviated breast MRI protocols, the necessary kinetic information for an accurate breast cancer diagnosis can be provided along with morphologic information.
Beyond Contrast
Due to its excellent sensitivity, DCE-MRI is currently the backbone of any given breast MRI protocol. Most recently, it has been demonstrated that the administration of gadolinium-based contrast agents (GBCAs) can cause MRI signal changes in the deep nuclei of the brain. While some linear contrast agents seem to cause greater MRI signal changes than some macrocyclic GBCAs, deposition of gadolinium has nevertheless been observed for macrocyclic GBCAs (65–73). To date, the clinical significance of the brain retention of GBCAs is unknown and there is no scientific evidence of adverse clinical effect. Nevertheless, this recent controversy about the safety of GBCAs has sparked the recommendation to use GBCAs only when essential diagnostic information cannot be obtained with unenhanced scans (66,74). This is particularly relevant for the field of breast imaging where healthy women are screened for breast cancer with DCE-MRI. Therefore, there are considerable efforts to develop unenhanced MRI techniques with equal sensitivity for breast MRI screening.
Amongst all investigated unenhanced MRI techniques, diffusion-weighted imaging (DWI) has currently emerged as the most robust and valuable. DWI using apparent diffusion coefficient (ADC) mapping is reported to have a sensitivity of up to 96% for breast cancer detection and a specificity of up to 100% for breast tumor characterization (75–77). Given the fact that DWI is robust, reliable and fast to perform with scan times usually ranging from 2–3 minutes, the use of DWI for abbreviated unenhanced screening is of interest.
Several authors have investigated abbreviated unenhanced protocols with different combinations of T1-weighted and/or T2-weighted with either DWI or DWI with background suppression, with encouraging results (64,78–83). Shin et al. investigated an abbreviated protocol using either an unenhanced protocol including high b-value DWI and T1-weighted imaging or an enhanced protocol including early DCE-MRI sequences in 129 lesions; both abbreviated protocols achieved similar detection rates and diagnostic accuracy (80). Baltzer et al. compared a DWI protocol with a DCE-MRI and T2-weighted imaging protocol; both protocols achieved similar results with high diagnostic performance and inter-reader agreement (84) (Figure 8). Bickelhaupt et al. compared an unenhanced diagnostic abbreviated MRI protocol (consisting of maximum intensity projections from DWI with background suppression and unenhanced morphologic sequences with an acquisition time of less than 7 minutes) with an abbreviated DCE-MRI protocol as well as a full diagnostic MRI protocol to predict the likelihood of malignancy in patients with a suspicious finding on screening mammography. In this study, the abbreviated unenhanced MRI protocol was able to exclude malignancy in these patients with an negative predictive value of 0.92 (83). In summary, in all these studies except for one study (79), the sensitivity of abbreviated unenhanced MRI was equal (78) or superior to mammography (64,80–83).
Figure 8.
Comparison of morphologic assessment on NC-MRI and CE-MRI. Panel A shows a circumscribed round lesion on the b850 s/mm2 image (dashed arrow) while panel B visualizes a noncircumscribed, rather spiculated lesion on the b850 s/mm2 image (arrow). The lesion from panel A appears with circumscribed margins on the early contrast-enhanced subtraction (C, dashed arrow) and was histologically proven as a fibroadenoma while the lesion from panel B appears noncircumscribed with heterogeneous internal structure and a feeding vessel on the early contrast-enhanced subtraction (D, arrow) and corresponds to an invasive ductal carcinoma G2. Reprinted with permission from: Baltzer PAT, Bickel H, Spick C, et al. Potential of Noncontrast Magnetic Resonance Imaging with Diffusion-Weighted Imaging in Characterization of Breast Lesions: Intraindividual Comparison With Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Invest Radiol. 2018;53(4):229–235.
While these results are encouraging and highlight the potential of DWI as a promising MRI technique for an abbreviated unenhanced protocol, a recent study comparing DCE-MRI, DWI as a stand-alone parameter for breast cancer detection, and combined DCE-MRI and DWI, demonstrated that DCE-MRI remains the most sensitive protocol for breast cancer detection. A current limitation of DWI is that its sensitivity is limited in lesions smaller in lesions ≤12mm or presenting as diffuse NME (63) (Figure 9). However, research to improve the spatial resolution of DWI is ongoing. Hence, it can be expected that further advances are possible to overcome its current limitations. Several studies have demonstrated that the combination of DCE-MRI and DWI maintains a high sensitivity, increases specificity and maximizes diagnostic accuracy (44) (Figure 10); therefore, it seems that there is also potential for the application of abbreviated MRI protocols with combined DCE-MRI and DWI in breast cancer screening.
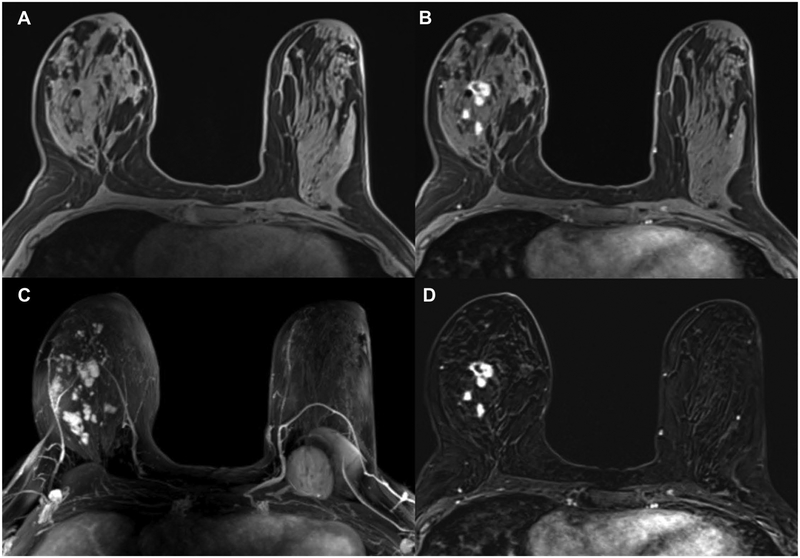
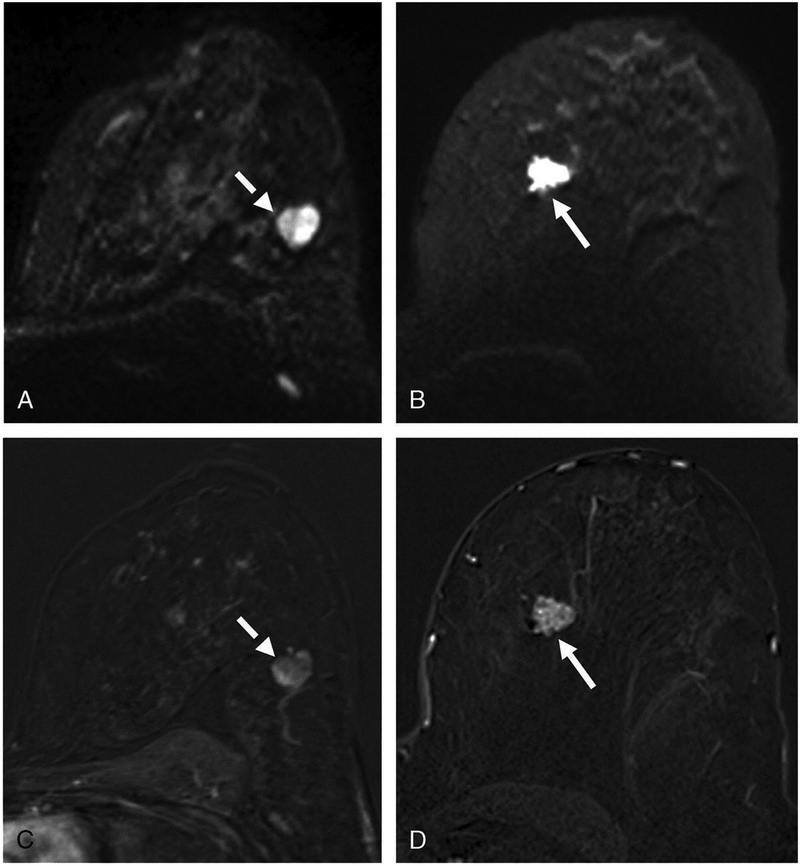
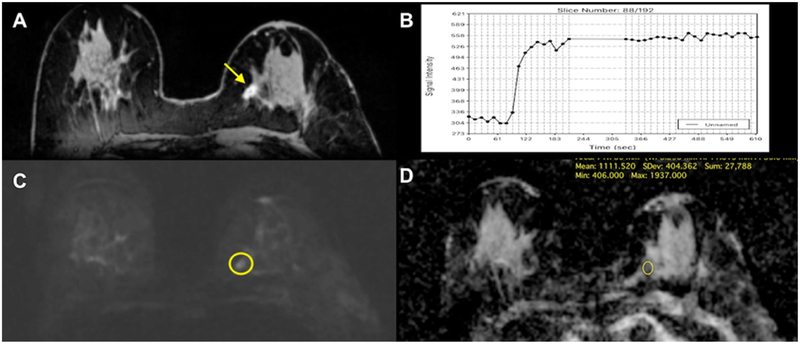
Figure 9.
Invasive ductal carcinoma (IDC) G1 in the left breast medial in a 71-year-old woman. (A) On DCE-MRI there is a 10mm irregular-shaped and marginated lesion (arrow) with (B) an initial fast/plateau enhancement (II); DCE-MRI findings were classified as suspicious for malignancy (BI-RADS 4). DWI was false negative as none of the readers called this lesion on DWI alone. However, when read as mpMRI combining DCE-MRI and DWI, readers identified a (C) hyperintense correlate (circle) with (D) ADC values measuring 1.111×10–3 mm2/s, which further confirmed malignancy.
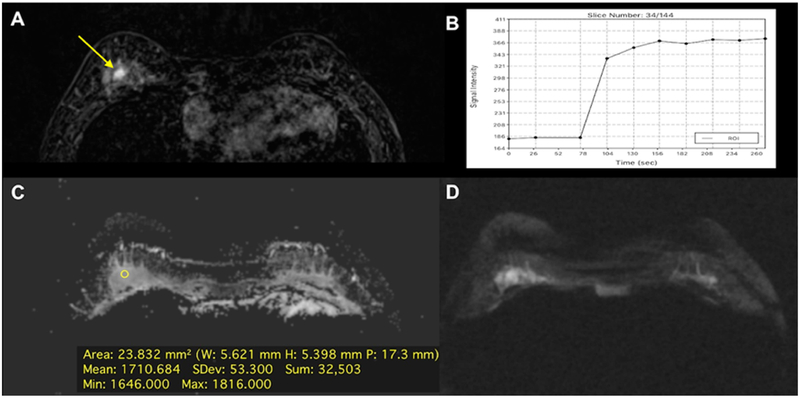
Figure 10.
Fibroadenoma in a 39-year-old woman, central in the right breast: (A) The irregular-shaped and partially irregularly marginated 7 mm mass demonstrates (B) an initial medium/persistent (II) slightly homogenous contrast enhancement and was classified as suspicious (BI-RADS 4). (C) On DWI there is no focal restricted diffusivity and (D) no decreased ADC values (1.710 ×10–3 mm2/s). DCE-MRI and DWI were discordant. According to the BI-RADS-adapted reading algorithm, the BI-RADS assessment category assigned based on DCE-MRI was overruled. Multiparametric MRI correctly classified the mass as benign and would have obviated an unnecessary breast biopsy.
Abbreviated MRI as a Valuable Initiative
The abbreviated MRI exam has been embraced in the radiology community as an important and valuable initiative. Current studies focusing on the role of MRI in screening and surveillance have developed specialized protocols that are often abbreviated with applications in the prostate and liver (85–92). Variable imaging protocols have been used and in general show a similar diagnostic performance compared with the full protocol for the detection of occult malignancies. Other novel applications include using abbreviated MRI in the pediatric population to decrease the duration of necessary sedation, and as a rapid and accurate imaging tool in the musculoskeletal setting to detect fractures and increase patient through-put in an emergency setting (93–95).
The above studies reflect the increasing scrutiny on the disproportionate contribution of radiology to the rising overall healthcare expenditures. As a result, value-based healthcare is the new paradigm that has quickly replaced the fee-for-service model (96–101). Healthcare policy makers have focused on curbing the use of advanced imaging examinations, especially CT and MRI, while promoting the quality and appropriateness of imaging. These policy changes have led to a variety of new metrics that are being imposed on radiology providers. Further, clinical decision support tools have been introduced, many of which focus on the limiting the use of these advanced imaging tools.
An important cornerstone of value-based healthcare is that value is defined as the patient’s outcome over costs (101). A correct diagnosis is the first outcome that matters to patients, and new metrics have been developed to measure the radiologist’s impacts on patient outcomes. These metrics will measure a radiologist’s contribution to reducing costs and improving patient outcomes with the intention of making reimbursement commensurate with adherence to these metrics (96). The concept of a fast, abbreviated MRI exam is therefore appealing given its high diagnostic accuracy coupled with the possibility of marked reducing the cost of a MRI examination. In addition, abbreviated MRI exams will enable higher volume patient throughput thereby generating more revenue and being more cost-effective.
Multiple investigators are evaluating if these rapid MRI exams are cost-effective enough to provide the initial diagnosis (20,22,25,27–31,33–38,83,85,86,93). In breast imaging, one of the goals is to evaluate if an abbreviated breast MRI may be a first-line exam and compete with other imaging exams such as 2D screening mammograms, digital breast tomosynthesis, and whole breast screening ultrasound exam. Current studies are limited by the heterogeneous imaging protocol and variable inclusion criteria for the patients. Most studies have shown a similar diagnostic accuracy to a full MRI exam. Will it be acceptable if an abbreviated MR exam is not as thorough as a standard MR exam, but good enough to triage patients more cost effectively? This topic is currently subject to intense research, aided by new technologies such as synthetic MRI reconstruction, MR fingerprinting and the use of deep learning tools for MR reconstruction.
Challenges and Outlook
An unresolved issue is the reimbursement for an abbreviated breast MRI exam and the way it will be implemented into the clinical workflow. This limitation applies to abbreviated protocols that are being evaluated for all organ systems, including the breasts. To our knowledge, the cost of an abbreviated MRI exam has yet to be determined because such an exam is currently being evaluated with respect to feasibility and/or is being validated by other studies. Therefore, important issues such as the length of the abbreviated MRI exam and whether gadolinium contrast agent is necessary need to be resolved before the issue of the cost of the exam may be addressed. From an operations viewpoint, it is clear that ‘scan time’ is not equivalent to ‘table time’. This information is critical to estimating the price point for an abbreviated MR exam. Operational improvements to maximize patient through put haven’t been explored since abbreviated exams are mainly performed in the research setting. Novel ideas, such as multi-head MRI scanners will improve the efficiencies of the abbreviated MRI exam.
Paving the way to address the issue of cost is the ECOG-ACRIN 1141 Trial (57) to compare abbreviated breast MRI and digital breast tomosynthesis for the detection of breast cancer in average-risk women with dense breasts. The primary aim of the study is to compare the invasive cancer detection rates of both exams. One of the secondary aims of this multicenter study is to perform a comparative cost analysis of both exams. Although the hypothetical cost of an abbreviated breast MRI is still being explored, a reasonable number would be a low-cost exam that is competitive with other breast imaging screening exams such as mammography, digital breast tomosynthesis and screening breast ultrasound.
Although results from previous studies investigating abbreviated MRI protocols are promising, they might not be generalizable to a broad population. To date, these protocols have only been investigated for breast cancer screening and still have to be evaluated for their value in the diagnostic setting or for neoadjuvant chemotherapy response assessment. In addition, the calculated time for these protocols may not reflect the entire time of the examination as there are other workflow considerations such as setup, room turnover, safety screening, IV placement, that must be considered. However, with training of personnel and work-flow optimization an abbreviated MRI examination will still be substantially shorter than one with a full protocol and allow higher volume patient throughput. Another aspect that needs to be considered is that although in the study setting reading times can be substantially shortened, it has to be seen who this will translate into clinical reality where an MRI interpretation may also include reviewing patient’s history and priors.
Abbreviated MRI protocols still need refinement, standardization across sites and validation by prospective multicenter trials before they can be implemented into clinical routine. Nevertheless, it can be expected that abbreviated MRI protocols will play an important role in breast imaging in the future.
Conclusion
MRI of the breast is undisputedly the most sensitive test for breast cancer detection and outperforms conventional imaging with mammography, tomosynthesis and ultrasound yet to date is only routinely implemented in the screening of women at an increased risk of breast cancer. Its widespread use as a screening tool for women at average risk of breast cancer is currently limited by its relatively high costs, long examination and reading times. Recently, abbreviated DCE-MRI protocols have been introduced that substantially shorten image acquisition and interpretation time, allow higher volume patient throughput thus being more cost-effective while maintaining a high diagnostic accuracy. With respect to the controversy to address concerns that DCE-MRI may cause unnecessary costly breast biopsies, unenhanced MRI parameters such as DWI are under investigation to be added to abbreviated MRI protocols to mitigate this limitation. Although further larger-scale studies and rigorous standardization is necessary, initial results suggest that it seems feasible to offer a cost-effective screening breast DCE-MRI to a broader population.
Acknowledgements:
The authors acknowledge the support in manuscript writing and editing from Joanne Chin.
Grant Support:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 of Memorial Sloan Kettering Cancer Center, the Breast Cancer Research Foundation grant of Memorial Sloan Kettering Cancer Center, and through the NIH P41 EB017183 of New York University,
References
- 1.Tabar L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011;260(3):658–663. [DOI] [PubMed] [Google Scholar]
- 2.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005;353(17):1773–1783. [DOI] [PubMed] [Google Scholar]
- 3.Mann RM, Balleyguier C, Baltzer PA, et al. Breast MRI: EUSOBI recommendations for women’s information. Eur Radiol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. European journal of cancer 2010;46(8):1296–1316. [DOI] [PubMed] [Google Scholar]
- 5.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 6.Sardanelli F, Podo F, Santoro F, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk italian 1 study): final results. Invest Radiol 2011;46(2):94–105. [DOI] [PubMed] [Google Scholar]
- 7.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004;292(11):1317–1325. [DOI] [PubMed] [Google Scholar]
- 8.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004;351(5):427–437. [DOI] [PubMed] [Google Scholar]
- 9.Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005;23(33):8469–8476. [DOI] [PubMed] [Google Scholar]
- 10.Lehman CD, Blume JD, Weatherall P, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer 2005;103(9):1898–1905. [DOI] [PubMed] [Google Scholar]
- 11.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307(13):1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freitas V, Scaranelo A, Menezes R, Kulkarni S, Hodgson D, Crystal P. Added cancer yield of breast magnetic resonance imaging screening in women with a prior history of chest radiation therapy. Cancer 2013;119(3):495–503. [DOI] [PubMed] [Google Scholar]
- 13.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 2005;365(9473):1769–1778. [DOI] [PubMed] [Google Scholar]
- 14.Passaperuma K, Warner E, Causer PA, et al. Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer 2012;107(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol 2011;29(13):1664–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. Journal of the American College of Radiology : JACR 2018;15(3 Pt A):408–414. [DOI] [PubMed] [Google Scholar]
- 17.Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental Breast MR Imaging Screening of Women with Average Risk of Breast Cancer. Radiology 2017;283(2):361–370. [DOI] [PubMed] [Google Scholar]
- 18.Cott Chubiz JE, Lee JM, Gilmore ME, et al. Cost-effectiveness of alternating magnetic resonance imaging and digital mammography screening in BRCA1 and BRCA2 gene mutation carriers. Cancer 2013;119(6):1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowry KP, Lee JM, Kong CY, et al. Annual screening strategies in BRCA1 and BRCA2 gene mutation carriers: a comparative effectiveness analysis. Cancer 2012;118(8):2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 2014;32(22):2304–2310. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez JE, Strigel RM, Johnson KM, Henze Bancroft LC, Reeder SB, Block WF. Feasibility of high spatiotemporal resolution for an abbreviated 3D radial breast MRI protocol. Magn Reson Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oldrini G, Derraz I, Salleron J, Marchal F, Henrot P. Impact of an abbreviated protocol for breast MRI in diagnostic accuracy. Diagn Interv Radiol 2018;24(1):12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldrini G, Fedida B, Poujol J, et al. Abbreviated breast magnetic resonance protocol: Value of high-resolution temporal dynamic sequence to improve lesion characterization. Eur J Radiol 2017;95:177–185. [DOI] [PubMed] [Google Scholar]
- 24.Heacock L, Melsaether AN, Heller SL, et al. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: Correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol 2016;85(4):815–823. [DOI] [PubMed] [Google Scholar]
- 25.Choi BH, Choi N, Kim MY, Yang JH, Yoo YB, Jung HK. Usefulness of abbreviated breast MRI screening for women with a history of breast cancer surgery. Breast Cancer Res Treat 2018;167(2):495–502. [DOI] [PubMed] [Google Scholar]
- 26.Dogan BE, Scoggins ME, Son JB, et al. American College of Radiology-Compliant Short Protocol Breast MRI for High-Risk Breast Cancer Screening: A Prospective Feasibility Study. AJR Am J Roentgenol 2018;210(1):214–221. [DOI] [PubMed] [Google Scholar]
- 27.Sheth D, Abe H. Abbreviated MRI and Accelerated MRI for Screening and Diagnosis of Breast Cancer. Top Magn Reson Imaging 2017;26(5):183–189. [DOI] [PubMed] [Google Scholar]
- 28.Kuhl CK. Abbreviated breast MRI for screening women with dense breast: The EA1141 Trial. Br J Radiol 2017:20170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romeo V, Cuocolo R, Liuzzi R, et al. Preliminary Results of a Simplified Breast MRI Protocol to Characterize Breast Lesions: Comparison with a Full Diagnostic Protocol and a Review of the Current Literature. Acad Radiol 2017;24(11):1387–1394. [DOI] [PubMed] [Google Scholar]
- 30.Panigrahi B, Mullen L, Falomo E, Panigrahi B, Harvey S. An Abbreviated Protocol for High-risk Screening Breast Magnetic Resonance Imaging: Impact on Performance Metrics and BI-RADS Assessment. Acad Radiol 2017;24(9):1132–1138. [DOI] [PubMed] [Google Scholar]
- 31.Chen SQ, Huang M, Shen YY, Liu CL, Xu CX. Application of Abbreviated Protocol of Magnetic Resonance Imaging for Breast Cancer Screening in Dense Breast Tissue. Acad Radiol 2017;24(3):316–320. [DOI] [PubMed] [Google Scholar]
- 32.Petrillo A, Fusco R, Sansone M, et al. Abbreviated breast dynamic contrast-enhanced MR imaging for lesion detection and characterization: the experience of an Italian oncologic center. Breast Cancer Res Treat 2017;164(2):401–410. [DOI] [PubMed] [Google Scholar]
- 33.Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat 2017;162(2):283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An Abbreviated Protocol for High-Risk Screening Breast MRI Saves Time and Resources. Journal of the American College of Radiology : JACR 2016;13(4):374–380. [DOI] [PubMed] [Google Scholar]
- 35.Machida Y, Shimauchi A, Kanemaki Y, Igarashi T, Harada M, Fukuma E. Feasibility and potential limitations of abbreviated breast MRI: an observer study using an enriched cohort. Breast Cancer 2017;24(3):411–419. [DOI] [PubMed] [Google Scholar]
- 36.Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated Combined MR Protocol: A New Faster Strategy for Characterizing Breast Lesions. Clinical breast cancer 2016;16(3):207–211. [DOI] [PubMed] [Google Scholar]
- 37.Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS. Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol 2015;22(9):1157–1162. [DOI] [PubMed] [Google Scholar]
- 38.Mango VL, Morris EA, David Dershaw D, et al. Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur J Radiol 2015;84(1):65–70. [DOI] [PubMed] [Google Scholar]
- 39.Pineda FD, Medved M, Wang S, et al. Ultrafast Bilateral DCE-MRI of the Breast with Conventional Fourier Sampling: Preliminary Evaluation of Semi-quantitative Analysis. Acad Radiol 2016;23(9):1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platel B, Mus R, Welte T, Karssemeijer N, Mann R. Automated characterization of breast lesions imaged with an ultrafast DCE-MR protocol. IEEE transactions on medical imaging 2014;33(2):225–232. [DOI] [PubMed] [Google Scholar]
- 41.Mann RM, Mus RD, van Zelst J, Geppert C, Karssemeijer N, Platel B. A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol 2014;49(9):579–585. [DOI] [PubMed] [Google Scholar]
- 42.Abe H, Mori N, Tsuchiya K, et al. Kinetic Analysis of Benign and Malignant Breast Lesions With Ultrafast Dynamic Contrast-Enhanced MRI: Comparison With Standard Kinetic Assessment. AJR Am J Roentgenol 2016;207(5):1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Tang M, Min Z, Lu J, Lei X, Zhang X. Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: a meta-analysis. Acta Radiol 2016;57(6):651–660. [DOI] [PubMed] [Google Scholar]
- 45.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004;351(5):427–437. [DOI] [PubMed] [Google Scholar]
- 46.Brekelmans CT, Seynaeve C, Bartels CC, et al. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol 2001;19(4):924–930. [DOI] [PubMed] [Google Scholar]
- 47.Schrading S, Kuhl CK. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology 2008;246(1):58–70. [DOI] [PubMed] [Google Scholar]
- 48.Obdeijn IM, Winter-Warnars GA, Mann RM, Hooning MJ, Hunink MG, Tilanus-Linthorst MM. Should we screen BRCA1 mutation carriers only with MRI? A multicenter study. Breast Cancer Res Treat 2014;144(3):577–582. [DOI] [PubMed] [Google Scholar]
- 49.Riedl CC, Luft N, Bernhart C, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol 2015;33(10):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krammer J, Pinker-Domenig K, Robson ME, et al. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 2017;163(3):565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuhl CK, Schild HH, Morakkabati N. Dynamic bilateral contrast-enhanced MR imaging of the breast: trade-off between spatial and temporal resolution. Radiology 2005;236(3):789–800. [DOI] [PubMed] [Google Scholar]
- 52.Pinker K, Grabner G, Bogner W, et al. A combined high temporal and high spatial resolution 3 Tesla MR imaging protocol for the assessment of breast lesions: initial results. Invest Radiol 2009;44(9):553–558. [DOI] [PubMed] [Google Scholar]
- 53.D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System.: Reston, VA, American College of Radiology: 2013. [Google Scholar]
- 54.Clauser P, Mann R, Athanasiou A, et al. A survey by the European Society of Breast Imaging on the utilisation of breast MRI in clinical practice. Eur Radiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Expert Panel on Breast I, Mainiero MB, Moy L, et al. ACR Appropriateness Criteria((R)) Breast Cancer Screening. Journal of the American College of Radiology : JACR 2017;14(11S):S383–S390. [DOI] [PubMed] [Google Scholar]
- 56.Haygood TM, Wang J, Atkinson EN, et al. Timed efficiency of interpretation of digital and film-screen screening mammograms. AJR Am J Roentgenol 2009;192(1):216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. http://ecog-acrin.org/clinical-trials/ea1141-educational-materials. Volume 2018.
- 58.Sung JS, Stamler S, Brooks J, et al. Breast Cancers Detected at Screening MR Imaging and Mammography in Patients at High Risk: Method of Detection Reflects Tumor Histopathologic Results. Radiology 2016;280(3):716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris EA. Rethinking breast cancer screening: ultra FAST breast magnetic resonance imaging. J Clin Oncol 2014;32(22):2281–2283. [DOI] [PubMed] [Google Scholar]
- 60.Oldrini G, Bedri A, Happi Ngankou E, Marchal F, Henrot P. The usefulness of high temporal resolution breast MRI sequences. Presse Med 2018;47(1):87–88. [DOI] [PubMed] [Google Scholar]
- 61.Tsao J, Kozerke S. MRI temporal acceleration techniques. J Magn Reson Imaging 2012;36(3):543–560. [DOI] [PubMed] [Google Scholar]
- 62.Goto M, Ito H, Akazawa K, et al. Diagnosis of breast tumors by contrast-enhanced MR imaging: comparison between the diagnostic performance of dynamic enhancement patterns and morphologic features. J Magn Reson Imaging 2007;25(1):104–112. [DOI] [PubMed] [Google Scholar]
- 63.Pinker K, Moy L, Sutton EJ, et al. Diffusion-Weighted Imaging With Apparent Diffusion Coefficient Mapping for Breast Cancer Detection as a Stand-Alone Parameter: Comparison With Dynamic Contrast-Enhanced and Multiparametric Magnetic Resonance Imaging. Invest Radiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baltzer PAT, Bickel H, Spick C, et al. Potential of Noncontrast Magnetic Resonance Imaging With Diffusion-Weighted Imaging in Characterization of Breast Lesions: Intraindividual Comparison With Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Invest Radiol 2018;53(4):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Runge VM. Critical Questions Regarding Gadolinium Deposition in the Brain and Body After Injections of the Gadolinium-Based Contrast Agents, Safety, and Clinical Recommendations in Consideration of the EMA’s Pharmacovigilance and Risk Assessment Committee Recommendation for Suspension of the Marketing Authorizations for 4 Linear Agents. Invest Radiol 2017;52(6):317–323. [DOI] [PubMed] [Google Scholar]
- 66.Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB, International Society for Magnetic Resonance in M. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 2017;16(7):564–570. [DOI] [PubMed] [Google Scholar]
- 67.Runge VM. Safety of the Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging, Focusing in Part on Their Accumulation in the Brain and Especially the Dentate Nucleus. Invest Radiol 2016;51(5):273–279. [DOI] [PubMed] [Google Scholar]
- 68.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270(3):834–841. [DOI] [PubMed] [Google Scholar]
- 69.Kang KM, Choi SH, Hwang M, Yun TJ, Kim JH, Sohn CH. T1 Shortening in the Globus Pallidus after Multiple Administrations of Gadobutrol: Assessment with a Multidynamic Multiecho Sequence. Radiology 2018;287(1):258–266. [DOI] [PubMed] [Google Scholar]
- 70.Cao Y, Huang DQ, Shih G, Prince MR. Signal Change in the Dentate Nucleus on T1-Weighted MR Images After Multiple Administrations of Gadopentetate Dimeglumine Versus Gadobutrol. AJR Am J Roentgenol 2016;206(2):414–419. [DOI] [PubMed] [Google Scholar]
- 71.Radbruch A, Weberling LD, Kieslich PJ, et al. High-Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-Weighted Images: Evaluation of the Macrocyclic Gadolinium-Based Contrast Agent Gadobutrol. Invest Radiol 2015;50(12):805–810. [DOI] [PubMed] [Google Scholar]
- 72.Bjornerud A, Vatnehol SAS, Larsson C, Due-Tonnessen P, Hol PK, Groote IR. Signal Enhancement of the Dentate Nucleus at Unenhanced MR Imaging after Very High Cumulative Doses of the Macrocyclic Gadolinium-based Contrast Agent Gadobutrol: An Observational Study. Radiology 2017;285(2):434–444. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Cao Y, Shih GL, Hecht EM, Prince MR. Extent of Signal Hyperintensity on Unenhanced T1-weighted Brain MR Images after More than 35 Administrations of Linear Gadolinium-based Contrast Agents. Radiology 2017;282(2):516–525. [DOI] [PubMed] [Google Scholar]
- 74.Dekkers IA, Roos R, van der Molen AJ. Gadolinium retention after administration of contrast agents based on linear chelators and the recommendations of the European Medicines Agency. Eur Radiol 2018;28(4):1579–1584. [DOI] [PubMed] [Google Scholar]
- 75.Dorrius MD, Dijkstra H, Oudkerk M, Sijens PE. Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: a systematic review and meta-analysis. Eur Radiol 2014;24(11):2835–2847. [DOI] [PubMed] [Google Scholar]
- 76.Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZL. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC cancer 2010;10:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bogner W, Pinker-Domenig K, Bickel H, et al. Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology 2012;263(1):64–76. [DOI] [PubMed] [Google Scholar]
- 78.Trimboli RM, Verardi N, Cartia F, Carbonaro LA, Sardanelli F. Breast cancer detection using double reading of unenhanced MRI including T1-weighted, T2-weighted STIR, and diffusion-weighted imaging: a proof of concept study. AJR Am J Roentgenol 2014;203(3):674–681. [DOI] [PubMed] [Google Scholar]
- 79.McDonald ES, Hammersley JA, Chou SH, et al. Performance of DWI as a Rapid Unenhanced Technique for Detecting Mammographically Occult Breast Cancer in Elevated-Risk Women With Dense Breasts. AJR Am J Roentgenol 2016;207(1):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin HJ, Chae EY, Choi WJ, et al. Diagnostic Performance of Fused Diffusion-Weighted Imaging Using Unenhanced or Postcontrast T1-Weighted MR Imaging in Patients With Breast Cancer. Medicine (Baltimore) 2016;95(17):e3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yabuuchi H, Matsuo Y, Sunami S, et al. Detection of non-palpable breast cancer in asymptomatic women by using unenhanced diffusion-weighted and T2-weighted MR imaging: comparison with mammography and dynamic contrast-enhanced MR imaging. Eur Radiol 2011;21(1):11–17. [DOI] [PubMed] [Google Scholar]
- 82.Baltzer PA, Benndorf M, Dietzel M, Gajda M, Camara O, Kaiser WA. Sensitivity and specificity of unenhanced MR mammography (DWI combined with T2-weighted TSE imaging, ueMRM) for the differentiation of mass lesions. Eur Radiol 2010;20(5):1101–1110. [DOI] [PubMed] [Google Scholar]
- 83.Bickelhaupt S, Laun FB, Tesdorff J, et al. Fast and Noninvasive Characterization of Suspicious Lesions Detected at Breast Cancer X-Ray Screening: Capability of Diffusion-weighted MR Imaging with MIPs. Radiology 2016;278(3):689–697. [DOI] [PubMed] [Google Scholar]
- 84.Baltzer PAT, Bickel H, Spick C, et al. Potential of Noncontrast Magnetic Resonance Imaging With Diffusion-Weighted Imaging in Characterization of Breast Lesions: Intraindividual Comparison With Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Invest Radiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ueno Y, Tamada T, Takahashi S. Detection of Clinically Significant Prostate Cancer by Using Abbreviated Biparametric Prostate MR Imaging. Radiology 2018;286(3):1093–1094. [DOI] [PubMed] [Google Scholar]
- 86.Kuhl CK, Bruhn R, Kramer N, Nebelung S, Heidenreich A, Schrading S. Abbreviated Biparametric Prostate MR Imaging in Men with Elevated Prostate-specific Antigen. Radiology 2017:170129. [DOI] [PubMed] [Google Scholar]
- 87.Kaji Y, Inamura K. Diagnostic Ability with Abbreviated Biparametric and Full Multiparametric Prostate MR Imaging: Is the Use of PI-RADS Version 2 Appropriate for Comparison? Radiology 2018;286(2):726–727. [DOI] [PubMed] [Google Scholar]
- 88.Cunha GM, Villela-Nogueira CA, Bergman A, Lobo Lopes FPP. Abbreviated mpMRI protocol for diffuse liver disease: a practical approach for evaluation and follow-up of NAFLD. Abdom Radiol (NY) 2018. [DOI] [PubMed] [Google Scholar]
- 89.Tillman BG, Gorman JD, Hru JM, et al. Diagnostic per-lesion performance of a simulated gadoxetate disodium-enhanced abbreviated MRI protocol for hepatocellular carcinoma screening. Clin Radiol 2018;73(5):485–493. [DOI] [PubMed] [Google Scholar]
- 90.Lee JY, Huo EJ, Weinstein S, et al. Evaluation of an abbreviated screening MRI protocol for patients at risk for hepatocellular carcinoma. Abdom Radiol (NY) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Besa C, Lewis S, Pandharipande PV, et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol (NY) 2017;42(1):179–190. [DOI] [PubMed] [Google Scholar]
- 92.Marks RM, Ryan A, Heba ER, et al. Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid-enhanced MRI for hepatocellular carcinoma surveillance. AJR Am J Roentgenol 2015;204(3):527–535. [DOI] [PubMed] [Google Scholar]
- 93.Ahmad R, Hu HH, Krishnamurthy R, Krishnamurthy R. Reducing sedation for pediatric body MRI using accelerated and abbreviated imaging protocols. Pediatric radiology 2018;48(1):37–49. [DOI] [PubMed] [Google Scholar]
- 94.May LA, Chen DC, Bui-Mansfield LT, O’Brien SD. Rapid Magnetic Resonance Imaging Evaluation of Femoral Neck Stress Fractures in a U.S. Active Duty Military Population. Mil Med 2017;182(1):e1619–e1625. [DOI] [PubMed] [Google Scholar]
- 95.Khurana B, Okanobo H, Ossiani M, Ledbetter S, Al Dulaimy K, Sodickson A. Abbreviated MRI for patients presenting to the emergency department with hip pain. AJR Am J Roentgenol 2012;198(6):W581–588. [DOI] [PubMed] [Google Scholar]
- 96.Sarwar A, Boland G, Monks A, Kruskal JB. Metrics for Radiologists in the Era of Value-based Health Care Delivery. Radiographics 2015;35(3):866–876. [DOI] [PubMed] [Google Scholar]
- 97.Rubin GD. Costing in Radiology and Health Care: Rationale, Relativity, Rudiments, and Realities. Radiology 2017;282(2):333–347. [DOI] [PubMed] [Google Scholar]
- 98.McGinty G Is Value-Driven Health Care an Unfunded Mandate for Radiologists? AJR Am J Roentgenol 2016;206(2):280–282. [DOI] [PubMed] [Google Scholar]
- 99.Duong PA, Pastel DA, Sadigh G, et al. The Value of Imaging Part II: Value beyond Image Interpretation. Acad Radiol 2016;23(1):23–29. [DOI] [PubMed] [Google Scholar]
- 100.Duong PA, Bresnahan B, Pastel DA, et al. Value of Imaging Part I: Perspectives for the Academic Radiologist. Acad Radiol 2016;23(1):18–22. [DOI] [PubMed] [Google Scholar]
- 101.European Society of R. ESR concept paper on value-based radiology. Insights into imaging 2017;8(5):447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]