Abstract
There is abundant evidence that the pathophysiology of Parkinson’s disease (PD) is not confined to the nigrostriatal dopaminergic pathway but propagates along the cortico-basal ganglia-thalamo-cortical neural network. A critical node in this functional circuit impacted by PD is the primary motor cortex (M1) which plays a key role in generating neural impulses that regulate movements. The past several decades have lay witness to numerous in vivo neuroimaging techniques that provide a window into the function and structure of M1. A consistent observation from numerous studies is that during voluntary movement, but also at rest, the functional activity of M1 is altered in PD relative to healthy individuals, and it relates to many of the motor signs. Although this abnormal functional activity can be partially restored with acute dopaminergic medication, it continues to deteriorate with disease progression and may predate structural degeneration of M1. The current review discusses the evidence that M1 is fundamental to the pathophysiology of PD, as measured by neuroimaging techniques such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), electroencephalography (EEG), magnetoencephalography (MEG), and functional and structural magnetic resonance imaging (MRI). While novel treatments that target the cortex will not cure PD, they could significantly slow down and alter the progressive course of the disease and thus improve clinical care for this degenerative disease.
1. Introduction
Cell death in the substantia nigra and shortage of dopamine in the striatum have been known as key developments that affect motor control in Parkinson’s disease (PD).1–7 Over the years, however, it has become apparent that PD is associated with more complex changes across several key regions of the motor network, including the primary motor cortex (M1).7–10 The dopaminergic cell loss in PD is believed to lead to an increased inhibition of the motor thalamic nuclei, and decreased excitation of the cerebral cortex which contributes to abnormal motor output.11, 12 There is also increasing evidence on abnormal neuronal oscillations within and between motor regions in PD, and these abnormal oscillations are thought to impair motor function and account for some of the clinical signs.13–16
One way to probe in vivo the functional and structural integrity of key nodes of the cortico-basal ganglia motor circuit is by using brain imaging and electrophysiological techniques.8, 17, 18 Therefore, the aim of this paper is to provide a comprehensive overview of the involvement of M1 in the pathophysiology of PD as revealed by modern brain imaging technologies.
2. The primary motor cortex and basal ganglia
M1 is located anterior to the central sulcus and can be distinguished from adjacent premotor and somatosensory areas by the presence of a thick cortical descending output layer 5 packed with large pyramidal (Betz) cells and a near-absent layer 4.19 M1 is the target of output from both the basal ganglia and cerebellum and the site where part of the corticospinal descending pathway originates.20 The ventrolateral nucleus of the thalamus relays signals from the basal ganglia and provides input to M1, supplementary motor area (SMA), and premotor cortex (PMC).21 The SMA, dorsal PMC, and ventral PMC are connected and provide input to M1, while M1 has reciprocal feedback with SMA and dorsal PMC.21 Similar to M1, SMA and PMC have projections to the spinal cord through the corticospinal tract.22, 23 M1 projects back to the basal ganglia primarily to the dorsolateral putamen compared to the input from SMA which is located more medially in the putamen.24 Templates exist to provide brain maps of the cortical motor and premotor regions, and the descending tracts for imaging and electrophysiological studies.22, 25
3. Functional changes in the motor cortex of PD
3.1. Positron emission tomography (PET)
Nuclear imaging offers the possibility of investigating alterations in cerebral perfusion and metabolism in PD.17 A few regional cerebral blood flow (rCBF) studies have shown that during rest the activation pattern in M1 in PD appears to be comparable to that observed in healthy individuals.26, 27 However, when patients are asked to move, PD patients tend to present with decreased activity in M1,28 even when carefully controlling for group differences in kinematics.29 Because of the significant cell death in the brainstem nuclei (substantia nigra pars compacta and locus coeruleus), neurochemical signaling in the cortex in PD is thought to be disrupted.30 Monoaminergic deficits in M1 were found at rest using 18F-dopa PET in a large group of PD, and across subgroups with different levels of striatal 18F-dopa uptake.31 Newer PET ligands such as 11C-MeNER have recently provided the first evidence of a decline in noradrenergic function in M1 in PD that seems to be more pronounced in the advanced stages of the disease.32 Together, these studies confirm postmortem immunohistochemical analyses of altered neurochemical projections from the midbrain to M1 in PD33, and suggest an involvement of multiple neurotransmitters that could influence future treatments.
The PET literature is not spared, however, from controversy about the direction of functional activity of M1. For instance, a study with PET and H215O during a unimanual task in hemiparkinsonian PD found an increased rCBF in M1 compared to controls when patients executed the task with their impaired hand.34 While tasks may influence the pattern of activity, it could well be that specific patterns of activity in M1 are related to different clinical phenotypes. For instance, tremor-dominant PD patients implanted with a deep brain stimulation (DBS) device in the ventral intermediate thalamic nucleus had increased metabolic activity in M1 and cerebellum in the absence of stimulation, and the level of activity in M1 was positively related to UPDRS ratings of tremor and accelerometer measurements of tremor.35 Subsequent thalamic stimulation led to a reduction in the M1 activity and an improvement of tremor ratings. M1 hyperactivity does not only seem to be closely linked to tremor characteristics,36 but is also a key finding in DBS patients with more severe motor signs, and PD with motor signs affecting other body parts (e.g., vocal musculature).37, 38 Interventions such as intracortical stimulation of STN and non-invasive measures like vocal training/treatment were successful in modulating the hyperactive state of M1 in these cohorts.38, 39 Given that restoration of striatal dopamine with oral levodopa provides symptom relief in patients with PD, it becomes imperative to elucidate whether dopaminergic therapy is sufficient for restoring the metabolic state of M1. PET imaging in early stage PD showed that IV levodopa infusion could improve UPDRS motor ratings and decrease the regional glucose metabolism in several subcortical and cortical motor structures, including M1,40 and that similar results can be obtained with acute oral medication.41 However, PD treated chronically with dopaminergic medication have a different response to an acute dose of medication than drug naïve patients, with the latter group having only a small response to the intervention. These results suggest that the severity of the disease as well as chronic exposure to levodopa may influence the responsiveness of M1, however the mechanisms by which this could happen are not clear.
3.2. Single photon emission computed tomography (SPECT)
This technique allows the investigation of in vivo metabolic and neurochemical changes,42, 43 and revealed an increase in rCBF in the ipsilateral sensorimotor cortex during a unimanual task in PD with motor fluctuations tested on medication, in the absence of group differences in task performance.44 This unexpected finding was attributed to potentially involuntary movements of the other hand which may or may not be induced by levodopa. Interestingly, in a study of PD with levodopa-induced dyskinesia, M1 was hyperactive bilaterally in patients compared to controls,45 complementing PET findings and suggesting that an overdrive of M1 may underlie hyperkinetic movements (i.e. tremor, dyskinesia) in PD. Exploring ways to modulate M1 activity may open therapeutic avenues in PD with drug-induced dyskinesia.
3.3. Functional magnetic resonance imaging (fMRI)
Task-based fMRI.
Functional brain abnormalities in multiple regions of the cortex, including M1, have been reported in several cross-sectional studies, and across a wide range of motor tasks. Reduced functional activity of M1 was found in both drug naïve PD and PD tested off dopaminergic medication, using motor tasks such as unimanual grip force production, finger tapping, and finger opposition.46–53 These results seem to support the basal ganglia-thalamo-cortical circuit model in which dopamine depletion leads to reduced excitatory thalamic outflow to the cortical motor areas which may result in the decrease of functional activity of M1.54 Nevertheless, there are a number of studies in drug naïve PD but also PD receiving dopaminergic treatment which have shown an increased activation in M1.55–58 The hyperactivity of M1 tends to be interpreted as a result of reorganization of the motor system in order to compensate for dysfunction of the basal ganglia. This is an interesting hypothesis worth exploring. However, the longitudinal nature of a process like compensation makes inferences from cross-sectional data less robust. An alternative hypothesis is that M1 hyperactivity as detected by fMRI, (also PET/SPECT imaging), may be driven by the prevalence of certain motor signs, such as tremor, rigidity, or dyskinesia. It has been long recognized that PD is a heterogeneous disease including hypokinetic and hyperkinectic features.59 Among the main subtypes in PD, we have the tremor dominant (TD) and the postural instability and gait difficulty (PIGD) subtypes,60 and an fMRI study paired with EMG measurements showed that PD with tremor presented with increased tremor-related activity in M1.61 Other motor signs such as upper limb rigidity and freezing of gait were also associated with an overactive M1, and suggest that M1 dysfunction is key in the pathophysiology of PD.55, 62 Similar to PET, task-based fMRI shows that M1 is responsive to dopaminergic medication,47, 58 but this effect highlights the importance of a careful control of medication state across patients in functional imaging studies. A less tight control of variables such as the duration of the withdrawal period prior to imaging could influence results in one direction or another (i.e., hyperactivity or hypoactivity).
In addition to changes in the magnitude of brain activity in M1, it has become clearer that PD patients also exhibit disruptions of the pattern of functional connectivity between cortical and subcortical motor regions.63, 64 The abnormal cortico-subcortical connectivity can be boosted, with recent results demonstrating that an improvement in pedaling rate in PD who had undergone exercise therapy was related to an increase in functional connectivity between M1 and thalamus.65 A linear increase in motor-related connectivity between the putamen and M1 was also detected following levodopa intake in a cohort of PD who later developed levodopa-induced dyskinesia.66 This particular case suggests that an increase in functional connectivity is necessary, but if maintained for a long time could lead to dyskinesia. The plasticity of functional connectivity in response temporary motor practice and acute levodopa highlights the need for more long-term interventions targeting the cortex that may help relieve some of the motor signs. Collectively, fMRI and task-fMRI connectivity findings strengthen those obtained with PET by showing that although these measures reflect different characteristics of neural activity, they are feasible for detecting functional abnormalities in M1 that are responsive to intervention.
Resting-state fMRI (rs-fMRI).
Over the past two decades, rs-fMRI has become a popular approach to study abnormalities of spontaneous neuronal activity in the brain in vivo in the absence of task performance.67 This method has revealed changes in the cortico-subcortical functional connectivity, with reduced connectivity between the putamen and M1 in both drug naïve and treated PD.68, 69 Interestingly, the pattern of resting-state connectivity with the sensorimotor cortex is not the same across the basal ganglia. Unlike the putamen, STN was consistently found to have increased functional connectivity with M1 in both de novo PD and moderate stage PD tested off medication, and the amount of connectivity appears to scale with disease severity.70, 71 This abnormally increased connectivity between STN and M1 also persists despite the effects of drug therapies, with PD tested on medication presenting a similar interconnectivity pattern, though not as severe.72 The temporal correlation of the fMRI signal from STN and M1 may indicate an overactivity of the hyperdirect pathway, which can be improved by STN stimulation.73
Analytic approaches of resting-state data that examine the functional relationship between remote brain regions (e.g., graph theory, seed-based connectivity) have revealed changes such as decreased nodal centrality which is inversely correlated with UPDRS motor scores, decreased inter-hemispheric M1 connectivity, and increased functional connectivity with other cortical regions.74–76 On the other hand, imaging studies that assess changes in the local resting-state fMRI fluctuations reported reduced regional homogeneity (ReHo) across the basal ganglia as well as M1, and further declines in this measure with disease progression.77, 78 However, there is also evidence that in some patients there is an increase in ReHo in M1, and this aberrant signal can be normalized with the administration of levodopa.79 Furthermore, antiparkinsonian medication was also found to modulate the amplitude of local low-frequency fluctuations (e.g., fALFF), by reducing the initially elevated resting-state activity detected in the off state.80 Together, rs-fMRI data suggest that M1 in PD is abnormally engaged in both long-distance connections with remote regions and short-distance connections.
3.4. Electroencephalography (EEG)
Brain activity is characterized by synchronized oscillations between populations of neurons, and cortical motor regions such as M1 are known for having prominent oscillations in the alpha and beta frequency band (i.e., 8–12 Hz, 13–30 Hz).81, 82 Typically, when a motor task is performed there is an attenuation of brain oscillations in the alpha and beta band and this process is known as desynchronization.83 In PD, a number of studies revealed an aberrant pattern of synchronization/desynchronization in the beta rhythm across the basal ganglia-thalamo-cortical circuit.81, 84–86 It is believed that the parkinsonian brain is characterized by an impairment in switching between akinetic (beta band) and prokinetic (gamma band) oscillations in the sensorimotor cortex of PD patients,82 and abnormal levels of beta activity within and between the cortex and basal ganglia that correlate with motor deficits but respond to interventions.87–89 For instance, high frequency stimulation of STN in PD reduced STN beta activity and decreased the sensorimotor cortical–STN coherence in the beta band.89 Acute treatment with antiparkinsonian medication is also successful as it was found to attenuate the alpha and beta rhythms over the cortical motor areas during wrist movements, and this effect correlated with improvements in bradykinesia.90 Positive effects of medication were also observed in the level of desynchronization in M1 prior to movement.91 Together, these studies suggest that abnormal beta oscillations may indeed underlie not all but many of the motor deficits observed in PD. One of the motor states least understood in PD is levodopa-induced dyskinesia. A study focusing on a rodent lesion model of PD found that dyskinesia was always accompanied by a strong narrowband oscillation at ~80 Hz in the motor cortex of the lesioned hemisphere.92 Interestingly, data collected over 1 year in two PD patients permanently implanted with an electrocorticography (ECoG) strip recording potentials over M1 found similar results with dyskinesia being associated with a narrowband gamma oscillation in M1 between 60 and 90 Hz.93 Collectively, studies of cortical oscillations in PD point to perturbed low frequency oscillatory activity within motor regions that could serve as a potential therapeutic target in future intervention studies.
3.5. Magnetoencephalography (MEG)
MEG can record task-related activity in the brain as well as brain activity at rest. Using MEG, it has been shown that PD patients have increased sensorimotor cortical power in the beta band frequency compared to controls at rest and during upper limb movements.94 Moreover, MEG recordings confirm EEG findings showing abnormal beta desynchronization occurring not only during movement but also prior to and after movement.95 Based on coherence estimates derived from simultaneous multi-site cortical MEG and local field potential recordings from STN, we know that there is a strong coherence in beta oscillations between the sensorimotor cortex and the basal ganglia, and between the sensorimotor cortex and other cortical motor regions.96 Acute dopaminergic medication appears to reduce this abnormal coupling.97 Brain activity in M1 was found to be coherent not only with activity of other regions but also with tremulous EMG suggesting that abnormal oscillations in M1 contribute to resting tremor in PD.98 MEG results complement findings derived from other imaging modalities (PET, fMRI) showing that M1 hyperactivity is important in a tremor-related network.
4. Structural changes in the motor cortex of PD
4.1. Morphometry
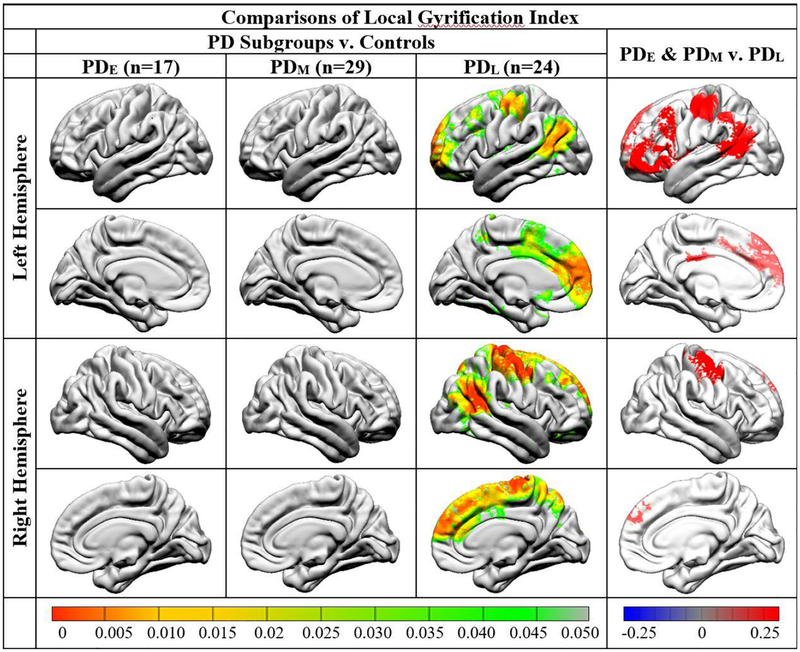
3D-T1 weighted MRI is widely used to study changes in brain structure in various neurological diseases as it provides good gray-white matter contrast. In diseases that affect the basal ganglia, this type of sequence is more challenging because the contrast of many nuclei is relatively poor due to the rich iron content, making accurate delineation of these structures difficult.9, 99 From a methodological standpoint, assessing cortical changes in PD using T1-based morphometry methods is less challenging. Using voxel-based morphometry (VBM) of gray matter we have learned that cortical morphometry in non-demented PD patients is typically normal or with minor differences compared to controls.9, 99, 100 By contrast, in PD with mild cognitive impairment or dementia, there are more robust gray matter changes in parieto-temporal regions.9 Changes in cortical motor regions are not very common, with a few observations of reduced gray matter volume in the precentral gyrus in PD with cognitive impairment,101–104 and in the postural instability and gait difficulty PD subtype.105 Alternatives to VBM that allow the estimation of gray matter changes (e.g. surface-based morphometry),106, 107 have revealed cortical thinning in the sensorimotor cortex in non-demented PD compared to controls, and reduced cortical gyrification in M1 in those PD with more advanced disease duration (Figure 1).108, 109 Although based on the majority of T1-based studies structural changes in M1 are not a signature of PD, the latest results using surface-based morphometry raise the possibility that changes in structure could occur once patients reach the more moderate to advanced stages of the disease, and may explain emerging signs such as the progressive loss of balance and increasing gait difficulty. Gray matter changes as picked up by the current macrostructural methods may be small and evolve slowly, highlighting the need for longitudinal designs and the development of more sensitive metrics.
Fig. 1.
Figure reprinted with permission: Sterling NW, Wang M, Zhang L, et al. Stage-dependent loss of cortical gyrification as Parkinson disease “unfolds”. Neurology 2016; 86(12):1143–1151. Comparison of local gyrification index between Parkinson disease (PD) subgroups and controls (left) and among PD subgroups (right) at baseline. In this study PD patients were grouped based on the number of years since diagnosis into PD-early (< 1 year, baseline UPDRS-III on medication of 11.4 ± 8.2, baseline Hoehn and Yahr stage 1.4 ± 0.6), PD-middle (1–5 years, baseline UPDRS-III on medication of 19.8 ± 10.4, baseline Hoehn and Yahr stage 1.4 ± 0.6), and PD-late (> 5 years, baseline UPDRS-III on medication of 20.4 ± 11.9, baseline Hoehn and Yahr stage 2.2 ± 0.6). PD with a long disease duration demonstrated significantly reduced gyrification bilaterally in the inferior parietal, precentral and postcentral, and superior frontal areas, compared to controls at baseline visit (left). These patients also had reduced gyrification in several neocortical areas, compared to PD with short and medium disease duration at baseline (right).
4.2. Diffusion MRI
Diffusion MRI can be used to quantify microstructural changes in parts of the brain which are unremarkable on routine structural MRI scans.110 Fractional anisotropy (FA) is a common index derived from diffusion MRI data that reflects the degree of anisotropic motion of water molecules within a voxel that can be modulated by the complex brain tissue environment.111 In PD, it has been widely used in evaluating structural changes in the substantia nigra,112–116 but its applications are not restricted to subcortical structures. Reduced FA was detected in the precentral gyrus and SMA in PD, while another study assessing diffusion MRI changes across motor tracts found increased FA in the corticospinal and thalamus-motor cortex tracts in patients compared to controls.117, 118 These rather unexpected findings highlight the possibility of microstructural changes being far more extensive than previously thought, encompassing complex pathways involved in the initiation and control of movement. However, future cross-sectional and longitudinal diffusion MRI studies are needed to confirm these observations and assess their relation to motor signs in PD. At the same time, we recommend caution in interpreting the significance of cortical motor changes since diffusion metrics such as FA, while sensitive to microstructural changes, lack specificity with respect to the underlying biological mechanisms (e.g., neuronal loss, compensatory axonal sprouting, changes in myelination and fiber density, neuroinflammation).119 For now, determining whether the microstructural changes detected in M1 and the motor tracts based on diffusion MRI are neurodegenerative or compensatory in nature remains an important challenge.
5. PD progression and changes in the motor cortex
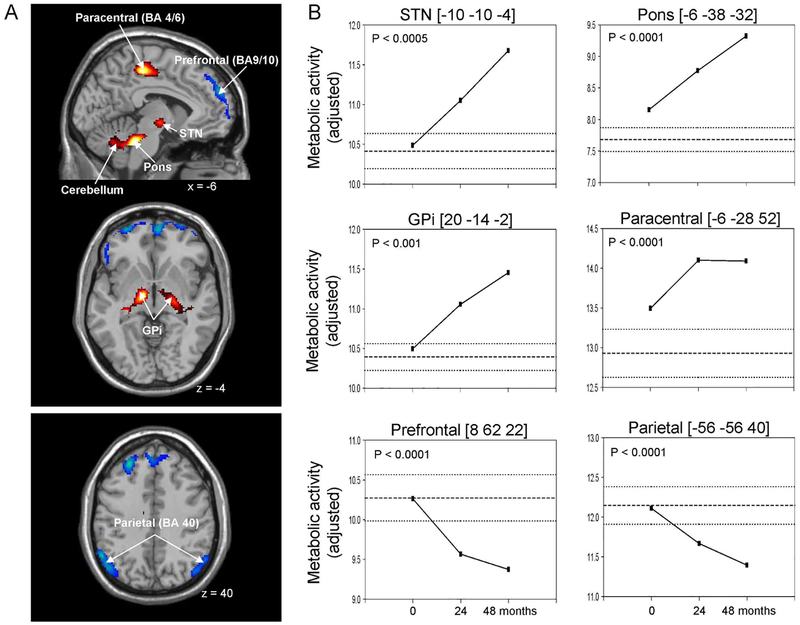
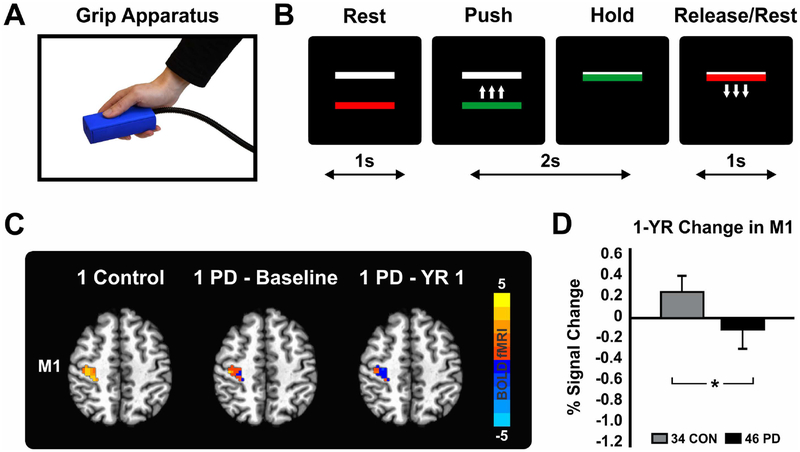
While the PD neuroimaging literature addressing longitudinal changes in M1 is limited, it is crucial to gain a better understanding of the rate of progression of M1 changes in order to develop therapeutic approaches. A 4-year multitracer PET imaging study in early stage PD patients revealed time-dependent increases in metabolism in several brain regions including M1 (Figure 2).120 Unlike other regions with a steady increase in glucose consumption, the regional metabolism in M1 did not rise continuously over time, but plateaued after 2 years. An interesting observation was that tremor ratings followed a similar pattern which suggests that the observed metabolic changes in M1 may reflect progression of specific motor signs such as tremor. Longitudinal changes in M1 were also detected in fMRI studies. Cohorts of PD patients followed up over 2 years presented with a decline at rest in the local amplitude of low-frequency fluctuations and regional synchrony of low-frequency fluctuations.78, 121 The level of activity of M1 during movement also deteriorated over time, with PD followed-up for 1 year showing reduced fMRI signal compared to controls (Figure 3).122 The results were observed in a large sample and in the absence of any group differences in task performance. The lack of a time effect in the control group highlights the potential of task-based fMRI as a progression marker that could aid in testing therapeutic strategies aimed at slowing down the progression of the disease. Furthermore, if confirmed, the sensitivity of task-based fMRI to the progression of PD may hold promise for the prodromal phase of the disease. It is known that brain changes in PD begin long before the onset of clinical signs, and during the prodromal period individuals present with a combination of subtle motor and non-motor signs that are currently not sufficient to diagnose the disease.123 Based on the involvement of M1 in the pathophysiological profile of PD, it could be that people at risk for PD present with abnormalities in this structure in the absence of overt motor signs. A recent multimodal imaging study of healthy adults who do not have motor symptoms but carry a risk genotype/variant in the alpha synuclein gene (SNCA rs356219) associated with increased odds of developing PD, revealed functional changes in the brain of carriers that mimic those observed in patients with PD (i.e. M1 hypoactivity).124 Changes in M1 were also found in prodromal individuals with REM sleep behavior disorder (RBD) followed-up clinically to assess PD conversion.125 The PD-related covariance pattern (PDRP) of metabolic activity at rest known to include different patterns of activity such as increases in M1 was elevated in the group at risk for PD.
Fig. 2.
Figure reprinted with permission: Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson’s disease. Brain 2007; 130(7):1834–1846. (A) Voxel-based analysis of longitudinal changes in regional metabolic activity. Metabolic increases with disease progression are displayed using a red–yellow scale. Progressive metabolic declines are displayed using a blue–purple scale. (B) Displays of the metabolic data for these individual regions at each timepoint. The coordinates refer to the Montreal Neurological Institute (MNI) standard space. Abbreviations: GPi = internal globus pallidus, STN = subthalamic nucleus, BA = Brodmann area.
Fig. 3.
Figure adapted from Burciu RG, Chung JW, Shukla P, et al. Functional MRI of disease progression in Parkinson disease and atypical parkinsonian syndromes. Neurology 2016;87(7):709–717. (A) Grip apparatus used to produce force. PD patients performed the task with the more affected hand (B) Task consisting of a series of 2 seconds of force production and 1 second of rest. Force target was set at 15% of maximum voluntary contraction. (C) Functional MRI signal during grip force production in the contralateral M1 in one healthy control along with task-based fMRI signal at baseline and 1 year later in one PD patient. (C) Group statistics showing a reduction in force-related fMRI activity in M1 in PD over the course of 1 year. Data represents the 1-year difference adjusted for the following variables at baseline: age, sex, Montreal Cognitive Assessment Test, and percent signal change in M1. Abbreviations: CON = controls, M1 = primary motor cortex, PD = Parkinson’s disease, YR = year.
As for structural imaging measures, these may also help gauge disease progression in PD. A recent 3-year progression study of PD without dementia showed reduced cortical gyrification in the precentral and postcentral areas in more advanced stages of the disease, and a decline in this measure with disease progression.109 While structural changes in M1 are not a hallmark of PD, they raise the hypothesis that functional cortical changes in PD could be followed by structural changes. In a study that compared cortical metabolism and measures of cortical atrophy in PD with intact cognition, PD with mild cognitive impairment, and PD with dementia, results showed a gradient in cortical changes that suggests that functional changes precede structural changes.104 Together, the existing longitudinal studies reinforce the importance of M1 in the progression of the disease. The development of new treatment options (whether pharmacological or not) is important because dopaminergic medication is not sufficient to slow down changes in M1.
6. Imaging the parkinsonian brain: what have we learned about the motor cortex?
Collectively, human brain imaging studies have proven useful in investigating the many facets of PD, contributing to a better understanding of the pathophysiological changes in M1. A summary of the main findings obtained with different imaging techniques can be found in Supplementary Figure 1. Importantly, M1 abnormalities in PD are often functional in nature, emerge across a wide range of motor paradigms and at rest, across all stages of the disease, and are partially restored by interventions. Structural changes in M1 are not as common and seem to be present in a subgroup of patients who are either in a more advanced stage of the disease or present with additional signs (e.g., posture and gait instability, cognitive deficits). When the functional neuroimaging studies are examined as a whole, two activation patterns in M1 emerge (i.e., hypoactivation vs. hyperactivation). When interpreting these patterns, one should keep in mind an important issue in the functional imaging field, and that is group differences in task performance and how much the task controls behavioral output. In experiments that do not account for task performance, any difference in activation observed between controls and PD might be due to a less successful performance of the patient group rather than a fundamental difference in the way the parkinsonian brain processes the motor task. For instance, parameters such as rate and amplitude of movements, and number of iterations within the measured interval can vary between PD and controls, which may account for differences in activation patterns. In button pressing tasks, the force applied to the button is not measured, and thus PD could be pressing with greater force and this would not be known. It is well known that increasing muscle force increases firing rates of pyramidal tract neurons in M1.126 Also, functional imaging experiments have shown that increasing force is associated with increases in fMRI signal and rCBF in M1.127, 128 In a joystick movement task, commonly used in fMRI, the experimenter does not know if the amount of force applied to the joystick is similar between patients and controls. However, records of joystick movements can be analyzed and integrated in group statistics. For instance, in the PET study of Turner and colleagues examining brain activity in PD during a visuomotor tracking task, movement velocity was used as a covariate to factor out major between-group differences in performance. Their analysis revealed several regions involved in motor control, including M1, had reduced activity in PD.29 Similarly, in studies that do use a force control task and the level of force is controlled along with the duration and number of force pulses, there is a highly consistent and replicated pattern of reduced fMRI activity in the M1 for PD compared with controls.48–51, 53, 122 In general, across PET and task-based fMRI modalities, the common observation is that the activity of M1 during movement in those studies that controlled the way the task was executed was reduced in PD compared to controls. However, there are also several studies that reported increases in metabolic activity as well as fMRI signal in PD compared to controls. Of particular importance is that hyperactivity of M1 both at rest and during movement seems to be specific to a certain clinical phenotype, PD with tremor or dyskinesia. An important aspect that one has to bear in mind when interpreting patterns of activity in M1 is the fact that M1 is the target of somatotopically organized outputs from both the basal ganglia and cerebellum.20 While it is not clearly understood how the basal ganglia and cerebellum interact with each other, there is increasing evidence on the involvement of the cerebellum in resting tremor in PD,61, 129 and it could be that information processing along this parallel pathway connecting the cerebellum to cortical motor areas contributes to and modulates the pathophysiological state of M1 in PD. Finally, while the existing dichotomy of functional activity in M1 as revealed by nuclear imaging and MRI may seem somewhat contradictory, it also raises the question whether PD may be associated with a patterned combination of hypo- and hyperactivity. However, the current imaging data acquired with different methods and spatial resolutions cannot provide an answer. Another open and interesting question that remains to be answered is whether the overdrive of M1 as detected by functional imaging in some cohorts of PD could be a form of early compensation that counteracts the effect of low dopamine levels in the brain, but longitudinal studies in early PD are needed to address this issue.
From the other functional imaging modalities (rs-fMRI, EEG, and MEG) we have learned more about the different properties of the motor brain network as a whole in PD. Although they differ in temporal and spatial resolution, by corroborating information derived from these different techniques, it becomes clearer that changes in PD in M1 are complex and can be characterized by abnormalities in the beta band synchronization, local and long-range coherence of neural oscillations, and dynamics of spontaneous BOLD fluctuations. While data supports the pathological neural oscillations model,130, 131 it also suggests that changes motor signs could result from a conglomerate of abnormalities ranging from changes in firing rate, pattern, and synchrony of neural populations.
As for structural changes in M1, it is worth noting that current models of PD assume the absence of any overt pathology in cortical motor regions. Although there is not sufficient evidence on structural abnormalities in M1 in PD based on non-invasive imaging, a few findings raise an intriguing hypothesis that M1 dysfunction in PD is not exclusively a consequence of basal ganglia malfunctioning. Prior work has reported microstructural changes in PD including Lewy bodies in the Betz cells of M1 and a reduction in dopaminergic innervation to layer 1.33, 132 Whether or not structural changes emerge later in the disease, it is plausible to assume that structural changes could have an impact on M1 activity independently of any modulatory changes coming from the basal ganglia. At the same time, the presence of structural changes in M1 as detected by structural MRI highlights an important issue in the field and that is the reliability of diagnosis in PD. The clinical diagnosis in PD is still challenging due to difficulties in recognizing atypical parkinsonian syndromes early on.133 Atypical parkinsonian patients present with extensive cortical atrophy including changes in motor regions when compared to PD,134, 135 so the accuracy of diagnosis should be considered when interpreting structural data. Every effort must be made to ensure that research studies recruit PD who have had a stable diagnosis. Also, the body of existing literature on cortical changes in PD suggests that many of these changes are related to a variety of clinical signs that prompt the need to address heterogeneity of PD in imaging studies, and validation on these findings in larger cohorts of subjects. In general, a better understanding of what sometimes appears to be conflicting evidence may be resolved by a careful evaluation of the research cohorts, the presence or absence of a control group, sample size, heterogeneity of motor signs, duration of withdrawal from antiparkinsonian medication which could alter the degree of functional activity of M1, statistical methods and limitations of the imaging modalities.
In summary, a variety of neuroimaging techniques provide complementary information and demonstrate predominantly functional changes in M1 in PD, and prompt for more research geared toward the development of therapeutics and interventions that go beyond replenishing dopamine depletion and target the motor cortex, potentially slowing down disease progression.
Supplementary Material
Supplementary Fig. 1. Summary of the functional and structural neuroimaging literature on the involvement of M1 in the pathophysiology of PD. Abbreviations: DBS = deep brain stimulation, FA = fractional anisotropy, M1 = primary motor cortex, rCBF = regional cerebral blood flow.
Acknowledgments
Funding: Supported by the National Institutes of Health (R01NS052318, R01NS075012, U01NS102038).
Financial disclosures:
Roxana G. Burciu – Reports no disclosures.
David E. Vaillancourt – Reports grants from NIH, NSF, and Tyler’s Hope Foundation during the conduct of the study, and personal honoraria from NIH, National Parkinson’s Foundation, and Northwestern University unrelated to the submitted work. He is Co-Founder of Neuroimaging Solutions, LLC.
References
- 1.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 1991;114 (Pt 5):2283–2301. [DOI] [PubMed] [Google Scholar]
- 2.Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013;136(Pt 8):2419–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankovic J Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008;79(4):368–376. [DOI] [PubMed] [Google Scholar]
- 4.Mazzoni P, Shabbott B, Cortes JC. Motor control abnormalities in Parkinson’s disease. Cold Spring Harb Perspect Med 2012;2(6):a009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog Neurobiol 2000;62(1):63–88. [DOI] [PubMed] [Google Scholar]
- 6.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst 2002;18(8):386–404. [DOI] [PubMed] [Google Scholar]
- 7.Obeso JA, Marin C, Rodriguez-Oroz C, et al. The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol 2008;64 Suppl 2:S30–46. [DOI] [PubMed] [Google Scholar]
- 8.Brooks DJ. Technology insight: imaging neurodegeneration in Parkinson’s disease. Nature clinical practice Neurology 2008;4(5):267–277. [DOI] [PubMed] [Google Scholar]
- 9.Broski SM, Hunt CH, Johnson GB, Morreale RF, Lowe VJ, Peller PJ. Structural and functional imaging in parkinsonian syndromes. Radiographics : a review publication of the Radiological Society of North America, Inc 2014;34(5):1273–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol 2007;64(1):20–24. [DOI] [PubMed] [Google Scholar]
- 11.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in neurosciences 1989;12(10):366–375. [DOI] [PubMed] [Google Scholar]
- 12.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends in neurosciences 1990;13(7):244–254. [DOI] [PubMed] [Google Scholar]
- 13.Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord 2006;21(10):1566–1577. [DOI] [PubMed] [Google Scholar]
- 14.Geng X, Zhang J, Jiang Y, et al. Comparison of oscillatory activity in subthalamic nucleus in Parkinson’s disease and dystonia. Neurobiol Dis 2017;98:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Wijk BCM, Pogosyan A, Hariz MI, et al. Localization of beta and high-frequency oscillations within the subthalamic nucleus region. Neuroimage Clin 2017;16:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witcher M, Moran R, Tatter SB, Laxton AW. Neuronal oscillations in Parkinson’s disease. Frontiers in bioscience (Landmark edition) 2014;19:1291–1299. [DOI] [PubMed] [Google Scholar]
- 17.Brooks DJ. Assessment of Parkinson’s disease with imaging. Parkinsonism Relat Disord 2007;13 Suppl 3:S268–275. [DOI] [PubMed] [Google Scholar]
- 18.Pavese N, Brooks DJ. Imaging neurodegeneration in Parkinson’s disease. Biochim Biophys Acta 2009;1792(7):722–729. [DOI] [PubMed] [Google Scholar]
- 19.Ebbesen CL, Brecht M. Motor cortex - to act or not to act? Nat Rev Neurosci 2017;18(11):694–705. [DOI] [PubMed] [Google Scholar]
- 20.Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci 1999;19(4):1446–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anatomy and embryology 2000;202(6):443–474. [DOI] [PubMed] [Google Scholar]
- 22.Archer DB, Vaillancourt DE, Coombes SA. A Template and Probabilistic Atlas of the Human Sensorimotor Tracts using Diffusion MRI. Cereb Cortex 2017:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 1991;11(3):667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp Brain Res 1998;120(1):114–128. [DOI] [PubMed] [Google Scholar]
- 25.Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage 2006;31(4):1453–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berding G, Odin P, Brooks DJ, et al. Resting regional cerebral glucose metabolism in advanced Parkinson’s disease studied in the off and on conditions with [(18)F]FDG-PET. Mov Disord 2001;16(6):1014–1022. [DOI] [PubMed] [Google Scholar]
- 27.Hilker R, Voges J, Weisenbach S, et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson’s disease. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2004;24(1):7–16. [DOI] [PubMed] [Google Scholar]
- 28.Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson’s disease. Brain 1999;122 (Pt 3):483–495. [DOI] [PubMed] [Google Scholar]
- 29.Turner RS, Grafton ST, McIntosh AR, DeLong MR, Hoffman JM. The functional anatomy of parkinsonian bradykinesia. Neuroimage 2003;19(1):163–179. [DOI] [PubMed] [Google Scholar]
- 30.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 2003;60(3):337–341. [DOI] [PubMed] [Google Scholar]
- 31.Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in Parkinson’s disease: an 18F-dopa PET study. Neurobiol Dis 2008;29(3):381–390. [DOI] [PubMed] [Google Scholar]
- 32.Michael S, K HA, Peter P, et al. Decreased noradrenaline transporter density in the motor cortex of Parkinson’s disease patients. Movement Disorders;0(0). [DOI] [PubMed] [Google Scholar]
- 33.Gaspar P, Duyckaerts C, Alvarez C, Javoy-Agid F, Berger B. Alterations of dopaminergic and noradrenergic innervations in motor cortex in Parkinson’s disease. Ann Neurol 1991;30(3):365–374. [DOI] [PubMed] [Google Scholar]
- 34.Thobois S, Dominey P, Decety J, Pollak P, Gregoire MC, Broussolle E. Overactivation of primary motor cortex is asymmetrical in hemiparkinsonian patients. Neuroreport 2000;11(4):785–789. [DOI] [PubMed] [Google Scholar]
- 35.Mure H, Hirano S, Tang CC, et al. Parkinson’s disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage 2011;54(2):1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuda M, Barnes A, Simon ES, et al. Thalamic stimulation for parkinsonian tremor: correlation between regional cerebral blood flow and physiological tremor characteristics. Neuroimage 2004;21(2):608–615. [DOI] [PubMed] [Google Scholar]
- 37.Thobois S, Dominey P, Fraix V, et al. Effects of subthalamic nucleus stimulation on actual and imagined movement in Parkinson’s disease : a PET study. J Neurol 2002;249(12):1689–1698. [DOI] [PubMed] [Google Scholar]
- 38.Liotti M, Ramig LO, Vogel D, et al. Hypophonia in Parkinson’s disease: neural correlates of voice treatment revealed by PET. Neurology 2003;60(3):432–440. [DOI] [PubMed] [Google Scholar]
- 39.Haslinger B, Kalteis K, Boecker H, Alesch F, Ceballos-Baumann AO. Frequency-correlated decreases of motor cortex activity associated with subthalamic nucleus stimulation in Parkinson’s disease. NeuroImage 2005;28(3):598–606. [DOI] [PubMed] [Google Scholar]
- 40.Feigin A, Fukuda M, Dhawan V, et al. Metabolic correlates of levodopa response in Parkinson’s disease. Neurology 2001;57(11):2083–2088. [DOI] [PubMed] [Google Scholar]
- 41.Hershey T, Black KJ, Carl JL, McGee-Minnich L, Snyder AZ, Perlmutter JS. Long term treatment and disease severity change brain responses to levodopa in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2003;74(7):844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Zhang Q, Li H, Zhang H. SPECT molecular imaging in Parkinson’s disease. J Biomed Biotechnol 2012;2012:412486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks DJ. PET and SPECT studies in Parkinson’s disease. Bailliere’s clinical neurology 1997;6(1):69–87. [PubMed] [Google Scholar]
- 44.Rascol O, Sabatini U, Chollet F, et al. Normal activation of the supplementary motor area in patients with Parkinson’s disease undergoing long-term treatment with levodopa. J Neurol Neurosurg Psychiatry 1994;57(5):567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rascol O, Sabatini U, Brefel C, et al. Cortical motor overactivation in parkinsonian patients with L-dopa-induced peak-dose dyskinesia. Brain 1998;121 (Pt 3):527–533. [DOI] [PubMed] [Google Scholar]
- 46.Mohl B, Berman BD, Shelton E, Tanabe J. Levodopa response differs in Parkinson’s motor subtypes: A task-based effective connectivity study. The Journal of comparative neurology 2017;525(9):2192–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. Pharmacologically modulated fMRI--cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain 2003;126(Pt 2):451–461. [DOI] [PubMed] [Google Scholar]
- 48.Spraker MB, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE. Basal ganglia hypoactivity during grip force in drug naive Parkinson’s disease. Hum Brain Mapp 2010;31(12):1928–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Planetta PJ, Kurani AS, Shukla P, et al. Distinct functional and macrostructural brain changes in Parkinson’s disease and multiple system atrophy. Hum Brain Mapp 2015;36(3):1165–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burciu RG, Ofori E, Shukla P, et al. Distinct patterns of brain activity in progressive supranuclear palsy and Parkinson’s disease. Mov Disord 2015;30(9):1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prodoehl J, Spraker M, Corcos D, Comella C, Vaillancourt D. Blood oxygenation level-dependent activation in basal ganglia nuclei relates to specific symptoms in de novo Parkinson’s disease. Mov Disord 2010;25(13):2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prodoehl J, Planetta PJ, Kurani AS, Comella CL, Corcos DM, Vaillancourt DE. Differences in brain activation between tremor- and nontremor-dominant Parkinson disease. JAMA neurology 2013;70(1):100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neely KA, Kurani AS, Shukla P, et al. Functional Brain Activity Relates to 0–3 and 3–8 Hz Force Oscillations in Essential Tremor. Cereb Cortex 2015;25(11):4191–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in brain research 1990;85:119–146. [PubMed] [Google Scholar]
- 55.Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 2007;35(1):222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabatini U, Boulanouar K, Fabre N, et al. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 2000;123 (Pt 2):394–403. [DOI] [PubMed] [Google Scholar]
- 57.Eckert T, Peschel T, Heinze HJ, Rotte M. Increased pre-SMA activation in early PD patients during simple self-initiated hand movements. J Neurol 2006;253(2):199–207. [DOI] [PubMed] [Google Scholar]
- 58.Haslinger B, Erhard P, Kampfe N, et al. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 2001;124(Pt 3):558–570. [DOI] [PubMed] [Google Scholar]
- 59.Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson’s disease: clinical and prognostic implications. Neurology 1985;35(4):522–526. [DOI] [PubMed] [Google Scholar]
- 60.Eggers C, Pedrosa DJ, Kahraman D, et al. Parkinson subtypes progress differently in clinical course and imaging pattern. PLoS One 2012;7(10):e46813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Helmich RC, Bloem BR, Toni I. Motor imagery evokes increased somatosensory activity in Parkinson’s disease patients with tremor. Hum Brain Mapp 2012;33(8):1763–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vercruysse S, Spildooren J, Heremans E, et al. The neural correlates of upper limb motor blocks in Parkinson’s disease and their relation to freezing of gait. Cereb Cortex 2014;24(12):3154–3166. [DOI] [PubMed] [Google Scholar]
- 63.Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. Neuroimage 2011;55(1):204–215. [DOI] [PubMed] [Google Scholar]
- 64.Wu T, Liu J, Zhang H, Hallett M, Zheng Z, Chan P. Attention to Automatic Movements in Parkinson’s Disease: Modified Automatic Mode in the Striatum. Cereb Cortex 2015;25(10):3330–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah C, Beall EB, Frankemolle AM, et al. Exercise Therapy for Parkinson’s Disease: Pedaling Rate Is Related to Changes in Motor Connectivity. Brain connectivity 2016;6(1):25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herz DM, Haagensen BN, Christensen MS, et al. Abnormal dopaminergic modulation of striato-cortical networks underlies levodopa-induced dyskinesias in humans. Brain 2015;138(6):1658–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine 1995;34(4):537–541. [DOI] [PubMed] [Google Scholar]
- 68.Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb Cortex 2010;20(5):1175–1186. [DOI] [PubMed] [Google Scholar]
- 69.Luo C, Song W, Chen Q, et al. Reduced functional connectivity in early-stage drug-naive Parkinson’s disease: a resting-state fMRI study. Neurobiol Aging 2014;35(2):431–441. [DOI] [PubMed] [Google Scholar]
- 70.Baudrexel S, Witte T, Seifried C, et al. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson’s disease. Neuroimage 2011;55(4):1728–1738. [DOI] [PubMed] [Google Scholar]
- 71.Kurani AS, Seidler RD, Burciu RG, et al. Subthalamic nucleus--sensorimotor cortex functional connectivity in de novo and moderate Parkinson’s disease. Neurobiol Aging 2015;36(1):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen B, Gao Y, Zhang W, et al. Resting State fMRI Reveals Increased Subthalamic Nucleus and Sensorimotor Cortex Connectivity in Patients with Parkinson’s Disease under Medication. Frontiers in aging neuroscience 2017;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kahan J, Urner M, Moran R, et al. Resting state functional MRI in Parkinson’s disease: the impact of deep brain stimulation on ‘effective’ connectivity. Brain 2014;137(Pt 4):1130–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sang L, Zhang J, Wang L, et al. Alteration of Brain Functional Networks in Early-Stage Parkinson’s Disease: A Resting-State fMRI Study. PLoS One 2015;10(10):e0141815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo C, Guo X, Song W, et al. Decreased Resting-State Interhemispheric Functional Connectivity in Parkinson’s Disease. Biomed Res Int 2015;2015:692684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu T, Long X, Wang L, et al. Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp 2011;32(9):1443–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan P, Zhan H, Xia M, Zhang Y, Guan D, Xu Y. Aberrant regional homogeneity in Parkinson’s disease: A voxel-wise meta-analysis of resting-state functional magnetic resonance imaging studies. Neuroscience and biobehavioral reviews 2017;72:223–231. [DOI] [PubMed] [Google Scholar]
- 78.Zeng Q, Guan X, Law Yan Lun JCF, et al. Longitudinal Alterations of Local Spontaneous Brain Activity in Parkinson’s Disease. Neuroscience bulletin 2017;33(5):501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu T, Long X, Zang Y, et al. Regional homogeneity changes in patients with Parkinson’s disease. Hum Brain Mapp 2009;30(5):1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwak Y, Peltier SJ, Bohnen NI, Muller ML, Dayalu P, Seidler RD. L-DOPA changes spontaneous low-frequency BOLD signal oscillations in Parkinson’s disease: a resting state fMRI study. Front Syst Neurosci 2012;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends in neurosciences 2011;34(12):611–618. [DOI] [PubMed] [Google Scholar]
- 82.Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 2005;6(4):285–296. [DOI] [PubMed] [Google Scholar]
- 83.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 1999;110(11):1842–1857. [DOI] [PubMed] [Google Scholar]
- 84.George JS, Strunk J, Mak-McCully R, Houser M, Poizner H, Aron AR. Dopaminergic therapy in Parkinson’s disease decreases cortical beta band coherence in the resting state and increases cortical beta band power during executive control. Neuroimage Clin 2013;3:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eusebio A, Brown P. Synchronisation in the beta frequency-band--the bad boy of parkinsonism or an innocent bystander? Exp Neurol 2009;217(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chung JW, Burciu RG, Ofori E, et al. Beta-band oscillations in the supplementary motor cortex are modulated by levodopa and associated with functional activity in the basal ganglia. NeuroImage: Clinical 2018;19:559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Hum Neurosci 2012;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Devos D, Labyt E, Derambure P, et al. Subthalamic nucleus stimulation modulates motor cortex oscillatory activity in Parkinson’s disease. Brain 2004;127(Pt 2):408–419. [DOI] [PubMed] [Google Scholar]
- 89.Kuhn AA, Kempf F, Brucke C, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci 2008;28(24):6165–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown P, Marsden CD. Bradykinesia and impairment of EEG desynchronization in Parkinson’s disease. Mov Disord 1999;14(3):423–429. [DOI] [PubMed] [Google Scholar]
- 91.Wang HC, Lees AJ, Brown P. Impairment of EEG desynchronisation before and during movement and its relation to bradykinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1999;66(4):442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Halje P, Tamte M, Richter U, Mohammed M, Cenci MA, Petersson P. Levodopa-induced dyskinesia is strongly associated with resonant cortical oscillations. J Neurosci 2012;32(47):16541–16551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swann NC, de Hemptinne C, Miocinovic S, et al. Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease. J Neurosci 2016;36(24):6445–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pollok B, Krause V, Martsch W, Wach C, Schnitzler A, Sudmeyer M. Motor-cortical oscillations in early stages of Parkinson’s disease. J Physiol 2012;590(13):3203–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heinrichs-Graham E, Wilson TW, Santamaria PM, et al. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson’s disease. Cereb Cortex 2014;24(10):2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirschmann J, Ozkurt TE, Butz M, et al. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson’s disease. Neuroimage 2011;55(3):1159–1168. [DOI] [PubMed] [Google Scholar]
- 97.Heinrichs-Graham E, Kurz MJ, Becker KM, Santamaria PM, Gendelman HE, Wilson TW. Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson’s disease: a pharmaco-magnetoencephalography study. J Neurophysiol 2014;112(7):1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain 2003;126(Pt 1):199–212. [DOI] [PubMed] [Google Scholar]
- 99.Pyatigorskaya N, Gallea C, Garcia-Lorenzo D, Vidailhet M, Lehericy S. A review of the use of magnetic resonance imaging in Parkinson’s disease. Ther Adv Neurol Disord 2014;7(4):206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sterling NW, Lewis MM, Du G, Huang X. Structural Imaging and Parkinson’s Disease: Moving Toward Quantitative Markers of Disease Progression. J Parkinsons Dis 2016;6(3):557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang J, Zhang YT, Hu WD, Li L, Liu GY, Bai YP. Gray matter atrophy in patients with Parkinson’s disease and those with mild cognitive impairment: a voxel-based morphometry study. International journal of clinical and experimental medicine 2015;8(9):15383–15392. [PMC free article] [PubMed] [Google Scholar]
- 102.Chen B, Wang S, Sun W, et al. Functional and structural changes in gray matter of parkinson’s disease patients with mild cognitive impairment. European journal of radiology 2017;93:16–23. [DOI] [PubMed] [Google Scholar]
- 103.Goldman JG, Stebbins GT, Dinh V, et al. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson’s disease with hallucinations. Brain 2014;137(Pt 3):849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gonzalez-Redondo R, Garcia-Garcia D, Clavero P, et al. Grey matter hypometabolism and atrophy in Parkinson’s disease with cognitive impairment: a two-step process. Brain 2014;137(Pt 8):2356–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosenberg-Katz K, Herman T, Jacob Y, Giladi N, Hendler T, Hausdorff JM. Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology 2013;80(16):1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hutton C, De Vita E, Ashburner J, Deichmann R, Turner R. Voxel-based cortical thickness measurements in MRI. Neuroimage 2008;40(4):1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 2009;48(2):371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koshimori Y, Segura B, Christopher L, et al. Imaging changes associated with cognitive abnormalities in Parkinson’s disease. Brain Struct Funct 2015;220(4):2249–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sterling NW, Wang M, Zhang L, et al. Stage-dependent loss of cortical gyrification as Parkinson disease “unfolds”. Neurology 2016;86(12):1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Le Bihan D Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 2003;4(6):469–480. [DOI] [PubMed] [Google Scholar]
- 111.Lenfeldt N, Larsson A, Nyberg L, Birgander R, Forsgren L. Fractional anisotropy in the substantia nigra in Parkinson’s disease: a complex picture. Eur J Neurol 2015;22(10):1408–1414. [DOI] [PubMed] [Google Scholar]
- 112.Burciu RG, Ofori E, Archer DB, et al. Progression marker of Parkinson’s disease: a 4-year multi-site imaging study. Brain 2017;140(8):2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Du G, Lewis MM, Sen S, et al. Imaging nigral pathology and clinical progression in Parkinson’s disease. Mov Disord 2012;27(13):1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seppi K, Schocke MF. An update on conventional and advanced magnetic resonance imaging techniques in the differential diagnosis of neurodegenerative parkinsonism. Curr Opin Neurol 2005;18(4):370–375. [DOI] [PubMed] [Google Scholar]
- 115.Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP. Diffusion tensor imaging of nigral degeneration in Parkinson’s disease: A region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. Neuroimage Clin 2013;3:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 2009;72(16):1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhan W, Kang GA, Glass GA, et al. Regional alterations of brain microstructure in Parkinson’s disease using diffusion tensor imaging. Mov Disord 2012;27(1):90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mole JP, Subramanian L, Bracht T, Morris H, Metzler-Baddeley C, Linden DE. Increased fractional anisotropy in the motor tracts of Parkinson’s disease suggests compensatory neuroplasticity or selective neurodegeneration. European radiology 2016;26(10):3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage 2013;73:239–254. [DOI] [PubMed] [Google Scholar]
- 120.Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson’s disease. Brain 2007;130(Pt 7):1834–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu XF, Zhang JQ, Jiang XM, et al. Amplitude of low-frequency oscillations in Parkinson’s disease: a 2-year longitudinal resting-state functional magnetic resonance imaging study. Chinese medical journal 2015;128(5):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Burciu RG, Chung JW, Shukla P, et al. Functional MRI of disease progression in Parkinson disease and atypical parkinsonian syndromes. Neurology 2016;87(7):709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barber TR, Klein JC, Mackay CE, Hu MTM. Neuroimaging in pre-motor Parkinson’s disease. Neuroimage Clin 2017;15:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burciu RG, Seidler RD, Shukla P, et al. Multimodal neuroimaging and behavioral assessment of alpha-synuclein polymorphism rs356219 in older adults. Neurobiol Aging 2018;66:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holtbernd F, Gagnon JF, Postuma RB, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology 2014;82(7):620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol 1968;31(1):14–27. [DOI] [PubMed] [Google Scholar]
- 127.Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol 2007;98(2):821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dettmers C, Fink GR, Lemon RN, et al. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol 1995;74(2):802–815. [DOI] [PubMed] [Google Scholar]
- 129.Lefaivre SC, Brown MJN, Almeida QJ. Cerebellar involvement in Parkinson’s disease resting tremor. Cerebellum & Ataxias 2016;3(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hutchison WD, Dostrovsky JO, Walters JR, et al. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J Neurosci 2004;24(42):9240–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Little S, Brown P. The functional role of beta oscillations in Parkinson’s disease. Parkinsonism Relat Disord 2014;20 Suppl 1:S44–48. [DOI] [PubMed] [Google Scholar]
- 132.Wakabayashi K, Mori F, Oyama Y, et al. Lewy bodies in Betz cells of the motor cortex in a patient with Parkinson’s disease. Acta Neuropathol 2003;105(2):189–192. [DOI] [PubMed] [Google Scholar]
- 133.Pfeiffer RF. Differentiating atypical parkinsonian syndromes--a way forward? Brain and behavior 2015;5(6):e00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heim B, Krismer F, De Marzi R, Seppi K. Magnetic resonance imaging for the diagnosis of Parkinson’s disease. Journal of Neural Transmission 2017;124(8):915–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shi HC, Zhong JG, Pan PL, et al. Gray matter atrophy in progressive supranuclear palsy: meta-analysis of voxel-based morphometry studies. Neurol Sci 2013;34(7):1049–1055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Summary of the functional and structural neuroimaging literature on the involvement of M1 in the pathophysiology of PD. Abbreviations: DBS = deep brain stimulation, FA = fractional anisotropy, M1 = primary motor cortex, rCBF = regional cerebral blood flow.