Abstract
Background:
Parkinson’s disease (PD) psychosis is often associated with cognitive impairment, including dementia, and involves dopaminergic, serotonergic, and cholinergic mechanisms.
Objective:
To evaluate the differential effect of the antipsychotic pimavanserin, a selective serotonin 2A receptor inverse agonist, in PD psychosis patients with versus without cognitive impairment and in those receiving versus not receiving cognitive-enhancing medications.
Methods:
Data from the pivotal randomized clinical trial of pimavanserin for PD psychosis were stratified by (1) screening MMSE score as cognitively impaired (21 to 24) versus unimpaired (≥25) and (2) concomitant use versus non-use of cognitive-enhancing medications. The primary outcome measure was change in the PD-adapted Scale for the Assessment of Positive Symptoms.
Results:
The mean (pimavanserin versus placebo) change from baseline was larger in the cognitively impaired (n=50) (-6.62 vs. -0.91; p=0.002) vs. the cognitively unimpaired (n=135) (−5.50 vs. −3.23; p=0.046) group. The comparable change was −6.04 vs. −2.18 (p=0.012) and −5.66 vs. −3.15 (p=0.041) in patients treated (n=69) and not treated (n=116) with concomitant cognitive-enhancing medication. Pimavanserin was similarly tolerated across all cognitive groups with no additional safety concerns identified. Overall adverse event rates were comparable across the concomitant cognitive-enhancing medication groups; however, rates of serious adverse events and discontinuations due to adverse events were increased in patients taking cholinesterase inhibitors.
Conclusions:
The antipsychotic effect of pimavanserin is robust in PD patients with cognitive impairment and may be enhanced by concomitant cognitive-enhancing medication use. Future prospective studies are needed to confirm these preliminary findings.
Keywords: Parkinson disease psychosis, Pimavanserin, Cognitive impairment, Cognitive-enhancing medications, Cholinesterase inhibitors, Memantine
INTRODUCTION
Parkinson disease psychosis (PDP), characterized by hallucinations and/or delusions, is a leading cause of disability and nursing home placement for Parkinson disease (PD) patients and impacts quality of life for both patients and their caregivers.1–3 PDP has a lifetime prevalence of up to 60% among PD patients.3,4 Risk factors include older age; greater disease severity; affective disorder; greater autonomic symptom burden; disorders of sleep and wakefulness, including REM sleep behavior disorder; and cognitive impairment.5–7 In addition to psychosis, cognitive impairment, including dementia, is another common and disabling non-motor symptom in PD.8–12 Numerous studies have reported a strong association between cognitive impairment and PDP.8,9,11,13 Thus, it is important that an antipsychotic used to treat PDP be effective, well tolerated, and safe across the cognitive spectrum and when used in combination with cognitive-enhancing medications.
Pimavanserin, a selective 5-HT2A inverse agonist, has demonstrated efficacy in a randomized, placebo-controlled trial and is the only antipsychotic specifically approved in the U.S. to treat hallucinations and delusions associated with PDP.14 In addition to serotonergic dysregulation,15,16 there is evidence that dopaminergic and cholinergic mechanisms are involved in the etiology of PDP.6 For example, dopaminergic medications, especially dopamine agonists, can induce or worsen hallucinations.17 Similarly, psychosis can be precipitated by anticholinergic medications.18 In addition, PDP is magnified in the setting of dementia (in part, a cholinergic-deficiency state in PD),19 and there is preliminary evidence that cognitive-enhancing procholinergic medications may also have antipsychotic effects.20–22
All antipsychotics approved by the U.S. Food and Drug Administration include a boxed warning for an increased risk of death in elderly patients with dementia-related psychosis.23 Although in the pivotal pimavanserin trial a formal diagnosis of dementia was exclusionary,14 it allowed a Mini-Mental State Examination (MMSE)24 score of ≥21, thus likely including a subset of patients with either mild cognitive impairment or mild dementia. We hypothesized that pimavanserin would have similar efficacy and tolerability in cognitively impaired PDP patients compared with cognitively unimpaired patients, and that PDP patients treated concomitantly with pimavanserin and a cognitive-enhancing medication would experience greater antipsychotic benefit than those taking pimavanserin alone.
METHODS
For this study, we used data from a 6-week randomized, double-blind, placebo-controlled, Phase 3 trial of pimavanserin (PIM) 34 mg (equivalent to 40 mg pimavanserin tartrate) in patients with PDP (ClinicalTrials.gov NCT01174004; Study ACP-013–020).14 In brief, adults aged ≥40 years and satisfying diagnostic criteria for PDP,25 with symptoms of psychosis present for at least one month and severe enough to require antipsychotic drug treatment, were randomized (1:1) to receive either PIM 34 mg daily or placebo. Patients were required to have an MMSE score ≥21 at screening. The primary outcome was the change in the 9-item Scale for the Assessment of Positive Symptoms adapted for PD (SAPS-PD; scores 0–45, higher scores indicating greater severity of psychosis).26 Although the original SAPS27 was designed for use in schizophrenia, it is one of only four scales recommended by the Movement Disorders Society Task Force on Rating Scales in PD for the assessment of response of psychosis to new treatments.28 Studies using the SAPS in clinical trials of PDP have shown that it is sensitive to change in response to treatment.29–31 The SAPS-PD is a shortened version that contains only items relevant to PDP while retaining the reliability and sensitivity to change of the larger scale.26 In a previous analysis, a 1-unit change in clinical global impression correlated to a 2.33-point change in SAPS-PD, a magnitude reflecting the minimal clinically important change in response to treatment.26
For this analysis, patients were stratified by MMSE score into cognitively impaired (21 to 24; N=50) and cognitively unimpaired (≥25; N=135). The cutoff utilized for this analysis is slightly more stringent than the MMSE score ≤25 recommended by the Movement Disorders Society Taskforce on Dementia in PD to screen for dementia.32 The primary outcome was the change in SAPS-PD score at Day 4314,26 and secondary outcomes were CGI-I score and tolerability. We also assessed efficacy and tolerability based on the concomitant use of cognitive-enhancing medications (i.e., either acetylcholinesterase inhibitors [ChI] or memantine [Mem]).
Statistical analysis
The safety analysis set included all patients who received at least one dose of study medication. The efficacy analysis set included all patients who had at least one post-baseline SAPS-PD assessment (modified intent-to-treat). The SAPS-PD change from baseline was analyzed with the mixed model repeated measures (MMRM) method. The model included fixed categorical effects of treatment (PIM or placebo), visit (days 15, 29, or 43), and treatment-by-visit interaction, and the continuous fixed covariate of baseline score. Missing values were not imputed. CGI-I analyses evaluated observed cases only. Significance (α<0.05) was determined based on the 2-sided p-value for treatment difference at specified visits from MMRM analysis. To evaluate the treatment effect over placebo between the subgroups with and without cognitive impairment or with and without cognitive-enhancing medications, an additional MMRM was performed using the aforementioned model with an additional subgroup factor and its associated 2-way and 3-way interaction terms. All statistical analyses we performed using SAS/STAT software Version 9.4 for Windows Server 2012.
RESULTS
The overall efficacy population included 185 participants: 95 in the PIM group and 90 in the placebo group. The safety population included 198 participants: 104 in the PIM group and 94 in the placebo group.
Baseline Demographics
Age, sex, PD duration, and duration of psychosis were comparable across the cognitive groups and regardless of coadministered cognitive-enhancing medication (Table 1). For the entire cohort, participants were in their early 70s with an MMSE score of ~26. Among PIM-treated patients, those in the cognitively impaired group compared with the overall study population were less likely to be male, had a longer duration of PD, and had a higher baseline SAPS-PD score, but the differences were not statistically significant. A total of 69 subjects (37%) were taking cognitive-enhancing medications, with 12 patients taking more than one. There was a lower proportion of women on Mem in the placebo group. Since there was no correlation between MMSE scores and cognitive-enhancing medication usage (Supplemental Figure 1) (rpb=−0.0751, p=0.309), an analysis of the use of cognitive-enhancing medication use stratified by MMSE was not performed.
Table 1.
Baseline demographic and clinical features
| Efficacy Analysis Set | MMSE 21–24 | MMSE ≥25 | Any Cognitive-enhancing Medication | Any acetylcholinesterase inhibitor | Memantine | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | PIM | Placebo | PIM | Placebo | PIM | Placebo | PIM | Placebo | PIM | Placebo | PIM | Placebo |
| (N=95) | (N=90) | (n=29) | (n=21) | (n=66) | (n=69) | (N=33) | (N=36) | (N=31) | (N=32) | (N=6) | (N=12) | |
| Age (yrs) | 72.4 (6.6) | 72.4 (7.9) | 73.4 (6.4) | 72.5 (9.1) | 72.0 (6.6) | 72.3 (7.6) | 73.1 (6.10) | 72.9 (8.4) | 72.9 (6.0) | 74.1 (7.9) | 73.2 (9.2) | 72.5 (9.2) |
| Sex (%), Male | 64 (67.4) | 52 (57.8) | 18 (62.1) | 16 (76.2) | 46 (69.7) | 36 (52.2) | 21 (63.6) | 27 (75.0) | 20 (64.5) | 25 (78.1) | 3 (50.0) | 10 (83.3) |
| PD duration (yrs) | 9.7 (6.5) | 10.6 (6.7) | 11.1 (6.9) | 12.0 (7.2) | 9.0 (6.3) | 10.2 (6.5) | 10.7 (7.7) | 8.4 (5.0) | 11.0 (7.8) | 8.9 (5.0) | 9.8 (7.3) | 9.0 (4.1) |
| PDP duration (mo) | 30.9 (30.0) | 36.4 (39.6) | 30.8 (36.1) | 34.2 (21.8) | 30.9 (27.3) | 37.1 (43.7) | 37.8 (36.0) | 32.6 (26.8) | 39.7 (36.3) | 33.1 (27.6) | 29.2 (21.6) | 31.3 (17.0) |
| SAPS-PD | 15.9 (6.1) | 14.7 (5.6) | 16.9 (6.0) | 14.2 (5.1) | 15.5 (6.2) | 14.9 (5.7) | 16.8 (6.5) | 14.6 (5.9) | 16.8 (6.6) | 15.1 (5.9) | 18.0 (6.3) | 12.1 (2.8) |
| CGI Severity | 4.3 (0.9) | 4.3 (0.9) | 4.5 (0.9) | 4.8 (0.8) | 4.2 (0.9) | 4.2 (0.9) | 4.0 (0.8) | 4.5 (0.9) | 4.0 (0.8) | 4.6 (1.0) | 4.2 (0.8) | 4.5 (1.0) |
| MMSE | 26.0 (2.6) | 26.6 (2.4) | 22.8 (1.1) | 23.1 (1.1) | 27.4 (1.6) | 27.6 (1.5) | 25.9 (2.4) | 26.1 (2.6) | 26.0 (2.5) | 25.9 (2.7) | 25.2 (1.9) | 26.4 (2.35) |
PIM: Pimavanserin; MMSE: Mini-Mental State Examination; CGI: Clinical Global Impression; PD: Parkinson’s Disease; PDP Parkinson’s Disease Psychosis
Mean (standard deviation)
SAPS-PD changes in cognitively impaired versus cognitively unimpaired
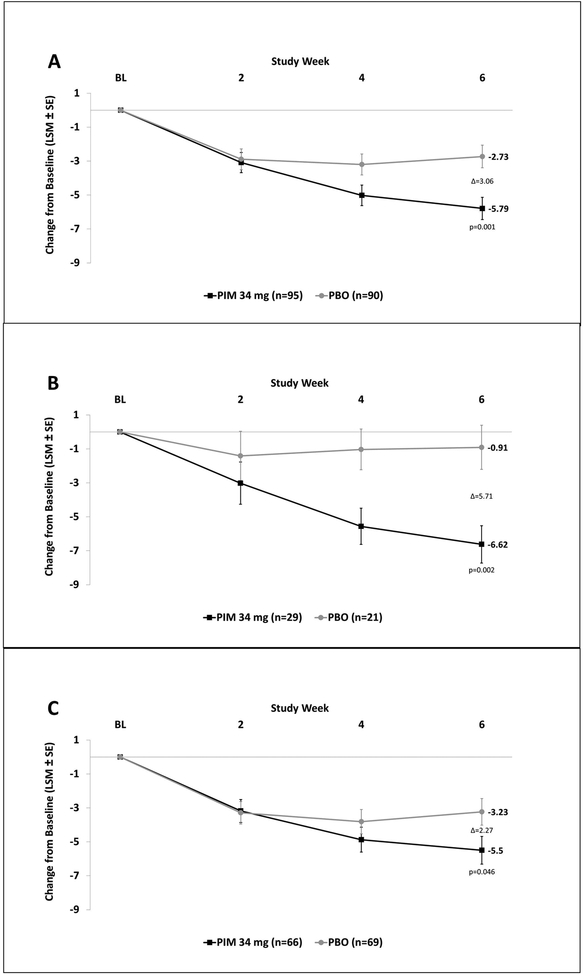
In the overall study population, the mean difference in SAPS-PD score change from baseline for PIM vs. placebo was −3.06 at Day 43 (p=0.001) (Figure 1A). PIM was superior to placebo in both the cognitively impaired and normal cognition subgroups. The least squares (LS) mean change from baseline in SAPS-PD scores at Day 43 in the cognitively impaired group was −6.62 with PIM vs. −0.91 with placebo (PIM minus placebo = −5.71 (95% CI, −9.17 to −2.24; p=0.002)) (Figure 1B). In the cognitively unimpaired group, the LS mean change in SAPS-PD from baseline to Day 43 was −5.50 with PIM vs. −3.23 with placebo (PIM minus placebo = −2.27 (95% CI, −4.50 to −0.04; p=0.046)) (Figure 1C). The between-group difference in SAPS-PD score change from baseline for those with and without cognitive impairment was not statistically significant.
Figure 1. SAPS-PD change from baseline stratified by baseline MMSE score.
SAPS-PD changes in: (A) overall population, (B) MMSE 21–24, (C) MMSE ≥25. (LSM: Least Squares Mean; SE: Standard Error; PIM: Pimavanserin; PBO: Placebo)
CGI-I in cognitively impaired versus cognitively unimpaired
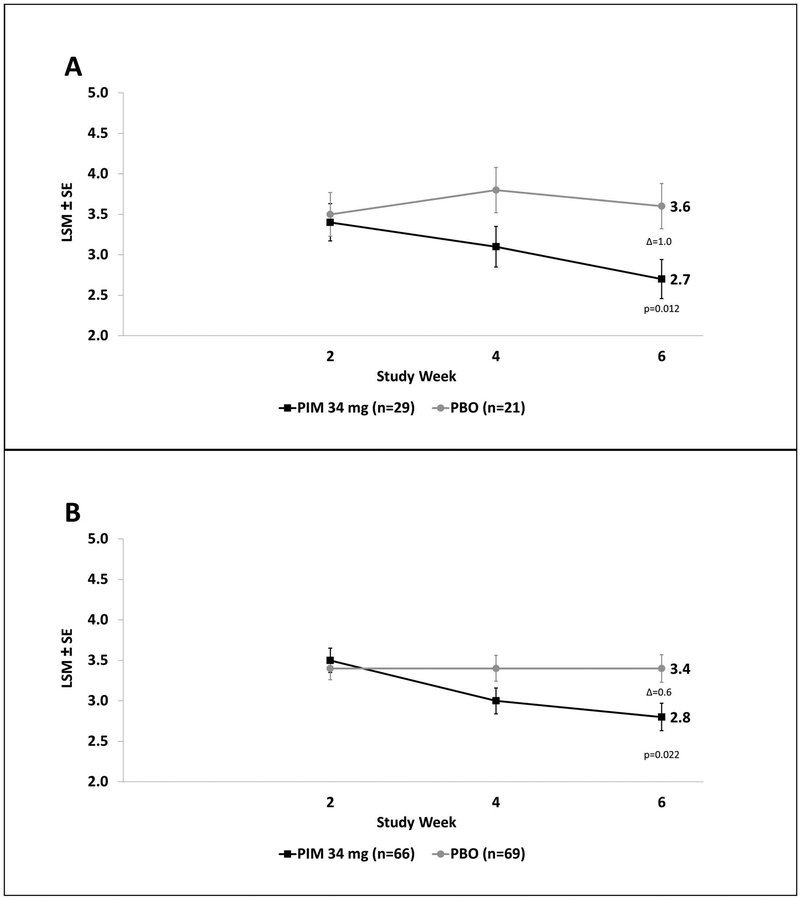
Among patients with cognitive impairment the mean difference in CGI-I score at Day 43 for pimavanserin vs. placebo was −1.0 (p=0.012), and for the cognitively unimpaired group the mean difference for pimavanserin vs. placebo was −0.6 (p=0.022). (Figure 2). The between-group difference in CGI-I for those with and without cognitive impairment was not statistically significant.
Figure 2.
CGI-I score in: (A) MMSE 21–24, and (B) MMSE ≥25. (LSM: Least Squares Mean; SE: Standard Error; PIM: Pimavanserin; PBO: Placebo)
SAPS-PD changes with concomitant cognitive-enhancing medications
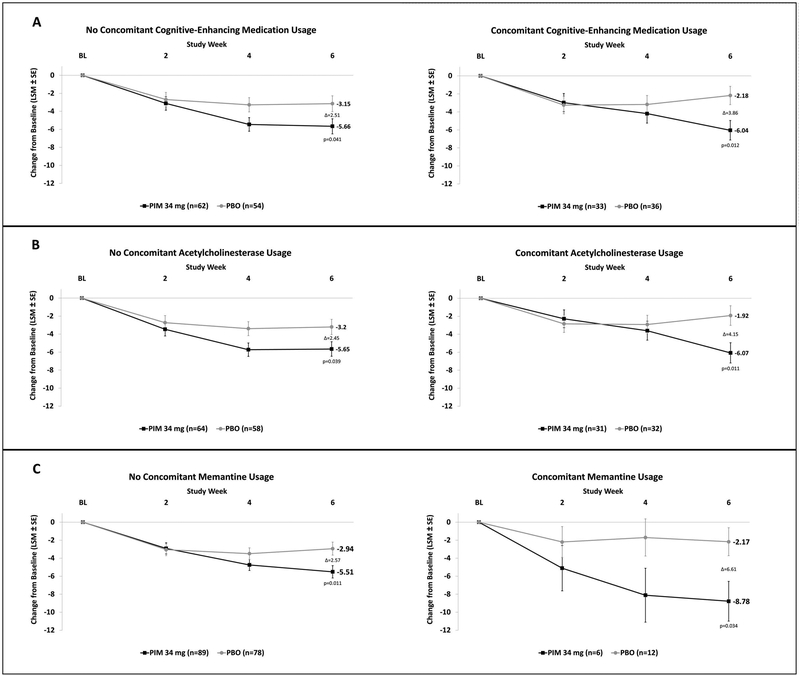
In the concomitant cognitive-enhancing medication group, the least squares (LS) mean change from baseline in SAPS-PD scores at Day 43 was −6.04 for PIM + cognitive-enhancing medication vs. −2.18 for placebo + cognitive-enhancing medication, a difference of −3.86 (p=0.012), compared to −5.66 and −3.15 in the PIM and placebo groups not taking cognitive-enhancing medication, a difference of −2.51 (p=0.041) (Figure 3A). The between-group difference in SAPS-PD score change from baseline for those treated with vs. without concomitant cognitive-enhancing medication was not statistically significant. Similarly, there was also a greater change from baseline in SAPS-PD for patients taking PIM with ChI or with Mem when compared to those not taking these concomitant medications (Figure 3B and 3C).
Figure 3. SAPS-PD change from baseline in patients with versus without cognitive-enhancing medications.
The change in SAPS-PD in subjects taking pimavanserin (PIM) or placebo (PBO), with or without concomitant cognitive-enhancing medication (A), any cholinesterase inhibitor (B), and memantine (C). (LSM: Least Squares Mean; SE: Standard Error)
CGI-I with concomitant cognitive-enhancing medications
Among patients taking concomitant cognitive-enhancing medications, the mean difference in CGI-I score at Day 43 for pimavanserin vs. placebo was −0.8 (p=0.01); for patients not taking them, the mean difference was −0.6 (p=0.03) (Supplemental Figure 2). The between-group difference in improvement in CGI-I for those treated with vs. without concomitant cognitive-enhancing medication was not statistically significant. Greater improvements in CGI-I were also seen for patients taking PIM with ChI or with MEM when compared to those not taking these concomitant medications.
Tolerability and Safety
The incidence of adverse events (AEs) leading to discontinuation was numerically higher in PIM versus placebo across all cognitively-defined groups: full population (9.6% vs. 3.2%), cognitive impairment (9.4% vs. 8.7%), and normal cognition (9.9% vs. 1.4%) (Table 2). The most common AEs, occurring in >5% of PIM treated patients overall, were urinary tract infection, fall, peripheral edema, hallucinations, nausea, and confusional state. The latter was reported more often in the pimavanserin group across all three groups: full population (5.8% vs. 3.2%), cognitive impairment (6.3% vs. 0.0%), and cognitively unimpaired (5.6% vs. 4.2%). Falls were reported more often in the pimavanserin group: full population (10.6% vs. 8.5%), cognitively impaired (12.5% vs. 8.7%), and cognitively unimpaired (9.9% vs. 8.5%).
Table 2.
Incidence (%) of adverse events (AE) based on baseline MMSE and concomitant cognitive-enhancing medication
| Safety Analysis Set | MMSE 21–24 | MMSE ≥25 | Any Cognitive-enhancing Medication | Any acetylcholinesterase inhibitor | Memantine | No Cognitive-enhancing Medication | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIM | Placebo | PIM | Placebo | PIM | Placebo | PIM | Placebo | PIM | Placebo | PIM | Placebo | PIM | Placebo | |
| (N=104) | (N=94) | (n=32) | (n=23) | (n=71) | (n=71) | (N=39) | (N=39) | (N=37) | (N=35) | (N=8) | (N=13) | (N=65) | (N=55) | |
| Most Common AEs* | ||||||||||||||
| Urinary tract infection | 14 (13.5) | 11 (11.7) | 2 (6.3) | 2 (8.7) | 12 (16.9) | 9 (12.7) | 6 (15.4) | 5 (12.8) | 6 (16.2) | 3 (8.6) | - - | 3 (23.1) | 8 (12.3) | 6 (10.9) |
| Fall | 11 (10.6) | 8 (8.5) | 4 (12.5) | 2 (8.7) | 7 (9.9) | 6 (8.5) | 8 (20.5) | 3 (7.7) | 8 (21.6) | 3 (8.6) | 1 (12.5) | - - | 3 (4.6) | 5 (9.1) |
| Peripheral edema | 7 (6.7) | 3 (3.2) | 2 (6.3) | - - | 5 (7.0) | 3 (4.2) | 1 (2.6) | 1 (2.6) | 1 (2.7) | 1 (2.9) | - - | - - | 6 (9.2) | 2 (3.6) |
| Hallucination | 7 (6.7) | 1 (1.1) | 1 (3.1) | - - | 6 (8.5) | 1 (1.4) | 4 (10.3) | 1 (2.6) | 4 (10.8) | 1 (2.9) | 1 (12.5) | - - | 3 (4.6) | - - |
| Nausea | 6 (5.8) | 6 (6.4) | 3 (9.4) | 4 (17.4) | 3 (4.2) | 2 (2.8) | 3 (7.7) | 4 (10.3) | 2 (5.4) | 4 (11.4) | 1 (12.5) | 1 (7.7) | 3 (4.6) | 2 (3.6) |
| Confusional state | 6 (5.8) | 3 (3.2) | 2 (6.3) | - - | 4 (5.6) | 3 (4.2) | 2 (5.1) | 1 (2.6) | 2 (5.4) | 1 (2.9) | - - | - - | 4 (6.2) | 2 (3.6) |
| Insomnia | 5 (4.8) | 4 (4.3) | 2 (6.3) | 1 (4.3) | 3 (4.2) | 3 (4.2) | 2 (5.1) | 2 (5.1) | 1 (2.7) | 2 (5.7) | 1 (12.5) | 1 (7.7) | 3 (4.6) | 2 (3.6) |
| Constipation | 4 (3.8) | 2 (2.1) | 1 (3.1) | - - | 3 (4.2) | 2 (2.8) | 2 (5.1) | 1 (2.6) | 2 (5.4) | - - | - - | 1 (7.7) | 2 (3.1) | 1 (1.8) |
| Arthralgia | 3 (2.9) | 2 (2.1) | - - | 1 (4.3) | 3 (4.2) | 1 (1.4) | 2 (5.1) | 1 (2.6) | 2 (5.4) | 1 (2.9) | - - | - - | 1 (1.5) | 1 (1.8) |
| Psychotic disorder | 3 (2.9) | 2 (2.1) | 2 (6.3) | 2 (8.7) | 1 (1.4) | - - | 2 (5.1) | 1 (2.6) | 2 (5.4) | 1 (2.9) | - - | - - | 1 (1.5) | 1 (1.8) |
| Back pain | 3 (2.9) | 1 (1.1) | - - | - - | 3 (4.2) | 1 (1.4) | 1 (2.6) | - - | 1 (2.7) | - - | - - | - - | 2 (3.1) | 1 (1.8) |
| Contusion | 3 (2.9) | 1 (1.1) | 3 (9.4) | 1 (4.3) | - - | - - | 2 (5.1) | 1 (2.6) | 2 (5.4) | 1 (2.9) | - - | - - | 1 (1.5) | - - |
| Diarrhea | 3 (2.9) | 1 (1.1) | 1 (3.1) | - - | 2 (2.8) | 1 (1.4) | 3 (7.7) | 1 (2.6) | 2 (5.4) | - - | 1 (12.5) | 1 (7.7) | - - | - - |
| Dehydration | 3 (2.9) | - - | 1 (3.1) | - - | 2 (2.8) | - - | 2 (5.1) | - - | 2 (5.4) | - - | - - | - - | 1 (1.5) | - - |
| Any AE | 74 (71.2) | 59 (62.8) | 22 (68.8) | 12 (52.2) | 52 (73.2) | 47 (66.2) | 28 (71.8) | 24 (61.5) | 26 (70.3) | 20 (57.1) | 4 (50.0) | 8 (61.5) | 46 (70.8) | 35 (63.6) |
| Any Serious AE | 11 (10.6) | 4 (4.3) | 3 (9.4) | 2 (8.7) | 8 (11.3) | 2 (2.8) | 6 (15.4) | 2 (5.1) | 6 (16.2) | 2 (5.7) | 1 (12.5) | 1 (7.7) | 5 (7.7) | 2 (3.6) |
| AE Leading to d/c | 10 (9.6) | 3 (3.2) | 3 (9.4) | 2 (8.7) | 7 (9.9) | 1 (1.4) | 6 (15.4) | 2 (5.1) | 6 (16.2) | 2 (5.7) | 1 (12.5) | - - | 4 (6.2) | 1 (1.8) |
| Fatal AE | 2 (1.9) | 1 (1.1) | 1 (3.1) | 1 (4.3) | 1 (1.4) | - - | 2 (5.1) | 1 (2.6) | 2 (5.4) | 1 (2.9) | - - | - - | - - | - - |
AEs occurring in > 2 of the PIM treated patients in the overall safety analysis group
(One patient did not have an MMSE score so could not be categorized for the MMSE analysis)
Adverse event rates were comparable across the concomitant cognitive-enhancing medication groups; however, rates of serious adverse events and discontinuations due to adverse events were increased in patients taking a ChI (Table 2). The rate of study discontinuation due to adverse events was 16.2% in the PIM+ChI group, 12.5% in the PIM+Mem, and 6.2% in the PIM-without concomitant cognitive-enhancing medication. The corresponding values in the placebo groups were 5.7%, 0.0%, and 1.8%. The mean (SD) heart rate-corrected QT interval (QTcF) change from baseline to the last assessment in PIM+ChI was +8.2 ms (23.5) ms, compared to +5.3 (20.5) ms in PIM without ChI, and +4.7 (18.2) ms in PIM+Mem, compared to +6.4 (21.8) ms in PIM without Mem. The corresponding values in the placebo groups were −0.6 (18.5) ms, +0.3 (16.3) ms, −1.2 (10.1) ms, and +0.2 (17.9) ms. There was no observed QTcF>500 ms in any group.
With PIM treatment one death each occurred in the cognitively impaired and normal cognition subgroups; in the placebo group, one death occurred in the cognitively impaired subgroup. All deaths were regarded as unrelated to study drug.14
DISCUSSION
In this subgroup analysis, pimavanserin demonstrated significant improvement in SAPS-PD and CGI-I scores among patients with PDP regardless of baseline cognition, but with larger responses in patients with impaired cognitive performance at baseline. Additionally, in participants taking concomitant cognitive-enhancing medications, there was also a larger numerical SAPS-PD effect compared to those not taking cognitive-enhancing medications, although not statistically significant given the low power of this post-hoc analysis. AEs were similar across the subgroups based on MMSE scores, but discontinuations due to an adverse event were more common in patients taking cognitive-enhancing medications.
Although psychotic symptoms in patients with PDP have a marked impact on patients and their caregivers,33–35 few treatment options are both safe and effective. Atypical antipsychotic drugs are commonly used for treating PDP but have been associated with increased morbidity.36 Furthermore, aside from clozapine and PIM,29,37 no other agents have been deemed efficacious by International Parkinson and Movement Disorder Society.38 To compound the difficulty, PDP is often associated with comorbid dementia, for which all antipsychotics have a boxed warning.23 Further, there is very limited data evaluating the safety and efficacy of antipsychotics in PDP patients with dementia compared to PDP patients without dementia. The only published study of which we are aware is a retrospective chart review of patient responses to quetiapine which included 20 PDP patients with dementia and 19 without, based on diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, revised third edition. In this study, improvement in psychosis was reported in a similar proportion of patients, but a greater number of demented patients experienced a worsening of motor symptoms.39 Our analysis suggests that PIM may provide a safe and efficacious treatment option for PDP patients with cognitive impairment, as well as those on cognitive-enhancing medications.
Further studies of PIM in PDP with a broader range of cognitive abilities (i.e., normal cognition, cognitive impairment, and dementia) are needed to replicate and further evaluate these findings of efficacy, tolerability, and safety in PD patients with cognitive impairment. Because previous studies have suggested antipsychotic effects for ChI and Mem,22,40,41 future studies should include stratification by ChI and Mem use to ensure a thorough analysis of the effects of these medications on psychosis. A recent study for PIM in patients with Alzheimer’s disease psychosis showed benefit,42 and another study of PIM for psychosis in multiple dementing disorders is ongoing (NCT03325556).
In addition to the post-hoc nature of this analysis, additional caveats need consideration. The definition of “cognitive impairment” was based upon the MMSE score (21–24), which is a relatively insensitive screening instrument for diagnosing mild cognitive impairment in PD43. To put the MMSE score in context, the corresponding Montreal Cognitive Assessment (MoCA) scores for the cognitively impaired group would be 16–20. In addition, patients with more significant cognitive impairment (i.e., MMSE score <21) were excluded. Finally, the six-week duration of the study prevents conclusions regarding long term tolerability and safety of PIM in patients with cognitive impairment or on cognitive-enhancing medication.
In conclusion, we provide preliminary evidence that the effect of pimavanserin may be robust in cognitively impaired PD patients, and raise the possibility that concomitant use of cognitive-enhancing medication may provide additional antipsychotic benefit. Future prospective randomized controlled trials should evaluate the efficacy and tolerability of pimavanserin in PD patients with a formal comorbid diagnosis of dementia, as well as in patients taking concomitant cognitive-enhancing medications to evaluate possible synergistic effects.
Supplementary Material
Supplemental Figure 1. Percent of patients taking cognitive-enhancing medications by baseline MMSE Score.
Supplemental Figure 2 CGI-I score in patients taking concomitant (A), any cholinesterase inhibitor (B), and memantine (C). (LSM: Least Squares Mean; SE: Standard Error; PIM: Pimavanserin; PBO: Placebo)
Acknowledgment:
Documentation of Author Roles
Research project: A. Conception, B. Organization, C. Execution;
Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
Manuscript: A. Writing of the first draft, B. Review and Critique.
AJE, DW: 1A, 1B, 2C, 3A, 3B
MTG, JN: 1B, 1C, 2C, 3B
NL, BC, JAV, CA, DF, CB, SAF, JHF, AEL: 2A, 3B
Funding sources
The study for this post-hoc analyses was funded by ACADIA Pharmaceuticals Inc.
Footnotes
Financial disclosure related to research covered in this article
AJE has served as Scientific Advisor for ACADIA.
MTG, JN, NJL, BC, CA, and DF are all employees of ACADIA.
Financial Disclosures (preceding 12 months)
AJE has received grant support from the NIH, Great Lakes Neurotechnologies and the Michael J. Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, TEVA, Impax, ACADIA, Acorda, Cynapsus/Sunovion, Lundbeck, and USWorldMeds; publishing royalties frocom Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from Abbvie, UCB, USWorldMeds, Lundbeck, ACADIA, the American Academy of Neurology, and the Movement Disorders Society.
MTG, JN, NL, BC, CA, DF, and CB are all employees of ACADIA Pharmaceuticals Inc.
JAV has nothing to declare.
SAF has received research grants from Ipsen, Medtronics, Teva, US World Meds, Sunovion Therapeutics, Solstice, Vaccinex, Voyager, Bristol Myers Squibb, Jazz Pharmaceuticals, CHDI Foundation, Michael J. Fox Foundation, NIH; personal honoraria from Neurocrine, Lundbeck, Teva, Avanir, Sunovion, Biogen, Prexton Therapeutics, Adamas; and royalties from Demos, Blackwell Futura for textbooks, and Uptodate.
JHF is a paid consultant to ACADIA Pharmaceuticals; received royalties from Springer Press and Cambridge University Press; research funds from NIH and the Michael J. Fox Foundation.
AEL has served as an advisor for Abbvie, Acorda, Biogen, Bristol Myers Squibb, Janssen, Sun Pharma, Merck and Corticobasal Degeneration Solutions; received honoraria from Sun Pharma, Medichem, Medtronic, AbbVie and Sunovion; received grants from Brain Canada, Canadian Institutes of Health Research, Corticobasal Degeneration Solutions, Edmond J Safra Philanthropic Foundation, Michael J. Fox Foundation, the Ontario Brain Institute, National Parkinson Foundation, Parkinson Society Canada, and W. Garfield Weston Foundation; received publishing royalties from Elsevier, Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press.
DW has received research funding or support from Michael J. Fox Foundation for Parkinson’s Research, National Institutes of Health (NINDS), Novartis Pharmaceuticals, Department of Veterans Affairs, Avid Radiopharmaceuticals, Alzheimer’s Disease Cooperative Study, and the International Parkinson and Movement Disorder Society; honoraria from AbbVie, ACADIA, Biotie, Clintrex LLC, Janssen, Novartis, Pfizer, Teva Pharmaceuticals, UCB, and the CHDI Foundation; license fee payments from the University of Pennsylvania for the QUIP and QUIP-RS; royalties from Wolters Kluweland; and fees for legal consultation for lawsuit related to antipsychotic prescribing in a patient with Parkinson’s disease.
References
- 1.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48(8):938–942. [DOI] [PubMed] [Google Scholar]
- 2.Factor SA, Feustel PJ, Friedman JH, et al. Longitudinal outcome of Parkinson’s disease patients with psychosis. Neurology. 2003;60(11):1756–1761. [DOI] [PubMed] [Google Scholar]
- 3.Fenelon G, Alves G. Epidemiology of psychosis in Parkinson’s disease. J Neurol Sci. 2010;289(1–2):12–17. [DOI] [PubMed] [Google Scholar]
- 4.Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67(8):996–1001. [DOI] [PubMed] [Google Scholar]
- 5.Factor SA, Scullin MK, Freeman A, Bliwise DL, McDonald WM, Goldstein FC. Affective Correlates of Psychosis in Parkinson’s Disease. Mov Disord Clin Pract. 2017;4(2):225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahodne LB, Fernandez HH. Pathophysiology and treatment of psychosis in Parkinson’s disease: a review. Drugs Aging. 2008;25(8):665–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett MJ, Blair JC, Sperling SA, Smolkin ME, Druzgal TJ. Baseline symptoms and basal forebrain volume predict future psychosis in early Parkinson disease. Neurology. 2018;90(18):e1618–e1626. [DOI] [PubMed] [Google Scholar]
- 8.Lenka A, Hegde S, Arumugham SS, Pal PK. Pattern of cognitive impairment in patients with Parkinson’s disease and psychosis: A critical review. Parkinsonism Relat Disord. 2017;37:11–18. [DOI] [PubMed] [Google Scholar]
- 9.Aarsland D, Creese B, Politis M, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13(4):217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pigott K, Rick J, Xie SX, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology. 2015;85(15):1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: a cohort study. Lancet Neurol. 2017;16(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weintraub D, Simuni T, Caspell-Garcia C, et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. Mov Disord. 2015;30(7):919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinkle JT, Perepezko K, Bakker CC, et al. Domain-specific cognitive impairment in non-demented Parkinson’s disease psychosis. Int J Geriatr Psychiatry. 2018;33(1):e131–e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–540. [DOI] [PubMed] [Google Scholar]
- 15.Albin RL, Koeppe RA, Bohnen NI, Wernette K, Kilbourn MA, Frey KA. Spared caudal brainstem SERT binding in early Parkinson’s disease. J Cereb Blood Flow Metab. 2008;28(3):441–444. [DOI] [PubMed] [Google Scholar]
- 16.Ballanger B, Strafella AP, van Eimeren T, et al. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67(4):416–421. [DOI] [PubMed] [Google Scholar]
- 17.Diederich NJ, Fenelon G, Stebbins G, Goetz CG. Hallucinations in Parkinson disease. Nat Rev Neurol. 2009;5(6):331–342. [DOI] [PubMed] [Google Scholar]
- 18.Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium - theory, evidence and practice. Br J Clin Pharmacol. 2016;81(3):516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005;20(10):1255–1263. [DOI] [PubMed] [Google Scholar]
- 20.Sawada H, Oeda T, Yamamoto K, et al. Trigger medications and patient-related risk factors for Parkinson disease psychosis requiring anti-psychotic drugs: a retrospective cohort study. BMC Neurol. 2013;13:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Factor SA, McDonald WM, Goldstein FC. The role of neurotransmitters in the development of Parkinson’s disease-related psychosis. Eur J Neurol. 2017;24(10):1244–1254. [DOI] [PubMed] [Google Scholar]
- 22.Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351(24):2509–2518. [DOI] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration (FDA). FDA Public Health Advisory. April 11, 2015.
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 25.Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22(8):1061–1068. [DOI] [PubMed] [Google Scholar]
- 26.Voss T, Bahr D, Cummings J, Mills R, Ravina B, Williams H. Performance of a shortened Scale for Assessment of Positive Symptoms for Parkinson’s disease psychosis. Parkinsonism Relat Disord. 2013;19(3):295–299. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen NC. Iowa CityScale for the assessment of positive symptoms (SAPS).
- 28.Fernandez HH, Aarsland D, Fenelon G, et al. Scales to assess psychosis in Parkinson’s disease: Critique and recommendations. Mov Disord. 2008;23(4):484–500. [DOI] [PubMed] [Google Scholar]
- 29.Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N Engl J Med. 1999;340(10):757–763. [DOI] [PubMed] [Google Scholar]
- 30.Pintor L, Valldeoriola F, Bailles E, Marti MJ, Muniz A, Tolosa E. Ziprasidone versus clozapine in the treatment of psychotic symptoms in Parkinson disease: a randomized open clinical trial. Clin Neuropharmacol. 2012;35(2):61–66. [DOI] [PubMed] [Google Scholar]
- 31.Goetz CG, Blasucci LM, Leurgans S, Pappert EJ. Olanzapine and clozapine: comparative effects on motor function in hallucinating PD patients. Neurology. 2000;55(6):789–794. [DOI] [PubMed] [Google Scholar]
- 32.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314–2324. [DOI] [PubMed] [Google Scholar]
- 33.Aarsland D, Bronnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. 2007;78(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G. What predicts mortality in Parkinson disease?: a prospective population-based long-term study. Neurology. 2010;75(14):1270–1276. [DOI] [PubMed] [Google Scholar]
- 35.Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Caregiver-burden in parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006;12(1):35–41. [DOI] [PubMed] [Google Scholar]
- 36.Weintraub D, Chiang C, Kim HM, et al. Antipsychotic use and physical morbidity in Parkinson disease. Am J Geriatr Psychiatry. 2017;25(7):697–705. [DOI] [PubMed] [Google Scholar]
- 37.Jethwa KD, Onalaja OA. Antipsychotics for the management of psychosis in Parkinson’s disease: systematic review and meta-analysis. BJPsych Open. 2015;1(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seppi K, Chahine L, Chaudhuri RK, et al. International Parkinson and Movement Disorder Society Evidence-Based Medicine Review: Update on Treatments for the Non-Motor Symptoms of Parkinson’s Disease. 2018; https://www.movementdisorders.org/MDS-Files1/Resources/PDFs/EBM_NMS_22062018.WEB.pdf. Accessed July 2, 2018. [DOI] [PubMed]
- 39.Reddy S, Factor SA, Molho ES, Feustel PJ. The effect of quetiapine on psychosis and motor function in parkinsonian patients with and without dementia. Mov Disord. 2002;17(4):676–681. [DOI] [PubMed] [Google Scholar]
- 40.Gauthier S, Loft H, Cummings J. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer’s disease by memantine: a pooled data analysis. Int J Geriatr Psychiatry. 2008;23(5):537–545. [DOI] [PubMed] [Google Scholar]
- 41.Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry. 2008;69(3):341–348. [DOI] [PubMed] [Google Scholar]
- 42.Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213–222. [DOI] [PubMed] [Google Scholar]
- 43.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Percent of patients taking cognitive-enhancing medications by baseline MMSE Score.
Supplemental Figure 2 CGI-I score in patients taking concomitant (A), any cholinesterase inhibitor (B), and memantine (C). (LSM: Least Squares Mean; SE: Standard Error; PIM: Pimavanserin; PBO: Placebo)