Abstract
Purpose:
Segmentation of cardiac medical images, an important step in measuring cardiac function, is usually performed either manually or semi-automatically. Fully automatic segmentation of the left ventricle (LV), the right ventricle (RV) as well as the myocardium of 3D magnetic resonance (MR) images throughout the entire cardiac cycle (4D), remains challenging. This study proposes a deformable-based segmentation methodology for efficiently segmenting 4D (3D+t) cardiac MR images.
Methods:
The proposed methodology first used the Hough transform and the local Gaussian distribution method (LGD) to segment the LV endocardial contours from cardiac MR images. Following that a novel level set-based shape prior method was applied to generate the LV epicardial contours and the RV boundary.
Results:
This automatic image segmentation approach has been applied to studies on 17 subjects. The results demonstrated that the proposed method was efficient compared to manual segmentation, achieving a segmentation accuracy with average Dice values of 88.62±5.47%, 87.35±7.26%, and 82.63±6.22% for the LV endocardial, LV epicardial and RV contours, respectively.
Conclusions:
We have presented a method for accurate LV and RV segmentation. Compared to three existing methods, the proposed method can successfully segment the LV and yields the highest Dice value. This makes it an option for clinical assessment of the volume, size and thickness of the ventricles.
Keywords: left ventricle, right ventricle, segmentation, level set method
I. INTRODUCTION
Cardiovascular disease consistently ranks among the leading causes of morbidity and mortality. In 2008, 17.3 million people died due to cardiovascular disease worldwide, accounting for 30% of total deaths12. Of these cases, about 7.3 million were due to coronary heart disease, and 6.2 million were due to stroke12.
In diagnosing, monitoring, and treating these pathologies numerous cardiac imaging modalities are available, including: magnetic resonance (MR) imaging, computed tomography (CT), echocardiography (ECHO), and nuclear medicine36. Time-resolved MRI studies have been proven to be effective in this setting38. Conventionally, multi-slice 2D cardiac cine MRI is applied with one or two slices per breath-hold. This requires multiple breath-holds to provide volumetric coverage of the heart. The cardiac contractility can be quantified from measurements of ventricle volumes, ejection fraction, and wall thickening. Left ventricular myocardial mass can also be quantified. These quantification methods require segmenting the left ventricle (LV) and the right ventricle (RV) on the cardiac MR cine images. Compared to 2D imaging, 3D imaging provides an increase in the signal-to-noise ratio (SNR), contiguous volumetric coverage, and thinner slices, and allows for more accurate measurement of cardiac volumes and function, reduced co-registration errors, and more flexible postprocessing. Free-breathing cardiac MRI techniques improve patients’ comfort during the scan and enable image acquisition that is not constrained by the length of the breath-hold permitting more advantageous MRI parameter settings. Free-breathing 4D (3D+t) cardiac imaging has been demonstrated to be promising for evaluating cardiac function11,40,42,44,72.
However, the slice-wise manual delineation of the LV and RV from 3D cardiac cine MR images is time-consuming, tedious, and since even a skilled cardiologist or radiologist requires more than 30 minutes to process one study, is prone to fatigue errors45,49. Development of an efficient, computer-aided method for cardiac segmentation is highly desirable for replacing the subjective manual contouring conducted by radiologists. That capability would provide post-processing that is more time-efficient, and not subject to tedium and consequent error, and could also provide more accurate delineation of the boundary of cardiac structures.
A. Previous studies
A number of segmentation approaches have been developed for the delineation of car-diac contours on cine MR images, including thresholding, clustering18, the active contour model20,47, the active shape model21,22, the active appearance model14,23, sparse shape composition24,25, the atlas or registration-based model13,14,16 and the deep learning based segmentation2,3,37,54. Most of these methods use priors and need a set of training shapes, patterns of variation in shapes, and spatial relationships of structures. The active shape model applies principal component analysis to a set of training shapes in order to extract a certain number of modes that represent shape variations. In the study of Andreopoulos23, a generalized active appearance model was proposed that combined both shape and appearance variations into a unified model for the segmentation of cardiac images. The sparse shape representation was proposed to approximate the shape of a target image using a sparse linear combination of other shape instances, which had been previously annotated. For the atlas-based methods49, the labeled atlas is registered to the target image and then the resultant transformation is applied to the atlas to obtain the final segmentation.
The methods mentioned above that use priors can, in many cases, achieve good performance. However, their robustness is limited because of the dependence on the training data. To date, most segmentation methods have been proposed and tested for evaluation of 2D cardiac cine MR images, while there has been relatively little work (i.e. Lin et al17 and Zhang et al24) that was proposed for 3D cardiac cine MR images. However, Lin et al. only detected the LV center and RV insertion points, not the LV and RV contours. Zhang et al. used a landmark-based method to segment the LV and RV, which may be sensitive to the landmark template. In this study, we developed a segmentation method that has been applied to data from a highly accelerated 4D (3D+t) cardiac cine MRI approach developed by our team33, that achieves whole heart coverage in a scan time of ~2.5 minutes during free-breathing. This study investigated a segmentation method without use of training data for achieving cardiac functional measurements based on 4D cardiac MRI data acquired in a short scan time, an advance that we believe could be helpful in clinical practice.
B. Contributions
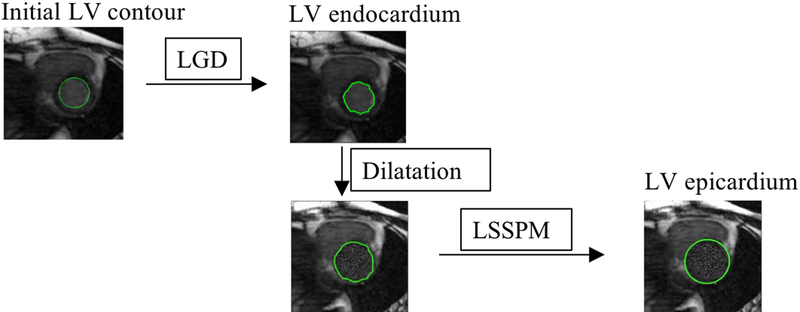
We propose to segment the heart by delineating both the endocardial and the epicar-dial borders of the LV chamber and the contour of the RV chamber. The latter is usually too thin to differentiate the endocardial and epicardial borders. We first segmented the LV endocardium using the Hough transfrom55 and the local Gaussian distribution method (LGD)29; once the endocardium was segmented, we segmented the LV epicardial and RV contours using the proposed level set-based shape prior method (LSSPM). This method included an elliptically refined term and a similarity term in a narrow band level set model. In this study, the segmentation accuracy was assessed using manual segmentation as the ground-truth. The segmented geometries can be used in biomechanical simulation66,68–71. Cardiac functional measurements, including chamber volumes, stroke volume, ejection fraction, cardiac output and myocardial wall thickness, were calculated based on the generated segmentations.
II. METHODOLOGY
A. Image pre-processing
We applied fully automatic pre-processing including cropping the volume to the region of interest, denoising, and contrast enhancement to improve the segmentation efficiency and reliability51.
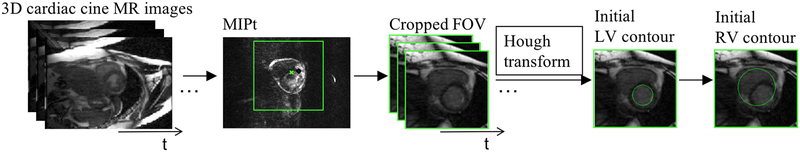
Based on the 3D cardiac cine images, a cropped field of view (FOV), centered on and including the heart, was first established before segmentation, as follows. In cardiac cine images, the majority of signal changes through the cardiac cycle are within the region surrounding the heart. A series of gradient images between adjacent time frames were calculated to detect signal changes. A maximum intensity projection through the time (MIPt) was applied to those gradient images. This produced images that have high intensities in the region of the heart. The calculated center of mass on the so-called MIPt images was then identified as the center of the heart (marked as a green star in FIG. 1), and was chosen as the center of the region of interest (ROI or cropped FOV) for segmentation (see FIG. 1). With an assumption for the size of the heart (~8×10×12 cm3) and with the known image spatial resolution (1.3×1.8×4 mm), an ROI of ~10×10 cm2 was chosen, with the center selected as described above. In this present study, linear image enhancement52 was used to increase the contrast of the images in the cropped FOV.
FIG. 1.
Diagram of the image pre-processing, including generated MIPt image, cropped images from 4D MR images, the initial LV and RV contours using the Hough transform.
B. Initial contour using the Hough transform
The LV endocardial contour has high contrast between the blood pool and the myocardium and is generally circular on the cardiac cine images acquired in the short-axis view. This allows us to apply the Hough transform to derive an initial circular contour for the LV endocardium. Automatic detection of the maximum voted circle of accumulator from the Hough transform was performed to find the center and radius of the circle. The only parameter in the Hough transform is the radius56 which is unknown. In this study, an initial radius for the Hough transform was estimated within a range of [1/8, 1/2] ∗ length of FOV. We first performed this process on a cropped FOV of the middle slice of the LV for the first-time frame (FIG. 1), following that the detected initial contour was used in the subsequent steps to segment the LV.
We enlarged (double the size) the initial contour of the LV to cover the RV. Although the shape of the RV is not circular, by applying the LSSPM algorithm and Boolean operation, the initial circular contour then deforms to fit the RV chamber (more details can be found in D. RV segmentation scheme). Similar to the LV segmentation, we started on a middle slice and propagated to the other slices sequentially.
C. LV segmentation scheme
The proposed LV segmentation methodology applies the Hough transform55 and LGD to segment the LV endocardial region from the 4D (3D+t) cardiac MR images, and then segments the epicardial boundary. The epicardial region is a diffuse object with low contrast relative to neighboring tissues, as opposed to the endocardial contour which separates high signal intensity blood from low signal intensity myocardium. It is therefore more difficult to delineate the epicardial contour with the same methods that work for segmenting endocardial contours. Similar to our previous work on segmenting the outer wall of the abdominal aortic aneurysms41, we here propose to replace the signal intensities of the voxels within the LV chamber with the mean value of a one-voxel-thick layer outside the LV chamber. This yields a more efficient and reliable delineation of the epicardial border using LSSPM and the dilated LV chamber as an initial contour.
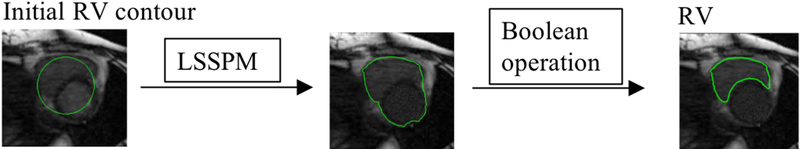
D. RV segmentation scheme
The active contour methods have been used on RV segmentation from 2D/3D MR images57,58. However, segmentation of the RV from 4D MR images is more challenging because of thinner imaging slice thickness and lower contrast between the myocardium and blood. Considering that the intensity of the LV and RV is similar and different from the surrounding tissue, we propose to segment the entire ventricular wall (LV + RV lateral wall) using LSSPM, and then to extract the RV by using Boolean operation on the entire ventricular contour and the LV contour obtained from the previous segmentation step. The RV segmentation scheme is shown in FIG. 3 and more details are described in the following sections.
FIG. 3.
Schematic diagram of RV segmentation: The LSSPM was used to separate the entire ventricular wall from the surrounding tissues, and the boolean opearation was used to separate the RV and LV region.
E. LGD segmentation
The Local Gaussian distribution model is an implicit active contour method based on the local intensity distribution29. We assume the image domain can be partitioned into two regions inside and outside the zero level set, which are marked as i = 1 and i = 2, respectively. The energy formulation for LGD segmentation can be written as follows:
| (1) |
where I is the image to be processed, ϕ is the distance function, H is the Heaviside function, Pi;x(I(y)) is the probability density, ω is a non-negative weighting function, chosen as a truncated Gaussian Kernel, and x denotes a point in the image and y represents a point within a circular neighborhood around x with a small radius ρ. To model the probability densities, we assume the mean and variance of the local Gaussian distribution are spatially varying parameters:
| (2) |
where ui(x) and σi(x) are local intensity means and standard deviations, respectively. The formulas of ui(x) and σi(x)2 were presented in several publications29,59,60:
| (3) |
| (4) |
where M1(ϕ(y)) = H(ϕ(y)) and M2(ϕ(y)) = 1 − H(ϕ(y)). Minimization of the energy function can be achieved by solving the following gradient descent flow equation:
| (5) |
where δ(ϕ) is Dirac function, ν and μ are positive regularization parameters, e1 − e2 denotes the image-based term that is independent of scale of local intensities caused by intensity inhomogeneity:
| (6) |
| (7) |
According to Wang and Li29,30, the kernel ω in Eq. (1) is truncated as a (2ρ+1)×(2ρ+1) mask, where ρ is no less than 2σ. We used σ = 3, ρ = 6, time step δt = 0:1, μ = 1:0, and ν = 0:0001 × 255 × 255 for all cases in our LGD segmentation29.
F. LSSPM segmentation
Given the assumption that the healthy epicardial region is bounded by an elliptical-liked contour, we added an ellipse term and a similarity term to the narrow band level set method to refine the segmentation. We adopt the following expression for the proposed LSSPM segmentation energy formulation form:
| (8) |
where Elevel set represents the level set term, Eellipse embeds the ellipse-like shape prior, and Esimilarity represents the similarity term. α and β are the positive hyper-parameters that balance the influence of the two terms.
In LSSPM segmentation, the weight α and β are the parameters for balancing three terms. When α and β is low, for example, in the extreme case α = 0 and β = 0, this amounts to using the traditional narrow band segmentation; when α is high, the segmentation contour will be closer to an ellipse; in the extreme case α = +∞, predominant weighting is given to the ellipsoid shape; for intermediate α values (~ 1), local variations of the final contour are closer to those of actual contours. In all the experiments presented herein, α and β were set at 0.5 and 0.2, which has been observed to provide contours that follow the actual ones.
As Alessandrini6 pointed out, Elevel set could be any of the data attachment terms, and we adopt the narrow band active contour as the data attachment term. The minimization of Elevel set with respect to ϕ leads to the following level set equation:
| (9) |
where f(x) is given by the following equation:
| (10) |
| (11) |
where y is a spatial variable in a user-de ned neighborhood N(x) at point x. The quantities ux and vx correspond to the average intensity values measured inside and outside the region N(x) respectively. The annular shape constraint on the level set framework is developed by minimizing the following energy criterion:
| (12) |
| (13) |
| (14) |
where ϕe(x) represents the Sampson distance of a point x from the annular shape determined by the parameter λ6, and F (x, λ) corresponds to the algebraic distance of a point x = (x1, x2) to an ellipse that is calculated with the standard quadratic equation for conic sections. The following equations are derived by obtaining the minimization of energy function defined in Eq.(10), subject to finding the zero level set:
| (15) |
| (16) |
Esimilarity represents the shape term which is incorporated in the energy functional measuring the non-overlapping areas between the initial prior shape and the evolving shape. ϕi(x) and ϕ(x) denote the distance function of the prior shape and the evolving level set shape representation. This energy function can be expressed as
| (17) |
where H is the Heaviside function.
G. Cardiac functional measurements
In this study, we applied the proposed segmentation method to obtain the cardiac measurements that are of clinical importance, including ventricular chamber volumes, stroke volume, ejection fraction, cardiac output and left ventricular myocardial wall thickness measurements.
Left Ventricular End-Diastolic and End-Systolic Volumes (LVEDV and LVESV) are measurements of the amount of blood in the chamber, encompassed by the myocardial tissue, Left Ventricular Stroke Volume (LVSV) is the amount of blood ejected from the heart during each contraction (LVSV = LVEDV - LVESV). Left Ventricular Ejection Fraction (LVEF) is a ratio of the blood pumped out of the heart in each beat (LVEF = LVSV / LVEDV), and Cardiac Output (LVCO) is the amount of blood pumped by the heart per minute (LVCO = LVSV × heart rate).
Left Ventricular Wall Thickness (LVWT) is the thickness of the myocardium and is typically measured on end-diastolic images in the sagittal view. The American Heart Association (AHA) 17-segment model is used to analyze wall thickness, and can be used to relate abnormalities to disease of the relevant coronary arteries48. Advances in 3D cine MRI technology allow us to provide more information than the traditional AHA 17-segment model. For each image slice, two contours were extracted, representing the inner and outer myocardial surfaces. Thicknesses were calculated at 360 locations (per degree) per image slice where rays radiating from the center of the myocardium intersected those contours. Thickness measurements were obtained at both end-systole and end-diastole to determine the myocardial thickening during the cardiac cycle.
III. EXPERIMENTS AND RESULTS
The segmentation experiments were performed on an OS X system with an Intel Core i7 CPU and 8 GB RAM using MATLAB®-based software developed in-house. Manual segmentation of the LV and RV, was performed independently by three trained experts (nine-, six- and four-year experience in medical imaging respectively) using MeVisLab software (http://www.mevislab.de/).
A. MRI data acquisition
This study was approved by the Institutional Review Board at UCSF. Written informed consent was obtained from all subjects after study procedures were fully explained. Data were acquired from 17 subjects (8 females, 9 males, age 37.6±15.1 years and heart rate = 64.3±8.8 bpm) on a 3.0T MR scanner (GE Medical Systems, Milwaukee, WI) with an 8-channel cardiac coil. The acquisition parameters for 3D imaging were: FOV=34.0×25.5cm2, slice thickness=4.0–5.0 mm, image matrix=256×144, and number of slices=30~32, TR/TE=4.1/1.7ms, flip angle=60°, bandwidth=±125kHz, temporal resolution =40ms, and scan time =2.5±0.3 minutes33.
B. Manual Segmentation
Manual segmentation was used as the ground-truth to evaluate the automatic segmentation32,34,67, where the Dice value is commonly used for measuring the overlap between manual and automatic segmentation. A MevisLab-based tool was implemented to help our readers draw the manual contours. The manual segmentation contours for end-diastolic and end-systolic slices of all subjects were performed in a blinded manner: all three readers were blinded to the automated segmentation results. An element was labelled as 1 if more than two out of the three readers assigned it as a ground-truth element. In this study, one reader drew manual contours two times in 3 cases and the intra-observer variability was 4.54±1.3%.
C. Evaluation criteria
The results were evaluated by comparing the proposed segmentation with the ground-truth using the “Dice coefficient”, which is defined as the ratio of the intersection of RA and RM to their sum4
| (18) |
where RA is the proposed segmentation result and RM is the ground-truth from manual segmentation in our study, and | | denotes the number of pixels in the corresponding volume. The greater the Dice coefficient is, the better the match of segmentation results between two methods (1 indicates perfect overlap, and 0 means no overlap). Moreover, the coefficient of variation (CV) was calculated, as a statistical measure of the precision of segmentation,
| (19) |
where d represents the standard deviation and μ is the average Dice value. The volume difference ϒ was also measured, and is de ned as follows
| (20) |
where VA is the volume of the proposed segmentation result and VM is the volume of the ground-truth.
D. Results
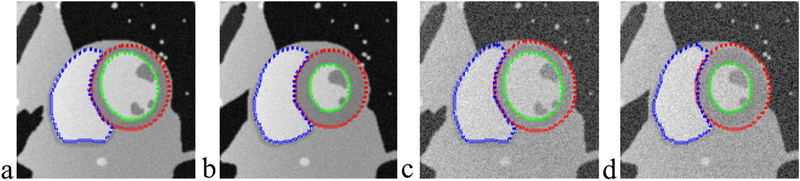
A simulated dynamic cardiac acquisition was used to evaluate the results on both LV and RV segmentation. MRXCAT cine phantom61 was segmented by the proposed method. Total 24 phases throughout the entire cardiac cycle were generated for segmentation. Two SNRs (signal-to-noise ratios) (50 and 25, blood signal versus background noise) were tested. Fig. 4a&b show the segmentation results at the end-diastolic and end-systolic phases. The contours obtained from LSSPM match well with the phantom image boundaries (the ground truth), with high Dice coefficients of 98.72±1.75% for LV endocardium, 97.12±2.12% for LV epicardium and 98.96±0.52% for RV, based on SNR of 50, and with 97.63±1.51%, 96.13 0.89% and 96.73±1.28% accordingly, based on much lower SNR of 25.
FIG. 4.
Segmentation of LV endocardium (green), LV epicardium (red) and RV (blue) on numerical phantom images using LSSPM, at end-diastole (a&c) and end-systole (b&d). Two SNRs were tested (a&b: 50; c&d: 25).
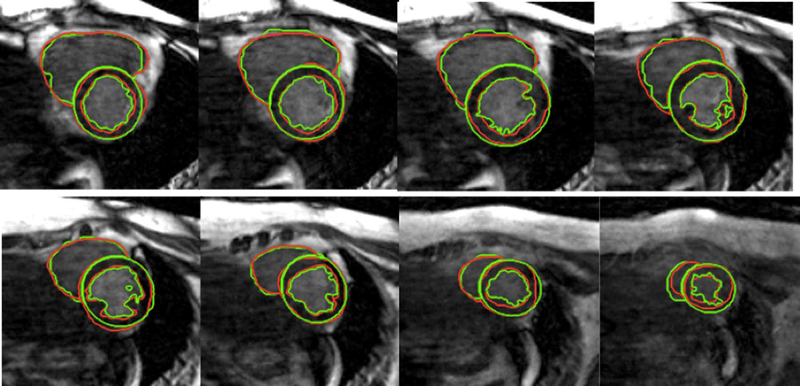
We applied the above-illustrated segmentation algorithm (as shown in FIG. 1–3) to 17 cases (each has about 20 time points and 15–20 processed slices, resulting in a total of over five thousand slices). LV endocardial, epicardial and RV contours were successfully segmented using the proposed method and were similar to those defined by manual delineation, as illustrated in FIG. 5. The quantitative comparisons between the manual segmentations and the proposed automatic segmentation are shown in TABLE I. The average Dice and CV values reached 88.62±5.47%, 87.35±7.26%, and 82.63±6.22% respectively, which demonstrates good and stable segmentation has been achieved with the proposed method on those 17 cases (Table I). An experienced clinician takes of the order of 1 minute to segment one slice, while the algorithm can achieve similar contours in less than 30 seconds (on a 2.2 GHz Intel laptop with 8GB RAM).
FIG. 5.
LV and RV segmentation. Green contours were generated by the proposed method, and red contours were obtained with manual segmentation.
TABLE I.
Quantitative comparison between different manual segmentation and the proposed segmentation method.
| Dice±CV(%) value | LV endocardium | LV epicardium | RV |
|---|---|---|---|
| Reader 1 | 98.26±1.92 | 97.23±2.56 | 95.66±2.35 |
| Reader 2 | 98.37±1.78 | 97.47±2.21 | 97.78±2.30 |
| Reader 3 | 97.64±2.51 | 97.22±2.70 | 95.31±3.17 |
| Proposed method | 88.62±5.47 | 87.35±7.26 | 82.63±6.22 |
Although the manual segmentation has been used as the reference for evaluating the proposed segmentation algorithm, we also applied three previously developed segmentation algorithms for comparison. Those methods have previously been used for LV segmentation, and include a combined CNN and level set method (CNN+LS)3, a LGD method, and a FCN-based deep learning method (FCNM)54. In the deep learning approach, the consensus manual segmentation results were used as the ground truth to train the datasets. The method was performed on 17 cases (80% of the slices were for training datasets while 20% were used for testing). Cross-validation was performed by taking 10 of the cases, training on 80% of the slices and testing on 20%. As summarized in Table II, compared to the manual segmentation, the proposed method achieved similar results on both the endocadium and epicardium, while the LGD did not locate the epicardium and FCNM had obvious errors for both endocardial and epicardial segmentation, which may be due to the low number of training datasets. The CNN+LS method performed well with Dice coefficients of 84.62% and 83.21% for endocardial and epicardial contours, respectively. Overall, the proposed method achieved the highest Dice value.
TABLE II.
Quantitative comparison between different segmentation methods and the manual segmentation.
| Dice value | LV endocardium | LV epicardium |
|---|---|---|
| CNN+LS | 84.62 | 83.21 |
| LGD | 88.62 | 76.87 |
| FCNM | 60.04 | 71.76 |
| Proposed | 88.62 | 87.35 |
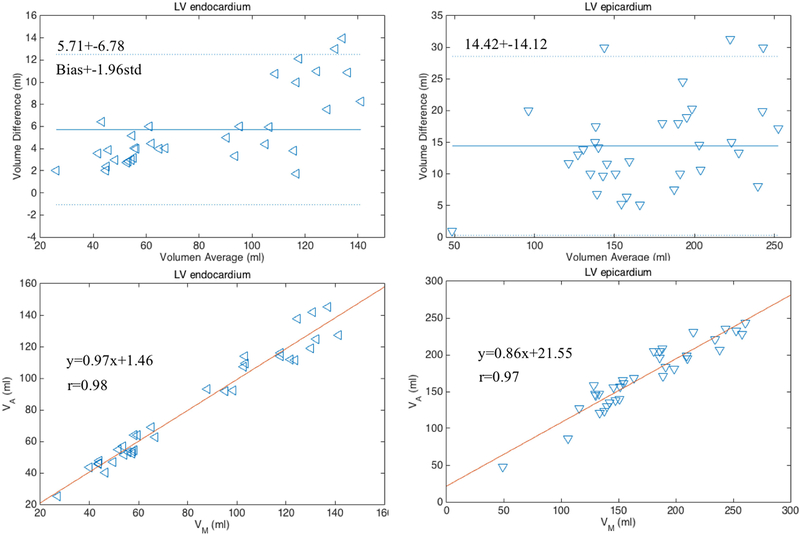
The Bland-Altman35 and the linear regression plots of the volume measurements were performed to evaluate the agreement and difference between the two segmentation results. The correlation coefficients, linear fitting slope and offsets, mean bias and confidence intervals (±1.96×std) were calculated and are reported in FIG. 6. As shown, the P-values were greater than the significance level of 0.05, thus the differences of the measurements between two methods are not significant.
FIG. 6.
Bland-Altman and linear regression plots of the measurements of LV endocardial and epicardial volumes obtained with manual and proposed segmentation methods on 17 cases at two cardiac phases (end-diastole and end-systole).
Table III summarizes the cardiac measurements of LVEDV, LVESV, LVSV, LVEF, and LVCO for all subjects.
TABLE III.
Quantitative measurements of wall thickness. EDWT: End-Diastolic Wall Thickness, ESWT: End-Systolic Wall Thickness.
| EDWT (mm) | ESVT (mm) | Change in Thinkness (mm) | |
|---|---|---|---|
| Thickness | 10.56±1.87 | 13.52±2.31 | 2.96±1.33 |
| Error (%) | 4.63 | 6.25 | 7.86 |
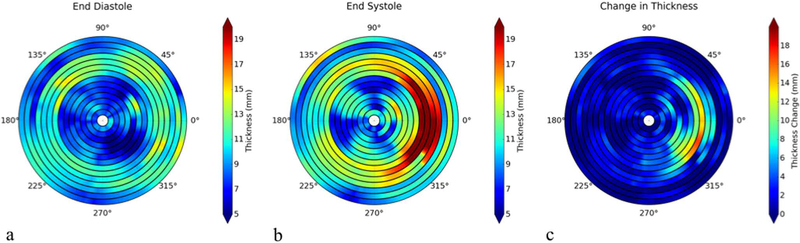
Left ventricular wall thickness and LV wall thickening can be calculated based on the segmentation results (Table III). FIG. 7a and b show the wall thickness measurements from a representative case, which provides wall thickness at 360 locations (per degree) at each slice at end-diastole and end-systole, respectively. FIG. 7c is the plot of the wall thickening (changes in thickness) between those two cardiac phases. The quantitative measurements of wall thickness are shown in TABLE IV. The errors of the thickness between manual and LSSPM segmentations are 4.63% and 6.25% for images at end-diastole and end-systole respectively.
FIG. 7.
Wall thickness measurements at end-diastole (a) and end-systole (b), and the changes between the two phases (c).
TABLE IV.
Cardiac functional measurements.
| Case | LVESV (ml) | LVEDV (ml) | LVSV (ml) | LVEF (%) | LVCO(L/min) |
|---|---|---|---|---|---|
| Average | 50.55 | 111.72 | 61.16 | 54.14 | 3.92 |
| Error (%) | 4.37 | 7.21 | 8.58 | 3.05 | 8.58 |
IV. DISCUSSION
In order to calculate cardiac structural and functional indices, delineation of the boundaries of the heart chambers is essential. In this study, we proposed an active contour based segmentation method to assess geometric characteristics of the heart including the LV and RV from 3D cardiac cine MR images. The main difficulty in segmentation is excluding tissues that surround the LV epicardial border from the myocardium. The proposed LSSPM was developed to solve this problem. The obtained measurements of LV endocardial, epicar-dial and RV contours demonstrate that the proposed segmentation method was comparable to those with manual segmentation. Manually contouring an entire volume (15–20 slices) would involve 20 minutes of active operator engagement. The computation time for automatic segmentation was about 30s per slice (both the LV and RV segmentation), although more powerful computing, such as using high performance servers, graphics processing unit (GPU) or parallel computing, could greatly shorten the processing time.
The proposed method provided higher accuracy than three previously proposed methods, including two learning-based methods. The deep learning methods have addressed the segmentation issue by estimating more complex shape using an annotated training set. However, it requires the training set to be large and rich. The active contour based segmentation uses constraints for image segmentation, which are usually based on image intensity and shape that use a small number or no annotated training sets. However, several parameters need to be provided, which may influence the segmentation results. The learning-based methods are trained to learn the parameters, which is an advantage compared to the proposed method.
For the learning-based approach, the Dice value is low may because of the small number of datasets. We used a pre-trained model (online datasets from MICCAI LV segmentation challenge) as the pre-processing for the FCNM. The results give slightly better Dice values of 65.36% and 73.24%. The images from the MICCAI datasets are 2D cine images, which do have differences (such as contrast, slice thickness, temporal resolution and so on) compared to our 4D datasets. To achieve better results with the FCNM, more 4D datasets are desired.
As reported in previous studies, the individual reader errors were 3~7% for LV segmentation65 and 5~11% for RV segmentation62,63. In this study, our readers have followed a great consensus on segmentation and achieved relatively small individual errors of 3~5% on LV&RV segmentation (Table I). The proposed segmentation results have an error of ~ 12% (Table I) off from the Dice coefficient with the three-reader consensus, which needs to be further improved such as by combining the LSSPM and learning-based method. The clinical parameters (such as LVESV, LVEDV, LVEF) calculated based on the segmentations actually have smaller errors of 3~7%, which would be acceptable in the clinic (errors reported in range of 3.9% ~ 10.2%64.
Our current method still has some limitations. Fifirstly, the heart moves along the longitudinal axis during the cardiac cycle, so that the number of slices that cover the heart could vary in different cardiac phases and have been manually determined for calculating the ventricular volumes. In the future, we plan to improve the current method by adding segmentation in the longitudinal direction to automatically obtain the LV and RV contours, which could help with determining the slices for volume measurements. Secondly, in this study, we chose 15–20 slices that covered the entire ventricles from the apex to the base (with closed region of LV), under the guidance of the radiologists. This is a limitation that our current algorithm does not provide automatic detection of the cut off slices (i.e. the slices containing the outflow tract). Third, our segmentation is fully automated using a circular initial contour. In future, an initial contour obtained using fully-automated deep learning methods may further improve the accuracy.
V. CONCLUSIONS
Automatic segmentation on the LV and RV throughout the cardiac cycle remains a challenge for cardiac cine MRI especially due to the low-contrast in the epicardial region. We have proposed an active contour based method to efficiently delineate the LV and RV contours throughout the entire cardiac cycle from 4D imaging of the heart in 17 subjects (15 volunteers and 2 patients). Our results have demonstrated high accuracy in segmenting both the LV (endocardial as well as epicardial) and RV. Qualitative and quantitative agreement is favorable compared to the manual segmentations (Table IV and Table S-1).
Supplementary Material
FIG. 2.
Schematic diagram of LV segmentation: LGD was used to obtain LV endocardium, and the LSSPM was used to get the LV epicardium.
ACKNOWLEDGMENTS
This study is supported by NIH grants K25EB014914 (JL) and R56HL133663 (JL).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts to disclose.
Contributor Information
Yan Wang, Department of Radiology; University of California San Francisco; CA94121; United States..
Yue Zhang, Department of Surgery; University of California San Francisco; CA94121; United States. Veteran Affaira Medical Center; San Francisco; CA94121; United States..
Wanling Xuan, The Ohio State University Wexner Medical Center; Ohio 43210, United States..
Evan Kao, Department of Radiology; University of California San Francisco; CA94121; United States. University of California Berkeley; CA94720; United States..
Peng Cao, Department of Radiology; University of California San Francisco; CA94107; United States..
Bing Tian, Department of Radiology, Changhai Hospital; Shanghai 200433, China..
Karen Ordovas, Department of Radiology; University of California San Francisco; CA94122; United States..
David Saloner, Department of Radiology; University of California San Francisco; CA94121; United States. Veteran Affairs Medical Center; San Francisco; CA94121; United States..
Jing Liu, Department of Radiology; University of California San Francisco; CA94108; United States..
REFERENCES
- 1.Al-amri SS, Kalyankar NV and Khamitkar SD, “Linear and non-linear contrast enhancement image,” International Journal of Computer Science and Network Security, 10, 139–143 (2010). [Google Scholar]
- 2.Avendi MR, Kheradvar A and Jafarkhani H, “A combined deep-learning and deformable-model approach to fully automatic segmentation of the left ventricle in cardiac MRI,” Medical image analysis, 30, 108–119 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Avendi MR, Kheradvar A and Jafarkhani H, “Automatic segmentation of the right ventricle from cardiac MRI using a learning-based approach,” Magnetic Resonance in Medicine, (2017). [DOI] [PubMed] [Google Scholar]
- 4.Dice LR, “Measures of the amount of ecologic association between species,” Ecology, 26, 297–302 (1945). [Google Scholar]
- 5.Saba L, Gao H, Raz E, Sree SV, Mannelli L, Tallapally N, Molinari F, Bassareo PP, R Acharya U, Poppert H, and Suri JS, “Variational B-spline level-set: a linear filtering approach for fast deformable model evolution,” Image Processing, IEEE Transactions on, 18, 1179–1191 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Bernard O, Friboulet D, Thvenaz P and Unser M, “Using a geometric formulation of annular-like shape priors for constraining variational level-sets,” Pattern Recognition Letters, 32, 1240–1249 (2011). [Google Scholar]
- 7.Bernard O, Friboulet D, Thvenaz P and Unser M, “Variational B-spline level-set: a linear filtering approach for fast deformable model evolution,” IEEE Transactions on Image Processing, 18, 1179–1191 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Delgado-Gonzalo R, Thevenaz P, Seelamantula CS and Unser M, “Snakes With an Ellipse-Reproducing Property,” Image Processing, IEEE Transactions on, 21, 1258–1271 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Alwan A, “Global status report on noncommunicable diseases 2010,” (2011). [Google Scholar]
- 10.Peng P, Lekadir K, Gooya A, Shao L, Petersen SE and Frangi AF, “A review of heart chamber segmentation for structural and functional analysis using cardiac magnetic resonance imaging,” Magnetic Resonance Materials in Physics, Biology and Medicine, 29, 155–195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earls JP, Ho VB, Foo TK, Castillo E and Flamm SD, “Cardiac MRI: recent progress and continued challenges,” Journal of magnetic resonance imaging, 16, 111–127 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Mendis S, Puska P and Norrving B, “Global atlas on cardiovascular disease prevention and control,” (2011). [Google Scholar]
- 13.Lorenzo-Valds M, Sanchez-Ortiz GI, Elkington AG, Mohiaddin RH and Rueckert D, “Segmentation of 4D cardiac MR images using a probabilistic atlas and the EM algorithm,” Medical Image Analysis, 8, 255–265 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Zhuang X, Rhode KS, Razavi RS, Hawkes DJ and Ourselin S, “A registration-based propagation framework for automatic whole heart segmentation of cardiac MRI,” IEEE transactions on medical imaging, 29, 1612–1625 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Long J, Shelhamer E and Darrell T, “Fully convolutional networks for semantic segmentation,” 3431–3440 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Bai W, Shi W, O’Regan DP, Tong T, Wang H, Jamil-Copley S, Peters NS and Rueckert D, “A probabilistic patch-based label fusion model for multi-atlas segmentation with registration refinement: application to cardiac MR images,” IEEE transactions on medical imaging, 32, 1302–1315 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Lin X, Cowan BR and Young AA, “Automated detection of left ventricle in 4D MR images: experience from a large study,” 728–735 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Cocosco CA, Niessen WJ, Netsch T, Vonken EJP, Lund G, Stork A and Viergever MA, “Automatic image-driven segmentation of the ventricles in cardiac cine MRI,” Journal of Magnetic Resonance Imaging, 28, 366–374 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Paragios N, “A level set approach for shape-driven segmentation and tracking of the left ventricle,” IEEE transactions on medical imaging, 22, 773–776 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Schaerer J, Casta C, Pousin J and Clarysse P, “A dynamic elastic model for segmentation and tracking of the heart in MR image sequences,” Medical Image Analysis, 14, 738–749 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Mitchell SC, Bosch JG, Lelieveldt BP, Van der Geest RJ, Reiber JH and Sonka M, “3-D active appearance models: segmentation of cardiac MR and ultrasound images,” IEEE transactions on medical imaging, 21, 1167–1178 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Papademetris X, Sinusas AJ and Duncan JS, “Segmentation of the left ventricle from cardiac MR images using a subject-specific dynamical model,” IEEE Transactions on Medical Imaging, 29, 669–687 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LAndreopoulos A and Tsotsos JK, “Efficient and generalizable statistical models of shape and appearance for analysis of cardiac MRI,” Medical Image Analysis, 12, 335–357 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Wahle A, Johnson RK, Scholz TD and Sonka M, “4-D cardiac MR image analysis: left and right ventricular morphology and function,” IEEE transactions on medical imaging, 29, 350–364 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Zhan Y, Dewan M, Huang J, Metaxas DN and Zhou XS, “Towards robust and effective shape modeling: Sparse shape composition,” Medical image analysis, 16, 265–277 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Zhan Y and Metaxas DN, “Deformable segmentation via sparse representation and dictionary learning,” Medical Image Analysis, 16, 1385–1396 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Li K, Wu X, Chen DZ and Sonka M, “Optimal surface segmentation in volumetric images-a graph-theoretic approach,” IEEE transactions on pattern analysis and machine intelligence, 28, 119–134 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hough PV, “Method and means for recognizing complex patterns,” US Patent 3,069,654, (1962).
- 29.Wang L, He L, Mishra A and Li C, “Active contours driven by local Gaussian distribution fitting energy,” Signal Processing, 89, 2435–2447 (2009). [Google Scholar]
- 30.Li C, Kao CY, Gore JC and Ding Z, “Minimization of region-scalable fitting energy for image segmentation,” IEEE transactions on image processing, 17, 1940–1949 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T and Verani MS, “Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart a statement for healthcare professionals from the cardiac imaging committee of the Council on Clinical Cardiology of the American Heart Association,” Circulation, 105, 539–542 (2002). [DOI] [PubMed] [Google Scholar]
- 32.LChen Y, Navarro L, Wang Y and Courbebaisse G, “Segmentation of the thrombus of giant intracranial aneurysms from CT angiography scans with lattice Boltzmann method,” Medical image analysis, 18, 1–8 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Feng L, Shen HW, Zhu C, Wang Y, Mukai K, Brooks GC, Ordovas K and Saloner D, “Highly-accelerated self-gated free-breathing 3D cardiac cine MRI: validation in assessment of left ventricular function,” Magnetic Resonance Materials in Physics, Biology and Medicine, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Zhang Y, Navarro L, Eker OF, Corredor Jerez RA, Chen Y, Zhu Y and Courbebaisse G, “Multilevel segmentation of intracranial aneurysms in CT angiography images,” Medical physics 43, 1777–1786 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Bland JM and Altman DG, “Measuring agreement in method comparison studies,” Statistical methods in medical research, 8, 135–160 (1999). [DOI] [PubMed] [Google Scholar]
- 36.El gih IA and Henein MY, “Non-invasive imaging in detecting myocardial viability:Myocardial function versus perfusion,” IJC Heart & Vasculature, 5, 51–56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngo TA, Lu Z and Carneiro G, “Combining deep learning and level set for the auto-mated segmentation of the left ventricle of the heart from cardiac cine magnetic resonance,” Medical image analysis, 35, 159–171 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Shors SM, Cotts WG, Pavlovic-Surjancev B, Franois CJ, Gheorghiade M and Finn JP, “Heart failure: evaluation of cardiopulmonary transit times with time-resolved MR angiography,” Radiology, 229, 743–748 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Nijveldt R, Hofman MB, Hirsch A, Beek AM, Umans VA, Algra PR, Piek JJ and van Rossum AC, “Assessment of microvascular obstruction and prediction of short-term remodeling after acute myocardial infarction: cardiac MR imaging study,” Radiology, 250, 363–370 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Spincemaille P, Codella NC, Nguyen TD, Prince MR and Wang Y, “Res-piratory and cardiac self-gated free-breathing cardiac CINE imaging with multiecho 3D hybrid radial SSFP acquisition,” Magnetic resonance in medicine, 63, 1230–1237 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Seguro F, Kao E, Zhang Y, Faraji F, Zhu C, Haraldsson H, Hope M, Saloner D and Liu J, “Segmentation of Lumen and Outer wall of Abdominal Aortic Aneurysms from 3D Black-Blood MRI with a Registration Based Geodesic Active Contour Model,” Medical Image Analysis, 40, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finn JP, Nael K, Deshpande V, Ratib O and Laub G, “Cardiac MR imaging: State of the technology 1,” Radiology, 241, 338–354 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Ronneberger O, Fischer P and Brox T, “U-net: Convolutional networks for biomedical image segmentation,” 234–241 (2015). [Google Scholar]
- 44.Nijveldt R, Hofman MB, Hirsch A, Beek AM, Umans VA, Algra PR, Piek JJ and van Rossum AC, “Assessment of microvascular obstruction and prediction of short-term remodeling after acute myocardial infarction: cardiac MR imaging study 1,” Radiology 250, 363–370 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Ltjnen JM, Jrvinen VM, Cheong B, Wu E, Kivist S, Koikkalainen JR, Mattila JJ, Kervinen HM, Muthupillai R, Sheehan FH and Lauerma K, “Evaluation of cardiac biventricular segmentation from multiaxis MRI data: a multicenter study,” Journal of Magnetic Resonance Imaging 28, 626–636 (2008). [DOI] [PubMed] [Google Scholar]
- 46.van Rikxoort EM, Isgum I, Arzhaeva Y, Staring M, Klein S, Viergever MA, Pluim JP and van Ginneken B, “Adaptive local multi-atlas segmentation: Application to the heart and the caudate nucleus,” Medical image analysis, 14, 39–49 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Gao Y, Kikinis R, Bouix S, Shenton M and Tannenbaum A “A 3D interactive multi-object segmentation tool using local robust statistics driven active contours,” Medical image analysis, 16, 1216–1227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T and Verani MS, “Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart,” Circulation, 105, 539–542 (2002). [DOI] [PubMed] [Google Scholar]
- 49.van Rikxoort EM, Isgum I, Arzhaeva Y, Staring M, Klein S, Viergever MA, Pluim JP and van Ginneken B, “Adaptive local multi-atlas segmentation: Application to the heart and the caudate nucleus,” Medical image analysis, 14, 39–49 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Simonyan K and Zisserman A, “Very deep convolutional networks for large-scale image recognition,” arXiv preprint arXiv:1409.1556 (2014). [Google Scholar]
- 51.Vera M, Bravo A, Garreau M and Medina R, “Similarity enhancement for automatic segmentation of cardiac structures in computed tomography volumes,” Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE, 8094–8097 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-amri SS, Kalyankar NV and Khamitkar SD, 2010 “Linear and non-linear contrast enhancement image,” International Journal of Computer Science and Network Security, 10, 139–143 (2010). [Google Scholar]
- 53.“Cardiovascular Disease: Global Atlas on Cardiovascular Disease Prevention and Control, 2012,” World Health Organization: Geneva, (2012). [Google Scholar]
- 54.Tran PV, “A fully convolutional neural network for cardiac segmentation in short-axisMRI,” arXiv preprint arXiv:1604.00494, (2016). [Google Scholar]
- 55.Hough PV, “Method and means for recognizing complex patterns,” US Patent 3,069,654, (1962).
- 56.Yuen HK, Princen J, Illingworth J and Kittler J, “Comparative study of Hough transform methods for circle finding,” Image and vision computing, 8, 71–77 (1990). [Google Scholar]
- 57.Soomro S, Akram F, Munir A, Lee CH and Choi KN, “Segmentation of Left and Right Ventricles in Cardiac MRI Using Active Contours,” Computational and mathematical methods in medicine (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nambakhsh CMS, “Automated Segmentation of Left and Right Ventricles in MRI and Classification of the Myocarfium Abnormalities,” (2013). [Google Scholar]
- 59.Rosenhahn B, Brox T and Weickert J, “Three-dimensional shape knowledge for joint image segmentation and pose tracking,” International Journal of Computer Vision 73, 243–262 (2007). [Google Scholar]
- 60.Brox T, Rosenhahn B and Weickert J, “Three-dimensional shape knowledge for joint image segmentation and pose estimation,” Joint Pattern Recognition Symposium, 109–116 (2005). [Google Scholar]
- 61.Wissmann L, Santelli C, Segars WP and Kozerke S, “MRXCAT: Realistic numerical phantoms for cardiovascular magnetic resonance,” Journal of Cardiovascular Magnetic Resonance, 16, 63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alfakih K, Plein S, Bloomer T, Jones T, Ridgway J and Sivananthan M, “Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging,” Journal of Magnetic Resonance Imaging, 18, 25–32 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Prakken NH, Velthuis BK, Vonken EJ, Mali WP and Cramer MJ, “Cardiac MRI: standardized right and left ventricular quantification by brie y coaching inexperienced personnel,” Open Magn Reson J, 1, 104–111 (2008). [Google Scholar]
- 64.Luijnenburg SE, Robbers-Visser D, Moelker A, Vliegen HW, Mulder BJ and Helbing WA, “Intra-observer and interobserver variability of biventricular function, volumes and mass in patients with congenital heart disease measured by CMR imaging,” The international journal of cardiovascular imaging, 26, 57–64 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pedrosa J, Queirs S, Bernard O, Engvall J, Edvardsen T, Nagel E and Dhooge J, “Fast and Fully Automatic Left Ventricular Segmentation and Tracking in Echocardiography Using Shape-Based B-Spline Explicit Active Surfaces,” IEEE transactions on medical imaging, 36, 2287–2296 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Nol R, Ge F, Zhang Y, Navarro L and Courbebaisse G, “Lattice Boltzmann method for modelling of biological phenomena,” Signal Processing Conference (EUSIPCO), 2017 25th European, 2654–2658 (2017). [Google Scholar]
- 67.Wang Y, Navarro L, Zhang Y, Kao E, Zhu Y and Courbebaisse G, “Intracranial Aneurysm Phantom Segmentation Using a 4D Lattice Boltzmann Method,” Computing in Science & Engineering, 19, 56–67 (2017). [Google Scholar]
- 68.Malaspinas O, Turjman A, de Sousa DR, Garcia-Cardena G, Raes M, Nguyen PT, Zhang Y, Courbebaisse G, Lelubre C, Boudjeltia KZ and Chopard B, “A spatio-temporal model for spontaneous thrombus formation in cerebral aneurysms,” Journal of theoretical biology, 394, 68–76 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Courbebaisse G, “Hemodynamic investigation and thrombosis modeling of intracranial aneurysms,” INSA Lyon (2015). [Google Scholar]
- 70.Zhang Y, “Hemodynamic investigation and thrombosis modeling of intracranial aneurysms,” INSA de Lyon, (2015). [Google Scholar]
- 71.Morgan AE, Zhang Y, Tartibi M, Goldburg S, Kim JJ, Nguyen TD, Guccione J, Ge L, Weinsaft JW and Ratcliffe MB, “Ischemic mitral regurgitation: abnormal strain overestimates nonviable myocardium,” The Annals of thoracic surgery, 105, 1754–1761 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J, Koskas L, Faraji F, Kao E, Wang Y, Haraldsson H, Kefayati S, Zhu C, Ahn S, Laub G and Saloner D, “Highly accelerated intracranial 4D ow MRI: evaluation of healthy volunteers and patients with intracranial aneurysms,” Magnetic Resonance Materials in Physics, Biology and Medicine, 31, 295–307 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.