Abstract
Background
Glaucoma is a leading cause of blindness worldwide. It results in a progressive loss of peripheral vision and, in late stages, loss of central vision leading to blindness. Early treatment of glaucoma aims to prevent or delay vision loss. Elevated intraocular pressure (IOP) is the main causal modifiable risk factor for glaucoma. Aqueous outflow obstruction is the main cause of IOP elevation, which can be mitigated either by increasing outflow or reducing aqueous humor production. Cyclodestructive procedures use various methods to target and destroy the ciliary body epithelium, the site of aqueous humor production, thereby lowering IOP. The most common approach is laser cyclophotocoagulation.
Objectives
To assess the effectiveness and safety of cyclodestructive procedures for the management of non-refractory glaucoma (i.e. glaucoma in an eye that has not undergone incisional glaucoma surgery). We also aimed to compare the effect of different routes of administration, laser delivery instruments, and parameters of cyclophotocoagulation with respect to IOP control, visual acuity, pain control, and adverse events.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2017, Issue 8); Ovid MEDLINE; Embase.com; LILACS; the metaRegister of Controlled Trials (mRCT) and ClinicalTrials.gov . The date of the search was 7 August 2017. We also searched the reference lists of reports from included studies.
Selection criteria
We included randomized controlled trials of participants who had undergone cyclodestruction as a primary treatment for glaucoma. We included only head-to-head trials that had compared cyclophotocoagulation to other procedural interventions, or compared cyclophotocoagulation using different types of lasers, delivery methods, parameters, or a combination of these factors.
Data collection and analysis
Two review authors independently screened search results, assessed risks of bias, extracted data, and graded the certainty of the evidence in accordance with Cochrane standards.
Main results
We included one trial (92 eyes of 92 participants) that evaluated the efficacy of diode transscleral cyclophotocoagulation (TSCPC) as primary surgical therapy. We identified no other eligible ongoing or completed trial. The included trial compared low-energy versus high-energy TSCPC in eyes with primary open-angle glaucoma. The trial was conducted in Ghana and had a mean follow-up period of 13.2 months post-treatment. In this trial, low-energy TSCPC was defined as 45.0 J delivered, high-energy as 65.5 J delivered; it is worth noting that other trials have defined high- and low-energy TSCPC differently. We assessed this trial to have had low risk of selection bias and reporting bias, unclear risk of performance bias, and high risk of detection bias and attrition bias. Trial authors excluded 13 participants with missing follow-up data; the analyses therefore included 40 (85%) of 47 participants in the low-energy group and 39 (87%) of 45 participants in the high-energy group.
Control of IOP, defined as a decrease in IOP by 20% from baseline value, was achieved in 47% of eyes, at similar rates in the low-energy group and the high-energy groups; the small study size creates uncertainty about the significance of the difference, if any, between energy settings (risk ratio (RR) 1.03, 95% confidence interval (CI) 0.64 to 1.65; 79 participants; low-certainty evidence). The difference in effect between energy settings based on mean decrease in IOP, if any exists, also was uncertain (mean difference (MD) −0.50 mmHg, 95% CI −5.79 to 4.79; 79 participants; low-certainty evidence).
Decreased vision was defined as the proportion of participants with a decrease of 2 or more lines on the Snellen chart or one or more categories of visual acuity when unable to read the eye chart. Twenty-three percent of eyes had a decrease in vision. The size of any difference between the low-energy group and the high-energy group was uncertain (RR 1.22, 95% CI0.54 to 2.76; 79 participants; low-certainty evidence). Data were not available for mean visual acuity and proportion of participants with vision change defined as greater than 1 line on the Snellen chart.
The difference in the mean number of glaucoma medications used after cyclophotocoagulation was similar when comparing treatment groups (MD 0.10, 95% CI −0.43 to 0.63; 79 participants; moderate-certainty evidence). Twenty percent of eyes were retreated; the estimated effect of energy settings on the need for retreatment was inconclusive (RR 0.76, 95% CI 0.31 to 1.84; 79 participants; low-certainty evidence). No data for visual field, cost effectiveness, or quality-of-life outcomes were reported by the trial investigators.
Adverse events were reported for the total study population, rather than by treatment group. The trial authors stated that most participants reported mild to moderate pain after the procedure, and many had transient conjunctival burns (percentages not reported). Severe iritis occurred in two eyes and hyphema occurred in three eyes. No instances of hypotony or phthisis bulbi were reported. The only adverse outcome that was reported by the treatment group was atonic pupil (RR 0.89 in the low-energy group, 95% CI 0.47 to 1.68; 92 participants; low-certainty evidence).
Authors’ conclusions
There is insufficient evidence to evaluate the relative effectiveness and safety of cyclodestructive procedures for the primary procedural management of non-refractory glaucoma. Results from the one included trial did not compare cyclophotocoagulation to other procedural interventions and yielded uncertainty about any difference in outcomes when comparing low-energy versus high-energy diode TSCPC. Overall, the effect of laser treatment on IOP control was modest and the number of eyes experiencing vision loss was limited. More research is needed specific to the management of non-refractory glaucoma.
Plain language summary
Laser surgery for glaucoma that lowers eye pressure by destroying a part of the eye responsible for production of fluid inside the eye
What is the aim of this review?
The aim of this Cochrane Review was to find out how laser procedures compare to other approaches for lowering the pressure in the eye for people with glaucoma not previously treated with surgery. Cochrane researchers sought and analyzed all relevant studies to answer these questions but found only one study.
Key messages
We do not know whether this type of laser surgery is safer or more effective than other surgeries for treating glaucoma. We included only one study in this review. This study compared low-energy versus high-energy diode lasers, and the results were too similar between treatment groups to draw any conclusions. Additionally, this study did not compare destructive laser surgery to other surgical approaches. More research is needed to understand the usefulness of destructive laser procedures for primary glaucoma treatment.
What was studied in this review?
Glaucoma is a progressive disease of the optic nerve causing loss of vision. It is a common cause of blindness worldwide. When treated early, vision loss may be delayed or prevented.
Intraocular pressure (IOP) is the main treatable risk factor for glaucoma. The ciliary body epithelium produces fluid that builds up pressure in the eye. It is thought that procedures that destroy the ciliary body epithelium, known as cyclodestructive procedures, may reduce IOP as a treatment for glaucoma. Different methods of cyclodestructive procedures are available; the most common is laser. The purpose of this review was to assess laser treatments that destroy the ciliary body epithelium. The review focused on the effectiveness and safety of the included procedures by assessing IOP control, vision, pain control, and side effects.
What are the main results of the review?
We found one study covering 92 people with glaucoma. The study compared low-energy versus high-energy diode transscleral cyclophotocoagulation, a laser procedure to stop production of the fluid in the eye. The trial was conducted in Ghana, and participants were followed for 13 months on average.
Overall, 47% of eyes treated with transscleral cyclophotocoagulation experienced IOP lowering of 20% or more, and there were no differences between the low-energy group and the high-energy group for any of the reported outcomes. IOP control was similar in both treatment groups. The number of medications used after treatment was also similar in both groups. Side effects were not reported separately by treatment group. Information on other important outcomes was not reported.
Based on this review, there is not enough evidence to determine whether transscleral cyclophotocoagulation is an appropriate primary surgical treatment for non-refractory glaucoma, nor whether low-energy or high-energy diode settings are safer or more effective in treating glaucoma.
How up-to-date is this review?
Cochrane researchers searched for studies that had been published up to 7 August 2017.
Background
Description of the condition
Glaucoma is a group of diseases that result in a progressive loss of retinal ganglion cells and their axons, leading to characteristic optic nerve head change and retinal nerve fiber layer (RNFL) damage. Visual function deteriorates as a result, with progressive loss of peripheral vision and, in late stages, loss of central vision and blindness. Intraocular pressure (IOP) is the main modifiable known risk factor. The goal of therapy, regardless of the disease mechanism, is reduction of IOP. Several major randomized clinical trials have demonstrated a clear benefit of lowering IOP in the prevention of the development and progression of optic nerve and visual field deterioration (AGIS 2000; CNTGS 1998; Coleman 2004; Garway-Heath 2015; Leske 2003; Lichter 2001). Currently, IOP can be lowered with medications, laser procedures, and incisional surgery.
Epidemiology
Glaucoma is the leading cause of irreversible blindness worldwide, affecting over 64 million people and causing bilateral blindness in over 8.4 million people (Quigley 2006; Tham 2014). It is estimated that in 2020 about 3.4 million people over the age of 40 in the USA will have open-angle glaucoma (OAG), the most prevalent form of the disease in North America (Friedman 2004).
Presentation and diagnosis
The glaucomas are a heterogeneous group of diseases, characterized by progressive or impending death of retinal ganglion cells associated with subsequent thinning of the neuroretinal rim of the optic nerve head and progressivevisual field loss. Elevation of IOP to a level at which the retinal ganglion cells are susceptible to damage is the main causal risk factor. Aqueous outflow obstruction is the main cause of elevated IOP. Glaucoma is an asymptomaticdisease until the very late stages. It has been estimated that more than 50% of all prevalent glaucoma cases are undiagnosed (Hennis 2007; Quigley 1996; Topouzis 2008; Weih 2001); diagnosis is commonly a result of screening during routine eye examinations. Glaucoma diagnosis is based on structural assessment of the optic nerve head and retinal nerve fiber layer (RNFL) clinically, and with the use of optical coherence tomography and the functional evaluation of the mid-peripheral visual field. Other relevant clinical examinations include IOP evaluation, central corneal thickness measurement, and gonioscopy.
Description of the intervention
The goal of glaucoma treatment is to preserve visual function and the related quality of life of the patient. Lowering IOP is the only approach known to reduce the risk of disease progression (EGS 2014). IOP can be lowered with medication, laser procedures or surgery (Burr 2012). Laser procedures such as cyclophotocoagulation (CPC) target the ciliary body to decrease the production of aqueous humor. In 1972, Beckman and colleagues introduced a laser methodfor CPC that uses a Hruby laser (Beckman 1972). Over the years, laser CPC has become the main form of cyclodestructive treatment; other methods have been used to coagulate the ciliary body, such as diathermy, cryotherapy and ultrasound (Coleman 1985; Meyer 1948).
CPC procedures include transpupillary CPC, transvitreal CPC, endoscopic CPC and transscleral CPC, based on the different paths used to approach the ciliary body. For example, transscleral CPC coagulates the ciliary body through the sclera and can be performed using a neodymium:yttrium-alluminum-garnet (ND:YAG) laser with a sapphire-tipped contact probe, or a semi-conductor diode laser equipped with disposable probes (G-probe). Traditionally, CPC is indicated for people with refractory glaucoma who have failed filtration procedures, such as trabeculectomy and aqueous tube shunt procedures, for people with limited useful vision and elevated IOP on maximum tolerated medical therapy, and for people with no visual potential in need of pain relief (Ansari 2007; Beckman 1972; Bloom 1997; Hauber 2002; Lin 2008; Murphy 2003; Pastor 2001). More recently, the use of this procedure has been extended to those with non-refractory glaucoma and good vision (Ansari 2007; Sinchai 2008). Most evidence supporting the use of lasers for ciliary body destruction comprises non-comparative case studies (Lin 2008; Pastor 2001). Fewer studies have evaluated the most effective laser parameters for accepted lasers or the different modes of delivery (Murphy 2003).
Although cyclodestructive procedures are described as safe in many of the observational case series, serious postoperative complications have been reported, including prolonged inflammation, intraocular hemorrhage, hypotony (pathologically-low IOP), loss of vision and, in some cases intractable ocular pain or phthisis bulbi (an end-stage process characterized by atrophy, shrinkage, and disorganization of the eye and intraocular contents; Pastor 2001). Conjunctival burns havebeen observed with the transscleral approach (Pastor 2001), and sympathetic ophthalmia (severe inflammation inthe untreated fellow eye) has been observed with Nd:YAG cyclophotocoagulation (Bechrakis 1994; Edward 1989; Lam 1992; Pastor 1993). Reports of these serious complications have limited the use of Nd:YAG lasers regardless of the mode of delivery, and studies on diode CPC have focused on its use in refractory glaucoma, not considering it as a primary surgical option. However, in recent publications, diode laser use in non-refractory glaucoma has been reported, with the main methods of delivery being transscleral and endoscopic.
How the intervention might work
The non-pigmented layer of the ciliary body epithelium is the site of production of aqueous humor for the eye. Aqueous humor is an ultrafiltrate of blood serum and is produced by one or more of the following processes: ultrafiltration, simple diffusion, and active transport across the ciliary body epithelium. Laser energy targeting the epithelium and ciliary processes destroys these tissues and causes a reduction in the production of aqueous humor. Thus, the goal of cyclodestructive procedures is to reduce IOP by destroying the ciliary body epithelium, the site of aqueous humor production. The thermal effect of the laser has been demonstrated to induce coagulative necrosis of the pars plicata (the area of the ciliary body that produces most aqueous humor), thereby lowering IOP, as well as undesired necrosis of surrounding structures, such as sclera, iris and pars plana. There is also evidence that transscleral cyclophotocoagulation may enhance uveal scleral outflow of aqueous humor (Liu 1994).
Why it is important to do this review
Cyclodestructive procedures have been used most commonly in people with poor vision and refractory glaucoma in which medication or other surgeries or both have failed, due to the risk of additional vision loss occurring after the destruction of the ciliary body. However, their use has more recently been expanded to include individuals with good visual acuity and non-refractory glaucoma, which represent clinical scenarios traditionally treated by other medical and surgical options. Cyclodestructive procedures may be especially important in low-income regions (such as parts of Africa and Asia) where access to medical treatment is difficult, making surgery – particularly non-invasive procedures like transscleral CPC - simpler and less expensive than continual medical therapy with eye drops.
Keeping this in mind, it is important to demonstrate a consistent effect across studies and to analyze effects on visual acuity as an important outcome measure in addition to IOP control when CPC is used in people with good visual acuity. An evaluation of cyclodestructive procedures for people with refractory glaucoma is covered in a separate Cochrane review (Chen 2016).
Objectives
To assess the effectiveness and safety of cyclodestructive procedures for the management of non-refractory glaucoma (i.e., glaucoma in an eye that has not undergone incisional glaucoma surgery). We also aimed to compare the effect of different routes of administration, laser delivery instruments, and parameters of cyclophotocoagulation with respect to IOP control, visual acuity, pain control, and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized clinical trials.
Types of participants
We included trials of participants who had undergone laser cyclodestruction as a primary surgical treatment for glaucoma (i.e. had not received any prior incisional surgery for glaucoma). We imposed no restriction regarding the underlying cause or mechanism of glaucoma or age of the participant. We excluded trials limited to those who had undergone palliative treatment for end-stage glaucoma, as these trials are covered in another Cochrane Review (Chen 2016).
Types of interventions
We included only head-to-head trials that evaluated CPC using different types of lasers, delivery methods, parameters, or a combination of these factors. We included all types of lasers (e.g. Nd:YAG, diode), routes of administration (transpupillary, transvitreal, non-contact and contact transscleral, and endoscopic), and laser settings (including power, number of applications, extent of treatment). We included trials in which CPC was performed alone or in combination with another procedure. We excluded trials in which endoscopic cyclophotocoagulation was compared with non-cyclodestructive glaucoma treatments, as these trials are covered in another Cochrane Review (Tóth 2017).
Types of outcome measures
We selected outcomes for this review based on those reported by Tseng 2017.
Primary outcomes
The primary outcome of this review was control of IOP at one year, defined as a decrease in IOP by 15% from baseline value (or by another percentage of IOP reduction, as reported by included trials). We also assessed mean change in IOP measurements by any recording device; contact or non-contact tonometry, collected prior to intervention and at one year.
Secondary outcomes
Preservation of visual acuity at one year postintervention. We considered the proportion of participants with a loss of no more than 1 line of visual acuity at one year to have had stable vision and the proportion of participants with greater than 1 line of vision loss to have had decreased vision.
Stability of visual field measured by automated perimetry (mean deviation and pattern standard deviation, measured as continuous variables), as available, throughout follow-up and at study end.
Total number of glaucoma medications, both topical and systemic, prescribed as adjuncts to surgery throughout the study period.
The proportion of participants who required additional glaucoma surgery throughout the study period.
Pain control as reported by the participant or amount of pain medication prescribed from baseline throughout the study period.
Adverse outcomes
We documented adverse outcomes as reported by included trials. Adverse outcomes of particular interest included: intractable ocular pain, prolonged inflammation, intraocular hemorrhage, hypotony, loss of vision, phthisis bulbi, and loss of an eye.
Economic data
We reviewed cost effectiveness data whenever reported.
Quality of life data
We compared quality-of-life outcomes when available.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 7 August 2017, with the exception of mRCT which is no longer in service.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 9) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 7 August 2017) (Appendix 1);
MEDLINE Ovid (1946 to 7 August 2017) (Appendix 2);
Embase.com (1980 to 7 August 2017) (Appendix 3);
LILACS (1982 to 7 August 2017) (Appendix 4);
metaRegister of Controlled Trials (mRCT) (last searched 28 June 2013) (Appendix 5);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 7 August 2017) (Appendix 6).
Searching other resources
We searched the references of reports from included trials for additional relevant trials, without restriction by language or date of publication.
Data collection and analysis
Selection of studies
Two review authors independently assessed all records identified by the electronic and manual searches as in the Criteria for considering studies for this review. Each review author classified the titles and abstracts as ‘definitely relevant’, ‘possibly relevant’ or ‘definitely not relevant’. We resolved discrepancies through discussion. We retrieved the full-text report for those records classified as ‘definitely relevant’ or ‘possibly relevant’. We grouped the reports by study and each review author assessed each study as ‘include, ‘exclude’ or ‘unsure’, resolving discrepancies through discussion. We did not need to contact study authors for further information, as described in the protocol for this review, because we classified no study as ‘unsure’ after review of the full-text. For studies excluded after review of the full-text, we documented the reasons for exclusion. We were unmasked to the report authors, institutions and trial results during these assessments.
Data extraction and management
Two review authors independently extracted data for included studies onto paper data collection forms developed in collaboration with Cochrane Eyes and Vision, and resolved discrepancies by discussion and consensus. We collected data related to trial methods, characteristics of participants and interventions, and outcomes. One review authorentered data into Review Manager 5 (RevMan 5) (Review Manager 2014) and a second review author verified the values.
Assessment of risk of bias in included studies
Two review authors independently assessed the risks of bias of the included studies as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We examined six main criteria: sequence generation, allocation concealment before randomization, masking, incomplete outcome data, selective reporting, and other potential sources of bias (such as funding source). Each review author assessed each included trial as having a low risk of bias, an unclear risk of bias (lack of information or uncertainty over the potential for bias), or a high risk of bias. We resolved discrepancies through discussion.
Measures of treatment effect
Dichotomous outcomes
We analyzed dichotomous outcomes as summary risk ratios (RRs) with 95% confidence intervals (CIs). Dichotomous outcomes included the proportions of participants with IOP control, stable visual acuity, decreased visual acuity, those needing additional glaucoma surgery, and those with adverse events.
Continuous outcomes
We analyzed continuous outcomes as summary mean differences (MDs) with 95% CIs. Continuous outcomes included mean change in IOP, mean visual acuity, mean and pattern standard deviations from visual field tests, mean numbers of glaucoma medications prescribed, degree of pain control reported by participants and amount of pain medication prescribed, cost effectiveness, and quality-of-life scores.
Unit of analysis issues
The unit of analysis was the individual (one study eye per person).
Dealing with missing data
We planned to contact trial investigators for missing details including study methods, effect estimates, standard deviations, and intention-to-treat (ITT) data when information was not reported or unclear. If there was no response within six weeks, we planned to use the available information reported in the study.
Assessment of heterogeneity
We did not assess heterogeneity as we included only one trial in the review. If more trials are included in updates of the review, we will assess clinical and methodological heterogeneity by examining potential variations in participant characteristics, inclusion/exclusion criteria and assessments of primary and secondary outcomes. When meta-analysis is appropriate, we will use the I2 statistic (%) to determine the proportion of variation due to heterogeneity, with a value above 50% suggesting substantial statistical heterogeneity. We will also examine the result of the Chi2 test for heterogeneity and the degree of overlap in confidence intervals of included studies; poor overlap of confidence intervals suggests heterogeneity.
Assessment of reporting biases
We planned to examine funnel plots of the intervention effect estimates for signs of asymmetry, to evaluate small study effects, if 10 or more studies were to be included in our meta-analysis. We assessed selective outcome reporting as part of the ‘Risk of bias’ assessment.
Data synthesis
We did not perform meta-analysis, as we included only one trial in this review. If more trials are included in updates of the review and neither the I2 statistic nor an inspection of the forest plot suggest substantial heterogeneity, we will combine the results of included trials in a meta-analysis using a random-effects model. We will use a fixed-effect model if there are fewer than three trials and there is no evidence of statistical, clinical or methodological heterogeneity. In this instance, the fixed-effect model will provide a more robust estimate of the treatment effect.
Subgroup analysis and investigation of heterogeneity
We did not conduct planned subgroup analysis due to insufficient data. When sufficient data become available, we will perform subgroup analysis comparing different types of cyclophotocoagulation: transpupillary, transvitreal endocyclophotocoagulation, transscleral (non-contact and contact Nd:YAG), semiconductor diode laser, and endoscopic. We will also conduct subgroup analysis for underlying causes of glaucoma (primary open-angle, angle-closure glaucoma, neovascular glaucoma, other secondary glaucoma), laser parameter settings (total power delivered per treatment) and number of treatments performed.
Sensitivity analysis
Due to lack of data, we did not conduct sensitivity analyses as specified in the protocol. When sufficient data become available, we will undertake sensitivity analyses to determine the impact of excluding studies at high risk of bias, industry-funded studies, and those studies providing only unpublished data.
Summary of findings
For each outcome, we assessed the certainty of evidence using the GRADE approach (GRADEpro GDT). The GRADE approach considers the following five criteria: risk of bias in individual trials, indirectness, heterogeneity, imprecision of estimate (wide confidence intervals), and publication bias. Two review authors independently judged the certainty of each outcome estimate according to the criteria as very low, low, moderate, or high. We resolved any discrepancy by discussion.
We summarized the main outcomes evaluated in this review in Summary of findings table 1, which presents the comparative effects between treatments. Because a ‘Summary of findings’ table was not part of the original Cochrane protocol, we chose the outcomes presented in the table post hoc. We based our selection on core outcomes for glaucoma research that have been proposed in the literature (Ismail 2016). The main outcomes for this review include:
Control of IOP at one year, defined as a decrease in IOP by 15% from baseline value (or by another percentage of IOP reduction, as reported by included trials)
Mean change in IOP measurements (by any recording device; contact or non-contact tonometry) collected prior to intervention and at one year
Proportion of participants with a loss of visual acuity at one year, defined as greater than 1 line of vision loss (or by more lines, as reported by included trials)
Mean visual field measured by automated perimetry (mean deviation and pattern standard deviation) at one year
Total number of glaucoma medications, both topical and systemic, prescribed as adjuncts to surgery throughout the study period to one year
Proportion of participants who required additional glaucoma surgery throughout the study period to one year
Proportion of participants with an adverse event to one year.
Results
Description of studies
Results of the search
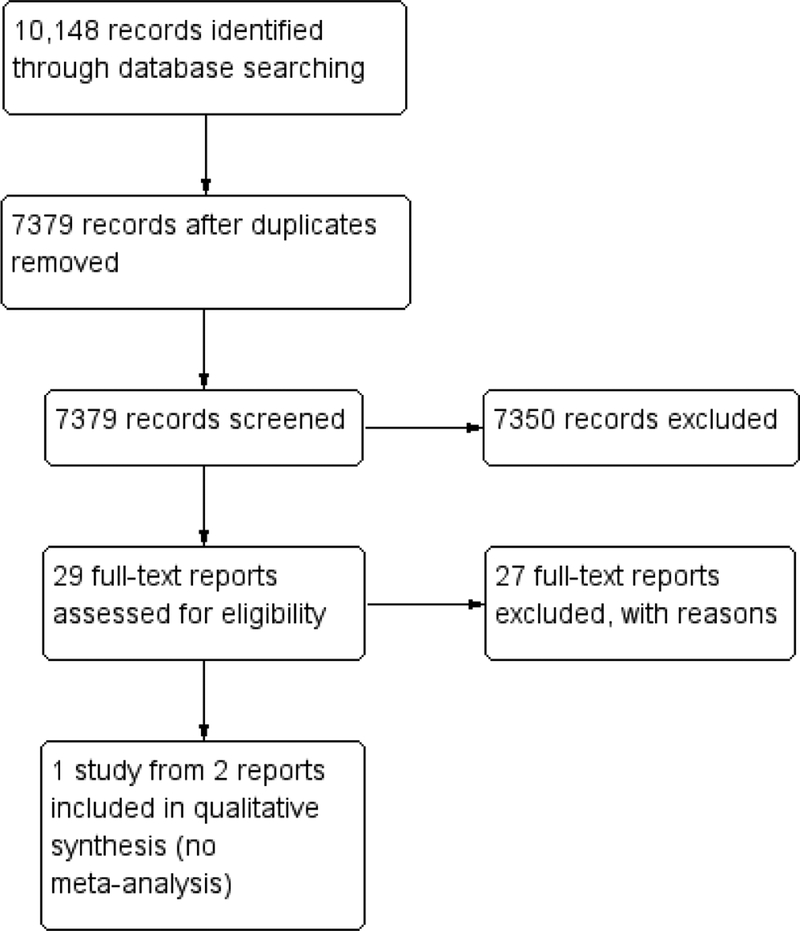
The electronic searches yielded 7379 unique records (Figure 1). We removed 7350 records by screening titles and abstracts, and excluded 27 records after reviewing the full-text report. We included two reports from one trial in this review (Egbert 2001). We identified no potentially relevant completed or ongoing trials from searching other sources.
Figure 1.
Study flow diagram.
Included studies
We include one trial (92 eyes of 92 participants) in this review. The trial was conducted in Ghana and enrolled adults with primary open-angle glaucoma. Although eyes with previous glaucoma surgery or no light perception were not eligible for the trial, most of the included eyes had very advanced glaucoma, as assessed using clinical examination of the optic nerve and measurement of IOP. Participants were treated with diode transscleral CPC and randomized to one of two energy settings with different power-to-exposure time ratios: low energy of 45.0 joules (1.5 watts for 1.5 seconds × 20 spots over 360 °) or high energy of 62.5 joules (1.25 watts for 2.5 seconds × 20 spots over 360 °). Retreatment was performed at the discretion of the treating ophthalmologist whenever the first treatment failed to lower IOP. Mean follow-up was 13.2 months after the first treatment. The trial investigators excluded participants with less than three months of follow-up from the analyses. The primary outcomes of the trial were change in IOP and reduction of medications; secondary outcomes included change in visual acuity, additional glaucoma surgery, and complications. No data for visual field, pain control, cost effectiveness, or quality-of-life outcomes were reported by the trial investigators. The primary analyses include 40 (85%) of 47 participants in the low-energy group and 39 (87%) of 45 participants in the high-energy group.
Excluded studies
We excluded 27 studies after review of the full-text report. The studies, with reasons for exclusion, are documented in the Characteristics of excluded studies table. We excluded 16 studies that were not randomized (case series, cohort studies) and 11 RCTs of cyclodestructive procedures in refractory glaucoma (covered in a separate Cochrane Review; Chen 2016).
Risk of bias in included studies
Allocation (selection bias)
We assessed risk of selection bias to be low, as randomization was performed adequately by coin toss and allocation was done after participants received retrobulbar anesthesia; the treatment assignment would therefore not have been known prior to an individual’s enrollment in the trial.
Masking (performance bias and detection bias)
Because no information was provided on masking of participants or trial personnel, we assessed the trial to have unclear risk of performance bias. We judged the trial to be at high risk of detection bias, due to outcome assessors being unmasked to the treatment assignments.
Incomplete outcome data (attrition bias)
We assessed the risk of attrition bias to be high, because trial investigators excluded 13 (14%) of 92 participants from analysis for all outcomes, except complications. When participants who were excluded for missing three months of follow-up were compared with those who were not excluded, the excluded participants were more often male and older than 50 years; however, preoperative IOP was similar in the excluded and non-excluded groups.
Selective reporting (reporting bias)
Results were reported for all outcomes specified in the Methods section of the published report. We therefore judged the trial to be at low risk of selective outcome reporting bias.
Other potential sources of bias
The laser used in the trial was provided by the manufacturer, and the trial had not been registered prospectively. For these reasons, we judged the trial to be at unclear risk of other potential sources of bias.
Effects of interventions
The one trial included in this review (Egbert 2001) reported control of IOP and burden of glaucoma medications after diode transscleral cyclophotocoagulation (TSCPC), and compared two energy settings for diode TSCPC. Because neither energy setting is considered the standard, we report the comparison of the two settings as documented in the trial: the low-energy group versus the high-energy group. All outcomes are reported for participants with at least three months of follow-up (mean13.2 months; 12.9 months in the low-energy group and 13.5 months in the high-energy group).
Control of IOP
Data for control of IOP, defined as a decrease in IOP of 15% from baseline value (our planned threshold), was not reported. However, data were reported for a decrease in IOP of 20% from baseline value; this level of control was seen in 37 of 79 eyes, 47%. Although the proportions of participants with a decrease in IOP by 20% were similar between the low-energy group (19 of 40 participants, 48%) and the high-energy group (18 of 39 participants, 46%), the small study size creates uncertainty in the effect estimate between energy settings (RR 1.03, 95% CI 0.64 to 1.65). We graded the certainty of evidence for this outcome as low, downgrading for risk of bias (−1) and imprecision (−1).
Egbert 2001 also reported a mean decrease in IOP of 3.3 mmHg (95% CI 0.70 to 5.90). The highest percentages of IOP reduction (57%) were observed in the subgroup of eyes having higher pretreatment IOP (> 22 mmHg). The mean difference (MD) in the mean decrease in IOP between energy settings was small, −0.50 mmHg (95% CI −5.79 to 4.79). Again, we graded the certainty of evidence for this outcome as low, downgrading for risk of bias (−1) and imprecision (−1).
Visual acuity
Egbert 2001 defined decreased vision as the proportion of participants with a decrease of 2 or more lines on the Snellen chart or one or more categories of visual acuity if unable to read the eye chart. Eighteen of 79 eyes demonstrated decreased vision. Considering eyes with good pretreatment visual acuity (20/60 or better), only one eye out of 19 (5%) experienced decreased vision after laser treatment. The proportions of participants with decreased vision were similar between the low-energy group (10 of 40 participants, 25%) and the high-energy group (eight of 39 participants, 21%); however, the effect estimate between energy settings was imprecise (RR 1.22; 95% CI 0.54 to 2.76). We graded the certainty of evidence for this outcome as low, downgrading for risk of bias (−1) and imprecision (−1).
Data were not available for mean visual acuity, proportion of participants with stable vision, or proportion of participants with decreased vision defined as greater than 1 line of vision loss.
Visual field
Egbert 2001 reported no visual field outcomes.
Number of glaucoma medications
The mean number of glaucoma medications used in the studied eyes fell from 1.8 ± 0.82 to 1.3 ± 1.18 after TSCPC treatment. The mean difference in the number of glaucoma medications used after TSCPC in the low-energy group (1.4; standard deviation (SD) 1.3) compared with the high-energy group (1.3; SD 1.1) was less than one (MD 0.10, 95% CI −0.43 to 0.63). We graded the certainty of evidence for this outcome as moderate, downgrading for risk of bias (−1).
Pain control
Egbert 2001 reported no outcomes related to pain control.
Additional glaucoma surgery
The numbers and proportions of participants undergoing retreatment were reported by Egbert 2001. Overall, 16 eyes (20%) were retreated. Seven (17%) of 40 participants in the low-energy group compared with nine (23%) of 39 participants in the high-energy group required retreatment (RR 0.76, 95% CI 0.31 to 1.84). We graded the certainty of evidence for this outcome as low, downgrading for risk of bias (−1) and imprecision (−1).
Adverse events
Most adverse events were reported for the total study population, rather than by treatment group. The authors of Egbert 2001 reported that “most patients experienced mild to moderate pain for a few days, but none complained of severe pain.” Many participants also had transient conjunctival burns (percentages not reported). Severe iritis occurred in two eyes and hyphema occurred in three eyes. No instances of hypotony or phthisis bulbi were reported. The only adverse event that was reported by treatment group was atonic pupil, which was observed in 13 (28%) of 47 eyes in the low-energy group and 14 (31%) of 45 eyes in the high-energy group (RR 0.89, 95% CI 0.47 to 1.68). We graded the certainty of evidence for this outcome as low, downgrading for risk of bias (−1) and imprecision (−1).
Economic data
Egbert 2001 reported no cost-effectiveness outcomes.
Quality of life data
Egbert 2001 reported no outcomes related to quality of life.
Discussion
Summary of main results
Our review to evaluate the effectiveness and safety of cyclodestructive procedures for the management of non-refractory glaucoma identified one eligible randomized clinical trial (Egbert 2001). Results from this trial did not compare cyclophotocoagulation to other procedural interventions, so we are unable to comment on the benefit of cyclodestructive procedures relative to other procedural therapies; this represents a knowledge gap in the glaucoma literature. The included trial compared two settings for diode transscleral CPC: low energy (45.0 joules) and high energy (62.5 joules). Mean follow-up was 13.2 months after the first treatment. The primary analyses included 40 (85%) of 47 participants in the low-energy group and 39 (87%) of 45 participants in the high-energy group. Due to the small number of participants analyzed, we can draw no conclusions about between-group differences for control of IOP, mean change in IOP, decrease in visual acuity, need for additional glaucoma surgery, and adverse events. The overall efficacy was modest, with IOP reduction by 20% or more in 47% of eyes. IOP control was highly variable and affected by pre-operative IOP, with the highest percentages of IOP reduction observed in the subgroup of eyes having higher pretreatment IOP (> 22 mmHg). Vision loss occurred in 23% of eyes, but in only 5% (one out of 19) of eyes having a pretreatment visual acuity of 20/60 or better. In both groups, the amount of laser energy applied was quite conservative and lower than the energy usually used in other studies investigating CPC procedures.
The number of glaucoma medications used was reduced after diode transscleral CPC treatment in both groups; however, the difference was less than one medication when comparing treatment groups. No data for visual field, pain control, cost effectiveness, or quality-of-life outcomes were reported in this trial.
Overall completeness and applicability of evidence
The included trial reported on IOP control as well as visual outcomes and glaucoma medication use following diode transscleral CPC. It compared low-energy versus high-energy diode transscleral CPC. We found no evidence comparing one type of laser with another (e.g. Nd:YAG versus diode) or different routes of administration (e.g. non-contact versus contact).
Most participants included in the trial were reported to have advanced glaucoma, although the proportion of 92 included eyes with this diagnosis was not given. Also, the definition of advanced glaucoma used in this trial did not include visual field findings. It is unclear whether similar effects would be observed in populations with less advanced glaucoma. Furthermore, the criteria used to decide to repeat diode CPC treatment of study participants were not reported, limiting the reproducibility of the trial.
Quality of the evidence
We graded the certainty of evidence for most outcomes as low, due to high risk of detection bias and attrition bias in the trial and imprecision in the effect estimates. Another limitation of the trial is that follow-up was not standardized among participants. Only mean follow-up time was reported for participants with at least three months of follow-up.
Potential biases in the review process
To minimize potential biases in the review process we designed a comprehensive, sensitive search strategy to identify relevant studies, and followed standard Cochrane methodology. We adhered closely to methods as specified in the protocol and noted any deviation from the protocol in the Differences between protocol and review section.
Agreements and disagreements with other studies or reviews
We identified no other systematic review on this topic. From the search results for this review we found few studies, randomized or non-randomized, that had evaluated cyclodestructive procedures in eyes with non-refractory glaucoma. Most of the research for cyclodestructive procedures has been conducted in eyes with refractory glaucoma (see Characteristics of excluded studies). More recently, non-randomized studies have been reported which evaluate cyclodestructive procedures in combination with phacoemulsification in eyes with glaucoma and cataract (Berke 2006; Janknecht 2005). Furthermore, individual studies have often reported findings for small samples of treated individuals and many are non-comparative case series that were not designed to assess the benefits and harms of one method over another.
Authors’ conclusions
Implications for practice
It is commonly accepted that CPC procedures are indicated in refractory glaucoma and in eyes with limited or no visual potential associated with elevated IOP or eye pain. Laser CPC has also been suggested and evaluated for treating non-refractory glaucoma in eyes with relatively good visual acuity, as an alternative to other surgical options in low-income countries. However, the cost/risk benefit of CPC as a primary surgical approach versus other surgical interventions is unknown due to lack of evidence, and has been studied minimally, perhaps due to concern for severe postoperative complications such as irreversible vision loss. It remains unclear whether vision loss observed in eyes after CPC treatment is related directly to the laser procedure or determined (at least partly) by the underlying disease process and the natural progression of end-stage glaucoma. Our review suggests that currently there is insufficient high-quality evidence to inform the use of CPC procedures for non-refractory glaucoma.
Implications for research
The results of the single included study are inconclusive and inadequate to achieve the aim of this review; more research is needed, specifically randomized clinical trials in participants with non-refractory glaucoma, to establish an evidence base.
Future trials should enroll a large number of participants, minimize losses to follow-up, and follow participants for longer (at least six months). To evaluate the effect of CPC on vision loss, participants at different stages of the disease should be targeted for enrollment and randomization should be stratified by the participants’ pretreatment visual acuity. Future trials should also use different levels of laser energy (less conservative than the study included in this review) and account for preand postoperative use of anti-glaucoma medications to adequately address the CPC effect on IOP changes. Most important will be direct comparison of cyclodestructive procedures versus other surgical glaucoma therapies, to establish their relative effectiveness and harms.
Included studies
Sources of support
Internal sources
• No sources of support provided
External sources
Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA Methodological support provided by the Cochrane Eyes and Vision US Satellite
National Institute for Health Research (NIHR), UK
Richard Wormald, Co-ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Differences between protocol and review
In the protocol (Jones 2011), the authors planned to include randomized and quasi-randomized controlled trials. However, we identified no quasi-randomized controlled trials, and will not consider quasi-randomized controlled trials in future updates to this review.
We included a ‘Summary of findings’ table and GRADE assessments in this review, neither of which were required under Cochrane standards at the time of the protocol.
Acknowledgements
We acknowledge Leslie S Jones (LSJ), Oluwatosin Smith (OS), Salman J Yousuf (SJY), and John Kwagyan (JK) for developing the protocol for this review. We thank Iris Gordon and Lori Rosman, Cochrane Eyes and Vision (CEV) Information Specialists, for developing the search strategy and executing the electronic searches. We also acknowledge the support of the CEV editorial team during the preparation of this review and thank the peer reviewers for their comments, and Nancy Fitton for editing the Plain Language Summary.
The contribution of the IRCCS - Fondazione Bietti in this paper was supported by the Italian Ministry of Health and by Fondazione Roma. The supporting organization had no role in the design or conduct of this research.
Appendices
1. CENTRAL search strategy
#1 MeSH descriptor Glaucoma
#2 glaucoma*
#3 MeSH descriptor Intraocular Pressure
#4 (ocular or intraocular or intra-ocular) near/1 (pressure*)
#5 MeSH descriptor Ocular Hypertension, this term only
#6 ocular hypertension
#7 IOP or OHT
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
#9 MeSH descriptor Lasers
#10 laser*
#11 MeSH descriptor Laser Coagulation, this term only
#12 photocoagulat*
#13 photo near/1 coagulat*
#14 coagulat* or argon or diode
#15 ND YAG
#16 cyclophotocoagulat* or cyclodestruct*
#17 ciliary body destruct*
#18 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17)
#19 (#8 AND #18)
2. MEDLINE Ovid search strategy
randomized controlled trial.pt.
(randomized or randomised).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1–7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
exp glaucoma/
glaucoma$.tw.
exp intraocular pressure/
((ocular or intraocular or intra-ocular) adj1 pressure$).tw.
Ocular Hypertension/
ocular hypertension.tw.
(IOP or OHT).tw.
or/13–19
exp lasers/
laser$.tw.
laser coagulation/
photocoagulat$.tw.
(photo adj1 coagulat$).tw.
(coagulat$ or argon or diode).tw.
ND YAG.tw.
(cyclophotocoagulat$ or cyclodestruct$).tw.
ciliary body destruct$.tw.
or/21–29
20 and 30
12 and 31
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
3. Embase.com search strategy
exp randomized controlled trial/
exp randomization/
exp double blind procedure/
exp single blind procedure/
random$.tw.
or/1–5
(animal or animal experiment).sh.
human.sh.
7 and 8
7 not 9
6 not 10
exp clinical trial/
(clin$ adj3 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
exp placebo/
placebo$.tw.
random$.tw.
exp experimental design/
exp crossover procedure/
exp control group/
exp latin square design/
or/12–21
22 not 10
23 not 11
exp comparative study/
exp evaluation/
exp prospective study/
(control$ or prospectiv$ or volunteer$).tw.
or/25–28
29 not 10
30 not (11 or 23)
11 or 24 or 31
exp glaucoma/
glaucoma$.tw.
exp intraocular pressure/
((ocular or intraocular or intra-ocular) adj1 pressure$).tw.
exp intraocular hypertension/
ocular hypertension.tw.
(IOP or OHT).tw.
or/33–39
exp lasers/
laser$.tw.
exp laser coagulation/
photocoagulat$.tw.
(photo adj1 coagulat$).tw.
(coagulat$ or argon or diode).tw.
ND YAG.tw.
(cyclophotocoagulat$ or cyclodestruct$).tw.
ciliary body destruct$.tw.
or/41–49
40 and 50
32 and 51
4. LILACS search strategy
glaucoma$ or intraocular pressure or ocular hypertension and laser$ or photocoagulat$ or cyclophotocoagulat$ or cyclodestruct$
5. meta Register of Controlled Trials search strategy
glaucoma and laser
6. ClinicalTrials.gov search strategy
Glaucoma AND Laser
Published notes
Characteristics of studies
Characteristics of included studies
| Sample size calculation: not reported | ||
| Diagnosis: participants had “very advanced” primary open-angle glaucoma; treated eye was the eye with more advanced glaucoma | ||
| Actual: mean 13.2 months; analysis included only participants with at least 3 months ollow-up | ||
| ntervals at which outcomes assessed: 1 day, 1 week, 3 weeks, and every 2 to 3 months | ||
| Reported subgroup analyses: none between treatment groups (only for total study population) | ||
| Bias | Authors’ judqement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “We randomly assigned patients to receive 1 of 2 energy settings. After the retrobulbar anesthesia and just before treatment, a nurse tossed the coin to determine the settings.” |
| Allocation concealment (selection jias) | Low risk | Quote: “After the retrobulbar anesthesia and just before treatment, a nurse tossed the coin to determine the settings.” Participants were randomized while under anesthesia, so allocation would not have been known prior to enrollment in the trial |
| Masking of participants and personnel (performance bias) | Unclear risk | No information provided on masking of participants or personnel |
| Masking of outcome assessment (detection bias) | High risk | Quote: “Examiners were not masked to the treatment.” |
| Incomplete outcome data (attrition bias) | High risk | 13 (14%) of 92 participants were excluded from analysis for all outcome measures, except for complications. In comparing those who were followed up for 3 months (and were included) with those who were not (excluded), the excluded were more likely to be male and more than 50 years old; preoperative IOP was similar between the 2 groups, but no postoperative IOP values were available. Comparisons between the treatment groups were not made for those that were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | Results were reported for all outcomes specified in the Methods section of published report |
| Other bias | Unclear risk | The laser used in the trial was provided by the manufacturer. The trial was not registered prospectively. |
Footnotes
IOP: intraocular pressure
J: joule
mg: milligram
W: watts
Characteristics of excluded studies
| Reason for exclusion | Refractory glaucoma: RCT of 60 eyes with refractory glaucoma treated with contact versus non-contact diode transscleral cyclophotocoagulation |
| Reason for exclusion | Case series: 18 eyes with neovascular glaucoma treated with contact diode transscleral cyclophotocoagulation |
| Reason for exclusion | Cohort study: 21 eyes with neovascular glaucoma treated with diode transscleral cyclophotocoagulation with laser on the ciliary body only; compared to a cohort of 21 eyes treated with diode transscleral cyclophotocoagulation with ablation of periphery retina |
| Reason for exclusion | Case series: 21 eyes with refractory glaucoma treated with continuous wave Nd:YAG cyclophotocoagulation using an artificial sapphire crystal contact probe |
| Reason for exclusion | Refractory glaucoma: RCT of 48 eyes with refractory glaucoma treated with micropulse versus continuous wave diode transscleral cyclophotocoagulation |
| Reason for exclusion | Cohort study: 626 eyes with cataract and medically-controlled glaucoma treated with phacoemulsification plus endoscopic cyclophotocoagulation; compared to a cohort of 81 eyes treated with phacoemulsification alone (conference abstract only, no full-length report) |
| Reason for exclusion | Refractory glaucoma: RCT of 31 eyes with intractable glaucoma treated with Nd:YAG cyclophotocoagulation versus cyclocryotherapy (conference abstract only, no full-length report) |
| Reason for exclusion | Case series: 10 eyes with neovascular glaucoma treated with semiconductor contact diode transscleral cyclophotocoagulation |
| Reason for exclusion | Case series: 36 eyes with neovascular glaucoma treated with Nd:YAG transscleral cyclophotocoagulation or pneumatically stented pars plana Baerveldt implants (conference abstract only, no full-length report) |
| Reason for exclusion | Case series: 54 eyes with neovascular glaucoma treated with Nd:YAG transscleral cyclophotocoagulation, endocyclophotocoagulation, or pars plana Baerveldt implants (conference abstract only, no full-length report) |
| Reason for exclusion | Cohort study: 7 eyes with refractory glaucoma treated with diode transscleral cyclophotocoagulation at 1.2 W for 3.5 seconds (4.25 J); compared to a cohort of 7 eyes treated with diode transscleral cyclophotocoagulation at 2.8 W for 1.5 seconds (4.25 J) |
| Reason for exclusion | Refractory glaucoma: RCT of 40 eyes with refractory glaucoma treated with noncontact Nd:YAG transscleral cyclophotocoagulation applied 1.5 versus 3.0 millimeters posterior to the comeoscleral limbus |
| iReason for exclusion | Letter to editor: discussion of diode versus Nd:YAG lasers for cyclophotocoagulation |
| Reason for exclusion | Case series: 21 eyes with refractory glaucoma treated with diode transscleral cyclophotocoagulation |
| Reason for exclusion | Cohort study: 32 eyes with refractory glaucoma treated with diode transscleral cyclophotocoagulation; compared to a cohort of 38 eyes treated with cyclocryotherapy |
| Reason for exclusion | Cohort study: 28 eyes with cataract and glaucoma treated with phacoemulsification plus cyclophotocoagulation; compared to a cohort of 28 eyes treated with phacoemulsification alone |
| Reason for exclusion | Cohort study: 22 eyes with diabetic neovascular glaucoma treated with diode transscleral cyclophotocoagulation; compared to a cohort of 16 eyes treated with cyclocryotherapy |
| Reason for exclusion | Cohort study: 29 eyes of children with secondary glaucoma treated with diode transscleral cyclophotocoagulation; compared to a cohort of 40 eyes treated with cyclocryotherapy |
| Reason for exclusion | Refractory glaucoma: RCT of 26 eyes with refractory glaucoma treated with cyclophotocoagulation versus cyclocryotherapy (conference abstract only, no full-length report) |
| Reason for exclusion | Refractory glaucoma: RCT of 72 eyes with refractory glaucoma treated with diode transscleral cyclophotocoagulation versus cyclocryotherapy |
| Reason for exclusion | Refractory glaucoma: RCT of 45 eyes with refractory glaucoma treated with 180 ° versus 360 ° Nd:YAG transscleral cyclophotocoagulation (conference abstract only, no full-length report) |
| Reason for exclusion | Refractory glaucoma: RCT of 22 eyes with uncontrolled glaucoma treated with 7 W versus 9 W Nd:YAG transscleral cyclophotocoagulation (conference abstract only, no full-length report) |
| Reason for exclusion | Case series: 14 eyes with refractory glaucoma treated with diode transscleral cyclophotocoagulation |
| Reason for exclusion | Refractory glaucoma: RCT of 89 eyes with intractable glaucoma treated with 4 J versus 8 J noncontact Nd:YAG transscleral cyclophotocoagulation |
| Reason for exclusion | Refractory glaucoma: RCT of 30 eyes with end-stage glaucoma treated with half-versus full-dose diode transscleral cyclophotocoagulation |
| Reason for exclusion | Refractory glaucoma: RCT of 66 eyes with neovascular glaucoma treated with contact diode transscleral cyclophotocoagulation versus Ahmed glaucoma valve implant |
| Reason for exclusion | Cohort study: 25 eyes with neovascular glaucoma treated with transscleral cyclophotocoagulation; compared to a cohort of 18 eyes treated with cyclocryotherapy |
Footnotes
J: joule
Nd:YAG: neodymium:yttrium-alluminum-garnet
RCT: randomized controlled trial
W: watts
Summary of findings tables
1 Low- versus high-energy diode trans-scleral cyclophotocoagulation for non-refractory glaucoma
| Low- versus high-energy diode transscleral cyclophotocoagulation for non-refractory glaucoma | ||||||
| Population: people with primary open-angle glaucoma and no previous glaucoma surgery | ||||||
| Settings: ophthalmology clinics | ||||||
| Intervention: low energy; 1.5 watts for 1.5 seconds × 20 spots over 360 ° (45.0 J) | ||||||
| Comparison: high energy; 1.25 watts for 2.5 seconds × 20 spots over 360 ° (62.5 J) | ||||||
| Outcomes* | Illustrative comparative risks** (95% Cl) | Relative (95% Cl) | No of participant (study) | buality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| High-energy diode transscleral cyclophotocoagulation | Low-energy diode transscleral cyclophotocoagulation | |||||
| Control of intraocular pressure | 462 per 1000 |
475 per 1000 (295 to 762) |
RR1.03 (0.64 to 1.65) | 79 (1 study) | ⊕⊕⊝⊝ low1,2 |
Control of intraocular pressure defined as a decrease in IOP by 20% from baseline value |
| Mean change in intraocular pressure | On average intraocular pressure in the high energy group dropped by 3 mmHg | On average intraocular pressure in the low-energy group was 0.5 mmHg lower than the IOP in the high-energy group (5.79 mmHg lower to 4.79 mmHg higher) | 79 (1 study) | ⊕⊕⊝⊝ low1,2 |
||
| Decrease in visual acuity | 205 per 1000 |
250 per 1000 (111 to 566) |
RR1.22 (0.54 to 2.76) |
79 (1 study) | ⊕⊕⊝⊝ low1,2 |
Decrease in visual acuity defined as a decrease of 2 or more lines on the Snellen chart or one or more categories of visual acuity if unable to read the eye chart |
| Mean visual Field | No visual field outcomes reported | - | - | - | - | - |
| Number of glaucoma medications after treatment | The mean number of glaucoma medications in the high energy group was 1.3 | The mean number of glaucoma medications in the low energy group was 0.10 more (0.43 Fewer to 0.63 more) | 79 (1 study) | ⊕⊕⊝⊝ Moderate 1 |
- | |
| Additional glaucoma surgery | 231 per 1000 |
175 per 1000 (72 to 425) |
RR 0.76 (0.31 to 1.84) |
79 (1 study) | ⊕⊕⊝⊝ low1,2 |
Additional glaucoma surgery defined as retreatment with cyclophotocoagulation according to randomized assignment |
| Adverse events: atonic pupil | 311 per 1000 |
277 per 1000 (146 to 523) |
RR 0.89 (0.47 to 1.68) |
92 (1 study) | ⊕⊕⊝⊝ low1,2 |
Atonic pupil was the only adverse event reported by treatment group. Trial authors noted that most participants had mild to moderate pain for a few days following the procedure and many also had transient conjunctival burns (number not reported). Severe iritis occurred in 2 eyes and hyphema occurred in 3 eyes. No instances of hypotony or phthisis bulbi were reported |
Footnotes
Downgraded for risk of bias in the trial (unmasked outcome assessors and 14% attrition).
Downgraded for imprecision in the effect estimate (wide confidence interval).
All outcomes are reported for participants with at least 3 months follow-up; mean follow-up was 13.2 months.
The basis for the assumed risk is the risk in the comparison group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). J: joule; CI: confidence interval; RR: risk ratio; mmHg: millimeter of mercury
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Footnotes
Declarations of interest
MM reports receiving payment for educational courses on methodology and glaucoma diagnostic accuracy from Polifarma and Santen. As a member of the International Glaucoma Panel (Allergan), mentored by Prof. David Garway-Heath, MM has received travel accommodations and meeting expenses for three ARVO meetings (2015 – 2017).
AKB: None known
KL: None known
References to studies
Included studies
- Egbert PR, Fiadoyor S, Budenz DL, Dadzie P, Byrd S. Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open-angle glaucoma. Archives of Ophthalmology 2001;119(3):345–50. [DOI] [PubMed] [Google Scholar]
- Egbert PR, Fiadoyor S, Budenz DL, Dadzie P, Byrd S. Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open-angle glaucoma. Evidence-Based Eye Care 2001;2(4):238–9. [DOI] [PubMed] [Google Scholar]
Excluded studies
- Agarwal HC, Gupta V, Sihota R. Evaluation of contact versus non-contact diode laser cyclophotocoagulation for refractory glaucomas using similar energy settings. Clinical and Experimental Ophthalmology 2004;32(1):33–8. [DOI] [PubMed] [Google Scholar]
- Alves AA Jr, Penna LB. Contact diode laser transcleral cyclophotocoagulation in neovascular glaucoma [Ciclolaser de diodo transescleral no tratamento do glaucoma secundário à isquemia retiniana]. Revista Brasileira de Oftalmologia 1997; 56(12):943–9. [Google Scholar]
- Alves AA Jr, Yamane R, Dos Santos Motta MM. Comparative study of diode transscleral cyclophotocoagulation associated or not with periphery retinal ablation in neovascular glaucoma. Revista Brasileira de Oftalmologia 2003;62(8):578–88. [Google Scholar]
- Ando F, Miyake K, Federman JL. Nd:YAG laser transscleral contact cyclophotocoagulation in refractory glaucoma. Lasers and Light in Ophthalmology 1990;3(2):119–22. [Google Scholar]
- Aquino MC, Barton K, Tan AM, Sng C, Li X, Loon SC, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clinical and Experimental Ophthalmology 2015;43(1):40–6. [DOI] [PubMed] [Google Scholar]
- Berke SJ, Sturm RT, Caronia RM, Nelson DB, D’Aversa G. Phacoemulsification combined with endoscopic cyclophotocoagulation in the management of cataract and glaucoma. American Academy of Ophthalmology 2006:184. [Google Scholar]
- Brooks AM, Dallison IW, Gillies WE, Guest CS, Taylor HR. Comparison of cycloablation with nd:yag cyclophotocoagulation and cyclocryotherapy. Investigative Ophthalmology and Visual Science 1993;34:ARVO E-Abstract 182. [Google Scholar]
- Cellini M, Pelle D, Sbrocca M, Possati GL, Caramazza N, Santiago L. Semiconductor diode laser cyclophotocoagulation in neovascular glaucoma treatment. 1994. Annali di Ottalmologia e Clinica Oculistica;120(10):629–35. [Google Scholar]
- Chalam KV, Lin N, Tripathi R. Advanced neovascular glaucoma: parsplana modified Baerveldt implant versus Nd:YAG transscleral cyclophotocoagulation. American Academy of Ophthalmology 1999:241. [Google Scholar]
- Chalam KV, Malkani SM, Tripathi RC, Ambati J. Neovascular glaucoma: pars plana baerveldt implant vs nd:yag transscleral cyclophotocoagulation vs yag endocyclophotocoagulation. American Academy of Ophthalmology 2001:168. [Google Scholar]
- Colvin Trucco R Diode laser in refracted glaucoma [Diodo láser en glaucoma refractario]. Archivos Chilenos de Oftalmologia 1995;52(2):35–7. [Google Scholar]
- Crymes BM, Gross RL. Laser placement in noncontact Nd:YAG cyclophotocoagulation. American Journal of Ophthalmology 1990;110(6):670–3. [DOI] [PubMed] [Google Scholar]
- Fankhauser F, Kwasniewska S, England C, Dürr V. Diode versus Nd:YAG laser for cyclodestructive procedures. Ophthalmic Surgery 1993;24(8):566–7. [PubMed] [Google Scholar]
- Gaasterland DE, Pollack IP, Spaeth GL, Coleman DJ, Wilensky JT. Initial experience with a new method of laser transscleral cyclophotocoagulation for ciliary ablation in severe glaucoma. Transactions of the American Ophthalmological Society 1992; 90:225–46. [PMC free article] [PubMed] [Google Scholar]
- Goldenberg-Cohen N, Bahar I, Ostashinski M, Lusky M, Weinberger D, Gaton DD. Cyclocryotherapy versus transscleral diode laser cyclophotocoagulation for uncontrolled intraocular pressure. Ophthalmic Surgery Lasers and Imaging 2005; 36(4):272–9. [PubMed] [Google Scholar]
- Janknecht P Phacoemulsification combined with cyclophotocoagulation. Klinische Monatsblatter fur Augenheilkunde 2005; 222(9):717–20. [DOI] [PubMed] [Google Scholar]
- Kato S, Ideta R, Kobayashi F, Shimizu E, Motegi Y, Funatsu H, et al. Treatment of diabetic neovascular glaucoma by cyclocryotherapy and transscleral cyclophotocoagulation with diode laser. Japanese Journal of Clinical Ophthalmology 1997; 51(10):1739–44. [Google Scholar]
- Koraszewska-Matuszewska B, Leszczyński R, Samochowiec-Donocik E, Nawrocka L. Cyclodestructive procedures in secondary glaucoma in children. Klinika Oczna 2004;106(1–2 Suppl):199–200. [PubMed] [Google Scholar]
- Korte P, Wirbelauer C, Haberle H, Pham DT. Cyclophoto- versus cyclocryo-coagulation for treatment of secondary glaucoma. Ophthalmologe 2002;99 (Suppl 1):S97. [Google Scholar]
- Liu G, Tang GL. Effect of transscleral diode laser cyclophotocoagulation and cyclocryosurgery in treatment of severe glaucoma. International Journal of Ophthalmology 2008;8(8):1673–4. [Google Scholar]
- Marcus C, Moster M, Wilson R. A four year follow up comparison of 180° vs. 360° neodymimium:yag transscleral cyclophotocoagulation. Investigative Ophthalmology and Visual Science 1992;33:ARVO E-Abstract 2876. [Google Scholar]
- Miller-Meeks M, Higginbotham EJ. Comparing energy levels of contact Nd:YAG transscleral laser cyclophotocoagulation (CTLC) in uncontrolled glaucoma. American Academy of Ophthalmology 1994:130. [Google Scholar]
- Montanari P, Italia A, Marangoni P, Pinotti D, Miglior M. Diode laser trans-scleral cyclophotocoagulation in refractory glaucoma treatment. Acta Ophthalmologica 1997;75(224):38. [DOI] [PubMed] [Google Scholar]
- Shields MB, Wilkerson MH, Echelman DA. A comparison of two energy levels for noncontact transscleral neodymium-YAG cyclophotocoagulation. Archives of Ophthalmology 1993;111(4):484–7. [DOI] [PubMed] [Google Scholar]
- Walland MJ. Diode laser cyclophotocoagulation: Dose-standardized therapy in end-stage glaucoma. Australian and New Zealand Journal of Ophthalmology 1998;26(2):135–9. [DOI] [PubMed] [Google Scholar]
- Yildirim N, Yalvac IS, Sahin A, Ozer A, Bozca T. A comparative study between diode laser cyclophotocoagulation and the Ahmed glaucoma valve implant in neovascular glaucoma: a long-term follow-up. Journal of Glaucoma 2009;18(3):192–6. [DOI] [PubMed] [Google Scholar]
- Zhang B Contrast of surgical effect of two different operations for neovascular glaucoma. International Journal of Ophthalmology 2010;10(4):671–3. [Google Scholar]
Other references
Additional references
- The Advanced Glaucoma Intervention Study Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. American Journal of Ophthalmology 2000; 130(4):429–40. [DOI] [PubMed] [Google Scholar]
- Ansari E, Gandhewar J. Long-term efficacy and visual acuity following transscleral diode laser photocoagulation in cases of refractory and non-refractory glaucoma. Eye 2007;21(7):936–40. [DOI] [PubMed] [Google Scholar]
- Bechrakis NE, Müller-Stolzenburg NW, Helbig H, Foerster MH. Sympathetic ophthalmia following laser cyclocoagulation. Archives of Ophthalmology 1994;112(1):80–4. [DOI] [PubMed] [Google Scholar]
- Beckman H, Kinoshita A, Rota AN, Sugar HS. Transscleral ruby laser irradiation of the ciliary body in the treatment of intractable glaucoma. Transactions of the American Academy of Ophthalmology and Otolaryngology 1972;76(2):423–36. [PubMed] [Google Scholar]
- Bloom PA, Tsai JC, Sharma K, Miller MH, Rice NS, Hitchings RA, et al. “Cyclodiode”. Trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology 1997;104(9):1508–19. [DOI] [PubMed] [Google Scholar]
- Burr J, Azuara-Blanco A, Avenell A, Tuulonen A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database of Systematic Reviews 2012, Issue 9 Art. No.: CD004399 DOI: 10.1002/14651858.CD004399.pub3. [DOI] [PubMed] [Google Scholar]
- Chen MF, Kim CH, Coleman AL. Cyclodestructive procedures for refractory glaucoma. Cochrane Database of Systematic Reviews 2016, Issue 6 Art. No.: CD012223 DOI: 10.1002/14651858.CD012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. American Journal of Ophthalmology 1998;126(4):498–505. [DOI] [PubMed] [Google Scholar]
- Coleman DJ, Lizzi FL, Driller J, Rosado AL, Chang S, Iwamoto T, et al. Therapeutic ultrasound in the treatment of glaucoma.I. Experimental model. Ophthalmology 1985;92(3):339–46. [DOI] [PubMed] [Google Scholar]
- Coleman AL, Gordon MO, Beiser JA, Kass MA. Ocular Hypertension Treatment Study. Baseline risk factors for the development of primary open-angle glaucoma in the Ocular Hypertension Treatment Study. American Journal of Ophthalmology 2004;138(4):684–5. [DOI] [PubMed] [Google Scholar]
- Edward DP, Brown SV, Higginbotham E, Jennings T, Tessler HH, Tso MO. Sympathetic ophthalmia following neodymium:YAG cyclotherapy. Ophthalmic Surgery 1989;20(8):544–6. [PubMed] [Google Scholar]
- European Glaucoma Society. European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 3: Treatment principles and options. Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 3 Treatment principles and options. British Journal of Ophthalmology 2017;101(6):130–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, Wolfs RC, O’Colmain BJ, Klein BE, Taylor HR, West S, et al. Prevalence of open-angle glaucoma among adults in the United States. Archives of Ophthalmology 2004;122(4):532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet 2015;385(9975):1295–304. [DOI] [PubMed] [Google Scholar]
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130–6. [PMC free article] [PubMed] [Google Scholar]
- GRADEpro GDT [Computer program]. Version accessed prior to 10 April 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015Available at gradepro.org. [Google Scholar]
- Hauber FA, Scherer WJ. Influence of total energy delivery on success rate after contact diode laser transscleral cyclophotocoagulation: a retrospective case review and meta-analysis. Journal of Glaucoma 2002;11(4):329–33. [DOI] [PubMed] [Google Scholar]
- Hennis A, Wu SY, Nemesure B, Honkanen R, Leske MC; Barbados Eye Studies Group. Awareness of incident open-angle glaucoma in a population study: the Barbados Eye Studies. Ophthalmology 2007;114(10):1816–1821. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
- Ismail R, Azuara-Blanco A, Ramsay CR. Consensus on outcome measures for glaucoma effectiveness trials: results from a Delphi and nominal group technique approaches. Journal of Glaucoma 2016;25(6):539–46. [DOI] [PubMed] [Google Scholar]
- Lam S, Tessler HH, Lam BL, Wilensky JT. High incidence of sympathetic ophthalmia after contact and noncontact neodymium:YAG cyclotherapy. Ophthalmology 1992;99(12):1818–22. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Archive of Ophthalmology 2003; 121(1):48–56. [DOI] [PubMed] [Google Scholar]
- Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, et al. ; CIGTS Study Group. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108(11):1943–53. [DOI] [PubMed] [Google Scholar]
- Lin SC. Endoscopic and transscleral cyclophotocoagulation for the treatment of refractory glaucoma. Journal of Glaucoma 2008;17(3):238–47. [DOI] [PubMed] [Google Scholar]
- Liu GJ, Mizukawa A, Okisaka S. Mechanism of intraocular pressure decrease after contact transscleral continuous-wave Nd:YAG laser cyclophotocoagulation. Ophthalmic Research 1994;26(2):65–79. [DOI] [PubMed] [Google Scholar]
- Meyer SJ. Diathermy cauterization of ciliary body for glaucoma. American Journal of Ophthalmology 1948;31(11):1504–6. [PubMed] [Google Scholar]
- Murphy CC, Burnett CA, Spry PG, Broadway DC, Diamond JP. A two centre study of the dose-response relation for transscleral diode laser cyclophotocoagulation in refractory glaucoma. British Journal of Ophthalmology 2003;87(10):1252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor SA, Iwach A, Nozik RA, Hetherington J, Fellman R. Presumed sympathetic ophthalmia following Nd: YAG transscleral cyclophotocoagulation. Journal of Glaucoma 1993;2(1):30–1. [PubMed] [Google Scholar]
- Pastor SA, Singh K, Lee DA, Juzych MS, Lin SC, Netland PA, et al. Cyclophotocoagulation: a report by the American Academy of Ophthalmology. Ophthalmology 2001;108(11):2130–8. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Number of people with glaucoma worldwide. British Journal of Ophthalmology 1996;80(5):389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology 2006;90(3):262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Review Manager 5 (RevMan 5) [Computer program]. Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- Sinchai PO, Vajaranant T, Wilensky JT, Hillman D. Outcomes of transscleral cyclophotocoagulation based on type of glaucoma. Investigative Ophthalmology and Visual Science 2008:ARVO E-Abstract 1233. [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121(11):2081–90. [DOI] [PubMed] [Google Scholar]
- Topouzis F, Coleman AL, Harris A, Koskosas A, Founti P, Gong G, et al. Factors associated with undiagnosed open-angle glaucoma: the Thessaloniki Eye Study. American Journal of Ophthalmology 2008;145(2):327–35. [DOI] [PubMed] [Google Scholar]
- Tseng VL, Coleman AL, Chang MY, Caprioli J. Aqueous shunts for glaucoma. Cochrane Database of Systematic Reviews 2017, Issue 7 Art. No.: CD004918 DOI: 10.1002/14651858.CD004918.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth M, Hu K, Bunce C, Gazzard G. Endoscopic cyclophotocoagulation (ECP) for open angle glaucoma and primary angle closure. Cochrane Database of Systematic Reviews 2017, Issue 8 Art. No.: CD012741 DOI: 10.1002/14651858.CD012741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih LM, Nanjan M, McCarty CA, Taylor HR. Prevalence and predictors of open-angle glaucoma: results from the Visual Impairment Project. Ophthalmology 2001;108(11):1966–72. [DOI] [PubMed] [Google Scholar]
Other published versions of this review
- Jones L, Smith O, Yousuf SJ, Kwagyan J. Cyclodestructive procedures for glaucoma. Cochrane Database of Systematic Reviews 2011, Issue 9 Art. No.: CD009313 DOI: 10.1002/14651858.CD009313. [Google Scholar]