Abstract
PURPOSE
Large-scale, population-based biobanks integrating health records and genomic profiles may provide a platform to identify individuals with disease-predisposing genetic variants. Here, we recall probands carrying familial hypercholesterolemia (FH)-associated variants, perform cascade screening of family members, and describe health outcomes affected by such a strategy.
METHODS
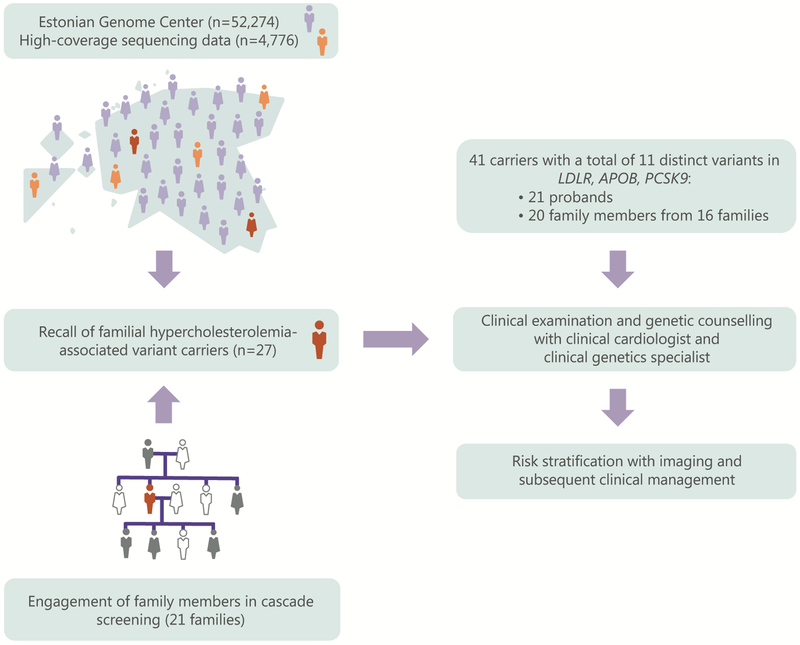
The Estonian Biobank of Estonian Genome Center, University of Tartu, comprises 52,274 individuals. Among 4,776 participants with exome or genome sequences, we identified 27 individuals who carried FH-associated variants in the LDLR, APOB or PCSK9 genes. Cascade screening of 64 family members identified an additional 20 carriers of FH-associated variants.
RESULTS
Via genetic counselling and clinical management of carriers, we were able to reclassify 51% of the study participants from having previously established non-specific hypercholesterolemia to having FH and identify 32% who were completely unaware of harbouring a high-risk disease-associated genetic variant. Imaging-based risk stratification targeted 86% of the variant carriers for statin treatment recommendations.
CONCLUSION
Genotype-guided recall of probands and subsequent cascade screening for familial hypercholesterolemia is feasible within a population-based biobank and may facilitate more appropriate clinical management.
Keywords: recall-by-genotype, population-based biobank, familial hypercholesterolemia, cascade screening, genomics-guided disease management
INTRODUCTION
With the plummeting costs of exome (WES) and genome sequencing (WGS), the collection of high-coverage genomic data is increasingly becoming routine in genetic research. Population-based biobanks, which combine such genomic data with electronic health records (EHR) and clinical phenotyping, provide an opportunity to study the population-specific landscape of clinically important phenotypes and enable recall by genotype (RbG) studies of individuals carrying genetic variant of interest, as well as their relatives1. The genotype-first approach (i.e. genetic variant ascertainment precedes phenotypic measurement) has been established as an effective strategy in human genetics, allowing the refinement of (endo)phenotypes and study of biological heterogeneity by overcoming initial phenotypic ascertainment biases2,3. Applying such strategy within a healthcare-associated biobank enables to additionally investigate whether the RbG approach benefits clinical disease management.
Familial hypercholesterolemia (FH) is as an ideal case in which to implement such a strategy, being one of the most common single-gene disorders (prevalence of 1 in 217 in Europe4) and having actionable treatment options5. FH, which primarily results from deleterious variants in the LDLR, APOB or PCSK9 genes, involves the dysfunction of the low-density lipoprotein (LDL) receptor and concomitant over-activity of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, resulting in elevated low-density lipoprotein cholesterol (LDL-C) levels and a substantially increased risk of premature coronary heart disease (CHD)6,7. While severely high LDL-C concentrations (>4.9 mmol/L) are commonly acknowledged in clinical FH diagnosis, recent studies highlight that FH-associated variant carriers display a wide spectrum of LDL-C levels, and the risk for CHD persists across the range of these values8,9.
The Estonian Genome Center’s population-based collection of comprehensive health data and genomic profiles on more than 50,000 individuals offers a valuable data source in which to assess the effectiveness of genotype-guided clinical management. Here, we apply the RbG approach coupled with cascade screening, genetic counselling and deep-phenotyping within the Estonian population-based biobank to FH, a condition that is linked to significant morbidity and mortality but is substantially modifiable when recognized and managed.
MATERIALS AND METHODS
Cohort overview
The Estonian Biobank is a population-based biobank of the Estonian Genome Center at the University of Tartu (EGCUT). The cohort of 52,274 individuals aged 18 and over closely reflects the age, sex and geographical distribution of the Estonian population. All participants have signed a broad informed consent form, which allows the continuous updating of epidemiologic data through periodical linking to national electronic databases and re-contacting of participants. Detailed overview of the biobank has been described previously10. High-coverage sequencing data was available for 10% of the cohort. The genomes of 2,535 individuals, selected randomly by county of birth to represent as much of the genetic diversity as possible, were sequenced at the Broad Institute, Cambridge, USA. Exomes of an additional subset of 2,500 individuals were sequenced at Nestlé Institute of Health Sciences, Lausanne. The latter sample set contained 878 healthy constitutionally thin individuals (BMI in the lowest 4% quantile at EGCUT after adjustment for age and sex) and 1,622 healthy BMI controls (BMI in 30–50% quantile at EGCUT after adjustment for age and sex) selected from the Estonian Biobank within the age range of 20–45 years. WGS followed a PCR-free sample preparation and sequenced on the Illumina HiSeq X Ten using 150 bp paired-end reads with mean coverage of 30×. WES samples were sequenced using the Agilent SureSelect Human All Exon V5+UTRs Kit according to the manufacturer’s recommendations with mean target coverage of 67×. Sequenced reads were aligned against the GRCh37/hg19 human reference genome using BWA-MEM11 v0.7.7, and sequence variants were annotated with Variant Effect Predictor12 version 87 (Gencode v19 on assembly GRCh37.p13) and ANNOVAR13. Baseline lipid parameters for the WGS subset were measured with either a conventional enzymatic colorimetric method at Tartu University Hospital or North Estonia Medical Centre (n=1,025), or with Vertical Auto Profile (VAP) based on density gradient ultracentrifugation by Atherotech14,15 (n=2,181; measured in mg/dl), and for the WES subset with the conventional method only. The effect of statin treatment in individuals, who had self-reported use at baseline lipid measurement, was taken into account by dividing LDL-C value by 0.7, as implemented previously16 (termed “statin-adjusted”) (Table S1).
The project was approved by the Research Ethics Committee of the University of Tartu (application number 253/T-14, December 2015 and 234/T-12, March 2014) and complies with the Declaration of Helsinki.
Identification of FH-associated variants
Rare (minor allele frequency (MAF) <0.5%) deleterious variants in three FH-associated autosomal dominant genes (LDLR, APOB, PCSK9) were ascertained in carriers with untreated baseline LDL-C level of ≥4.0 mmol/L within the WGS and WES datasets. The determined threshold corresponds to the lowest LDL-C value considered in the Dutch Lipid Clinic Network (DLCN) diagnostic criteria for familial hypercholesterolemia diagnosis17, suggested by the European Society of Cardiology and European Atherosclerosis Society for the management of dyslipidaemias18 (Table S2).
We considered variants annotated as loss-of-function (i.e., premature stop codon, disruption of an essential splice site, or frameshift of the reading frame), deleterious missense variants in LDLR, gain-of-function missense variants in PCKS9, and deleterious variants in exon 26 (encompassing the binding site of the LDL receptor)19 in APOB. A variant was considered as FH-associated if MAF <0.5%, identified in a carrier with LDL-C level of ≥4.0 mmol/L and 1) reported as pathogenic/likely pathogenic in NCBI-ClinVar20 and/or 2) determined pathogenic according to in silico prediction algorithms.
Management of probands and cascade screening
Carriers of the identified FH-associated variants (probands) were contacted via regular mail and upon positive response scheduled for an initial appointment with a clinical genetics specialist and clinical cardiologist either at Tartu University Hospital or North Estonia Medical Centre. At the initial appointment, family and medical history was specified, and a standard clinical examination including ascertainment of features specific to FH was performed. 50 ml of fasting blood from a peripheral vein was drawn for biochemical measurements (including enzymatic colorimetric assay-based lipid measurements) and for a DNA-based confirmation of the genetic finding. If the proband did not have clinical atherosclerotic cardiovascular disease (ASCVD), investigations for subclinical atherosclerosis were performed (computed tomography for coronary artery calcium (CAC) score, carotid ultrasound for intima-media thickness (IMT) assessment, and exercise electrocardiogram (ECG)). Sub-clinical ASCVD was determined as CAC (Agatston score) >0, or presence of atherosclerotic plaque in carotid artery (focal wall thickening >50% greater than the surrounding vessel wall or focal region with an IMT measurement ≥1.5 mm protruding into the lumen). After the confirmation of the identified variant by Sanger sequencing at a CLIA-certified laboratory, a feedback appointment was scheduled. During the visit, the specified genetic finding was disclosed, the pathophysiology of FH and the pattern of inheritance together with the probability for close relatives being affected was explained, and a summary of the clinical and imaging investigations and treatment or changes in treatment, if necessary, were provided. The investigation of the 1st and 2nd degree relatives invited to participate in cascade screening followed the same approach as for the probands. Only those individuals, who carried the variants identified in the family, were subjected to instrumental investigations. For extended details on cascade screening please refer to Supplementary Materials and Methods and Figure S1.
LDL-C level association analysis
To investigate the effect of FH-associated genetic variants on LDL-C levels, a subset of 978 FH-associated variant non-carriers from the WGS sample set with available conventionally measured LDL-C levels was formed. FH-associated variant carriers included 21 probands and 20 relatives, who participated in the study and had LDL-C measurements available at the initial appointment. Statin treatment was taken into account by dividing LDL-C value by 0.720 (termed “statin-adjusted”). A mixed linear model (package lme4qtl)21 in R (R Project for Statistical Computing22) version 3.4.1 was used to analyse the association between LDL-C levels and FH-associated variants: the presence of any FH-associated variant was defined as a binary variable in all samples, and the model was adjusted for age, age2 and sex, with relatedness between individuals taken into account as a kinship matrix. While the kinship matrix for FH-linked variant non-carriers (n=978) and index cases (n=21) was calculated based on genome-wide genetic data, the kinship of family members (n=20) was added such that the relatedness with FH-linked non-carriers was deemed zero, and with the respective family members as the coefficient of relationship based on the pedigree overview. As the significance effect is not reported in the abovementioned package, it was estimated on a sample set, where up to 3rd degree relatives had been removed (3 probands, all cascades and 53 non-carriers were excluded).
CAC score sensitivity analysis
The CAC scores of FH-associated carriers with subclinical disease and CAC >0 (n=19) were compared with the distribution of CAC scores in the Multi-Ethnic Study of Atherosclerosis (MESA) sub-cohort according to the CAC Score Reference Values23. The respective MESA cohort consisted of 2503 men and women of Caucasian ethnicity and free of symptomatic clinical ASCVD and treated diabetes. While the CAC scores at the 25th and the 75th percentiles were available for the age range of 45 to 84, the estimated percentiles for those <45 years of age were set at zero.
Please refer to Supplementary Material and Methods for expanded Methods section.
RESULTS
Upon screening for rare deleterious genetic variants in WGS (n=2,420) and WES (n=2,356) samples, we identified 27 probands who carry a total of 11 distinct heterozygous variants in FH-associated genes (LDLR, APOB, PCSK9) (Table S3). All variants were confirmed with Sanger sequencing prior to the call-back of probands with non-concordance rate of zero, indicating high reliability of deep-coverage WGS/WES for variant identification.
Study participants
Twenty-one (78%) of the twenty-seven FH-associated variant carriers (mean age 47.1 (SD 15.9), 43% were female) responded positively to the call-back and were scheduled for an appointment with a clinical cardiologist and clinical genetics specialist. Six individuals declined to participate either due to health issues or missing contact information (Figure S1). Of 21 participants, ten probands harboured a missense variant in APOB (p.Arg3527Gln), ten carried a total of eight distinct missense variants in LDLR, and one had a missense variant in the PCSK9 gene (Table S3). After clinical management and genetic counselling, all carriers were guided to engage their 1st and 2nd degree relatives in cascade screening. Of 112 invited family members, 64 relatives (57%, mean age 46.6 (SD 17.1), 52% were female) participated with at least one cascade joining from 16 families. Twenty relatives (mean age 47.3 (SD 17.5), 55% were female) were heterozygous for the FH-associated variant, yielding one new case per proband. Altogether, 41 carriers were identified via RbG and cascade screening, demonstrating high re-contact rate of probands (78%) and successful engagement of family members (76% of the invited families engaged) (Figure 1).
Figure 1.
Overview of recall by genotype approach for familial hypercholesterolemia within the Estonian Genome Center and subsequent clinical management.
Clinical status of FH-associated variant carriers
Prior to participating, among forty-one FH-associated variant carriers, twenty-one (51%) had non-specific hypercholesterolemia diagnosed or increased LDL-C level identified, three (7%) had a clinical FH diagnosis and four (10%) had ASCVD manifested. Seventeen individuals (41%) did not carry diagnoses for hypercholesterolemia or ASCVD. Of 41 FH-associated variant carriers, 6 individuals (15%) reported positive family history of premature ASCVD in a first-degree family member. While 19 FH-associated variant carriers (46%) had statins prescribed, 13 (32%) of the 19 carriers reported statin use and one was taking over-the-counter lipid-lowering supplements at the initial appointment. LDL-C levels varied from 3.22 mmol/L to 11.56 mmol/L (mean 5.78 mmol/L (SD 2.05)) among carriers not on treatment (n=28). The mean statin-adjusted LDL-C concentration in carriers on treatment (n=13) was 5.79 mmol/L (SD 1.41), ranging from 3.89 mmol/L to 9.10 mmol/L (Table 1). None of those on treatment had achieved the target of LDL-C <2.6 mmol/L recommended for FH cases without ASCVD according to the ESC guidelines18 (Table S5). Only two carriers (5%) had a visible physical sign (arcus cornealis) of FH (77- and 78-year-old brother and sister carrying APOB p.Arg3527Gln).
Table 1.
Characteristics of study participants.
| Characteristics | Probands | Participating family members | |
|---|---|---|---|
| Participants | Carriers | Non-carriers | |
| N | 21 | 20 | 44 |
| Age (years) | 47.7 (15.9) | 47.3 (17.5) | 46.25 (17.11) |
| Women | 9 (43%) | 9 (45%) | 22 (50%) |
| Blood pressure (mmHg) | |||
| systolic | 132.4 (25.0) | 135.2 (19.2) | 137.6 (22.1) |
| diastolic | 82. 9 (10.2) | 84.8 (12.1) | 84.5 (11.3) |
| LDL-C (mmol/L) | 5.3 (2.1) | 5.1 (1.8) | 3.4 (0.9) |
| TC (mmol/L) | 7.3 (2.2) | 6.8 (1.9) | 5.2 (0.9) |
| HDL-C (mmol/L) | 1.9 (0.8) | 1.6 (0.4) | 1.7 (0.4) |
| TG (mmol/L) | 1.2 (0.7) | 1.4 (0.9) | 1.1 (0.7) |
| Diabetes Mellitus | 0 (0%) | 1 (5%) | 3 (7%) |
| Hypercholesterolemia | 13 (62%) | 8 (40%) | 3 (7%) |
| FH | 3 (14%) | 0 (0%) | 0 (0%) |
| Prevalent ASCVD | 2 (10%) | 2 (10%) | 6 (14%) |
| Body mass index | 26.5 (9.2) | 27.8 (5.7) | 27.3 (5.3) |
| Current smokers | 7 (33%) | 4 (20%) | 13 (30%) |
| Lipid-lowering medication prescription | 13 (62%) | 6 (30%) | 8 (18%) |
| Lipid-lowering medication use | 8 (38%) | 5 (25%) | 9 (20%) |
| Antihypertensive medication use | 4 (19%) | 6 (30%) | 13 (30%) |
Data are number (%) or mean (SD). FH - familial hypercholesterolemia; ASCVD - atherosclerotic cardiovascular disease; TC - total cholesterol; TG - triglycerides.
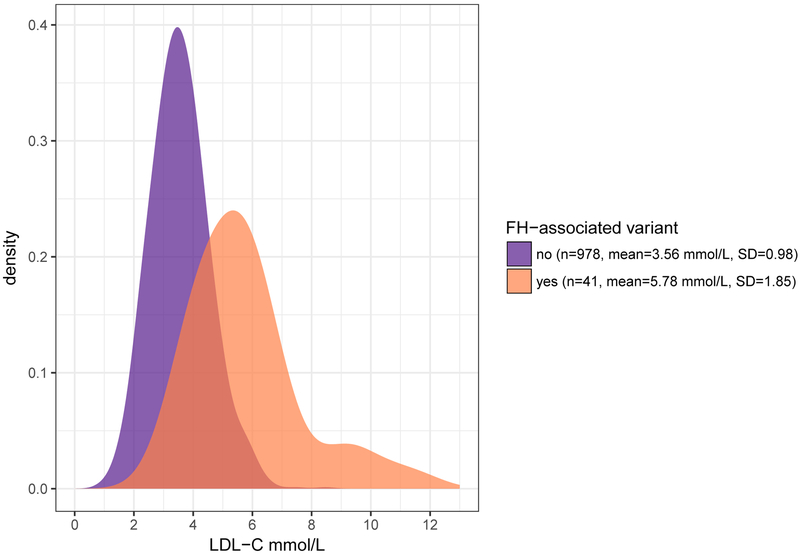
Compared to 978 non-carriers in the WGS sample set with conventionally measured LDL-C levels, the LDL-C value was increased on average by 2.33 mmol/L (SD 0.18, adjusted model p =1.55×10−21) in FH-associated variant carriers (n=41). APOB variant carriers had on average greater LDL-C concentrations (n=20, β =2.96 mmol/L, SD 0.25) than individuals with a variant in LDLR (n=20, β =1.73 mmol/L, SD 0.24), compared to non-carriers. LDL-C level of a PCSK9 variant carrier was 1.5 SD higher than in non-carriers. Despite higher LDL-C levels among FH-associated variant carriers, we observed substantial overlap in the LDL-C distributions between carriers and non-carriers (Figure 2), highlighting current clinical screening challenges. Furthermore, low statin use and LDL-C levels above treatment goals in FH-associated variant carriers indicate lack of genetic and clinical awareness of the disease.
Figure 2.
Distribution of statin-adjusted LDL-C values in FH-associated variant carriers (n=41, mean LDL-C 5.78 mmol/L, SD 1.85) and non-carriers (n=978, mean LDL-C 3.56 mmol/L, SD 0.98). The LDL-C level was increased by 2.33 mmol/L (SD 0.18) in individuals harbouring an FH-associated variant, compared to non-carriers.
Imaging-based phenotyping
To determine the presence of subclinical cardiovascular pathology, all probands and carrier relatives without prevalent ASCVD underwent imaging-based phenotyping (carotid ultrasound and computed tomography). While four FH-associated variant carriers (10%) had clinically manifested ASCVD (mean age 73.5 (SD 11.6), 50% were female) and three (7%) declined the procedures, twenty FH-associated variant carriers (49%) displayed sub-clinical disease (mean age 50.5 (SD 12.8), 46% were female, mean statin-adjusted LDL-C 6.31 (SD 1.76)) and fourteen (34%) did not (mean age 36.2 (SD 8.9), 43% were female, mean statin-adjusted LDL-C 4.55 (SD 1.06) (Table S5).
Of 20 individuals with sub-clinical atherosclerosis, five had plaques in carotid arteries and 19 CAC >0. Of the latter group, 16 (84%) were not expected to have sub-clinical atherosclerosis by the CAC score Reference Value model23, illustrating that the increased LDL-C levels due to a genetic defect predisposes to the premature progression of atherosclerosis. Three individuals, however, displayed expected CAC scores: 59-year-old male with LDLR p.Arg115Cys, and 51- and 61-year-old females harbouring APOB p.Arg3527Gln and LDLR p.Gly396Ala, respectively. The latter two had, however, demonstrable plaques in carotid arteries (Figure 3).
Figure 3.
The CAC scores of FH-associated variant carriers with sub-clinical disease and CAC >0 (n=19) (filled coloured circles) in comparison with the distribution of CAC scores in the MESA sub-cohort of Caucasian ethnicity and without symptomatic clinical ASCVD and treated diabetes. The rectangles represent the expected CAC score distribution between the 25th and 75th percentile in the MESA sub-cohort for every age and for men (blue) and women (red) separately, with age on the x-axis and CAC score on the y-axis. While 16 individuals were not expected to have subclinical ASCVD, three individuals did (indicated with black circles). However, the 61-year-old female with LDLR p.Gly396Ala and the 54-year-old female with APOB p.Arg3527Gln displayed plaques in carotid arteries. The 59-year-old male harboured LDLR p.Arg115Cys.
Clinical management of FH-associated variant carriers
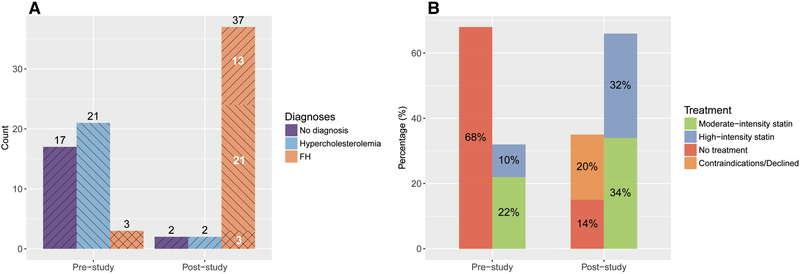
After clinical and imaging-based phenotyping, 37 individuals (90%) carried a diagnosis for FH. Twenty-one FH-associated carriers (51%) were reclassified from having non-specific hypercholesterolemia to having FH, while thirteen (32%) had completely gone unrecognized by the medical system (Figure 4A). Moderate-intensity statin treatment was started for fourteen (34%), statins were up-titrated for eleven (27%), left unchanged for two (5%) and not prescribed for six (14%). Eight participants (20%) either had contraindications or declined. (Figure 4B). All individuals were given recommendations for lifestyle modifications.
Figure 4.
A. Disease diagnoses in FH-associated variant carriers before and after the study. After clinical and imaging-based phenotyping, 37 participants were diagnosed with FH: 3 carried FH diagnosis before the study (crossed lines), 21 participants were reclassified from having non-specific hypercholesterolemia to FH (left-leaning diagonal lines), and 13 were newly diagnosed cases (right-leaning diagonal lines). B. Statin treatment in FH-associated variant carriers before and after the study. At the initial appointment, 13 (32%) participants reported statin use, while 28 (68%) did not. At the end of the study moderate-intensity statin treatment was started for 14 (34%), and up-titrated to or kept on high-intensity statin treatment for 13 (32%) carriers. Statin treatment was not started for 14 (34%) participants, including eight who either had contraindications or declined.
DISCUSSION
Recall of probands harbouring a high-risk FH-associated genetic variant and their 1st and 2nd degree family members allowed us to investigate the feasibility and clinical value of the RbG approach for risk estimation and potential risk reduction within a population-based biobank. This strategy led us to ascertain the genetic cause of non-specific hypercholesterolemia for 51% of the carriers, for whom increased LDL-C had clinically been identified, discover 32%, who were completely unaware of being at high risk for premature CHD, and allowed us to clinically identify 86%, who were targeted for pharmacological intervention.
By screening for FH-associated variants in high-coverage sequenced data (n=4,776), we identified 27 probands, of whom 78% participated in the study, and subsequently engaged 76% of the families for clinical evaluation. In total, 41 individuals harboured an FH-associated genetic variant that predisposes to premature CHD. While the increased LDL-C levels had clinically been identified for 51% of the participants prior to the study, only half of them were on statin treatment and none had achieved the guideline-recommended LDL-C levels18. These observations conform with the epidemiological studies denoting substantial underdiagnosis of the disease4,24.
The clinical examination of the variant carriers demonstrated insensitivity of current criteria used in FH diagnosis. First, we confirmed that FH-associated variant carriers display a wide spectrum of LDL-C levels as observed previously8,9: 14 (34%) of the 41 variant carriers had statin-adjusted LDL-C levels ≤4.9 mmol/L. Second, the presence of visible accumulations of lipid deposits, that are commonly considered in FH scores (Dutch Lipid Clinic Network17, Simon Broome25, MEDPED26), were detected in 5% of the carriers only. And third, we perceived significant heterogeneity in clinical expression, even in individuals carrying the same FH-associated variant. Variability in phenotype and paucity of physical FH features has been highlighted previously27,28, even among homozygous FH cases29, and thus, reinforces the requisite for genetic testing in FH diagnosis.
Via imaging-based phenotyping, we identified premature clinical or sub-clinical atherosclerosis in 59% of the participants. This corroborates with recent results that the disease is diagnosed late in life and most of the FH cases are characterized with the presence of ASCVD30–32. Participants without demonstrable atherosclerosis (34%) were considerably younger (mean age 36.2 (SD 8.9)) compared to those with sub-clinical disease (mean age 50.5 (SD.12.8)), and comprise, thus, a target group that should be kept under further clinical surveillance.
This study is among the first that utilizes the genomic and health data contained in a population-based biobank to evaluate the implications of genomics-guided disease management that can be directly translated to the clinic. While the RbG approach has been shown to be an effective tool to systematically investigate tissue-specific and mechanistic associations of single or multiple genetic variants with phenotypes of interest1,33,34, the assessment of clinical impacts of such a study design is thus far lacking. Here we demonstrate that biobank-contained resources hold the potential to determine disease- or phenotype-predisposing genetic variants, identify diagnostic shortcomings in current medical system and provide clinically applicable solutions to help fill these gaps. The high call-back rate of study participants and the identification of clinical and genetic unawareness of FH demonstrates the value of such approach and augments the power of biobank-based studies. Implementation of such strategy can ultimately result in more accurate risk estimation of other clinically important phenotypes and guide towards personalized and more appropriate preventative measures. However, we recognize that the value and essence of genetic testing and cascade screening need better communication to the general public and to various levels of healthcare providers. Integration of decision-support software into clinical setting can greatly benefit the healthcare professionals in terms of interpreting genetic risk.
The study had a number of limitations. First, the number of the identified FH-associated variant carriers was small. Although the yield of probands in the discovery platform reflects the prevalence of FH in Europe4,24, the ascertainment of additional disease-causing genetic variants was limited. While the participation rate of probands and family members demonstrated great interest in the study, the yield of one new case per proband reflects small family sizes in Estonia, illustrates an existing treatment gap in FH cases and highlights the need for increased disease awareness. Cascade screening based on genetic testing has been shown to be incrementally cost-effective for identifying individuals affected by FH, even with similar cascade screening yields35–37, and should, thus, be encouraged. Second, our analysis was limited to single nucleotide variant discovery. While no copy number variation >1000 base pairs (bp) was detected in the WGS sample set (n=2,420), smaller structural rearrangements <1000bp were identified, but not analysed. Given the enrichment of Alu elements in the LDLR gene locus and prior associations of DNA rearrangements with FH38,39, the structural variations can account for a number of FH cases.
Lastly, we acknowledge that in silico prediction algorithms for variant pathogenicity prediction do not work perfectly and can indicate false-positive associations. Moreover, given that >1,500 variants in the LDLR gene have been associated with FH38 and discrepancies do exist in NCBI-ClinVar regarding clinical significance of FH-associated variants20 we established a multi-level framework to minimize false-positive variant inclusion. First, we concentrated on rare variants annotated only as loss-of-function or deleterious missense within the FH-associated genes and/or gene domains. Second, we used an endo-phenotype (LDL-C level) as the intermediate step for filtering out putative false-positive variants that were deemed deleterious based on in silico prediction algorithms. Third, we examined the variant frequencies and prior estimates in databases that outline disease associations or list variants identified in sequencing results of different populations. Finally, variant pathogenicity estimation was leveraged based on deep clinical and imaging-based phenotyping as well as on segregation patterns within families. Our approach conforms with the aims of ClinGen40 to improve the understanding of variation within a population, provide expert-reviewed clinical validity and assess the clinical actionability of variants that can and should be incorporated into clinical setting.
In summary, we established that recalling participants by genotype within a biobank is feasible. Next, we identified the increased need for nation-wide testing in Estonia to identify those at increased risk for premature CHD, especially given the actionability of the disease. Finally, we conclude that implementing genomics-guided disease management utilizing the resources contained in a population-based biobank can facilitate clinical management in a more personalized and more effective manner.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by Estonian Research Council [IUT20–60, IUT24–6, PUT1660 to T.E., PUT736 to N.T.]; European Union Horizon 2020 [692145]; European Union through the European Regional Development Fund [2014–2020.4.01.15–0012 GENTRANSMED]; National Institute of Health R01 [MP1GV17428]; BroadNext10; European Research Area Network on Cardiovascular Diseases [Detectin-HF] and National Institute of Health/National Heart, Lung and Blood institute [K08HL140203 to P.N.].
We express our sincere thanks to the biobank participants and their relatives for participating in the study and visitestonia.com team for the contour map of Estonia. Data analyses were carried out in part in the High Performance Computing Center of University of Tartu.
Footnotes
Conflict of interest: none declared.
REFERENCES
- 1.Corbin LJ, Tan VY, Hughes DA, et al. Formalising recall by genotype as an efficient approach to detailed phenotyping and causal inference. Nat Commun 2018;9:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stessman HA, Bernier R, Eichler EE. A Genotype-First Approach to Defining the Subtypes of a Complex Disease. Cell 2014;156:872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stessman HAF, Xiong B, Coe BP, et al. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat Genet 2017;49:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benn M, Watts GF, Tybjærg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: Screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J 2016;37:1384–1394. [DOI] [PubMed] [Google Scholar]
- 5.Gidding SS, Champagne MA, Ferranti SD De, et al. The Agenda for Familial Hypercholesterolemia: A Scientific Statement from the American Heart Association. Circulation 2015;132:2167–2192. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein JK, Hobbs HH BM. Familial hypercholesterolemia In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds), The Metabolic & Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001. p. 2863–2913. [Google Scholar]
- 7.Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic Causes of Monogenic Heterozygous Familial Hypercholesterolemia : A HuGE. Am J Epidemiol 2004;160:407–420. [DOI] [PubMed] [Google Scholar]
- 8.Khera AV, Won HH, Peloso GM, et al. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia. J Am Coll Cardiol 2016;67:2578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abul-Husn NS, Manickam K, Jones LK, et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science 2016;354:aaf7000. [DOI] [PubMed] [Google Scholar]
- 10.Leitsalu L, Haller T, Esko T, et al. Cohort profile: Estonian biobank of the Estonian genome center, university of Tartu. Int J Epidemiol 2015;44:1137–1147. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009;25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaren W, Gil L, Hunt SE, et al. The Ensembl Variant Effect Predictor. Genome Biol Genome Biology 2016;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Wang K. Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat Protoc 2015;10:1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni KR, Garber DW, Marcovina SM, Segrest JP. Quantification of cholesterol in all lipoprotein classes by the VAP-II method. J Lipid Res 1994;35:159–168. [PubMed] [Google Scholar]
- 15.Kulkarni KR. Cholesterol Profile Measurement by Vertical Auto Profile Method. Clin Lab Med 2006;26:787–802. [DOI] [PubMed] [Google Scholar]
- 16.Peloso GM, Auer PL, Bis JC, et al. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am J Hum Genet 2014;94:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Defesche JC, Lansberg PJ, Umans-Eckenhausen MA KJ. Advanced method for the identification of patients with inherited hypercholesterolemia. Semin Vasc Med 2004;4:59–65. [DOI] [PubMed] [Google Scholar]
- 18.Catapano AL, Graham I, Backer G De, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J 2016;37:2999–3058l. [DOI] [PubMed] [Google Scholar]
- 19.Borén J, Lee I, Zhu W, Arnold K, Taylor S, Innerarity TL. Identification of the low density lipoprotein receptor-binding site in apolipoprotein B100 and the modulation of its binding activity by the carboxyl terminus in familial defective Apo-B100. J Clin Invest 1998;101:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landrum MJ, Lee JM, Riley GR, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids 2014;42:D980–D985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziyatdinov A, Vázquez-Santiago M, Brunel H, Martinez-perez A, Aschard H, Soria JM. Lme4Qtl : Linear Mixed Models With Flexible Covariance Structure for Genetic Studies of Related Individuals. BMC Bioinformatics 2018;19:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team (2013). R: A language and environment for statistical computing. Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- 23.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of Coronary Artery Calcium by Race, Gender, and Age. Circulation 2006;113:30 LP–37. [DOI] [PubMed] [Google Scholar]
- 24.Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease. Eur Heart J 2013;34:3478–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. Br Med J 1991;303:893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams RR, Hunt SC, Schumacher MC, et al. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am J Cardiol 1993;72:171–176. [DOI] [PubMed] [Google Scholar]
- 27.Bertolini S, Pisciotta L, Rabacchi C, et al. Spectrum of mutations and phenotypic expression in patients with autosomal dominant hypercholesterolemia identified in Italy. Atherosclerosis 2013;227:342–348. [DOI] [PubMed] [Google Scholar]
- 28.Isla LP De, Alonso R, Mata N, et al. Coronary heart disease, peripheral arterial disease, and stroke in familial hypercholesterolaemia: Insights from the SAFEHEART registry (Spanish familial hypercholesterolaemia cohort study). Arterioscler Thromb Vasc Biol 2016;36:2004–2010. [DOI] [PubMed] [Google Scholar]
- 29.Sjouke B, Kusters DM, Kindt I, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype–phenotype relationship, and clinical outcome. Eur Heart J 2015;36:560–565. [DOI] [PubMed] [Google Scholar]
- 30.Degoma EM, Ahmad ZS, O’Brien EC, et al. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States. Circ Cardiovasc Genet 2016;9:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krogh HW, Mundal L, Holven KB, Retterstøl K. Patients with familial hypercholesterolaemia are characterized by presence of cardiovascular disease at the time of death. Eur Heart J 2016;37:1398–1405. [DOI] [PubMed] [Google Scholar]
- 32.Perak AM, Ning H, Ferranti SD De, Gooding HC, Wilkins JT, Lloyd-Jones DM. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation 2016;134:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan GD, Neville MJ, Liverani E, et al. The in vivo effects of the Pro12Ala PPARγ2 polymorphism on adipose tissue NEFA metabolism: The first use of the Oxford Biobank. Diabetologia 2006;49:158–168. [DOI] [PubMed] [Google Scholar]
- 34.Tuomi T, Nagorny CLF, Singh P, et al. Increased Melatonin Signaling Is a Risk Factor for Type 2 Diabetes. Cell Metab 2016;23:1067–1077. [DOI] [PubMed] [Google Scholar]
- 35.U-EM AW, Defesche JC, Sijbrands EJG, Scheerder RLJM, Kastelein JJP. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 2001;357:165–168. [DOI] [PubMed] [Google Scholar]
- 36.Kerr M, Pears R, Miedzybrodzka Z, et al. Cost effectiveness of cascade testing for familial hypercholesterolaemia, based on data from familial hypercholesterolaemia services in the UK. Eur Heart J 2017;38:1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lázaro P, Pérez de Isla L, Watts GF, et al. Cost-effectiveness of a cascade screening program for the early detection of familial hypercholesterolemia. J Clin Lipidol 2017;11:260–271. [DOI] [PubMed] [Google Scholar]
- 38.Leigh S, Futema M, Whittall R, et al. The UCL low-density lipoprotein receptor gene variant database: pathogenicity update. J Med Genet 2017;54:217 LP–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldmann R, Tichý L, Freiberger T, et al. Genomic characterization of large rearrangements of the LDLR gene in Czech patients with familial hypercholesterolemia. BMC Med Genet 2010;11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rehm HL, Berg JS, Brooks LD, et al. ClinGen — The Clinical Genome Resource. N Engl J Med 2015;372:2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.