Abstract
BACKGROUND And Objective:
Shorter sleep duration is associated with childhood obesity. Few studies measure sleep quantity and quality objectively or examine cardiometabolic biomarkers other than obesity.
METHODS:
This cross-sectional study of 829 adolescents derived sleep duration, efficiency and moderate-to-vigorous physical activity from ≥5 days of wrist actigraphy recording for ≥10 hours/day. The main outcome was a metabolic risk score (mean of 5 sex-specific z-scores for waist circumference, systolic blood pressure, HDL-cholesterol scaled inversely, and log-transformed triglycerides and HOMA-IR), for which higher scores indicate greater metabolic risk. Secondary outcomes included score components and DXA fat mass. We measured socioeconomic status, race/ethnicity, pubertal status and obesity-related behaviors (television-viewing and fast food and sugar-sweetened beverage consumption) using questionnaires.
Results:
The sample was 51.5% female; mean (SD) age 13.2 (0.9) years, median (IQR) sleep duration was 441.1 (54.8) minutes/day and sleep efficiency was 84.0% (6.3). Longer sleep duration was associated with lower metabolic risk scores (−0.11 points; 95% CI: −0.19, −0.02, per inter-quartile range). Associations with sleep efficiency were similar and persisted after adjustment for BMI z-score and physical activity, television-viewing, and diet quality. Longer sleep duration and greater sleep efficiency were also favorably associated with waist circumference, systolic blood pressure, HDL-cholesterol, and fat mass.
Conclusions:
Longer sleep duration and higher sleep efficiency were associated with a more favorable cardiometabolic profile in early adolescence, independent of other obesity-related behaviors. These results support the need to assess the role of sleep quantity and quality interventions as strategies for improving cardiovascular risk profiles of adolescents.
Table of Contents Summary:
Using objective measurements, this study found that longer sleep duration and greater sleep efficiency are associated with more favorable cardiometabolic profiles in early adolescence.
BACKGROUND
Obesity and cardiovascular risk factors in childhood promote cardiovascular disease later in life.1–3 Sleep has emerged as a potential target for obesity prevention: accumulating evidence, including our prior work, has observed that shorter childhood sleep duration leads to higher body mass index (BMI).4–7 However, few studies examine whether child or adolescent sleep duration is associated with other aspects of cardiometabolic risk (e.g., blood pressure, blood lipids, and insulin resistance),8 and fewer yet examine the contribution of sleep quality (e.g., efficiency) to cardiometabolic risk.9,10 Further, most studies used subjectively-reported sleep characteristics. Those with objective measurements (e.g., polysomnography11–17 or actigraphy18–27) often had small sample sizes or could not control for confounders such as socioeconomic status or potential mediators such as physical activity, television viewing, or diet quality.
The present study fills these gaps by examining actigraphy-estimated sleep characteristics with cardiometabolic health in a large sample of children entering adolescence, a period with dramatic changes in sleep architecture and duration, high prevalence of inadequate sleep,28 and emerging cardiovascular risk profiles.1–3,29 In this cross-sectional study, we examine associations of sleep quantity and quality with a comprehensive range of measurements of adiposity and cardiometabolic risk factors including blood pressure, lipids, and insulin resistance.30
METHODS
Study Participants
Project Viva recruited pregnant women from their initial prenatal visit at Atrius Health in eastern Massachusetts (1999–2002) and has followed mother-child pairs since. After delivery, mothers reported their child’s race/ethnicity. Of the 2128 children enrolled, 1038 participated in the adolescent in-person visit (mean [SD] age was 13.2 [0.9] years; range: 11.9 to 16.6 years) and were eligible for the sleep exam. Of these, 829 provided valid actigraphy measurements. A smaller number completed blood draws and DXA scans; sample sizes for each outcome are in Table 1. Women provided written informed consent at each visit and children began providing verbal consent at mid-childhood. All procedures were approved by the relevant institutional review boards.
Table 1.
Parent, household and child characteristics according to actigraphy-estimated sleep duration in early adolescence. Data from 829 participants from Project Viva.
| Overall | <480 minutes/day | ≥480 minutes/day | |
|---|---|---|---|
| n=829 | n=692 | n=137 | |
| Mother/household characteristics | Mean (SD) | ||
| Maternal age at enrollment, years | 32.3 (5.0) | 32.3 (5.1) | 32.6 (4.7) |
| Mother graduated college | N (%) | ||
| No | 228 (27.5) | 198 (28.7) | 30 (21.9) |
| Yes | 600 (72.5) | 493 (71.3) | 107 (78.1) |
| Household income >$70,000/year at early teen visit | N (%) | ||
| No | 177 (22.2) | 154 (23.1) | 23 (17.4) |
| Yes | 622 (77.8) | 513 (76.9) | 109 (82.6) |
| Adolescents’ characteristics | |||
| Female | N (%) | ||
| No | 402 (48.5) | 357 (51.6) | 45 (32.8) |
| Yes | 427 (51.5) | 335 (48.4) | 92 (67.2) |
| Race/ethnicity | N (%) | ||
| Black | 131 (15.8) | 117 (16.9) | 14 (10.2) |
| Hispanic | 36 (4.3) | 33 (4.8) | 3 (2.2) |
| Asian | 22 (2.7) | 21 (3.0) | 1 (0.7) |
| White | 532 (64.3) | 428 (61.9) | 104 (75.9) |
| Other | 107 (12.9) | 92 (13.3) | 15 (10.9) |
| Season at early adolescent visit | N (%) | ||
| Winter | 170 (20.5) | 140 (20.2) | 30 (21.9) |
| Spring | 222 (26.8) | 187 (27.0) | 35 (25.5) |
| Summer | 275 (33.2) | 229 (33.1) | 46 (33.6) |
| Fall | 162 (19.5) | 136 (19.7) | 26 (19.0) |
| Mean (SD) | |||
| Tanner stage1 | 3.6 (1.0) | 3.6 (1.0) | 3.5 (1.1) |
| Age, years | 13.2 (0.9) | 13.2 (0.9) | 13.1 (0.8) |
| Television viewing, hours/day | 2.0 (1.4) | 2.1 (1.4) | 1.7 (1.1) |
| Fast food intake, servings/week | 0.7 (1.1) | 0.7 (1.1) | 0.6 (1.1) |
| Sugar sweetened beverage intake, servings/day | 0.8 (0.9) | 0.8 (0.9) | 0.7 (0.7) |
| BMI z-score | 0.36 (1.07) | 0.41 (1.06) | 0.11 (1.07) |
| Actigraphy measurements: | Median (IQR) | ||
| Average daily moderate-to-vigorous physical activity, minutes | 7.0 (17.0) | 7.0 (16.0) | 7.0 (16.5) |
| Average daily sleep duration, minutes | 441.1 (54.8) | 432.7 (46.0) | 495.8 (21.9) |
| Average daily sleep efficiency, % | 84.0 (6.3) | 83.5 (6.1) | 86.3 (4.9) |
| Average daily wake after sleep onset, minutes | 74.8 (36.2) | 75.9 (36.3) | 69.2 (33.9) |
| Day-to-day variability in sleep duration, standard deviation2 | 56.0 (35.4) | 55.7 (35.8) | 58.5 (34.1) |
| Adiposity outcomes | Mean (SD) | ||
| Body mass index z-score, n=827 | 0.36 (1.07) | 0.41 (1.06) | 0.11 (1.07) |
| Waist circumference, cm, n=829 | 72.8 (11.7) | 73.4 (11.9) | 69.7 (10.5) |
| Sum of skinfolds, mm, n=826 | 28.2 (13.7) | 28.6 (14.2) | 26.2 (11.2) |
| DXA fat mass index, kg/m2, n=619 | 6.3 (3.1) | 6.4 (3.2) | 5.9 (2.3) |
| DXA trunk fat mass index, kg/m2, n=619 | 2.4 (1.5) | 2.4 (1.5) | 2.2 (1.1) |
| Cardiometabolic outcomes | Mean (SD) | ||
| Metabolic risk, z-score3, n=493 | −0.02 (0.60) | 0.00 (0.61) | −0.12 (0.56) |
| HOMA-IR, n=496 | 3.1 (1.9) | 3.2 (2.0) | 2.8 (1.4) |
| Insulin, uU/ml n=560 | 15.9 (14.9) | 16.4 (15.9) | 12.9 (6.1) |
| Glucose, mg/dL n=513 | 92.7 (23.5) | 93.0 (25.1) | 91.1 (11.5) |
| Systolic BP, mmHg, n=823 | 107.2 (8.9) | 107.7 (8.9) | 104.9 (9.0) |
| Triglycerides, mg/dL, n=559 | 69.8 (31.3) | 69.1 (31.3) | 73.4 (31.3) |
| HDL-cholesterol, mg/dL, n=560 | 55.3 (13.1) | 55.1 (13.2) | 56.9 (12.5) |
Tanner stage pubic hair (5-point scale)
Standard deviation of daily time spent asleep (all days), minutes
Mean of 5 sex- and cohort-specific z-scores for waist circumference, systolic blood pressure, high-density lipoprotein (HDL)-cholesterol scaled inversely, and log-transformed triglycerides and homeostatic model assessment of insulin resistance (HOMA-IR); higher scores indicate greater metabolic risk.
Actigraphy Protocol & Sleep Exposures
We measured nighttime sleep and daytime physical activity using wrist actigraphy (Actigraph™ GT3X+) analyzed using ActiLife-6® software (ActiGraph, Inc., Pensacola, FL). Actigraphs collected activity data in 60-second epochs. We asked adolescents to wear the device on their non-dominant wrist for 7–10 consecutive days and nights and complete daily sleep logs. We identified each day’s main rest interval as the primary sleep period based on logs and observation of a sharp decrease in activity with subsequent increase. Participants with ≥5 days of recording with ≥10 hours of wear-time were included. If the device was removed for ≥1 hour within the in-bed interval, then the primary sleep period was considered invalid. We applied Cole-Kripke’s algorithm to classify sleep and wake periods.31
Our sleep exposures were from overnight sleep periods, averaged over all nights of valid recording: (1) duration (sleep time in minutes) and (2) maintenance efficiency (percentage of time between sleep onset and final awakening spent asleep). Secondary exposures included: (3) variability (standard deviation of sleep duration over all valid nights); and (4) wake after sleep onset (WASO, time awake after sleep onset in minutes).
Adiposity Outcomes
We measured children’s height using calibrated stadiometers (Shorr Productions, Olney, MD) and weight with calibrated Tanita scales (model TBF-300A, Tanita Corporation of America, Inc., Arlington Heights, IL). We calculated BMI in kilograms per meters squared (kg/m2) and age- and sex-specific z-scores using national reference data.32,33 We measured total body fat and trunk fat using dual-energy x-ray absorptiometry (DXA) and calculated fat mass indices (kg/m2). We measured waist circumference (cm) using a Lefkin woven tape, and subscapular and triceps skinfold thicknesses (mm) using Holtain calipers (Holtain LTD, Crosswell, UK) and calculated their sum. Trained research assistants performed all measurements following standardized techniques.34
Cardiometabolic biomarkers
We measured systolic blood pressure with a Dinamap Pro-100 (General Electric/Critikon, Inc., Tampa, FL). We obtained 5 measurements 1 minute apart and calculated mean systolic blood pressure.
A phlebotomist collected blood samples, which were stored at −70°C until they were assayed. Plasma fasting insulin was measured using an electro-chemiluminescence immunoassay on the Roche Modular system and fasting glucose was measured enzymatically using Roche Diagnostics reagents.35,36 We calculated the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR, insulin [μU/mL] × glucose [mg/dL]/405). Triglycerides and high-density lipoprotein (HDL) cholesterol were measured enzymatically.
We derived a metabolic risk score, described previously,37 as the mean of sex- and cohort-specific z-scores for waist circumference, systolic blood pressure, HDL-cholesterol (scaled inversely), log-transformed HOMA-IR, and log-transformed triglycerides. Higher scores indicate greater metabolic risk. While there is no consistent definition of the metabolic syndrome in children, prior research has utilized similar scores.38–40
Other Measures
We derived moderate-to-vigorous physical activity (MVPA) from actigraphy. From total activity (vertical axis counts/min) excluding sleep and non-wear time, we applied Chandler’s cut-points to derive average MVPA minutes/day.41 We defined season of measurement as “Spring” March 1st-May 31th; “Summer” June 1st-August 31st, “Autumn” September 1st-November 30th, or “Winter” December 1st-February 28th. Via questionnaire, mothers reported their educational attainment and household incomes and adolescents reported their pubertal status (Tanner stage derived from a validated 5-point rating scale on pubic hair growth, with higher values indicating greater pubertal development),42 the number of hours/day on weekdays and weekends spent watching television and the servings of fast food and sugar-sweetened beverages consumed per/day.
Statistical analysis
We performed all analyses using SAS version 9.4 (SAS Institute, Cary, NC). We examined correlations among the sleep exposures, and the normality of biomarker measurements. For comparability, we reported associations per inter-quartile range (IQR) of sleep exposures. Our multivariable linear regression models adjusted for confounding variables selected a priori from prior literature; all models included demographics (adolescents’ age, sex, and race/ethnicity), socioeconomic status (mothers’ education and household income), pubertal status, and season.
To examine the associations of sleep exposures independent of other obesity-related behaviors, we additionally adjusted models for MVPA, television-viewing, and fast food and sugar-sweetened beverages, which prior studies suggest mediate and/or confound the sleep-cardiometabolic risk relationship.9,10,43–46 Similarly, to examine associations independent of overall adiposity, we adjusted blood biomarker outcomes for BMI z-score.
To explore whether associations of sleep duration and efficiency were independent, we entered these exposures (Spearman correlation=0.41) into the same model and reported mutually-adjusted results.
We evaluated interactions of sex with sleep exposures through stratified analyses and product terms in multivariable models.
RESULTS
Mean (SD) age was 13.2 (0.9) years and 51.5% of participants were female (Table 1). Median (IQR) sleep duration was 441.1 (54.8) minutes/day, sleep efficiency percentage was 84.0 (6.3) %, WASO was 74.8 (36.2) minutes, and variability was 56.0 (35.4) minutes. Using actigraphy-estimated sleep duration, a minority (n=18, 2.2%) of adolescents met the lower bound of the National Sleep Foundation (NSF)’s recommended sleep duration (>480 minutes [8 hours]/day for 14–17-year olds and >540 minutes [9 hours]/day for 11–13-year olds).47 Very short sleep (<420 minutes/day) occurred in 31% of the sample (n=257). A majority were classified as having low sleep efficiency using the threshold ≤85% (n=484, 58.4%). Sleep characteristics are reported separately for weekdays and weekend recording in eTable 1.
Adiposity
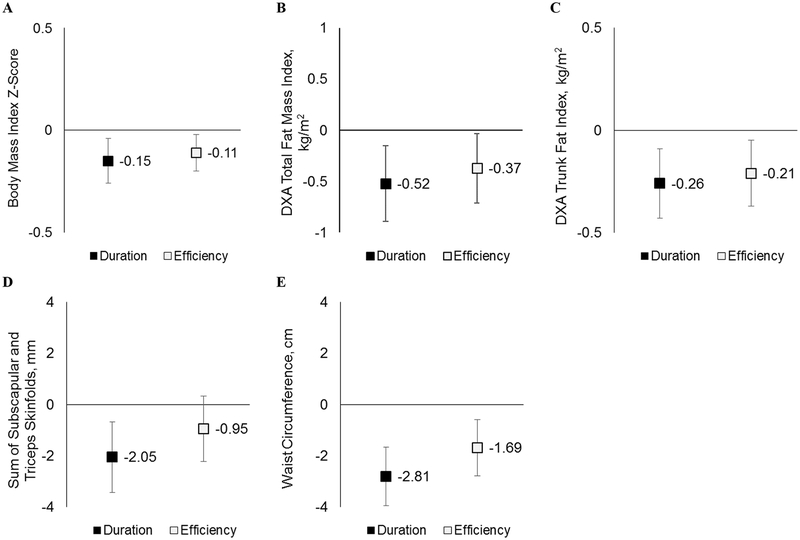
Each increment of sleep duration (55 minutes/day) was inversely associated with adiposity, independent of adjustment for sociodemographics, puberty and season (Figure 1), including BMI z-score (−0.15 per IQR; 95% CI: −0.26, −0.05), sum of skinfold thicknesses (−2.05 millimeters; 95% CI: −3.43, −0.68), DXA fat mass index (−0.52 kg/m2; 95% CI: −0.89, −0.15), and DXA trunk fat mass index (−0.26 kg/m2; 95% CI: −0.43, −0.08).
Figure 1. Multivariable-Adjusted associations of sleep duration and efficiency (per inter-quartile increase) with adiposity outcomes.
Results for adiposity outcomes (A. Body Mass Index Z-score, B. DXA Total Fat Mass Index, C. DXA Trunk Fat Index, D. Sum of Subscapular and Triceps Skinfolds, and E. Waist Circumference) are shown per inter-quartile range of sleep exposures: duration (55 minutes) and efficiency (6%). Models adjust for maternal education and household income, season of measurement, and adolescent age, sex, race/ethnicity, and puberty (Tanner stage).
Likewise, sleep efficiency (IQR: 6%) was inversely associated with adiposity measured by DXA (Figure 1), including total fat mass index (−0.37 kg/m2; 95% CI: −0.71, −0.03), and trunk fat mass index (−0.21 kg/m2; 95% CI: −0.37, −0.05).
Sleep variability (IQR: 35 minutes) and WASO (IQR: 36 minutes) had no association with any adiposity measures after multivariable adjustment (Table 2).
Table 2.
Multivariable-adjusted associations of sleep measures with adiposity and cardiometabolic outcomes
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Sleep Duration | |||
| (per 55 minutes) | |||
| Body mass index z-score | −0.23 (−0.33, −0.13) | −0.13 (−0.24, −0.03) | |
| Waist circumference, cm | −3.06 (−4.12, −2.00) | −2.66 (−3.84, −1.49) | |
| Sum of skinfolds, mm | −2.53 (−3.80, −1.26) | −1.85 (−3.22, −0.48) | |
| DXA fat mass index, kg/m2 | −0.67 (−1.01, −0.34) | −0.49 (−0.86, −0.11) | |
| DXA trunk fat index, kg/m2 | −0.33 (−0.49, −0.17) | −0.24 (−0.42, −0.05) | |
| Metabolic risk score | −0.11 (−0.18, −0.03) | −0.08 (−0.17, 0.00) | −0.06 (−0.13, 0.01) |
| HOMA-IR | −0.34 (−0.58, −0.10) | −0.16 (−0.44, 0.11) | −0.12 (−0.38, 0.14) |
| Insulin, uU/ml | −2.69 (−4.38, −1.01) | −0.81 (−2.76, 1.14) | −0.93 (−2.78, 0.93) |
| Glucose, mg/dL | −0.90 (−3.76, 1.97) | 0.09 (−3.51, 3.70) | 0.37 (−3.22, 3.97) |
| Systolic blood pressure, mmHg | −1.73 (−2.53, −0.93) | −1.49 (−2.40, −0.57) | −1.42 (−2.34, −0.51) |
| Triglycerides, mg/dL | 0.73 (−2.88, 4.34) | 0.68 (−3.50, 4.85) | 1.21 (−2.92, 5.34) |
| HDL cholesterol, mg/dL | 1.66 (0.16, 3.15) | 1.63 (−0.12, 3.37) | 1.44 (−0.25, 3.14) |
| Sleep Efficiency | |||
| (per 6%) | |||
| Body mass index z-score | −0.05 (−0.15, 0.04) | −0.10 (−0.20, 0.00) | |
| Waist circumference, cm | −1.16 (−2.18, −0.14) | −1.65 (−2.76, −0.55) | |
| Sum of skinfolds, mm | −0.57 (−1.78, 0.63) | −0.72 (−1.99, 0.56) | |
| DXA fat mass index, kg/m2 | −0.17 (−0.50, 0.15) | −0.30 (−0.65, 0.04) | |
| DXA trunk fat index, kg/m2 | −0.11 (−0.26, 0.05) | −0.18 (−0.34, −0.01) | |
| Metabolic risk score | −0.07 (−0.14, 0.00) | −0.09 (−0.17, −0.01) | −0.08 (−0.14, −0.02) |
| HOMA-IR | −0.08 (−0.31, 0.14) | −0.11 (−0.37, 0.14) | −0.10 (−0.34, 0.14) |
| Insulin, uU/ml | −1.80 (−3.35, −0.24) | −0.65 (−2.42, 1.13) | −0.62 (−2.31, 1.07) |
| Glucose, mg/dL | −2.71 (−5.38, −0.05) | −3.91 (−7.24, −0.58) | −3.80 (−7.12, −0.49) |
| Systolic blood pressure, mmHg | −1.17 (−1.93, −0.41) | −1.53 (−2.39, −0.68) | −1.46 (−2.32, −0.61) |
| Triglycerides, mg/dL | −2.68 (−5.99, 0.62) | −1.16 (−4.98, 2.65) | −0.84 (−4.60, 2.92) |
| HDL cholesterol, mg/dL | 1.47 (0.10, 2.84) | 1.65 (0.05, 3.24) | 1.46 (−0.08, 3.00) |
| Wake After Sleep Onset | |||
| (per 36 minutes) | |||
| Body mass index z-score | 0.00 (−0.10, 0.09) | 0.07 (−0.03, 0.16) | |
| Waist circumference, cm | 0.44 (−0.58, 1.46) | 1.03 (−0.07, 2.13) | |
| Sum of skinfolds, mm | −0.08 (−1.28, 1.12) | 0.18 (−1.08, 1.45) | |
| DXA fat mass index, kg/m2 | 0.02 (−0.30, 0.33) | 0.19 (−0.15, 0.53) | |
| DXA trunk fat index, kg/m2 | 0.03 (−0.12, 0.18) | 0.12 (−0.04, 0.29) | |
| Metabolic risk score | 0.04 (−0.03, 0.11) | 0.06 (−0.02, 0.13) | 0.06 (−0.01, 0.12) |
| HOMA-IR | −0.02 (−0.23, 0.20) | 0.04 (−0.20, 0.29) | 0.04 (−0.19, 0.28) |
| Insulin, uU/ml | 1.20 (−0.34, 2.73) | 0.51 (−1.24, 2.26) | 0.52 (−1.15, 2.18) |
| Glucose, mg/dL | 1.51 (−1.08, 4.10) | 2.72 (−0.52, 5.97) | 2.70 (−0.52, 5.93) |
| Systolic blood pressure, mmHg | 0.74 (−0.02, 1.50) | 1.13 (0.27, 1.98) | 1.08 (0.23, 1.93) |
| Triglycerides, mg/dL | 2.87 (−0.39, 6.12) | 1.15 (−2.60, 4.90) | 0.97 (−2.73, 4.67) |
| HDL cholesterol, mg/dL | −1.13 (−2.48, 0.22) | −1.30 (−2.87, 0.27) | −1.16 (−2.68, 0.36) |
| Sleep Duration Variability | |||
| (per 35 minutes) | |||
| Body mass index z-score | 0.12 (0.03, 0.22) | −0.01 (−0.11, 0.09) | |
| Waist circumference, cm | 0.90 (−0.13, 1.93) | −0.18 (−1.31, 0.95) | |
| Sum of skinfolds, mm | 2.06 (0.84, 3.27) | 0.79 (−0.51, 2.09) | |
| DXA fat mass index, kg/m2 | 0.33 (0.01, 0.65) | 0.05 (−0.30, 0.41) | |
| DXA trunk fat index, kg/m2 | 0.15 (0.00, 0.30) | 0.03 (−0.15, 0.20) | |
| Metabolic risk score | 0.03 (−0.04, 0.09) | −0.02 (−0.09, 0.06) | −0.01 (−0.08, 0.05) |
| HOMA-IR | 0.22 (0.00, 0.43) | −0.02 (−0.26, 0.23) | 0.00 (−0.23, 0.24) |
| Insulin, uU/ml | 0.89 (−0.67, 2.45) | −0.19 (−1.93, 1.55) | −0.23 (−1.88, 1.43) |
| Glucose, mg/dL | 3.03 (0.45, 5.60) | 2.60 (−0.62, 5.82) | 2.65 (−0.55, 5.86) |
| Systolic blood pressure, mmHg | 0.08 (−0.70, 0.85) | 0.07 (−0.82, 0.95) | 0.08 (−0.80, 0.95) |
| Triglycerides, mg/dL | −1.18 (−4.50, 2.13) | −2.04 (−5.76, 1.69) | −2.24 (−5.92, 1.44) |
| HDL cholesterol, mg/dL | 0.61 (−0.77, 1.99) | 1.36 (−0.20, 2.92) | 1.40 (−0.11, 2.91) |
Model 1. Adjusted for age and sex
Model 2. Model 1 + maternal education and household income, season of measurement, and adolescent race/ethnicity, puberty (Tanner stage), and obesity-behaviors (moderate-vigorous physical activity, television-viewing hours/day and fast food and sugar-sweetened beverage servings/day).
Model 3. Model 2 + BMI z-score (for cardiometabolic biomarkers only).
Most associations persisted after adjusting for MVPA, television-viewing, and sugar-sweetened beverage and fast food consumption (Table 2, Model 2).
Metabolic risk score, blood pressure, and blood lipids
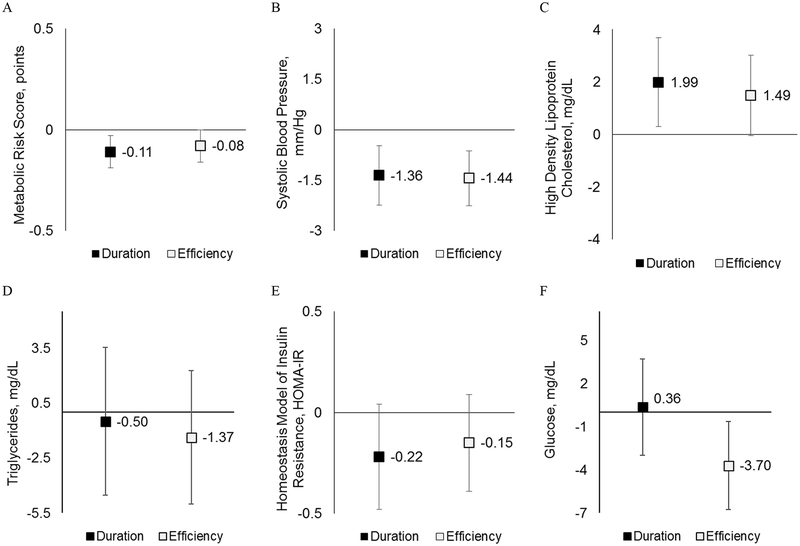
Longer sleep duration and higher sleep efficiency were associated with lower metabolic risk scores (Figure 2A): −0.11 points/IQR; 95% CI: −0.19, −0.02, and −0.08 points; 95% CI: −0.16, −0.01, respectively. Examining the score’s individual components, this finding was driven by smaller waist circumference (Figure 1E: −2.81 cm; 95% CI: −3.96, −1.65 and −1.69 cm; 95% CI: −2.78, −0.61, respectively), lower systolic blood pressure (Figure 2B: −1.36 mmHg; 95% CI: −2.24, −0.47 and −1.44 mmHg; 95% CI: −2.26, −0.61, respectively), and higher HDL-cholesterol (Figure 2C: 1.99 mg/dL; 95% CI: 0.30, 3.67 and 1.49 mg/dL; 95% CI: −0.04, 3.02). Associations with the triglyceride and HOMA-IR components were in the expected direction but confidence intervals were wide (Figure 2D–E). Associations of both sleep duration and efficiency with systolic blood pressure and of efficiency with the metabolic risk score and HDL-cholesterol persisted with additional adjustment for BMI z-score and MVPA, television-viewing, and diet quality (Table 2).
Figure 2. Multivariable-Adjusted associations of sleep duration and efficiency (per inter-quartile increase) with cardiometabolic biomarker outcomes.
Results for cardiometabolic outcomes (A. Metabolic Risk Score, B. Systolic Blood Pressure, C. High Density Lipoprotein [HDL]-Cholesterol, D. Triglycerides, E. Homeostatis Model Assessment of Insulin Resistance [HOMA-IR], and F. Glucose) are shown per inter-quartile range of sleep exposures: duration (55 minutes) and efficiency (6%). Models adjust for maternal education and household income, season of measurement, and adolescent age, sex, race/ethnicity, and puberty (Tanner stage).
Sex differences
The associations of sleep efficiency and WASO with triglycerides and variability with the adiposity measures varied by sex: there was a robust association in girls that was not found in boys (p-interactions<0.05, eTable 2). Otherwise, associations did not vary by sex.
Sleep quantity versus quality
When sleep duration and efficiency were in the same model, each was independently associated with systolic blood pressure. Associations of sleep duration with adiposity (BMI z-score and waist circumference) and the metabolic risk score were robust to adjustment for sleep efficiency, whereas associations of sleep efficiency with adiposity and the metabolic risk score attenuated towards the null once adjusted for sleep duration (eTable 3). Associations of WASO and variability with most outcomes remained null regardless of adjustment for sleep duration.
DISCUSSION
This study is one of the largest and most comprehensive to date, assessing multiple sleep characteristics and adiposity and cardiometabolic outcomes in adolescents. Inadequate sleep as estimated by actigraphy was highly prevalent: 31% of adolescents slept <420 minutes and 42% had sleep efficiencies <85%. While few children met the NSF-recommended sleep duration, self or parent-reported sleep duration over-estimates sleep compared to actigraphy;48 additional research is needed to establish normative data using objective sleep assessments. Adolescents with longer sleep duration and higher sleep efficiency had a more favorable cardiometabolic profile, including components of the metabolic syndrome—namely—central adiposity, systolic blood pressure, and HDL-cholesterol. Greater sleep efficiency was associated with lower metabolic risk scores independent both of other obesity-related behaviors and of BMI z-score. These findings are clinically important: each of the outcomes examined influences not only current cardiometabolic health but also future cardiovascular risk.
Adiposity
Moving beyond the existing literature on sleep duration and BMI,49–66 we found that both shorter sleep duration and lower efficiency were associated with central adiposity (waist circumference and DXA trunk fat), which strongly predicts risk of diabetes and other metabolic derangements.67 A limited number of actigraphy studies examined sleep duration and abdominal adiposity in children or adolescents;22,23,27,68,69 even fewer examined sleep efficiency or DXA trunk fat.12,19,68,70 Adding new rigor to this evidence, we demonstrate that associations of inadequate sleep with abdominal adiposity are independent of confounders (socioeconomic status) and other obesity-related behaviors.
Metabolic risk
Sleep duration and efficiency were associated with our aggregate measure of cardiometabolic risk, the metabolic risk score. While few studies are directly comparable,68 in adults, both short and extended duration of sleep have longitudinal associations with metabolic risks independent of BMI, including diabetes,71 hypertension72 and hyperlipidemia.73,74 Meanwhile, our prior longitudinal work in younger children found that the association between parent-reported sleep duration and mid-childhood metabolic risk was mediated through mid-childhood adiposity (mean age 7 years).37 Here, we examine the same cohort as they enter adolescence (mean age 13 years) and estimate sleep duration and efficiency using actigraphy. As before, the association of sleep duration with the metabolic risk score is dependent on BMI z-score; however, sleep efficiency was associated with metabolic risk independent of BMI z-score, suggesting that adolescent sleep quality, as measured by increased waking during the sleep period, contributes to cardiometabolic health through pathways other than BMI.
Blood pressure and blood lipids
We observed associations of sleep duration and efficiency with higher systolic blood pressure independent of BMI z-score and obesity-related behaviors. While the literature on sleep and blood pressure is sparse among children and adolescents,20,24,25,75 the few existing studies that objectively-measured sleep confirm our findings: an early study of this topic found that low efficiency and short duration were both associated with higher odds of pre-hypertension among adolescents.17 With respect to blood lipids, we found that longer sleep was associated with higher HDL-cholesterol in all subjects and with lower triglycerides in girls only. Though some studies with objectively-measured sleep found inverse associations of sleep duration with triglycerides,27,76 most have not observed associations with HDL-cholesterol.68,76 This makes our finding that greater sleep efficiency is associated with higher HDL-cholesterol independent of other obesity-related behaviors of particular interest.
Glucose homeostasis
The associations of sleep duration with glucose homeostasis are under-studied and inconsistent among children and adolescents, perhaps due to the challenge of obtaining fasting blood samples.22,68 While we found no statistically-significant associations with HOMA-IR or insulin, higher sleep efficiency was associated with lower glucose. Other actigraphy studies reported an inverse association of sleep duration and HOMA-IR,22,68 or a U-shaped relationship,21 depending on participant age and study design: for example, a Danish study found no cross-sectional association of HOMA-IR, but longitudinally positive changes in sleep duration were associated with beneficial changes in HOMA-IR.68
Sex differences
We found associations of sleep efficiency and WASO with triglycerides and variability with adiposity only among girls; others have also found effect modification by sex when examining sleep characteristics. For example, the Cleveland Study found that short sleep’s association with dietary fat intake was stronger in girls,77 while its association with BMI and glucose homeostasis was stronger in boys.22 Adult experimental studies also report that females have a greater inflammatory and immune response to sleep restriction than males.78 Sex may modify the physiological response to sleep disturbance. The complex inter-relationship of sex hormones, sleep and obesity-related behaviors in adolescence warrants further investigation.
Sleep quantity versus sleep quality
The associations of sleep duration and efficiency with systolic blood pressure were robust to mutual-adjustment, underscoring that both quantity and quality/continuity of sleep are both important to cardiometabolic health in adolescence. Typically, sleep duration and efficiency are examined in separate models; very few studies reported independent associations.20 For example, in Danish children, sleep efficiency was associated with waist circumference after adjustment for sleep duration,23 and others have found that sleep efficiency23 and variability,19 but not duration, are associated with greater adiposity. This runs counter to our finding that while duration was associated with BMI z-score and waist circumference was independent of efficiency, associations of efficiency with adiposity outcomes attenuated after adjusting for duration.
Proposed mechanisms
Several proposed mechanisms explain the association between sleep duration and quality and cardiometabolic health79–81 including physiologic and behavioral changes that impact energy intake and expenditure. In adults,82–91 and, to a limited extent, in children92 and adolescents,93,94 experimental research suggests that inadequate sleep duration leads to increased energy intake, altered food choices, changes in the brain’s response to reward stimuli,80 lowered leptin and increased ghrelin.84 Inadequate quantity and quality of sleep have also been associated with decreased physical activity, increased screen time,95,96 decreased fat oxidation,80 and abnormalities in the autonomic nervous system and hypothalamus-pituitary-adrenocortical axis.80,81 These, in turn, influence the metabolic outcomes examined in this study including abdominal adiposity, insulin resistance, dyslipidemia, and elevated blood pressure. While this web of bi-directional relationships cannot be disentangled in a cross-sectional study, it is striking that many of the associations were independent both of BMI z-score and of obesity-related behaviors including MVPA, television-viewing, and fast food and sugar-sweetened beverage intake.
Clinical Implications
Though causality cannot be determined from cross-sectional data, pediatricians should be aware that poor sleep quality, e.g. frequent awakenings – not just insufficient duration of sleep – is associated with increased cardiometabolic risk. Optimizing children’s health should include strategies for addressing child and adolescent sleep, including duration and efficiency, and screening for sleep problems and disorders. While greater clarity is needed on the independent effects of sleep quantity and quality, our findings suggest that both sleep duration and efficiency associate with cardiometabolic health in early adolescence. Intervention trials have attempted to extend sleep duration, but very few target sleep efficiency or other aspects of sleep quality. In adults, exercise increases sleep efficiency,97,98 and in children television-viewing and other forms of screen time reduce sleep efficiency. Preventive interventions to improve sleep health should target behavioral and environmental factors that influence sleep continuity as well as duration, such as screen time,99 stress, noise, caffeine and exercise.98 Such multimodal interventions are likely to improve multiple aspects of sleep.100
Strengths and Limitations
Among its strengths, this study objectively-measured sleep quantity as well as sleep quality in relation to a composite metabolic risk score and its components. The use of data from a well-established cohort of adolescents followed since birth allowed us to address limitations of prior studies through control for sociodemographic confounders and obesity-related behaviors. However, our study was cross-sectional, a key limitation that complicates its interpretation: longitudinal studies with repeated, objective measures are needed to establish the temporal order of inadequate sleep and metabolic risk. Further, while actigraphy is a practical method to measure sleep over multiple days with minimal participant burden,101 actigraphy differs from the gold-standard (polysomnography)48 and there are important exposures (e.g., sleep stage distribution) not captured by actigraphy.81
CONCLUSION
Sleep quantity and quality are associated with a more favorable cardiometabolic profile in early adolescence. Though associations vary by sleep parameter or metabolic marker examined, they are strongest and most consistent for sleep duration and efficiency in relationship to central adiposity and blood pressure. BMI and obesity-related behaviors do not fully account for the associations, suggesting there may be additional physiologic pathways such as abnormalities in hypothalamic–pituitary–adrenal axis and the autonomic nervous system that account for sleep’s influence on cardiometabolic health in early adolescence. Sleep quantity and quality are pillars of health alongside diet and physical activity, and future studies should examine intervention strategies to improve sleep quality as well as quantity in adolescents.
Supplementary Material
WHAT’S KNOWN ON THIS SUBJECT: Current evidence, composed primarily of cross-sectional studies with self-reported sleep duration, suggests that short sleep increases childhood obesity risk. Limited evidence, mainly in adults, links poor sleep quality, including sleep fragmentation, and increased variability in sleep duration, to cardiometabolic risk.
WHAT THIS STUDY ADDS: Longer sleep duration and higher sleep efficiency – measured using actigraphy - are associated with a more favorable cardiometabolic profile, including lower abdominal adiposity, lower systolic blood pressure and higher HDL-cholesterol. Many associations were independent of obesity-related behaviors and BMI.
Acknowledgments
Funding source: Research reported in this publication was 100% supported by the National Institute of Child Health and Human Development, the National Institute of Diabetes and Digestive and Kidney Diseases, and National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers U54CA116847, R01HD034568, UG3OD023286, P30 DK092924, K24 DK10589, and R35 HL135818. Dr. Quante was supported by a scholarship from the Tuebinger Program for the Advancement of Women in Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- BMI
Body mass index
- SD
Standard deviation
- IQR
Inter-Quartile Range
- WASO
Wake after sleep onset
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- HDL-cholesterol
High-density lipoprotein cholesterol
- DXA
dual-energy x-ray absorptiometry
- MVPA
moderate-to-vigorous physical activity
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose
REFERENCES
- 1.Aatola H, Koivistoinen T, Tuominen H, et al. Influence of Child and Adult Elevated Blood Pressure on Adult Arterial Stiffness: The Cardiovascular Risk in Young Finns Study. Hypertension (Dallas, Tex : 1979). 2017;70(3):531–536. [DOI] [PubMed] [Google Scholar]
- 2.Ajala O, Mold F, Boughton C, Cooke D, Whyte M. Childhood predictors of cardiovascular disease in adulthood. A systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2017. [DOI] [PubMed] [Google Scholar]
- 3.Nuotio J, Pitkanen N, Magnussen CG, et al. Prediction of Adult Dyslipidemia Using Genetic and Childhood Clinical Risk Factors: The Cardiovascular Risk in Young Finns Study. Circulation Cardiovascular genetics. 2017;10(3). [DOI] [PubMed] [Google Scholar]
- 4.Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2015;16(2):137–149. [DOI] [PubMed] [Google Scholar]
- 5.Cappuccio FP, Taggart FM, Kandala N-B, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taveras EM, Gillman MW, Pena MM, Redline S, Rifas-Shiman SL. Chronic sleep curtailment and adiposity. Pediatrics. 2014;133(6):1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cespedes EM, Hu FB, Redline S, et al. Chronic insufficient sleep and diet quality: Contributors to childhood obesity. Obesity (Silver Spring, Md). 2016;24(1):184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaput JP, Gray CE, Poitras VJ, et al. Systematic review of the relationships between sleep duration and health indicators in school-aged children and youth. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2016;41(6 Suppl 3):S266–282. [DOI] [PubMed] [Google Scholar]
- 9.Matthews KA, Pantesco EJ. Sleep characteristics and cardiovascular risk in children and adolescents: an enumerative review. Sleep medicine. 2016;18:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quist JS, Sjödin A, Chaput J-P, Hjorth MF. Sleep and cardiometabolic risk in children and adolescents. Sleep medicine reviews. 2016;29:76–100. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Mendoza J, Baker JH, Vgontzas AN, Gaines J, Liao D, Bixler EO. Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain, behavior, and immunity. 2017;61:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klingenberg L, Christensen LB, Hjorth MF, et al. No relation between sleep duration and adiposity indicators in 9–36 months old children: the SKOT cohort. Pediatric obesity. 2013;8(1):e14–18. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Li AM, Au CT, et al. Association between sleep architecture and glucose tolerance in children and adolescents. Journal of Diabetes. 2014;7(1):10–15. [DOI] [PubMed] [Google Scholar]
- 14.Koren D, Levitt Katz LE, Brar PC, Gallagher PR, Berkowitz RI, Brooks LJ. Sleep Architecture and Glucose and Insulin Homeostasis in Obese Adolescents. Diabetes Care. 2011;34(11):2442–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flint J, Kothare SV, Zihlif M, et al. Association between Inadequate Sleep and Insulin Resistance in Obese Children. The Journal of pediatrics. 2007;150(4):364–369. [DOI] [PubMed] [Google Scholar]
- 16.Armitage R, Lee J, Bertram H, Hoffmann R. A preliminary study of slow-wave EEG activity and insulin sensitivity in adolescents. Sleep medicine. 2013;14(3):257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archbold KH, Vasquez MM, Goodwin JL, Quan SF. Effects of Sleep Patterns and Obesity on Increases in Blood Pressure in a 5-Year Period: Report from the Tucson Children’s Assessment of Sleep Apnea Study. The Journal of pediatrics. 2012;161(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall MH, Lee L, Matthews KA. Sleep duration during the school week is associated with C-reactive protein risk groups in healthy adolescents. Sleep medicine. 2015;16(1):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He F, Bixler EO, Liao J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep medicine. 2015;16(12):1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118(10):1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. The Journal of pediatrics. 2011;158(4):617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews KA, Dahl RE, Owens JF, Lee L, Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. 2012;35(10):1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNeil J, Tremblay MS, Leduc G, et al. Objectively-measured sleep and its association with adiposity and physical activity in a sample of Canadian children. Journal of sleep research. 2015;24(2):131–139. [DOI] [PubMed] [Google Scholar]
- 24.Meininger JC, Gallagher MR, Eissa MA, Nguyen TQ, Chan W. Sleep duration and its association with ambulatory blood pressure in a school-based, diverse sample of adolescents. American journal of hypertension. 2014;27(7):948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mezick EJ, Hall M, Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension (Dallas, Tex : 1979). 2012;59(3):747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park H, Tsai KM, Dahl RE, et al. Sleep and Inflammation During Adolescence. Psychosomatic medicine. 2016;78(6):677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung V, Beebe DW, Vandyke R, et al. Does sleep duration predict metabolic risk in obese adolescents attending tertiary services? A cross-sectional study. Sleep. 2011;34(7):891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colrain IM, Baker FC. Changes in sleep as a function of adolescent development. Neuropsychology review. 2011;21(1):5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111(2):302–307. [DOI] [PubMed] [Google Scholar]
- 30.Zimmet P, Alberti KGM, Kaufman F, et al. The metabolic syndrome in children and adolescents–an IDF consensus report. Pediatric diabetes. 2007;8(5):299–306. [DOI] [PubMed] [Google Scholar]
- 31.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. [DOI] [PubMed] [Google Scholar]
- 32.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 Growth Charts for the United States: Improvements to the 1977 National Center for Health Statistics Version. Pediatrics. 2002;109(1):45–60. [DOI] [PubMed] [Google Scholar]
- 33.Barlow SE. Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics. 2007;120(Supplement):S164–S192. [DOI] [PubMed] [Google Scholar]
- 34.Louer AL, Simon DN, Switkowski KM, Rifas-Shiman SL, Gillman MW, Oken E. Assessment of Child Anthropometry in a Large Epidemiologic Study. Journal of visualized experiments : JoVE. 2017(120). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Annals of Epidemiology. 2014;24(11):793–800.e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatzi L, Rifas-Shiman SL, Georgiou V, et al. Adherence to the Mediterranean diet during pregnancy and offspring adiposity and cardiometabolic traits in childhood. Pediatric obesity. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cespedes EM, Rifas‐Shiman SL, Redline S, Gillman MW, Peña MM, Taveras EM. Longitudinal associations of sleep curtailment with metabolic risk in mid‐childhood. Obesity. 2014;22(12):2586–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viitasalo A, Lakka TA, Laaksonen DE, et al. Validation of metabolic syndrome score by confirmatory factor analysis in children and adults and prediction of cardiometabolic outcomes in adults. Diabetologia. 2014;57(5):940–949. [DOI] [PubMed] [Google Scholar]
- 39.Eisenmann JC, Laurson KR, DuBose KD, Smith BK, Donnelly JE. Construct validity of a continuous metabolic syndrome score in children. Diabetology & Metabolic Syndrome. 2010;2(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Ferranti SD, Osganian SK. Epidemiology of paediatric metabolic syndrome and type 2 diabetes mellitus. Diabetes and Vascular Disease Research. 2007;4(4):285–296. [DOI] [PubMed] [Google Scholar]
- 41.Chandler J, Brazendale K, Beets M, Mealing B. Classification of physical activity intensities using a wrist‐worn accelerometer in 8–12‐year‐old children. Pediatric obesity. 2016;11(2):120–127. [DOI] [PubMed] [Google Scholar]
- 42.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. Journal of youth and adolescence. 1980;9(3):271–280. [DOI] [PubMed] [Google Scholar]
- 43.Zhang G, Wu L, Zhou L, Lu W, Mao C. Television watching and risk of childhood obesity: a meta-analysis. European journal of public health. 2016;26(1):13–18. [DOI] [PubMed] [Google Scholar]
- 44.Cespedes EM, Gillman MW, Kleinman K, Rifas-Shiman SL, Redline S, Taveras EM. Television viewing, bedroom television, and sleep duration from infancy to mid-childhood. Pediatrics. 2014;133(5):e1163–e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marinelli M, Sunyer J, Alvarez-Pedrerol M, et al. Hours of television viewing and sleep duration in children: a multicenter birth cohort study. JAMA pediatrics. 2014;168(5):458–464. [DOI] [PubMed] [Google Scholar]
- 46.Marinelli M Impact of television on the quality of sleep in preschool children. Sleep medicine. 2016;20:138–139. [DOI] [PubMed] [Google Scholar]
- 47.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. [DOI] [PubMed] [Google Scholar]
- 48.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27(1):158–165. [DOI] [PubMed] [Google Scholar]
- 49.Yu Y, Lu BS, Wang B, et al. Short Sleep Duration and Adiposity in Chinese Adolescents. Sleep. 2007;30(12):1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sokolovic N, Kuriyan R, Kurpad AV, Thomas T. Sleep and birthweight predict visceral adiposity in overweight/obese children. Pediatric obesity. 2013;8(3):e41–e44. [DOI] [PubMed] [Google Scholar]
- 51.Shaikh W, Patel M, Singh SK. Association of sleep duration with arterial blood pressure profile of Gujarati Indian adolescents. Indian Journal of Community Medicine. 2010;35(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narang I, Manlhiot C, Davies-Shaw J, et al. Sleep disturbance and cardiovascular risk in adolescents. Canadian Medical Association Journal. 2012;184(17):E913–E920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNeil J, Tremblay MS, Leduc G, et al. Objectively-measured sleep and its association with adiposity and physical activity in a sample of Canadian children. Journal of sleep research. 2014;24(2):131–139. [DOI] [PubMed] [Google Scholar]
- 54.Lee JA, Park HS. Relation between sleep duration, overweight, and metabolic syndrome in Korean adolescents. Nutrition, Metabolism and Cardiovascular Diseases. 2014;24(1):65–71. [DOI] [PubMed] [Google Scholar]
- 55.Kuriyan R, Thomas T, Sumithra S, et al. Potential factors related to waist circumference in Urban South Indian children. Indian Pediatrics. 2011;49(2):124–128. [DOI] [PubMed] [Google Scholar]
- 56.Jiang YR, Spruyt K, Chen WJ, et al. Associations between parent-reported sleep duration and adiposity in Chinese early adolescents. Journal of Public Health. 2014;37(2):277–285. [DOI] [PubMed] [Google Scholar]
- 57.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of Short and Long Sleep Durations with Insulin Sensitivity in Adolescents. The Journal of pediatrics. 2011;158(4):617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarrin D, McGrath J, Drake C. Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents. Sleep medicine. 2013;14:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hitze B, Bosy-Westphal A, Bielfeldt F, et al. Determinants and impact of sleep duration in children and adolescents: data of the Kiel Obesity Prevention Study. European Journal of Clinical Nutrition. 2008;63(6):739–746. [DOI] [PubMed] [Google Scholar]
- 60.Guo X, Zheng L, Li Y, et al. Association Between Sleep Duration and Hypertension Among Chinese Children and Adolescents. Clinical Cardiology. 2011;34(12):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garaulet M, Ortega FB, Ruiz JR, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes (Lond). 2011;35(10):1308–1317. [DOI] [PubMed] [Google Scholar]
- 62.Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Québec en Forme’ Project. Int J Obes (Lond). 2006;30(7):1080–1085. [DOI] [PubMed] [Google Scholar]
- 63.Biggs SN, Dollman J. Association between sleep, BMI and waist girth in children and adolescents: a retrospective analysis. Acta Paediatrica. 2007;96(12):1839–1840. [DOI] [PubMed] [Google Scholar]
- 64.Azadbakht L, Kelishadi R, Khodarahmi M, et al. The association of sleep duration and cardiometabolic risk factors in a national sample of children and adolescents: The CASPIAN III Study. Nutrition (Burbank, Los Angeles County, Calif). 2013;29(9):1133–1141. [DOI] [PubMed] [Google Scholar]
- 65.Altenburg TM, Chinapaw MJM, van der Knaap ETW, Brug J, Manios Y, Singh AS. Longer Sleep – Slimmer Kids: The ENERGY-Project. PLoS ONE. 2013;8(3):e59522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Hazzaa HM. Joint associations of body mass index and waist-to-height ratio with sleep duration among Saudi adolescents. Annals of Human Biology. 2013;41(2):111–117. [DOI] [PubMed] [Google Scholar]
- 67.Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(6):1039–1049. [DOI] [PubMed] [Google Scholar]
- 68.Hjorth MF, Chaput JP, Damsgaard CT, et al. Low physical activity level and short sleep duration are associated with an increased cardio-metabolic risk profile: a longitudinal study in 8–11 year old Danish children. PLoS One. 2014;9(8):e104677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garaulet M, Ortega FB, Ruiz JR, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes (Lond). 2011;35(10):1308–1317. [DOI] [PubMed] [Google Scholar]
- 70.Hjorth MF, Chaput JP, Ritz C, et al. Fatness predicts decreased physical activity and increased sedentary time, but not vice versa: support from a longitudinal study in 8- to 11-year-old children. Int J Obes (Lond). 2014;38(7):959–965. [DOI] [PubMed] [Google Scholar]
- 71.Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–537. [DOI] [PubMed] [Google Scholar]
- 72.Jackowska M, Steptoe A. Sleep and future cardiovascular risk: prospective analysis from the English Longitudinal Study of Ageing. Sleep medicine. 2015;16(6):768–774. [DOI] [PubMed] [Google Scholar]
- 73.Petrov ME, Kim Y, Lauderdale D, et al. Longitudinal associations between objective sleep and lipids: the CARDIA study. Sleep. 2013;36(11):1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kinuhata S, Hayashi T, Sato KK, et al. Sleep duration and the risk of future lipid profile abnormalities in middle-aged men: the Kansai Healthcare Study. Sleep medicine. 2014;15(11):1379–1385. [DOI] [PubMed] [Google Scholar]
- 75.Martikainen S, Pesonen AK, Feldt K, et al. Poor sleep and cardiovascular function in children. Hypertension (Dallas, Tex : 1979). 2011;58(1):16–21. [DOI] [PubMed] [Google Scholar]
- 76.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127(2):e345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The Association of Sleep Duration with Adolescents’ Fat and Carbohydrate Consumption. Sleep. 2010;33(9):1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep medicine reviews. 2012;16(2):137–149. [DOI] [PubMed] [Google Scholar]
- 79.Gozal D, Khalyfa A, Qiao Z, Akbarpour M, Maccari R, Ottana R. Protein-Tyrosine Phosphatase-1B Mediates Sleep Fragmentation-Induced Insulin Resistance and Visceral Adipose Tissue Inflammation in Mice. Sleep. 2017. [DOI] [PubMed] [Google Scholar]
- 80.Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. The Lancet Diabetes & Endocrinology. 2015;3(1):52–62. [DOI] [PubMed] [Google Scholar]
- 81.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences. 2013;110(14):5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. American Journal of Clinical Nutrition. 2011;94(2):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spiegel K, Tasali E, Penev P, Cauter EV. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Annals of Internal Medicine. 2004;141(11):846. [DOI] [PubMed] [Google Scholar]
- 85.Spaeth AM, Dinges DF, Goel N. Sex and race differences in caloric intake during sleep restriction in healthy adults1,3. American Journal of Clinical Nutrition. 2014;100(2):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep. 2013;36(7):981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Capers PL, Fobian AD, Kaiser KA, Borah R, Allison DB. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obesity Reviews. 2015;16(9):771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calvin AD, Carter RE, Adachi T, et al. Effects of Experimental Sleep Restriction on Caloric Intake and Activity Energy Expenditure. Chest. 2013;144(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Broussard JL, Kilkus JM, Delebecque F, et al. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity. 2015;24(1):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. American Journal of Clinical Nutrition. 2010;91(6):1550–1559. [DOI] [PubMed] [Google Scholar]
- 91.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of Partial Sleep Deprivation on Energy Balance and Insulin Sensitivity in Healthy Women. Obesity Facts. 2008;1(5):266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mullins EN, Miller AL, Cherian SS, et al. Acute sleep restriction increases dietary intake in preschool-age children. Journal of sleep research. 2017;26(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klingenberg L, Chaput JP, Holmback U, Jennum P, Astrup A, Sjodin A. Sleep restriction is not associated with a positive energy balance in adolescent boys. American Journal of Clinical Nutrition. 2012;96(2):240–248. [DOI] [PubMed] [Google Scholar]
- 94.Beebe DW, Simon S, Summer S, Hemmer S, Strotman D, Dolan LM. Dietary Intake Following Experimentally Restricted Sleep in Adolescents. Sleep. 2013;36(6):827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hart CN, Hawley N, Davey A, et al. Effect of experimental change in children’s sleep duration on television viewing and physical activity. Pediatric obesity. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. The American journal of clinical nutrition. 2009;90(6):1476–1482. [DOI] [PubMed] [Google Scholar]
- 97.Dolezal BA, Neufeld EV, Boland DM, Martin JL, Cooper CB. Interrelationship between Sleep and Exercise: A Systematic Review. Advances in preventive medicine. 2017;2017:1364387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Med Rev. 2015;22:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hale L, Guan S. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. 2015;21:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep medicine. 2010;11(8):735–742. [DOI] [PubMed] [Google Scholar]
- 101.Quante M, Kaplan ER, Rueschman M, Cailler M, Buxton OM, Redline S. Practical considerations in using accelerometers to assess physical activity, sedentary behavior, and sleep. Sleep Health: Journal of the National Sleep Foundation. 2015;1(4):275–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.