Abstract
The uterine microenvironment during the first 7 days after ovulation accommodates and facilitates sperm transit to the oviduct and constitutes the sole source of nutrients required for development of preimplantation embryos. Knowledge of the composition of uterine fluid is largely incomplete. Using untargeted mass spectrometry, we characterized the uterine metabolome during the first 7 days of the estrous cycle. Bovine uteri were collected on day 0 (N=4), 3 (N=4), 5 (N=3) and 7 (N=4) relative to ovulation and flushed with Dulbecco’s phosphate-buffered saline. A total of 1,993 molecular features were detected of which 184 peaks with putative identification represent 147 unique metabolites, including amino acids, benzoic acids, lipid molecules, carbohydrates, purines, pyrimidines, vitamins, and other intermediate and secondary metabolites. Results revealed changes in the uterine metabolome as the cow transitions from ovulation to day 7 of the estrous cycle. The majority of metabolites reached maximum intensity on either day 5 or day 7 relative to ovulation. Moreover, several metabolites found in uterine fluid have signaling capabilities and some have been shown to affect preimplantation embryonic development. In conclusion, the metabolome of the bovine uterus changes during early stages of the estrous cycle and is likely to participate in the regulation of preimplantation embryonic development. Data reported here will serve as basis for future studies aiming to evaluate maternal regulation of preimplantation embryonic development and optimal conditions for culture of embryos.
Keywords: metabolome, histotroph, endometrium, maternal-embryo crosstalk
1. INTRODUCTION
The uterus is a dynamic organ that undergoes large-scale changes in morphology and function during the life cycle of the female. This is true for the period encompassing ovulation, mating and early embryonic development. Coincident with ovulation and mating, the uterus accommodates and facilitates sperm transit to the oviduct (Hawk, 1983). At the same time, the sperm entering the uterus trigger an inflammatory response leading to neutrophil invasion and sperm phagocytosis (Schuberth et al., 2008; Katila et al., 2012). Anti-microbial defenses in the uterus are also optimal around ovulation (Wira et al., 2014). Following fertilization in the oviduct, the newly-formed embryo enters the uterus several days later (4–5 days in the cow; Hackett et al., 1993) where its survival is dependent on the uterine environment for growth, differentiation, and placentation. An inadequate uterine environment, such as associated with inflammatory disease (Ribeiro et al., 2016), can lead to embryonic death.
The uterine milieu in which sperm transport, bacterial clearance and early embryonic development take place is established in large part by secretions from endometrial cells as well as molecules from blood. Transfer of molecules from blood to the uterine lumen involves active and selective transport of molecules such as amino acids and specific ions (Fahning et al., 1967; Schultz et al., 1971; McRae, 1988). In the cow, the species under investigation here, changes in endometrial gene expression (Mitko et al., 2008; França et al., 2017; Tribulo et al., 2018) and protein secretion (Malayer et al., 1988) during the first 7–8 days after estrus have been reported. There is also some information about changes in the presence of small molecules present in the uterine fluid during this period, especially for amino acids (Fahning et al., 1967; Roussel & Loe, 1973; Hugentobler et al., 2007; 2010; França et al., 2017) and select ions and sugars (Schultz et al., 1971; Hugentobler et al., 2010).
The objective of the present study was to characterize the metabolome of the lumen of the uterus of the cow during the first 7 days after ovulation. It was hypothesized that the uterine metabolome changes during the first 7 days after ovulation to meet the changing role of the uterus in sperm transport, immune defense, and early embryonic development. A second objective was to identify metabolites in uterine fluid during this time known to affect preimplantation embryonic development. Such molecules might be useful targets for efforts to improve cow fertility or for addition to culture media in systems for in vitro production of embryos.
2. MATERIALS AND METHODS
2.1. Synchronization of the estrous cycle
Non-lactating Holstein cows were also used for a previously-reported study (Tribulo et al., 2018). A total of 20 cows with a detectable corpus luteum as determined by transrectal ultrasonography were subjected to a hormonal protocol to synchronize ovulation. On day −18 (day of expected ovulation = day 0), cows were injected, i.m., with 25 mg prostaglandin (PGF, Lutalyse®, Zoetis, Florham Park, NJ, USA) followed by 100 μg gonadorelin (GnRH; Cystorelin®, Merial Inc., Duluth, GA, USA) on day −16. A second, identical injection of GnRH was given on day −9 and a progesterone-containing controlled internal drug release device device (CIDR®, Zoetis) was inserted intravaginally. Subsequently, at day −4, each cow was given 25 mg PGF, i.m., and the intravaginal device was removed. Another 25 mg PGF was injected at day −3 and 100 μg GnRH was injected i.m. at day −2, i.e 24 h after PGF. Transrectal ultrasonography of ovaries was performed on days −4, −1 and 0 to confirm ovulation. A total of 15 cows were successfully synchronized and were slaughtered at day 0 (n=4), 3 (n=4), 5 (n=3) or 7 (n=4) relative to the expected day of ovulation.
Uterine flushings
Reproductive tracts were obtained immediately after slaughter and placed on ice. Processing of all organs was completed within 4 hours. Side of the reproductive tract was identified as being ipsilateral or contralateral to the side of ovulation. Ovulation of cows slaughtered at day 0 was confirmed by absence of a preovulatory follicle in 3 of 4 cows. For the remaining cow, ovaries were lost during processing and ovulation could not be confirmed.
Mesometrium was removed and the uterine horn ipsilateral to the site of ovulation was clamped near the uterine body. The oviduct was removed by dissecting its end at the uterotubal junction. The uterine wall in this opening was handled with two hemostats to guide the opening to a 50 ml tube (Sarstedt AG & Co., Numbrecht, Germany). Using an 18 ga needle, 30 ml of Dulbeccos’s phosphate-buffered saline (DPBS) at room temperature were flushed into the uterine horn from the end near the clamp and the fluid propelled by massage along the uterine horn through the opening at uterotubal junction. Recovered fluid was kept in ice. After centrifugation at 3,000 × g for 15 min at 4°C, the supernatant fraction was obtained and stored at −20°C.
2.2. Metabolomics
Metabolites present in uterine flushings from the side ipsilateral to ovulation were identified by liquid chromatography and mass spectrophotometry (LC-MS) at the Southeast Center for Integrated Metabolomics at the University of Florida. Analyses were performed on a Thermo Q-Exactive High Resolution Mass Spectrometer coupled with a Dionex UHPLC and autosampler (ThermoFisher, Waltham, MA, USA). The instrument runs both positive and negative ion modes with heated electrospray ionization. Mass resolution was 70,000 at m/z 200 with mass accuracy of less than 5 ppm in positive mode and less than 10 ppm in negative mode. Separation of metabolites was achieved on an Ace C18-PFP column (100 × 2.1 mm, 2 μm; Advanced Chromatography Technologies, Aberdeen, Scotland) with 0.1% (v/v) formic acid in water as mobile phase A and acetonitrile as mobile phase B and with a column temperature of 25°C.
Samples were prepared by mixing 100 μl of each thawed uterine fluid sample with 20 μl of internal standard mix (creatine-D3, L-leucine-D10, L-tryptophan-D3 and caffeine-D3) and 800 μl of 8:1:1 acetonitrile:methanol:acetone (v/v/v) solution. Samples were then vortexed for 10 min and cooled in a refrigerator for 30 min. The tubes were centrifuged at 20,000 g for 10 min to pellet the protein. The supernatant was then transferred to a new tube and dried down with nitrogen. The dried material was reconstituted by adding 100 μl of 0.1% (v/v) formic acid in LC-MS grade water.
Flow rate was set to 350 μl/min with a total run time of 20 min. A total of 2 μl of each sample was injected for positive ion mode analysis and 4 μl was injected for negative ion mode analysis. The LC-MS data were processed using MZmine 2 software (Pluskal et al., 2010) for peak picking and alignment, and for generation of intensity tables for statistical analyses. MZmine (freeware; http://mzmine.github.io/) was used to identify features, deisotope, align features and perform gap filling to fill in any features that may have been missed in the first alignment algorithm. All sodiated or potassiated adducts of the protonated ion and dimer or trimer complexes of the same ion were identified and removed from the data set. Metabolites were first identified by use of an in-house curated retention time metabolite library (Ulmer et al., 2015). Subsequently, additional external databases such as the human metabolome database (www.hmdb.ca) were used to expand the number of identified metabolites.
Statistical analysis
Multivariate analysis was performed in Metaboanalyst (version 3.0; Xia and Wishart, 2016) using partial least-squares discriminant analysis (PLS-DA) evaluating positive and negative ion data separately. The data were sum-normalized, log transformed and autoscaled prior to PLS-DA analysis. The 184 features with putative identification were also subjected to analysis of variance using the GLM procedure of SAS for Windows, version 9.4 (SAS Institute Inc., Cary, NC, USA) with day as a fixed effect in the statistical model. Orthogonal contrasts were calculated to identify the pattern of variation over days, including linear, quadratic, and cubic effects of day. For analysis of variance, data were log transformed to meet the assumptions of normal distribution. Least squares means were back-transformed and SEM calculated as outlined by Jørgensen and Pedersen (1998). Level of significance was established at P<0.05 and statistical tendency was considered when 0.05>P<0.10.
A canonical analysis of populations (CAP) was performed upon merging positive and negative data sets. The CAP is a methodology based on the Mahalanobis distance that uses a linear combination of predictor variables to identify new canonical components that maximize the variability among groups relative to the variability within group while maximizing the following function:
Where v is a vector of values that maximizes the variability of the vector of original variables; vT is the transpose of v, Hv is the covariance matrix among groups, and Sv is the covariance matrix within groups. Thus, g(v) is a relationship between variance among groups (numerator) and variance within groups (denominator) imposing the latter as equal to 1 (Amaro et al., 2004; Krzanowski, 1989). Using this approach, statistically significant variables (P <0.05) were retained and used for graphing.
3. RESULTS
3.1. Characteristics of identified molecules
There were a total of 1419 features detected in the positive ion mode and 574 features in the negative ion mode. Of these, identification was established for 108 peaks in the positive mode and 76 peaks in the negative ion mode (Table S1, Supplementary Material). Those 184 peaks with putative identification represent 147 unique metabolites.
Features identified in the negative and positive ion modes were classified in 10 superclasses, 25 classes and 38 subclasses based on the human metabolome data base (http://www.hmdb.ca). Superclasses represented by features assigned a metabolite were: benzenoids; inorganic compounds; lipids and lipid-like molecules; nucleosides, nucleotides and analogues; organic acids and derivatives; organic nitrogen compounds; organic oxygen compounds; organoheterocyclic compounds; organooxygen compounds and phenylpropanoids and polyketides. Subclasses represented by features assigned a metabolite are listed in Table S2 (Supplementary Material).
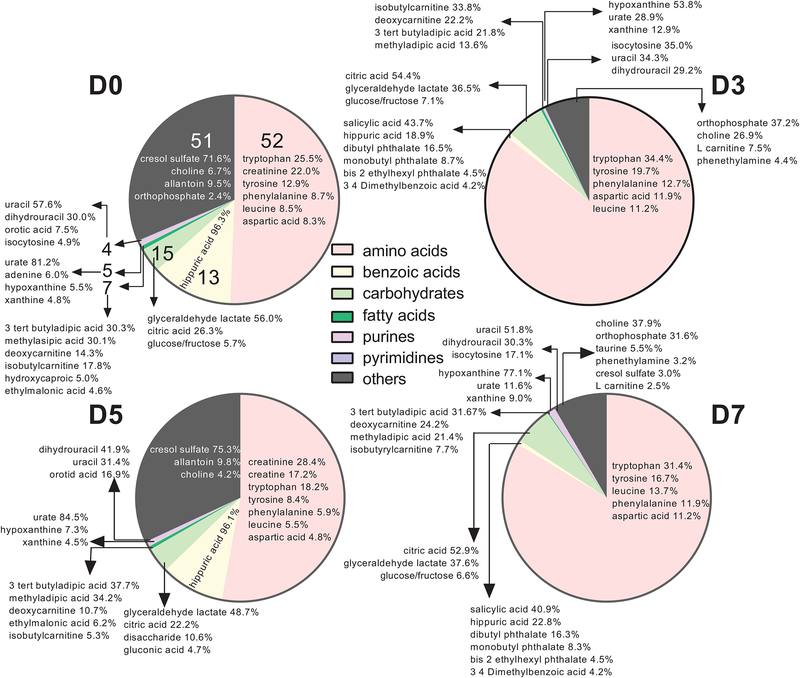
All metabolites were present on all days. The peak intensity (i.e., proportional to the concentration) of the features identifying these metabolites, however, varied across time. The total intensity of identified features for each subclass of metabolite on days 0, 3, 5 and 7 relative to ovulation was estimated by dividing the sum of intensities of identified features within a subclass by the sum of intensities of all 184 identified features. Results are shown in Figure 1. The most abundant subclass of metabolites at all times was the amino acids, peptides and analogues subclass. A total of 52 features were assigned to this subclass, with tryptophan, tyrosine, phenylalanine, leucine and aspartic acid being within the 5 to 7 most abundant amino acids for each day.
FIGURE 1.
Pie graph representing the proportion of identified metabolites in uterine flushings assigned to specific subclasses of the human metabolome data set. Each pie represents the sum of intensities of identified metabolites within a subclass as a percent of the sum of intensities of all 184 identified features for day 0 (D0), day 3 (D3), day 5 (D5) and day 7 (D7) relative to ovulation. Numbers on each pie slice for day 0 represent the total number of metabolites identified for each subclass (number of metabolites per subclass were the same for each day). The group referred to as “others” includes subclasses with 1 to 3 metabolites. The percent data within each slice indicates the proportion of the total intensity in a subclass (i.e. sum of all intensity peaks for each subclass) represented by a given metabolite.
The second most abundant subclass of metabolites present in the uterine fluid for days 0 and 5 was benzoic acids and derivatives, with hippuric acid representing 96% of the total intensity of metabolites in this category. For days 3 and 7, the second most abundant subclass of metabolites was carbohydrates and carbohydrate conjugates. Citric acid, glyceraldehyde lactate, and glucose or fructose were the most abundant metabolites of the carbohydrates and carbohydrates conjugates at all days. Glyceraldehyde lactate was the most abundant metabolite in this subclass at days 0 and 5, representing 56.0% and 48.7%, respectively, of the total intensity of metabolites for the subclass. On days 3 and 7, citric acid was the predominant metabolite for this subclass representing 54.4% and 52.9%, respectively, of the total intensity of metabolites for the subclass.
A total of seven fatty acids were detected. Among the most abundant were 3 tert butyladipic acid, methyladipic acid, deoxycarnitine and isobutylcarnitine. There were 5 purines and 4 pyrimidines detected. Urate was predominant on days 0 and 5 constituting 81.2% and 84.5% of the purine subclass, respectively. Hypoxanthine was predominant on days 3 and 7, constituting 53.8% and 77.1% of the purine subclass, respectively. Pyrimidines were mainly represented by uracil on days 0 and 7, constituting 57.6% and 51.8% of the subclass. Dihydrouracil was abundant as well, constituting 29.9%, 29.2%, 41.9% and 30.3% on days 0, 3, 5 and 7, respectively. Isocytosine represented 35.0% of the pyrimidines in uterine fluid on day 3 but was lower than 15% of the pyrimidines at the other times.
Subclasses that contained only one to three metabolites were grouped together and denominated “others”. Choline was within the top 3 to 6 most abundant molecules on all days and represented up to 37.9% of the total intensity of molecules of this category. Although not abundant on day 3 or 7, cresol sulfate represented 71.6% and 75.3% of the total intensity of molecules of this category on days 0 and 5, respectively.. Orthophosphate represented 37.2% and 31.6% of the total intensity of molecules of this category on days 3 and 7, respectively.
3.2. Use of multivariate analyses to discriminate between days relative to ovulation
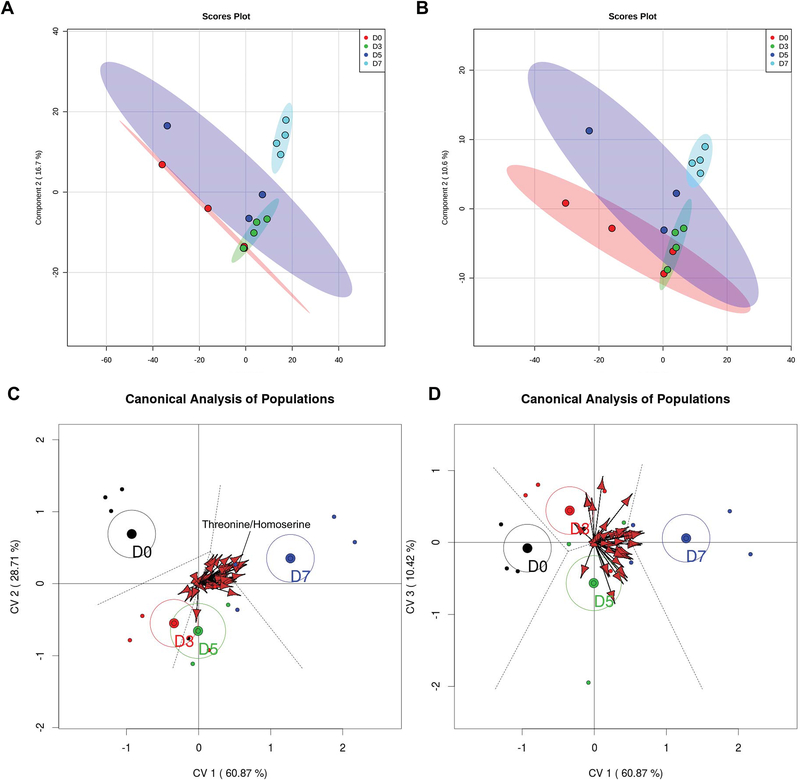
Results are shown in Figure 2. The PLS-DA showed separation of the data with component 1 and component 2 describing 60.6% (positive mode; Figure 2A) and 61.3% (negative mode; Figure 2B) of the variance. There was less variation among samples on day 7 than on other days. Moreover, there was partial overlap in the characteristics of the metabolome on days 0, 3 and 5. The permutation test for PLS-DA results yielded a p-value of 0.019 (positive mode) and 0.015 (negative mode) for two components.
FIGURE 2.
Partial least-squares discriminant analysis (PLS-DA). Panels A and B represent the PLS-DA of the positive (A) and negative (B) data sets including all features while panels C and D are biplot representations of a canonical analysis of populations from merged positive and negative data sets. Panel C represents the relationship between canonical variables 1 and 2. Panel D represents the relationship between canonical variables 2 and 3. Vectors represent statistically significant (p < 0.05) features across days relatives to ovulation. The vectors point towards the day in which intensity of the feature increases and length of the vector reflects the magnitude of variance explained by that feature. One vector, for threonine/homoserine, is labeled for illustrative purposes.
Canonical analysis of populations also yielded statistically significant separation of uterine metabolomes according to the day relative to ovulation (Figures 2C and 2D). Results showed that all four days were well described using three canonical variables. The first canonical variable retained 60.9% of the variance, the second retained 28.7% and the third variable retained 10.4% of the variance. Centroids and confidence interval ellipses (95%) were calculated using individuals. The groups with confidence ellipses that do not overlap between each other are significantly different. By this criterion, the metabolome on day 0 differs from days 3, 5 and 7, days 3 and 5 are similar to each other (differences not captured in canonical variables 1 or 2 but captured in canonical variable 3), and days 3 and 5 were different from day 7. When looking at the canonical variable 3, which explains 10% of the variation, day 3 and 5 are separated.
There were 46 statistically significant variables represented by vectors in the plot (Figures 2C and 2D) and their correlation with the canonical variables were studied to determine their relationship with the day relative to ovulation. Only 10 of the 46 variables have a putative identification, representing 7 unique metabolites. In order of significance, those metabolites were threonine/homoserine, uridine, hypoxanthine, methionine sulfoxide, D-ribose, L-glutamine, and glutatione disulfide. The complete list of significant metabolites, with their respective relative quality of representation related to each canonical variable, is available in Table S3 (Supplementary Material).
3.3. Analysis of variance to identify molecules whose intensity changed with day relative to ovulation
Of the features with a designated identity, there were a total of 39 in the positive mode and 19 in the negative mode that were affected by day (Table S4; Supplementary Material). An additional 15 features in the positive mode and 14 in the negative mode tended to be affected by day. A total of 12 metabolites that changed or tended to be affected by day were identified twice based on different features. Except for one case (taurine), in each of the 12 cases, the same pattern of change was seen for both features.
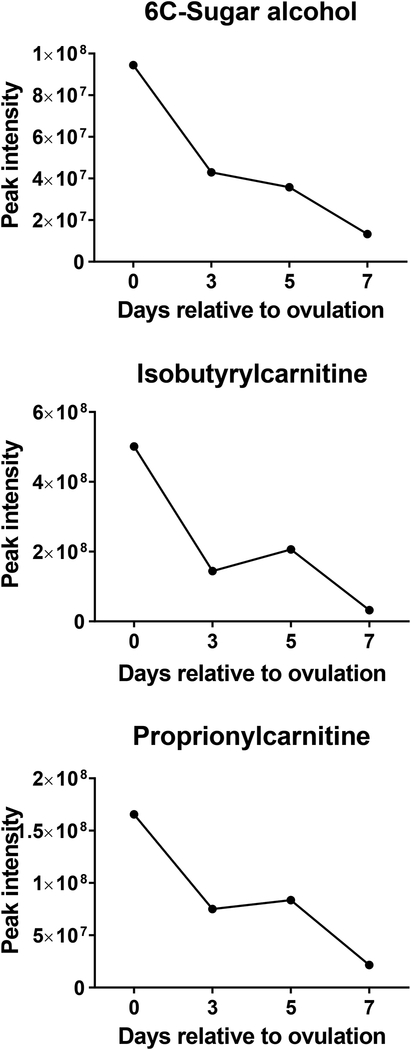
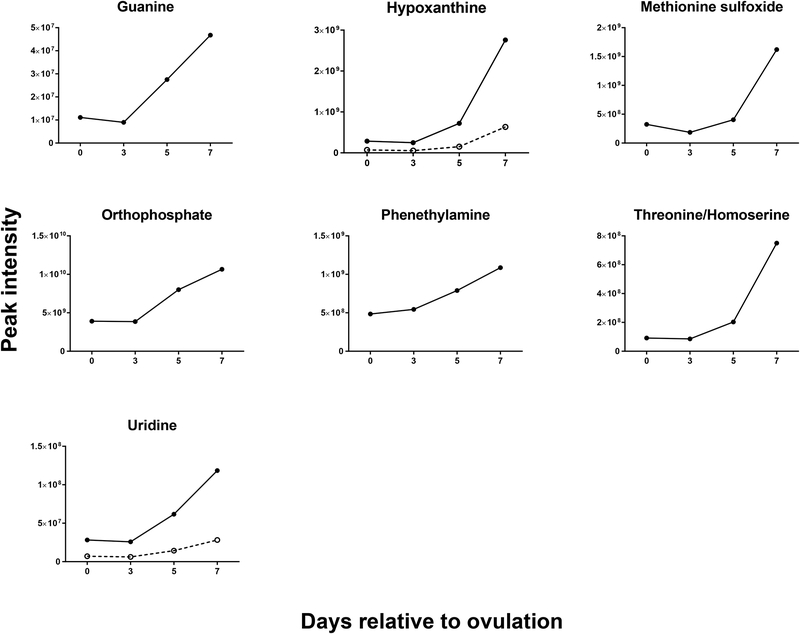
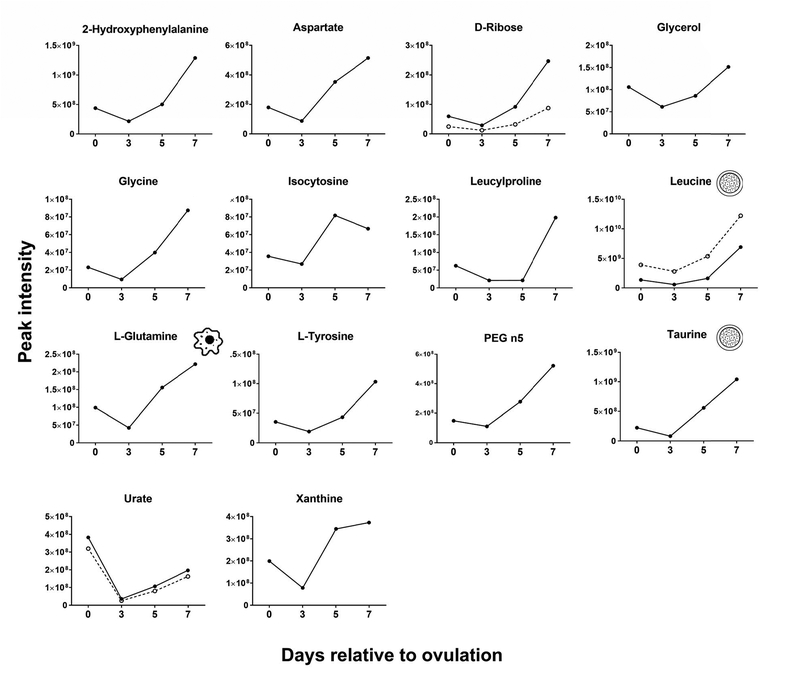
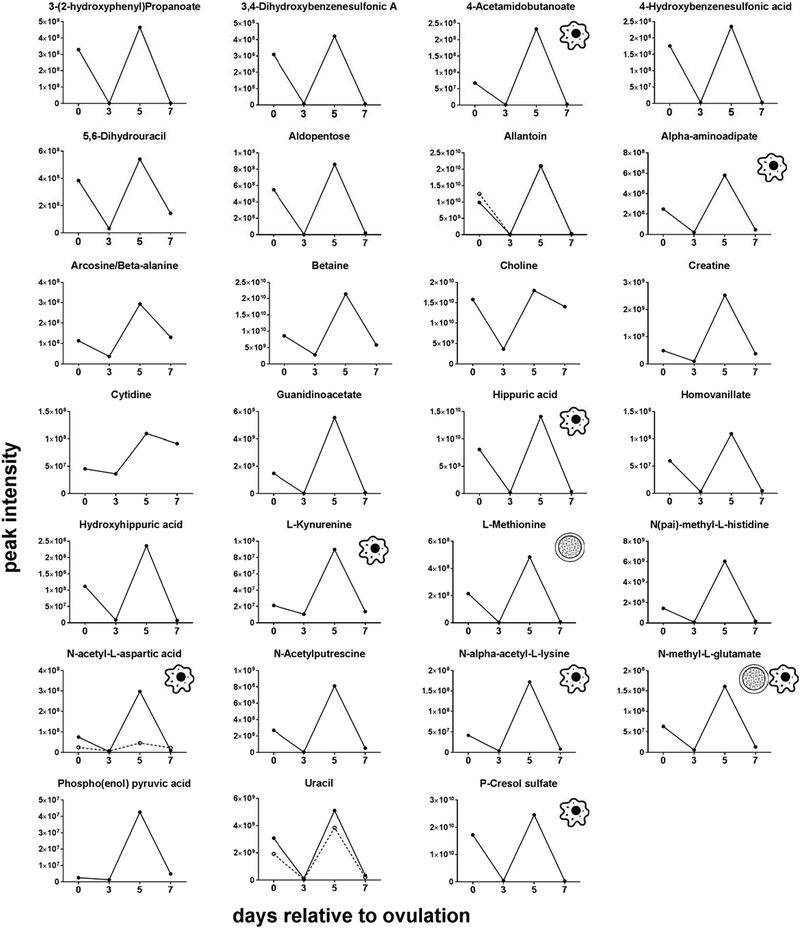
Changes of the intensity of the features significantly affected by day were characterized by four different patterns. In the first pattern, represented by isobutyrylcarnitine, proprionylcarnitine, and 6C-sugar alcohol, there was a linear decrease from day 0 to day 7 (Figure 3). The second pattern, represented by guanine, hypoxanthine, methionine sulfoxide, orthophosphate, phenethylamine, threonine/homoserine, and uridine, was characterized by little change in intensity from day 0 to day 3 and an increase from day 3 to day 7 (Figure 4). The third pattern, represented by 14 metabolites, was a decrease in intensity from day 0 to day 3 and then an increase from day 3 to day 7. Metabolites in this group were 2-hydroxyphenylalanine, aspartate, D-ribose, glycerol, glycine, isocystosine, leucylproline, leucine, L-glutamine, L-tyrosine, polyethylene glycol n5 (PEG n5), taurine, urate and xanthine (Figure 5). Finally, the pattern with the most metabolites represented was characterized by a decrease in intensity from day 0 to day 3, and increase to a maximum intensity on day 5, and a decrease thereafter on day 7. Metabolites following this pattern were 3-(2-hydroxyphenyl)propanoate, 3,4-dihydroxybenzenesulfonic A, 4-acetamdobutanoate, 4-hydroxybenzenesulfonic acid, 5,6-dihydrouracil, aldopentose, allantoin, alpha-aminoadipate/N-methyl-L, arcosine/beta-alanine, betaine, choline, creatine, cytidine, guanidinoacetate, hippuric acid, homovanillate, hydroxyhippuric acid, L-kynurenine, L-methionine, N(pai)-methyl-L-histidine, N-acetyl-L-aspartic acid, N-acetylputrescine, N-alpha-acetyl-L-lysine, N-methyl-L-glutamate, phosphor(enol) pyruvic acid, uracil, p-cresol sulfate (Figure 6).
FIGURE 3.
Changes in concentration during the first 7 days after ovulation for molecules where the intensity decreased from day 0 to day 7 relative to ovulation (P<0.05). Data are least-squares means. P values for linear, quadratic and cubic effects of day and the SEM are reported in Table S4 (Supplementary Material).
FIGURE 4.
Changes in concentration during the first 7 days after ovulation for molecules where the intensity was similar at day 0 and day 3 but then increased from day 3 to day 7 relative to ovulation (P<0.05). Metabolites with more than one feature are indicated by two lines. The cell icon placed within individual graphs indicate that the metabolite has been reported to activate one or more cellular signaling pathways. Data are least-squares means. P values for linear, quadratic and cubic effects of day and the SEM are reported in Table S4 (Supplementary Material).
FIGURE 5.
Changes in concentration during the first 7 days after ovulation for molecules where the intensity decreased from day 0 to day 3 and then increased from day 3 to day 7 relative to ovulation (P<0.05). Metabolites with more than one feature are indicated by two lines. Icons placed within individual graphs indicate that the metabolite has been reported to activate one or more cellular signaling pathways (cell icon) or to affect preimplantation embryonic development (morula icon). Data are least-squares means. P values for linear, quadratic and cubic effects of day and the SEM are reported in Table S4 (Supplementary Material).
FIGURE 6.
Changes in concentration during the first 7 days after ovulation for molecules where the intensity decreased from day 0 to day 3, increased to a maximum on day 5 relative to ovulation, and decreased afterwards (P<0.05). Metabolites with more than one feature are indicated by two lines. Icons placed within individual graphs indicate that the metabolite has been reported to activate one or more cellular signaling pathways (cell icon) or to affect preimplantation embryonic development (morula icon). Data are least-squares means. P values for linear, quadratic and cubic effects of day and the SEM are reported in Table S4 (Supplementary Material).
3.4. Molecules identified in uterine flushings with known role in cell signaling
Thirty-one unique metabolites (revealed by 36 features) possess a known signaling function (Table 1). Among these metabolites, there were amino acids, intermediate metabolites of oxidative respiration and amino acid metabolism. Of the metabolites with signaling function, L-glutamine, methionine sulfoxide and phenethylamine changed during the first 7 days relative to ovulation (P<0.05). In particular, these three metabolites increased from day 3 to day 7 (Figures 4 and 5). In addition, 4-acetamidobutanoate, alpha-aminoadipate, choline, L-kynurenine, N-acetyl-L-aspartic acid, N-alpha-acetyl-L-lysine, N-methyl-L-glutamate, and P-cresol sulfate changed during the first 7 days relative to ovulation with maximum intensity occurring on day 5 (P<0.05; Figure 6).
TABLE 1.
Metabolites with a potential signaling function that were present in flushings from the uterine horn ipsilateral to the side of ovulation within the first 7 days relative to ovulation
| Putative identification |
|---|
| Cysteine sulfinate |
| 4-Oxoproline |
| Kynurenic acid |
| L-Glutamic acid |
| L-Glutamine |
| N-Acetylaspartylglutamic acid |
| N-Acetyl-L-aspartic acid |
| N-Acetyl-L-leucine |
| Orotic acid |
| P-Cresol sulfate |
| Succinate |
| Succinic acid-13C4 |
| Urocanate |
| 2’,4’-Dihydroxyacetophenone |
| 4-Acetamidobutanoate |
| 4-Aminobutanoate (GABA) |
| 5-Hydroxymethyl-2-furaldehyde |
| 5-Oxo-L-proline/pyroglutamic acid |
| Alpha-aminoadipate/N-methyl-L-glutamate |
| Choline |
| Diethanolamine |
| Guanidinoacetate |
| Kynurenic acid |
| L-Glutamine |
| L-Proline |
| L-Valine/5-aminopentanoate/L-norvaline |
| Methionine sulfoxide |
| N-Acetyl-L-aspartic acid |
| N-Alpha-acetyl-L-Lysine |
| Noradrenaline |
| Phenethylamine |
| Proprionyl-L-carnitine |
| Stachydrine |
| Succinate |
| Triethyl phosphate |
| Urocanate |
3.5. Molecules identified in uterine flushings with reported role in regulation of embryonic development
Of all features with a putative ID, there were 14 metabolites (identified with a total of 20 features) that have been reported to affect preimplantation embryonic development in either the cow or one or more other mammals (Table 2). Three of the metabolites were phthalates which could have been introduced into the flushings from the plastic tubing used for fluid collection. Four metabolites (N-methyl-L-glutamate, taurine, leucine and L-methionine) changed during the first 7 days relative to ovulation. Leucine and taurine increased linearly from the day of ovulation to day 7 (Figure 5) whereas N-methyl-L-glutamate, and L-methionine decreased from day 0 to day 3, peaked on day 5 and decreased by day 7 (P<0.05; Figure 6).
TABLE 2.
Metabolites reported to affect preimplantation development present in uterine horn ipsilateral to the side of ovulation within the first 7 days relative to ovulation
| Mode | Mass | Time | Putative identification | Action on preimplantation developmemt (species) |
|---|---|---|---|---|
| (−) | 152.0 | 0.80 | 3-Sulfino-L-alanine | Increase development to blastocyst stage (rats)1 |
| (−) | 215.0 | 0.71 | Glucose/fructose | Phosphorylates mTORC1 and ribosomal S6 kinase1 (rabbit)2; Increased reactive oxygen species (mouse)3 |
| (−) | 217.0 | 0.71 | Glucose/fructose | Phosphorylates mTORC1 and ribosomal S6 kinase1 (rabbit)2; Increased reactive oxygen species (mouse)3 |
| (−) | 133.0 | 1.04 | Malate | Improve development to 8-cell stage (hamster)4 |
| (−) | 133.0 | 1.19 | Malate | Improve development to 8-cell stage (hamster)4 |
| (−) | 116.0 | 1.16 | N-Acetylglycine | Increase development to blastocyst stage (rats)1 |
| (−) | 116.0 | 1.16 | N-Acetylglycine | Increase development to blastocyst stage (rats)1 |
| (−) | 160.1 | 0.89 | N-Methyl-L-glutamate | Increase development to blastocyst stage (rats)1 |
| (−) | 124.0 | 0.71 | Taurine | Increased embryo viability (bovine)5; Increase development to blastocyst stage (rats)1 |
| (+) | 157.0 | 0.98 | (R)-Malate | Improve development to 8-cell stage (hamster)4 |
| (+) | 132.1 | 0.77 | 5-Aminolevulinic acid | Impaired development to the blastocyst stage (mouse)6 |
| (+) | 137.0 | 6.07 | Allopurinol | Inhibits morula and blastocyst formation (rabbit)7 |
| (+) | 391.3 | 16.57 | Bis(2-ethylhexyl) phthalate | Impaired development to the blastocyst stage (mouse)8 |
| (+) | 279.2 | 14.66 | Dibutyl phthalate | Impaired development to the blastocyst stage (mouse)8 |
| (+) | 162.1 | 0.94 | L-Carnitine | Decrease size of lipid bodies (mice)9; Increased rate of ATP:ADP conversion (bovine)10 |
| (+) | 132.1 | 2.51 | Leucine | Altered gene expression in mTORC dependent manner (rabbit)2 |
| (+) | 132.1 | 2.24 | Leucine | Regulated TE motility (mouse)11 |
| (+) | 150.1 | 1.62 | L-Methionine | Increase lipid accumulation and reduces methylation (bovine)12 |
| (+) | 223.1 | 12.40 | Monobutyl phthalate | Impaired development to the blastocyst stage (mouse)8 |
| (+) | 126.0 | 0.71 | Taurine | Increased embryo viability (bovine)5; Increase development to blastocyst stage (rats)1 |
Ain et al., 1997;
Chu et al., 2014;
4. DISCUSSION
This paper is one of the first reports to characterize the metabolome of the uterine lumen of any mammalian species (Romero et al., 2017). As expected, the composition of the uterine lumen varied with time, with large differences between the metabolome on day 0 and day 7 and with day 3 and 5 being intermediate. These changes accompany alterations in the endocrine regulation of the uterus, from an organ regulated by estrogen on day 0 to one dominated by actions of progesterone on day 7. In addition, changes in the metabolome are coincident with changes in the function of the uterus from one of post-mating sperm transport and bacterial removal on day 0 to support for development of the embryo on day 5 and 7. Many of the metabolites found in the uterus are known to regulate cell signaling or have been reported to affect embryonic development. These may be important molecules for survival of the embryo in vivo and in vitro.
Modifications in the constituents of the uterine metabolome from day 0 to 7 after ovulation probably reflect, in part, hormone-driven changes in vascular blood flow and permeability. Estrogen can increase endometrial edema and this action is likely to involve increased blood flow (Koos, 2011) as well as paracellular leakage of the endothelium because of reduced formation of tight junctions (Aberdeen et al., 2008; Hata et al., 2014). One mechanism by which estradiol increased vascular permeability is through increased endometrial synthesis of vascular endothelial growth factor (Albrecht et al., 2003). Progesterone also increases vascular permeability (Goddard et al., 2014). In the cow, timed-average maximum velocity of blood flow through uterine arteries was greater on day −1 relative to ovulation than on day 7 after ovulation (Bollwein et al., 2010) but there were no differences in measurements of blood flow in uterine arteries between day 3 and 6 after estrus (Honnens et al., 2008).
Another mechanism by which steroids can regulate the composition of the metabolome is through regulation of secretory activity of endometrial cells. Concentrations of amino acids in uterine fluid were largely greater than concentrations in blood serum (Fahning et al., 1967). In the cow, supplementation with progesterone increased endometrial expression of the amino acid transporter genes SLC1A1, SLC1A4, SLC7A1, SLC7A7, SLC38A7, and SLC43A14 on day 7 of the estrous cycle while decreasing expression of SLC1A5 (Forde et al., 2014). Similarly, manipulation of follicular growth to produce a large ovulatory follicle increased endometrial expression of SLC6A6, SLC7A4, SLC17A5, SLC38A1, and SLC38A7 on day 4 of the estrous cycle (França et al., 2017). These transporters mediate transport of aspartic acid, glucose, glutamate, leucine, and taurine that were found in abundant quantities in the present study.
Multivariate analysis indicated that the metabolome on days 0, 3 and 5 were more similar to each other than the metabolome on day 7. Moreover, there were only 3 metabolites that were highest in intensity on day 0 as compared to other days but there were 20 metabolites that had highest intensity on day 7. One possible interpretation of this result is that the metabolome is programmed to change in composition to meet the needs of the developing embryo, which first enters the uterus on day 4–5 after ovulation (Hackett et al., 1993) but does not need to be preprogrammed to allow for sperm transport or bacterial removal coincident with mating on day 0. Perhaps, the uterine metabolome does not need to change greatly to allow sperm to survive in the uterus or for the inflammatory responses associated with semen deposition to occur. It may also be that sperm transport or bacterial removal is facilitated by concentrations of specific metabolites being low. Interestingly, two of the three metabolites found to have greatest intensity on day 0, isobutyrylcarnitine and proprionylcarnitine, increase in intensity at the utero-tubal junction during sperm storage in the tropical bat, Scotophilus heathi (Roy & Krishna, 2013). It is also possible that additional metabolites important for uterine events accompanying semen deposition accumulate in the uterine lumen as part of the inflammatory response to sperm.
The potential importance of specific constituents of the uterine metabolome for optimal development of the embryo is indicated by two findings. First, embryos produced in vitro, in the absence of most of the molecules ordinarily present in the uterine lumen, have a wide array of abnormal phenotypes (Hansen et al., 2010). Such disordered development could be a consequence of absence of critical regulatory or nutritive molecules. Secondly, a large number of molecules identified in the metabolome of the uterine lumen have been reported to regulate embryonic development (excluding phthalates, n=11) or affect cell signaling more generally (n=31). Most of the molecules reported to affect embryonic development did not change in intensity with days after ovulation. However, leucine and taurine had highest intensity on day 7 and N-methyl-L-glutamate and methionine was highest on day 5. N-methyl-L-glutamate has been reported to increase developmental competence to the blastocyst stage in the rat (Nakamura et al., 2016). Taurine has similar actions in the rat (Nakamura et al., 2016) and cow (Ealy et al., 1992), and feeding of methionine increased lipid content and reduced DNA methylation in embryos produced by superovulation (Acosta et al., 2016). In the rabbit preimplantation embryo, leucine can activate mTOR signaling and increase expression of genes involved in cell cycle, proliferation, translation, and amino acid transport (Gürke et al., 2016). Leucine also activates mTOR signaling in the mouse preimplantation embryo and has been implicated in activation of changes in the trophoblast leading to implantation (González et al., 2012).
Among the categories of metabolites present in the uterine lumen, that of amino acids, peptides and analogues were the most abundant type of molecule at each day examined. In contrast to few reports on uterine concentration of amino acids at similar times of the estrous cycle, in which the most abundant amino acids were glycine, taurine, and alanine (Fahning et al., 1967; Elhassan et al., 2001; Hugentobler et al., 2007) or threonine, arginine and glutamic acid (Roussel et al., 1973), the most abundant amino acids found in our study included tryptophan, tyrosine, phenylalanine and leucine. It is worth noticing that the aforementioned studies did not flush the uterus. These methodological methods may be responsible for the discrepancy in the results.
Only seven fatty acids were identified in our study. It might be expected that metabolomics analysis using mass spectrometry in the present study would identify more lipids and lipid derivatives, especially when compared to the characterization of a large number of phospholipids in the bovine uterine lumen (Belaz et al., 2016). In the study of Belaz et al., (2016), ionization of the samples prior to MS was performed using a matrix-assisted laser desorption/ionization, a technique that minimizes molecule fragmentation during this ionization. In addition, only a small fraction of the features identified in the current study were assigned a metabolite and an unknown number of lipids could be present in the fraction of features that were unidentified.
Metabolites involved in bioenergetics processes such as cellular respiration and β-oxidation have been shown to affect embryonic development and were found in the uterus including glucose/fructose, malate, and L-canitine. None of these molecules changed over time.
Two very abundant metabolites were hippuric acid and p-cresol sulfate. Although these two metabolites are typically observed in urine, they are also present in several other fluids (Dame et al., 2015; Kolho et al., 2017) and circulate in blood plasma (Hoffmann et al., 1993). Their presence in uterine flushings probably represents transudation from blood plasma. Other metabolites found were various phthalates, which were likely present because of contamination of the flush fluid from constituents in the plastic tube used for flushing.
The most common pattern of temporal change in intensity of the features was characterized by a peak on day 5 followed by a decreased by day 7. It is possible that this pattern represents interactions between progesterone and estrogen in their actions on endometrial function. Indeed, effects of progesterone on endometrial secretion of proteins can be either enhanced or inhibited by estradiol (Fazleabas et al., 1982; Hansen et al., 1985). Perhaps actions of estrogen on the uterus at day 5 enhance the stimulatory effect of progesterone on secretion of metabolites but the decline of estrogen influence by day 7 results in a lack of action of progesterone on secretion by that day.
Our data provide evidence of changes in the metabolome of the uterine fluid during the period when the embryo transitions from the morula to the blastocyst stage of development, with many metabolites reaching highest intensity between days 5 and 7 after ovulation. Many of the metabolites found in the uterus are known to regulate cell signaling or have been reported to affect embryonic development. These may be important molecules for future studies aiming to characterize the maternal microenvironment of preimplantation embryos; the first step toward a systematic characterization of maternal secretions involved in regulation of embryonic development.
Supplementary Material
5. ACKNOWLEDGEMENTS
The authors thank owners and employees of Central Beef Packing Co. (Center Hill, FL) for facilitating reproductive tract collection; staff from the Sumter County Extension Office of University of Florida for providing access to their facility for sample collection, and Dr. Timothy Garret from the Southeast Center for Integrated Metabolomics at the University of Florida for performing the metabolome analysis.
Research was supported by Agriculture and Food Research Initiative Competitive Grant Number 2011–67015–30688 from USDA National Institute of Food and Agriculture, NIH R01 HD088352 and the L.E. “Red” Larson Endowment.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- Aberdeen GW, Wiegand SJ, Bonagura TW Jr., Pepe GJ, & Albrecht ED (2008). Vascular endothelial growth factor mediates the estrogen-induced breakdown of tight junctions between and increase in proliferation of microvessel endothelial cells in the baboon endometrium. Endocrinology 149, 6076–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta DAV, Denicol AC, Tribulo P, Rivelli MI, Skenandore C, Zhou Z, Luchini D, Corrêa MN, Hansen PJ, & Cardoso FC (2016). Effects of rumen-protected methionine and choline supplementation on the preimplantation embryo in Holstein cows. Theriogenology 85,1669–1679. [DOI] [PubMed] [Google Scholar]
- Ain R, & Seshagiri PB (1997). Succinate and malate improve development of hamster eight‐cell embryos in vitro: Confirmation of viability by embryo transfer. Mol. Reprod. Dev 47, 440–447. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Aberdeen GW, Niklaus AL, Babischkin JS, Suresch DL, & Pepe GJ (2003). Acute temporal regulation of vascular endothelial growth/permeability factor expression and endothelial morphology in the baboon endometrium by ovarian steroids. J. Clin. Endocrinol. Metab 88, 2844–2852. [DOI] [PubMed] [Google Scholar]
- Amaro IR, Vicente-Villardón JL, & Galindo-Villardón MP (2004). Manova Biplot para arreglos de tratamientos con dos factores basado en modelos lineales generales multivariantes. Interciencia 29, 26–32. [Google Scholar]
- Belaz KRA, Tata A, Franca MR, Santos da Silva MI, Vendramini PH, Fernandes AMAP, D’Alexandri FL, Eberlin MN, & Binelli M (2016). Phospholipid profile and distribution in the receptive oviduct and uterus during early diestrus in cattle. Biol. Reprod 95, 127. [DOI] [PubMed] [Google Scholar]
- Bollwein H, Prost D, Ulbrich SE, Niemann H, & Honnens A (2010). Effects of a shortened preovulatory follicular phase on genital blood flow and endometrial hormone receptor concentrations in Holstein-Friesian cows. Theriogenology 73, 242–249. [DOI] [PubMed] [Google Scholar]
- Chu DP, Tian S, Sun DG, Hao CJ, Xia HF, & Ma X (2013). Exposure to mono-n-butyl phthalate disrupts the development of preimplantation embryos. Reprod. Fertil. Dev 25, 1174–1184. [DOI] [PubMed] [Google Scholar]
- Dame ZT, Aziat F, Mandal R, Krishnamurthy R, Bouatra S, Borzouie S, Guo AC, Sajed T, Deng L, Lin H, & Liu P (2015). The human saliva metabolome. Metabolomics 11, 1864–1883. [Google Scholar]
- Ealy A, Drost M, Barros CM, & Hansen PJ (1992). Thermoprotection of preimplantation bovine embryos from heat shock by glutathione and taurine. Cell Biol. Int. Rept 16, 125–131. [DOI] [PubMed] [Google Scholar]
- Elhassan YM, Wu G, Leanez AC, Tasca RJ, Watson AJ, & Westhusin ME (2001). Amino acid concentrations in fluids from the bovine oviduct and uterus and in KSOM-based culture media. Theriogenology 55, 1907–1918. [DOI] [PubMed] [Google Scholar]
- Fahning ML, Schultz RH, & Graham EF (1967). The free amino acid content of uterine fluids and blood serum in the cow. J. Reprod. Fertil 13, 229–236. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Bazer FW, & Roberts RM (1982). Purification and properties of a progesterone-induced plasmin/trypsin inhibitor from uterine secretions of pigs and its immunocytochemical localization in the pregnant uterus. J. Biol. Chem 257, 6886–6897. [PubMed] [Google Scholar]
- Forde N, Simintiras CA, Sturmey R, Mamo S, Kelly AK, Spencer TE, Bazer FW, & Lonergan P (2014). Amino acids in the uterine luminal fluid reflects the temporal changes in transporter expression in the endometrium and conceptus during early pregnancy in cattle. PLoS One 9, e100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França MR, da Silva MIS, Pugliesi G, Van Hoeck V, & Binelli M (2017) Evidence of endometrial amino acid metabolism and transport modulation by peri-ovulatory endocrine profiles driving uterine receptivity. J. Anim. Sci. Biotechnol 8, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard LM, Murphy TJ, Org T, Enciso JM, Hashimoto-Partyka MK, Warren CM, Domigan CK, McDonald AI, He H, Sanchez LA, Allen NC, Orsenigo F, Chao LC, Dejana E, Tontonoz P, Mikkola HK, & Iruela-Arispe ML (2014). Progesterone receptor in the vascular endothelium triggers physiological uterine permeability preimplantation. Cell 156, 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González IM, Martin PM, Burdsal C, Sloan JL, Mager S, Harris T, & Sutherland AE (2012). Leucine and arginine regulate trophoblast motility through mTOR-dependent and independent pathways in the preimplantation mouse embryo. Dev. Biol 361, 286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürke J, Schindler M, Pendzialek M, Thieme R, Grybel KJ, Heller R, Spengler K, Fleming T, Fischer B, & Santos AN (2016). Maternal diabetes promotes mTORC1 downstream signaling in rabbit preimplantation embryos. Reproduction 151, 465–476. [DOI] [PubMed] [Google Scholar]
- Hackett AJ, Durnford R, Mapletoft R, & Marcus G (1993). Location and status of embryos in genital tract of superovulated cows 4 to 6 days after insemination. Theriogenology 40, 1147–1153. [Google Scholar]
- Hansen PJ, Bazer FW, & Roberts RM (1985). Appearance of β-hexosaminidase and other lysosomal-like enzymes in the uterine lumen of gilts, ewes and mares in response to progesterone and oestrogens. J. Reprod. Fertil 73, 411–424. [DOI] [PubMed] [Google Scholar]
- Hansen PJ, Block J, Loureiro B, Bonilla L, & Hendricks KE (2010). Effects of gamete source and culture conditions on the competence of in vitro-produced embryos for post-transfer survival in cattle. Reprod. Fertil. Dev 22, 59–66. [DOI] [PubMed] [Google Scholar]
- Hata M, Yamanegi K, Yamada N, Ohyama H, Yukitatsu Y, Nakasho K, Okamura H, & Terada N (2014). Estrogen decreases the expression of claudin-5 in vascular endothelial cells in the murine uterus. Endocr. J 61, 705–715. [DOI] [PubMed] [Google Scholar]
- Hawk HW (1983). Sperm survival and transport in the female reproductive tract. J. Dairy Sci 66, 2645–2660. [DOI] [PubMed] [Google Scholar]
- Hoffmann GF, Meier-Augenstein W, Stöckler S, Surtees R, & Nyhan WL (1993). Physiology and pathophysiology of organic acids in cerebrospinal fluid. J. Inher. Met. Dis 16, 648–669. [DOI] [PubMed] [Google Scholar]
- Honnens A, Voss C, Herzog K, Niemann H, Rath D, & Bollwein H (2008). Uterine blood flow during the first 3 weeks of pregnancy in dairy cows. Theriogenology 70, 1048–1056. [DOI] [PubMed] [Google Scholar]
- Hugentobler SA, Diskin MG, Leese HJ, Humpherson PG, Watson T, Sreenan JM, & Morris DG (2007). Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Mol. Reprod. Dev 74,445–454. [DOI] [PubMed] [Google Scholar]
- Hugentobler SA, Sreenan JM, Humpherson PG, Leese HJ, Diskin MG, & Morris DG (2010). Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood. Reprod. Fertil. Dev 22, 684–694. [DOI] [PubMed] [Google Scholar]
- Jørgensen E, & Pedersen AR (1998). How to obtain those nasty standard errors from transformed data and why they should not be used Biometry Res. Unit, Int. Rep. 7. Danish Inst. Ag. Sci., Aarhus, Denmark. [Google Scholar]
- Katila T (2012). Post-mating inflammatory responses of the uterus. Reprod. Domest. Anim 47, 31–41. [DOI] [PubMed] [Google Scholar]
- Kolho KL, Pessia A, Jaakkola T, de Vos WM, & Velagapudi V (2017). Faecal and serum metabolomics in paediatric inflammatory bowel disease. J Crohns Colitis 11, 321–334. [DOI] [PubMed] [Google Scholar]
- Koos RD (2011). Minireview: Putting physiology back into estrogens’ mechanism of action. Endocrinology 152, 4481–4488. [DOI] [PubMed] [Google Scholar]
- Krzanowski WJ (1989). On confidence regions in canonical variate analysis. Biometrika 76, 107–116. [Google Scholar]
- Kyvelidou C, Sotiriou D, Antonopoulou T, Tsagkaraki M, Tserevelakis GJ, Filippidis G, & Athanassakis I (2016). L-Carnitine affects preimplantation embryo development toward infertility in mice. Reproduction 152, 283–291. [DOI] [PubMed] [Google Scholar]
- Malayer JR, Hansen PJ, & Buhi WC (1988). Effect of day of the oestrous cycle, side of the reproductive tract and heat shock on in-vitro protein secretion by bovine endometrium. J. Reprod. Fertil 84, 567–578. [DOI] [PubMed] [Google Scholar]
- McRae AC (1988). The blood-uterine lumen barrier and exchange between extracellular fluids. J. Reprod. Fertil 82:857–873. [DOI] [PubMed] [Google Scholar]
- Mitko K, Ulbrich SE, Wenigerkind H, Sinowatz F, Blum H, Wolf E, & Bauersachs S (2008). Dynamic changes in messenger RNA profiles of bovine endometrium during the oestrous cycle. Reproduction 135, 225–240. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kuo TC, Dharmarajan AM, Atlas SJ, & Wallach EE (1989). In vivo administration of allopurinol affects ovulation and early embryonic development in rabbits. Am. J. Obstet. Gynecol 161, 1709–1714. [DOI] [PubMed] [Google Scholar]
- Morin-Dore L, Blondin P, Vigneault C, Grand F, Labrecque R & Sirard M-A (2017). Transcriptomic evaluation of bovine blastocysts obtained from peri-pubertal oocyte donors. Theriogenology 93, 111–123. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morimoto K, Shima K, Yoshimura Y, Kazuki Y, Suzuki O, MAtsuda J, & Ohbayashi T (2016). The effect of supplementation of amino acids and taurine to modified KSOM culture medium on rat embryo development. Theriogenology 86, 2083–2090. [DOI] [PubMed] [Google Scholar]
- Nasr-Esfahani MH, & Johnson MH (1991). The origin of reactive oxygen species in mouse embryos cultured in vitro. Development 113, 551–561. [DOI] [PubMed] [Google Scholar]
- Pluskal T, Castillo S, Villar-Briones A & Oresic M (2010). MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics 11, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro ES, Gomes G, Greco LF, Cerri RLA, Vieira-Neto A, Monteiro PLJ, Lima FS, Bisinotto RS, Thatcher WW, & Santos JEP (2016). Carryover effect of postpartum inflammatory diseases on developmental biology and fertility in lactating dairy cows J. Dairy Sci 99, 2201–2220. [DOI] [PubMed] [Google Scholar]
- Romero JJ, Liebig BE, Broeckling CD, Prenni JE, & Hansen TR (2017). Pregnancy-induced changes in metabolome and proteome in ovine uterine flushings. Biol. Reprod 97, 273–287. [DOI] [PubMed] [Google Scholar]
- Roussel JD, & Loe WC (1973). Effects of heat stress and melengestrol acetate on amino acid pattern of uterine fluid from dairy heifers. Int. J. Biometeorol 17, 153–156. [Google Scholar]
- Roy VK, & Krishna A (2013). Changes in glucose and carnitine levels and their transporters in utero-tubal junction in relation to sperm storage in the vespertilionid bat, Scotophilus heathi. J. Exp. Zool. A Ecol. Genet. Physiol 319, 517–526. [DOI] [PubMed] [Google Scholar]
- Schuberth HJ, Taylor U, Zerbe H, Waberski D, Hunter R, & Rath D (2008). Immunological responses to semen in the female genital tract. Theriogenology 70, 1174–1181. [DOI] [PubMed] [Google Scholar]
- Schultz RH, Fahning ML, & Graham EF (1971) A chemical study of uterine fluid and blood serum of normal cows during the oestrous cycle. J. Reprod. Fertil 27, 355–367. [DOI] [PubMed] [Google Scholar]
- Sutton-McDowall ML, Feil D, Robker RL, Thompson JG & Dunning KR (2012). Utilization of endogenous fatty acid stores for energy production in bovine preimplantation embryos. Theriogenology 77, 1632–1641. [DOI] [PubMed] [Google Scholar]
- Tríbulo P, Siqueira LGB, Oliveira LJ, Scheffler T & Hansen PJ (2018). Identification of potential embryokines in the bovine reproductive tract. J. Dairy Sci 101, 690–704. [DOI] [PubMed] [Google Scholar]
- Ulmer CZ, Yost RA, Chen J, Mathews CE, & Garrett TJ (2015). Liquid chromatography-mass spectrometry metabolic and lipidomic sample preparation workflow for suspension-cultured mammalian cells using Jurkat T lymphocyte cells. J. Proteomics Bioinform 8, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Rodriguez-Garcia M, Shen Z, & Patel MV (2014) Regulation of mucosal immunity in the female reproductive tract: the role of sex hormones in immune protection against sexually transmitted pathogens. Am J Reprod Immunol 72, 236–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JZ, Greer PA, Van Vugt DA, & Reid RL (1995). Treatment with 5-aminolevulinic acid and photoactivating light causes destruction of preimplantation mouse embryos. Fertil. Steril 63, 1088–1093. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.