Abstract
Background:
Microinvasive breast cancer is an uncommon pathologic entity. Due to the rarity of this condition, the surgical axillary management and overall prognosis of this entity remain controversial.
Methods:
A total of 310 patients with microinvasive ductal carcinoma in situ (DCIS) underwent surgery for invasive breast cancer at the European Institute of Oncology, Milan between 1998–2010. Out of these 257 (83%) patients underwent axillary staging through sentinel lymph node biopsy and were included in the study.
Results:
Of the 257 patients undergoing sentinel lymph node biopsy, 226 (87.9%) had negative sentinel lymph nodes (SLNs) and 31 had metastatic SLNs. Out of these, 12 patients had isolated tumour cells (ITCs), 14 micrometastases, and 5 macrometastases in the sentinel nodes. Axillary lymph node dissection was performed in 16 of 31 patients with positive SLNs. After 11 years median follow-up, only one regional first event was observed in the positive-SLN group without axillary lymph node dissection. There were no regional first events in the positive-SLN group treated with axillary dissection.
Conclusion:
Good DFS and OS were found in patients with positive sentinel nodes and microinvasive DCIS. This study is in line with studies showing that SLNB in microinvasive DCIS may not be useful, and supports the evidence that less surgery can provide the same level of OS with better quality of life.
Keywords: microinvasive breast cancer, ductal carcinoma in situ, sentinel lymph node biopsy
Introduction
Microinvasive breast cancer is an uncommon pathologic entity accounting for approximately 1% of all breast cancer 1, 2, and the definition of microinvasive breast cancer has varied over time3, 4. Recently, the definition of microinvasion as given by the 7th Edition of the American Joint Committee on Cancer (AJCC) Staging Manual is the extension of cancer cells beyond the basement membrane into the adjacent tissue with no focus more than 1 mm in greatest dimension, has gained common acceptance. As a result, the term “T1mic” has now been added to the TNM staging system5, 6.
Due to the rarity of this condition, questions remain regarding the surgical management of the axilla and the overall prognosis of this entity. In the literature, a large incidence spectrum of axillary metastasis is found. This can be attributable to differing definitions of microinvasive ductal carcinoma in situ (DCIS) over the years and to the varying techniques used to analyse the sentinel node. These differences are likely responsible for the different recommendations on how to manage the axilla in microinvasive DCIS7.
DCIS is a disease devoid of invasive behaviour and thus without potential for spread to the axillary lymph nodes. Current practice is to perform SLNB in DCIS only in selected cases when there is a substantial risk of upgrade of the lesion at final pathology, such as a mass lesion highly suggestive of invasive cancer at imaging and physical examination, or in cases of a large DCIS area at imaging (≥ 5 cm) and when mastectomy is indicated26. However, evidence for this recommendation is inadequate because of the scarcity of data analysed in the literature, which is also characterized by a lack of long-term follow-up studies and still subject to controversial scientific analysis27.
If staging the axilla in DCIS is accepted globally in the above-described conditions, what remains controversial is the real utility of staging the axilla with SLNB in microinvasive DCIS4, 6, 7, 22, 23, 28–47 (as reviewed in Table 1). The incidence of axillary metastasis in sentinel nodes varies in studies between approximately 2% and 20%. This is likely due to the different pathology methods used to examine the sentinel node, as well as differences in the methodology used to section the breast tissue. Factors correlated with axillary nodal positivity in patients with DCIS and microinvasive DCIS are younger age, size of DCIS lesion, histologic grade, receptor status, HER2 overexpression, and lymphovascular invasion6, 40, 41.
Table 1.
Literature review (selected studies of microinvasive ductal carcinoma in situ patients who underwent sentinel lymph node biopsy)
| Without defined SLNB status | |||||||
|---|---|---|---|---|---|---|---|
| Type of metastases (AJCC criteria) | |||||||
| Author | Year | Total patients with axillary staging | Patients submitted SNL | ITC | Micro | Macro | SNLB positivity (%) |
| Cox | 2001 | 15 | 15 | NS | NS | NS | 3 (20) |
| Camp | 2005 | 13 | 13 | NS | NS | NS | 2 (15) |
| Wilkie | 2005 | 51 | 51 | 5 | NS | NS | 7 (14) |
| Tunon-de-Lara | 2008 | 45 | 45 | 0 | NS | NS | 2 (5) |
| Fortunato | 2008 | 77 | 77 | NS | NS | NS | 6 (8) |
| Vieira | 2010 | 17 | 14 | NS | NS | NS | 1 (6) |
| Parikh | 2012 | 46 | 4 | NS | NS | NS | 1 (2) |
| Without defined SLNB status | |||||||
|---|---|---|---|---|---|---|---|
| Type of metastases (AJCC criteria) | |||||||
| Author | Year | Total patients with axillary staging | Patients submitted SNL | ITC | Micro | Macro | SNLB positivity (%) |
| Zavotsky | 1999 | 14 | 14 | 1 | 0 | 1 | 2 (14) |
| Klauber-DeMore | 2000 | 31 | 31 | 0 | 2 | 1 | 3 (10) |
| Intra | 2003 | 41 | 41 | 0 | 2 | 2 | 4 (10) |
| Katz | 2006 | 21 | 21 | 0 | 1 | 1 | 2(10) |
| Leidenius | 2006 | 11 | 11 | 1 | 0 | 0 | 1 (9) |
| Zavagno | 2007 | 43 | 43 | 0 | 1 | 3 | 4 (9) |
| Gray | 2007 | 79 | 77 | 2 | 2 | 2 | 6 (7) |
| Guth | 2008 | 44 | 20 | 2 | 0 | 3 | 5 (11) |
| Sakr | 2008 | 20 | 20 | 0 | 2 | 0 | 2 (10) |
| Lyons | 2012 | 112 | 112 | 6 | 5 | 3 | 14 (12) |
| Ko | 2012 | 293 | 180 | 6 | 12 | 4 | 22 (8) |
| Kapoor | 2013 | 45 | 31 | 4 | 4 | 1 | 9 (20) |
| Margalit | 2013 | 68 | 53 | 4 | 3 | 0 | 7 (10) |
| Matsen | 2014 | 414 | 414 | 0 | 26 | 6 | 32 (7,7) |
| Hanna | 2014 | 81 | 64 | 2 | 0 | 0 | 2 (3) |
| Orzalesi | 2016 | 126 | 126 | 10 | 3 | 5 | 18 (14,3) |
AJCC, American Joint Committee on Cancer; SNL, sentinel lymph node; ITC, isolated tumour cells; Micro, micrometastases; Macro, macrometastases
To contribute to a better understanding of this surgical aspect as well as to the prognostic implications of microinvasion, this retrospective observational study examines 257 patients with microinvasive breast cancer who underwent axillary staging through sentinel lymph node (SLN) biopsy.
Methods
After institutional review board approval, a database of 22,120 patients who underwent surgery for invasive breast cancer at the European Institute of Oncology, Milan, Italy between 1998 and 2010 was analysed, and 310 patients with microinvasive DCIS were identified. Fifty-three were excluded on the basis of no axillary surgery. The remaining 257 patients with microinvasive breast cancer undergoing axillary staging through SLNB were included in the analysis.
Sentinel lymph node identification was usually performed using radiocolloid technique (99m Tc-labeled colloidal particles of human albumin). Intraoperative lymph node analysis was conducted utilizing haematoxylin-eosin (H&E)-stained sections and, when necessary, was aided by immunohistochemical staining, as has been previously reported8.
Based on AJCC classification criteria, axillary lymph node metastases were defined as follows: macrometastases (> 2.0 mm), micrometastases (0.2–2.0 mm), or isolated tumour cells (ITCs) (< 0.2 mm)5. Systemic adjuvant therapy was recommended according to the contemporary St. Gallen treatment guidelines9–13.
The following parameters were used in the analysis; clinical (year of surgery/ age/ menopausal status), pathology (tumour histology/tumour grade/tumour subtype), and type of treatment (local treatment/systemic treatment) (Table 2). Long-term outcomes were studied via follow-up data recording the first recurrence events, classified as local (ipsilateral breast and chest), regional (ipsilateral axillary or supraclavicular lymph nodes), distant metastases, contralateral breast cancer, other primary tumour, and deaths as the first-reported event.
Table 2.
Patient characteristics by sentinel lymph node status
| SLN status | All patients | ||||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | ||||||
| n | %r | n | %r | P | n | %c | |
| All patients | 226 | 87.9 | 31 | 12.1 | - | 257 | 100.0 |
| Year of surgery | 0.915 | ||||||
| Before 2003 | 42 | 89.4 | 5 | 10.6 | 47 | 18.3 | |
| 2003–2006 | 111 | 88.1 | 15 | 11.9 | 126 | 49.0 | |
| 2007–2010 | 73 | 86.9 | 11 | 13.1 | 84 | 32.7 | |
| Age class, years | 0.079 | ||||||
| < 50 | 91 | 82.7 | 19 | 17.3 | 110 | 42.8 | |
| 50–59 | 69 | 90.8 | 7 | 9.2 | 76 | 29.6 | |
| 60+ | 66 | 93.0 | 5 | 7.0 | 71 | 27.6 | |
| Menopausal status | 0.030 | ||||||
| Premenopausal | 99 | 83.2 | 20 | 16.8 | 119 | 46.3 | |
| Postmenopausal | 127 | 92.0 | 11 | 8.0 | 138 | 53.7 | |
| Histology | 0.177 | ||||||
| Ductal | 203 | 86.8 | 31 | 13.2 | 234 | 91.1 | |
| Lobular | 8 | 100.0 | 0 | - | 8 | 3.1 | |
| Other | 15 | 100.0 | 0 | - | 15 | 5.8 | |
| Grade | 0.511 | ||||||
| G1 | 36 | 87.8 | 5 | 12.2 | 41 | 15.9 | |
| G2 | 87 | 89.7 | 10 | 10.3 | 97 | 37.8 | |
| G3 | 83 | 84.7 | 15 | 15.3 | 98 | 38.1 | |
| Unknown | 20 | 95.2 | 1 | 4.8 | 21 | 8.2 | |
| Subtype | 0.387 | ||||||
| Unknown | 23 | 88.5 | 3 | 11.5 | 26 | 10.1 | |
| Luminal A | 70 | 90.9 | 7 | 9.1 | 77 | 30.0 | |
| Luminal B (Ki67 ≥ 20%) | 22 | 81.5 | 5 | 18.5 | 27 | 10.5 | |
| Luminal B (HER2 positive) | 22 | 88.0 | 3 | 12.0 | 25 | 9.7 | |
| HER2 positive | 66 | 90.4 | 7 | 9.6 | 73 | 28.4 | |
| Triple negative | 23 | 79.3 | 6 | 20.7 | 29 | 11.3 | |
| Local treatment | 0.206 | ||||||
| Mastectomy w/o RT | 49 | 81.7 | 11 | 18.3 | 60 | 23.3 | |
| Mastectomy w RT | 27 | 87.1 | 4 | 12.9 | 31 | 12.1 | |
| Quadrantectomy w RT | 150 | 90.4 | 16 | 9.6 | 166 | 64.6 | |
| Systemic treatment | < 0.001 | ||||||
| Nil | 109 | 93.2 | 8 | 6.8 | 117 | 45.5 | |
| ET | 110 | 89.4 | 13 | 10.6 | 123 | 47.9 | |
| CT | 5 | 35.7 | 9 | 64.3 | 14 | 5.4 | |
| CT+ET | 2 | 66.7 | 1 | 33.3 | 3 | 1.2 | |
Abbreviations: SLN, sentinel lymph node; RT, radiotherapy; ET, endocrine therapy; CT, chemotherapy.
Statistical Analysis
Demographic and clinical characteristics of the study sample were analysed using descriptive statistics. The association between SLN status, and demographic and clinical variables was evaluated using the Chi-square test. The cumulative incidences of the first observed relapse (categorized as local recurrence, regional recurrence, or distant metastasis) were assessed from the date of surgery to the date of event. In case of no event, the observation was censored at the last follow-up visit. Cumulative incidence functions were estimated according to the method described by Kalbfleisch and Prentice14, taking into account the competing causes of relapse. Gray’s test15 was used to assess cumulative incidence differences between groups.
Overall survival (OS) was defined as the time from the date of surgery until the date of death due to any cause; disease-free survival (DFS) was defined, according to the standardized definitions for efficacy end points (STEEP) criteria16, as the time from surgery to events such as relapse (including ipsilateral breast tumour recurrence), appearance of a second primary cancer (including contralateral breast cancer), or death, whichever occurred first. OS and DFS curves were estimated using the Kaplan-Meier method. The log-rank test was used to assess OS or DFS differences between groups.
Median follow-up was calculated using the reverse Kaplan-Meier method17. All analyses were performed using SAS software v. 9.4 (SAS Institute, Cary, NC). All statistical tests were two sided.
Results
Of the 257 (83%) patients undergoing sentinel lymph node biopsy, 161 (62%) patients had only one SLN; 57 (22%) had two SLNs, and 26 (10%) had three SLNs. In 13 patients (5%), more than three SLNs were removed.
Sentinel node metastasis and tumour characteristics
Negative SLNs were found in 226 (87.9%) patients. In one patient out of the 226, axillary dissection was performed due to the presence of a micrometastasis in an additional level 1 lymph node removed at the time when this still was an institutional criterion for axillary dissection. A total of 31 patients presented with metastatic SLNs. Twelve of these presented with ITCs, 14 with micrometastases, and five with macrometastases. Thus, the overall rate of metastasis in the SLN was 12.1% with macrometastasis in 2%, micrometastasis in 5.4%, and ITCs in 4.7% (Supplementary Table 1). All patients with metastatic SLNs had ductal histology of the breast cancer. A higher percentage of patients with positive SLNs were found in the luminal B (30.5%) and triple negative (20.7%) subtypes when compared to the other subtypes.
Axillary surgery
Axillary dissection was performed in 16 of 31 patients with positive SLNs. Out of these in one patient with ITCs, 10 with micrometastasis and five with macrometastasis. The five patients with macrometastasis had no more than 3 positive lymph nodes at final histological examination (pN1a). The remaining 11 patients with ITCs and four with micrometastasis at SLN were diagnosed in the later period (from 2004 onwards) and were thus not subjected to axillary dissection. Table 2 shows the clinical and pathological characteristics of the patients in the study, subdivided by lymph node status.
Breast surgery
Breast conserving surgery (BCS) was performed in 166 patients (64.6%) and of these, 150 (90.4%) had negative SLNs and 16 (9.6%) had positive SLNs. A total of 91 patients (35.4%) underwent mastectomy, with conservation of the nipple-areola complex and immediate reconstruction in most cases. Of these, 76 patients (83,5%) had negative SLNs and 15 (16,5%) had positive SLNs. Of the 31 patients who underwent nipple-sparing mastectomy and received intraoperative radiotherapy of the nipple areola complex, 27 patients had negative SLNs and 4 had positive SLNs.
Adjuvant treatment
Adjuvant endocrine treatment alone was administered in 123 of the 257 patients who underwent SLNB (47.9%), 14 patients received chemotherapy alone (5.4%) and three patients received both chemotherapy and endocrine therapy (1.2%). The distribution by SLN status is described in Supplementary Table 1.
Out of the who did not undergo SLNB, 21 of the 53 received endocrine therapy alone; eight of them were classified as Luminal A. Four received chemotherapy alone, one luminal B with Ki67≥20%, one patient with a HER2+ cancer, one triple negative and in one patient information was missing to derive the tumour subtype. Five patients received endocrine therapy plus chemotherapy and these were two luminal A, one luminal B (HER2+), one triple negative and one patient without enough information to derive the tumour subtype classification. The remaining 23 patients did not receive adjuvant treatment in accordance with pathological tumour stage.
Recurrences and survival
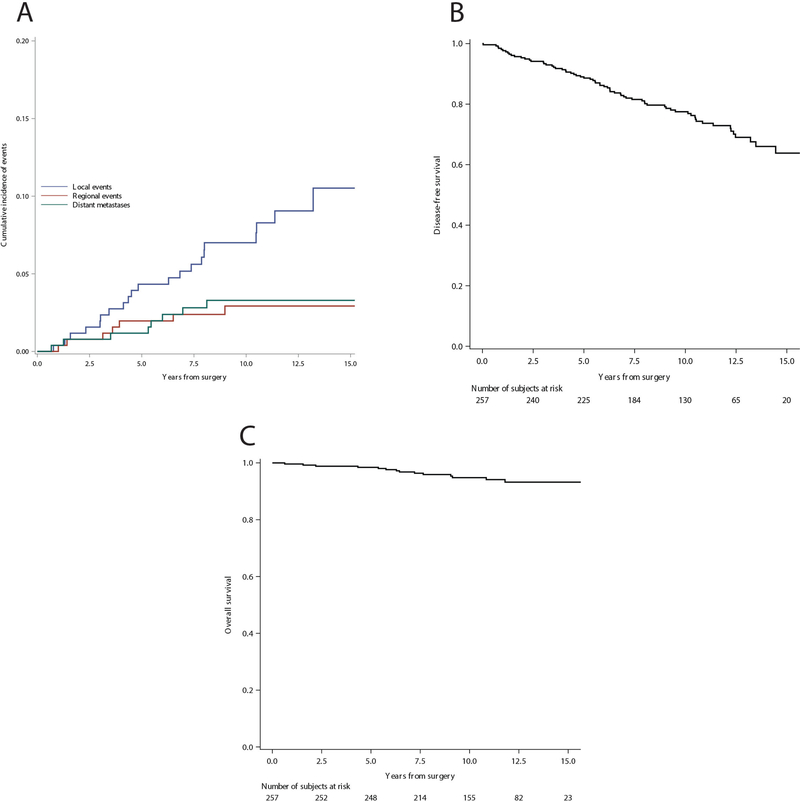
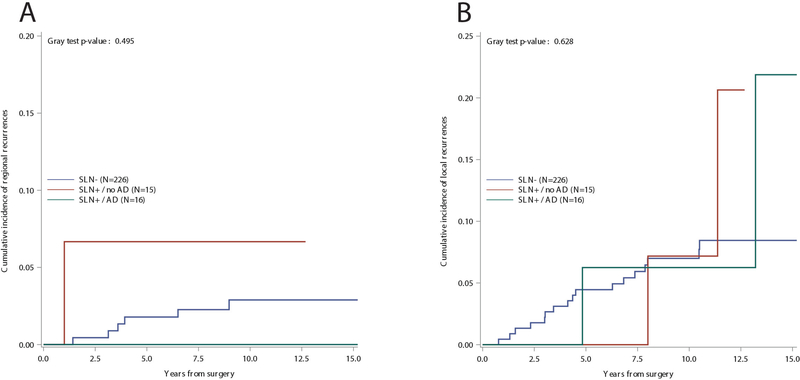
The median follow-up time was 11 years with 2765 cumulative person-years. At median follow-up, 14 deaths and 69 first events were observed. Seventeen local recurrences, six regional recurrences, and six distant metastases were observed among the 226 SLN negative patients. In the SNL positive group without further axillary dissection, two local events, one regional event, and one case of distant metastases were observed, while in the SNL positive SLN group followed by axillary dissection, two local events and one case of distant metastases were observed. Fig. 1a shows the cumulative incidence of events over 15 years of follow-up. The estimated 10-year cumulative incidence of local, regional, and distant recurrences was 7%, 2.9%, and 3.2%, respectively. Fig. 1b shows the overall disease-free survival, and Fig. 1c shows overall survival. The estimated 10-years DFS was 77.5%, and the estimated 10-years OS was 94.8%. The cumulative incidence of regional (panel A) and local (panel B) recurrences in relation to the SLN status and its correlating surgical axillary treatment (SLN negative/SLN positive followed or not followed by axillary dissection) is shown in Figure 2.
Fig. 1.
Cumulative incidence of a local, regional, and distant events, b disease-free survival, c overall survival
Fig. 2.
a Cumulative incidence of regional events by sentinel lymph node status and axillary dissection, and b cumulative incidence of local events by sentinel lymph node status and axillary dissection
Discussion
Microinvasive breast cancer is a rare form of breast cancer defined by the presence of 1 mm of invasive cancer in a background of DCIS, and comprises 0.6%−3.4 % of all breast cancer1, 15, 17.In the AJCC staging system, it is considered a subset of T1 disease (T1mi)15. A precise and more complete definition is the World Health Organization Classification of clearly separate microscopic foci of infiltration of tumour cells into the mammary stroma, each ≤ 1 mm in size. No further extension beyond the specialized intralobular stroma is required, the number of invasive foci and their percentage among all the carcinoma cells are irrelevant, and sizes of different foci are not to be added together18. Invasive cells are generally found in the context of DCIS in the background with microinvasive cancer found in 10%−20% of DCIS cases2. This consideration could justify the fact that it is often defined as DCIS with microinvasion7, 19. The sole presence of an invasive breast carcinoma 1 mm or less, without any in situ background, is very rare and should be regarded as an invasive carcinoma of that specific diameter1.
Recent studies have investigated the histopathological characteristics and clinical outcomes of microinvasive DCIS6, 20–24 as whether the survival and biological behaviour of this rare form of breast carcinoma differs from DCIS remain controversial. Microinvasive DCIS is frequently found in a high nuclear grade comedo DCIS setting, and less frequently with other types of DCIS or lobular carcinoma in situ25.
In this study, the incidence of SLNB metastasis was 12.1% in microinvasive breast cancer, which falls within the range described in the literature. The rate of macrometastasis (2%) was low, with major incidence of micrometastasis and ITC. Moreover the long-term outcomes were favourable (median follow-up time of 11 years) with a very low rate of regional recurrence in patients with positive SLNs. There was only one regional recurrence among patients with positive SLNs without axillary dissection, which did not statistically differ from the group of patients with axillary dissection after positive SLNB. No correlation was found between the incidence of SNL metastasis and type of breast surgery, conservation of the breast with or without radiation therapy, or mastectomy without radiation therapy. Most interesting is the discovery of a higher rate of regional recurrence in patients with microinvasive DCIS with negative SLNs, but with a specific molecular pattern.
The findings of this study supports that SLNB may not be useful in microinvasive DCIS due to the low risk of lymph node metastasis and good prognosis. The good prognosis may be explained by the theory that33 the major rate of positivity could correspond to an iatrogenic transit of tumour/epithelial cells to lymph nodes, without the significance of real metastasis. Level 1 evidence shows that in SLN positive breast cancer patients, axillary dissection may be avoided in cases involving low axillary metastatic burden (Z0011)48 and in patients undergoing BCS with radiation, which also supports the conclusion that SLNB in microinvasive DCIS may not be useful. In particular, in the group of patients in this study who underwent BCS and axillary dissection for positive sentinel nodes, the total number of positive nodes including sentinel nodes was less than 3, including those patients meeting ACOSOG Z0011 criteria.
An important consideration in staging the axilla in these patients is the possible implication for systemic therapy. In this study, adjuvant treatment was however largely decided based on cancer biology. The findings of this study, of low positive SLN rates in patients with good DFS and OS, and the lack of influence on selecting adjuvant treatment, are in line with other studies showing that SLNB in microinvasive DCIS may not be useful. This study supports the evidence that less surgery, combined with adequate pre-surgical clinical/histological information allowing for the planning of a correct, personalized clinical pathway for each patient, may provide the same level of OS with better patient quality of life.
Supplementary Material
Acknowledgements
The preparation of this study was funded in part by NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Sources of funding: The preparation of this study was funded in part by NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Footnotes
Disclosure: The authors declare no conflicts of interest
Statement of Ethics
The manuscript was approved by the Institutional Review Board of the European Institute of Oncology, Milan. Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Bianchi S, Vezzosi V. Microinvasive carcinoma of the breast. Pathol Oncol Res 2008;14(2): 105–111. [DOI] [PubMed] [Google Scholar]
- 2.Fang Y, Wu J, Wang W, Fei X, Zong Y, Chen X, Huang O, He J, Chen W, Li Y, Shen K, Zhu L. Biologic behavior and long-term outcomes of breast ductal carcinoma in situ with microinvasion. Oncotarget 2016;7(39): 64182–64190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagios MD, Westdahl PR, Margolin FR, Rose MR. Duct carcinoma in situ. Relationship of extent of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer 1982;50(7): 1309–1314. [DOI] [PubMed] [Google Scholar]
- 4.Orzalesi L, Casella D, Criscenti V, Gjondedaj U, Bianchi S, Vezzosi V, Nori J, Cecconi L, Meattini I, Livi L, Bernini M. Microinvasive breast cancer: pathological parameters, cancer subtypes distribution, and correlation with axillary lymph nodes invasion. Results of a large single-institution series. Breast Cancer 2016;23(4): 640–648. [DOI] [PubMed] [Google Scholar]
- 5.Edge S, Byrd DR, Compton CC, Fritz GA, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th Edition. Springer; New York: 2009. [Google Scholar]
- 6.Vieira CC, Mercado CL, Cangiarella JF, Moy L, Toth HK, Guth AA. Microinvasive ductal carcinoma in situ: clinical presentation, imaging features, pathologic findings, and outcome. Eur J Radiol 2010;73(1): 102–107. [DOI] [PubMed] [Google Scholar]
- 7.Lyons JM 3rd, Stempel M, Van Zee KJ, Cody HS 3rd. Axillary node staging for microinvasive breast cancer: is it justified? Ann Surg Oncol 2012;19(11): 3416–3421. [DOI] [PubMed] [Google Scholar]
- 8.Veronesi U, Zurrida S, Mazzarol G, Viale G. Extensive frozen section examination of axillary sentinel nodes to determine selective axillary dissection. World J Surg 2001;25(6): 806–808. [DOI] [PubMed] [Google Scholar]
- 9.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26(8): 1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin Oncol 2001;19(18): 3817–3827. [DOI] [PubMed] [Google Scholar]
- 11.Goldhirsch A, Glick JH, Gelber RD, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. J Natl Cancer Inst 1998;90(21): 1601–1608. [DOI] [PubMed] [Google Scholar]
- 12.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 2009;20(8): 1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22(8): 1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Hoboken, NJ: Wiley & Sons Ltd; 1980. [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16: 1141–1154. [Google Scholar]
- 16.Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, Sparano JA, Hunsberger S, Enos RA, Gelber RD, Zujewski JA. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 2007;25(15): 2127–2132. [DOI] [PubMed] [Google Scholar]
- 17.Altman DG, De Stavola BL, Love SB, Stepniewska KA. Review of survival analyses published in cancer journals. Br J Cancer 1995;72(2): 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinder SE, Ellis IO, Schnitt SJ, Tan PH, Rutgers E, Morrow M. Microinvasive carcinoma In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van de vijvair MJ, editors. WHO classification of tumours of the breast. Lyon: IARC Press; 2012. p. 96–7. [Google Scholar]
- 19.Yang M, Moriya T, Oguma M, De La Cruz C, Endoh M, Ishida T, Hirakawa H, Orita Y, Ohuchi N, Sasano H. Microinvasive ductal carcinoma (T1mic) of the breast. The clinicopathological profile and immunohistochemical features of 28 cases. Pathol Int 2003;53(7): 422–428. [DOI] [PubMed] [Google Scholar]
- 20.Cavaliere A, Scheibel M, Bellezza G, Colella R, Vitali R, Gori S, Aristei C, Rulli A, Sidoni A. Ductal carcinoma in situ with microinvasion: clinicopathologic study and biopathologic profile. Pathol Res Pract 2006;202(3): 131–135. [DOI] [PubMed] [Google Scholar]
- 21.de Mascarel I, MacGrogan G, Mathoulin-Pelissier S, Soubeyran I, Picot V, Coindre JM. Breast ductal carcinoma in situ with microinvasion: a definition supported by a long-term study of 1248 serially sectioned ductal carcinomas. Cancer 2002;94(8): 2134–2142. [DOI] [PubMed] [Google Scholar]
- 22.Margalit DN, Sreedhara M, Chen YH, Catalano PJ, Nguyen PL, Golshan M, Overmoyer BA, Harris JR, Brock JE. Microinvasive breast cancer: ER, PR, and HER-2/neu status and clinical outcomes after breast-conserving therapy or mastectomy. Ann Surg Oncol 2013;20(3): 811–818. [DOI] [PubMed] [Google Scholar]
- 23.Parikh RR, Haffty BG, Lannin D, Moran MS. Ductal carcinoma in situ with microinvasion: prognostic implications, long-term outcomes, and role of axillary evaluation. Int J Radiat Oncol Biol Phys 2012;82(1): 7–13. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Zhu W, Du F, Luo Y, Xu B. The Demographic Features, Clinicopathological Characteristics and Cancer-specific Outcomes for Patients with Microinvasive Breast Cancer: A SEER Database Analysis. Sci Rep 2017;7: 42045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemoto T, Castillo N, Tsukada Y, Koul A, Eckhert KH, Jr., Bauer RL. Lobular carcinoma in situ with microinvasion. J Surg Oncol 1998;67(1): 41–46. [DOI] [PubMed] [Google Scholar]
- 26.Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, Benson AB 3rd, Bosserman LD, Burstein HJ, Cody H 3rd, Hayman J, Perkins CL, Podoloff DA, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2014;32(13): 1365–1383. [DOI] [PubMed] [Google Scholar]
- 27.Gojon H, Fawunmi D, Valachis A. Sentinel lymph node biopsy in patients with microinvasive breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol 2014;40(1): 5–11. [DOI] [PubMed] [Google Scholar]
- 28.Camp R, Feezor R, Kasraeian A, Cendan J, Schell S, Wilkinson E, Copeland E, Lind S. Sentinel lymph node biopsy for ductal carcinoma in situ: an evolving approach at the University of Florida. Breast J 2005;11(6): 394–397. [DOI] [PubMed] [Google Scholar]
- 29.Cox CE, Nguyen K, Gray RJ, Salud C, Ku NN, Dupont E, Hutson L, Peltz E, Whitehead G, Reintgen D, Cantor A. Importance of lymphatic mapping in ductal carcinoma in situ (DCIS): why map DCIS? Am Surg 2001;67(6): 513–519; discussion 519–521. [PubMed] [Google Scholar]
- 30.Fortunato L, Santoni M, Drago S, Gucciardo G, Farina M, Cesarini C, Cabassi A, Tirelli C, Terribile D, Grassi GB, De Fazio S, Vitelli CE. Sentinel lymph node biopsy in women with pT1a or “microinvasive” breast cancer. Breast 2008;17(4): 395–400. [DOI] [PubMed] [Google Scholar]
- 31.Gray RJ, Mulheron B, Pockaj BA, Degnim A, Smith SL. The optimal management of the axillae of patients with microinvasive breast cancer in the sentinel lymph node era. Am J Surg 2007;194(6): 845–848; discussion 848–849. [DOI] [PubMed] [Google Scholar]
- 32.Guth AA, Mercado C, Roses DF, Darvishian F, Singh B, Cangiarella JF. Microinvasive breast cancer and the role of sentinel node biopsy: an institutional experience and review of the literature. Breast J 2008;14(4): 335–339. [DOI] [PubMed] [Google Scholar]
- 33.Hanna MG, Jaffer S, Bleiweiss IJ, Nayak A. Re-evaluating the role of sentinel lymph node biopsy in microinvasive breast carcinoma. Mod Pathol 2014;27(11): 1489–1498. [DOI] [PubMed] [Google Scholar]
- 34.Intra M, Zurrida S, Maffini F, Sonzogni A, Trifiro G, Gennari R, Arnone P, Bassani G, Opazo A, Paganelli G, Viale G, Veronesi U. Sentinel lymph node metastasis in microinvasive breast cancer. Ann Surg Oncol 2003;10(10): 1160–1165. [DOI] [PubMed] [Google Scholar]
- 35.Kapoor NS, Shamonki J, Sim MS, Chung CT, Giuliano AE. Impact of multifocality and lymph node metastasis on the prognosis and management of microinvasive breast cancer. Ann Surg Oncol 2013;20(8): 2576–2581. [DOI] [PubMed] [Google Scholar]
- 36.Katz A, Gage I, Evans S, Shaffer M, Fleury T, Smith FP, Flax R, Drogula C, Petrucci P, Magnant C. Sentinel lymph node positivity of patients with ductal carcinoma in situ or microinvasive breast cancer. Am J Surg 2006;191(6): 761–766. [DOI] [PubMed] [Google Scholar]
- 37.Klauber-DeMore N, Tan LK, Liberman L, Kaptain S, Fey J, Borgen P, Heerdt A, Montgomery L, Paglia M, Petrek JA, Cody HS, Van Zee KJ. Sentinel lymph node biopsy: is it indicated in patients with high-risk ductal carcinoma-in-situ and ductal carcinoma-in-situ with microinvasion? Ann Surg Oncol 2000;7(9): 636–642. [DOI] [PubMed] [Google Scholar]
- 38.Ko BS, Lim WS, Kim HJ, Yu JH, Lee JW, Kwan SB, Lee YM, Son BH, Gong GY, Ahn SH. Risk factor for axillary lymph node metastases in microinvasive breast cancer. Ann Surg Oncol 2012;19(1): 212–216. [DOI] [PubMed] [Google Scholar]
- 39.Leidenius M, Salmenkivi K, von Smitten K, Heikkila P. Tumour-positive sentinel node findings in patients with ductal carcinoma in situ. J Surg Oncol 2006;94(5): 380–384. [DOI] [PubMed] [Google Scholar]
- 40.Matsen CB, Hirsch A, Eaton A, Stempel M, Heerdt A, Van Zee KJ, Cody HS 3rd, Morrow M, Plitas G Extent of microinvasion in ductal carcinoma in situ is not associated with sentinel lymph node metastases. Ann Surg Oncol 2014;21(10): 3330–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pimiento JM, Lee MC, Esposito NN, Kiluk JV, Khakpour N, Carter WB, Han G, Laronga C. Role of axillary staging in women diagnosed with ductal carcinoma in situ with microinvasion. J Oncol Pract 2011;7(5): 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross DS, Hoda SA. Microinvasive (T1mic) lobular carcinoma of the breast: clinicopathologic profile of 16 cases. Am J Surg Pathol 2011;35(5): 750–756. [DOI] [PubMed] [Google Scholar]
- 43.Sakr R, Bezu C, Raoust I, Antoine M, Ettore F, Darcourt J, Kerrou K, Darai E, Rouzier R, Uzan S. The sentinel lymph node procedure for patients with preoperative diagnosis of ductal carcinoma in situ: risk factors for unsuspected invasive disease and for metastatic sentinel lymph nodes. Int J Clin Pract 2008;62(11): 1730–1735. [DOI] [PubMed] [Google Scholar]
- 44.Tunon-de-Lara C, Giard S, Buttarelli M, Blanchot J, Classe JM, Baron M, Monnier B, Houvenaeghel G. Sentinel node procedure is warranted in ductal carcinoma in situ with high risk of occult invasive carcinoma and microinvasive carcinoma treated by mastectomy. Breast J 2008;14(2): 135–140. [DOI] [PubMed] [Google Scholar]
- 45.Wilkie C, White L, Dupont E, Cantor A, Cox CE. An update of sentinel lymph node mapping in patients with ductal carcinoma in situ. Am J Surg 2005;190(4): 563–566. [DOI] [PubMed] [Google Scholar]
- 46.Zavagno G, Belardinelli V, Marconato R, Carcoforo P, Franchini Z, Scalco G, Burelli P, Pietrarota P, Mencarelli R, Marconato G, Nitti D. Sentinel lymph node metastasis from mammary ductal carcinoma in situ with microinvasion. Breast 2007;16(2): 146–151. [DOI] [PubMed] [Google Scholar]
- 47.Zavotsky J, Hansen N, Brennan MB, Turner RR, Giuliano AE. Lymph node metastasis from ductal carcinoma in situ with microinvasion. Cancer 1999;85(11): 2439–2443. [DOI] [PubMed] [Google Scholar]
- 48.Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252(3): 426–432; discussion 432–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.