Abstract
Objectives:
This limited review examines the role of the reticular activating system (RAS), especially the pedunculopontine nucleus (PPN), one site of origin of bottom-up gamma, in the symptoms of bipolar disorder (BD).
Methods:
The expression of neuronal calcium sensor protein 1 (NCS-1) in the brains of BD patients is increased. It has recently been found that all PPN neurons manifest intrinsic membrane beta/gamma frequency oscillations mediated by high threshold calcium channels, suggesting that it is one source of bottom-up gamma. This review specifically addresses the involvement of these channels in the manifestation of BD.
Results:
Excess NCS-1 was found to dampen gamma band oscillations in PPN neurons. Lithium, a first line treatment for BD, was found to decrease the effects of NCS-1 on gamma band oscillations in PPN neurons. Moreover, gamma band oscillations appear to epigenetically modulate gene transcription in PPN neurons, providing a new direction for research in BD.
Conclusions:
This is an area needing much additional research, especially since the dysregulation of calcium channels may help explain many of the disorders of arousal in, elicit unwanted neuroepigenetic modulation in, and point to novel therapeutic avenues for, BD.
Keywords: Arousal, binding, Ca2+ channels, neuroepigenetics, gamma oscillations, lithium, NCS-1, preconscious awareness, REM sleep, waking
1. INTRODUCTION
1.1. Sleep dysregulation in bipolar disorder (BD)
The wake-sleep patterns manifested in the EEG of bipolar disorder (BD) patients include fragmented sleep, decreased slow wave sleep, increased vigilance, and increased REM sleep drive1–3. More specifically, adults with BD manifest reduced sleep duration and delayed sleep onset during episodes of mania, which show decreased REM sleep latency and increased REM sleep duration. On the other hand, during episodes of depression, BD patients experience insomnia or hypersomnia, also with decreased REM sleep latency and increased REM sleep density1,3–5. Recent analyses described fragmented sleep (characterized by increased sleep onset, waking after sleep onset, and overall sleep efficiency) in BD6,7. These patients also show decreased habituation of the arousal-related P50 potential in a paired stimulus paradigm8,9, indicative of a sensory gating deficit, along with increased startle response10, and dysregulation of blink reflexes11, indicative of exaggerated reflexes. All of these symptoms are suggestive of hyper-arousal as a hallmark of the disease. Importantly, reduced maintained gamma band activity has been reported in BD patients12. That is, while patients have hyper-arousal, waking is marked by erratic maintenance of high frequency activity. As we will see below, hyper-arousal and decreased maintenance of gamma band activity can account for many of the symptoms in BD, including wake-sleep, arousal, and cognitive symptoms.
1.2. Tonic and Phasic Arousal and Hyper-arousal.
The reticular activating system (RAS) exhibits both phasic and tonic actions. On the one hand, it is in charge of relaying sudden or startling stimuli to elicit both cortical activation and reticulospinal motor readiness, and on the other hand, it is in charge of keeping us awake on a continuous basis13. The first description of the RAS suggested that it specifically participates in “tonic or continuous” arousal14. In addition, it was found that lesions of this region could eliminate tonic arousal15. Therefore, these tonic mechanisms help maintain gamma band activity for prolonged periods and are critically important for such functions as perception, learning, and memory, not to mention consciousness. It has been suggested that consciousness is associated with continuous gamma band activity, as opposed to an interrupted pattern of activity16. The lack of consistent gamma band activity would alter waking and REM sleep drive, decrease binding of perceptions, decrease the ability to attend, etc., thus accounting for a number of symptoms in BD. Transitions between mania and depression could well be related to shifts in arousal levels, as would erratic energy and activity levels. In addition, sudden stimuli activate ascending RAS projections to the thalamus and then the cortex to induce cortical arousal, and simultaneously activate descending projections that influence the spinal cord in the form of postural changes in tone resulting from the startle response, as well as trigger locomotor events in fight-vs-flight responses13. Dysregulation of this system thus can elicit a host of abnormal sensory and motor (reflex) responses, both tonic and phasic, that can account for a number of symptoms expressed in BD.
2. METHODS
We have reviewed a limited series of articles describing both basic science and clinical data related to the underlying mechanisms of BD. In addition, cross-references were searched from identified papers related to calcium channels involved in intrinsic gamma oscillations, especially neuronal calcium sensor protein 1 (NCS-1). Papers not addressing the role of calcium channels in BD were excluded based on initial screening of titles and abstracts, and subsequently selection was based on the concept of “bottom-up gamma” and new experiments describing a mechanism of action of lithium, and gamma oscillations and their epigenetic modulation in the PPN.
2. RESULTS
3.1. Bottom-up gamma
3.1.i. What does it do?
Recent studies suggest that feed forward signals from the brainstem and feedback signaling from the cortex use different frequency channels, specifically gamma and beta frequencies, respectively17. On the one hand, bottom-up gamma or feed forward brain processes depend on sensory events that activate lower brain centers and the information rises to succeeding higher centers to promote perception. On the other hand, top-down beta or feedback processing refers to the influence imposed by higher centers on the perception of and attention to sensory stimuli. Gamma/beta oscillations are known to participate in perception, problem solving, and memory18–21. Synchronous or coherent beta/gamma band activation in thalamocortical networks22, and other neuronal groups is thought to contribute to the merger, or “binding”, of information originating from separate regions, necessary for unified perception23. Moreover, beta/gamma oscillation deficits have been suggested as a pathophysiologic feature of diseases like schizophrenia and Alzheimer’s disease24,25.
3.1.ii. Where does it come from?
Gamma oscillations are thought to emerge from the dynamic interaction between intrinsic neuronal and synaptic properties of thalamocortical networks22. That is, synaptic connections alone may not be able to maintain firing at gamma frequencies (~30–90 Hz), so that intrinsic membrane properties also appear essential to the maintenance of gamma band activity26,27. Thus, the ability of cells with intrinsic membrane properties, coupled with synaptic interactions, is what allows the circuit as a whole to fire at a preferred frequency, and is essential to maintaining frequencies in the beta/gamma range. At the thalamic level, thalamocortical excitatory neurons have intrinsic properties needed to generate subthreshold gamma band membrane oscillations28.
However, the cortex and thalamus are not the only regions capable of generating beta/gamma frequency oscillations. For example, the hippocampus and cerebellum have the intrinsic and synaptic properties necessary to generate gamma band oscillatory activity. Hippocampal oscillatory activity in the gamma range (30–60 Hz) has been extensively described to be functional associated with entorhinal cortex afferents29. Gamma band activity in the hippocampal cornu ammonis 1 (CA1) subfield was divided into fast (>65 Hz) and slow (~25–60 Hz) frequency components differentially coupling CA1 and CA3 subfields30. These different frequency bands have been proposed to “bind” CA1 fast gamma oscillations with very high frequency activity from entorhinal cortex (in charge of providing information about object and place recognition in rodents31), whereas CA1 slow gamma oscillations would be locked to the slower frequencies present in the CA3 area in charge of memory storage32.
Gamma band power also has been described in the olivocerebellar system33,34. Cortico-cerebellar coherence at gamma frequencies is evident in monkeys during performance of a manual precision grip task35, and cerebello-thalamic activity is synchronized with neocortical activity at gamma frequencies36. Thus, it has been proposed that both cerebellar and thalamocortical networks might oscillate at the same frequencies to enable information exchange and processing among these brain areas34.
Importantly, it has been shown that beta/gamma band activity in the motor cortex lags behind coherent activity in the basal ganglia37. This led to the suggestion that motor cortex beta/gamma synchronization reflects a momentary arousal-related event for enabling the initiation of movement38,39. That is, structures such as the RAS, that mediates arousal, and thalamus, that carries afferent information, may play an early permissive role in the control of movement. Moreover, there are several other regions generating gamma band activity besides the cortex and thalamus, including the hippocampus, cerebellum, basal ganglia, and importantly, the RAS.
3.2. Gamma during waking vs during REM sleep
3.2.i. Two types of high threshold calcium channels.
The PPN is the only nucleus in the RAS that is active during states of high frequency (beta/gamma) EEG activity as in waking and REM sleep. Paradoxically, the EEG during the two markedly different states is identical, but, is beta/gamma band activity during waking really the same as during REM sleep? In general, recordings of PPN neurons during sleep-wake states revealed neurons that were active during waking and REM sleep, referred to as “Wake-REM on” cells, others were active only during REM sleep, referred to as “REM on” cells, and yet others were active only during waking, referred to as “Wake on” cells, and none were more active during slow wave sleep40–43. Gamma band activity has been observed in the cortical EEG of the cat in vivo when the animal is active44; in the region of the PPN in humans during stepping, but not at rest45; and firing at low frequencies ~10 Hz at rest in the primate, but firing at gamma band frequencies when the animal woke up, or when the animal began walking on a treadmill46. Thus, the same cells were involved in both arousal and motor control in the PPN in vitro, in vivo, and across species, including man.
Our findings established that all PPN neurons fired maximally at beta/gamma frequencies47, that all PPN neurons manifested beta/gamma frequency intrinsic membrane oscillations48, and that these oscillations were mediated by high threshold, voltage-dependent N- and P/Q-type calcium channels48–50. We found that these channels were distributed along the dendrites of PPN neurons51, and that some cells exhibited both N- and P/Q-type calcium channels, some had only N-type channels, and some had only P/Q-type channels52,53. We suggested that these three cell types represented neurons that were active in relation to waking and REM sleep (N+P/Q cells = “Wake-REM on” cells), only during REM sleep (N-only = “REM on” cells), or only during waking (P/Q only = “Wake on” cells), respectively52–55.
3.2.ii. Two intracellular pathways.
In freely moving animals, injections of glutamate into the PPN increased both waking and REM sleep56, but injections of NMDA, one glutamatergic receptor agonist, increased only waking57, while injections of kainic acid (KA), another glutamatergic receptor agonist, increased only REM sleep58. These data indicated a differential control of waking vs REM sleep by different glutamatergic receptor subtypes. Protein kinase C (PKC), which modulates KA receptors, enhances N-type channel activity and has no effect on P/Q-type channel function59, however, calmodulin dependent kinase II (CaMKII), which modulates NMDA receptors, was shown to modulate P/Q-type channel function60. These results suggest that the two high threshold calcium channel types are modulated by different intracellular pathways, N-type by the cAMP/PK pathway, and P/Q-type via the CaMKII pathway. The implications from all of these results is that there is a “waking” pathway mediated by CaMKII and P/Q-type channels and a “REM sleep” pathway mediated by cAMP/PK and N-type channels, moreover, different PPN cells fire during waking (those with N+P/Q and only P/Q-type) or REM sleep (those with N+P/Q and only N-type) or both (N+P/Q)13,54,55.
3.2.iii. Is gamma from the PPN manifested in the cortical EEG?
Recent findings showed that gamma band activity at the level of the cortex during waking is characterized by coherence across regions, but gamma band activity in the cortex during REM sleep has an absence of coherence61. Thus, since the brainstem is the origin of REM sleep drive, it is clear that the manifestation of gamma band activity during REM sleep at the level of the cortex begins in the brainstem. We assume that the manifestation of gamma band activity during waking is at least in part originating in the brainstem as well. Moreover, injections of the cholinergic agonist carbachol induced REM sleep with cataplexy that is characterized by decreased gamma band coherence in the cortex62. Therefore, this line of evidence suggests that, not only do brainstem centers drive gamma band activity that is manifested in the cortical EEG, but during waking, brainstem-thalamic projections include coherence across regions, and during REM sleep, which is controlled by the Subcoeruleus region (lesion of this region eliminates REM sleep, and injection of carbachol into it induces REM sleep), drives cortical EEG rhythms without coherence63,64.
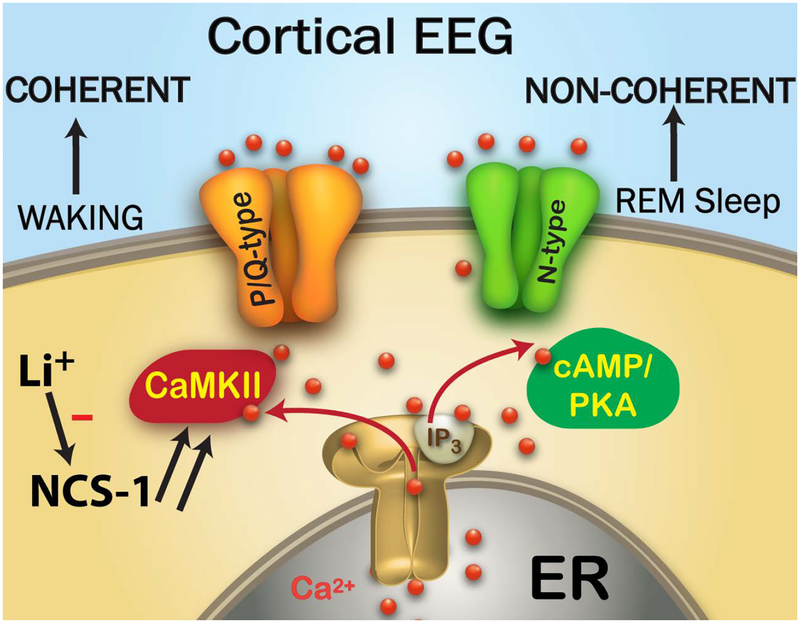
Figure 1 shows the proposed organization of the differential control of beta/gamma band activity during waking through P/Q-type calcium channels that are modulated by intracellular CaMKII, while activity during REM sleep through N-type channels is modulated by cAMP/PKA. As far as the cortical EEG is concerned, waking drive from the PPN through the intralaminar thalamus induces coherent cortical gamma band activity, but REM sleep drive, presumably through the Subcoeruleus nucleus, induces cortical gamma band activity without coherence across regions. Included in this figure is the role of neuronal calcium sensor protein 1 (NCS-1) in modulating P/Q-type channels, and that of lithium, which inhibits the action of NCS-1.
Figure 1. Waking vs REM sleep Gamma Mechanisms.
The Cortical EEG looks the same during waking and REM sleep, but it is coherent across regions during waking and non-coherent during REM sleep. The cortical EEG, therefore, is driven by the RAS, which regulates waking and sleep. Cells in the PPN modulate gamma oscillations during waking through P/Q-type calcium channels that are under the control of CaMKII, while cells in the PPN that modulate REM sleep do so through N-type calcium channels that are under the control of cAMP/PKA. In BD, NCS-1 is over expressed (note double arrows to denote over expression) and its excess decreases CaMKII and P/Q-type driven gamma oscillations to decrease the maintenance of gamma band activity during waking. This may also lead to excessive REM sleep drive. Li+ inhibits the action of over expressed NCS-1 in order to restore the generation of gamma band oscillations during waking.
3.3. Neuronal calcium sensor protein 1 (NCS-1)
3.3.i. Role of NCS-1.
NCS-1 is expressed in the brain and heart, where it has been identified as a regulator of cardiac Ca2+ signaling65,66. Furthermore, a study on immature hearts reported that NCS-1 interacts with inositol 1,4,5-triphosphate receptor protein (InsP3R)66. NCS-1 is known to enhance the activity of InsP3Rs67, thus amplifying Ca2+ signaling through these receptors. Importantly, InsP3Rs are present in the PPN68. Stimulation of InsP3 levels resulted in phosphorylation of CaMKII, which was enhanced by NCS-1 over expression. These results indicate that a functional link exists between NCS-1, InsP3 function, and CaMKII activation that potentially affects global Ca2+ signaling65,66. However, NCS-1 is known to interact with many other target proteins in the brain, including 1-phosphatidylinositol 4-kinase69,70, dopamine D2 receptors71, as well as voltage-gated Ca2+72–74, and K+ channels65,75.
We discovered that intracellular NCS-1 had a concentration-dependent effect on beta/gamma oscillatory activity in PPN neurons76,77. While lower levels of NCS-1 enhanced oscillation amplitude at long latency, higher levels of NCS-1 had an early enhancing effect of oscillation amplitude that was followed by an inhibitory effect, ultimately reducing the amplitude of gamma oscillations. Strikingly, the time course of the calcium current block was similar to the high level NCS-1 effect on oscillation amplitude, but both of these effects were faster than the one observed for the enhancement of gamma band amplitude, suggesting that multiple intracellular mechanisms may mediate the NCS-1 effects on PPN oscillations. We concluded that NCS-1 at optimal concentrations will help maintain gamma band oscillations dependent on P/Q-type calcium channels, but excessive levels of NCS-1 will lead to a decrease or interrupted pattern of waking-related (P/Q-type channel-mediated) gamma band activity. However, since NCS-1 is also known to down regulate N-type calcium channels78, we assumed that these are the channels being blocked by NCS-1 to reduce calcium currents. Therefore, we suggested that NCS-1 will also down regulate N-type calcium channel REM sleep-related gamma oscillations76,77. This implies a push-pull effect in which appropriate levels of NCS-1 will promote P/Q-type channel oscillations as in waking, and suppress N-type channel oscillations as in REM sleep.
3.3.ii. Over expression in BD.
In addition to the symptoms described at the beginning of this article, the symptoms of BD also include disturbances in cognition. These include sleep and circadian disturbances, but also emotional dysregulation, and cognitive impairments1,79–81. For example, attention and memory deficits, impairment in verbal recall and fine motor skills, and disturbance of sustained attention are present during depressive episodes, whereas during mania, episodes of dysfunction in attention, complex processing, memory, and emotional processing are manifested80. Cognitive deficits are evident even during euthymia, when executive function, verbal memory, sustained attention, visual memory, and verbal fluency are disturbed81. Many of these symptoms, however, can be accounted for by dysregulation of gamma band activity.
Human postmortem studies reported increased expression of high affinity, low capacity NCS-1 in the brains of some BD and schizophrenic patients82. However, although the average levels were elevated, some patients manifested NCS-1 levels close to normal values. Therefore, either the expression levels are chronically elevated only in some patients, or death in some patients occurred when levels were close to normal, implying that levels fluctuate. Which option may be correct remains to be determined. Given the paucity of research in this area, we can only speculate, but there are a number of potentially productive lines of research that could lead to better therapeutic options.
Given the foregoing, we assume that during normal waking, normal levels of NCS-1 will promote P/Q-type channel function and suppress N-type channel function, thus helping maintain the awake state. During sleep, NCS-1 levels probably decrease, removing waking drive and disinhibiting N-type channel activity, which presumably promotes REM sleep. What happens if NCS-1 levels are increased such as in over expression? Excessive NCS-1 levels during waking would decrease waking drive through suppression of gamma activity. At the same time, there may be some inactivation of the inhibitory effects of NCS-1 on N-type channels, permitting REM sleep drive to increase. Under these circumstances, perception and consciousness would be interrupted during waking, leading to attentional and cognitive disturbances. In addition, REM sleep drive may be increased during waking, leading to misperception and perhaps hallucinations. Therapies that normalize sleep-wake states would certainly help reduce some of these symptoms.
3.3.iii. Effects of lithium.
Lithium was by chance found to treat the mood disturbances in BD, and it is an ion that remains one of the best treatment options, although it is limited by side effects83. Lithium may act by inhibiting the interaction between NCS-1 and InsP384. Our findings demonstrated that lithium suppressed the effects of NCS-1 on gamma oscillations in a concentration-dependent manner76,77. These results provide a physiological mechanism (gamma band dysregulation) that can account for many of the symptoms of the disease, as well as a rationale for the effective treatment of BD with lithium by down regulating NCS-1, and promoting the maintenance of gamma band activity. If lithium levels are too high, then the suppression would lead to sleepiness and other adverse effects, if too low, there would be insufficient suppression of over expressed NCS-1 (See Figure 1). Such a mechanism helps explain the need for therapeutic levels of lithium to be monitored in order to remain at an effective range. A number of other agents are also used successfully, including anticonvulsants and antipsychotics, which are beyond the scope of the present article. Fortunately, for many patients such therapies do allow them to function more normally and lead productive lives.
3.3.iv. Neuroepigenetic modulation of PPN neurons
Epigenetic mechanisms, that is, histone post-translational modification and DNA methylation, play a role in regulating gene expression in response to environmental stimuli, such as learning, stress, or drugs of abuse85. Acetylation of histones is associated with the modulation of transcription by relaxing chromatin structure that would increase the accessibility of transcription factors to their target genes, and also by direct acetylation of transcription factors and other proteins86. Histone acetylation is controlled by the balance between two families of enzymes, the histone acetyltransferases (HATs) and the histone deacetylases (HDACs). The antagonism between these enzymes is a key regulatory mechanism for many cellular processes and disease states. HDACs have been classified into four classes, with Class I HDACs localized in the nucleus, but Class IIs shuttle between the nucleus and the cytoplasm where they can modify non-histone proteins. HDAC inhibitors are candidates for treating cancer and several neurodegenerative disorders87. However, these produce a range of side effects, including fatigue, seizures, somnolence, and gait problems88. That is, these HDAC inhibitors may affect arousal.
We studied whether PPN intrinsic gamma oscillations that are involved in arousal are affected by inhibition of histone deacetylation. Our results showed that, a) acute in vitro exposure to the histone acetylation inhibitor trichostatin A (TSA) led to the elimination of high threshold, voltage-dependent Ca2+ channel-mediated intrinsic membrane oscillations, specifically in the gamma band range but not lower frequencies, b) pre-incubation with TSA led to a similar decrease specifically in gamma band oscillations, c) a significant reduction in Ca2+ currents was elicited by the same histone acetylation inhibitor, d) a HDAC Class I inhibitor, MS275, failed to affect intrinsic gamma oscillations in PPN neurons, and e) a HDAC Class IIb inhibitor, MC1568, blocked gamma oscillations89. These results suggest that there is a specific effect on gamma band oscillations when histone deacetylation via HDACIIb is blocked, suggesting that gamma oscillations related to arousal could be modulated by the balance between histone acetylation and deacetylation.
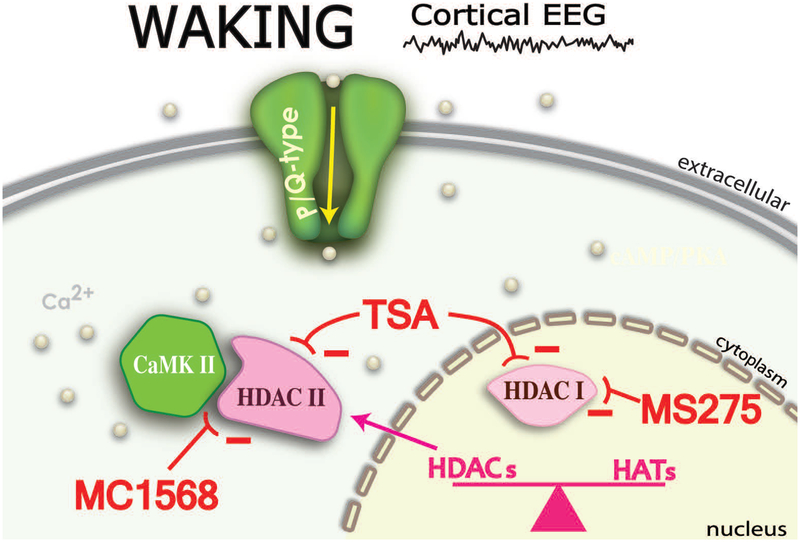
Acetylation and deacetylation of histone proteins in the brain represents one of the most significant neuroepigenetic mechanisms for the control of gene expression. The fact that the PPN modulates both waking and REM sleep, the two states with prominent EEG gamma band activity, suggests that TSA blockade of intrinsic membrane gamma oscillations eliminates a major process subserved by gamma band frequency activity, arousal. Our discovery suggests that this constant flow of continuous sensory information through the induction of gamma oscillations may be modulating the transcription of genes in PPN neurons89. This represents a potential neuroepigenetic mechanism not previously considered, but which carries significant implications. The constant flow of afferent information, termed bottom-up gamma63, may be providing ongoing remodeling of the neurons in this part of the RAS, a kind of daily update of sensory experience. Figure 2 is a diagram of the relationship between P/Q-type Ca2+ channels, CaMKII, and the interaction with HDAC Class IIs. Table 1 summarizes the effects of the agents used in this study89.
Figure 2. P/Q-type channels, CaMKII, and HDAC Class II interactions.
During waking, gamma oscillations mediated by P/Q-type Ca2+ channels lead to Ca2+ entry and interaction with CaMKII. In turn, CaMKII leads to phosphorylation of HDAC Class IIs that have left the nucleus (while HDAC Class Is remain in the nucleus). The complex between CaMKII and HDAC Class IIs is maintained by Ca2+ entry, in the case of P/Q-type channels, specifically during waking. Deacetylation of histones facilitates transcription. MC1568, a HDAC Class IIb inhibitor blocked oscillations, while MS275, a HDAC Class I inhibitor did not affect oscillations89. The minus (−) signs denote inhibition by TSA of HDAC class I and HDAC class II; inhibition of HDAC class I by MS275’ and inhibition of HDAC class II by MC1568.
Table 1.
Neuroepigenetic effects of histone deacetylases in PPN gamma oscillations89.
| Agent | Conc’n Role | Effect | |
|---|---|---|---|
| Trichostatin A (TSA) | 1 uM | Inhibits HDAC class I | No effect on oscillations |
| Inhibits HDAC class II | Blocks oscillations | ||
| MC1568 | 1 uM | Inhibits HDAC class IIa | Blocks oscillations |
| MS275 | 500 nM | Inhibits HDAC class I | No effect on oscillations |
| Tubastatin A | 150 nM | Inhibits HDAC class IIb | No effect on oscillations |
| KN-93 | 1 uM | Inhibits CaMKII | Reduced effects of TSA |
Note: HDAC class Is remains in the nucleus while HDAC class IIs can move to cytoplasm.
In BD, therefore, maintaining an appropriate level of gamma oscillations is essential to the sculpting of neurons taking place through genetic modification. Long-term dysregulation of this process may physically transform neurons exposed to over expression of NCS-1 and its effects on gamma oscillations, requiring extended treatment to correct these distortions. Stabilizing the manifestation of gamma oscillations during waking is essential to combating the debilitating effects of decreased or interrupted gamma band activity.
4. DISCUSSION
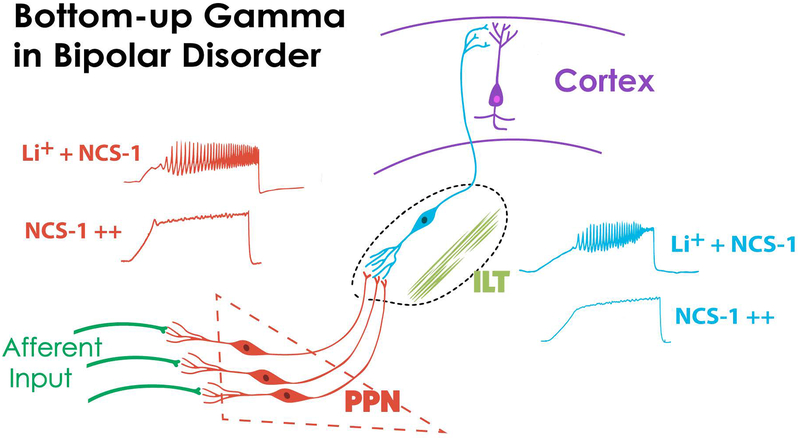
Figure 3 depicts one origin of bottom-up gamma via sensory afferents that induce gamma oscillations in PPN neurons. These PPN cells project to the parafascicular nucleus in the intralaminar thalamus (ILT), that also manifests N- and P/Q-type calcium channel-mediated gamma oscillations90, and these channels are located all along the dendrites of Pf neurons91. The ILT projects arousal-related input to upper layers of the cortex, providing the context of sensory perception22,23. In BD, the effects of over expressed NCS-1 in suppressing intrinsic membrane gamma oscillations in the PPN, and presumably in its ascending target, the Pf, would decrease gamma maintenance at the level of the cortex. However, with lithium treatment, appropriate gamma oscillation amplitudes are presumably restored in the PPN and Pf, among other regions. These suggestions provide a direction for determining, for example, the role of N-type and P/Q-type calcium channel expression in BD, which has not been explored. The role of intracellular pathways through CaMKII and cAMP/PKA in BD require further attention, as does the modulation of levels of NCS-1 and InsP3. For example, are the effects of NCS-1 mediated by its interaction with InsP3? Will blockade of InsP3 have similar effects as blocking NCS-1? These and additional questions remain, but the discoveries described herein provide, a) a physiological mechanism that accounts for many of the symptoms of the disease, and b) multiple novel directions for developing more effective treatments for BD.
Figure 3. Bottom-up Gamma in Bipolar Disorder.
Afferent input helps activate PPN neurons that manifest intrinsic gamma band oscillations through high threshold, voltage-dependent calcium channels. These cells project to the intralaminar thalamus (ILT)90,91 that also manifests these calcium channels to drive cortical activity by terminating in layers I and II. When NCS-1 is over expressed (NCS-1 with multiple ++), these oscillations are reduced, but Li+ therapy (Li+ + NCS-1) will restore intrinsic gamma band oscillations, perhaps both in the PPN (left side, red records) and ILT (right side, blue records), among other regions. We assume that too much Li+ will over inhibit NCS-1 and decrease oscillations, while too little Li+ will not overcome the suppression of oscillations by over expressed NCS-1. This suggests, in keeping with standard therapy, that concentrations of Li+ should be maintained at an optimal level.
Such efforts are important because disturbances in gamma band maintenance at the level of the PPN will disrupt a basic survival process. The ubiquitous generation of gamma band activity by all PPN neurons has been proposed, not to participate in the temporal process of sensory binding as in the cortex, but to underlie the more basic process of preconscious awareness13,27. Briefly, the act of waking up has a complex role since it needs to integrate the world with ourselves, while we use other parts of our brains to formulate our plans and desires. Preconscious awareness is a process in which we may not be paying attention to some of these plans and desires, that is, we are not consciously attending to a mass of information that we nevertheless process preconsciously. That is, the RAS is involved in subliminally formulating movements and actions of which we are not consciously (but only preconsciously) aware. We suggested that the manifestation of this process is “bottom-up gamma”26,27. This expands the role of the background of activity in the RAS as not only allowing afferent information to flow into the brain, but in establishing the background of activity on which we superimpose volition and free will, preconsciously. If this process is impaired in BD, the distortions in perception, attention, and formulation of actions will be massively disturbing.
5. CONCLUSION
PPN gamma activity may participate in a process that provides the essential stream of information necessary for the formulation of many of our actions. Preconscious awareness is the state that allows us to reliably assess the world around us on a continuous basis, providing a unifying picture and promoting survival. Although this process does not involve selective attention to specific stimuli, it provides the background upon which we assess our environment and act upon it. Without this function, our very survival is at risk. Interruptions in this process, therefore, will markedly disrupt perception as well as planning of motor strategies, adding uncertainty to the wildly shifting world of the BD patient. Dysregulation of preconscious awareness may not only disturb but create the rollercoaster mood swings in this condition.
Acknowledgments
FUNDING
Supported by NIH award P30 GM110702 from the IDeA program at NIGMS to the CTN, allowing the center to generate over $100 million in grant support for its members over the last 15 years. EGR would also like to express profound gratitude to all of the Federal funding agencies, especially NIH and NSF that have continuously funded his lab for the last 40 years. In addition, this work was supported by grants from FONCYT-Agencia Nacional de Promoción Científica y Tecnológica; Préstamo BID 1728 OC.AR. PICT-2016–1728 and Proyecto Unidad Ejecutora-Idea Proyecto P-UE # 22920170100062CO (to Dr. Urbano).
Footnotes
CONFLICT OF INTEREST
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.
REFERENCES
- 1.Harvey AG, Talbot LS, Gershon A. Sleep disturbances in bipolar disorder across the lifespan. Clin Psychol. 2009;16: 256–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadrmas A, Winokur G.Manic depressive illness and EEG abnormalities. J Clin Psychiat. 1979;40: 306–307. [PubMed] [Google Scholar]
- 3.Kupfer DJ, Foster FG, Coble P, McPartland RJ, Ulrich RF. The application of EEG sleep for the differential diagnosis of affective disorders. Amer J Psychiat. 1978;135: 69–74. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan KA, Williams R. Hypersomnia: an overlooked, but not overestimated, sleep disturbance in bipolar disorder. Evid based Mental Health. 2017;20: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hegerl U, Hensch T. The vigilance regulation model of affective disorders and ADHD. Neurosci Biobehav Rev. 2014;44:45–57. [DOI] [PubMed] [Google Scholar]
- 6.Ng TH, Chung KF, Ho FY, Yeung WF, Yung KP, et al. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: A systematic review and meta-analysis. Sleep Med Rev. 2015;20:46–58. [DOI] [PubMed] [Google Scholar]
- 7.Geoffroy PA, Scott J, Boudebesse C, Lajnef M, Henry C, et al. Sleep in patients with remitted bipolar disorders; a meta-analysis of actigraphy studies. Acta Psychiat Scand. 2015;131:89–99. [DOI] [PubMed] [Google Scholar]
- 8.Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. Amer J Psychiat 2005;162:43–49. [DOI] [PubMed] [Google Scholar]
- 9.Schulze KK, Hall M, McDonald C, Marshall N, Walshe M, et al. P50 auditory evoked potential suppression in bipolar disorder patients with psychotic features and their unaffected relatives. Biol Psychiat. 2007;62:121–128. [DOI] [PubMed] [Google Scholar]
- 10.Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiat. 2001;50:418–424. [DOI] [PubMed] [Google Scholar]
- 11.Depue RA, Arbisi P, Krauss S, Iacono WG, Leon A, et al. Seasonal independence of low prolactin concentration and high spontaneous blink rates in unipolar and bipolar II seasonal affective disorder. Arch Gen Psychiat. 1990;47:356–364. [DOI] [PubMed] [Google Scholar]
- 12.Ozerdem A, Guntenkin B, Atagun I, Turp B, Basar E. Reduced long distance gamma (28–48 Hz) coherence in euthymic patients with bipolar disorder. J Affect Disord. 2011;132:325–332. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Rill E Waking and the Reticular Activating System. Academic Press, New York, USA, 2015;pp. 330. [Google Scholar]
- 14.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroenceph Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 15.Watson RT, Heilman KM, Miller BD. Neglect after mesencephalic reticular formation lesions. Neurology 1974;24:294–298. [DOI] [PubMed] [Google Scholar]
- 16.Vanderwolf CH. Are neocortical gamma waves related to consciousness? Brain Res. 2000;855:217–224. [DOI] [PubMed] [Google Scholar]
- 17.Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, et al. Visual areas exert feedforward and feedback through distinct frequency channels. Neuron 2015;85:390–340. [DOI] [PubMed] [Google Scholar]
- 18.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, et al. Coherent oscillations: a mechanism of feature linking in the visual system? Biol Cybern. 1988;60:121–130. [DOI] [PubMed] [Google Scholar]
- 19.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Nat Acad Sci USA. 1989;86:1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philips S, Takeda Y. Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient then efficient visual search in human EEG. Int J Psychophysiol. 2009;73:350–354. [DOI] [PubMed] [Google Scholar]
- 21.Palva S, Monto S, Palva JM. Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage 2009;49:3257–3268. [DOI] [PubMed] [Google Scholar]
- 22.Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci USA. 2002;99:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llinás RR, Paré D. Of dreaming and wakefulness. Neuroscience 1991;44:521–535. [DOI] [PubMed] [Google Scholar]
- 24.Stam CJ, van Cappellen van Walsum AM, Pijnenburg YA, Berendse HW, et al. Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19:562–574. [DOI] [PubMed] [Google Scholar]
- 25.Uhlhaas PJ, Singer W. High-frequency oscillations and the neurobiology of schizophrenia. Dial Clin Neurosci. 2013;15:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Rill E, Kezunovic N, D’Onofrio S, Luster B, Hyde J, et al. Gamma band activity in the RAS-intracellular mechanisms. Exp Brain Res. 2014;232:1509–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbano FJ, D’Onofrio SM, Luster BR, Hyde JR, Bisagno V, et al. Pedunculopontine nucleus gamma band activity-preconscious awareness, waking, and REM sleep. Frontiers in Sleep and Chronobiol. 2014;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedroarena C, Llinás RR. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc Natl Acad Sci USA. 1997;94:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charpak S, Paré D, Llinás RR. The entorhinal cortex entrains fast CA1 hippocampal oscillations in the anaesthetized guinea-pig: role of the monosynaptic component of the perforant path. Eur J Neurosci. 1995;7:1548–1557. [DOI] [PubMed] [Google Scholar]
- 30.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 2009;462:353–357. [DOI] [PubMed] [Google Scholar]
- 31.Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci. 1999;19:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology (Bethesda) 2010;25:319–329. [DOI] [PubMed] [Google Scholar]
- 33.Lang EJ, Sugihara I, Llinás RR. Olivocerebellar modulation of motor cortex ability to generate vibrissal movements in rat. J Physiol (Lond). 2006;571:101–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Middleton SJ, Racca C, Cunningham MO, Traub RD, Monyer H, et al. High-frequency network oscillations in cerebellar cortex. Neuron 2008;58:763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soteropoulos DS, Baker SN. Cortico-cerebellar coherence during a precision grip task in the monkey. J Neurophysiol. 2006;95:1194–1206. [DOI] [PubMed] [Google Scholar]
- 36.Timofeev I, Steriade M. Fast (mainly 30–100 Hz) oscillations in the cat cerebellothalamic pathway and their synchronization with cortical potentials. J Physiol (Lond). 1997;504:153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalo E, Thobois S, Sharott A, Polo G, Mertens P, et al. Patterns of bidirectional communication between cortex and basal ganglia during movement in patients with Parkinson disease. J Neurosci. 2008;28:3008–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheyne G, Ferrari P. MEG studies of motor cortex gamma oscillations: evidence for a gamma “fingerprint” in the brain? Frontiers Human Neurosci. 2013;7:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkinson N, Kuhn AA, Brown P. Gamma oscillations in the human basal ganglia. Exp Neurol. 2013;245:72–76. [DOI] [PubMed] [Google Scholar]
- 40.Steriade M, Paré D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10: 2560–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kayama Y, Ohta M, Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. [DOI] [PubMed] [Google Scholar]
- 42.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:79–82. [DOI] [PubMed] [Google Scholar]
- 43.Datta S, Siwek DF, Stack EC. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neuroscience. 2009;163:397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fraix V, Bastin J, David O, Goetz L, Ferraye M, et al. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLOS ONE. 2013;8:e83919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goetz L, Piallat B, Bhattacharjee M, Mathieu H, David O, et al. The primate pedunculopontine nucleus region: towards a dual role in locomotion and waking state. J Neural Transm. 2016;123:667–678. [DOI] [PubMed] [Google Scholar]
- 47.Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, et al. Gamma band unit activity and population responses in the pedunculopontine nucleus. J Neurophysiol. 2010;104:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, et al. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN). Eur J Neurosci. 2011;34:404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Rill E, Kezunovic N, Hyde J, Beck P, Urbano FJ. Coherence and frequency in the reticular activating system (RAS). Sleep Med Rev. 2013;17:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urbano FJ, D’Onofrio SM, Luster BR, Hyde JR, Bisagno V, et al. Pedunculopontine nucleus gamma band activity-preconscious awareness, waking, and REM sleep. Frontiers Sleep Chronobiol. 2014;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hyde JR, Kezunovic N, Urbano FJ, Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol. 2013;115:1402–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luster B, D’Onofrio S, Urbano FJ, Garcia-Rill E. High-Threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN). Physiol Rep. 2015;3:e12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luster B, Urbano FJ, Garcia-Rill E. Intracellular mechanisms modulating gamma band activity in the pedunculopontine nucleus (PPN). Physiol Rep. 2016;4:e12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Rill E, Luster B, D’Onofrio S, Mahaffey S, Bisagno V, et al. (2015) Implications of gamma band activity in the pedunculopontine nucleus. J Neural Transm 123: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Rill E, Luster B, Mahaffey S, MacNicol M, Hyde JR, et al. Pedunculopontine gamma band activity and development. Brain Sci. 2015;5:546–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Datta S, Spoley EE. Patterson, E.H. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Amer J Physiol Reg Integ Comp Physiol. 2001; 280:R752–R759. [DOI] [PubMed] [Google Scholar]
- 57.Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental nmda receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2002;66:109–116. [DOI] [PubMed] [Google Scholar]
- 58.Datta S Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol. 2002;87:1790–1798. [DOI] [PubMed] [Google Scholar]
- 59.Stea A, Soomg TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal Ca2+ channels. Neuron 1995;15:929–940. [DOI] [PubMed] [Google Scholar]
- 60.Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, et al. Modulation of Cav2.1 channels by Ca+/calmodulin-dependent kinase II bound to the C-terminal domain. Proc Nat Acad Sci USA. 2008;105:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castro S, Falconi A, Chase M, Torterolo P. Coherent neocortical 40-Hz oscillations are not present during REM sleep. Eur J Neurosci. 2013;37: 1330–1339. [DOI] [PubMed] [Google Scholar]
- 62.Torterolo P, Castro-Zaballa S, Cavelli M, Chase M, Falconi A. Neocortical 40 Hz oscillations during carbachol-induced rapid eye movement sleep and cataplexy. Eur J Neurosci. 2015;281:318–325. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Rill E, D’Onofrio S, Mahaffey S. Bottom-up Gamma: the Pedunculopontine Nucleus and Reticular Activating System. Transl Brain Rhythm. 2016;1:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia-Rill E, Virmani T, Hyde JR, D’Onofrio S, Mahaffey S. Arousal and the control of perception and movement. Curr Trends Neurol. 2016;10:53–64. [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura TY, Sturm E, Pountney DJ, Orenzoff B, Artman M, et al. Developmental expression of NCS1 (frequenin), a regulator of Kv4 K+ channels, in mouse heart. Pediatr Res. 2003;53:554–557. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura TY, Jeromin A, Mikoshiba K, Wakabayashi S. Neuronal calcium sensor-1 promotes immature heart function and hypertrophy by enhancing Ca2+ signals. Circ Res. 2011;109:512–523. [DOI] [PubMed] [Google Scholar]
- 67.Kasri NN, Holmes AM, Bultynck G, Parys JB, Bootman MD, et al. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J. 2004;23:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodrigo J, Suburo AM, Bentura ML, Fernández T, Nakade S, et al. Distribution of the inositol 1,4,5-trisphosphate receptor, P400, in adult rat brain. J Comp Neurol. 1993;337:493–517. [DOI] [PubMed] [Google Scholar]
- 69.Hendricks KB, Wang BQ, Schnieders EA, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol. 1999;1:234–241. [DOI] [PubMed] [Google Scholar]
- 70.Rajebhosale M, Greenwood S, Vidugiriene J, Jeromin A, Hilfiker S. Phosphatidylinositol 4-OH Kinase is a downstream target of neuronal calcium sensor 1 in enhancing exocytosis in neuroendocrine cells. J Biol Chem. 2003;278:6075–6084. [DOI] [PubMed] [Google Scholar]
- 71.Lian LY, Pandalaneni SR, Patel P, McCue HV, Hayesn LP, et al. Characterisation of the interaction of the C-Terminus of the Dopamine D2 Receptor with Neuronal CalciumSensor-1. PLoS ONE. 2011;6:e27779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rousset M, Cens T, Gavarini S, Jeromin A, Charnet P. Down-regulation of voltage-gated Ca2+ channels by neuronal calcium sensor-1 is beta subunit-specific. J Biol Chem. 2003;278:7019–7026. [DOI] [PubMed] [Google Scholar]
- 73.Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 2002;295:2276–2279. [DOI] [PubMed] [Google Scholar]
- 74.Weiss JL, Archer DA, Burgoyne RD. NCS-1/frequenin functions in an autocrine pathway regulating Ca2+ channels in bovine adrenal chromaffin cells. J Biol Chem. 2000;275:40082–40087. [DOI] [PubMed] [Google Scholar]
- 75.Guo W, Malin SA, Johns DC, Jeromin A, Nerbonne JM. Modulation of Kv4-encoded K+ currents in the mammalian myocardium by neuronal calcium sensor-1. J Biol Chem. 2002;277:26436–26443. [DOI] [PubMed] [Google Scholar]
- 76.D’Onofrio S, Kezunovic N, Hyde JR, Garcia-Rill E. Modulation of gamma oscillations in the pedunculopontine nucleus (PPN) by neuronal calcium sensor protein-1 (NCS-1): relevance to schizophrenia and bipolar disorder. J Neurophysiol. 2015;113:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.D’Onofrio S, Urbano FJ, Messias E, Garcia-Rill E. Lithium decreases the effects of neuronal calcium sensor protein 1 in pedunculopontine neurons. Physiol Rep. 2016;4: e12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Handley MT Lian LY, Haynes LP, Burgoyne RD. Structural and functional deficits in a neuronal calcium sensor-1 mutant identified in a case of autistic spectrum disorder. PLoS ONE. 2010;5:E10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leboyer M, Kupfer DJ. Bipolar disorder; new perspectives in health care and prevention. J Clin Psychiatry. 2010;71:1689–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldberg JF, Chengappa KN. Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disord. 2009;11:123–137. [DOI] [PubMed] [Google Scholar]
- 81.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. [DOI] [PubMed] [Google Scholar]
- 82.Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic P, et al. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci USA. 2003;100:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brown KM, Tracy DK. Lithium: the pharmacodynamics actions of the amazing ion. Ther Adv Psychopharmacol. 2012;3:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schlecker C, Boehmerle W, Jeromin A, DeGray B, Varshney A, et al. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of Li+. J Clin Invest. 2006;116:16668–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cadet JL. Epigenetics of Stress, Addiction, and Resilience: Therapeutic Implications. Mol Neurobiol. 2016;53:545–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Didonna A, Opal P. The promise and perils of HDAC inhibitors in neurodegeneration. Ann Clin Transl Neurol. 2015;2:79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Subramanian S, Bates SE, Wright JJ, Espinoza-Delgado I, Piekarz RL. Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals (Basel). 2010;3:2751–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Urbano FJ, Bisagno V, Mahaffey S, Garcia-Rill E. Class II histone deacetylases require P/Q-type Ca2+ channels and CaMKII to maintain gamma oscillations in the pedunculopontine nucleus. Sci Rep. 2018;8:13156.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kezunovic N, Hyde J, Simon C, Urbano FJ, Williams DK, et al. Gamma band activity in the developing parafascicular nucleus (Pf). J Neurophysiol. 2012;107:772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Visualization of fast calcium oscillations in the parafascicular nucleus. Pflugers Arch. 2013;465:1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]