Executive summary
Inspired by unprecedented improvements in human health and development in recent decades, our world has embarked on a quest that only a generation ago would have been considered unreachable—achieving sustainable health and development for all. Improving the health and wellbeing of the world’s people is at the core of the Sustainable Development Goals (SDGs), reflected in targets that call for ending the epidemics of AIDS, tuberculosis, and malaria; achieving enormous improvements in maternal and child health; and tackling the growing burden of non-communicable diseases (NCDs). Attaining universal health coverage is the means by which these ambitious health targets are to be achieved.
Although on their face, the SDGs reflect an unprecedented level of global solidarity and resolve, the trends that increasingly define our world in 2018 are inconsistent with both the sentiments that underlie the SDGs and the ethos that generated such striking health and development gains in recent years. Democracy is in retreat, and in many countries the space for civil society is declining and the human rights environment deteriorating. Official development assistance for health has stalled, as an inward-looking nationalism has in many places supplanted recognition of the need for global collaboration to address shared challenges. The loss of momentum on global health ignores the urgent need to strengthen health systems to address the steady growth of NCDs, which now account for seven of ten deaths worldwide.
Recent trends in the HIV response are especially concerning. Although the number of new HIV infections and AIDS-related deaths have markedly decreased since the epidemic peaked, little progress has been made in reducing new infections in the past decade. Without further reductions in HIV incidence, a resurgence of the epidemic is inevitable, as the largest ever generation of young people age into adolescence and adulthood. Yet where vigilance and renewed efforts are needed, there are disturbing indications that the world’s commitment is waning. Allowing the HIV epidemic to rebound would be catastrophic for the communities most affected by HIV and for the broader field of global health. If the world cannot follow through on HIV, which prompted such an extraordinary global mobilisation, hopes for achieving the ambitious health aims outlined in the SDGs will inevitably dim.
At this moment of uncertainty for the future of the HIV response and for global health generally, the International AIDS Society and The Lancet convened an international Commission of global experts and stakeholders to assess the future of the HIV response in the context of a more integrated approach to health. A central finding of the Commission is that the HIV epidemic is not on track to end and that existing tools are insufficient. Although antiretroviral therapy (ART) has transformed the HIV response by averting deaths, improving quality of life, and preventing new HIV infections, HIV treatment alone will not end the epidemic. The UNAIDS 90–90-90 approach must be accompanied by a similarly robust commitment to scaled-up primary HIV prevention and to the development of a preventive vaccine and a functional cure for HIV. Ironically, the diminishing energy on HIV is occurring at the moment when lessons learned during the HIV response could serve as pathfinders in the quest for sustainable health for all.
From its inception, the HIV response was a unique undertaking, apart from the broader health system. Although elements of a disease-specific approach will and should be retained, the future of the HIV response will also depend on finding opportunities for integrating HIV services more closely within health systems. Wholesale abandonment of vertical HIV funding would involve considerable risks, as the laser-like focus on a single disease accounts in large measure for the HIV response’s successes. Unique attributes that have defined the HIV response (including its multisectoral and inclusive approach, engagement of civil society, emphasis on equity and human rights, galvanisation of scientific innovation, and foundation of global collaboration and problem solving) must be preserved and mainstreamed across global health practice.
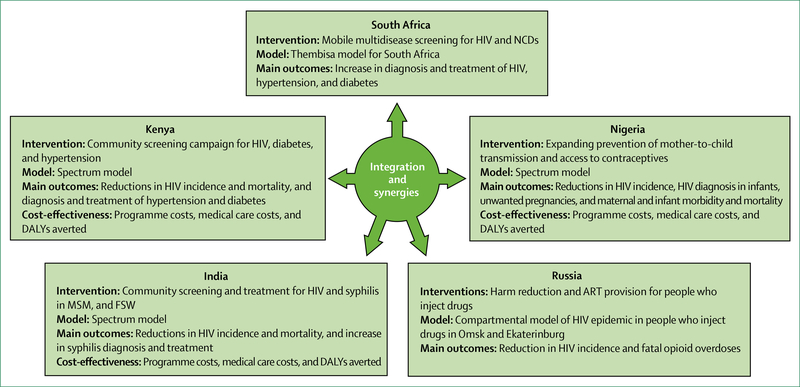
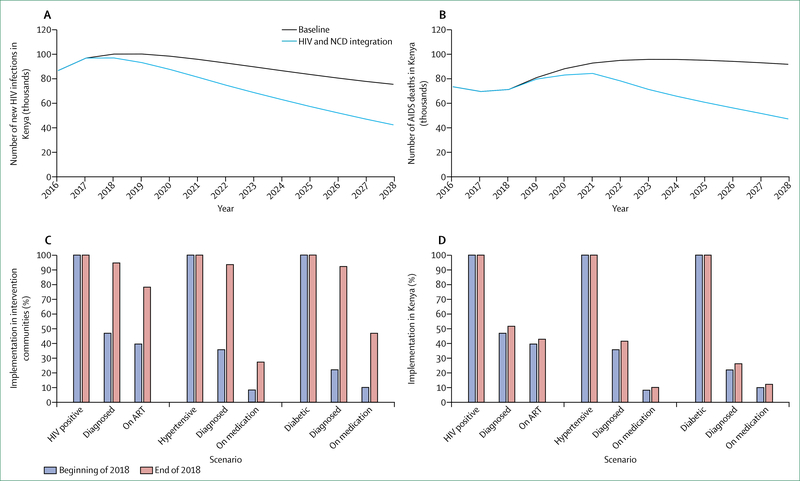
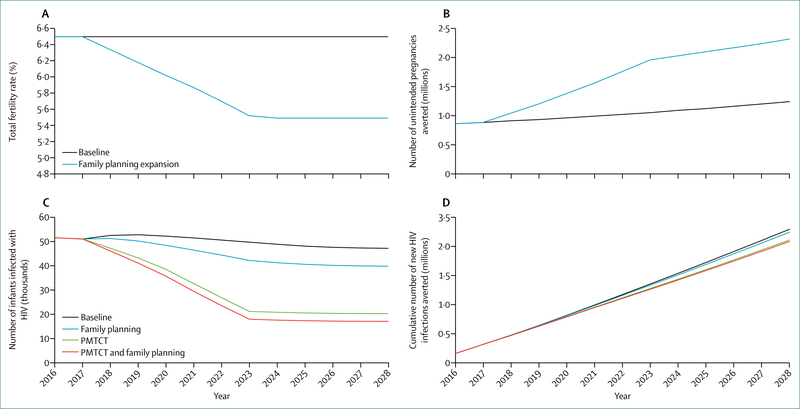
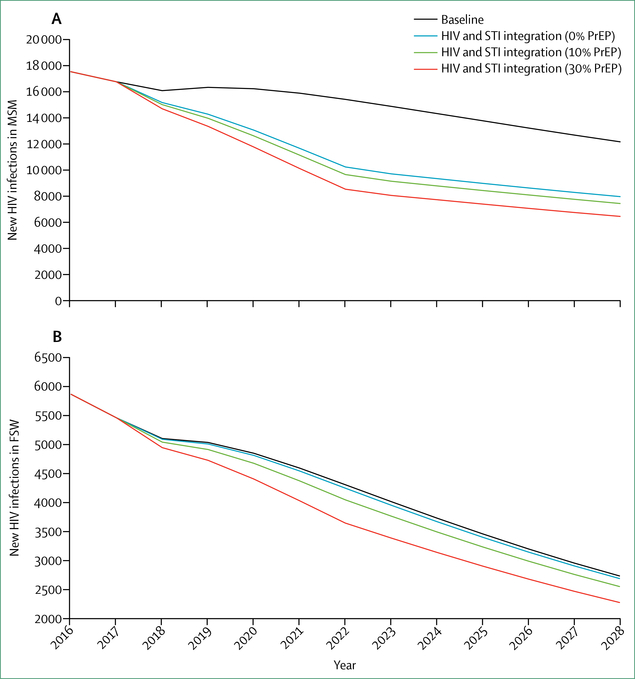
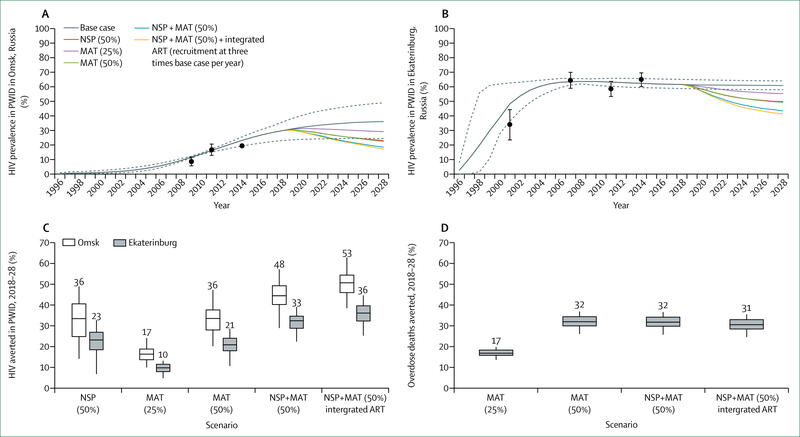
Whether to integrate HIV within broader health systems is not an either-or choice, and optimal paths will differ between settings, populations, and services. To be effective, more integrated approaches must yield improvements both to HIV-related and non-HIV-related health outcomes. In most cases, approaches to integration will and should be incremental, allowing learning by doing. To assess the health and financial benefits of such win-win scenarios, the Commission engaged modellers to examine different scenarios for incremental integration of HIV-related and non-HIV-related services. These include: models in South Africa and Kenya for screening of HIV alongside screening for diabetes, hypertension, and other NCDs; integration of HIV in reproductive health services in Nigeria; integrated management of HIV and sexually transmitted infections in India; and integration of harm reduction and overdose services and ART for people who use drugs in Russia. In each of these scenarios, integrated approaches generated concrete improvements in HIV and broader health outcomes. With one exception (antiretroviral pre-exposure prophylaxis [PrEP] in India), integrated models were consistently found to be cost-effective.
The HIV community must make common cause with the global health field— to make universal health coverage a reality, to substantially increase the share of resources devoted to health, and to build worldwide recognition of health as key to progress across the breadth of the SDGs. The global health field must take a leading role in resisting the turn towards authoritarianism, xenophobia, and austerity with respect to essential public health investments. In a time of fragmentation and uncertainty, the global health field can aid in reminding all of us of our common humanity. Health systems must be designed to meet the needs of the people they serve, including having the capacity to address multiple health problems simultaneously. No one can be left behind in our efforts to achieve sustainable health. Recognising health as an investment, major new resources (from national governments, the international community, and the private sector, involving innovative financing mechanisms) must be mobilised to support stronger, sustainable, and people-centred health systems.
Global health and HIV
The SDGs sharply elevate global health and development aspirations, contemplating a world that is far more prosperous, secure, healthy, and equitable, where human rights and dignity are universally respected, and where human development unfolds in a manner that preserves the natural environment. Yet, the 3 years that have passed since the SDGs were agreed have dimmed prospects for achieving many of these visionary aims. By contrast with the international solidarity, shared commitment, and increased investments that characterised the era of the Millennium Development Goals (MDGs), much of the world has, since 2015, turned inward and toward authoritarianism, repression, a diminished role for civil society, a policy of austerity for public investments, and suspicion of international cooperation. As a result of civil conflicts that have yet to elicit an appropriate international response, more people than ever have been forced from their homes and countries. At a time when the fruits of scientific advances are so evident, denial in many quarters of the role of humankind in the degradation of our environment threatens the very health and wellbeing of our planet and our civilisations.
Among the reasons why the world opted for such an ambitious agenda for the SDGs was the success of the HIV response. As a result of a worldwide mobilisation, the incidence of HIV infections peaked and began to decrease in all parts of the world, and AIDS-related mortality decreased from 1–9 million in 2005 to 1–0 million in 2016.1 The HIV response has not been an unalloyed story of achievement, as the world’s capacity to respond effectively to the epidemic has been undermined by 15 years of relative inaction in the epidemic’s early stages, an approach to epidemic management that has undervalued primary prevention, and the enduring stigma associated with HIV.
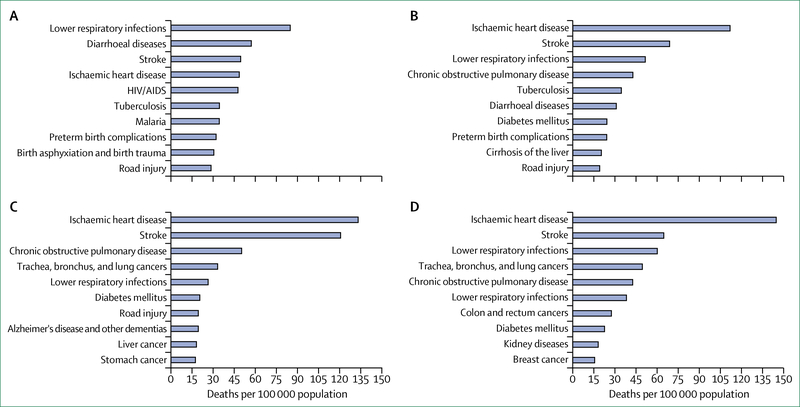
The broader global health community, facing both historic opportunities and profound challenges, could potentially benefit from lessons learned from the successes and failures of the global HIV response. With its multisectoral and inclusive approach, mobilisation of political commitment, engagement of civil society at every level, emphasis on equity and human rights, galvanisation of scientific innovation, and foundation of global collaboration and problem solving, the HIV response has properly been cited as a model for the future of global health.2 The global health challenge remains immense, with millions of people in low-income and middle-income countries (LMICs) dying each year from causes that have either been largely eradicated or are decreasing in prevalence in high-income countries (figure 1).3 Whether the world is prepared to meet these challenges is unclear. Although the incidence of communicable, maternal, neonatal, and nutritional diseases have decreased worldwide since 1980,3 the most recent projections indicate that financial resources available for health programmes in LMICs are likely to fall far short of amounts needed to reach the health targets set forth in the SDGs.4 Persistent weaknesses of health systems undermine prospects for progress in addressing the full panoply of health challenges.
Figure 1: Ten leading causes of death in 2015.
(A) Low-income economies. (B) Low-middle-income economies. (C) Upper-middle-income economies. (D) High-income economies. Source: WHO.
At the very moment when HIV could serve as a pathfinder for global health, there are signs that global commitment to build on the gains achieved against HIV thus far is waning. From 2013 to 2016, international HIV assistance was reduced by roughly 20%, from almost US$10 billion to US$8·1 billion.5
Relinquishing the fight against HIV before it is over would have disastrous consequences, both for people affected by HIV and for the broader global health community. Unless further investments are made to accelerate expansion of HIV prevention and treatment programmes, the HIV epidemic is likely to rebound and grow far more serious in the coming years, especially as the world’s largest-ever cohort of young people age into adolescence and young adulthood.6 Notwithstanding the enormous progress that has been made in the HIV response, HIV remains “the epidemic of our time”.7 In 2015–16, an estimated 36·7–38·8 million people were living with HIV worldwide, including 1·9–2·5 million newly infected in 2015.1,8 More than 35 million people have died of AIDS-related causes; 1·0 million of these deaths were in 2016.9 A refusal to follow through to achieve long-term control of the epidemic would merely repeat a longstanding pattern in global health, when failure to sustain a surge in global interest in combating particular health threats allows these epidemics to return in force. The history of malaria elimination efforts is a case in point, as the failure to sustain malaria-related control programmes, funding, and research investments led to an abandonment of the global malaria elimination campaign in 1969 and subsequent increases in the global malaria burden.10 At a moment when the means to improve human health are greater than ever, allowing a resurgence of HIV through neglect and apathy could deal a blow from which the broader cause of global health could need decades to recover.
The relationship between the HIV response and the broader global health field is multilayered and bidirectional. Even as the HIV response offers important lessons from which global health can learn, it is also clear that controlling the HIV epidemic will depend in large measure on the broader global health and development fields. However, the exceptionalist approach to the HIV epidemic, in which the HIV response has often unfolded as a vertical undertaking, distinct from other health programmes, has achieved historic results and should not be jettisoned lightly.
Both the HIV response and the broader global health field share a commitment to the development of health systems that are capable of addressing several health challenges at the same time. In many settings, robust, if still flawed, service systems have been developed for certain populations (eg, pregnant women, children) or for priority health conditions (eg, maternal and child health, HIV, and other communicable diseases). However, health systems as a whole are largely unprepared for providing care that is holistic, universal, and well coordinated. In the still-early years of the SDG era, the gap between reality and the vision of sustainable health remains gaping.
In the midst of uncertainty about the long-term feasibility of an exceptionalist HIV approach and the prospects for achieving the lofty health targets in the SDGs, the International AIDS Society (IAS)-Lancet Commission on the Future of Global Health and the HIV Response was established in 2016 to critically examine future prospects for global health and the HIV response. The Commission was tasked with assessing the future of the HIV response in a more integrated global health and development agenda, with the aim of advising how best to achieve global control of the HIV pandemic in an era in which health and development priorities are proliferating. The Commission studied the history of the HIV response to discern how experience in responding to HIV might inform and strengthen global health more broadly. Modelling exercises were undertaken to assess the effect of various approaches to improve integration of HIV and non-HIV-related services. The most salient threats to global health and to the goal of universal health coverage were identified.
With this report, we summarise the findings of the Commission, and we seek to articulate a vision for the future of the HIV response and global health that builds common cause across health and development movements and sectors. Rather than despair over the trends and patterns of the past several years, we must instead look to the extraordinary achievements of the past two decades to embolden us and reinforce our resolve to rejuvenate the HIV response and strengthen the broader cause of global health. By taking on board the lessons of the HIV response, the global health field can be made fit for the purpose of realising the vision of sustainable health for all. Global health can serve as a driving force to repudiate and discredit the continuing retreat from international solidarity, human rights, reason, scientific evidence, and open societies. Global health can serve as a pioneer in a re-engineering of the development project, from one based on charity from the high-income countries to one that tackles the central determinants of global health inequities. Just as the HIV response has demonstrated that global problems demand global solutions, the future health and wellbeing of our planet relies on us to recognise, celebrate, and build on our common humanity.
With respect to the flagging response to HIV, the Commission hopes that this report serves as a wake-up call. Without a thorough rejuvenation of the HIV response and a change of course, we are likely to see a resurgence of the epidemic. After such history-making successes from unprecedented global solidarity and collaboration, the world can and must do better.
To realise the vision of sustainable health for all, we must ensure that health systems are equipped to bring communicable diseases under control and to respond effectively to the growing burden of NCDs. A focused response and categorical HIV funding will remain crucial to avoid a resurgence of HIV and to bring the global pandemic under control. However, immediate and incremental steps are needed to strategically integrate HIV services into co-located primary care platforms and toward the longer-term goal of creating fully integrated, co-located, and patient-centred health-service systems.
Global Health in 2018: new ambitions and growing threats
The Agenda for Sustainable Development envisages “a world free from poverty, hunger, disease and want, where all life can thrive.”11 SDG 3 calls for concerted action to ensure healthy lives and promote wellbeing for all at all ages.11 SDG 3 also calls for ending the epidemics of AIDS, tuberculosis, malaria, and neglected tropical diseases; eliminating preventable deaths in children younger than 5 years; and reducing by a third the number of deaths from NCDs.11 Under the Agenda for Sustainable Development, universal health coverage serves as the primary vehicle for continuing and fully leveraging the momentum on health.11
The health targets of SDG 3 build on historic gains made under the MDGs (panel 1).12 The health gains during the MDG era coincided with, and were enabled by, advances across the broader development agenda. Whereas nearly half of the population in LMICs lived on less than $1·25 per day in 1990, this proportion had fallen to 14% by 2015.12 Primary school attendance worldwide increased between 2000 and 2015, and differences in secondary school attendance between boys and girls diminished or disappeared altogether in some regions.12
Panel 1: Health targets for Sustainable Development Goal 3.
By 2030, reduce the global maternal mortality ratio to less than 70 deaths per 100 000 livebirths
By 2030, end preventable deaths of newborn babies and children younger than 5 years, with all countries aiming to reduce neonatal mortality to at least as low as 12 deaths per 1000 livebirths and under-5 mortality to at least as low as 25 deaths per 1000 livebirths
By 2030, end the epidemics of AIDS, tuberculosis, malaria, and neglected tropical diseases and combat hepatitis, water-borne diseases, and other communicable diseases
By 2030, reduce by a third premature mortality from non-communicable diseases through prevention and treatment, and promote mental health and wellbeing
Strengthen the prevention and treatment of substance abuse, including narcotic drug abuse and harmful use of alcohol
By 2020, halve the number of deaths and injuries from road traffic accidents globally
By 2030, ensure universal access to sexual and reproductive health-care services, including for family planning, information, and education and ensure the integration of reproductive health into national strategies and programmes
Achieve universal health coverage, including financial risk protection, access to quality and essential health-care services, and access to safe, effective, quality, and affordable essential medicines and vaccines for all
By 2030, substantially reduce the number of deaths and illnesses from hazardous chemicals and air, water, and soil pollution and contamination
Strengthen the implementation of the WHO Framework Convention on Tobacco Control in all countries, as appropriate
Support the research and development of vaccines and medicines for the communicable and non-communicable diseases that primarily affect developing countries, provide access to affordable essential medicines and vaccines, in accordance with the Doha Declaration on the TRIPS Agreement and Public Health, which affirms the right of developing countries to use to the full the provisions in the Agreement on Trade-Related Aspects of Intellectual Property Rights regarding flexibilities to protect public health, and, in particular, provide access to medicines for all
Substantially increase health financing and the recruitment, development, training, and retention of the health workforce in developing countries, especially in least developed countries and small-island developing states
Strengthen the capacity of all countries, in particular developing countries, for early warning, risk reduction, and management of national and global health risks
Decreasing prominence of health on the global political stage
Whereas health occupied three of the eight MDGs, health is specifically addressed in only one of 17 SDGs and ten of 169 SDG targets. Effective measures to improve global health outcomes draw on principles of international solidarity and shared responsibility, recognising that all of us, irrespective of where we live, have a stake in improving the health of our planet and its people. Yet growing hostility towards globalisation, which has become especially pronounced in many countries that have long served as global health donors, poses a potential threat to all forms of international and multilateral cooperation, including global collaboration to address health and development challenges. As threats to global solidarity have intensified, stresses on such multilateral institutions as the European Union (EU) have become more apparent.
Substantial additional health investments will be necessary to achieve the SDG 3 targets. Yet prospects for mobilising such sums appear in doubt as global assistance for health has flattened since 2010.13 Although domestic investments in health by LMICs have generally increased since 2000,14 these investments frequently fall short of leaders’ stated ambitions. In the Abuja Declaration of 2001, African heads of state committed to allocate 15% of government budget to health, yet most countries are not reaching this agreed benchmark.15 Economic growth in LMICs (estimated at 3·9% in 201616), if sustained, has the potential to expand the fiscal space for investments in health if sufficient political commitment exists. However, most low-income countries will for the foreseeable future lack the capacity to fully finance health and development initiatives on their own, underscoring the continued need for robust and sustained international assistance.17
Weak and dysfunctional health systems and the future of global health
Health systems in much of the world appear unprepared to realise the vision of sustainable health for all. As recently as 2013, 37 of 49 countries in Africa did not have a single medical laboratory that satisfied international quality assurance standards.18 In 2013, the number of health workers needed to provide essential health services fell 17·4 million short of the number needed, with the most severe shortages occurring in Africa and southeast Asia.19 In Africa, health worker shortages are expected to worsen in the coming years.19
Weak and overburdened health systems that are already struggling to provide basic services will confront vastly more serious stresses in the coming years. The global population is projected to grow from 7·6 billion people in 2017, to 9 8 billion people by 2050, with the sharpest increase set to occur in Africa, where the population will roughly triple in size.20 Many health systems are unprepared to carry through on the SDG 3 target of reducing NCD-associated premature mortality by a third. Although LMICs are home to 70% of global cancer deaths, they account for only 5% of cancer spending world-wide.21 Some countries in Africa have no clinical oncologists, whereas India reportedly had only 29 treatment centres to manage cancer for more than 1 billion people in 2010.22
Confronting the inherent inequities of the international order
The narrowing of economic disparities between rich and poor countries represents one of the signal achievements of our era, but yawning inequities in access to resources between countries and regions persist. In 2016, the per-capita gross domestic product (GDP) in North America was more than 34 times higher than in south Asia or sub-Saharan Africa and nearly 92 times higher than in low-income countries generally.23 These extraordinary disparities in access to the fruits of the global economy are inevitably reflected in health outcomes because lower socioeconomic status is closely correlated with poorer health.24
In addition to differences between countries, persistent social and economic inequalities within countries increase vulnerability to disease and diminish service access. In diverse countries, lower-income households consistently have poorer access to health care than the more affluent and experience comparatively greater morbidity and mortality.25
Existing global health mechanisms and practices fail to ensure ready, equitable access to international public goods such as medicines and diagnostics. Although the 2001 Doha Declaration on TRIPS and Public Health recognised the flexibility of countries under international law to obtain access to essential medicines, the global community has yet to find a workable balance between trade and the right to health. Unfortunately, the HIV response, which achieved a 99% reduction in the annual cost of first-line ART between 2000 and 2015, largely remains an outlier with respect to medicines access in resource-limited settings. Affordable diagnostic tools are also often in short supply. Nearly half of African countries have no cancer radiotherapy services, leaving four of five Africans without access to radiotherapy.26
The 2014 Ebola outbreak in west Africa underscored the ongoing crisis of global health governance.27 Although the International Health Regulations were designed in part to discourage restrictive and coercive responses to health emergencies, nearly a quarter of the WHO member states imposed restrictions on trade and travel in response to the Ebola outbreak.28 WHO, a cornerstone for global health governance, remains chronically underfunded and hobbled by a decentralised structure, which often inhibits rapid and effective responses to emerging health crises.29
Deteriorating environment for human rights, sound governance, and global cooperation
The underlying political and social environment is shifting in ways that are inimical to good health and to the development of sound and sustainable health systems. After years in which the proportion of the world’s people living in free and democratic societies increased, freedom is now in retreat, with one global freedom index reporting the 11th consecutive year of decline in 2017.30 Accompanying this democratic retreat is growing official hostility toward civil society in many countries.30
The increase in authoritarianism and the decrease in adherence to democratic norms also risk normalising human rights violations and degrading universal human rights commitments. The global retreat on human rights has exacted an especially heavy toll on migrants and other disenfranchised groups. In 2015, the global population of displaced people was greater than ever (21 million people) as raging conflicts in Syria and elsewhere have compelled millions of people to flee their homeland.31 More recently, government-sanctioned violence against the Rohingya minority in Myanmar has caused hundreds of thousands of people to seek sanctuary in neighbouring countries.
Discriminatory practices within health systems, which frequently mirror prejudices prevalent in the broader population, prevent many from accessing the most basic health services. Migrants, indigenous populations, and ethnic minorities often encounter hostility from health providers (including, in the case of migrants, formalized exclusion of non-citizens from health-care services).25 Lesbian, gay, bisexual, and transgender people worldwide experience considerable difficulties in accessing good quality and non-judgmental health services.32
Threats to planetary health
While political, social, and economic patterns threaten the foundation of global health, continued deterioration of the physical environment potentially poses an existential threat to the planet.33 The planet is warming because of human activity, and failure to take swift action to arrest or slow the growing concentration of manmade greenhouse gases is projected to lead to further devastating increases in temperature in the coming decades. Threatening the very habitability of our planet, these trends will have powerful effects on health, affecting food production, susceptibility to heat-related illnesses and natural disasters, and patterns for vector-borne and water-borne diseases.
The world’s response to climate change in many respects serves as a fundamental test case for international action to address shared threats. In this respect, recent trends are not promising. In 2018, authoritative reports on climate trends indicated that no developed country is on track to meet its pledges under the Paris Agreement and that current warming trends suggest that the global temperature will rise in coming decades well beyond the maximum threshold set by the Paris Agreement.34
Towards sustainable health for all: a status report
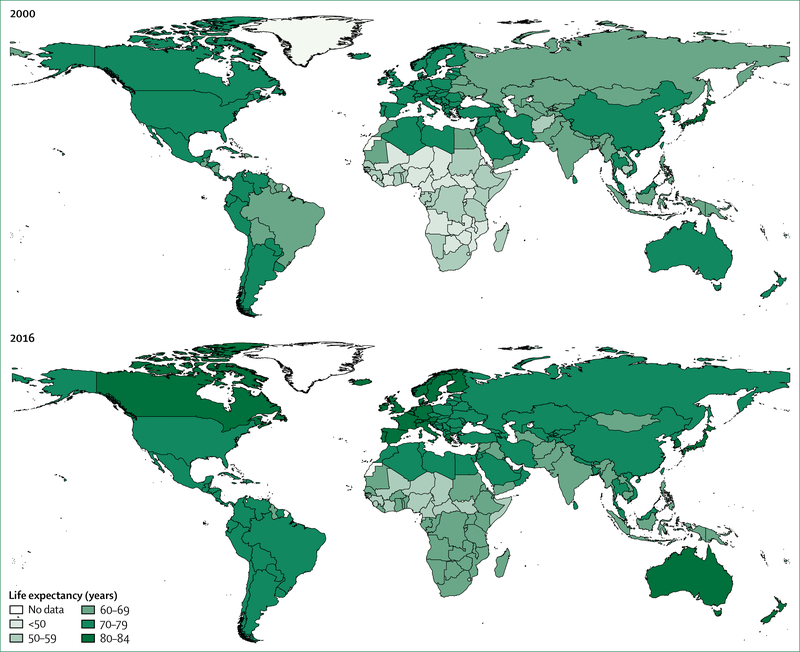
As a result of remarkable increases in health spending,35 the much healthier since 1990. Under-5 mortality decreased from 90 deaths per 1000 livebirths in 1990, to 43 deaths per 1000 livebirths in 2015, the proportion of children younger than 5 years who are underweight decreased from 25% to 14%, and maternal mortality decreased by 45% worldwide.12 Adolescent mortality is estimated to have decreased by about 17% since 2000. Notable reductions in the incidence of new infections and deaths associated with HIV, tuberculosis, and malaria between 2000 and 2015 led the UN to declare that these epidemics had been halted and reversed.12 Globally, life expectancy increased faster in 2000–15 than in half a century, increasing on average a full 5 years. The greatest increase in life expectancy occurred in Africa, which had previously seen sharp reductions in life expectancy as a result of the HIV epidemic (figure 2). With three of the eight MDGs specifically focused on health, the MDG agenda catalysed a remarkable increase in official development assistance for health.
Figure 2: Life expectancy in 2000 and 2016.
Source: WHO
An enormous gap persists between current reality and the vision of sustainable health for all. Although numerous LMICs have made important strides in expanding health coverage,36 400 million people do not have access to essential health services, and 6% of people in LMICs are impoverished or pushed deeper into poverty by household medical expenses.37
More than 15 000 children younger than 5 years die every day, largely from preventable communicable diseases and malnutrition.38 45% of childhood deaths are associated with undernutrition, 80% are associated with low birthweight, and NCDs are increasing as a major cause of death in children.39 In 2015, 1·2 million adolescents died, largely from preventable causes.40 Adolescents are at high risk of sexually transmitted infections (including HIV41), unintended pregnancy, unmet contraceptive need, and contraceptive failure.42
NCDs (eg, cancers, cardiovascular disease, hypertension, and diabetes) account for 70% of deaths worldwide, and more than 75% of NCD-related deaths occur in LMICs.43 In the future, LMICs will account for up to 80% of the anticipated global increase in cancer cases and deaths.44 NCDs are projected to exact economic costs of $47 trillion in the next two decades,45 and progress in combatting NCDs will affect the world’s ability to attain at least nine of the 17 SDGs.46
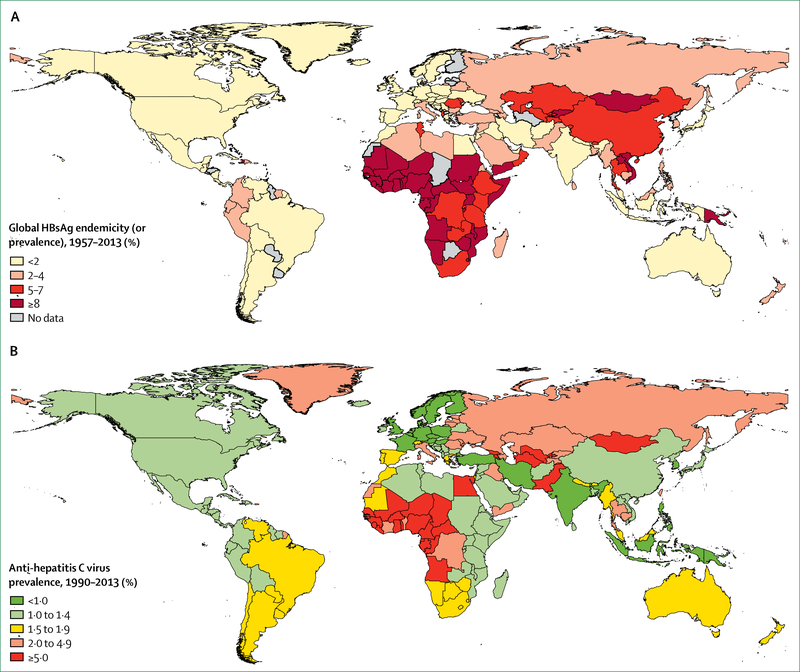
While the world mobilises to address NCDs as the primary driver of mortality and disability, the global community must follow through on its commitments to make communicable diseases a thing of the past. Tuberculosis, for example, is the leading cause of death by single infectious agents, the ninth leading cause of death overall (accounting for 1·7 million deaths in 201638), and the leading cause of death in people living with HIV.47 Africa accounts for roughly 80% of all cases of and death from combined HIV and tuberculosis. Unlike HIV, tuberculosis is a curable infection. Nearly half of the world’s population is at risk for malaria, and no meaningful progress in reducing malaria cases and deaths was seen in 2016.48 Likewise, the challenge of addressing viral hepatitis remains considerable (figure 3). An estimated 71 million people worldwide have chronic hepatitis C virus (HCV) infection, and 2·7 million of these people have HIV infection.51 An estimated 1·34 million people die from viral hepatitis each year, and this includes an estimated 399 000 deaths related to HCV infection.51
Figure 3: Global burden of hepatitis B virus and hepatitis C virus.
(A) Prevalence of hepatitis B virus infection between 1957 and 2013. (B) Prevalence of anti-hepatitis C virus between 1990 and 2013. Adapted from Schweitzer et al (2015)49 and from Gower et al (2014),50 by permission of Elsevier.
Alcohol and drug use disorders are associated with extensive morbidity, disability, and mortality52 and are also closely linked with HIV transmission through the sharing of syringes and the effects of specific drugs (eg, amphetamines) on condom use and sexual HIV transmission.53 In 2016, mental health disorders accounted for nearly 16% of years lived with disability worldwide.52
HIV and global health: what have we learned, and where do we go from here?
The global HIV response serves as one of the most inspiring undertakings in the history of global health. The escalating toll of AIDS across the world in the 1980s and 1990s, especially in sub-Saharan Africa, resulted in “the single greatest reversal in human development” in history.54 However, a genuinely multi-sectoral and multidimensional global mobilization uniting and synergising a diverse set of actors from all across the world achieved what some considered unattainable: halting and beginning to reverse the epidemic.55 These health advances have had profoundly positive effects on households, communities, and societies. In South Africa, home to nearly one in five people living with HIV, sharply reduced AIDS-related mortality stemming from a remarkable expansion of HIV treatment services caused average life expectancy to increase from 52 years to 61 years in less than a decade.56
An unprecedented expansion of access to ART
The greatest achievement of the HIV response to date has been the unprecedented global expansion of access to ART. Although only 680 000 people were receiving ART in 2000 (most of them in high-income countries), by June 2017, this number had risen to 20·9 million people (18·4 million–21·7 million), or 57% of all people living with HIV worldwide.57 In 2016, an estimated 1·6 million deaths were averted worldwide as a result of ART.1
The history of ART and its effect on the global HIV epidemic is one of multidisciplinary collaboration, continual improvements in the quality and durability of treatment regimens, ever-increasing aspirations for service coverage, and passionate advocacy to overcome the resistance that HIV treatment expansion elicited. Public and private sector researchers combined to generate the therapies capable of halting viral replication; civil society advocacy and competition from and within the generic pharmaceutical industry coalesced to produce marked reductions in the prices of ART; robust funding, primarily at the outset from international donors, enabled rapid introduction and expansion of treatment programmes; and programme planners and implementers (as well as affected communities) have helped to improve the efficiency and effectiveness of HIV treatment service delivery. As the scientific evidence expanded, normative guidance on HIV treatment evolved, and in 2015, WHO recommended the initiation of ART for all people living with HIV, irrespective of disease stage, both for clinical benefit to the individual living with HIV and for the prevention benefits of successful viral suppression on onward transmission.58
Substantial scientific advances have also been achieved in primary HIV prevention. Principally due to implementation of ART for pregnant women and other measures to prevent vertical HIV transmission, the number of children newly infected with HIV decreased from 470 000 children in 2002, to 160 000 children in 2016.1 Since results of clinical trials more than a decade ago showed that medical male circumcision reduces the risk of female-to-male sexual HIV transmission by about 60%,59 nearly 15 million men in sub-Saharan Africa have been circumcised.60 PrEP has substantial HIV prevention efficacy,61 and cash transfers might reduce young people’s HIV risk behaviours or the odds of acquiring HIV, or both.62
The future of HIV: an unfinished agenda with an uncertain outcome
The fight against HIV is far from over. In eastern Europe and central Asia, the incidence of new HIV infections increased by 60% between 2010 and 2016,5 and the incidence is also trending upwards in populous countries such as Philippines and Ghana.1 Progress in reducing new HIV infections in adults has largely stalled worldwide. Although UNAIDS estimates that new HIV infections in adults decreased by a modest 11% between 2010 and 2016,5 the Global Burden of Disease Study 2015 investigators found no meaningful decrease in new HIV infections in the previous decade.8 The coming demographic wave, as children become adolescents and young adults, threatens major expansions of the epidemic. In 2016, 43% of the population in low-income countries, 43% of the population in all of sub-Saharan Africa (excluding high-income countries), and 31% of the population in lower-middle income countries were younger than 15 years.63 In a recent modelling study, the failure to build on existing prevention and treatment coverage gains was found to result in a rebound of the HIV epidemic in the coming years.64
The 90–90-90 approach: its promise and its limitations
As part of the SDGs, UN member states have pledged to end the AIDS epidemic as a public health threat by 2030, which has been defined as reducing the number of new HIV infections and AIDS-related deaths by 90% relative to 2010. The cornerstone of this global undertaking is the UNAIDS 90–90-90 approach, which follows that by 2020, 90% of all people living with HIV will know their HIV status, 90% of people with an HIV diagnosis will receive ART, and 90% of people receiving ART will achieve viral suppression.65 The 90–90-90 approach is grounded in the considerable evidence that ART sharply reduces the risk of HIV transmission.66 Within the Fast-Track strategy proposed by UNAIDS to reduce the incidence of new HIV infections to less than 200 000 annually by 2030, UNAIDS estimates that scaled-up ART will account for 60% of HIV infections averted during this period.64
However, it is increasingly clear that the 90–90-90 approach on its own will be inadequate to end the epidemic. Even as ART coverage has steadily increased and as the HIV response has adopted an almost singular focus on HIV treatment expansion, the rate at which the incidence of new HIV infections is decreasing remains far too slow to achieve epidemic control. A case in point is Botswana, which might already have achieved the 90–90-90 target,67 but the decrease in new HIV infections is far too modest to end the country’s epidemic.1
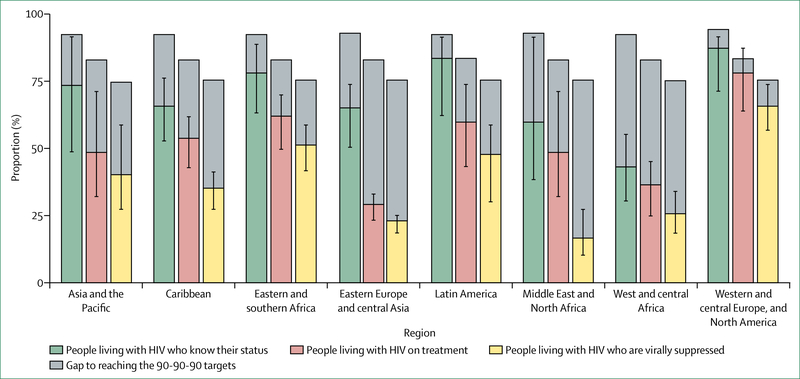
Outcomes across the HIV treatment continuum in eastern and southern Africa, Latin America, and high-income countries are cause for optimism that the 90–90-90 target might be achieved by 2020 in these settings (figure 4). However, in other regions, the proportion of people living with HIV who had suppressed virus in 2016 was far off the pace for the 73% target viral suppression benchmark for 2020 under the 90–90-90 approach (viral suppression was achieved for 34% of people with HIV in the Caribbean, 25% in west and central Africa, 22% in eastern Europe and central Asia, and 16% in the Middle East and north Africa).1 Ensuring widespread viral suppression is also a challenge in many high-income countries such as the USA, where only 49% of people with HIV were virally suppressed in 2014.68
Figure 4: Knowledge of HIV status, treatment coverage, and viral load suppression by region in 2016.
Comparison of HIV testing and treatment cascades by region reveals different patterns of progress. Western and central Europe and North America are approaching global targets. Latin America and eastern and southern Africa show high levels of achievement across the cascade. Eastern Europe and central Asia, the Middle East and North Africa, and western and central Africa are clearly off track. Source: UNAIDS special analysis, 2017.
The prevention benefits of expanded ART would be substantially enhanced by a similarly vigorous scale-up of other strategic prevention interventions. In Uganda, the combination of steady expansion in ART coverage, a near-doubling of the rate with which HIV viral suppression is achieved, and roll-out of medical male circumcision was associated with a 42% reduction in HIV incidence in 10 years.69 In cities in high-income countries, the prioritised roll-out of PrEP as a complement to the test-and-treat approach has contributed to sharp decreases in the incidence of new HIV infections, especially in gay and bisexual men.70,71 Although the so-called combination prevention approach has long been recommended as the optimal means to reduce the risk of new HIV infections, the approach has seldom been implemented at scale because national HIV responses have increasingly come to be dominated by an overwhelming emphasis on HIV treatment. Less than one in five people at risk of HIV acquisition have access to prevention programmes because of chronic underfunding of HIV prevention.6
An alternative future for the HIV epidemic: persistent settings and communities with heavy HIV burdens
Far from putting the world on course to vanquish AIDS, existing approaches are leaving numerous populations behind. In sub-Saharan Africa, young people and men of all ages consistently have suboptimal outcomes along the HIV treatment continuum.5 Various marginalized populations at increased risk of HIV infection, including gay and bisexual men, people who inject drugs, sex workers, transgender people, and the sex partners of people in these groups, accounted for 44% of new HIV infections worldwide (80% of new infections outside sub-Saharan Africa).5
Contrary to optimistic expectations of ending AIDS, these trends point toward the likelihood of a much more concerning scenario. Athough the desired benefits of population-level viral suppression could be realised in settings and populations where access to HIV testing and treatment services is widespread, those people living in countries or belonging to marginalised populations in which services are difficult or impossible to obtain will remain highly vulnerable to HIV acquisition.
Already, evidence suggests that the epidemic is sustained in substantial high-prevalence and high-incidence geographical areas in large measure by the inability of service systems to reach and engage specific populations. For example, even as the incidence of HIV infections in the USA have decreased overall since 2008,72 black gay and bisexual men now have a 50% lifetime chance of acquiring HIV (figure 5).73 Rates of viral suppression in people living with HIV in the USA have steadily improved,74 but black gay and bisexual men living with HIV are markedly less likely than other people living with HIV to receive ART, remain engaged in care, and achieve viral suppression.75 Major pockets of endemic HIV are also apparent in Russia, where HIV infections are heavily concentrated in six subnational regions (referred to as Federal Subjects) in eastern and western Siberia, along principal overland heroin and opium trafficking routes out of Afghanistan. In the case of people who inject drugs, in Russia and elsewhere, access to prevention and treatment services is extremely limited.76 A similar clustering of susceptibility to HIV is apparent in the KwaZulu-Natal province of South Africa, where adolescent girls and women younger than 25 years are roughly three times more likely than men younger than 25 years to be living with HIV.77
Figure 5: African American men who have sex with men have an increased lifetime acquisition probability of HIV infection in 2018.
Credit: © NASTAD.
The persistence of large, high-prevalence, and high-incidence areas in the midst of epidemics that are large and small, decreasing and increasing, is a global phenomenon. Compared with the population as a whole, gay men and other men who have sex with men (MSM), people who inject drugs, sex workers, and transgender women are 19 times,78 13·5 times,79 22 times,80 and 49 times,81 more likely to be living with HIV, respectively. Yet although these marginalised populations have a disproportionate and growing HIV burden, they are often the groups least likely to obtain access to HIV prevention, treatment, and care services.78
With the failure of existing service approaches to address persistent susceptibility or to reach those people who are in greatest need, it is clear that new and scaled-up service delivery and community engagement strategies will be needed. The transmission-preventing potential of scaled-up ART must be matched with an equally strong reduction in the risk of HIV acquisition and transmission.7,82 And programmes for the delivery of prevention and treatment services must be coupled with renewed efforts to address the social and structural factors that increase risk and susceptibility and diminish utilisation of essential services.
Activism and the future of the HIV response
Grassroots political activists have had a defining role in the HIV response. In the epidemic’s early years, gay communities in several countries mobilised to resist the imposition of coercive measures in what was then a new and frightening epidemic. Across the world, chapters of the AIDS Coalition to Unleash Power (ACT UP) marched in the streets and infiltrated decision making bodies to demand greater investments in HIV research and expedited, people-centred approaches to the evaluation and approval of new medicines. In South Africa, activism by Treatment Action Campaign and other groups led to dramatic changes in national HIV policies, which have reverberated around the world. In every region, activists have emerged to challenge high prices for HIV treatments and to insist on approaches to intellectual property that take into account the needs of people with life-threatening diseases.
Today, inclusion of civil society and people living with HIV is standard practice of many HIV-related decision making bodies. Non-governmental representatives, including from organisations and networks of people living with HIV, are now routinely involved in national HIV-related advisory bodies. Vigorous activism persists in many countries and regions. However, as the incidence of HIV infections and AIDS-related mortality have decreased, and as funding for civil society activities has diminished, so have the magnitude and intensity of AIDS activism. In particular, the kind of cohesive activist voice that united the global north and south and laid the foundation for the HIV treatment revolution is no longer evident. In some cases, experiences in the HIV response have led some activists to concentrate more on broader health and human rights issues. For some, a focus on service delivery in their own communities has taken the place of activism for systemic change.
Standard inclusion of civil society in national and global bodies is one of the great achievements of the HIV response and one of its defining features, yet the voices of grassroots activists are at risk of being silenced or muffled. As a class of international and non-governmental HIV professionals has developed, questions have emerged about a potential disconnect between civil society spokespeople at the global level and those working in communities. The inclusion of civil society in decision-making bodies, especially at the global level, is often more tokenistic than substantive.
How to rejuvenate and empower a new wave of activism on HIV remains a topic of considerable discussion and debate within the HIV community (figure 6). What is clear is that the loss of a strong activist presence diminishes both the accountability of the HIV response and the likelihood that our policies, programmes, and approaches will respond to the communities being left behind. Few advocacy community equivalents to HIV exist in other domains of global health. Infusing the broader global health field with the grassroots energy and leadership that has defined the HIV response could revolutionise global health and elevate it on the global political agenda, but a key first step towards this end is to reinvigorate HIV activism.
Figure 6: Activism on HIV in India.
Students recognise International AIDS Candlelight Memorial Day with painted faces at the Centre for Social Work, Panjab University, Chandigarh, India. Credit: © 2016 Gaurav Gaur, Photoshare.
The future of HIV exceptionalism
From the earliest recognition of the epidemic in the early 1980s, the HIV response adopted an exceptionalist approach. Rather than look to often-overburdened health systems to manage HIV as one of many health problems, specific vertical funding mechanisms and frequently disease-specific service delivery channels were established to respond to the global emergency. In 2000–01, diverse global stakeholders spearheaded the creation of the Global Fund to Fight AIDS, Tuberculosis and Malaria, which, by the end of 2016, had allocated more than $17 billion for HIV programmes in more than 100 countries, while also providing much needed support to chronically neglected tuberculosis and malaria.83 Created in 2003, the US President’s Emergency Plan for AIDS Relief (PEPFAR) is the largest bilateral health programme ever devoted to a single disease. The UN also took a distinctly unorthodox approach to HIV, vesting UN leadership on the epidemic, not with the designated health agency WHO, but in the Joint United Nations Programme on HIV/AIDS (UNAIDS), a multidisciplinary and cosponsored programme.84
Whether such an exceptionalist approach will remain feasible is unclear. Overall investments in the HIV response, taking into account both domestic and international sources, has remained relatively flat in recent years, at about $19.1 billion, roughly $7 billion short of the amounts needed to achieve 90–90-90 and the broader UNAIDS Fast-Track strategy.5 In 2016, total resources for HIV programmes actually decreased by 7%.85 In the midst of growing insecurity and disorder in the regions that have historically led in mobilising international financing for the HIV response (namely, North America and Europe), HIV seems less frightening than in earlier times. African leadership in the HIV response, so crucial to success under the MDGs, is also less visible. In a globalised world where attention spans are limited, the temptation to pretend that HIV is a problem that has been solved has proven to be powerful.
As the HIV response grapples with an uncertain future and works to identify optimal strategies for sustainability, it faces a pivotal challenge in defining, for the long-term, its relationship to the global health field. Appropriately integrating HIV within broader health systems will probably be essential to achieve ambitious global HIV aims, sustain treatment access in decades to come, and spread costs associated with controlling the epidemic. However, there are genuine risks to wholesale relinquishment of the exceptionalist approach to HIV. Notwithstanding the many criticisms of vertical health programmes, there is little dispute that HIV exceptionalism has worked, mobilising extraordinary new financial, technical, and human resources, uniting diverse stakeholders, focusing global attention on concrete results, driving and benefiting from scientific innovation, and engaging communities in far-reaching ways. Abandonment of an approach that has been groundbreaking in its success should not be done without a rigorous interrogation of the risks and benefits of mainstreaming the HIV response into national health systems.
Heeding the lessons of the HIV response
Even as the HIV response will inevitably depend on health and social systems to achieve its ambitious global target for 2030, lessons from the HIV response are also instructive for the future of global health. The UNAIDS-Lancet Commission on Defeating AIDS—Advancing Global Health in 2015 outlined the salient attributes of the HIV response that have contributed to its success and that should inform broader global health efforts. These include the sustained leadership of civil society and people living with HIV, the multistakeholder nature of the response, the extraordinary degree of political leadership for the fight against HIV, the centrality of human rights, gender equity, and social justice to the response, and a commitment to global and local-level accountability and transparency.6 Similarly pivotal has been the close link between scientific research and efforts to strengthen HIV programmatic efforts.
An emphasis on innovation has typified the HIV response from its earliest days and is in large measure responsible for bringing the world to the point where HIV as a medical problem can be effectively managed. Continuing research investments have made possible first-line HIV treatment regimens that are more durable, more tolerable, and less expensive than the regimens that have already saved millions of lives. Additional innovations that are anticipated in the coming years, including long-acting antiretroviral regimens for both HIV treatment and PrEP, are among the reasons for optimism that even greater progress against HIV is achievable in the coming years, including the ultimate goals of a preventive vaccine and a functional cure for HIV.
Infusing the HIV response with a renewed energy and sense of purpose will be challenging, but the future health and wellbeing of countless millions of people require that we meet this challenge. Decision makers must be made aware of the profound and indisputable humanitarian and economic stakes at play in the future of the HIV epidemic. While fully acknowledging the daunting challenges we face, the HIV community must reinvigorate itself and embrace the scientist-activist zeal that yielded such historic achievements.
Opportunities for integration and synergy in the global health and HIV responses
Linking global health and HIV responses more closely and integrally across health programmes and practice is hardly a new idea. Experience in integrating HIV in broader health systems underscores both the benefits and potential risks associated with a more integrated and less exceptionalist approach to HIV, illustrates the challenges confronting greater linkage and integration, and highlights potentially valuable opportunities to rethink further how the HIV response fits within the broader global health enterprise. This experience further shows that the options for future programmatic approaches to HIV are not confined to a binary choice between exceptionalism or wholesale integration, but rather that several opportunities exist in the immediate-term for more incremental integration and for learning by doing. In all cases, steps toward integration should be undertaken thoughtfully, retaining the key principles that have defined the HIV response.
People and communities
To advance the SDGs’ goal of leaving no one behind and addressing the needs of the most susceptible first, innovative and rights-based approaches are needed to realise the vision of sustainable health for all. The particular health needs of different populations, outlined here, illustrate diverse ways in which the HIV response and the global health field can benefit and learn from each other.
Children’s health: ensuring that every child has the best chance at healthy adulthood
Comparatively well funded and vertical disease programmes have had a clear effect on child health. Childhood vaccination programmes, including programmes supported by G AVI, the Vaccine Alliance, have generated exceptionally high and increasing rates ofimmunisation.39 Between 2000 and 2014, measles vaccination is estimated to have saved 17·1 million lives.86 Between 2010 to 2015, the mortality from malaria in children younger than 5 years decreased by 35%87 because of dramatic increases in the coverage of key malaria interventions.39
The HIV response illustrates both the potential of focused global action to improve the health of children as well as the persistent failures of health systems to address children’s needs. As the proportion of pregnant women living with HIV who receive antiretroviral medicines has increased, the incidence of new HIV infections in children has sharply decreased. In 2016, the 160 000 new HIV infections in children represented a 47% decrease since 2010, and a 66% decrease since 2005.1
By contrast, little progress has been made with respect to integrated interventions that rely on regular contacts with the health system. Comparing utilisation data for 2000–08 with that of 2009–14, met demand for family planning services increased only modestly, from 54% to 64%, the proportion of pregnant women who visited antenatal care at least four times increased only from 50% to 56%, the share of births with a skilled attendant increased only incrementally, from 55% to 65%, and similarly modest increases (less than 10%) were reported for exclusive breastfeeding, oral rehydration therapy, and care-seeking for pneumonia.39 Shortcomings in health systems that undermine health outcomes for children include weak laboratory systems, inadequate systems for monitoring mother-child pairs from antenatal to paediatric care settings, fragmented service delivery systems that invite discontinuity of care, and frequent drug stock-outs.
These deficiencies in health systems are evident with respect to HIV treatment for children. HIV treatment coverage in children (46% as of June, 2017) remains far lower than in adults (58%), resulting in notably higher morbidity and mortality in children than in adults.1 Although children accounted for 6% of all people living with HIV worldwide in 2016, they made up 12% of all AIDS-related deaths.1 The persistent coverage gap between children and adults has long been excused by complexities associated with diagnosing and treating HIV in children, including the need for virological testing to confirm HIV infection in children and the historic shortage of child-appropriate antiretroviral reformulations. However, as a result of technological advances in recent years, the means to address these complexities now include point-of-care early infant diagnostic testing platforms, innovative methods to reduce turnaround times for standard virological testing by remote central laboratories, and striking progress in optimising treatment options for children.
Effectively integrating service delivery for children will meet children’s health needs in a holistic manner and provide co-located services that are effective, efficient, and of good quality. Health-service integration for children spans not only the levels of care (eg, community, primary, and referral) but also the child’s life (pre-pregnancy to childhood). The Global Action Plan for the Prevention and Control of Pneumonia is a valuable approach to service integration, providing a framework for coordinated and integrated actions to improve feeding and nutrition for infants and young children, access to safe drinking water and sanitation, hand washing with soap, reduction in indoor air pollution, immunisation, HIV prevention, and treatment of pneumonia and diarrhoea.88
Adolescent health
Health risks are especially acute for adolescent girls (figure 7). The risk of new HIV infections in women living in high-burden countries in sub-Saharan Africa peaks at age 15–24 years, about 10 years earlier than in their male peers, and AIDS persists as the fourth leading cause of death in adolescent girls and young women in sub-Saharan Africa.89 The disproportionate health burden in adolescent girls stems from a confluence of factors, including the persistently low socioeconomic status of women in many societies, lack of educational and economic opportunities, and sharply limited access to family planning and adolescent-friendly health services.
Figure 7: Sexual and emotional health in adolescents in India.
A young girl attends a candlelit march for sexual and emotional health for young teens in Udaipur, India. Credit: © 2016 Arvind Jodha/UNFPA, Photoshare.
One in four women worldwide become married before age 19 years, and 6% of women are married before age 15 years.90 Early marriage is linked with lower nutritional status, lower educational attainment, increased mortality for women,91 increased risk of HIV acquisition,92 intimate partner violence,93 and reduced health, nutritional status, and socioeconomic outcomes for their children.91
Particular risks are experienced by the estimated 21 million girls aged 15–19 years and the 2 million girls younger than 15 years who give birth each year.94 Pregnancy during adolescence increases the risk of complications, and childbirth is the leading cause of death in girls aged 15–19 years.91 Giving birth during adolescence also increases health risks for newborn children, including low birthweight, preterm delivery, and poor long-term developmental outcomes.95 Minimising health risks for adolescent girls, including but not limited to HIV infection, will rely on an unprecedented, well resourced, and multisectoral effort. Keeping girls in school, through cash transfers and other strategies, is an overriding priority.
The HIV response has galvanised interdisciplinary work to clarify young people’s reasons for engaging in unprotected sex and effective means to reduce sexual risks. Data from extensive research have conclusively shown that abstinence is a failed strategy for HIV prevention.96 Effective prevention of HIV and sexually transmitted infections and advancement of broader sexual and reproductive health in young people necessitates evidence-based, tailored, flexible, and client-centred approaches to sexual harm reduction and comprehensive sexual education (figure 8).97 Ready access to contraception and other family planning services is essential because half of pregnancies in girls aged 15–19 years are unintended.98
Figure 8: HIV testing, family planning education and referrals, and deworming kits in Uganda.
A dance troupe with Public Health Ambassadors Uganda perform in the Kasana Market in Luwero to call attention to a pop-up health clinic providing HIV testing, family planning education and referrals, and deworming kits.
Credit: © 2016 David Alexander, Photoshare.
Changing lifestyles mean that an increasing number of adolescents are susceptible to the health risks associated with poor diet, tobacco, alcohol and substance abuse, malnutrition (including anaemia and obesity), and NCDs such as diabetes and cancer. In turn, these risks are associated with preventable mortality in adulthood. Mental health issues are among the top five causes of adolescent disability-adjusted life-years (DALYs) lost, with more than 75% of mental illnesses originating before age 24 years.99
All adolescents have the right to accessible health services. Helping youth to prioritise health and engage in services is challenging when low mortality and good health is expected in young people, prior experience in health-care interaction is limited, and concern about confidentiality breaches is high.99 Among the key lessons learned in the HIV response is that health services must be tailored to young people’s needs and preferences and should offer an integrated combination of services. Integrated service packages must include comprehensive health checks relevant to adolescents and take into consideration other priorities such as education and economic opportunities, which, if unaddressed, lead to poor health and wellbeing.89
Achieving universal health coverage demands the availability of adolescent-focused health services in all health facilities and in other more accessible multipurpose spaces. Adolescent-friendly services offer operating hours outside of work or school, create a friendly and non-judgmental environment, engage peers in support functions, promote health in ways that appeal to young people, and use modern technologies and innovations already incorporated in the daily lives of adolescents.100,101
Meeting the needs of women
Women’s health needs often differ from those of men. Whereas breast cancer is not a leading cause of death in men, the breast is the most common site of cancer diagnosis in women (accounting for 25% of all cases in 2012).44 Cervical cancer is the fourth leading cancer diagnosis in women and the fourth leading cancer-related cause of death in women.44 Although maternal mortality has decreased, an estimated 830 women die daily from preventable causes associated with pregnancy and childbirth.102
In addition to heightened physiological vulnerabilities to many diseases and health conditions, women’s health risks are often closely linked with their unequal status in societies. For example, an estimated one in three women worldwide will experience physical or sexual violence, and those women who do are roughly twice more likely to experience depression or have an alcohol use disorder and 16% more likely to have a baby with low birthweight than those women who do not.103
Because of both increased physiological susceptibility to HIV acquisition during penile-vaginal intercourse and because of gender-related challenges in negotiating safer sex, women are especially susceptible to HIV, accounting for 56% of all people living with HIV in sub-Saharan Africa.1 Women’s experience of intimate partner violence has been correlated with a 50% increase in the risk of HIV acquisition,104 and emotional abuse by partners is linked with rapid progression of HIV disease in women.105
The HIV response has long joined forces with the broader women’s health agenda through political advocacy for gender equality, programmes to promote healthy gender norms, and strategies to prevent gender-based violence. At national, regional, and global levels, women living with HIV have organised networks to provide mutual support and to increase recognition of HIV as a women’s health issue. Substantial resources have been invested in condom programming and other interventions to reduce women’s sexual risks and in research to develop prevention methods that women can control on their own.
The successful global scale-up of ART in women has shown the feasibility of achieving broad access to care for women. Among people living with HIV, women are more likely than men to know their HIV status, receive ART, and achieve viral suppression.5 The proportion of pregnant women living with HIV who receive effective antiretroviral medicines increased from 47% in 2010, to 76% in 2016.5
There is growing recognition that the women’s health agenda must move beyond its traditional focus on sexual and reproductive health and tackle women’s growing burden of NCDs.105 The Pink Ribbon Red Ribbon partnership, working in six countries in sub-Saharan Africa and Latin America, aims to leverage momentum and infrastructure from the HIV response to reduce the cancer burden in women. Through the third quarter of 2017, the partnership reported that it had screened more than 465 000 women for cervical cancer and more than 34 000 women for breast cancer, administered the vaccine for human papillomavirus to nearly 148 000 girls, and provided treatment services to almost 30 000 women diagnosed with cervical cancer.106
The gender gap in health: services for men
Health outcomes are consistently worse in men than in women. In 2015, mean life expectancy was 5·8 years higher in women than in men (74·8 years [uncertainty interval 74·4—75.2] for women vs 69–0 [68·6–69·4] for men), and this gender gap in life expectancy is increasing over time.107 These patterns are vividly reflected in HIV-related outcomes; in 2016, men accounted for less than half of all adults (>15 years) living with HIV but for an estimated 58·4% of AIDS-related deaths.1
A key driver in gender differences in health outcomes appears to be men’s comparatively lower utilisation of health services. In a national survey in 2013,108 men in the USA were markedly less likely than women (72% vs 86%) to report a regular source of care or to report having seen a health-care provider in the previous 2 years (75% vs 91%). Across regions, men are consistently less likely than women to know their HIV status, to receive ART, and to remain engaged in care once they initiate HIV treatment.5
In many countries, especially in LMICs, the best developed health-service delivery platforms (eg, antenatal, maternal, paediatric, sexual and reproductive services) are often designed primarily for use by women or children, or both. In many settings, few, if any, health-service delivery channels have been developed specifically with men in mind. This has led to the common belief that clinics are not meant for men,109 deterring men from accessing health services. Often, health services are accessible only during working hours, further discouraging male workforce participants from obtaining the services they need. Popular notions of masculinity might also disfavor receipt of health services as a sign of weakness.110
In view of the severe gender gap in health, it is striking how little attention the HIV response or the global health field has given to men’s health issues. To close the gender gap, decision makers must expressly value men’s lives and prioritise action to increase men’s access to and use of health services. In the case of HIV, a growing body of evidence suggests that community-centred programmes that specifically work to engage men and address their needs and preferences (including flexible clinic hours, mobile services, workplace programmes, and private access) can effectively diminish the gender gap in men’s utilisation of health services.111 To reach nearly 15 million men with voluntary medical male circumcision in 14 African countries in 2008–16, and thereby avert more than 500 000 new HIV infections through 2030,60 circumcision programmes worked with men and local communities to forge new social norms, offered services at hours that corresponded to men’s needs, used a variety of service delivery approaches (eg, mobile outreach), and integrated circumcision services with multiple other health services.112
Innovative service models could diminish or eliminate gender gaps in HIV service utilisation (figure 9). SEARCH, a community-centred research project in 32 rural communities in Kenya and Uganda, integrated HIV testing in multidisease health fairs and used innovative approaches to deliver ART through co-located services. To help avoid a gender gap in testing utilisation, SEARCH created men-only discussion groups that invited men to discuss HIV and sexual health issues. After 2 years of the intervention, gender gaps across the 90–90-90 cascade had effectively disappeared, with men achieving the first two 90s and 88% viral suppression.113
Figure 9: HIV testing services in Kenya.
A counsellor provides HIV testing services to livestock herders during community outreach services in the Eremit area of Kajiado County, Kenya. Credit: © 2012 George N Obanyi/FHI 360, Photoshare.
People who inject drugs
One of the great ironies with respect to the prohibitionist approach to substance use, which treats drug use primarily as a law enforcement and national security matter and continues to have widespread and negative implications for HIV114 and global health more generally, is that it was initiated in the name of public health. A further irony is that these failed policies also spawned one of the most successful of all HIV prevention strategies. Harm reduction is a practical and rights-based approach that aims to mitigate the negative consequences of behaviours, including when cessation of the behavior is infeasible or not desired by the individual. This approach has potential applicability to a host of health problems beyond blood-borne pathogens such as reducing alcohol-related problems, minimising road trauma, and preventing skin cancers. Harm reduction needs be seen as a core global health promotion strategy.
For prevention of HIV and viral hepatitis, harm reduction has traditionally referred to a range of services that include the provision of clean needles and syringes (NSPs). Combined with access to medication-assisted therapy (MAT), NSPs continue to be the most effective strategy to reduce new HIV infections in people who inject drugs. Harm-reduction services have the added public health benefit of facilitating linkages to other HIV-related services, drug treatment, and primary health care. Harm reduction is increasingly being adopted in diverse settings, although large gaps in the scale and coverage of harm-reduction programmes limit the ability of many countries to halt HIV and HCV epidemics.115 The USA, long a leading proponent of the global war on drugs, appears poised to undertake a rapid expansion of MAT in response to a surge in opioid addiction, offering potential opportunities to alter the global discourse on harm reduction.
To reduce the negative consequences associated with drug use, expansion of harm-reduction services also needs to be coupled with broad-based reform of laws and policies. Incremental progress has been made in reforming UN drug conventions to incorporate certain health and human rights considerations, but a wholesale paradigm shift in the global approach to drug use has yet to occur.116 Needed legal reforms (eg, decriminalisation, proportional sentencing, or diversion to health and social services for non-violent offenders) will not only facilitate greater access to health and social services for susceptible communities but also sharply reduce prison populations. Reductions in the prison population might free up resources for scaling up ART, drug treatment, and primary health care in prison settings and reduce the incidence and risk of infectious diseases. In pursuing locally tailored drug policy reform, nation states and states within nation states can help build a compelling evidence base that can guide and inform reform efforts, unlock new efficiencies in prison systems, and build new constituencies for drug policy reform. To transcend polarising political debates that have limited its support, harm reduction itself needs to expand and mainstream its ethos and more integrate its application beyond drug use across a wider spectrum of stakeholders and public health issues.
Gay and bisexual men
In 2016, 12% of all new HIV infections worldwide and 22% of all incident infections outside sub-Saharan Africa were in gay men and other MSM.117 Among HIV risk behaviours, HIV acquisition from condomless and receptive anal intercourse is twice more likely than from needle sharing during injecting drug use and more than 17 times more likely than from receptive penile-vaginal intercourse.118
However, gay men’s disproportionate HIV burden cannot be disentangled from their profound (and in many settings, worsening) social disadvantages. In 2017, more than 70 countries criminalised consensual same-sex relations,119 and the legal and policy barriers confronted by gay men have only increased in recent years, including through enactment of Russia’s so-called gay propaganda law and a draconian anti-gay law in Nigeria that went into effect in 2014. The past 2 years witnessed a sharp increase in the number of arrests of gay men in Indonesia120 and the forced closure of drop-in clinics providing HIV and other health and social services to gay men in Tanzania.121
Such a climate of fear and hostility inevitably increases gay men’s susceptibility to HIV and reduces their access to essential prevention and treatment services. In India and in Moscow, only a small minority of gay men living with HIV have achieved viral suppression, with lack of knowledge of HIV status serving as the most serious service bottleneck.122,123 Coverage of PrEP, a potentially transformative prevention tool for gay man and other marginalised populations, is only minimal outside of high-income countries.5
A crucial barrier to a more effective HIV response for gay men (and an unfortunate reflection of the neglect of gay men’s needs) is the shortage of robust evidence for action in this population. Although findings from focused studies continue to demonstrate extraordinarily high HIV prevalence and incidence in gay men in broadly diverse settings, evidence on gay men from standard surveillance systems is extremely limited, primarily because these systems focus on adults of reproductive age, especially in high-burden settings. Many countries still have no estimates of the size of the gay population, and half of those countries with estimations employ methodologies that call the reliability of these estimates into question.124
Migrants and the displaced
The number of people living in a country other than the one in which they were born (244 million in 2015) is larger than the population of all but four countries.125 In addition to cross-border migration, many people move for work or other reasons to different states or provinces within their own country; In China alone, this affects more than 280 million rural migrants.126 Worldwide, the migrant population includes 65·6 million forcibly displaced people, 22·5 million refugees, and 10 million stateless people.127
Addressing the health needs of the world’s massive and growing population of migrants is a health and human rights imperative.128 However, public health insurance schemes often exclude or limit services for migrants, especially for those who are undocumented. In part through regional cooperative compacts, more countries are taking steps to introduce health insurance approaches that explicitly cover migrants, yet implementation of these approaches remains incomplete129 and many migrant workers in such settings are unaware of the health benefits to which they are ostensibly entitled.130
The stigmatisation and scapegoating of immigrants, including the risk of death or injury from increasingly coercive border control practices and acts of anti-immigrant violence, contribute immeasurably to their susceptibility. Migration could lead to discontinuity and fragmentation of health care, a particularly serious problem for people with chronic diseases. Health risks are compounded when migration is the forced result of military conflict or humanitarian emergency, which can create especially chaotic conditions and prove particularly disruptive to service systems. In 2018, results of a phylogenetic analysis in Ukraine suggested that migration resulting from civil conflict in eastern Ukraine led to a rapid redistribution of HIV infection westward and an apparent increase in HIV drug resistance.131
In an increasingly mobile world, steps to address the health needs of people on the move are crucial to the future of global health. In the push to achieve universal health coverage, countries need to take on board lessons learned from Thailand’s pioneering provision of comprehensive health coverage for migrants living within its borders.132 In regions with extensive cross-border migration, innovative regional efforts will be needed to ensure continuity of care. Migrants should have access to health navigators and other health services in their own language. In close collaboration with other civil society partners, health advocates should energetically oppose demagogic efforts to stigmatise migrant communities.
Related health issues
To date, the greatest focus on linking HIV with the broader global health field has occurred with respect to specific health problems that overlap with HIV. These include diseases that share modes of transmission as well as comorbidities that often accompany HIV infection.
HIV, NCDs, and the future of global health
The future of the HIV response is intrinsically linked with the future of global efforts to address NCDs. Due to the effectiveness of ART, the HIV population is steadily ageing over time, with the number of people older than 50 years who are living with HIV increasing by 36% between 2012 and 2016.1 In 2016, sub-Saharan Africa was home to 3·7 million people (65 %) of the world’s people living with HIV who are older than 50 years.1 As people live longer with HIV and depend on ART for decades, the care of people living with HIV will increasingly focus on prevention and management of NCDs. People living with HIV have an increased risk of many age-associated diseases,133 including cardiovascular diseases,134 neurocognitive disorders,135 end-stage renal disease, and non-AIDS-defining cancers.136 NCD risks appear to be less pronounced for people who initiate HIV treatment at higher CD4 counts and, presumably, for those using modern ART regimens with fewer known negative side-effects.137
As diverse stakeholders coalesce to create a global movement to address NCDs, experiences in the HIV response (including its advocacy successes, community engagement, models of care, and innovation to ensure access to essential diagnostics and medicines) potentially hold important lessons for the NCD community.138 Already, the global attention to NCDs has surged, as reflected in the creation of the NCD Alliance and the Lancet NCD Action Group, the upcoming Third High Level Meeting on NCDs at the UN’s General Assembly in 2018, the WHO-commissioned Independent High Commission on NCDs, and grassroots movements to increase cancer awareness and funding for cancer research. However, much greater political commitment, and the willingness to stand up to entrenched financial interests such as the tobacco and food industries,139 will be needed if NCDs are to receive the attention they warrant.140
The NCD agenda has a clear potential to catalyse action to address the structural determinants of health. Structural approaches (ensuring smoke-free environments, banning or restricting advertising for tobacco or alcohol products, using taxes to discourage use of tobacco or alcohol, reducing salt and sugar content in packaged foods and beverages, and replacing trans-fats with healthier alternatives) are pivotal to efforts to reduce NCDs.141 Given the powerful degree to which socio-economic status influences susceptibility to NCDs,142 strategies to alleviate poverty also support prevention of NCDs. As structural action to address NCDs requires action in multiple domains (eg, social protection, trade, agriculture, taxation and finance, agriculture, manufacturing, labour), it is important that health routinely be taken into account in policy development processes. Toward the goals of more integrated and universally accessible health systems and sustainable health for all, policy development should prioritise structural approaches that generate health benefits for diverse populations and health conditions. As prevention of NCDs involve substantial shifts in social norms and practices (eg, smoking and alcohol consumption, diet, indoor cooking practices), engagement of communities is essential.
For some NCDs, prevention technologies must be brought to scale, actively promoted, and made broadly accessible. These include nicotine replacement and other smoking cessation tools, as well as vaccines for the hepatitis B virus and human papilloma virus. Cognitive behavioural interventions, akin to the behaviour change theories and approaches that have been associated with reduced HIV risk behaviours and new HIV infections,7 have a role in reducing the modifiable risk behaviours that might increase NCD risks.
Experience in the HIV epidemic has taught that efforts to prevent a disease are often less effective if treatment for the disease is unavailable. Until recently, the global health discourse on NCDs focused almost exclusively on prevention, implicitly assuming that treatments would remain out of reach for the foreseeable future. The growing global movement on NCDs, as in the HIV response that preceded it, is rightly insisting on urgent action to make NTD treatments affordable and widely available.143
Although the HIV response is justifiably proud of having created service systems capable of delivering ART to millions of people, the health-systems challenges associated with NCDs are even greater than they were for HIV. Effectively addressing NCDs will require reconfiguration of health programmes and individual clinics and will demand new investments in health workforce training and development144 to increase expertise in management of cardiovascular disease, oncology, geriatric medicine, radiotherapy, and other NCD-related disciplines. Comprehensive and integrated care needs to be established as the working norm for health systems, with primary care as the cornerstone. Models of community-based and community-delivered care, which prioritise self-care and differentiated services for people living with HIV, have potentially broad applicability to the management of NCDs.145 Capacitating health systems to address multiple health problems in an integrated manner, including through co-located services, will improve efficiencies and aid in destigmatising both HIV and NCDs.
In joining hands with the NCD field, the HIV community will also need to evolve. Moving forward, the HIV response must transcend its singular, if imperative, focus on viral suppression and recognise that prevention and control of NCDs are equally pivotal measures of success. Vertical health programmes have driven much of the expansion of health capacity in LMICs in recent years; they have also created professional silos that are often jealously guarded. Strong action will be needed to overcome these tendencies and persuade diverse healthcare cadres and constituencies to understand the transformative potential of more integrated and comprehensive approaches that preserve or improve quality. For both national governments and international health donors, new funding formulae might be necessary to incentivise integrated and multidisease service models.
Multidisease health screening models have already proven effective in addressing not only HIV but also hypertension, diabetes, and childhood illnesses.146 To expand the evidence base on the benefits and costs of integrating HIV and NCD programmes, the Commission conducted modelling exercises to examine the health and financial effect of multidisease screening programmes for HIV and NCDs in South Africa and Kenya.
Integration of sexual and reproductive health services
The rationale for integrating HIV and sexual and reproductive health and rights (SRHR) services is compelling,147 as individuals seeking or needing SRHR and HIV services are likely to face interrelated risks (eg, of sexually transmitted infections and HIV infection, unintended pregnancy, high-risk pregnancy, perinatal transmission of HIV, gender-based violence) and have interrelated needs (for sexually transmitted infections and HIV detection and treatment, contraception, antenatal or postnatal care, prevention of mother-to-child transmission, pregnancy termination, and sexuality education and counselling). For such patients, the ability to receive integrated care (in a single setting, and in a single day) offers substantial benefits in terms of convenience and cost. In practice, however, for a range of political, strategic, and funding reasons, efforts to integrate HIV with other SRHR services have been minimal for much of the pandemic’s history.
Funding and structures created to respond to the global AIDS crisis, while critical to saving lives, contributed to the separation of HIV and SRHR: the establishment of UNAIDS in 1996, which anchored HIV firmly outside of the SRHR framework; the MDGs in 2000, which ignored SRHR altogether and defined a separate goal for HIV/ AIDS; and the Global Fund, which has been a lifeline to millions of people around the world, but, at the time of its creation in 2002 and until relatively recently,148 focused little attention or funding on SRHR, except with respect to services to prevent vertical transmission of HIV.149
The separation of HIV from SRHR in these structures and funding mechanisms contributed to an over-medicalisation of the HIV response and shifted attention away from addressing the epidemic in the context of sex and sexuality. Consequently, other elements of SRHR (eg, abortion, contraception, and comprehensive sexuality education for youth) became easy targets for political agendas already seeking to eliminate access to such services. Moreover, this separation has undermined efforts to firmly establish HIV and SRH service delivery in a human rights framework, prioritising bodily autonomy, sexual rights, and access to information and services,150 which has been a contributing factor to violations of sexua and reproductive rights of women living with HIV, such as the use of forced or coerced sterilisation or abortion.151
Although notable, if uneven, progress has been made in integrating services for the prevention of mother-to-child HIV transmission in broader SRHR approaches,152 progress in addressing the SRHR needs of women, girls, and sexual minorities remains wholly inadequate. Even in high-burden settings in sub-Saharan Africa, where a substantial number of women need services to prevent both unintended pregnancy and HIV, integrated services are rare.153 Marginalised populations at increased risk of HIV infection (including sexual minorities) have additional SRHR needs that are often unaddressed by SRHR services that primarily cater to married or coupled women of reproductive age.153
Integrated definition of sexual and reproductive health and rights
The Guttmacher-Lancet Commission on Sexual and Reproductive Health and Rights154 defines sexual and reproductive health as “a state of physical, emotional, mental, and social well-being in relation to all aspects of sexuality and reproduction, not merely the absence of disease, dysfunction, or infirmity. As such, a positive approach to sexuality and reproduction should recognize the role played by pleasurable sexual relationships, trust, and communication between partners in promoting self-esteem and overall well-being”. The Commission defined an essential package of sexual and reproductive health interventions and emphasised that the achievement of sexual and reproductive health depends on realisation of sexual and reproductive rights, which are based on the human rights of all individuals to: have their bodily integrity, privacy, and personal autonomy respected; freely define their own sexuality, including sexual orientation and gender identity and expression; decide whether and when to be sexually active and choose their sexual partner (or partners); decide whether, when, and by what means to have a child or children, and how many children to have; and have access over their lifetimes to the information, resources, services, and support necessary to achieve all the above, free from discrimination, coercion and violence.
Effective integration of HIV and SRHR services requires action in diverse health service domains, including contraception, maternal and newborn care, and abortion services. In 2017, 43% of the 206 million pregnancies in LMICs were unintended, and 84% of these unintended pregnancies were in women who had an unmet need for modern contraception.98 In 2017, 37% of women who gave birth in developing countries did not receive the recommended minimum of four antenatal care visits, 28% gave birth outside a health facility, and 308 000 women in developing countries died from pregnancy-related causes.98
HIV-SRHR service integration can occur either by introducing HIV prevention and testing into the current suite of contraceptive, maternal and child care, and abortion services or by improving access to these types of SRHR services for people living with HIV. When HIV and SRHR services are functionally integrated, important improvements can be achieved in HIV testing rates, condom use, and selected indicators of quality of care.153 We modelled these interactions for the potential benefit of integrated services in Nigeria (figure 10).
Figure 10: Prevention of mother-to-child transmission of HIV in Nigeria.
Mothers and children in Alausa Area of Lagos, Nigeria, during a state-sponsored educational programme on prevention of mother-to-child transmission of HIV. Credit: © 2014 Kunle Ajayi, Photoshare.
The important intersections between use of specific hormonal contraceptive methods and various HIV-related risks further buttress the rationale for integration of contraceptive and HIV services. Observational data suggest a potential association between use of the progestin-only injectable contraceptive depot medroxy-progesterone acetate (DMPA) and risk of HIV acquisition in women exposed to the virus.155 In updated guidelines, WHO in February, 2017, recommended that women considering use of progestin-only injectables be advised about these concerns, the uncertainty over whether the association is causal, and how to minimise their risk of acquiring HIV.156
Services for the prevention, diagnosis, and treatment of sexually transmitted infections offer an important platform for integrating HIV in broader SRHR services. Fully capturing the potential synergies and complementarities between sexually transmitted infections and HIV prevention will necessitate a more holistic and context-specific approach to sexual health. As an example of HIV-STI integration, the Dean Street Express clinic in London, UK, provides a comfortable drop-in facility where individuals input sexual risk information in kiosks, privately self-collect their oral, rectal, and genital specimens for detection of sexually transmitted infection, have specimens delivered via pneumatic tube to an onsite laboratory, and receive their results via text messaging within several hours. Another approach to enhance sexual health is the New York City PlaySure sexual health programme, which educates at-risk persons that HIV medications used for treatment, PrEP, or post-exposure prophylaxis (PEP) can protect the health of individuals irrespective of serostatus. Paired with an integrated package of sexual health services that include routine sexual health screenings and rapid initiation of antiretroviral medications for either prevention or treatment, New York’s Status Neutral model uses sexual health services as a gateway to transition from safety net services to more longitudinal and comprehensive care.
Scaling up an integrated and synergistic approach to sexual health confronts important obstacles that will need to be overcome. Notably, many primary care providers do not routinely ask important questions about sexual health. Because medical and professional schools often omit sexual history taking in medical training, health workers often miss important opportunities for prevention and fail to provide their patients with good-quality services.
Transcending the historic separation between HIV and sexual health will rely on bold leadership, innovative approaches, new funding models, and new partnerships that help dissolve the siloes in which these related efforts have operated. Integrating HIV and SRHR will help ground these linked enterprises in a human rights framework that prioritises bodily autonomy, sexual rights, and access to information and services.
Global health emergencies: Ebola, Zika, pandemic flu, and antimicrobial resistance
Global health emergencies related to infectious diseases in recent years have highlighted the pressing need for all countries to be prepared for the advent of health emergencies and have the capacity to respond rapidly and effectively. In recent years, the Ebola, Zika, and pandemic influenza viruses moved through specific regions quickly, with devastating effects on people’s lives, communities, and economies. With increasing population pressure, urbanisation, travel and displacement, climate change, habitat destruction, and global antimicrobial use, the world will inevitably see more frequent health emergencies related to infectious diseases.157 Some of these emergencies are highly likely, such as pandemic influenza, which will always reappear.158 Others emergencies such as Middle East respiratory syndrome or severe acute respiratory syndrome might be entirely new infections that will require rapid collection of data to understand the natural history. New presentations could be of known infections but in non-immune communities, as recently seen with the Zika virus outbreak, or the emergence of drug-resistant strains of known pathogens, including Mycobacterium tuberculosis and HIV.159
Many disease-control interventions are relevant irrespective of the particular health challenge, including community-based care delivery, the requirement for and training of a diverse health workforce able to handle infectious diseases, control of nosocomial spread, research methodology, and infrastructure for effective community engagement (figure 11). All infectious disease emergencies require strong laboratory and surveillance systems to detect and understand the rapidly changing epidemiology and drive an evidence-informed and country-specific response. The HIV response has been an example of community engagement and empowerment that has led to accelerated research and more effective programme implementation in many parts of the world. The strengths of the HIV response can therefore inform responses to global health emergencies.
Figure 11: Monitoring babies for signs of congenital Zika syndrome in Honduras.
At Tela Hospital in El Progreso City, Honduras, Ileana Mayes Flores, the only geneticist in the public sector of Honduras, examines Aylin Meja, a baby aged 3 months whose mother, Sandra Meja aged 19 years, was infected with the Zika virus during pregnancy. The medical staff is closely monitoring her baby for signs of congenital Zika syndrome. Credit: © 2017 Brendan Bannon/USAID, Photoshare.
Although modes of transmission might vary in each health emergency, comparable groups are often at risk of multiple diseases, and similar interventions are available to block transmission. For example, both Zika and Ebola virus infections were sexually transmissible and transmissible from mother to child. As in the case of HIV, fears and suspicion of people infected with Ebola virus, and to a lesser extent Zika virus, have prompted calls for quarantine, with associated paranoia and misinformation.160 Often, infections and their associated stigma disproportionately affect the reproductive rights of women. Dispelling stigma and ensuring a non-discriminatory and evidence-informed response is important to balance the rights of individuals with appropriate disease control measures.
Although there is much common ground, health emergencies differ in important respects from HIV. First, whereas the HIV epidemic is a long-wave event that has unfolded over many years, health emergencies in infectious diseases are often acute, leading to major short-term disruption. Second, a very rapid political response is needed, often with mobilisation of vast amounts of money, which might shift resources away from HIV programmes. Third, there is a limited research base, and few or no known interventions at the time an emergency response is needed.
In the absence of an activist community, inappropriate decisions in health emergencies can be made for and not with affected communities. In the HIV response, these communities have been built, strengthened, and sustained over decades, not days or weeks. The affected community might not always be easily defined, as, for example, seen in pandemic influenza epidemic or in the case of co-infection and overlaps in disease susceptibility. Infectious disease emergencies can profoundly disrupt health services and supply chains, leading to cascading health-system failures that affect people with a wide range of diseases.161
Integration of HIV and global health responses could be assisted by a high-level political platform bringing people and organisations together to transcend administrative and academic silos and to build broadly responsive and effective health systems. Identifying key stakeholders in emergency response preparedness and resource mapping of relevant capabilities in laboratory diagnostics, surveillance and epidemiology, clinical care, clinical trials capabilities, ethics, and target communities will facilitate integration. Investment in communication across sectors could enable the development of a business model for rapid mobilisation of resources and effective responses to global health emergencies.
Tuberculosis
The basic approach to controlling the linked epidemics of HIV and tuberculosis is deceptively straight-forward. People with HIV-tuberculosis co-infection who develop active tuberculosis disease need to be found and treated promptly with tuberculosis drugs and ART. Co-infected individuals at risk of developing tuberculosis must be treated with ART and with isoniazid preventive therapy (IPT).
In reality, however, controlling tuberculosis in the context of HIV has proven to be a major global health challenge. Globally, less than half of tuberculosis cases are diagnosed before death, and only about half of all patients with tuberculosis know their HIV status.162 Less than half of all people living with HIV who have tuberculosis are estimated to be receiving ART, but HIV treatment coverage is 77% among notified tuberculosis cases.162 In 2016, 60% of countries with high HIV-tuberculosis burden did not report the provision of IPT.162 Despite these challenges, the improvements in HIV care and treatment, recognition that both diseases coexist, and a move to more active case finding led to a reduction in annual mortality from tuberculosis in people living with HIV by 33% between 2005 and 2015.1
The global failures to address tuberculosis in the context of the HIV epidemic demonstrate the challenges of service integration, even when the biology of pathogens and the clinical realities of disease interactions clearly argue for integration. Tuberculosis and HIV programme siloes are object lessons in the difficulties of providing better and more streamlined care for patients in dual epidemics. Inadequate HIV-tuberculosis integration results in suboptimal service coverage, limited access, missed opportunities for prevention, diagnostic and therapeutic intervention, and inefficient use of resources.
Although undoubtedly beneficial, integration of HIV and tuberculosis services is not always easy to achieve. Infection control, particularly in health-care settings, is necessary but often difficult under existing conditions, with multidrug-resistance tuberculosis and extreme multidrug-resistance tuberculosis posing lethal risks for people living with HIV. Health workforce shortages undermine effective management and control of HIV-tuberculosis co-infection, and health workers addressing HIV-tuberculosis co-infection are also at substantial risk of occupational exposure to tuberculosis. As HIV and tuberculosis programmes were developed separately, with different funding streams and constituencies, ensuring ongoing communication and collaboration between these programmes might be hindered by resistance from turf-conscious programme implementers and planners. Tuberculosis and HIV-tuberculosis co-infection are particularly challenging issues for incarcerated persons worldwide.163 According to a review of HIV, viral hepatitis, and tuberculosis burden in prisoners and detainees, the most effective way to reduce these infections in prisoners and in the broader communities from which they come is to reduce mass incarceration for people who inject drugs.163
Numerous efforts worldwide, often extremely successful, have been made to improve HIV-tuberculosis collaboration and linkages. In Khayelitsha, South Africa, HIV and tuberculosis programmes were physically merged in 2003 to enable so-called one-stop shopping for people with HIV-tuberculosis co-infection.164 In Tanzania, PASADA, a faith-based organisation providing comprehensive HIV care, collaborated with national tuberculosis authorities to integrate tuberculosis services and improve laboratory capacity, harmonise staff training and reporting, dedicate a room within the clinic for patients with tuberculosis, and link services with a strong social support system. In India, collaboration at the national level has driven systematic implementation of collaborative HIV-tuberculosis activities across the country.
Notwithstanding these promising examples, HIV and tuberculosis programmes continue to operate independently in most parts of the world. Joint efforts are warranted for coordination of national responses to HIV and tuberculosis, including strengthened surveillance, programme planning, and monitoring and evaluation. Intensified tuberculosis case finding, scaled-up IPT, and a strengthened emphasis on infection control are needed to reduce the tuberculosis burden in people living with HIV. Further efforts are needed to ensure routine HIV testing, access to HIV prevention methods, ART, and HIV care and support for patients with tuberculosis. Collaborative programmes for HIV and tuberculosis should build on well developed community systems and civil society engagement and incorporate interventions for poverty alleviation and social protection as well as specific measures to reduce the HIV and tuberculosis burden in prisoners and migrants. Collaborative HIV-tuberculosis programmes must be prepared to manage the most common co-occurring health conditions in individuals with HIV-tuberculosis co-infection such as diabetes, alcohol and drug use disorders, health conditions related to tobacco smoking, and lung diseases. These strengthened collaborative programmatic efforts should be supported by robust investments in research and innovation that will support the discovery, development, and rapid uptake of new tools and interventions as well as the research to optimise implementation and catalyse further innovation.
Viral hepatitis
The links and synergies between HIV and viral hepatitis are evident, in part because of overlapping routes of transmission, overlapping populations at highest risk of acquisition and transmission, and common approaches to prevention and treatment. HCV infection, however, by marked contrast with HIV, is now curable. With a relatively short course and well tolerated oral therapy, a cure is reliably achieved in more than 95% of infected persons.165 Isolation of HCV and development of a curative regimen are recent developments, and there is reason for hope that rapid gains can be made in diminishing the burden associated with the infection. Hepatitis B virus was isolated much earlier than Hepatitis C virus, and global coverage of the vaccine, which is cheap and effective, for newborn babies reached 84% in 2016.166
The HIV epidemic, including the desperation for effective therapies it elicited in its early years and the changes it wrought to drug development, regulatory policies, and the global infrastructure for clinical trial enrolment, laid the foundation for the development of the highly effective, safe, all-oral, curative therapies that have emerged for HCV infection. As in the case of HIV, originator companies have entered voluntary license agreements with generic manufacturers to produce and distribute direct-acting antivirals, which has substantially lowered treatment costs in the LMICs covered by these agreements. Due to differences in epidemiological burden between HIV and HCV, with substantial concentration of HCV infection in many upper-middle-income countries and high-income countries that are excluded from voluntary licenses for direct-acting antivirals, costs of these drugs remain prohibitive in many parts of the world.167
Community mobilisation has had an essential role for responses to both HIV and HCV. Civil society mobilisation to expand treatment access for HCV infection is rapidly increasing to demand development of national treatment guidelines, innovative use of intellectual property law to lower the cost of direct-acting antivirals, and routine availability of diagnostic and therapeutic tools.
Treatment as prevention, a concept made feasible by the advent of effective therapies to achieve HIV control, has the potential to be realised for HCV infection through expanded use of direct-acting antivirals, the first highly effective curative treatment for a chronic viral disease. Elimination of HCV would inspire the global health field and generate important lessons pertinent to efforts to control other infectious diseases through the delivery of safe, effective, and short-term treatment to marginalised populations.
To realise the elimination goal for viral hepatitis, a major shift will be required in the management of these diseases. Here, too, lessons from the HIV response are instructive, notably the evolution in HIV treatment from a focus on the sickest to recognition of the benefits of treatment for all people living with HIV. Treatment for HCV infection is often reserved for the sickest patients, and many people who could benefit from treatment have been denied therapy because of risk behaviours (eg, ongoing injection drug use). As the right to treatment has proved such a powerful and animating force in the HIV response, a similar rights-based approach is needed to ensure the access of all people with chronic HCV infection to treatment services.
In yet another echo of experience with HIV, the HCV treatment revolution has the potential to draw marginalised people into health services that have historically been hostile or unwelcoming. Health systems must adapt to provide the needed array of health and social services for vulnerable populations. The HCV response, like the HIV response, brings into sharp relief the importance of ensuring investment in research and development of new medicines, maintaining sustainable health budgets, and leveraging social and health policy to prevent the spread of infectious diseases.
Differentiated care for HIV
Models of service delivery developed for the rapid scale-up and maintenance of ART are potentially useful for informing care strategies for other diseases, including the growing burden of NCDs. Taking what began as a standardised and somewhat rigid approach to the delivery of HIV treatment, people living with HIV and the communities in which they live pioneered new approaches to service delivery.
Although these adaptations are diverse, they share the central idea of responding to client and health systems needs. In rural Mozambique, groups of clients took turns travelling to the health centre to collect refills of antiretroviral medicines to avert the need for individual patients to travel long distances for monthly refills.168 Networks of people living with HIV in Uganda opened community drug distribution points closer to communities to enhance access.169 In urban South Africa, adherence clubs offered peer-based adherence support and reduced the frequency with which virally suppressed clients needed to attend clinic, helping decongest overcrowded clinics.145 Importantly, these service delivery innovations not only increase client convenience and minimise health-system burdens, but they also generate excellent health outcomes, as measured by retention in care and rates of viral suppression.170
Together, these various service delivery adaptations have become known as differentiated service delivery. Consistent with a people-centred approach to health care, differentiated service delivery recognises and responds to the diversity of client needs.171 In 2016, WHO formalized the paradigm shift in HIV service delivery, replacing the standardised one-size-fits-all model, which had long been recommended for HIV services, with distinct packages of care for different groups of people living with HIV.172 Differentiated service delivery illustrates not only how service delivery innovations can improve efficiency and effectiveness but also how communities can shape and inform health systems.
Substance use disorders
Marginalised communities, including but not limited to sex workers and sexual minorities, often experience addiction in the context of multiple health and social disadvantages (eg, mental health disorders, abuse, police harassment, homophobia, and transphobia).173,174 In particular, the criminalisation of substance use and of substance users has long aided and abetted transmission of HIV and other diseases.175 The regions where the HIV pandemic continues to expand (eastern Europe and central Asia, the Middle East, and north Africa) share substantial HIV epidemics in people who inject drugs and have harsh prohibitionist drug regimes.175 In Russia, home to the largest epidemic in eastern Europe and central Asia, all opioid substitution therapy is illegal and the country instead relies heavily on coercive detention for rehabilitation (figure 12).76 In the USA, an expanding opioid epidemic has led to outbreaks of HIV and HCV, especially in rural communities where a high proportion of new cases are young, white women.176
Figure 12: Patients undergo drug detoxification while handcuffed to their beds at a programme for heroin addiction in Ekaterinburg, Russia.
Credit: Brendan Hoffman.
Untreated substance use disorders are associated with reduced retention in HIV care and worsened HIV-related health outcomes.177 Drug dependency requires intensive, personalised treatment such as MAT with methadone, buprenorphine, and other opioid agonists. These services for drug addiction are fully compatible with HIV prevention and treatment services, including HIV testing and provision of ART, PrEP, syringes, condoms, and overdose prevention (eg, naloxone). A team in northeast USA developed culturally tailored programmes that use innovative means to provide MAT, work with correctional systems, and understand the interplay between substance use, violence and victimisation, sex work, and behavioural health morbidities.178 This approach was subsequently adapted to other settings, including Ukraine and Malaysia.
Globally, integration of HIV and addiction services is scarce, in part because of stigma. People with substance use disorders are often excluded from MAT or other services if they have a comorbid psychiatric disorder (and vice versa), an approach that not only worsens health outcomes but also compounds the marginalization experienced by people with these disorders. In many settings, treatment for substance use and HIV operate in silos, resulting in fragmented care and suboptimal clinical outcomes. Meaningful integration of services for people with substance use and HIV requires a health workforce that is culturally competent and professionally skilled to create clinical environments that promote service access, engagement, retention, and medical adherence. Modelling exercises on behalf of the Commission examined the epidemiological and cost effect of such a one-stop shopping approach for people who inject drugs in Russia.
Mental health disorders
There are multiple reasons for the frequent co-occurrence of mental health disorders and HIV, with many stemming from the internalised stigma and stress that are often associated with behaviours that can result in HIV transmission (eg, sex between men, gender variance, or injecting drug use).179 Internalised stigma associated with HIV is common not only in marginalized populations but also in settings with high-burden epidemics in heterosexual people, linked in many cases to shunning or shaming by families, friends, and social arbiters, including faith leaders. In one recent US cohort, more than one in five patients had clinically significant depression, 22% of the cohort had an anxiety disorder, and a comparable proportion of the cohort had a clinically significant substance use disorder.180
The linkages and overlaps between HIV and mental health are multifaceted. Depression, especially when it occurs alongside substance use and other health and social conditions, can contribute to risky sexual behaviours.181 Untreated depression is also correlated with reduced capacity for self-care and retention in HIV care.182 These associations are hardly unique to HIV as untreated depression and other psychiatric disorders are strongly linked with non-adherence in patients with cardiovascular and other NCDs.183 One of the most striking features of the intersection between HIV and mental health is the common co-occurrence of multiple coexisting psychosocial and clinical challenges in people with or at risk of HIV.184 The presence of these syndemic conditions potentiates sexual transmission risk182 and non-adherence to antiretroviral medication regimens.172
Because of the centrality of mental health to the management of a broad array of health conditions, mental health services should be fully integrated into the health system and ideally co-located with other related health services such as treatment and care for HIV, NCDs, and substance use disorders.185 However, as confirmed by findings from a situation analysis in five LMICs, most health systems are not prepared to provide an integrated and scaled-up access to mental health services.186 Although mental health disorders are one of the leading causes of disability worldwide52 and one in four people will need mental health services at some point in their lives, many countries invest as little as 2% of total health spending on mental health services.187 Immediate efforts are needed to implement the actions outlined in WHO’s mental health action plan 2013–20.188 Implementation of these action steps will require major new investments in mental health training for health personnel and a decentralisation of mental health services to increase access to community-based services.189
Health systems and structural change
Perhaps more than any health problem of our time, HIV has shown both the importance of strong health systems and how social, legal, economic, and structural factors affect susceptibility to disease and the capacity of societies to mount effective public health responses. To date, however, the success of the HIV response in addressing these social and structural factors has been imperfect at best, in part due to the failure to translate key insights into meaningful policies and programmes. The Agenda for Sustainable Development, which is premised on the indivisibility of the broader development agenda, affords new opportunities to integrate attention to social and structural issues as a fundamental pillar of global health. Indeed, although health-specific targets are clustered under SDG 3, the future of global health is integrally linked with the vision of SDG 16 to “promote peaceful and inclusive societies for sustainable development, provide access to justice for all and build effective, accountable and inclusive institutions at all levels”. Such important change will not be easily achieved, but the long-terms benefits could be substantial.
Just as disease-specific and population-specific funding for health can create silos that impede greater integration, having separate budgets for health and other social sector programmes makes it difficult to address the social and structural issues that increase susceptibility to disease and diminish use of health services. Health outcomes are affected by outcomes from other sectors such as education,190 housing,191 human rights, gender and women’s rights, and law enforcement. Other sectors also have a crucial role in delivering services that address the needs of people affected by HIV or other health problems; for example, social protection that provides cash transfers or undertakes programmes to address the needs of susceptible households.
Overcoming these silos will need a clear and strong political mandate for diverse sectors and ministries that maximise and fully leverage policy and programmatic synergies as well as a strong commitment of each of these sectors to work together. Incorporating multi-sectoral approaches in budgetary and strategic planning is a potentially valuable avenue for addressing social and structural issues. Under this approach, key sectors or ministries participate in formal health planning processes, receive clear budgetary outlays associated with concrete deliverables, and participate in monitoring, evaluation, and accountability systems to ensure that these social interventions achieve desired health outcomes.
Building strong health systems
Across diverse populations and disease priorities, health system weaknesses impede efforts to improve health and wellbeing and to advance towards the goal of universal health coverage.
Well functioning health systems will be essential to sustaining ART in the decades to come and to promoting holistic, client-centred, one-stop-shopping models that integrate diverse services. New service delivery models that prioritise patient self-care and decongest clinics will be needed, and innovative approaches will be necessary to maximise outcomes from available health personnel.
Urgent and sustained efforts are needed to build and preserve human resources for health. In addition to essential new investments in traditional medical education, the shifting or sharing of clinical tasks from higher-cadre to lower-cost health workers will be needed to improve health-service efficiency and expand the reach of health services, especially for remote and marginalized communities. Increased use of trained community health workers offers an important task-shifting strategy for rationalising health spending and bringing services closer to the communities that need them. Compensation and training costs for community health workers are much less than for higher-cadre health workers, and the availability of community health workers enables doctors and nurses to focus more time on the skills for which they are specially trained.192 Numerous models for community health workers are already in operation in diverse regions, including in high-income countries. Ideally, community health workers are drawn from the communities they serve, receive extensive training that enables them to diagnose and address multiple health conditions, and are compensated, supervised, and fully integrated into health systems. Integrating community health workers into health systems has been shown to increase immunisation rates, enhance access to diverse health services, and reduce childhood mortality.193–195
The financing of health care can have a major effect on the efficiency of health services. To improve efficiency and quality of care, policy makers are advised to optimise the mix of payment strategies (eg, capitation, case-based reimbursement, fee-for-service, per diem) that incentivizes service access, quality of care, satisfaction of provider and client, and other system priorities.196 Evidence suggests that carefully designed financial incentives to patients could increase use of preventive care, which improves health outcomes and potentially averts more costly therapeutic interventions in the future.
The HIV response has undoubtedly generated benefits for health systems. An independent evaluation of PEPFAR found that the bilateral programme had increased national capacity for commodity forecasting, procurement, storage, distribution, and tracking.197 Beginning in 2013, the Global Fund began soliciting proposals not only for specific disease programmes but also for health-systems strengthening. Global Fund grants have underwritten a variety of activities to buttress procurement and supply management, including expanded warehousing capacity in Tanzania, digitising and streamlining supply logistics in Laos, and revising and updating of the essential medicines list in Cambodia (Shakarishvili G, Global Fund to Fight AIDS, Tuberculosis and Malaria, personal communication). Despite these strategic investments, stock-outs of antiretroviral medicines remain a persistent problem in sub-Saharan Africa,198 especially with respect to new regimens.199 Weaknesses in procurement and supply management are not confined to Africa or to HIV medicines but instead represent a systemic challenge that demands high-level and sustained attention. For example, in the Mekong region, where a rapid surge in malaria resistance has caused global alarm, fake or substandard anti-malaria medicines are common.200 Maintaining separate disease-specific systems for procurement and supply chain management wastes money, reduces incentives for service integration within health systems and facilities, and siphons scarce funding away from needed systemic investments.
Comparable actions are needed to strengthen and sustain laboratory systems. Independent evaluators found that PEPFAR’s impact on national laboratory capacity has been “fundamental and substantial”.197 Investments by PEPFAR, the Global Fund, and other donors have resulted in the training of tens of thousands of laboratory professionals, the purchase of new laboratory equipment, and upgrading the degree of management and organisation in laboratory medicine, especially in sub-Saharan Africa.
Although HIV has served as a shot in the arm for many laboratory systems, these functions remain badly under-resourced. Maintenance of vertical funding streams for laboratory medicine undermines efforts to apply laboratory technology and skills across diseases. For example, although management of HIV and tuberculosis is critically linked, laboratory services for the diseases often remain distinct and unintegrated. This represents an important missed opportunity because certain laboratory tools, such as the nucleic acid diagnostic networks created to support early infant HIV diagnosis, have the potential to enable diagnosis of a range of diseases (Peter T, Clinton Health Access Initiative, personal communication).
Structural change and health in lesbian, gay, bisexual, transgender, and intersex (LGBTI) communities
In a singular achievement, the global HIV response has yielded new opportunities for gay and bisexual men and others in LGBTI communities to be recognised as citizens, rights holders, and beneficiaries of public health programmes in their own countries. Donor funding has played a pivotal part in supporting LGBTI communities to engage in HIV leadership and in developing programming in gay and bisexual men and other marginalized populations. Among 93 countries reporting the existence of HIV services for gay and bisexual in 2005–13, 67 countries said these programmes were wholly dependent on international funding.
Bringing an end to HIV exceptionalism risks rolling back this historic progress in recognising and responding to the health and related needs of LGBTI people. For example, replacing disease-specific and population-specific funding streams with general health-system funding could jeopardise the sustainability of community-led organisations in the many countries whose governments have proven either indifferent or openly hostile to gay and bisexual men. Innovative models of service delivery, which communities of gay and bisexual men have developed in the face of stigma and discrimination, could well be lost.
Universal health coverage offers new opportunities to right many of these wrongs associated with the longstanding underprioritisation of the health needs of LGBTI people. However, while coverage is expanded to all, irrespective of demographic status, health systems also must be transformed to respond to the diversity of the people they serve. This will require the routine collection and analysis of disaggregated data to understand health status in LGBTI sub-populations, to measure programme performance, and to assess LGBTI communities’ satisfaction with services.
Opportunities for integration: key conclusions of the Commission
In disease-specific and population-specific reviews, the Commission has identified numerous opportunities (both in the immediate term and longer term) for synergising HIV with the broader global health agenda and for advancing intersectoral action towards universal health access (table; panel 2).201
Table:
Immediate opportunities for synergies through co-located services, by service platform
| Population (or populations) | |
|---|---|
| Co-located HIV and SRHR services | Women, adolescent girls, sexual minorities |
| Co-located HIV and antenatal services | Pregnant women |
| Co-located HIV and paediatric health services | Children living with HIV |
| Co-located and jointly planned HIV and tuberculosis screening and treatment services | People with or at risk of HIV-tuberculosis co-infection |
| Co-located HIV and HCV screening and treatment services | People with or at risk of HIV-HCV co-infection |
| Co-located drug treatment or rehabilitation for HIV, NSP, and MAT | People who inject drugs |
| Screening for HIV and NCDs | General population in high-burden settings |
| Management of NCDs in HIV service platforms | People living with HIV and people with or at high risk of NCDs |
SRHR=sexual and reproductive health and rights. HCV=hepatitis C virus. NSP=provision of clean needles and syringes.MAT=medication-assisted therapy. NCDs=non-communicable diseases.
Panel 2: Longer-term opportunities to maximise synergies.
Integrated care in mainstream health services
Key action items:
Health systems strengthening interventions (eg, payment systems, patient incentives, procurement and supply management, workforce training, laboratory strengthening)
Progressive integration of treatment services for HIV and non-communicable diseases
Steps to ensure that all health services are adolescent-friendly
Clinic and community interventions to increase men’s use of health-care facilities
Capacitation of health systems to address the needs of marginalised populations, including sexual minorities and migrants
Steady progress towards universal health coverage (with HIV progressively folded into broader health insurance schemes)
Addressing social and structural issues
Key action items:
Repeal of criminalisation laws
Multisectoral budgeting, planning, and accountability for results
Following through on women’s equality and LGBTI agenda
Steps to ensure an enabling environment
LGBTI=lesbian, gay, bisexual, transgender, and intersex.
Integration of HIV and other health services: modelled scenarios
As indicated by the multiple domains in which integration of HIV and broader health, social, and structural approaches is warranted, the opportunities for integration are myriad. Actions to integrate HIV and global health aim to achieve win-win results, whereby both HIV-related and non-HIV-related health outcomes are enhanced in a manner that is cost-effective. Movements to integrate HIV into health systems will and should be incremental at the outset, with integrated approaches tailored to national conditions. Rather than attempt a rapid and wholesale transfer of vertical programmes to national health systems, initial efforts might focus on more fully integrating HIV with services to address other health issues (eg, sexual health, NCDs, tuberculosis). Especially for marginalised populations that are not well served by mainstream health services, early steps towards integration might focus on integrating HIV services with services that specifically serve these populations (such as harm reduction services in the case of people who use drugs).
Successful integration of HIV services with other health services requires an in-depth understanding of setting-specific factors that drive the potential effect of new programmes at a national level. Given the diversity of HIV epidemics and health systems, we studied the potential effect of HIV integration in five countries with different HIV epidemic profiles and health-system characteristics—thus requiring different approaches to integration of HIV with other health services (figure 13). In each country scenario, we estimated the population-level effect of integration, not only in terms of HIV-specific outcomes but also in terms of potential effect on other health conditions. In select countries, we additionally estimated the cost-effectiveness of these programmes.
Figure 13: A multicountry modelling exercise to study the effect of HIV integration in various settings.
NCDs=non-communicable diseases. MSM=men who have sex with men. FSW=female sex workers. ART=antiretroviral therapy.
South Africa: mobile multidisease screening for HIV and NCDs
The world’s largest HIV epidemic is in South Africa, an upper-middle-income country, where an estimated 7·1 million people have HIV, and the prevalence of HIV infection is 18–9%7 South Africa also has a burgeoning epidemic of NCDs. 30% of women aged 55–64 years have diabetes,202 and the diabetes-specific mortality has beensteadily increasing since 1997,203 reaching 3·6% of all deaths in 2012.204 Cardiovascular disease accounted for 18·5% of all deaths in South Africa in 2012,204 and an estimated 31·8% of South African adults have hypertension, the leading risk factor for cardiovascular disease.205 Altogether, HIV, diabetes, and cardiovascular disease accounted for 51% of all deaths in South Africa in 2012.204 Although high levels of HIV diagnosis have been achieved in South Africa,206 only half of diabetes and hypertension cases have been diagnosed, and disease is seldom effectively controlled (figure 14). Platforms that integrate screening for these three diseases have the potential to address the urgent need for improved diagnosis of diabetes and hypertension.
Figure 14: South African health statistics and the potential effects of multidisease screening.
(A) HIV estimates are obtained from the Thembisa model estimates for 2016, whereas diabetes and hypertension estimates are from the 2012 South African National Health and Nutrition Examination Survey. Controlled on treatment is defined in the case of HIV as having a viral load less than 400 copies per mL, and in the case of diabetes as a HbA1c concentration less than 7%. Estimates of treatment coverage and control rates among hypertension cases are obtained from a national survey in 2010. (B) The expected fraction of screened individuals testing positive, the expected fraction of newly diagnosed individuals, and the expected fraction of individuals linking to treatment services in the context of a multidisease screening programme implemented between 2018 and 2028.
Several mobile multidisease screening interventions have been piloted in South Africa207 (figure 15) and other African countries.208,209 To assess the potential effect and cost of introducing a mobile screening service for diabetes, hypertension, and HIV in South Africa, we extended an HIV model that was previously developed for South Africa, to incorporate diabetes and hypertension screening (panel 3). The model estimates that if such a multidisease screening programme were introduced in 2018–28, and reached 10% of South African adults each year, the programme would diagnose 492 000 new HIV cases, 1·21 million new diabetes cases, and 6·35 million new hypertension cases. Expressed as a fraction of individuals screened, the proportion newly diagnosed is expected to be lowest in the case of HIV and highest in the case of hypertension (figure 14). The total number of new treated HIV, diabetes, and hypertension cases would be expected to increase by 197 000, 543 000, and 2·1 million, respectively. As a result of the increase in HIV diagnosis and treatment rates, the incidence of adult HIV infection is expected to decrease by 3·5% in 2018–28, which is equivalent to 69 000 infections averted.
Figure 15: Differented care in South Africa—The Tutu Tester mobile screening programme for HIV, tuberculosis, diabetes, hyptertension, and body-mass index.
Credit: Desmond Tutu HIV Research Foundation/Alexis Dominguez
Panel 3: Modelling HIV, diabetes, and hypertension screening in South Africa.
Thembisa is an integrated demographic and HIV model developed for South Africa. The process of calibrating the model to data on HIV prevalence, HIV diagnosis, and mortality has previously been described.206,210 In this analysis, the prevalence of diabetes (defined as a concentration of HbA1c ≥6·5%) and hypertension (defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or current use of blood pressure medication) was estimated from the age-specific and sex-specific prevalences measured in the 2012 South African National Health and Nutrition Examination Survey (SANHANES).205 Age-specific and sex-specific rates of diagnosis for diabetes and hypertension were estimated from the same source. The average annual proportion of the adult population (aged 15 years and older) screened was assumed to be to 10%.208,209,211,212 This average proportion was scaled by age-specific and sex-specific adjustment factors based on similar screening programmes in Uganda.209 Uptake of screening was assumed to be independent of disease status and further assumed to be constant over the period from July 1, 2018, to June 30, 2028. The proportion of newly-diagnosed individuals who linked to care and started treatment soon after diagnosis was set to 40% in the case of HIV,213 45% in the case of diabetes, and 33% in the case of hypertension.210 A more detailed description of the model is provided in the appendix, as are sensitivity analyses with lower programme coverage and lower sensitivity and specificity of screening for hypertension and diabetes.
Kenya: screening for HIV with hypertension and diabetes
Kenya has the joint fourth largest HIV epidemic in the world, with 1·6 million people living with HIV in 2016.1 Although the prevalence of HIV in adults (5·4%) is lower than in South Africa (18·9%),1 the proportion of HIV that remains undiagnosed (30%)214 is higher in Kenya than in South Africa (13%). As is the case across sub-Saharan Africa, Kenya is experiencing an increasing burden of NCDs, which account for 27% of all deaths and 50% of hospital admissions.215 Much of this increase can be attributed to lack of screening and early detection of NCDs, which in turn leads to late diagnosis and poor treatment outcomes. For example, 56% of people in Kenya have never had their blood pressure measured, and only 22% of individuals with increased blood pressure or hypertension diagnosis report taking treatment.215 These enormous gaps in screening and treatment of NCDs are occurring in the context of an overwhelmed health system and an HIV epidemic that has yet to be brought under control.
A modelling scenario for integrated screening for HIV, hypertension, and diabetes in Kenya is shown in panel 4. At baseline and in the absence of additional intervention, the incidence of HIV infection in Kenya is estimated to decrease by 13% in the next decade. The integration scenario models a gradual increase in HIV screening and coverage of ART over time, reaching a population-level coverage of 78% in 2028. This corresponds to an additional 4 117 912 person-years on ART in the next decade. With this intervention, the number of new HIV infections is projected to decrease by 44% by 2028, corresponding to 216 655 new infections and 244 374 AIDS deaths averted (figure 16). Sensitivity analyses of ART uptake suggested that even with only 40% treatment initiation, the integration scenario would reduce the incidence of HIV infection by 23%. Given the longer life expectancy of HIV-positive individuals receiving ART, the intervention results in only marginal improvements in HIV prevalence (1·4% reduction by 2028), despite the substantial reductions in new infections.
Panel 4: Modelling scenario for integrated screening for HIV, hypertension, and diabetes in Kenya.
The Spectrum package was used to explore the potential effect of a country-wide campaign to integrate screening for HIV, diabetes, and hypertension.
Baseline ART coverage in 2016 was maintained at a fixed level from 2018 to 2028.
Following the framework of project SEARCH,146 we modelled a joint community-outreach campaign for HIV and NCDs screening in the next decade (2018–28). We assumed that each year, a geographically distinct area of Kenya comprising 10% of the population would receive the programme, and that 90% of eligible adults aged 15 years or older would be successfully screened for HIV, diabetes, and hypertension.
To model the potential effect on HIV-related outcomes, we ambitiously assumed that this intervention could successfully initiate and retain 81% of newly diagnosed individuals on ART (ie, assuming a target level of 90% linkage and 90% engagement in HIV care after diagnosis). This corresponds to a 73% reduction in unmet need for ART in people living with HIV in the next decade, modelled as a linear increase in the ART coverage in each subgroup from 2018 to 2028.
Using a representative national survey,215 we estimated the proportion of adults with, aware of, and receiving medication for hypertension and diabetes. Based on the number of adults screened and the expected uptake of treatment, we estimated the effect of the integrated intervention on awareness of and treatment for hypertension and diabetes.
To assess cost-effectiveness, intervention costs were based on those reported in the SEARCH study,216 and the number of people receiving ART and HIV-related DALYs were obtained from Spectrum. As most treatment costs and DALYs are incurred later in life for NCDs, we report the change in discounted lifetime treatment costs and DALYs with and without intervention. These estimates are based on published treatment cost and DALY estimates,217 and are discounted at 3% per year. More details about the experimental scenarios are provided in the appendix.
Sensitivity analyses with less ambitious programme coverage (5% vs 10% of the national population per year), lower antiretroviral therapy uptake, and lower sensitivity and specificity of screening for hypertension and diabetes are also presented in the appendix.
NCD=non-communicable disease. ART=antiretroviral therapy. DALYs=disability-adjusted life-years.
Figure 16: Projected effects of HIV and NCD integration scenarios in Kenya.
(A-B) The projected effect of the intervention on the number of new HIV infections and AIDS deaths in Kenya in the next decade (2018–28). (A) Effects of the care cascade in intervention communities in 2018, and similar patterns of improvement in cascades are expected for each new set of intervention communities. (B) Improvements in care cascades in 2018 averaged for the whole country. National care cascades are expected to continue to improve through the end of programme in 2028. (C-D) The care cascades for HIV, hypertension, and diabetes in adults before and after first year (2018) of implementation in intervention communities and in Kenya, respectively. Intervention communities constitute representative 10% of the adult population (aged ≥15 years), with assumption of 90% screening uptake and treatment uptake after diagnosis of 81% for HIV, 23% for hypertension, and 45% for diabetes. NCD=non-communicable disease. ART=antiretroviral therapy.
Pairing NCD screening with HIV testing has important effects on the continuum of NCD care. Immediately after the first year of screening campaigns, treatment in the intervention communities increased from 10% to 47% for diabetes and from 8% to 27% for hypertension. First year effects would be less dramatic at the national level, but the national care cascades for hypertension, diabetes, and HIV will continue to improve as more communities are covered under this programme. Over the ensuing decade, this integrated screening and linkage-to-care intervention is projected to identify 686 000 individuals with previously untreated diabetes and 7·57 million individuals with previously untreated hypertension.
The per-person costs of the community health campaign are estimated at $21·03 for community census and HIV testing and $1·19 for hypertension and diabetes screening, all valued in 2016 $US. With an estimated 4·51 million individuals aged 15 years or older who were screened in 2018, total costs for community mobilization would be $56·8 million for HIV testing and $3·2 million for NCD testing. In 2018, the number of people receiving ART would increase by 67 000 at an additional cost of $16·9 million, and by 2028, 659 000 additional people would receive ART for $346·3 million in additional treatment costs (assuming no further price reductions for ART). Through 2028, the HIV-related components of the community health campaign would avert 6·5 million DALYs at a discounted cost of $1480 million, equivalent to $227 per DALY averted, making the intervention highly cost-effective relative to 2016 GDP per capita of $3155.
We estimate that 590 000 individuals with moderate or high blood pressure not currently on treatment will be identified in 2018. Assuming an uptake of hypertension treatment at 23%215 and discounted incremental lifetime health-care costs of $149 and $486 for moderate-risk and high-risk hypertension treatment, respectively, the hypertension screening and treatment would avert 160 000 DALYs at a cost of $37·2 million, corresponding to $232 per DALY averted. Over the 10-year modelling scenario, the population would increase, as would the costs and benefits, reaching $49·8 million and 215 000 DALYs averted in 2028.
Assuming a prevalence of 2·2% for diabetes in adult Kenyans,202 we estimate that blood glucose screening in conjunction with the HIV community health campaign would identify 53 000 individuals with diabetes at a marginal cost of $54 per case identified, with this cost jointly shared by hypertension screening. With standard glycaemic control for individuals with diabetes, the cost per DALY averted is about $944.218 As with the HIV-related components, the hypertension and diabetes components are estimated to be highly cost-effective.
From a costing perspective, integrated programmes will probably be cost-effective with respect to both HIV-related and NCD-related outcomes. From a policy perspective, in the context of limited resources at the national level and competing interests for allocation of available funding to different health sectors, integration of services for HIV and NCDs offers a promising solution to diagnose HIV and NCDs. Nonetheless, increased diagnosis will probably lead to increased demand for health-care services in both Kenya and South Africa and will require substantial resources to meet the demand.
Nigeria: integration of HIV with reproductive health services
With more than 3·2 million people infected with HIV, Nigeria has the second largest HIV burden in the world. Unlike South Africa and Kenya, where more than 95% and 80% of eligible women receive prevention of mother-to-child transmission (PMTCT) services, respectively,1 PMTCT coverage in Nigeria has stagnated at 30%, resulting in an estimated 75 000 new infant HIV infections per year.219 Similarly, only 15% of Nigerian women aged 15–49 years use contraception for limiting and spacing of births. On average, a Nigerian woman gives birth to 6·5 children in her lifetime. As 16% of currently married women have an unmet need for family planning services, linking all married women who wish to space or limit their children to contraceptives would increase the prevalence of contraceptive use to 31%.220
The modelling scenario for integration of HIV and reproductive health services in Nigeria is shown in panel 5. In the absence of additional intervention, the baseline model estimates a 37% increase in the number of women of reproductive age in the next decade (2018–28) because of population growth, resulting in a growing need for family planning services in Nigeria. Expansion of contraceptive use to 31% was estimated to result in a 15·5% reduction in the total fertility rate (ie, from 6·5 children per woman at baseline to 5·49 children per woman in 2028; figure 17). This reduction in fertility would avert more than 8 million cumulative unintended pregnancies between 2018 and 2028 when compared with a baseline level of contraceptive use of 15%. Increasing PMTCT coverage to 90% of HIV-positive pregnant women in Nigeria by 2023 is estimated to reduce the number of infants acquiring HIV by 56% (figure 17), averting more than 237 500 vertical HIV transmissions cumulatively in the next decade. Expansion of PMTCT services along with family planning resulted in a further estimated 12% reduction in HIV incidence at the national level, which is equivalent to averting more than 295 500 new HIV infections by 2028 (figure 17) and a 3·5% reduction in population-level HIV prevalence.
Panel 5: Modelling scenario for integration of HIV and reproductive health services in Nigeria.
Spectrum’s demproj, AIM/GOALs, and FamPlan modules were used to create three scenarios describing integration of HIV and reproductive health services in Nigeria.
At baseline, the 2016 PMTCT coverage (about 26% of HIV-positive pregnant women) and contraceptive use prevalence (15%) were maintained at fixed levels from 2018 through 2028. We further modelled the transition away from PMTCT Option B (provision of ART during pregnancy and breastfeeding) by year 2020, and replaced that with an equal coverage through Option B+ programmes (providing lifelong ART for all pregnant women living with HIV).
For family planning, the prevalence of contraceptive use was scaled up linearly from 15% in 2018, to 31% in 2023 (to meet the unmet need for contraceptives by married Nigerian women),220 and was maintained at that fixed level until 2028.
For PMTCT, the proportion of HIV-positive pregnant women receiving ART through Option B+ was scaled up linearly from 16% in 2016, to an ambitious target of 90% coverage in 2023 (in accordance to UNAIDS and PEPFAR targets) for 95% PMTCT coverage221 and was maintained at that target level until 2028.
For combined PMTCT and family planning, starting in 2018, both PMTCT coverage and contraceptive use were scaled up linearly as described for each individual scenario above.
To assess cost-effectiveness, PMTCT costs were based on PEPFAR estimates.222 HIV-related treatment costs and DALYs were estimated as described for Kenya (panel 4). The effects of increased family planning access were estimated using the Impact Now model.223 Family planning costs were based on a person-year cost of US$3·48 for a mix of contraceptive methods. Per-user family planning costs ($3·48), maternal and infant health-care costs averted ($3·15), and DALYs averted (0·35) were derived from the ImpactNow model.
PMTCT=prevention of mother-to-child-transmission. ART=antiretroviral therapy. DALYs=disability-adjusted life-years.
Figure 17: Projected effects of HIV and reproductive health services integration scenarios in Nigeria.
(A) Total fertility rate. (B) Number of unintended pregnancies averted. (C) Number of infants infected with HIV. (D) Cumulative number of new HIV infections in Nigeria. Scenarios include expansion of family planning services by increasing the prevalence of contraceptive use from 16% at baseline to 31% in 2023, and expansion of PMTCT by increasing the coverage to reach 90% of HIV-positive pregnant women in Nigeria by year 2023, compared against baseline levels in Nigeria. PMTCT=prevention of mother-to-child transmission.
Increasing access to PMTCT would require $31·3 million in 2018, which includes $17·3 million in screening costs and $14·0 million in PMTCT costs. With full scale-up in 2023, $172·2 million would be required to identify and deliver PMTCT to an additional 142 000 HIV- positive pregnant women. Although the number of women receiving PMTCT would decrease through 2028, the costs would increase slightly to $181·0 million because of the larger number of pregnant women requiring screening as the prevalence of HIV decreases. From 2018 to 2028, the scale-up of PMTCT would avert 8·6 million DALYs at a cost of $169 per DALY averted and, compared with the GDP per capita of $5861 in 2016, would be highly cost-effective.
An increase in contraceptive use from 16% to 31% would require an additional investment of $4·8 million in 2019, increasing to $27·4 million in 2023 with greater coverage and population growth, and increasing further to $32·3 million in 2028 with continued population growth. About 90% of the increased investments needed for scaled-up contraceptive services would be offset by maternal and infant health-care costs averted through family planning. 484 000 DALYs averted per year in 2019 would increase to 3·2 million DALYs averted per year in 2028, with a total of 22·9 million DALYs averted. Increased access to family planning is highly cost-effective and approaches the cost-saving threshold, with a net cost of about $0·95 per DALY averted.
These results suggest that substantial improvements can be achieved by integrating HIV and reproductive health services for women in Nigeria, averting, in the case of HIV, more than 230 000 new infections in the next decade. A crucial first step for ensuring the successful integration of HIV with reproductive health services is addressing the underlying weaknesses of the existing health services in Nigeria because more than a third of pregnant Nigerian women do not attend antenatal care services during pregnancy,224 with especially low coverage in poor, rural, currently married, and less educated populations. Integration can aid in expanding the coverage of family planning services in Nigeria, which in turn can avert a large number of unwanted pregnancies and unsafe abortions.
India: Integrated management of HIV and sexually trainsmitted infections for men who have sex with men (MSM) and female sex workers (FSW)
With more than 2 million people with HIV and an estimated 62 000 AIDS-related deaths in 2016, India has the third largest HIV epidemic in the world.1 Although most HIV transmissions are driven by heterosexual sex (accounting for 87% of new infections in 2015), the epidemic is concentrated in high-risk groups, particularly in FSW and their clients, MSM, and people who inject drugs in some sub-national settings. In 2016, an estimated 2·2% of FSW in India had HIV, although there are considerable geographic variations, with a prevalence in this population as high as 17·9% in Maharashtra and 13·1% in Manipur in 2013. Similar variations are reported for other sexually transmitted infections, with the prevalence of syphilis ranging from 3·1% to 51·0% for FSW and from 3·5% to 28·0% in MSM and transgender people.225
India is committed to the prevention and control of epidemics of sexually transmitted infections and HIV. The Avahan initiative is estimated to have averted 202 000 HIV infections in 4 years.226 Respondent-driven sampling has been used to engage MSM and people who inject drugs in testing services for sexually transmitted infections and HIV in India.227 In addition to expanding the reach of these screening and prevention programmes, integration of sexually transmitted infections and HIV can also facilitate linkage to, and engagement in, HIV care through a combination of medical, social, and economic interventions targeting key populations. We applied the Spectrum model to estimate the epidemiological and economic effect of such an approach on outcomes related to HIV and sexually transmitted infections in MSM and FSW in India (panel 6).
Panel 6: Modelling scenario for the integration of HIV and sexually transmitted infections in India: MSM and FSW.
The Spectrum package was used to model HIV transmission in MSM and FSM in India. An intervention scenario was modelled to represent integration of services for HIV and sexually transmitted infections for these populations.
At baseline, ART coverage was maintained at the estimated 2016 level from 2018 to 2028.
Using data from the Avahan programme, we modelled a joint community-outreach campaign for HIV and sexually transmitted infection screening in MSM over a 5-year period (2018–23). On the basis of reported levels of population coverage from Avahan, we assumed that this programme could reach 60% of MSM and 90% of FSM and will provide an infrastructure for maintaining all improvements in HIV treatment and PrEP coverage over the subsequent 5 years (2024–28). To model the potential effect on HIV-related outcomes, we assumed that this integrated intervention can successfully deliver ART to 81% of individuals with diagnosed HIV who are not currently receiving HIV treatment (ie, 90% linkage and 90% engagement). This corresponds to a 54% and 81% reduction in the unmet need for ART in HIV-positive MSM and FSM, respectively. We modelled this effect as a linear increase in the rate of ART coverage from 2018 to 2023. Furthermore, to estimate the potential effect of PrEP, we modelled two additional scenarios for implementation of oral PrEP in these two populations at low (10%) and high (30%) levels of coverage. In these scenarios, PrEP coverage was increased linearly from 2018 to 2023 (continuing at fixed levels through 2028), and PrEP adherence was assumed at 50% for those individuals on PrEP.
Because of a lack of representative data on the incidence and prevalence of sexually transmitted infections in these two populations, we limited our analysis to study the effect of integrated programmes for screening and treating syphilis infection. Moreover, because of the complexity of modelling the co-epidemics of syphilis and HIV infections in India, we chose a simplified approach for estimating the effect of the intervention on programme participants only and did not estimate the effect on secondary infections averted. Using an array of available estimates from the scientific literature, we estimated the national prevalence of syphilis at 5·8% of FSW and 3·5% of MSM (appendix).
Following the methodology proposed by Solomon and colleagues,227 we modelled a scenario for reaching these two populations through respondent-driven sampling, whereby members of these populations are incentivised to visit programme sites and refer others to programme sites, at an estimated cost of US$20·33 per person tested. ART costs were estimated at $126·06 per person-year. PrEP costs of $91 per person-year were based on Stover,228 with updated drug cost estimates for tenofovir disoproxil fumarate plus lamivudine.229 Syphilis testing and treatment costs for FSW and MSM were estimated at $3·50 and $9·30, respectively.230 DALYs per prevalent syphilis case (0·64) were calculated using the method by Korenromp and colleague,230 using estimates of the global DALYs for syphilis231 and the global prevalence of syphilis.232
MSM=men who have sex with men. FSW=female sex workers. ART=antiretroviral therapy. PrEP=pre-exposure prophylaxis. DALYs=disability-adjusted life-years.
The integrated intervention is projected to gradually increase coverage of ART in the next 5 years, reaching coverage of 73% of MSM (compared with 43% at baseline) and 81% of FSW (compared with 29% at baseline). This corresponds to an additional 1 180 000 person-years on ART in the next decade. Through this intervention, the number of new HIV infections in MSM is projected to fall by 34% by 2028, corresponding to 43 000 new infections averted (figure 18), and 59 000 AIDS-related deaths will be averted. Given the projected rapid decrease in HIV incidence in FSW at baseline, the intervention is estimated to reduce HIV incidence only marginally (1·6% reduction in 10 years) but AIDS-related deaths substantially (by 81%), averting 6200 deaths in FSW. At a national level, this intervention reduces the number of new transmissions in the population by 7%, averting 51 000 new infections and 81 000 AIDS-related deaths in the next decade. In sensitivity analyses with 40% (vs 81%) uptake of ART, HIV incidence was roughly halved, with HIV incidence falling by 17% in MSM, by 0·4% in FSW, and by 3–2% at a national level.
Figure 18: Projected effects of HIV and sexually transmitted infection services integration scenarios for MSM and FSW in India.
The number of new HIV infections in (A) MSM and (B) FSW. Intervention scenarios include expansion of ART coverage in MSM (from 43% to 73%) and FSW (from 29% to 81%) with no PrEP, at a low PrEP coverage of 10%, and at a high PrEP coverage of 30%. Each scenario is modelled as a gradual increase in coverage of ART and PrEP in MSM and FSW from 2018 to 2023, continued at a fixed coverage afterward (2024 to 2028). MSM=men who have sex with men. FSW=female sex workers. PrEP=pre-exposure prophylaxis. STI=sexually transmitted infections.
Implementation of PrEP will further improve the epidemiological outcome, although the extent of PrEP-related benefits is limited by the preventive effects of expanded ART coverage. For example, provision of PrEP at a low-coverage or high-coverage level (corresponding to more than 11·1 million and 33·6 million person-years of PrEP from 2018 to 2028, respectively) will avert 6800 and 20 000 additional new HIV infections in MSM and FSW compared with ART scale-up alone. With respect to other sexually transmitted infections, the integrated HIV-syphilis programme is projected to diagnose and treat more than 510 000 cases of syphilis in FSW (5·8% prevalence) and MSM (3·5% prevalence) in 2018 alone. The number of cases detected and treated in subsequent years will depend in part on the rate of infection and reinfection in individuals who were previously treated.
In the intervention scenarios, recruitment and HIV testing and referral costs would increase from $34·1 million in 2018 to $190·4 million in 2028. With 81% uptake of ART by individuals who are diagnosed, the number of people receiving ART would increase over the baseline by 55 000 in 2018 and by 107 000 in 2028. Excluding PrEP, the programme of testing and reatment would be highly cost-effective, averting 2·7 million DALYs at $656 per DALY averted (per-capita GDP of $6571 in 2017), with an incremental cost of $1797 million over the base-case cost of $1·315 million. Neither of the PrEP scenarios (10% or 30% coverage) would be cost-effective, with an incremental cost of $21 531–58 812 per DALY averted compared with the no-PrEP scenario.
The addition of syphilis screening and treatment to the HIV testing and treatment (but no-PrEP) scenario would probably be highly cost-effective. In 2018, the programme would test 1·68 million individuals and diagnose and treat 76 000 cases at an incremental cost of $6·6 million. At an estimated 0·64 DALYs per prevalent case, we estimate that this scenario would avert 49 000 DALYs in the first year at $135 per DALY averted.
Our results suggest that the modelled interventions would lessen the burden of HIV and syphilis infection in MSM and FSW in India. Increasing the coverage of ART in MSM to 54% would avert substantial numbers of new HIV infections and AIDS-related deaths. Further addition of PrEP can provide marginal improvements in HIV-related outcomes, although the population-level implementation of PrEP in these populations in India is not cost-effective. As suggested by previous studies, PrEP should be tailored to high-risk populations who stand to benefit the most from it. On the other hand, despite assuming a higher level of achievable coverage in FSW (up to 90%), similar programmes resulted in smaller improvements to HIV incidence over time in this population, primarily stemming from the decreasing prevalence of HIV in FSW in India. Integrated programmes provide an opportunity for mass screening and treatment of other sexually transmitted infections, including syphilis. Even ignoring the potential effect for averting future syphilis transmissions and reducing the transmissibility of HIV infection in coinfected cases, the integrated programme was cost-effective.
Our results for these scenarios in India are limited by several restricting assumptions. First, we excluded the existing geographical heterogeneities in patterns of the prevalence of sexually transmitted infections and HIV in key populations in India (as evident by a wide range of reported HIV prevalences in MSM, ranging from 2·5% in Tamil Nadu to 14% in Andhra Pradesh in 2016), which can have important implications for the estimated effect and costs of integrated programmes in different part of the country. Future research could build on our findings by investigating the effect of integration in various Indian cities and states representative of different epidemiological profiles and health-system infrastructures. Further-more, our simplified approach to estimating the effect of intervention on sexually transmitted infections is limited by restricting assumptions that might underestimate the effect of interventions on the sector. Future studies applying transmission models of HIV-sexually transmitted infection co-epidemics in India might provide more representative estimates of epidemiological outcomes.
Russia: integrated harm reduction and ART for people who inject drugs
An estimated 1% of the Russian population has HIV, but the prevalence of HIV is much higher in people who inject drugs, with city-level estimates for this population ranging from 3% to 64%. 233 Several regions in the Ural and Siberian Federal Districts have the highest incidences of new HIV diagnoses in the country, most of which are in people who inject drugs.76 People who inject drugs in Russia are also at high risk of overdose (estimated at 2 overdoses per 100 person-years).234
Although harm reduction and HIV treatment services would clearly benefit the country’s estimated 1·88 million people who inject drugs, considerable barriers prevent these services from being scaled up. Non-naltrexone MAT remains illegal, despite evidence that it reduces HIV transmission risk,235 fatal overdose,236 drug-related criminal behaviour,237 and discontinuation of ART;238 and NSPs are legal but badly underfunded, providing on average only 1–3 syringes per person who injects drugs annually.115 Coverage of ART in people who inject drugs is low,239 with evidence of denial of therapy to active users of drugs in some settings,240 and 2017 Russian clinical guidelines recommend possibly delaying treatment initiation in patients with evidence of severe drug addiction,241 which could act as a barrier for the scale-up of ART in people who inject drugs.
We examined the effect of policy changes enabling the expansion of integrated harm reduction and HIV services on HIV and fatal overdose among people who inject drugs in two Russian settings. A dynamic transmission model of HIV among people who inject drugs (panel 7) was developed to assess the effect of scaled-up harm reduction (NSP or non-naltrexone MAT, or both), alone or integrated with scaled-up ART for people who inject drugs. For illustrative purposes, we modeled two different HIV epidemic settings (figure 19): (1) Omsk, a major city in the Siberian Federal District with evidence suggestive of a growing epidemic; and (2) Ekaterinburg, Russia’s fourth largest city located in the Ural Federal district with a stable and high-prevalence epidemic in people who inject drugs.
Panel 7: Modelling methods: integrated harm reduction and ART in Russia.
We used a dynamic epidemic model of HIV in people who inject drugs to assess the potential effect of integrated harm reduction and HIV services for this population in Russia. Full methodological details are in the appendix.
A dynamic and deterministic model of HIV transmission in this population was developed, incorporating injecting and sexual transmission in people who inject drugs. People who inject drugs enter due to injecting initiation and exit due to cessation of injecting or death (overdose, HIV-related death, or non-HIV background mortality). The model was stratified by HIV disease stage (uninfected, acute, latent, pre-AIDS, AIDS), ART status, risk (low or high, defined as history of incarceration on the basis of survey data for both settings), sex, and harm reduction access (off or on). We assumed no baseline coverage of any harm reduction and minimal ART coverage (26% of HIV-infected people who inject drugs in 2014). We modelled harm reduction scale-up through exploring different scenarios of harm reduction programme availability: high coverage NSP only, non-naltrexone MAT only, or combined NSP and MAT service, either alone or integrated with expanded ART for people who inject drugs. NSP and non-naltrexone MAT were assumed to reduce injecting-related HIV transmission risk.
Non-naltrexone MAT was additionally assumed to increase ART recruitment rates, reduce treatment drop-out, and reduce rates of fatal overdose. However, we also incorporated increased mortality risk within the first 4 weeks of MAT initiation and discontinuation. ART was assumed to reduce HIV-related mortality, as well as sexual and injection-related transmission.
The model was calibrated to two cities with differing epidemic profiles in Russia: Omsk (high but expanding HIV epidemic in people who inject drugs) and Ekaterinburg (very high but stable HIV epidemic). For each setting, the model was calibrated to multiple timepoints of HIV prevalence in people who inject drugs, stratified by sex and risk (incarceration history, when available), ART coverage in 2014, proportion high risk (ever incarcerated), and the estimated proportion of transmission related to sexual risk. Because of uncertainty, most parameters were sampled from underlying distributions, generating 100 parameter sets. For each parameter set, the model was calibrated to the data using an optimisation solver and minimising the sum log likelihood. For each of these 100 calibrated parameter sets, model scenarios were run and projections presented as means and 2·5–97·5% intervals.
We examined the effect of the following intervention scale-up scenarios: (1) no harm reduction base-case; (2) NSP for 50% of people who inject drugs; (3) MAT for 25% of people who inject drugs; (4) MAT for 50% of people who inject drugs; (5) combination NSP-MAT for 50% of people who inject drugs; and (6) combination NSP-MAT for 50% of people who inject drugs integrated with scaled-up ART for HIV-infected people who inject drugs on harm reduction (recruited at three times current rates). We tracked the effect on HIV infections and fatal overdoses in people who inject drugs.
The percentage of new HIV infections and fatal overdoses in people who inject drugs averted in the next decade was calculated by comparing the cumulative projected number of events from 2018 to 2028 with intervention scale-up compared with the base-case.
ART=antiretroviral therapy. NSP=provision of clean needles and syringes. MAT=medication-assisted therapy. HCV=hepatitis C virus.
Figure 19: Model projections of the effect of integrated harm reduction and HIV services in two Russian cities.
Model projections of (A) median HIV prevalence in PWID in Omsk, Russia, (B) median HIV prevalence in PWID in Ekaterinburg, Russia, (C) proportion HIV infections averted in PWID in 2018–28, and (D) proportion of fatal overdoses averted in PWID in 2018–28 with various levels of intervention scale-up. Scenarios include no harm reduction or ART (base case), expansion of NSPs to 50% coverage in PWID (NSP 50%), expansion of MAT to 25% or 50% of PWID (MAT 25%; MAT 50%), combination MAT and NSP to 50% of PWID (MAT+NSP 50%), or combination MAT and NSP to 50% of PWID integrated with ART recruited at three times the base-case recruitment rate of HIV-infected PWID on harm reduction per year from 2018. Observed HIV prevalence data in PWID shown as black circles and 95% CI; dashed lines are 2·5–97% uncertainty bounds. Box plots indicate the median (middle line) projections, 25–75% percentile range (boxes), and 2·5–97·5% percentile range (whiskers), and median estimates reported above box plots. PWID=people who inject drugs. ART=antiretroviral therapy. MAT=medication-assisted therapy. NSP=needle and syringe programme.
Removal of structural and funding barriers in harm reduction provision could have a substantial effect on both HIV and fatal overdose. Expanding high coverage NSP to 50% of people who inject drugs could avert 36% (2·5–97·5th percentile 15–51%) and 23% (7–33%) of new HIV infections in people who inject drugs in the next decade in Omsk and Ekaterinburg, respectively (figure 19). A legal change allowing non-naltrexone MAT would avert both HIV infections and prevent fatal overdose. Scaling up non-naltrexone MAT to 50% coverage could avert 36% (2·5–97·5th percentile 21–49%) and 21% (11–29%) of HIV infections in Omsk and Ekaterinburg, respectively, and prevent about 32% (26–37%) of fatal overdoses in 10 years in Omsk and Ekaterinburg. About half the effect on HIV and overdoses would be achieved if MAT were only scaled-up to 25%. In combination, scale-up of MAT and NSP at 50% coverage could avert 48% (2·5–97·5th percentile 28–60%) and 33% (23–39%) of HIV infections in people who inject drugs at 10 years in Omsk and Ekaterinburg, respectively.
Scale-up of combination MAT plus NSP to 50% of people who inject drugs and integrated ART (recruiting HIV-infected people who inject drugs in harm reduction at three times the current recruitment rates) would avert 53% (2·5–97·5th percentile 40–65%) and 36% (25–45%) of new HIV infections in 10 years in Omsk and Ekaterinburg, respectively.
Given the paucity of existing HIV prevention services for people who inject drugs, the economic implications of the expansion scenarios described are uncertain. However, methadone is cost-effective in neighbouring Ukraine242 and Kazakhstan,243 at an estimated annual cost of US$250–415 per person who injects drugs (2017 US$). During the period when the Global Fund invested in HIV programmes in Russia, the cost per needle exchanged in the country was $0·38.244 Scaling up MAT and NSPs to 50% of the 1·88 million people who inject drugs in Russia could cost $333–521 million annually. Additionally, the cost of ART regimens varies from $3050–4290 per year in Russia,245 and modelling has predicted ART provision for people who inject drugs would be cost-effective in Russia.
Our work supports findings from previous studies, which indicate that removing policy and programmatic barriers and expanding HIV and harm reduction services to people who inject drugs in Russia would have a substantial effect on HIV,246 and reveals marked benefits in preventing fatal overdose. Our analysis does not take into account likely additional benefits that scaled-up harm reduction services would have on preventing HCV transmission or recidivism, which are likely to be substantial, as an estimated 69% and 40% of people who inject drugs in Russia have a history of HCV infection and incarceration, respectively.178,247,248
These modelling exercises show multiple health benefits that could be achieved through integrating services for HIV and other health conditions, and they also highlight the crucial need for setting-specific approaches to integration. In settings such as Kenya and South Africa, with generalised HIV epidemics and increasing burden of underdiagnosed and undertreated NCDs, integrated community-based screening could yield substantial benefits in identifying and treating hypertension, diabetes, and HIV. The realisation of such benefits, however, will be limited by the capacity of the health-care system to provide appropriate treatment. In settings like Nigeria with low antenatal care coverage and substantial mother to child HIV transmission, integration of PMTCT and family planning services could reduce HIV infections in infants and in the population as a whole as well as prevent unintended pregnancies. Increasing PMTCT coverage will require both access to and uptake of care. The former might require substantial infrastructure and human resources investments, and the latter could benefit from scale-up of peer support or congregation-based interventions.
In countries with concentrated epidemics in FSW and MSM, integrating testing and treatment for sexually transmitted infections and HIV should be considered. Modelling in India suggests that this approach would be cost-effective in addressing HIV and syphilis infections in both sex workers and MSM. Although we have modelled a streamlined approach, a more comprehensive intervention such as Avahan could prove more cost-effective. In some countries, few or no services are available for integration for highly affected populations. For example, in Russia, where there is a high concentration of HIV in people who inject drugs, the harm reduction, drug treatment, and HIV services for this population are either illegal or reach few in need. As such, there is an urgent need in Russia first for legal changes and capacity building to provide basic health services for people who inject drugs, ideally in an integrated programme that could reduce HIV, HCV, fatal overdose, and mass incarceration. Future work should consider the optimal integration strategy in each country based on HIV epidemiology and the health-care system, and models of integration will be improved through increased understanding of barriers and facilitators of integration and will depend on how well different integration scenarios can achieve economies of scope and scale. The potential benefits of integration are substantial, but they are only realisable with the bridgingof now-siloed health-care strategies.
The future of global health and HIV: governance and financing
Capturing and sustaining the benefits of a more integrated approach to HIV and global health, as demonstrated in the Commission’s modelling studies, will demand governance approaches that enable the world to give health the priority it deserves. Additionally, substantial, new, and reliable financing will be necessary to lay the foundation for sustainable health for all.
Governance for sustainable global health
The path towards a robust governance system for global health suitable for the era of sustainable health and development will inevitably be complicated, involve diverse stakeholders, and require honest dialogue about the strengths, weaknesses, and value added of all actors that have a role in health governance. For the new era of global health solidarity, a review of governance architecture is warranted. New institutional frameworks or approaches might be needed to elevate health as a central global priority and to usher into place comprehensive, integrated, and people-centred health systems that provide universal health access. To this end, the Commission urgently calls for an independent effort, authorised by an appropriate resolution of the UN General Assembly, to outline new institutional architecture and arrangements to carry forward the new era of global health solidarity envisaged by the Commission. A similar approach has been used before, when a UN resolution authorised the deliberative process that resulted in the closure of the WHO Global Programme on AIDS and the launch of UNAIDS.84 As a key component of the work of this independent governance review, actionable recommendations should be developed to make HIV governance less duplicative and costly and optimally accelerate progress toward ending the pandemic.
HIV and health governance
The HIV response arguably has the best developed mechanisms for governance of any aspect of the global health agenda. The institutional governance structure for HIV was developed rapidly, beginning in the mid-1990s at the global level, in response to the recognition of HIV as a global health emergency. Key actors in the pluralistic HIV governance approach include national health ministries, participatory national coordinating bodies (eg, national and provincial AIDS councils, country coordinating mechanisms), international governance bodies (eg, UNAIDS Secretariat and Cosponsors, including WHO), key donors (eg, Global Fund, PEPFAR, Bill & Melinda Gates Foundation, and other philanthropic entities), and organisations that focus on specific HIV priorities and functions (eg, Unitaid for market-shaping, Medicines Patent Pool for the issuance of voluntary licensing).
HIV governance includes elements that can inform the evolution of global health governance more generally, including its engagement of civil society and affected communities, institutional emphasis on a multisectoral response, and ethos of mutual accountability for achievement of concrete targets. HIV remains unusual within the global health field with respect to the extent of its formalised engagement with civil society (figure 20). Global health governance will be more responsive, effective, and politically potent if it includes strong participation from diverse non-governmental stakeholders. The greater integration of affected communities in global health governance, should it occur, will be one of the lasting legacies of HIV activism.
Figure 20: Community-led prevention providing combination prevention including pre-exposure prophylaxis to their peers in Thailand.
Credit: © Richard Nyberg, USAID.
However, the HIV experience also offers warning signs, as governance of the HIV response over time has become bloated and overly costly. The elaborate mechanisms for governance of the HIV response have not prevented a premature withering of commitment to the HIV fight. In addition to clearly outlining an overarching global health governance architecture, the independent panel proposed by the Commission should also examine how best to ensure that HIV governance is fit for the purpose of meeting current challenges and galvanising a sustainable response for the long term. Making HIV governance leaner, focusing governance on the overriding challenge of rebuilding political support and financing for HIV, and reviving African solidarity and leadership on HIV will be pivotal to hopes for rejuvenating the response.
Although efforts to strengthen governance of certain aspects of global health are growing, as reflected, for example, in the Third High-Level Meeting on NCDs planned at the UN in 2018, the gap in governance between HIV and non-HIV-related health issues is considerable. Neither NCDs, maternal and child health, nor any other single disease, for example, has advocacy benefits, partnership cultivation, and focused governance structure that is comparable to what UNAIDS brought to the HIV response. By contrast with HIV, key weaknesses of the global fight against cancer and other NCDs include the lack of strong global leadership and an inclusive social movement.249 The new governance approach for a new era of global health solidarity must include both overarching governance structures that address health-system weaknesses and promote comprehensive approaches that integrate services for diverse health conditions as well as sufficiently resourced and high-visibility disease-focused advocacy and governance structures to drive progress against the leading causes of death and disability (eg, cancer, cardiovascular disease, maternal and childhealth, tuberculosis).
The multidimensional governance structure developed for HIV includes key functions that will be required to ensure sustainable health for all. For example, the Global Fund is a highly successful platform for pooling resources for results-driven national programmes for HIV, tuberculosis, and malaria. Likewise, outside the HIV arena, GAVI, the Vaccine Alliance has had a pivotal role in increasing the rates of childhood immunisation250 by pooling and distributing resources for vaccination programmes in resource-limited settings. To accelerate health access and achieve the ambitious health aims of SDG 3, one or more similar mechanisms will be needed to mobilise, pool, and distribute resources for health systems strengthening, universal and equitable access to health commodities, and programmes to address priority health conditions such as NCDs and sexual and reproductive health.
Market-shaping measures will be needed to facilitate access to affordable medicines, vaccines, and diagnostics. In the case of HIV, the Medicines Patent Pool issues voluntary licenses for the generic manufacture of priority medicines, whereas Unitaid undertakes market-shaping interventions to enhance the affordability and uptake of essential interventions. To achieve worldwide expansion of access to health commodities to treat other leading causes of deaths, these market-oriented functions must extend well beyond HIV. To date, the voluntary licensing route has been used for HIV medicines and recently for direct-acting antivirals for HCV but not for medicines to treat cancer, hypertension, diabetes, or other diseases.
A sound health system is necessary but not sufficient for ending the HIV epidemic. Although opportunities for synergies and greater integration must be seized, categorical programmes and a dedicated governance system will remain essential if the world hopes to make good on its many HIV commitments.
Towards sound governance for sustainable health: key principles
Governance mechanisms must ensure that communities and broader civil society are fully engaged as partners in the conceptualisation, implementation, and monitoring of people-centred health systems. Health governance must strive for equity in access to global health goods and in health outcomes. Ushering in an era of genuine global health solidarity will demand a shared understanding of global health as a common enterprise, with institutional governance arrangements that are inclusive, democratic, and take into account the perspectives of all stakeholders.
Governance mechanisms for health must be sufficiently resourced, transparent, and accountable. Health governance should be multidisciplinary to ensure appropriate attention to social and structural factors and to promote integrated and coordinated approaches to multiple health challenges. Governance for health must be forward-looking and build capacity at national, global, and regional levels to anticipate and respond to future problems such as health emergencies or national transition from eligibility for international assistance. Transitions away from international support must be undertaken over a reasonable period of time (at least 10 years), and flexibility in transition approaches will be essential, enabling course corrections if and when problems arise (such as the collapse of community service systems for marginalised groups). Eligibility for international assistance should continue for countries with limited domestic capacity, heavy disease burden, and high-prevalence, high-incidence settings or populations.
Consistent with the vision of sustainable health for all, global health governance must ensure prioritised attention to the most susceptible and marginalised people. Global health governance must reflect and promote principles of human rights and gender equity. The new era of global health solidarity will demand governance mechanisms that elevate scientific evidence over ideology. Effective health governance evinces a willingness to speak hard truths when necessary.
Effective health governance functions as a faithful steward of public resources, implementing measures to protect against corruption, diversion of resources, and conflicts of interest. Effective health governance also inevitably depends on sound governance more generally, underscoring the need for countries to adhere to principles of democracy, open societies, inclusive and participatory processes, transparency, and accountability.
Financing for sustainable global health
Achieving universal health coverage will require substantial resources over and above amounts presently spent on health.251 For both HIV and global health generally, a continuation of current trend lines in financing will leave the world without sufficient resources to achieve the ambitious health targets of SDG 3.5,252,253
A new era of global health solidarity is needed, augmenting and fully synergising the contributions of each essential financing sector (eg, national governments, the international community, and the private sector) and continually reinforcing health investments as central to the international development agenda. Key to this era of global health solidarity will be rapid creation of a reliable, sustained mechanism for the equitable distribution of global public goods supported by global-level mechanisms to generate and pool resources.
As the decision to allocate resources inevitably reflects political choices, a political sea change is needed if the financing required to control communicable diseases and to effectively manage NCDs is to be mobilised. Drawing on experiences from the 1990s and early 2000s, when the world was jolted out of its early and lethargic response to the HIV epidemic, the HIV community must urgently join together to focus the world’s attention on the very real risk that we will lose the fight against HIV without major new investments. African leaders, in particular, must become broadly re-engaged in rejuvenating the HIV response because failure risks undermining the sustainable development agenda. Together, the HIV community and the broader global health field must undertake a comprehensive advocacy effort to position health as central to the broader global development agenda.
Domestic resources, which already finance most of health spending in LMICs, must serve as a cornerstone of financing in the era of sustainable health.254 Health spending, both per capita and as a share of GDP, is increasing in LMICs, increasing faster than the economy as a whole in the past 15 years.254 However, per-capita amounts spent on health in some LMICs are too low to deliver even the most basic package of health services,255 and the failure of most African countries to meet the Abuja Declaration target for health spending highlights the need for greater domestic investments and political support for health. Economic growth offers an opportunity for many LMICs to increase investments in health,4 but national leaders must demonstrate sufficient political will to allocate these dividends to health services. A recent analysis by the Global Burden of Disease Health Financing Collaborator Network4 found per-capita health spending in Bangladesh and Pakistan would roughly triple and double, respectively, were these countries to allocate towards health the share of national budget spent by similarly situated countries that place greater priority on health. Realisation of the vision of sustainable health for all will require LMICs to dig much deeper into their own pockets, and the World Bank and regional development banks must aid by expanding incentives to invest in health. To maximise domestic financing for health, systematic efforts are needed to avoid inefficiencies linked to the diversion of scarce resources through rent-seeking, clientelism, and corruption and to increase the accountability of national and subnational governments.
Universal health coverage can aid in off-loading certain health-related costs from the public sector and better enable public programmes to target services for the most susceptible communities. Although categorical HIV funding will remain essential for the foreseeable future to rejuvenate the response, funding for HIV programmes over time should progressively be integrated into broad-based social insurance systems, pooling resources through prepayment mechanisms, and innovative funding methods will be needed to incentivise integrated and multidisease approaches.
Careful planning and bridge funding are essential for an effective transition from donor dependence to national funding. However, the reality for the foreseeable future is that many countries, irrespective of whether bridge funding is available, are unlikely on their own to finance programmes that benefit marginalised populations that are politically powerless and that, in some cases, are subject to official harassment, institutionalized discrimination, and criminalisation. Both for HIV and for other priority health issues, the international community must continue to support essential prevention and treatment programmes for populations that would otherwise be left behind.
In addition to increased domestic financing, the new era of global health solidarity will require more, not less, engagement of the international community to foster collective action. In particular, robust international support for health will be essential to fund public goods, address cross-border health issues, and address health needs in low-income countries, where per-capita health spending, fiscal space, and health-system capacity will remain limited.4,252 Africa accounts for the overwhelming majority of low-income countries that will continue to need extensive international health assistance.
The pivotal role of international donors in jump-starting the global HIV response has been widely heralded, but the reality is that high-income countries remain parsimonious with respect to global health and development assistance. Although developed countries have pledged to allocate 0·7% of gross national income to development assistance, in 2015, only six countries (Denmark, Netherlands, Norway, Luxembourg, Sweden, and the UK) reached this target.256 Official development assistance for health is projected to increase only marginally through 2030,4 and concern with recent reductions in international HIV assistance is growing.
Renewing the engagement of the international community on health issues will require new thinking and prioritisation by international decision makers. The growth in antimicrobial resistance257 and increasing concerns about the global capacity to respond to health emergencies are reminding international donors of their own stake in having strong and durable health systems in place in all countries. New political leadership in countries such as Canada, France, and South Africa offers opportunities to revive political support for global health.
Consistent with the Commission’s findings, financing efforts in the new era of global health solidarity must achieve two aims: follow through on commitments made on HIV and mobilise sufficient resources to build strong, durable and people-centred health systems; and achieve universal health access. A robust and well funded Global Fund will be essential to hopes for achieving major progress in reducing new cases of HIV, tuberculosis, and malaria and associated mortality from all three diseases, and similar mechanisms will be needed to finance health-systems strengthening and service integration and to focus efforts on preventing leading global causes of death.
The USA is by far the largest single donor for health assistance, although in other countries such as the UK, the proportion of GDP devoted to health assistance is greater than the USA.258 Although the Trump administration has proposed major reductions in international health assistance, it is heartening that a bipartisan coalition in the US Congress has rejected these proposed cuts and confirmed US leadership on global health. In the omnibus budget approved in March, 2018, for the fiscal year 2018, Congress provided 34% more funding for global health assistance than requested by President Trump, holding steady funding for PEPFAR and the Global Fund and increasing appropriations for global health security. The US Government should maintain support for PEPFAR as a singular contribution to global health and wellbeing. In particular, US decision makers should move beyond the singular focus on achieving epidemic control in its 13 priority countries, especially as feared funding cuts to PEPFAR have not materialised. As a shared global responsibility, financing for health will require greater engagement by the EU and cultivation of major new donors such as China.
The future of global health and the HIV response: recommendations for sustainability
Creating people-centred health systems that ensure universal access to essential health services will demand concerted action by the entire global community. Only a genuine global movement, engaging diverse communities, linking together the HIV community and the global health field, and uniting multiple sectors in a common undertaking to improve the health and wellbeing of all people can realise the vision of sustainable health for all. Towards realisation of this vision, the Commission offers the following recommendations for immediate action.
1. The world must take immediate steps to rejuvenate the HIV response and follow through on its HIV-related commitments
All HIV stakeholders must join together to revitalise the HIV response, recognising both the urgency and the long-term nature of the global fight against HIV. Towards averting a potential resurgence of the epidemic, the reduction in HIV categorical funding, including major new investments by domestic governments and international donors, must be reversed.
HIV prevention must be revitalised and brought to scale. Whereas redoubling efforts to maximise the preventive and therapeutic benefits of ART through achievement of the 90–90-90 benchmarks, national governments, international donors, and HIV advocates must place much higher priority on primary HIV prevention to avert a resurgence of the epidemic. Communities should be resourced to lead prevention efforts, and stronger, prioritised investments in research are needed to accelerate progress towards the development of a preventive vaccine and a cure for HIV.
2. HIV should be carefully and strategically integrated within primary care and the broader global health agenda, with the scale and pace of integration geared to national and subnational circumstances and the needs of the populations in greatest need of HIV services
Immediate efforts are required to integrate HIV services with services for diseases that clearly overlap or are closely linked with HIV, and strengthened integration of contraceptive services, maternal child heath, and women’s sexual health and reproductive rights, which is cost-saving and of vital importance to women’s health, is urgent for successful control of perinatal transmission of HIV. Co-located and client-centred primary care services (one-stop shopping) should be scaled up. Effective integration will involve both the integration of HIV interventions in mainstream primary care and other service systems as well as the leveraging of HIV service systems for the co-located integration of other health services.
Effective integration of HIV with broader health systems is only possible if key defining features of the HIV response are preserved and mainstreamed; these include a respect for human rights and gender equality, sufficiently resourced participatory mechanisms for community inclusion and engagement, an ironclad commitment to ensure equitable access and to leave no one behind, and multisectorality to address the social and structural determinants of health
Population-focused service integration should be prioritised to address the needs of underserved populations. Adolescents of all genders and sexual orientations need adolescent-friendly information and services that are relevant, comprehensive, and address their sexual and HIV risks in a broader framework that is focused on their wellbeing, rights, and needs. Especially in settings where marginalised populations are not well served by mainstream health services, integration and co-location of services for these populations is urgently needed to enhance access to good-quality and culturally appropriate care and support. Notably in Russia and the wider eastern Europe and central Asian region, and any other setting where substantial injecting opioid use occurs, immediate efforts are needed to scale-up and integrate harm reduction services, overdose prevention, evidence-based drug treatment, and HIV, tuberculosis, and HCV services for people who inject drugs.
Efforts to integrate the HIV response more broadly into national health systems should be undertaken thoughtfully and follow careful study. Models should be used to predict the effects and cost-effectiveness of service integration scenarios.
Additional financing will be necessary to sharply reduce new HIV infections. Robust financing of the Global Fund and continuation of PEPFAR will be crucial to future prospects for reducing new HIV infections and AIDS-related deaths. Categorical HIV funding will remain essential for the foreseeable future and while transiting toward greater integration, although incremental steps should thoughtfully incorporate HIV funding over time into broader health insurance schemes. Innovative formulae to fund co-located, multidisease, and integrated services should be explored immediately.
No one must be left behind in the HIV response. All countries, including upper-middle-income and high-income countries, must accept the responsibility to provide life-saving services for all segments of their population, but in cases where countries fail in this responsibility, strong and flexible international mechanisms must be available to fund community-based services for marginalised groups. The HIV response must take bolder action to remove social and structural barriers to good health; this includes dismantling the global war on drugs, repealing laws that criminalise same-sex relations, and eliminating laws that criminalise HIV exposure, non-disclosure, or transmission.
3. The HIV response must make common cause with the global health field to achieve sustainable health for all
Prioritised and sustained efforts are necessary to make health systems fit for the purpose of delivering sustainable and people-centred care. National governments, international donors, health professionals, and health advocates should work to create health systems that are capable of ensuring universal health access and managing multiple health problems simultaneously, with services tailored to the needs of individual patients. Major new investments by countries and by international donors will be essential and should focus on key elements of health systems, including strengthened primary care, medical education and training, laboratory systems, systems for procurement and supply management, and quality assurance and continual performance improvement. Health systems should be transformed to create user-friendly and outcome-oriented service delivery platforms for children, adolescents, and men as well as for marginalised populations such as sexual minorities and people who inject drugs.
As an early step toward the long-term goal of integrating HIV and NCD services, existing HIV service platforms should be adapted to enable the delivery of co-located and integrated services for NCDs. HIV service providers must move beyond their singular focus on viral suppression as the goal of HIV care and treatment and recognise (through adapted training programmes, recon-figured clinics, and expanded monitoring and evaluation indicators) prevention and management of NCDs as core HIV outcomes.
High-level global dialogue that includes the private sector is urgently needed to mainstream a new paradigm for the development and provision of public goods. Drawing on experience from the HIV response, originator pharmaceutical companies should, as a standard practice, routinely enter into voluntary licenses for the full array of their product portfolios. Voluntary licenses for medicines and other public goods should maximise the coverage in LMICs using mechanisms such as sliding (ie, reasonable royalty payments to address differences among countries in ability to pay). The viability of a robust and flexible generics pharmaceutical industry must be preserved through, for example, the protection of flexibilities under international intellectual property agreements to ensure access to essential medicines and other public goods.
A review of global health governance architecture is needed. With the authorisation of a resolution of the UN General Assembly, a high-level, time-limited, and independent effort should be launched to develop a new global health governance architecture to catalyse and guide a new era of global health solidarity. As a subset of this review of global health governance, this independent exercise should generate concrete recommendations for HIV governance, with the goals of eliminating duplication, enhancing coherence, and refocusing global HIV governance on the overriding task of reviving global support and commitment for the HIV response. Mechanisms for global health governance must be democratic, inclusive, transparent, accountable, and grounded in a respect for human rights and gender equity, with specific steps that ensure the input and engagement of community, civil society, and other non-governmental actors.
The Commission recognises the urgent need to translate this vision of a new era of global health solidarity into a concrete action plan. The Commission will work to develop a clear, time-bound plan for implementing these recommendations in the coming year.
Supplementary Material
Key messages.
The HIV pandemic is not on track to end, and the prevailing discourse on ending AIDS has bred a dangerous complacency and may have hastened the weakening of global resolve to combat HIV
Existing HIV tools and strategies are insufficient, and although dramatic gains can be made through maximizing existing prevention and treatment strategies, the HIV pandemic is likely to remain a major global challenge for the foreseeable future
Tens of millions of people will require sustained access to antiretroviral therapy for decades to come, vigilance will be needed to prevent a resurgence of the epidemic as the largest-ever generation of young people age into adolescence and young adulthood, and intensified efforts are required to address HIV among populations and settings that are being left behind
Allowing the pandemic to rebound after achieving such remarkable progress would not only increase the human and financial costs of HIV, but it would potentially demoralise the global health field and diminish support for similarly ambitious global health undertakings
A rejuvenated global effort on HIV is essential; to renew and strengthen the global HIV response, the world’s impressive commitment to the scaling up of HIV treatment services must be matched by a similarly robust commitment to expanded access to HIV prevention
The HIV response must make common cause with the broader global health field to herald a new era of global solidarity for health, and specific action is urgently needed to respond to the rapidly rising health toll associated with non-communicable diseases, including taking health into account in the development of public policies of all kinds. HIV services should, where feasible, be integrated with broader health services, in co-located sites where possible, with the aim of improving both HIV-related and non-HIV-specific health outcomes; greater integration of HIV and global health must preserve and build on key attributes of the HIV response, including participatory community and civil society engagement and an ironclad commitment to human rights, gender equality, and equitable access to health and social justice
The new era of global health solidarity should focus on the development of robust, flexible, people-centred health systems to end communicable diseases, develop effective measures to address the steady rise of non-communicable diseases, achieve universal health coverage, provide coordinated services tailored to the needs of health service users, and effectively address the social and structural determinants of health
Acknowledgments
Declaration of interests
This work has been supported, in part, by grants to the International AIDS Society and to the Center for Public Health and Human Rights at Johns Hopkins Bloomberg School of Public Health from The Bill & Melinda Gates Foundation, The Ford Foundation, amfAR The Foundation for AIDS Research, The Elton John AIDS Foundation, and the US National Institutes of Health to the Johns Hopkins Center for AIDS Research, CFAR (P30AI094189).
Contributor Information
Linda-Gail Bekker, International AIDS Society, Geneva, Switzerland; Desmond Tutu HIV Centre, University of Cape Town, South Africa.
George Alleyne, NCD Alliance, Office of the Director, Pan American Health Organization, Washington, DC, USA.
Stefan Baral, Centre for Public Health and Human Rights, Department of Epidemiology, Johns Hopkins University, Baltimore, MD, USA.
Javier Cepeda, Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California-San Diego, San Diego, CA, USA.
Demetre Daskalakis, New York City Department of Health, New York, NY, USA.
David Dowdy, Department of Epidemiology, Johns Hopkins University, Baltimore, MD, USA.
Prof Mark Dybul, Centre for Global Health and Quality, Georgetown University School of Medicine, Washington, DC, USA.
Prof Serge Eholie, Department of Dermatology and Infectious Diseases, Medical School, Felix Houphouet Boigny Universty Abidjan, Cote d’Ivoire.
Kene Esom, HIV, Health and Development Group, United Nations Development Programme, New York, NY, USA.
Prof Geoff Garnett, HIV Deliver, Bill & Melinda Gates Foundation, Washington, DC, USA.
Anna Grimsrud, International AIDS Society, Geneva, Switzerland.
Prof James Hakim, Department of Medicine, University of Zimbabwe College of Health Sciences, Harare, Zimbabwe.
Diane Havlir, Division of HIV, Infectious Diseases and Global Medicine, Department of Medicine, University of California-San Francisco, San Fransisco, CA, USA.
Michael T Isbell, International AIDS Society, Geneva, Switzerland.
Leigh Johnson, School of Public Health and Family Medicine, Faculty of Health Sciences, University of Cape T own, Cape Town, South Africa.
Prof Adeeba Kamarulzaman, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia.
Parastu Kasaie, Department of Health, Behaviour and Society, Johns Hopkins University, Baltimore, MD, USA.
Prof Michel Kazatchkine, UNAIDS and Global Health Center, Graduate Institute, Geneva, Switzerland.
Nduku Kilonzo, National AIDS Control Council for Kenya, Nairobi, Kenya.
Prof Michael Klag, Department of Epidemiology, Department of Health Policy and Management, Johns Hopkins University, Baltimore, MD, USA.
Marina Klein, Division of Infectious Diseases, Faculty of Medicine, McGill University Health Centre, Montreal, QC, Canada.
Sharon R Lewin, The Peter Doherty Institute for Infection and Immunity, The University of Melbourne and Royal Melbourne Hospital, Melbourne, VIC, Australia.
Chewe Luo, HIV/AIDS Section, United Nations Children’s Fund, New York City, NY, USA.
Keletso Makofane, Harvard T.H. Chan School of Public Health, Harvard University, Boston, MA, USA.
Natasha K Martin, Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California-San Diego, San Diego, CA, USA.
Kenneth Mayer, The Fenway Institute, Harvard Medical School, Boston, MA, USA.
Gregorio Millett, The Foundation for AIDS Research, New York City, NY, USA.
Prof Ntobeko Ntusi, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Loyce Pace, Global Health Council, Washington, DC, USA.
Carey Pike, Desmond Tutu HIV Centre, University of Cape Town, South Africa.
Prof Peter Piot, London School of Hygiene and Tropical Medicine, London, UK.
Anton Pozniak, HIV Services, Chelsea and Westminster NHS Foundation Trust Hospital, London, UK.
Thomas C Quinn, Centre for Global Health, Johns Hopkins University, Baltimore, MD, USA; International AIDS Society-National Institute for Drug Abuse, Johns Hopkins University, Baltimore, MD, USA; Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institute of Health, MD, USA.
Prof Jurgen Rockstroh, HIV Clinic, Department of Medicine, University Hospital Bonn, Bonn, Germany.
Jirair Ratevosian, Department of Epidemiology, Johns Hopkins University, Baltimore, MD, USA.
Owen Ryan, International AIDS Society, Geneva, Switzerland.
Serra Sippel, Center for Health and Gender Equity, Washington DC, USA.
Bruno Spire, Aix Marseille Univ, INSERM, IRD, SESSTIM, Sciences Economiques & Sociales de la Santé & Traitement de l’Information Médicale, Marseille, France.
Agnes Soucat, Health Systems, Governance and Financing, World Health Organisation, Geneva, Switzerland.
Ann Starrs, Guttmacher Institute, New York, NY, USA.
Steffanie A Strathdee, Global Health Sciences, Department of Medicine, University of California-San Diego, San Diego, CA, USA.
Nicholas Thomson, Centre for Public Health and Human Rights, Johns Hopkins University, Baltimore, MD, USA; Nossal Institute for Global Health, University of Melbourne, VIC, Australia.
Stefano Vella, Center for Global Health, Istituto Superiore di Sanità, Rome, Italy.
Prof Mauro Schechter, Department of Preventative Medicine, Universidade Federal do Rio de Janeiro, Rio de Janerio, Brazil.
Prof Peter Vickerman, School of Social and Community Medicine, Bristol Medical School, University of Bristol, Bristol, UK.
Brian Weir, Department of Health, Behaviour and Society, Johns Hopkins University, Baltimore, MD, USA.
Prof Chris Beyrer, International AIDS Society, Geneva, Switzerland; Centre for Public Health and Human Rights, Department of Epidemiology, Johns Hopkins University, Baltimore, MD, USA.
References
- 1.AIDSInfo. Joint United Nations Programme on HIV/AIDS. http://aidsinfo.unaids.org (accessed June 14, 2018).
- 2.Piot P, Quinn TC. Response to the AIDS pandemic—a global health model. N Engl J Med 2013; 368: 2210–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016:a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Burden of Disease Health Financing Collaborator Network. Future and potential spending on health 2015—40: development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet 2017; 389: 2005–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UN. Ending AIDS Progress towards the 90–90-90 targets. Geneva: Joint United Nations Programme on HIV/AIDS, 2017. [Google Scholar]
- 6.Piot P, Abdool Karim SS, Hecht R, et al. , and the UNAIDS-Lancet Commission. Defeating AIDS—advancing global health. Lancet 2015; 386: 171–218. [DOI] [PubMed] [Google Scholar]
- 7.Corey L, Gray GE. Preventing acquisition of HIV is the only path to an AIDS-free generation. Proc Natl Acad Sci USA 2017; 114: 3798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2015 HIV Collaborators. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV 2016; 3: e361–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Global Health Observatory (GHO) data. http://www.who.int/gho/hiv/en/ (accessed June 14, 2018).
- 10.Najera JA, Gonzalez-Silva M, Alonso PL. Some lessons for the future from the Global Malaria Eradication Programme (1955–1969). PLoS Med 2011; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UN. Resolution adopted by the General Assembly on 25 September 2015 Transforming our world: the 2030 Agenda for Sustainable Development (A/Res/70/1). New York: United Nations General Assembly, 2015. [Google Scholar]
- 12.UN. The Millennium Development Goals Report 2015. New York: United Nations, 2015. [Google Scholar]
- 13.Dieleman JL, Schneider MT, Haakenstad A, et al. Development assistance for health: past trends, associations, and the future of international financial flows for health. Lancet 2016; 387: 2536–4. [DOI] [PubMed] [Google Scholar]
- 14.Murray C, Heisel W, Leach-Kemon K, Schneider M, Zhang R, Gubbins P. Financing Global Health 2010: Development assistance and country spending in economic uncertainty. Seattle, WA: Institute for Health Metrics and Evaluation, 2010. [Google Scholar]
- 15.Agyepong IA, Sewankambo N, Binagwaho A, et al. The path to longer and healthier lives for all Africans by 2030: the Lancet Commission on the future of health in sub-Saharan Africa. Lancet 2018; 390: 2803–59. [DOI] [PubMed] [Google Scholar]
- 16.World Bank. GDP growth (annual %). https://data.worldbank.org/indicator/NY.GDP.MKTP.KD.ZG (accessed June 14, 2018).
- 17.Evans D, Elovairilo R, Humphreys G. Health systems financing: the patch to universal coverage. Geneva: World Health Organization, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder LF, Amukele T. Medical laboratories in sub-Saharan Africa that meet international quality standards. Am J Clin Pathol 2014; 141: 791–95. [DOI] [PubMed] [Google Scholar]
- 19.WHO Health workforce requirements for universal health coverage and the Sustainable Development Goals. Geneva: World Health Organization, 2016. [Google Scholar]
- 20.UN. World population prospects The 2017 revision, key findings and advance tables. New York, NY: United Nations, Department of Economic and Social Affairs, Population Division, 2017 [Google Scholar]
- 21.International Atomic Energy Agency; Inequity in cancer care: a global perspective. Vienna: International Atomic Energy Agency, 2010. [Google Scholar]
- 22.Sharma V, Kerr SH, Kawar Z, Kerr DJ. Challenges of cancer control in developing countries: current status and future perspective. Future Oncol 2011; 7: 1213–22. [DOI] [PubMed] [Google Scholar]
- 23.<World Bank. GDP per capita (current US$). https://data.worldbank.org/indicator/NY.GDP.PCAP.CD (accessed June 14, 2018).
- 24.Marmot M, Friel S, Bell R, Houweling TA, Taylor S, and the Commission on Social Determinants of Health. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet 2008; 372: 1661–69. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Closing the gap in a generation-health equity through action on the social determinants of health Final report of the Commission on Social Determinants of Health. Geneva: World Health Organization, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Wahab M, Bourque JM, Pynda Y, et al. Status of radiotherapy resources in Africa: an International Atomic Energy Agency analysis. Lancet Oncol 2013; 14: e168–75. [DOI] [PubMed] [Google Scholar]
- 27.Heymann DL, Chen L, Takemi K, et al. Global health security: the wider lessons from the west African Ebola virus disease epidemic. Lancet 2015; 385: 1884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worknop C Provoking barriers: the 2014 Ebola outbreak and unintended consequences of WHO’s power to declare a public health emergency. Glob Health Gov 2017; 11: 7–26. [Google Scholar]
- 29.Gostin L The World Health Organization’s historic moment of peril and promise: reimaging a global health agency fit for purpose in the 21st century. Glob Health Gov 2017; 11: 57–75. [Google Scholar]
- 30.Freedom House. Freedom in the world 2017. Washington, DC: Freedom House, 2017. [Google Scholar]
- 31.UN. Global trends. Forced displacement in 2015. Geneva: United Nations High Commissioner for Refugees, 2016. [Google Scholar]
- 32.Alencar Albuquerque G, de Lima Garcia C, da Silva Quirino G, et al. Access to health services by lesbian, gay, bisexual, and transgender persons: systematic literature review. BMC Int Health Hum Rights 2016; 16: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watts N, Amann M, Ayeb-Karlsson S, et al. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health. Lancet 2018; 391: 581–630. [DOI] [PubMed] [Google Scholar]
- 34.Climate Action Tracker. CAT warming project. Global update, 2017. http://climateactiontracker.org/decarbonisation/intro (accessed June 14, 2018).
- 35.Dieleman JL, Graves C, Johnson E, et al. Sources and focus of health development assistance, 1990–2014. JAMA 2015; 313: 2359–68. [DOI] [PubMed] [Google Scholar]
- 36.Cotlear D, Napgal S, Smith O, Tandon A, Cortez R. Going universal: how 24 developing countries are implementing Universal Health Coverage reforms from the bottom up. Washington, DC: World Bank Group, 2015. [Google Scholar]
- 37.WHO World Bank. Tracking universal health coverage: first global monitoring report. Geneva: World Health Organization, World Bank, 2015. [Google Scholar]
- 38.WHO. Children: reducing mortality Fact sheet. Geneva: World Health Organization, 2017. [Google Scholar]
- 39.Victora CG, Requejo JH, Barros AJ, et al. Countdown to 2015: a decade of tracking progress for maternal, newborn, and child survival. Lancet 2016; 387: 2049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. Adolescent deaths and burden of disease. http://www.who.int/maternal_child_adolescent/epidemiology/adolescent-deaths-burden-disease/en/ (accessed June 14, 2018).
- 41.Dehne K, Riedner G. Sexually transmitted infections among adolescents: the need for adequate health services. Geneva: World Health Organization, 2005. [DOI] [PubMed] [Google Scholar]
- 42.WHO. Adolescent pregnancy Fact sheet. 2014. http://www.who.int/maternal_child_adolescent/epidemiology/adolescent-deaths-burden-disease/en/ (accessed June 14, 2018).
- 43.WHO. Global Health Observatory (GHO) data NCD mortality and morbidity 2018. http://www.who.int/gho/en/ (accessed June 14, 2018).
- 44.International Center for Research on Cancer. World cancer report 2014.Lyon: International Center for Research on Cancer, 2014. [Google Scholar]
- 45.Bloom D, Chisholm D, Jane-Llopis E, Prettner K, Stein A, Fiegel A. From burden to best buys: reducing the economic impact of non-communicable diseases in low- and middle-income countries. Geneva: World Economic Forum, 2011. [Google Scholar]
- 46.Nugent R, Bertram MY, Jan S, et al. Investing in non-communicable disease prevention and management to advance the Sustainable Development Goals. Lancet 2018; published online April 5. 10.1016/S0140-6736(18)30667–6. [DOI] [PubMed] [Google Scholar]
- 47.WHO. TB/HIV facts 2015. Geneva: World Health Organization, 2017. [Google Scholar]
- 48.WHO. Malaria Fact sheet. Geneva: World Health Organization, 2018. [Google Scholar]
- 49.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015; 386: 1546–55. [DOI] [PubMed] [Google Scholar]
- 50.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61 (suppl): S45–57 [DOI] [PubMed] [Google Scholar]
- 51.WHO. Global hepatitis report, 2017 Geneva: World Health Organization, 2017 [Google Scholar]
- 52.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Degenhardt L, Mathers B, Guarinieri M, et al. Meth/amphetamine use and associated HIV: implications for global policy and public health. Int J Drug Policy 2010; 21: 347–58. [DOI] [PubMed] [Google Scholar]
- 54.United Nations Development Programme. Human development report 2005. International cooperation at a crossroads: aid, trade and security in an unequal world. New York, NY: United Nations, 2005. [Google Scholar]
- 55.Joint United Nations Programme on HIV/AIDS. How AIDS changed everything. Geneva: United Nations, 2016. [Google Scholar]
- 56.Statistics South Africa. Mid-year population estimates 2014. Pretoria: Statistics South Africa, 2014. [Google Scholar]
- 57.Joint United Nations Programme on HIV/AIDS. Right to health. Geneva: United Nations, 2017. [Google Scholar]
- 58.WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
- 59.WHO. Male circumcision for HIV prevention. http://www.who.int/hiv/topics/malecircumcision/en/ (accessed June 14, 2018).
- 60.WHO. Voluntary medical male circumcision for HIV prevention in 14 priority countries in East and Southern Africa. Geneva: World Health Organization, 2017. [Google Scholar]
- 61.Spinner CD, Boesecke C, Zink A, et al. HIV pre-exposure prophylaxis (PrEP): a review of current knowledge of oral systemic HiV PrEP in humans. Infection 2016; 44: 151–58. [DOI] [PubMed] [Google Scholar]
- 62.United Nations Development Programme. Cash transfers and HIV prevention: discussion paper. New York: United Nations, 2014. [Google Scholar]
- 63.World Bank. Population ages 0–14 (% of total). 2017. https://data.worldbank.org/indicator/SP.POP.0014.TO.zS (accessed June 14, 2018).
- 64.Joint United Nations Programme on HIV/AIDS. Fast-track. Ending the epidemic by 2030. Geneva: United Nations, 2014. [Google Scholar]
- 65.Joint United Nations Programme on HIV/AIDS. 90–90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: United Nations, 2014. [Google Scholar]
- 66.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaolathe T, Wirth KE, Holme MP, et al. Botswana’s progress toward achieving the 2020 UNAIDS 90–90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV 2016; 3: e221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.US Centres for Disease Control and Prevention. More people with HIV have the virus under control [press release]. July 27, 2017. Atlanta, GA: US Centres for Disease Control and Prevention, 2017 [Google Scholar]
- 69.Grabowski MK, Serwadda DM, Gray RH, et al. HIV prevention efforts and incidence of HIV in Uganda. N Engl J Med 2017; 377: 2154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nwokolo N, Hill A, McOwan A, Pozniak A. Rapidly declining HIV infection in MSM in central London. Lancet HIV 2017; 4: e482–83. [DOI] [PubMed] [Google Scholar]
- 71.Amatuli J New HIV cases in New York City hit record low, Health Department says. HuffPost, November 30, 2017 [Google Scholar]
- 72.US Centers for Disease Control and Prevention. HIV in the United States At a glance. Atlanta, GA: US Centres for Disease Control and Prevention, 2017. [Google Scholar]
- 73.Hess K, Hu K, Lansky A, Mermin J, eds. Estimating the lifetime risk of a diagnosis of HIV infection in the United States. 23rd Conference on Retroviruses and Opportunistic Infections; Boston, MA; Feb 22–25, 2016 Abstract 52. [Google Scholar]
- 74.Bradley H, Mattson C, Beer L, Huang P, Shouse R, eds. Increased HIV viral suppression among US adults receiving medical care, 2009–2013. 23rd Conference on Retroviruses and Opportunistic Infections; Boston, MA; Feb 22–25, 2016 Abstract 53. [Google Scholar]
- 75.Dailey AF, Johnson AS, Wu B. HIV care outcomes among blacks with diagnosed HIV—United States, 2014. MMWR Morb Mortal Wkly Rep 2017; 66: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beyrer C, Wirtz AL, O’Hara G, Léon N, Kazatchkine M. The expanding epidemic of HIV-1 in the Russian Federation. PLoS Med 2017; 14: e1002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Oliveira T, Kharsany AB, Grâf T, et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: a community-wide phylogenetic study. Lancet HIV 2017; 4: e41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med 2007; 4: e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joint United Nations Programme on HIV/AIDS. UNAIDS report on the global AIDS epidemic. Geneva: United Nations, 2012. [Google Scholar]
- 80.Baral S, Beyrer C, Muessig K, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 538–49. [DOI] [PubMed] [Google Scholar]
- 81.Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13: 214–22. [DOI] [PubMed] [Google Scholar]
- 82.Isbell MT, Kilonzo N, Mugurungi O, Bekker LG. We neglect primary HIV prevention at our peril. Lancet HIV 2016; 3: e284–85. [DOI] [PubMed] [Google Scholar]
- 83.The Global Fund. Results report 2017. Geneva: Global Fund to Fight AIDS, Tuberculosis and Malaria, 2017. [Google Scholar]
- 84.Merson M, Inrig S. The AIDS pandemic: searching for a global response. Cham, Switzerland: Springer Nature, 2018. [Google Scholar]
- 85.Joint United Nations Programme on HIV/AIDS. Report on progress in the implementation of the UNAIDS Joint Programme Action Plan. Strategic resource mobilization plan 2018—2021. Geneva: United Nations, 2017. [Google Scholar]
- 86.WHO. Measles vaccination has saved an estimated 17–1 million lives since 2000 [press release]. November 15, 2015. Geneva: World Health Organization, 2015. [Google Scholar]
- 87.WHO. World malaria report. Geneva: World Health Organization, 2016. [Google Scholar]
- 88.WHO, UNICEF. Global action plan for prevention and control of pneumonia (GAPP). Geneva: World Health Organization, United Nations Children’s Fund, 2009. [Google Scholar]
- 89.WHO. Global Acceleration Action for the Health of Adolescents. (AA-HA!): guidance to support country implementation. Summary. Geneva: World Health Organization, 2017. [Google Scholar]
- 90.UNICEF. Child marriage database. New York: United Nations Children’s Fund, 2017. [Google Scholar]
- 91.Marphatia AA, Ambale GS, Reid AM. Women’s marriage age matters for public health. A review of the broader health and social implications in South Asia. Front Public Health 2017; 5: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clark S Early marriage and HIV risks in sub-Saharan Africa. Stud Fam Plann 2004; 35: 149–60. [DOI] [PubMed] [Google Scholar]
- 93.Kidman R Child marriage and intimate partner violence: a comparative study of 34 countries. Int J Epidemiol 2017; 46: 662–75. [DOI] [PubMed] [Google Scholar]
- 94.WHO. Adolescent pregnancy Fact sheet. Geneva: World Health Organization, 2018. [Google Scholar]
- 95.Ganchimeg T, Ota E, Morisaki N, et al. Pregnancy and childbirth outcomes among adolescent mothers: a World Health Organization multicountry study. BJOB 2014; 121 (suppl 1): 40–8. [DOI] [PubMed] [Google Scholar]
- 96.Uzoigwe CE, Franco LCS. Abstinence in HIV prevention: science and sophistry. Lancet Glob Health 2017; 5: e30. [DOI] [PubMed] [Google Scholar]
- 97.Secor-Turner M, Randall B, Christensen K, Jacobson A, Loyola Melendez M. Implementing community-based comprehensive sexuality education with high-risk youth in a conservative environment: lessons learned. Sex Educ 2017; 17: 544–54. [Google Scholar]
- 98.Darroch J, Woog V, Bankole A, Ashford L. Adding it up: costs and benefits of meeting the contraceptive needs of adolescents. New York: Guttmacher Institute, 2016. [Google Scholar]
- 99.WHO. Adolescent health epidemiology http://www.who.int/maternal_child_adolescent/epidemiology/adolescence/en/ (accessed June 14, 2018).
- 100.WHO. Making health services adolescent friendly: developing national quality standards for adolescent friendly health services. Geneva: World Health Organization, 2012. [Google Scholar]
- 101.Bekker LG, Johnson L, Wallace M, Hosek S. Building our youth for the future. J Int AIDS Soc 2015; 18 (suppl 1): 20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.WHO. Maternal mortality. Fact sheet. Geneva: World Health Organization, 2016. [Google Scholar]
- 103.WHO. Global and regional estimates of violence against women. Geneva: World Health Organization, 2013. [Google Scholar]
- 104.Jewkes RK, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet 2010; 376: 41–48. [DOI] [PubMed] [Google Scholar]
- 105.Jewkes R, Dunkle K, Jama-Shai N, Gray G. Impact of exposure to intimate partner violence on CD4+ and CD8+ T cell decay in HIV infected women: longitudinal study. PLoS One 2015; 10: e0122001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pink Ribbon Red Ribbon. Our impact. http://pinkribbonredribbon.org/our-impact/ (accessed June 14, 2018).
- 107.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Henry J Kaiser Family Foundation. Gender differences in health care, status, and use. Spotlight on men’s health. Washington, DC: Henry J Kaiser Family Foundation, 2015. [Google Scholar]
- 109.Leichliter JS, Paz-Bailey G, Friedman AL, et al. ‘Clinics aren’t meant for men’: sexual health care access and seeking behaviours among men in Gauteng province, South Africa. Sahara J 2011; 8: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baker P, Dworkin SL, Tong S, Banks I, Shand T, Yamey G. The men’s health gap: men must be included in the global health equity agenda. Bull World Health Organ 2014; 92: 618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharma M, Barnabas RV, Celum C. Community-based strategies to strengthen men’s engagement in the HIV care cascade in sub-Saharan Africa. PLoS Med 2017; 14: e1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hines JZ, Ntsuape OC, Malaba K, et al. Scale-up of voluntary medical male circumcision services for HIV prevention-12 countries in Southern and Eastern Africa, 2013–2016. MMWR Morb Mortal Wkly Rep 2017; 66: 1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petersen M, Balzer L, Kwarsiima D, et al. Association of implementation of a universal testing and treatment intervention with HIV diagnosis, receipt of antiretroviral therapy, and viral suppression in east Africa. JAMA 2017; 317: 2196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DeBeck K, Cheng T, Montaner JS, et al. HIV and the criminalisation of drug use among people who inject drugs: a systematic review. Lancet HIV 2017; 4: e357–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health 2017; 5: e1208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rethinking Collins J. ‘flexibilities’ in the international drug control system: potential, precedents and models for reforms. Int J Drug Policy 2017; published online Jan 24. DOI: 10.1016/j.drugpo.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 117.WHO. The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en/index1.html (accessed June 4, 2018).
- 118.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS 2014; 28: 1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carroll A, Mendos L. State-sponsored homophobia—A world survey of sexual orientation laws: criminalisation, protection and recognition. Geneva: International Lesbian, Gay, Bisexual, Trans and Intersex Association, 2017. [Google Scholar]
- 120.Hutton J Indonesia’s crackdown on gay men moves from bars into the home. New York Times, December 20, 2017. [Google Scholar]
- 121.Green A Global Fund-supported programs suspended amid Tanzanian government crackdown on LGBT community. March 14, 2017 http://aidspan.org/gfo_article/global-fund-supported-programs-suspended-amid-tanzanian-government-crackdown-lgbt-0 (accessed June 14, 2018).
- 122.Mehta SH, Lucas GM, Solomon S, et al. HIV care continuum among men who have sex with men and persons who inject drugs in India: barriers to successful engagement. Clin Infect Dis 2015; 61: 1732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wirtz AL, Zelaya CE, Latkin C, et al. The HIV care continuum among men who have sex with men in Moscow, Russia: a cross-sectional study of infection awareness and engagement in care. Sex Transm Infect 2016; 92: 161–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beyrer C, Baral SD, Collins C, et al. The global response to HIV in men who have sex with men. Lancet 2016; 388: 198–206. [DOI] [PubMed] [Google Scholar]
- 125.United Nations Population Division. International migrant stock, 2015. New York: United Nations, 2016. [Google Scholar]
- 126.China Labour Bulletin. Migrant workers and their children. http://www.clb.org.hk/content/migrant-workers-and-their-children (accessed June 14, 2018).
- 127.UNHCR. Figures at a glance. 2017. http://www.unhcr.org/en-us/figures-at-a-glance.html (accessed June 14, 2018).
- 128.International Organization of Migration, WHO, United Nations Office of the High Commissioner on Human Rights. International migration, health and human rights. Geneva: International Organization of Migration, World Health Organization, United Nations Office of the High Commissioner on Human Rights, 2013. [Google Scholar]
- 129.Suphanchaimat R, Pudpong N, Tangcharoensathien V. Extreme exploitation in southeast Asia waters: challenges in progressing towards universal health coverage for migrant workers. PLoS Med 2017; 14: e1002441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ang JW, Chia C, Koh CJ, et al. Healthcare-seeking behaviour, barriers and mental health of non-domestic migrant workers in Singapore. BMJ Glob Health 2017; 2: e000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vasylyeva TI, Liulchuk M, Friedman SR, et al. Molecular epidemiology reveals the role of war in the spread of HIV in Ukraine. Proc Natl Acad Sci USA 2018; 115: 1051–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tangcharoensathien V, Thwin AA, Patcharanarumol W. Implementing health insurance for migrants, Thailand. Bull World Health Organ 2017; 95: 146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59: 1787–97. [DOI] [PubMed] [Google Scholar]
- 134.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173: 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25: 1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Althoff KN, McGinnis KAC, Wyatt CM, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2015; 60: 627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197: 126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Atun R, Jaffar S, Nishtar S, et al. Improving responsiveness of health systems to non-communicable diseases. Lancet 2013; 381: 690–97. [DOI] [PubMed] [Google Scholar]
- 139.Moodie R, Stuckler D, Monteiro C, et al. , and the Lancet NCD Action Group. Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet 2013; 381: 670–79. [DOI] [PubMed] [Google Scholar]
- 140.Alleyne G, Binagwaho A, Haines A, et al. , and the Lancet NCD Action Group. Embedding non-communicable diseases in the post-2015 development agenda. Lancet 2013; 381: 566–74. [DOI] [PubMed] [Google Scholar]
- 141.UN. Prevention and control of non-communicable diseases: report of the Secretary-General In: (A/66/83) UNGA, ed. New York: United Nations, 2011. [Google Scholar]
- 142.Niessen LW, Mohan D, Akuoku JK, et al. Tackling socioeconomic inequalities and non-communicable diseases in low-income and middle-income countries under the Sustainable Development agenda. Lancet 2018; published online April 5. 10.1016/S0140-6736(18)30482-3. [DOI] [PubMed] [Google Scholar]
- 143.Hogerzeil HV, Liberman J, Wirtz VJ, et al. Promotion of access to essential medicines for non-communicable diseases: practical implications of the UN political declaration. Lancet 2013; 381: 680–89. [DOI] [PubMed] [Google Scholar]
- 144.Mills EJ, Barnighausen T, Negin J. HIV and aging—preparing for the challenges ahead. N Engl J Med 2012; 366: 1270–73. [DOI] [PubMed] [Google Scholar]
- 145.Tsondai P, Brimsrug A, Mdlalo P, Ullauri A, Boulle A. High rates of retention and viral suppression in the scale-up of antiretroviral therapy adherence clubs in Cape Town, South Africa. J Int AIDS Soc 2017; 20 (suppl 4): 21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chamie G, Clark TD, Kabami J, et al. A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV 2016; 3: e111–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.WHO. Consolidated guideline on sexual and reproductive health and rights of women living with HIV. Geneva: World Health Organization, 2017. [PubMed] [Google Scholar]
- 148.The Global Fund. Technical brief: strengthening sexual, reproductive, maternal, newborn, child and adolescent health (SRMNCAH) in funding requests to the Global Fund. Geneva: Global Fund to Fight AIDS, Tuberculosis and Malaria, 2018. [Google Scholar]
- 149.Germain A, Dixon-Mueller R, Sen G. Back to basics: HIV/AIDS belongs with sexual and reproductive health. Bull World Health Organ 2009; 87: 840–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.World Association for Sexual Health. Declaration of sexual rights. 2014. http://www.worldsexology.org/wp-content/uploads/2013/08/declaration_of_sexual_rights_sep03_2014.pdf (accessed June 14, 2018).
- 151.Kendall T, Albert C. Experiences of coercion to sterilize and forced sterilization among women living with HIV in Latin America. J Int AIDS Soc 2015; 18: 19462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abrams EJ, Myer L, Rosenfield A, El-Sadr WM. Prevention of mother-to-child transmission services as a gateway to family-based human immunodeficiency virus care and treatment in resource-limited settings: rationale and international experiences. Am J Obstet Gynecol 2007; 197 (suppl): S101–06. [DOI] [PubMed] [Google Scholar]
- 153.Warren CE, Mayhew SH, Hopkins J. The current status of research on the integration of sexual and reproductive health and HIV services. Stud Fam Plann 2017; 48: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Starrs A, Exeh A, Barker G, al. e. Accelerate progress—sexual and reproductive health and rights for all: report of the Guttmacher-Lancet Commission on sexual and reproductive health and rights. Lancet 2018; published online May 9. 10.1016/S0140-6736(18)30293-9. [DOI] [PubMed] [Google Scholar]
- 155.Polis CB, Curtis KM, Hannaford PC, et al. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS 2016; 30: 2665–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.WHO. Hormonal contraceptive eligibility for women at high risk of HIV Guidance statement. Geneva: World Heatlh Organization, 2017. [PubMed] [Google Scholar]
- 157.Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature 2008; 451: 990–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Taubenberger JK, Morens DM. Influenza: the once and future pandemic. Public Health Rep 2010; 125 (suppl 3): 16–26. [PMC free article] [PubMed] [Google Scholar]
- 159.Beyrer C, Pozniak A. HIV drug resistance—an emerging threat to epidemic control. N Engl J Med 2017; 377: 1605–07. [DOI] [PubMed] [Google Scholar]
- 160.Gonsalves G, Staley P. Panic, paranoia, and public health—the AIDS epidemic’s lessons for Ebola. N Engl J Med 2014; 371: 2348–9. [DOI] [PubMed] [Google Scholar]
- 161.Reliefweb. Ebola hampers HIV/AIDS care in Liberia. November 21, 2014. https://reliefweb.int/report/liberia/ebola-hampers-hivaids-care-liberia (accessed June 14, 2018).
- 162.WHO. Tuberculosis. Key facts. 2018. http://www.who.int/en/news-room/fact-sheets/detail/tuberculosis (accessed June 14, 2018).
- 163.Dolan K, Wirtz AL, Moazen B, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet 2016; 388: 1089–102. [DOI] [PubMed] [Google Scholar]
- 164.Coetzee D, Hilderbrand K, Goemaere E, Matthys F, Boelaert M. Integrating tuberculosis and HIV care in the primary care setting in South Africa. Trop Med Int Health 2004; 9: A11–15. [DOI] [PubMed] [Google Scholar]
- 165.Foster G, Gane E, Asatryan A, et al. , eds. ENDURANCE03: safety and efficacy of glecaprevir/pibrentasvir compared to sofosbuvir plus declatasvir in treatment-naive HCV genotype 3-infected patients without cirrhosis. International Liver Congress; Amsterdam, Netherlands; April 19–23, 2017. [Google Scholar]
- 166.WHO. Immunization coverage Fact sheet. Geneva: World Health Organization, 2018. [Google Scholar]
- 167.Hill AM, Nath S, Simmons B. The road to elimination of hepatitis C: analysis of cures versus new infections in 91 countries. J Virus Erad 2017; 3: 117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Decroo T, Telfer B, Biot M, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete Province, Mozambique. J Acquir Immune Defic Syndr 2011; 56: e39–4. [DOI] [PubMed] [Google Scholar]
- 169.Okoboi S, Ding E, Persuad S, et al. Community-based ART distribution system can effectively facilitate long-term program retention and low-rates of death and virologic failure in rural Uganda. AIDS Res Ther 2015; 12: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bemelmans M, Baert S, Goemaere E, et al. Community-supported models of care for people on HIV treatment in sub-Saharan Africa. Trop Med Int Health 2014; 19: 968–77 [DOI] [PubMed] [Google Scholar]
- 171.Grimsrud A, Bygrave H, Doherty M, et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc 2016; 19: 21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd edn. Geneva: World Health Organization, 2016. [PubMed] [Google Scholar]
- 173.Poteat T, Scheim A, Xavier J, Reisner S, Baral S. Global epidemiology of HIV infection and related syndemics affecting transgender people. J Acquir Immune Defic Syndr 2016; 72 (suppl 3): S210–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Santos GM, Do T, Beck J, et al. Syndemic conditions associated with increased HIV risk in a global sample of men who have sex with men. Sex Transm Infect 2014; 90: 250–53. [DOI] [PubMed] [Google Scholar]
- 175.Csete J, Kamarulzaman A, Kazatchkine M, et al. Public health and international drug policy. Lancet 2016; 387: 1427–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Peters PJ, Pontones P, Hoover KW, et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014—2015. N Engl J Med 2016; 375: 229–39. [DOI] [PubMed] [Google Scholar]
- 177.Blashill AJ, Bedoya CA, Mayer KH, et al. Psychosocial syndemics are additively associated with worse ART adherence in HIV-infected individuals. AIDS Behav 2015; 19: 981–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Loeliger KB, Altice FL, Desai MM, Ciarleglio MM, Gallagher C, Meyer JP. Predictors of linkage to HIV care and viral suppression after release from jails and prisons: a retrospective cohort study. Lancet HIV 2018; 5: e96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mayer KH, Bekker LG, Stall R, Grulich AE, Colfax G, Lama JR. Comprehensive clinical care for men who have sex with men: an integrated approach. Lancet 2012; 380: 378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.O’Cleirigh C, Magidson JF, Skeer MR, Mayer KH, Safren SA. Prevalence of psychiatric and substance abuse symptomatology among HIV-infected gay and bisexual men in HIV primary care. Psychosomatics 2015; 56: 470–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.O’Cleirigh C, Newcomb ME, Mayer KH, Skeer M, Traeger L, Safren SA. Moderate levels of depression predict sexual transmission risk in HIV-infected MSM: a longitudinal analysis of data from six sites involved in a “prevention for positives” study. AIDS Behav 2013; 17: 1764–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr 2011; 58: 181–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kronish IM, Ye S. Adherence to cardiovascular medications: lessons learned and future directions. Prog Cardiovasc Dis 2013; 55: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stall R, Mills TC, Williamson J, et al. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am J Public Health 2003; 93: 939–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mimiaga MJ, O’Cleirigh C, Biello KB, et al. The effect of psychosocial syndemic production on 4-year HIV incidence and risk behavior in a large cohort of sexually active men who have sex with men. J Acquir Immune Defic Syndr 2015; 68: 329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hanlon C, Luitel NP, Kathree T, et al. Challenges and opportunities for implementing integrated mental health care: a district level situation analysis from five low- and middle-income countries. PLoS One 2014; 9: e88437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.WHO. Mental health atlas 2011. Geneva: World Health Organization, 2011. [Google Scholar]
- 188.WHO. Mental health action plan 2013–2030. Geneva: World Health Organization, 2013. [Google Scholar]
- 189.Saraceno B, van Ommeren M, Batniji R, et al. Barriers to improvement of mental health services in low-income and middle-income countries. Lancet 2007; 370: 1164–74. [DOI] [PubMed] [Google Scholar]
- 190.Hargreaves JR, Bonell CP, Boler T, et al. Systematic review exploring time trends in the association between educational attainment and risk of HIV infection in sub-Saharan Africa. AIDS 2008; 22: 403–14. [DOI] [PubMed] [Google Scholar]
- 191.Holtgrave DR, Wolitski RJ, Pals SL, et al. Cost-utility analysis of the housing and health intervention for homeless and unstably housed persons living with HIV. AIDS Behav 2013; 17: 1626–31. [DOI] [PubMed] [Google Scholar]
- 192.African Union Commission. 2 million African community health workers: harnessing the demographic dividend, ending AIDS and ensuring sustainabile health for all in Africa. Addis Ababa: African Union Commission, 2017. [Google Scholar]
- 193.Hall J Effective community-based interventions to improve exclusive breast feeding at four to six months in low- and low-middle-income countries: a systematic review of randomized controlled trials. Midwifery 2011; 27: 497–502. [DOI] [PubMed] [Google Scholar]
- 194.Pegurri E, Fox-Rushby JA, Damian W. The effects and costs of expanding the coverage of immunisation services in developing countries: a systematic literature review. Vaccine 2005; 23: 1624–35. [DOI] [PubMed] [Google Scholar]
- 195.Dawson P, Pradhan Y, Houston R, Karki S, Poudel D, Hodgins S. From research to national expansion: 20 years’ experience of community-based management of childhood pneumonia in Nepal. Bull World Health Organ 2008; 86: 339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ankhbayar Cashin C., Phuong H, et al. Asssessing health provider payment systems: a practical guide for countries working toward universal health coverage. Washington, DC: Joint Learning Network for Universal Health Coverage, 2015. [Google Scholar]
- 197.Institute of Medicine. Evaluation of PEPFAR. Washington, DC: National Academies Press, 2013. [Google Scholar]
- 198.Medicins Sans Frontieres, Rural Doctors Association of Southern Africa, Rural Health Advocacy Project, Treatment Action Campaign, SECTION27, Southern African HIV Clinicians Society. SSP stockouts national survey. June, 2016. https://www.groundup.org.za/media/uploads/documents/StopStockoutsSurvey2016.pdf (accessed June 14, 2018).
- 199.Gils T, Bossard C, Verdonck K, et al. Stockouts of HIV commodities in public health facilities in Kinshasa: Barriers to end HIV. PLoS One 2018; 13: e0191294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lamy M, Liverani M. Tackling substandard and falsified medicines in the Mekong: national responses and regional prospects. Asia Pac Policy Stud 2015: 2: 245–54. [Google Scholar]
- 201.Jamison D, Alwan A, Mock C, et al. Universal health coverage and intersectoral action for health: key messages from Disease Control Priorities, 3rd edition. Lancet 2017; 391: 1108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Atun R, Davies JI, Gale EAM, et al. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol 2017; 5: 622–67 [DOI] [PubMed] [Google Scholar]
- 203.Nojilana B, Bradshaw D, Pillay-van Wyk V, et al. Persistent burden from non-communicable diseases in South Africa needs strong action. S Afr Med J 2016; 106: 23–24. [DOI] [PubMed] [Google Scholar]
- 204.Msemburi W, Pillay-van Wyk V, Dorrington R, et al. Second national burden of disease study for South Africa: cause-of-death profile for South Africa, 1997–2012. Cape Town: South African Medical Research Council, 2016. [Google Scholar]
- 205.Shisana O, Labadarios D, Rehle T, et al. South African national health and nutrition examination survey (SANHANES-1). Cape Town: HSRC Press, 2014. [Google Scholar]
- 206.Johnson LF, Rehle TM, Jooste S, Bekker LG. Rates of HIV testing and diagnosis in South Africa: successes and challenges. AIDS 2015; 29: 1401–09. [DOI] [PubMed] [Google Scholar]
- 207.Govindasamy D, Kranzer K, van Schaik N, et al. Linkage to HIV, TB and non-communicable disease care from a mobile testing unit in Cape Town, South Africa. PLoS One 2013; 8: e80017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Labhardt ND, Motlomelo M, Cerutti B, et al. Home-based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster-randomized trial. PLoS Med 2014; 11: e1001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chamie G, Kwarisiima D, Clark TD, et al. Uptake of community-based HIV testing during a multi-disease health campaign in rural Uganda. PLoS One 2014; 9: e84317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Johnson LF, May MT, Dorrington RE, et al. Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: a mathematical modelling study. PLoS Med 2017; 14: e1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kranzer K, Govindasamy D, van Schaik N, et al. Incentivized recruitment of a population sample to a mobile HIV testing service increases the yield of newly diagnosed cases, including those in need of antiretroviral therapy. HIV Med 2012; 13: 132–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sweat M, Morin S, Celentano D, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis 2011; 11: 525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature 2015; 528: S77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ng’ang’a A, Waruiru W, Ngare C, et al. The status of HIV testing and counseling in Kenya: results from a nationally representative population-based survey. J Acquir Immune Defic Syndr 2014; 66 (suppl 1): S27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ministry of Health, Kenya Bureau of National Statistics, WHO. Kenya STEPwise Survey for non communicable diseases risk factors 2015 report. Nairobi: Ministry of Health, Kenya Bureau of National Statistics, World Health Organization, 2015. [Google Scholar]
- 216.Chang W, Chamie G, Mwai D, et al. Implementation and operational research: cost and efficiency of a hybrid mobile multidisease testing approach with high HIV testing coverage in east Africa. J Acquir Immune Defic Syndr 2016; 73: e39–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ngalesoni FN, Ruhago GM, Mori AT, Robberstad B, Norheim OF. Cost-effectiveness of medical primary prevention strategies to reduce absolute risk of cardiovascular disease in Tanzania: a Markov modelling study. BMC Health Serv Res 2016; 16: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ortegon M, Lim S, Chisholm D, Mendis S. Cost effectiveness of strategies to combat cardiovascular disease, diabetes, and tobacco use in sub-Saharan Africa and south east Asia: mathematical modeling study. BMJ 2012; 344: e607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Abiodun O, Sotunsa J, Ani A, Olaleye A, Taiwo A. Elimination of mother-to-child transmisson of HIV in Nigeria: the roles, preparedness and determinants of successful involvement of traditional birth attendants. J AIDS Clin Res 2015; 6: 481. [Google Scholar]
- 220.National Population Commission, ICF International. Nigeria Demographic and Health Survey 2013. Abuja: National Population Commission, ICF International, 2014. [Google Scholar]
- 221.Joint United Nations Programme on HIV/AIDS, PEPFAR. ‘Start Free, Stay Free, AIDS Free: a super fast track framework for ending AIDS among children, adolescents and young women by 2020’. Geneva: United Nations, United States President’s Emergency Plan for AIDS Relief, 2016. [Google Scholar]
- 222.PEPFAR. Report on pilot, expenditure analysis of PEPFAR programs in six countries. Washington, DC: United States President’s Emergency Plan for AIDS Relief, 2012. [Google Scholar]
- 223.Health Policy Project, United States Agency for International Development (USAID), and Marie Stopes International. ImpactNow manual: estimating the health and economic impacts of family planning use. Washington, DC: Futures Group, Health Policy Project, 2014. [Google Scholar]
- 224.Fagbamigbe AF, Idemudia ES. Barriers to antenatal care use in Nigeria: evidences from non-users and implications for maternal health programming. BMC Pregnancy Childbirth 2015; 15: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gupte S, Daly C, Agarwal V, Gaikwad SB, George B. Introduction of rapid tests for large-scale syphilis screening among female, male, and transgender sex workers in Mumbai, India. Sex Transm Dis 2011; 38: 499–502. [DOI] [PubMed] [Google Scholar]
- 226.Vassall A, Pickles M, Chandrashekar S, et al. , and the Charme India Group. Cost-effectiveness of HIV prevention for high-risk groups at scale: an economic evaluation of the Avahan programme in south India. Lancet Glob Health 2014; 2: e531–40. [DOI] [PubMed] [Google Scholar]
- 227.Solomon SS, McFall AM, Lucas GM, et al. Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: a cross-sectional, community-based analysis. PLoS Med 2017; 14: e1002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stover J, Hallett TB, Wu Z, et al. How can we get close to zero? The potential contribution of biomedical prevention and the investment framework towards an effective response to HIV. PLoS One 2014; 9: e111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Médians Sans Frontières. Untangling the web of antiretroviral price reductions, 18th edn. Geneva: Medicins Sans Frontieres, 2016. [Google Scholar]
- 230.Korenromp EL, Wi T, Resch S, Stover J, Broutet N. Costing of national STI program implementation for the Global STI Control Strategy for the Health Sector, 2016–2021. PLoS One 2017; 12: e0170773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Murray CJ, Barber RM, Foreman KJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 2015; 386: 2145–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10: e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Eritsyan K, Heimer R, Barbour R, et al. Individual-level, network-level and city-level factors associated with HIV prevalence among people who inject drugs in eight Russian cities: a cross-sectional study. BMJ Open 2013; 3: e002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Coffin P Overdose: a major cause of preventable death in central and eastern Europe and central Asia. Vilnius, Lithuania: Eurasian Harm Reduction Network, 2008. [Google Scholar]
- 235.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012; 345: e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 2011; 106: 32–51. [DOI] [PubMed] [Google Scholar]
- 237.Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction 1998; 93: 515–32. [DOI] [PubMed] [Google Scholar]
- 238.Low AJ, Mburu G, Welton NJ, et al. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. Clin Infect Dis 2016; 63: 1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mathers BM, Degenhardt L, Ali H, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet 2010; 375: 1014–28. [DOI] [PubMed] [Google Scholar]
- 240.Bangkok Samoilov D. 2004. Double discrimination: drug users living with HIV/AIDS. HIV AIDS Policy Law Rev 2004; 9: 83–85. [PubMed] [Google Scholar]
- 241.Ministry of Health of the Russian Federation. Clinical recommendations of HIV-infection in adults. Moscow: Ministry of Health of the Russian Federation, 2017. [Google Scholar]
- 242.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med 2011; 8: e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet 2010; 376: 355–66. [DOI] [PubMed] [Google Scholar]
- 244.Marseille E, Dandona L, Saba J, et al. Assessing the efficiency of HIV prevention around the world: methods of the PANCEA project. Health Serv Res 2004; 39: 1993–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Treatment Preparedness Coalition. ARV procurement in 2014: History of decentralization. Results of monitoring of ARV procurement and provision in the Russian Federation. St Petersburg: Treatment Preparedness Coalition, 2015. [Google Scholar]
- 246.Vickerman P, Platt L, Jolley E, Rhodes T, Kazatchkine MD, Latypov A. Controlling HIV among people who inject drugs in Eastern Europe and Central Asia: insights from modeling. Int J Drug Policy 2014; 25: 1163–73. [DOI] [PubMed] [Google Scholar]
- 247.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5: e1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Heimer R, Usacheva N, Barbour R, Niccolai LM, Uuskula A, Levina OS. Engagement in HIV care and its correlates among people who inject drugs in St Petersburg, Russian Federation and Kohtla-Jarve, Estonia. Addiction 2017; 112: 1421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Atun R, Cavalli F. The global fight against cancer: challenges and opportunities. Lancet 2018; 391: 412–13. [DOI] [PubMed] [Google Scholar]
- 250.UNICEF. Current status and progress: an estimated 1 in 7 children worldwide did not receive the required third dose of DPT in 2016. New York: United Nations Children’s Fund, 2018. [Google Scholar]
- 251.Jamison DT, Summers LH, Alleyne G, et al. Global health 2035: a world converging within a generation. Lancet 2013; 382: 1898–955. [DOI] [PubMed] [Google Scholar]
- 252.Dieleman JL, Templin T, Sadat N, et al. National spending on health by source for 184 countries between 2013 and 2040. Lancet 2016; 387: 2521–35. [DOI] [PubMed] [Google Scholar]
- 253.Stenberg K, Hanssen O, Edejer TT, et al. Financing transformative health systems towards achievement of the health Sustainable Development Goals: a model for projected resource needs in 67 low-income and middle-income countries. Lancet Glob Health 2017; 5: e875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.WHO. New perspectives on global health spending for universal health coverage. Geneva: World Health Organization, 2017 [Google Scholar]
- 255.Evans T, Pablos-Mendez A. Shaping of a new era for health financing. Lancet 2016; 387: 2482–84. [DOI] [PubMed] [Google Scholar]
- 256.OECD. Detailed final 2015 aid figures released by OECD/DAC. http://www.oecd.org/dac/financing-sustainable-development/development-finance-data/final-2015-oda.htm (accessed June 18, 2018).
- 257.WHO. Antimicrobial resistance Fact sheet. Geneva: World Health Organization, 2017. [Google Scholar]
- 258.Valentine A, Wexler A, Oum S, Kates J. Donor funding for health in low- & middle-income countries, 2002–2013. Washington, DC: Henry J Kaiser Family Foundation, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.