Abstract
Background:
Wheezing illnesses among young children are common, and are a risk factor for asthma. However, determinants of childhood bronchial reactivity, a key feature of asthma, are largely unknown. Aim of this study was to determine how patient characteristics during the first severe virus-induced wheezing episode are associated with pulmonary function at pre-school age.
Methods:
Study consisted of 76 children presenting with their first wheezing episode at the ages of 3 to 23 months. At study entry, viral etiology, rhinovirus genome load, atopic and clinical characteristics, and standardized questionnaire were analyzed. At 4-year follow-up visit, impulse oscillometry with exercise challenge was performed.
Results:
At study entry, the mean age of the children was 12 months (SD 6.0), 57 (75%) were rhinovirus positive and 22 (30%) were sensitized. At follow-up visit four years later, the mean age of the children was 60 months (SD 7.9) and 37 (49%) were using asthma medication regularly (discontinued before testing in 25 [68%] children). Bronchial reactivity (≥35% change in mean crude values of resistance) after exercise challenge or bronchodilation was present in 9 (12%) children. Children with atopic sensitization at the time of the first wheezing episode were more often likely to develop bronchial reactivity (odds ratio 8.8, P = .03) than the children without sensitization. No other significant associations were found.
Conclusions:
Atopic sensitization at the time of the first severe wheezing episode is an important early risk factor for increased bronchial reactivity at pre-school age.
Keywords: Bronchial reactivity, impulse oscillometry, pulmonary function, sensitization, wheezing
Introduction
Previous studies have shown that early wheezing induced by rhinovirus, and/or associated with sensitization are important risk factors for recurrent wheezing and asthma. They are likely to reveal the underlying airway inflammation and the weaknesses in viral defense mechanisms.1–3 However, the factors influencing bronchial reactivity in children are poorly characterized. Previous studies have shown, that early life factors, such as early onset atopy,4–7 family history of asthma,6,8 maternal smoking,4–6,8,9 exposure to traffic-related air pollution,10,11 preterm birth12–14 and low birth weight15 may be associated with decreased pulmonary function in later childhood. Two studies have shown that wheezing at young age, induced by rhinovirus, may be associated with decreased pulmonary function or increased airway reactivity later in childhood.8,16 Some,5,17,18 but not all9,19 studies have shown a comparable association between respiratory syncytial virus (RSV) induced wheezing and reduced pulmonary function. However, these earlier studies mainly included older children and there were no data from the first virus-induced wheezing episode.
Pulmonary function testing in young children is challenging. Impulse oscillometry (IOS) is a method for testing pulmonary function during tidal breathing.20–23 A key measurement in IOS is respiratory resistance (Rrs) which indicates the energy loss due to resistive forces to the airflow in the airways and is clinically interpreted as an indicator of obstruction. IOS is child-friendly as it requires a minimum co-operation and has successfully been used from the age of 2–3 years forward.21,24 In Finland, there are population-based reference values available for children aged 2–7 years.20,23
Secondary strategies to prevent asthma can be initiated at the time of the first wheezing episode.25–28 However, it is not known which patient characteristics at the time of the first acute wheezing episode predict abnormal pulmonary function during the later childhood. Thus, among children who had suffered from the first acute severe wheezing episode before the age of 2 years, we aimed to determine the pulmonary function at the age of 5 years and its reactivity to exercise or bronchodilation by IOS. We also tested for early-life factors predicting the exercise or bronchodilation induced bronchial reactivity. We hypothesized that patient characteristics, such as rhinovirus etiology and atopy at the time of the first severe wheezing episode would be associated with the pulmonary function and increased bronchial reactivity measured by IOS 4 years after the first wheezing episode.
Methods
Subjects
This study is a part of a larger Vinku2 study which was carried out in the Turku University Hospital (recruitment 6/2007–3/2009).27,29,30 The aim of the original study was to assess the effect of a 3-day course of oral prednisolone (first dose 2 mg/kg, then 2 mg/kg/day in 2 divided doses, maximum dose 60 mg/day, Prednisolon® 5 mg tablets, Leiras Takeda, Helsinki, Finland) in children with the first acute rhinovirus-induced wheezing episode (79% hospitalized; 21% treated at emergency room of tertiary hospital) using a randomized double-blind placebo-controlled paralleled design. The inclusion criteria were age 3–23 months, first acute wheezing episode (confirmed by parental report and medical records) and delivery at ≥36 weeks. Children with chronic non-atopic illnesses, need for intensive care unit treatment, or previous systemic or inhaled corticosteroid (ICS) treatment were excluded from the study. The study was approved by the Ethics Committee of the Turku University Hospital and was commenced after obtaining written informed consent from guardians. The trial was double-blinded until the 12-month follow-up.
Study protocol
At study entry, the guardian completed a standard questionnaire form, the child was physically examined, a nasopharyngeal aspirate sample was taken using a standardized procedure31 and blood sample was drawn. The study drug was initiated if nasopharyngeal aspirate was rhinovirus PCR positive. Pulmonary function was evaluated at the follow-up visit four years later (1–5/2012, 8–10/2012) by IOS (Jaeger GmbH, Würzburg, Germany, see below for more details). The guardians were instructed to discontinue the ICS treatment of the children 4 weeks before the 4-year follow-up visit. The study protocol was registered at ClinicalTrials.gov in August 2008 (ClinicalTrials.gov number NCT00731575)
Laboratory methods
At study entry, multiplex (Seeplex RV12 ACE Detection, Seegene, Seoul, Korea) or in-house single PCR tests were used for detecting adenovirus, coronavirus 229E, NL63 and HKU1, enteroviruses, human bocavirus, human metapneumovirus, influenza A and B virus, and parainfluenza virus types 1–3, rhinovirus types A, B and C and RSV A and B at the Department of Virology, University of Turku.29 Rhinovirus sequencing has previously been described and analyzed in collaboration with the University of Wisconsin-Madison.32,33 Human bocavirus-1 was also analyzed using serology, as previously described.34 To analyze the virus genome load, a sterile flocked swab (catalog number 502CS01, Copan, Brescia, Italy) was first dipped in the aspirate and then diluted with 1 ml of phosphate-buffered saline. The genome load was analyzed from RNA of rhinovirus positive samples by a quantitative RT-PCR, using known concentrations of rhinovirus-B14 plasmid. The plasmid was received from Glyn Stanway at the University of Colchester (Essex, United Kingdom) and has been described elsewhere.35 Serum levels of allergen-specific Immunoglobulin E (IgE, Phadiatop Combi®, Phadia, Uppsala, Sweden) and blood eosinophil counts (B-eos) were analyzed by the Central Laboratory of Turku University Hospital. Serum 25-hydroxyvitamin D (25-OH-D) measurements were done using liquid chromatography tandem mass spectrometry at Massachusetts General Hospital (Boston, USA).
Impulse oscillometry
The airway hyperresponsiveness and reversible airflow obstruction were measured using IOS.20–23 The impulse oscillatory signals of 5–35 Hz were used and output pressure and flow signals analyzed for their amplitude and phase difference, to determine the airway resistance (Rrs, variable that reflects the energy loss due to resistive forces to the airflow) and the reactance (Xrs, indicator of viscoelastic properties of the small airways). Rrs and Xrs both are components of total respiratory impedance (Zrs, results from phase and pressure changes of the airflow). The frequency dependency of resistance (dRrs/df) was determined by using linear regression through resistance data points Rrs5 and Rrs10. During obstruction Rrs increases resulting in increased dRrs/df. Xrs decreases due to peripheral stiffening as a result of obstruction. The technical realization of IOS is described in more detail in Online Repository.
After the baseline measurements, a 6-minute exercise test was conducted. The children were urged to run 6–8 minutes at an exercise level where the heart rate was held at 85–90% of their estimated maximum heart rate (205 – 1/2 × age), assessed with a heart rate monitor (Polar Sport Tester®, Polar Elektro Ltd, Kempele, Finland). IOS measurement was repeated 1, 5, and 10 minutes after the exercise testing.23,36,37 Finally, the reversible airflow obstruction was assessed with IOS 15 minutes after the bronchodilation with inhalation of 400 micrograms of salbutamol (Ventoline®) administered through a spacer (Babyhaler®); both products are from GlaxoSmithKline (Brentford, UK).20 The results were proportioned by the height of the child. Rrs at 5Hz was categorized as pathological if exercise-induced change in mean crude values was +35% or more2 or if bronchodilator-induced change in mean crude values was −35% or more.23,37(Malmberg 2008, Beydon 2007)
Definitions
Children had atopic sensitization if they had a positive test (cut-off level ≥0.35 kU/L) for IgE specific for any of tested allergens (codfish, cow’s milk, egg, peanut, soybean, wheat, cat, dog, horse, birch, mugwort, timothy, Cladosporium herbarum and Dermatophagoides pteronyssinus).29 Eczema at study entry was a physician-made diagnosis according to pruritus, typical morphology and chronicity of disease and it was defined as atopic if any sensitization was diagnosed.
Statistical methods
Baseline differences between groups of different characteristics were analyzed with two-sample t test (normally distributed variables), Mann-Whitney U test (skewed variables), χ2 test or Fisher exact test (categorical data). Results are expressed as mean (standard deviation, SD) when normally distributed, median (interquartile range, IQR) when they had skewed distribution and numbers (%) for categorical variables. The normality of the variables was assessed with Kolmogorov-Smirnov test. Natural logarithmic (ln) change was applied for Rrs 5 Hz before the multivariable regression analysis due to the positively skewed distribution. Logistic and linear regression analyses were used for multivariable analyses. Results of logistic regression analyses are expressed as odds ratios (OR) with 95% confidence interval (95%CI), and results of linear regression analyses as regression coefficient beta (β) with 95%CI. Multivariable models included age, oral corticosteroid (OCS) treatment and rhinovirus at study entry and depending on the dependent variable also atopic sensitization, seasonal sensitization, atopic eczema, hospitalization at study entry and/or ICS within 4 weeks. Data were analyzed using IBM SPSS version 24 (SPSS Inc, Chicago, Ill, USA). A statistical power analysis was not done for IOS outcomes.(Jartti 2015) A two-sided P <.05 was considered statistically significant.
Results
Study cohort
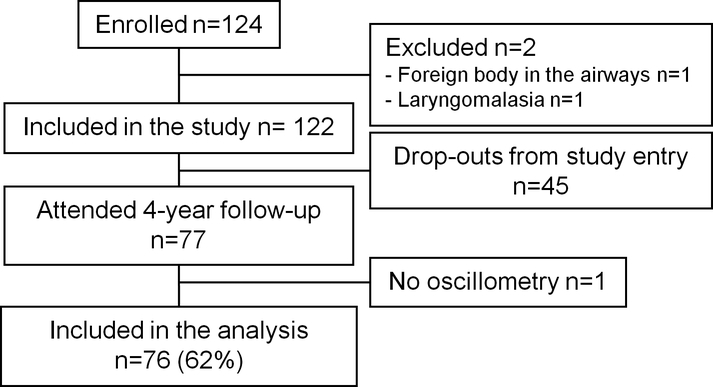
Seventy-seven (62%) of the 124 enrolled children attended the 4-year follow up visit (Fig. 1). IOS and bronchodilation tests were conducted for 76/77 (99%) children and exercise test for 73/77 (95%) children. Exercise test was not conducted due to refusal of running in two (3%) children and due to severe asthma symptoms in one (1%) child. All the children with bronchodilation and/or exercise test (n = 76) were included in the analysis. The included (n=76) and excluded (n=48) participants did not differ from one another concerning the patient characteristics shown in Table I (Online Repository Table E1).
Fig1.
Study Flow Chart
Table I.
Patient characteristics at study entry
| Characteristic | N = 76 |
|---|---|
| Age, months | 12 (6.0) |
| Male sex, no. | 50 (66%) |
| Hospitalized at study entry, no. | 62 (82%) |
| OCS treatment, no. | 21 (28%) |
| Atopic eczema, no. | 13 (17%) |
| B-eos, x109/L | 0.27 (0.16–0.58) |
| B-eos > 0.4 ×109/L, no. | 32/74 (43%) |
| Atopic sensitization, no. | 22/74 (30%) |
| Food sensitization, no. | 21/74 (28%) |
| Aeroallergen sensitization, no. | 11/74 (15%) |
| Seasonal sensitization, no. | 5/74 (7%) |
| Perennial sensitization, no. | 10/74 (14%) |
| Parental asthma, no. | 16 (21%) |
| Parental smoking, no. | 30 (40%) |
| Maternal smoking, no. | 15 (20%) |
| Virus positive, no. | 76 (100%) |
| Rhinovirus, no. | 57 (75%) |
| Rhinovirus repeated during the first-year follow-up, no. | 47/56 (83%) |
| Rhinovirus genome load, copies/mL | 4000 (49–17000) |
| Rhinovirus genome load >7000 copies/mL, no. | 20/50 (40%) |
| Rhinovirus genome load >9000 copies/mL, no. | 18/50 (36%) |
| RSV, no. | 20 (26%) |
| Other virus, no. | 30 (40%) |
| Co-infection, no. | 26 (34%) |
| 25-hydroxyvitamin-D, nmol/l | 88 (20) |
| 25-hydroxyvitamin-D >75nmol/l, no. | 52/73 (71%) |
| Regular ICS medication at 4-year follow-up | 37 (75%) |
| ICS discontinued ≥4 weeks before testing | 25/37 (68%) |
Data are expressed as mean (SD) when normally distributed and median (interquartile range) when not normally distributed, or number (%) unless otherwise noted.
B-eos, blood eosinophil count; OCS, oral corticosteroid; RSV, respiratory syncytial virus.
Patient characteristics
At study entry, the mean age of the 76 study subjects was 12 months (SD 6.0), 62 of them (82%) were hospitalized, and 50 (66%) children were boys. At least one virus was found in the airways of all of the children. The most common virus was rhinovirus, which was found in 57 (75%) children. RSV was found in 20 (26%) children and other virus in 30 (40%) children. At least two viruses were found in the airways of 26 (34%) children. During the first wheezing episode, 22/74 (30%) had atopic sensitization, 11/74 (15%) had aeroallergen sensitization and 21/74 (28%) had food sensitization (Table I).
At the 4-year follow-up visit, the mean age of the children was 60 months (SD 7.9 months). Thirty-seven (49%) children used ICS treatment during the 12 months preceding the 4-year follow-up visit. Asthma medication was discontinued ≥4 weeks prior to the exercise testing in 25 children (68% of the children using ICS) (Table I).
Baseline pulmonary function at 4-year visit
In baseline IOS measurements, one (1%) child had a pathological Rrs 5Hz (z-score >+1.65) value and one (1%) child a pathological Xrs 5 Hz (z-score < −1.65) value when compared to Finnish height-adjusted reference values. Absolute IOS values are shown in online repository Table E2. Children who had atopic eczema (β −0.74; 95%CI −1.4 to −0.11; P = .022) or seasonal atopic sensitization (β −1.1; 95%CI −2.1 to −0.14; P = .025) at study entry had lower Xrs 5Hz z-score at baseline measurement than the children with no atopic eczema or seasonal sensitization, respectively. These results, however, were not statistically significant in multivariable analyses which included age, OCS for the first wheezing episode, rhinovirus etiology and ICS use 4 weeks before the oscillometry. In multivariable analysis, age at study entry for acute wheezing was associated with decreased Xrs 5Hz (β −0.052; 95%CI −0.095 to −0.009; P = .019), i.e. the older the age at study entry, the lower the compliance of the lung tissue due to small airway obstruction. Other factors, such as virus etiology, shown in Table II were not associated with the baseline values of IOS (Online Repository Table E3). Baseline Rrs 10 values were not associated with any of the factors shown in Table II (data not shown).
Table II.
Patient characteristics vs. pulmonary function.
| Baseline Rrs 5 Hz (z-score) | Post-exercise Rrs 5Hz change ≥35%(n = 8) | |||
|---|---|---|---|---|
| Characteristic | Unadjusted | Multivariable | Unadjusted | Multivariable |
| Age at study entry, months | −0.008 (−0.046, 0.030) P = .69 |
−0.11 (−0.54, 0.32) P = .62 |
1.1 (0.97, 1.3) P = 0.15 |
0.98 (0.84, 1.1) P = .83 |
| Age at 4-year follow-up, months | −0.13 (−0.47, 0.22) P = .47 |
1.7 (0.55, 5.5) P = .35 |
||
| Male sex | −0.088 (−0.57, 0.39) P = .71 |
3.7 (0.43, 32) P = .23 |
||
| Hospitalized at study entry | 0.080 (−0.50, 0.66) P = .79 |
n/a P = 1.0 |
||
| OCS at study entry | −0.085 (−0.59, 0.42) P = .74 |
−3.1 (−8.7, 2.5) P = .28 |
2.9 (0.65, 13) P = .16 |
1.7 (0.34, 8.9) P = .51 |
| Atopic eczema at study entry | 0.33 (−0.28, 0.92) P = .28 |
14 (2.7, 72) P = .002 |
||
| B-eos > 0.4 ×109/L, at study entry | −0.36 (−0.82, 0.096) P = .12 |
3.6 (0.65, 20) P = .14 |
||
| Atopic sensitization at study entry | 0.18 (−0.32, 0.69) P = .48 |
−0.27 (−5.9, 5.4) P = .92 |
10 (1.8, 55) P = .008 |
8.8 (1.2, 64) P = .03 |
| Food sensitization at study entry | 0.16 (−0.35, 0.67) P = .54 |
11 (2.0, 60) P = .006 |
||
| Aeroallergen sensitization at study entry | 0.23 (−0.42, 0.88) P = .48 |
9.8 (1.9, 50) P = .006 |
||
| Seasonal sensitization at study entry | 1.0 (−1.5, 3.6) P = .32 |
11 (1.2, 88) P = .03 |
||
| Perennial sensitization at study entry | 0.29 (−0.38, 0.96) P = .40 |
5.9 (1.1, 31) P = .04 |
||
| Parental asthma | 0.080 (−0.47, 0.64) P = .77 |
0.49 (0.055, 4.3) P = .52 |
||
| Parental smoking at study entry | 0.22 (−0.24, 0.68) P = .34 |
0.92 (0.20, 4.2) P = .92 |
||
| Maternal smoking at study entry | −0.10 (−0.67, 0.47) P = .72 |
0.53 (0.060, 4.7) P = .57 |
||
| Rhinovirus at study entry | −0.17 (−0.69, 0.35) P = .52 |
−0.97 (−7.1, 5.2) P = .75 |
n/a P = 1.0 |
n/a P = 1.0 |
| Rhinovirus repeated during the first-year follow-up, no. | −0.10 (−0.86, 0.65) P = .78 |
1.2 (0.13, 12) P = .86 |
||
| Rhinovirus genome load >7000 copies/mL | −0.30 (−0.92, 0.32) P = .33 |
0.17 (0.019, 1.5) P = .11 |
||
| Rhinovirus genome load >9000 copies/mL | −0.34 (−0.97, 0.29) P = .29 |
0.20 (0.023, 1.8) P = .15 |
||
| RSV | 0.049 (−0.47, 0.56) P = .85 |
n/a P = 1.0 |
||
| Other virus | −0.057 (−0.52, 0.41) P = .81 |
0.48 (0.090, 2.6) P = .39 |
||
| Co-infection | −0.085 (−0.56, 0.39) P = .72 |
0.58 (0.11, 3.1) P = .53 |
||
| 25-hydroxyvitamin-D, nmol/l | 0.004 (−0.008, 0.015) P = .51 |
1.0 (0.96, 1.03) P = .88 |
||
| 25-hydroxyvitamin-D, >75nmol/l, no. | 0.22 (−0.30, 0.73) P = .41 |
3.2 (0.37, 28) P = .29 |
||
| ICS within 4 weeks | 0.096 (−0.52, 0.72) P = .76 |
4.4 (0.87, 22) P = .074 |
||
For baseline values, 2-sample t test and linear regression analysis were used and results are expressed as regression coefficient beta or mean difference and 95% confidence intervals. For post-exercise values, logistic regression analysis was used and results are reported as odds ratios and 95% confidence intervals. Multivariable model included age, OCS treatment, sensitization and rhinovirus etiology at study entry. Significant associations are shown bold and italic.
B-eos, blood eosinophil count; 25-OH-D, 25-hydroxyvitamin-D; ICS, inhaled corticosteroid; n/a, not applicable; OCS, oral corticosteroid; Rrs, resistance; RSV, respiratory syncytial virus.
Bronchial reactivity
Increased bronchial reactivity was diagnosed in 9/75 (12%) children. Eight (11%) of these changes were diagnosed in the exercise test as a Rrs 5Hz increase of ≥35% and one (1%) of the children had a Rrs 5 Hz decrease of ≥35% following administration for salbutamol. Increased bronchial reactivity induced by exercise was more common in children who had atopic eczema (OR 14; 95%CI 2.7 to 72; P = .002) or atopic sensitization (OR 10; 95%CI 1.8 to 55; P = .008) at the time of the first wheezing episode. The association with atopic sensitization (OR 8.8; 95%CI 1.2 to 64; P = .032) remained in multivariable analysis which included age at study entry, OCS for the first wheezing episode, atopic sensitization and rhinovirus etiology at study entry (Table II). The exercise-induced change in Rrs 5 Hz was significantly greater in children with atopic sensitization at study entry (β 0.74; 95%CI 0.28 to 1.2; P = .002), atopic eczema at study entry (β 0.90; 95%CI 0.35 to 1.4; P = .002) hospitalization at study entry (β 0.73; 95%CI 0.18 to 1.3; P = .010) or ICS use within 4 weeks (β 0.70; 95%CI 0.080 to 1.3; P = .028). In adjusted analysis, which included age at study entry, hospitalization, OCS use at study entry, ICS use within 4 weeks, atopic sensitization at study entry and rhinovirus etiology, atopic sensitization remained statistically significant (β 0.54; 95%CI 0.027 to 1.1; P = .027). The changes in post-bronchodilator values were not affected by any of the factors shown in Table II (Online Repository Table E3). Post-exercise or post-bronchodilator Rrs 10 values were not affected by any of the factors shown in table II (data not shown).
There were no statistically significant differences in bronchial reactivity depending on age, sex or virus etiology. However, all 8 children with pathological exercise-induced bronchial reactivity (≥35%) were rhinovirus-positive during the first wheezing episode. Likewise, other patient characteristics shown in Table I were not associated with bronchial reactivity.
The effect of corticosteroid treatment
The OCS treatment during the first wheezing episode did not affect the pulmonary function four years later. There were no significant differences in baseline IOS parameters (all P ≥ .13) or in exercise-induced bronchial reactivity (P ≥ .16) between the prednisolone and placebo groups. In the 12 children who were not able to discontinue the ICS use, the exercise-induced change in Rrs 5 Hz was greater than in non-ICS users (β 0.70 95%CI 0.080 to 1.3, P = .028), but this association did not remain in adjusted analysis (Table II, Online Repository Table E3) The results without the children using continuous ICS treatment before the follow-up visit were not markedly different (data not shown).
Discussion
To our knowledge, this is the first study to investigate the associations between the patient characteristics of the first acute severe virus-induced wheezing episode and the subsequent pulmonary function and bronchial reactivity later in childhood. As a main finding, atopic sensitization, both aeroallergen and food sensitization, during the first wheezing episode was associated with increased bronchial reactivity at pre-school age. We did not find any significant association between the rhinovirus or its genome load, other virus infections or other patient characteristics at study entry and the later pulmonary function or bronchial reactivity.
Previous studies have reported that the early atopic sensitization is an important predictor for reduced pulmonary function in later life.4,6,7 Illi et al4 first recognized that the pulmonary function was impaired from age 7 to 13 years in children with earlier atopic wheezing but not in children with earlier non-atopic wheezing. Turner et al6 demonstrated that the infant atopy is associated with the reduction of pulmonary function measured by rapid thoraco-abdominal compression technique or spirometry from the age of 1 month to 18 years. Moreover, Belgrave et al.7 found that number of early specific sensitizations correlated inversely with pulmonary function measured by plethysmography from the age of 3 to 11 years. Our finding extends these results by showing that atopic sensitization at the time of the first severe wheezing episode is associated with increased bronchial reactivity measured by IOS in addition to a reduction in pulmonary function in long-term follow-up to the age of 5 years. The link between allergic airway inflammation and subsequent increased bronchial reactivity is incompletely understood. The possible explanations are related to immune dysregulation and promotion of type 2 cytokines, e.g. IL-13, and counteracting Th1-mediated mechanisms, e.g. interferon expression, which are important in viral defence.1 Allergic airway inflammation may also weaken epithelial barrier function and thereby increase susceptibility to viral infections.38
Previous studies have shown parental asthma and maternal smoking to be associated with baseline pulmonary function and/or bronchial reactivity.5,8 Some studies have also found an association between these patient characteristics and decreasing pulmonary function during a follow-up until the age of 18 years.6,7 Vitamin D has been found to be potential in preventing infectious diseases and atopic illnesses through both innate and adaptive immune responses.39–41 In our study, patient characteristic data were carefully assembled and 25-OH-D levels were analyzed. However, we did not find associations between any of these patient characteristics and IOS parameters. Association between rhinovirus genome load and pulmonary function has not been investigated earlier. We did not find any association between viral load and IOS parameters as well. In our study, the mean age of the children during the pulmonary function testing was lower comparing to the earlier studies (mean 5 years vs. 7.2–11years), which may partly explain the negative results. The low sensitivity of IOS is another probable explanation for the negative results. Moreover, this is the first study concentrating on the first acute virus-induced wheezing episode, and thus, our results underline the role of sensitization as an early risk marker for later bronchial reactivity.
Earlier studies have shown decreased baseline pulmonary function in 20–29% of cases 6–8 years after virus-induced bronchiolitis.8,9,19 In our study, the proportion of pathological pulmonary function at baseline was low (3%). Our detection rate of exercise induced bronchial reactivity (11%) is also relatively low when compared to earlier studies (13–62%).8,9,19 In our study, twelve (32%) of the 37 children were not able to discontinue the ICS medication for asthma, which may partly explain the low detection rate of increased bronchial reactivity. However, in majority (68%) of the children using ICS, the medication was discontinued at least four weeks before the IOS testing. According to Finnish guidelines,42 which are in line with the U.S. National Asthma Education and Prevention Program guidelines,36 regular long-term asthma control medication is started relatively early in life. Although unproven, this may lead to better management of asthma already during early childhood and minor pulmonary function impairment at preschool age.
Pulmonary function measurement in young children is technically challenging. IOS is a relatively new method and that has been shown to be promising from the age 2–3 years onwards due to its requirements of minimum co-operation.20,21,24 Success rate of IOS in our study was 99% and exercise testing was conducted successfully in 96% of the children. This supports the role of IOS as a preferred pulmonary function testing method in young children and exercise test as a practicable method for bronchial reactivity testing already in pre-school aged children. Rrs is dependent on height of the child and frequency of the signal. We used the Finnish population-based height-adjusted reference values,20,23 which makes the results more reliable.
Strengths of our study include the prospective design, new focus on the first wheezing episode, comprehensive viral diagnostics, the use of IOS for pulmonary function testing, good quality of the IOS measurements and discontinuation of regular asthma controller medication in majority of asthmatic children before testing. Allergen specific IgE was used for characterization of atopic sensitization. The children were carefully examined by a physician and the standard questionnaire was used which provided detailed characterization of the children.43 However, our study has some limitations. Most patients were hospitalized during the first acute wheezing episode, which makes these results only applicable for the most severe end of illness. The relatively small study population precluded analysis of rhinovirus species or the associations of other respiratory viruses with spirometry outcomes. Reasonable challenge in exercise test was ensured by continuous heart rate monitoring, but ventilation was not measured for this purpose.
In conclusion, atopic sensitization diagnosed during the first acute severe wheezing episode was associated with increased bronchial reactivity and reduced lung function at preschool age. Clinically, these results suggest that testing for atopic sensitization at the time of first wheezing episode may have prognostic significance with respect to lung function. These results are also important to consider for the design of early intervention trials for secondary prevention of asthma.
Supplementary Material
Acknowledgements
We thank Mrs Tiina Peromaa for her valuable help in conducting the exercise tests, Heidi Jokinen, biomedical laboratory scientist, for her help in laboratory analyses, Dr Pekka Malmberg, PhD, for his guidance into the IOS methodology, and Mr Hans-Jüergen Smith (Jaeger, Würzburg, Germany) for providing us with the IOS device during this study.
Sources of funding: This study was supported by the Academy of Finland (grants 114034, 132595, and 267133), Helsinki; the Allergy Research Foundation, Helsinki; the Allergy Research Foundation of Southwest Finland, Turku; the Finnish Cultural Foundation, Turku and Helsinki; the Finnish Medical Foundation, Helsinki; the Foundation for Pediatric Research, Helsinki; Foundation of the Finnish Anti-Tuberculosis Association, Helsinki; Orion Research Foundation, Helsinki; the Research Foundation of the Pulmonary Diseases, Helsinki; the Sigrid Juselius Foundation, Helsinki; the Tampere Tuberculosis Foundation, Tampere; the Turku University Foundation, Turku; and the Paulo Foundation, Helsinki – all in Finland.
Abbreviations
- B-eos
blood eosinophil count
- CI
confidence interval
- dRrs/df
frequency dependency of resistance
- β
regression coefficient beta
- Fres
resonance frequency
- ICS
inhaled corticosteroids
- IQR
interquartile range
- IgE
immunoglobulin E
- IOS
impulse oscillometry
- OCS
oral corticosteroids
- PCR
polymerase chain reaction
- RSV
respiratory syncytial virus
- Rrs
resistance
- SD
standard deviation
- Xrs
reactance
- 25-OH-D
25-hydroxyvitamin-D
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest in connection with this paper.
References
- 1.Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol 2017;140:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubner FJ, Jackson DJ, Evand MD, Gangnon RE, Tisler CJ, Pappas TE et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol 2017;139:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukkarinen M, Koistinen A,Turunen R,Lehtinen P,Vuorinen T,Jartti T. Rhinovirus-induced first wheezing episode predicts atopic but not nonatopic asthma at school. J Allergy Clin Immunol 2017;140:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet 2006;368:763–70. [DOI] [PubMed] [Google Scholar]
- 5.Hyvärinen MK, Kotaniemi-Syrjänen A, Reijonen TM, Korhonen K, Korppi MO. Lung function and bronchial hyper-responsiveness 11 years after hospitalization for bronchiolitis. Acta Paediatr 2007;96:1464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner S, Fielding S, Mullane D, Cox DW, Goldblatt J, Landau L et al. A longitudinal study of lung function from 1 month to 18 years of age. Thorax 2014;69:1015–20. [DOI] [PubMed] [Google Scholar]
- 7.Belgrave DC, Buchan I, Bishop C, Lowe L, Simpson A, Custovic A Trajectories of lung function during childhood. Am J Respir Crit Care Med 2014;189:1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotaniemi-Syrjänen A, Reijonen TM, Korhonen K, Waris M, Vainionpää R, Korppi M. Wheezing due to rhinovirus infection in infancy: Bronchial hyperresponsiveness at school age. Pediatr Int 2008; 50:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauhkonen E, Koponen P, Nuolivirta K, Paassilta M, Toikka J, Korppi M. Lung function by impulse oscillometry at age 5–7 years after bronchiolitis at age 0–6 month. Pediatr Pulmonol 2015;50:389–95. [DOI] [PubMed] [Google Scholar]
- 10.Morales E, Garcia-Esteban R, de la Cruz OA, Basterrechea M, Lertxundi A, de Dicastillo MD et al. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax 2015;70:64–73. [DOI] [PubMed] [Google Scholar]
- 11.Schultz ES, Hallberg J, Gustafsson PM, Bottai M, Bellander T, Bergström A et al. Early life exposure to traffic-related air pollution and lung function in adolescence assessed with impulse oscillometry. J Allergy Clin Immunol 2016;138:930–2. [DOI] [PubMed] [Google Scholar]
- 12.Vollsӕter M, Røksund OD,Eide GE, Markestad T Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax 2013;68:767–76. [DOI] [PubMed] [Google Scholar]
- 13.Thunqvist P, Gustafsson PM, Schultz ES, Bellander T, Berggren-Brorström E, Norman M et al. Lung Function at 8 and 16 Years After Moderate-to-Late Preterm Birth: A Prospective Cohort Study. Pediatrics 2016;137:4. [DOI] [PubMed] [Google Scholar]
- 14.den Dekker HT, Sonnenschein-van der Voort AMM, de Jongste JC, Anessi-Maesano I, Arshad SH, Barros H et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J Allergy Clin Immunol 2016;137:1026–35. [DOI] [PubMed] [Google Scholar]
- 15.Örtqvist AK, Ullemar V, Lundholm C, Kuja-Halkola R, Magnusson PKE, Lichtenstein P et al. Fetal Growth and Childhood Lung Function in the Swedish Twin Study on Prediction and Prevention of Asthma. Ann Am Thorac Soc 2017;14:1147–53. [DOI] [PubMed] [Google Scholar]
- 16.Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton R et al. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol 2011;128:532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541–5. [DOI] [PubMed] [Google Scholar]
- 18.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010;65:1045–52. [DOI] [PubMed] [Google Scholar]
- 19.Fjaerli HO, Farstad T, Rød G, Ufert GK, Gulbrandsen P, Nakstad B. Acute bronchiolitis in infancy as risk factor for wheezing and reduced pulmonary function by seven years in Akershus County, Norway. BMC Pediatr 2005;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malmberg LP, Pelkonen A, Poussa T, Pohjanpalo A, Haahtela T, Turpeinen M. Determinants of respiratory system input impedance and bronchodilator response in healthy Finnish preschool children. Clin Physiol Funct Imaging 2002;22:64–71. [PubMed] [Google Scholar]
- 21.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse Oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol 2003;112:317–22. [DOI] [PubMed] [Google Scholar]
- 22.Oostveen E, MacLeod D, Lorino H, Farré R, Hantos Z, Desager K et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J 2003;22:1026–41. [DOI] [PubMed] [Google Scholar]
- 23.Malmberg LP, Mäkelä MJ, Mattila PS, Hammarén-Malmi S, Pelkonen AS. Exercise-induced changes in respiratory impedance in young wheezy children and non-atopic controls. Pediatr Pulmonol 2008;43:538–44. [DOI] [PubMed] [Google Scholar]
- 24.Dencker M, Malmberg LP, Valind S, Thorsson O, Karlsson MK, Pelkonen A et al. Reference values for respiratory system impedance by using impulse oscillometry in children aged 2–11 years. Clin Physiol Funct Imaging. 2006;26:247–50. [DOI] [PubMed] [Google Scholar]
- 25.Lukkarinen M, Lukkarinen H, Lehtinen P, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr Allergy Immunol. 2013;24:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukkarinen M, Vuorinen T, Lehtinen P, Ruuskanen O, Jartti T. Sensitization at the first wheezing episode increases risk for long-term asthma therapy. Pediatr Allergy Immunol. 2015;26:687–91 [DOI] [PubMed] [Google Scholar]
- 27.Jartti T, Nieminen R, Vuorinen T, Lehtinen P, Vahlberg T, Gern J et al. Short- and long-term efficacy of prednisolone for first acute rhinovirus-induced wheezing episode. J Allergy Clin Immunol 2015;135:691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koistinen A, Lukkarinen M, Turunen R, Vuorinen T, Vahlberg T, Camargo CA Jr, Gern J, Ruuskanen O, Jartti T. Prednisolone for the first rhinovirus-induced wheezing and 4-year asthma risk: A randomized trial. Pediatr Allergy Immunol. 2017;28:557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turunen R, Koistinen A, Vuorinen T, Arku B, Söderlund-Venermo M, Ruuskanen O et al. The first wheezing episode: respiratory virus etiology, atopic characteristics, and illness severity. Pediatr Allergy Immunol 2014;25:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukkarinen M, Koistinen A, Turunen R, Lehtinen P, Vuorinen T, Jartti T. Rhinovirus-induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J Allergy Clin Immunol. 2017;140:988–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jartti T, Lehtinen P, Vuorinen T, Osterback R, van den Hoogen B, Osterhaus AD et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis 2004;10:1095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improve molecular typing assay for rhinovirus species A, B and C. J Clin Microbiol 2014;52:2461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turunen R, Jartti T, Bochkov Y, Gern J, Vuorinen T. Rhinovirus species and clinical characteristics in the first wheezing episode in children. J Med Virol. 2016;88:2059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Söderlund-Venermo M, Lahtinen A, Jartti T, Hedman L, Kemppainen K, Lehtinen P et al. Clinical assessment and improved diagnosis of bocavirus-inducedwheezing in children, Finland. Emerg Infect Dis 2009;15:1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanway G, Hughes PJ, Mountford RC, Minor PD, Almond JW. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 1984;12:7859 –7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heart National, Lung and Blood Institute (NAEPP). Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. Full report. 2007 http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdin.pdf.
- 37.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P et al. An official American Thoracic Society/European Respiratory Society statement:pulmonary function testing in preschool children. Am J Respir Crit Care Med 2007;175:1304–45. [DOI] [PubMed] [Google Scholar]
- 38.Jakiela B, Brocman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker VP, Modlin RL. The Vitamin D connection to pediatric infections and immune function. Pediatr Res 2009;65:106–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullins RJ, Camargo CA. Latitude, sunlight, vitamin D, and childhood food allergy/anaphylaxis. Curr Allergy Asthma Rep 2012;12:64–71. [DOI] [PubMed] [Google Scholar]
- 41.Bergman P, Lindh AU, Björkhem-Bergman L, Lindh JD. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asthma. Current Care Guidelines. Working group set up by the Finnish Medical Society Duodecim, the Finnish Respiratory Society, Finnish Paediatric Society, and Finnish Society of Clinical Physiology, Helsinki: The Finnish Medical Society Duodecim, 2012. (referred November 13, 2017). Available online at: www.kaypahoito.fi.
- 43.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW and et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.