Abstract
Different GABAergic interneuron types have specific roles in hippocampal function, and anatomical as well as physiological features vary greatly between interneuron classes. Long-term plasticity of interneurons has mostly been studied in unidentified GABAergic cells and is known to be very heterogeneous. Here we tested whether cell type-specific plasticity properties in distinct GABAergic interneuron types might underlie this heterogeneity. We show that long-term potentiation (LTP) and –depression (LTD), two common forms of synaptic plasticity, are expressed in a highly cell type-specific manner at glutamatergic synapses onto hippocampal GABAergic neurons. Both LTP and LTD are generated in interneurons expressing parvalbumin (PV+), whereas interneurons with similar axon distributions but expressing cannabinoid receptor-1 (CB1R+) show no lasting plasticity in response to the same protocol. In addition, LTP or LTD occurs in PV+ interneurons with different efferent target domains. Perisomatic-targeting PV+ basket and axo-axonic interneurons express LTP, whereas glutamatergic synapses onto PV+ bistratified cells display LTD. Both LTP and LTD are pathway-specific, independent of NMDARs and occur at synapses with calcium-permeable AMPA receptors. Plasticity in interneurons with CP-AMPARs strongly modulates disynaptic GABAergic transmission onto CA1 pyramidal cells. We propose that long-term plasticity adjusts the synaptic strength between pyramidal cells and interneurons in a cell type-specific manner and in the defined CA1 interneurons shifts the spatial pattern of inhibitory weight from pyramidal cell dendrites to the perisomatic region.
Keywords: CCK (Cholecystokinin), basket cell, feedback, inhibition, network, synaptic plasticity, synchrony
INTRODUCTION
Synapses between cortical excitatory principal cells show relatively stereotypical activity-induced plasticity and may express either long-term potentiation (LTP) or long-term depression (LTD) depending on glutamate NMDA receptors. Concomitant with LTP in hippocampal pyramidal cells, the strength of local inhibition is often altered (Kullmann and Lamsa, 2007; McBain, 2008; Pelletier and Lacaille, 2008). Parallel modulation of inhibition is likely to be required to balance changes in the network excitability caused by plasticity between principal cells (Kullmann and Lamsa, 2007), as well as to maintain fidelity, threshold and gain of pyramidal cell responses to their excitatory inputs (Lamsa et al., 2005; Carvalho and Buonomano, 2009). Yet, plasticity reported in GABAergic interneurons is highly heterogeneous and varies between hippocampal areas and layers (McMahon and Kauer, 1997; Cowan et al., 1998; Alle et al., 2001; Perez et al., 2001; Lei and McBain, 2004; Lamsa et al., 2005; Lamsa et al., 2007; Galvan et al., 2008), possibly reflecting the high diversity of GABAergic cells in the hippocampus (Kullmann and Lamsa, 2007; McBain, 2008). Cell type-specific firing patterns of many interneurons in vivo suggest that distinct GABAergic cell types have highly specified roles in the hippocampal function (Klausberger et al., 2003; Klausberger et al., 2005). Excitatory glutamatergic synapses onto many hippocampal interneurons have calcium-permeable (CP-) AMPA receptors and these synapses often express NMDA receptor-independent LTP or LTD (Laezza et al., 1999; Perez et al., 2001; Laezza and Dingledine, 2004; Lei and McBain, 2004; Lamsa et al., 2007; Oren et al., 2009). Afferent stimulation of glutamatergic pathways which elicits LTP in principal cells in the hippocampal CA1 or dentate gyrus can induce parallel potentiation of GABAergic transmission (Buzsaki and Eidelberg, 1982; Kairiss et al., 1987; Lapointe et al., 2004; Lamsa et al., 2005). It has been suggested that this might result from LTP of excitatory glutamatergic synapses driving a subpopulation of local inhibitory interneurons (Kullmann and Lamsa, 2007; Pelletier and Lacaille, 2008). Equally, afferent stimulation of hippocampal mossy fibres can elicit either LTP or LTD in CA3 inhibitory cells in a layer-specific manner (Maccaferri et al., 1998; Lei and McBain, 2004; Galvan et al., 2008; McBain, 2008), but each hippocampal layer may contain several types of interneurons, as defined by their neurochemical markers and the pyramidal cell membrane domains that their axons target. To understand the nature and functional significance of heterogeneous interneuron plasticity in the hippocampus, it is therefore important to identify the interneurons studied in terms of their axonal projections and molecular expression profiles.
Here we test how consistent long-term plasticity is across five common interneuron types innervating different domains of pyramidal cells in the hippocampal CA1 area. We hypothesized that plasticity properties might systematically vary between individual GABAergic interneuron types. In the CA1 area, interneurons immunopositive for parvalbumin (PV+) or cannabinoid receptor 1 (CB1R+) collectively target the whole postsynaptic surface of local pyramidal cells, but individual cell types preferentially innervate one or more specific membrane domains of their targets. We demonstrate that there are cell type-specific rules of plasticity, which emphasize distinct roles of interneurons in hippocampal function.
METHODS
Hippocampal slice preparation
Three- to four- week old male Sprague-Dawley rats were killed according to the Animals (Scientific Procedures) Act 1986, and transverse hippocampal slices (350 μm thickness) were prepared as described in Oren et al. (2009) (see Supplemental methods). Hippocampal slices were placed in a recording chamber (Luigs & Neumann, Germany) mounted on the stage of an upright microscope (Olympus BX51WI, Japan), where they were held under a nylon mesh grid and superfused at 3-5 ml min−1 with artificial cerebrospinal fluid (ACSF) at 31 – 33°C. Slices were visualized using a 20x immersion objective with 2–4x zoom and infrared differential interference contrast (DIC) optics. A cut was made between CA1 and CA3 to prevent propagation of recurrent excitation from the CA3. The perfusion medium contained (mM): NaCl (119), KCl (2.5), CaCl2 (2.5), MgSO4 (1.3), NaH2PO4 (1.25), NaHCO3 (25), glucose (11); final pH 7.4 (equilibrated with 95% O2 / 5% CO2). Glutamate NMDARs were blocked in most experiments with DL-AP5 (100 μM) unless stated otherwise. The GABA receptor blockers picrotoxin (PiTX, 100 μM) and CGP 55845 (1 μM) were added to the solution for all experiments except those for recording IPSCs (Figs. 5 and 6, Supplemental figures S3 and S4). In experiments with cannabinoid receptor type 1 antagonist AM-251 (5-10 μM), slices were incubated with the drug for at least 1 h before recording and the drug was present in the perfusion solution. Glutamate receptor antagonists philanthotoxin-433 (PhTx, 10 μM) and NBQX (10 μM) were applied via perfusion. Chemicals were purchased from Sigma-Aldrich (UK) and drugs from Tocris Cookson (UK) or Ascent Scientific (UK).
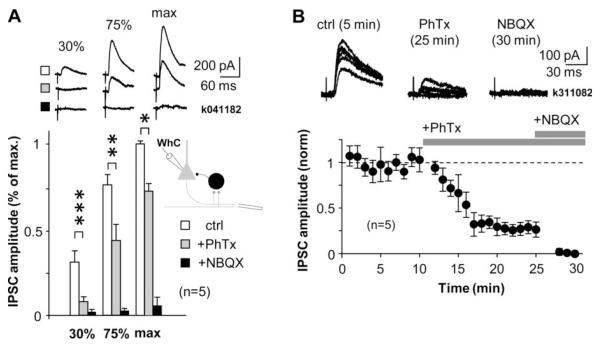
Figure 5.
Disynaptic GABAergic transmission in the CA1 involves activation of interneurons with CP-AMPARs. CP-AMPAR blocker PhTx (10 μM) strongly inhibits disynaptic IPSCs recorded in the pyramidal cell soma. The effect of PhTx was studied at three different IPSC amplitudes relative to the maximum (max, 75% and 30% amplitudes), and was most pronounced at lower amplitudes of disynaptic IPSCs, indicating strong contribution of interneurons with CP-AMPARs. A) Top: IPSCs averages in one experiment. Symbols indicate ctrl, +PhTx and +NBQX as below. Bottom: histogram shows IPSC amplitudes at three different stimulus intensities relative to the maximum disynaptic IPSC. IPSCs were evoked by extracellular stimulation in oriens/alveus close to subiculum. Schematic shows experimental design. Open bars show IPSCs (mean ± se) in control, and grey bars following wash-in of PhTx (15 min). (*P<0.05, **P<0.01, ***P<0.005, paired t-test). Disynaptic origin of IPSCs was confirmed at the end by NBQX application (10 μM, solid bars). B) Temporal properties of blocking disynaptic IPSCs by PhTx when 30% of maximum IPSC was used throughout experiment. Horizontal bars indicate timing of drug application as indicated. Top: Consecutive IPSCs in one experiment during baseline and following exposure to the drugs.
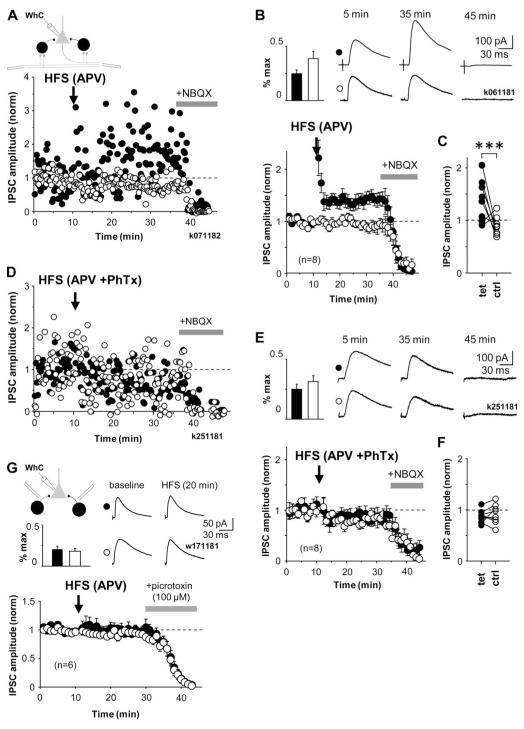
Figure 6.
LTP of disynaptic GABAergic transmission in str. oriens is NMDAR-independent, pathway-specific and requires interneurons with CP-AMPARs. A) HFS induces long-term potentiation in disynaptic IPSCs recorded in CA1 pyramidal cell somata. Two disynaptic pathways were stimulated with electrodes positioned in str.oriens/alveus. Schematic shows experimental design. IPSC amplitude was adjusted to approximately 30% of maximum. After a baseline, HFS was given to one of the two pathways (solid symbols), the other pathway was not tetanized and was used as a control (open symbols). LTP was observed for 25 min. Full blockade of IPSCs at the end by NBQX (wash-in indicated by horizontal bar) confirmed disynaptic nature of the IPSCs. Data are from one cell, in the presence of DL-APV (100 μM) and CGP55845 (1 μM). In addition cannabinoid receptors were blocked with AM-251 (10 μM). B) Similar recordings as in (A), averaged from eight cells showing mean ± se of disynaptic IPSC amplitude. Top left, bar histogram shows disynaptic IPSC strength relative to maximum in the pathways during baseline (mean ± se). Top right, averaged IPSCs at different time points in one cell as indicated. C) Comparison of averages of baseline-normalized IPSC amplitudes in all experiments 20 min following the HFS. Solid and open symbols indicate tetanized and control pathways as above (***P<0.005, unpaired t-test). D) LTP of disynaptic GABAergic transmission fails in the presence of the CP-AMPAR blocker PhTx. Similar experiment as in (A), but in the presence of PhTx (10 μM). E) Disynaptic IPSC mean ± se eight experiments in the presence of PhTx as in (D). Insets as in (B). F) Averages of baseline-normalized IPSCs in all experiments 20 min following the HFS. G) HFS does not change monosynaptic GABAergic IPSC. Monosynaptic IPSCs measured in CA1 pyramidal cell soma elicited by stimulation from str.oriens. IPSC amplitudes were adjusted on average below 30 % of maximum (Inset bar histogram). HFS was applied to one pathway (solid symbols) after a baseline period. Control pathway is shown by open symbols. HFS failed to induce lasting changes in the monosynaptic IPSC. Plot shows mean ± se of baseline-normalized IPSC amplitude in 6 cells. IPSCs were blocked by picrotoxin at the end. GABAB, ionotopic glutamate and CB1 receptors were blocked with CGP55845 (1 μM), NBQX (10 μM), DL-AP5 (100 μM) and AM-251 (10 μM). Averaged IPSCs are shown from one experiment during baseline and following HFS (20min).
Electrophysiological recordings
Perforated patch
(see Supplemental methods) Somatic perforated-patch recordings were made from neurons close to or inside CA1 stratum (str.) pyramidale using pipettes containing gramicidin (50-200 μg/ml). Electrodes were made from borosilicate glass capillaries. Pipette resistance was 8–15 MΩ for perforated patch. Filling solution contained (mM): K-gluconate (145), NaCl (8), K-HEPES (20–25), EGTA (0.2), and QX-314 Br (5); pH 7.2, osmolarity 295 mOsml−1. The electrode tip was filled with gramicidin-free solution. Recordings were started when series resistance was below 150 MΩ. Series resistance was continuously monitored but not recorded.
Re-patch
Upon completion of perforated patch recordings, the pipette was slowly retracted under infra-red DIC observation. Once the pipette detached from the cell, it was rapidly withdrawn from the slice. Next, the same cell was approached with a new pipette and re-patched in whole-cell configuration. Infra-red images of the cell at different magnification were obtained with a CCD camera during perforated patch recording and compared in whole-cell recordings to verify that the same cell was re-patched (Supplemental Fig. 1) (Lamsa et al., 2005; Lamsa et al., 2007; Oren et al., 2009). Voltage-clamp recording of EPSCs was performed in re-patched cells only if access resistance was <20 MΩ.
Whole-cell
(see Supplemental methods) The whole-cell recordings were made with a solution containing (mM) CsCl (145), HEPES (20), Cs-EGTA (0.2), NaCl (8), Mg-ATP (2), GTP (0.3), and QX-314 Br (5); pH 7.2, 290 mOsm. In some experiments Cs-methanesulphonate (145) was used instead of CsCl. Spermine tetrahydrochloride (0.5 mM, Tocris Cookson, UK) was included in the filling solution to maintain polyamine-mediated rectification of AMPA/kainate receptors during whole cell recording. In addition, neurobiotin (0.2-0.5%, Vector Laboratories, CA, USA) or biocytin (0.5%, Sigma-Aldrich) was included in the solution to enable post hoc anatomical analysis. Electrode input resistance for whole cell recordings was 4-6 MΩ.
Electrical stimulation
(see Supplemental methods) Monosynaptic excitatory postsynaptic potentials (EPSPs) or excitatory postsynaptic currents (EPSCs) were evoked by alternately stimulating (50-100 μs) in the s. oriens/alveus in area CA1 with 15 s inter-event interval via two concentric bipolar electrodes, connected to constant current isolated stimulators. In some experiments, the stimulation electrode for control pathway was positioned in str.radiatum (see Supplemental tables 1,2). Evoked EPSPs were recorded from the resting membrane potential or in some experiments during a brief (500 ms) hyperpolarizing step (5–10 mV) to avoid action potential generation. In whole cell recordings, EPSC current-voltage relationships were estimated over a postsynaptic membrane potential range from −90 mV to +60 mV using 1 s steps in voltage-clamp mode. The EPSC rectification index (RI) was obtained by dividing the amplitude of the EPSC recorded at +60 mV by that measured at −60 mV. A Multiclamp 700B amplifier was used for recording. In all recordings data were low-pass filtered (4 - 5 kHz) and acquired at 10 - 20 kHz on a PC for offline analysis. Data was analysed using LabView and pClamp 10. Disynaptic inhibitory currents (IPSCs) were evoked by stimulation in str.oriens/alveus, and voltage clamping the postsynaptic cell to between 0 and +10 mV (Cs-methanesulphonate–containing filling solution was used). Maximum disynaptic IPSC was screened by increasing stimulation intensity voltage using fixed duration (100-150 μs). Submaximal amplitude IPSCs were evoked by shortening the stimulation duration (up to 25 μs).
Statistics
Data are shown as mean ± s.e. Data on postsynaptic potentials/currents were baseline-normalized within each pathway and analyzed with Student’s paired t-test. Within each cell the change in the initial slope (3-5 ms from onset) of the paired pathway was compared to that of the control pathway with an unpaired t-test.
Anatomical analysis
Tissue processing
(see Supplemental methods) Neurons were filled with neurobiotin (Vector Laboratories, CA, USA) or biocytin (Sigma, UK) during whole cell recordings, fixed and examined as described in Oren et al. (2009). For illustration, selected cells were digitally photographed from one or two 70 μm thick sections using structured illumination microscopy (AxioImager ApoTome, Zeiss AxioImager.Z1, Carl Zeiss Microimaging Gmbh, Germany) with Zeiss 38HE filter, 40x oil immersion objective and AxioVision Release 4.7.1 software. Images were constructed from Z-stacks using ImageJ 1.42 software (NIH, USA) and inverted to show the cell on white background; NeuronJ program was used for neurite tracing at a preset line thickness and quantification. Dendrites were manually selected from microscopic examination and are shown in a colour different from the axon. Epifluorescent images were taken with the Zeiss AxioImager.Z1 microscope (Zeiss HE38 filter, 40x or 63x oil-immersion objective) using AxioVision software, and digital micrographs were constructed from Z-stacks with ImageJ software. Micrographs were not manipulated selectively, only brightness and contrast of the whole stacked image was adjusted.
Immunohistochemistry
(see Supplemental methods) Sections were blocked in normal horse serum (NHS, Vector Laboratories, CA, USA) for 1 h and incubated in mixtures of appropriate primary antibodies for 48 h at 4°C. A complete list of primary antibodies, their species, dilution, source and specificity references is given in Supplemental table 3. When fluorescence was not detectable in the relevant area of the section where similar parts of other unfilled cells were immunopositive, cells were considered immunonegative. If a decision about immunoreactivity could not be clearly made because the cell appeared to be negative, but the immunoreactivity in unrecorded cells of the slice was low or mostly absent, then the immunoreaction test does not inform about the presence or absence of the molecule in that specific cell. Furthermore, in cases of low immunoreactivity levels, when a conclusion could not be reached by several investigators, the test was considered inconclusive (indicated as reacted but not tested “nt” in the Supplemental tables 1 and 2).
Electron microscopy
Sections from two putative axo-axonic cells were prepared for electron microscopic analysis (see Supplemental table 1). After fixation and re-sectioning of slices (as above), selected sections were washed in 0.1M PB and then stored in 0.05% sodium azide with 0.1M PB. Following cryoprotection with sucrose and freeze-thaw to enhance penetration of reagents, the cells were revealed with HRP reaction (ABC Elite kit, Vector Laboratories; 0.05% DAB, Sigma, UK; 0.01% H2O2). The sections were treated with 1% OsO4 (in PB; TAAB Laboratory Equipment Ltd, UK) and 1% aqueous uranyl acetate, dehydrated and embedded in epoxy resin (Durcupan, Fluka, UK). The axons were examined for the identity of postsynaptic targets without lead staining (Klausberger et al., 2003).
RESULTS
Cell type-specific LTP in CA1 perisomatic-targeting interneurons
We studied long-term plasticity in glutamatergic afferents onto five different GABAergic interneuron types or groups in the hippocampal CA1 area, as defined by their axonal projections and molecular expression profiles (Klausberger and Somogyi, 2008). We patched putative interneurons inside or in the vicinity of str. pyramidale in the CA1 area of hippocampal slices. Perforated patch recording was used in order to minimize disruption of intracellular postsynaptic content of interneurons (Kullmann and Lamsa, 2007). Interneurons were then re-patched with a new pipette in whole cell mode, studied in voltage clamp and filled with biocytin for post hoc anatomical identification (Lamsa et al., 2007; Oren et al., 2009). Cells were identified on the basis of axonal pattern and neurochemical marker expression. Briefly, cell types were: axo-axonic cells innervating mainly or exclusively the axon initial segment of pyramidal cells; basket cells innervating somata and proximal dendrites as predicted from the concentration of their axon in str. pyramidale, and expressing either PV or CB1R; and bistratified cells innervating mainly dendrites in str. radiatum and oriens and expressing PV, neuropeptide-Y (NPY) and/or somatostatin (Halasy et al., 1996; Losonczy et al., 2002; Pawelzik et al., 2002; Klausberger et al., 2004; Baude et al., 2007). The fifth group of interneurons included several cell types in terms of synaptic targets, as predicted from the absence of axon concentration to str. pyramidale, and for brevity will be called “non-basket cell CB1 expressing cell type”, because in slice preparations often insufficient axon is revealed for accurate identification (Klausberger and Somogyi, 2008). Cells classified as CB1R+non-basket cells had their soma either in str. radiatum (n=11) or in str. oriens (n=3). They presented radially or diagonally oriented dendrites in strata oriens and radiatum, sometimes with small dendritic tufts into str. lacunosum moleculare, and large axon arbors in strata oriens and/or radiatum, hardly ever crossing the border to str. lacunosum moleculare. The proportion of innervation to str. oriens or str. radiatum differed between individual cells (for examples see Figures 2D and E). Long axon collaterals travelling across strata oriens and radiatum were characteristic of most CB1R+ non-basket cells. Others were more reminiscent of bistratified cells with denser axon clusters above and below str. pyramidale but the pyramidal layer itself always had sparse innervation in contrast to typical basket cells. Only cells that fit the criteria for one of the categories above were included in this study. Data for identification of all interneurons studied are presented in Supplemental tables 1 and 2.
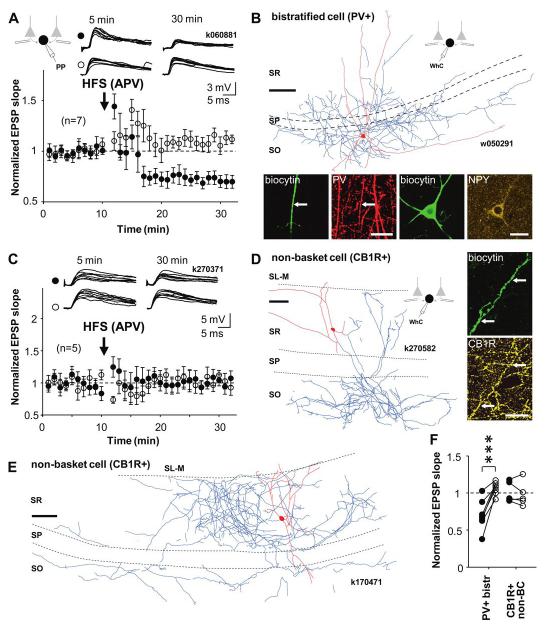
Figure 2.
LTP is specific to perisomatic-targeting PV+ cell types. EPSPs in PV+ bistratified cells which target to the dendritic domain of CA1 pyramidal cells show LTD. EPSPs in interneurons that express CB1R and innervate pyramidal cell dendrites show no lasting plasticity. Perforated patch (PP) recordings. A) Mean ± se of EPSP slope in 7 identified bistratified cells. HFS to one of the pathways (solid symbols) induced pathway-specific LTD. Open symbols show EPSP in the untetanized control pathway. Top, consecutive EPSP traces during baseline and 20 min following the HFS in the two pathways. Schematic shows experimental design. B) Visualization of one bistratified cell by digital rendering of fluorescent images (dendrites red and axon blue, from one 70 μm section) recorded in whole cell (WhC). Scale bar, 100 μm. Fluorescence micrographs demonstrating immunopositivity for PV (red, laser confocal images) as tested in a dendrite (indicated by arrow) and for neuropepetide Y (yellow, structured illumination microscope images) in the soma. Biocytin is shown in green, scale, 20 μm. C) Mean ± se of EPSP slope from 5 cells identified as dendrite-targeting CA1 interneurons expressing CB1 receptor. None of the cells showed significant lasting plasticity in the EPSP. Top, consecutive EPSPs in the two pathways during baseline and 20 min after HFS. D) Visualisation of a CB1R+ non-basket cell (k270582, reconstruction from two 70 μm thick sections), recorded in perforated and WhC mode. Axon ramifies in strata oriens and radiatum, but not in pyramidale. It was verified that the main axon originated 55 μm away from the soma at a point where the dendrite turned by 90 degrees. Scale, 100 μm. Fluorescence micrographs demonstrating immunopositivity for CB1R in an axon visualised with biocytin (green, indicated by arrow; structured illumination microscopic images). Scale, 20 μm. E) Another example of a non-basket cell (k170471, dendrites, red, from three 70 μm thick sections, axon, blue, from two sections) recorded in WhC mode that was also confirmed to be positive for CB1R (not shown but see Supplemental table 2). Note the rich axon arborisation in strata radiatum and oriens but the clear absence of axon concentration in str. pyramidale. Scale,100 μm. F) Comparison of baseline-normalized EPSP slopes following HFS (20 min) in the two types of dendrite-targeting interneurons. Solid and open symbols indicate tetanized and control pathways, respectively (***P<0.005, unpaired t-test).
We elicited EPSPs by alternately stimulating two electrodes in different locations in str.oriens/alveus to activate pyramidal cell axons (see Methods). GABA receptors were blocked by PiTX (100 μM) and CGP55845 (1 μM), glutamate NMDARs were blocked with DL-APV (100 μM). After a baseline period of at least 10 min, high frequency stimulation (HFS, 100 Hz for 1s, delivered twice, 20s interval) was delivered to one pathway whereas the other pathway served as a control. Unlike the long-term plasticity in pyramidal cells, LTP and LTD in many hippocampal interneurons is NMDAR-independent, and involves CP-AMPARs (Laezza et al., 1999; Lamsa et al., 2007; Oren et al., 2009). Postsynaptic somatic potential was voltage clamped to −70 mV (or more negative down to −90 mV) during HFS in order to maximize conductance of CP-AMPAR (Geiger et al., 1995) (Supplemental Fig. 1). Following the HFS, EPSPs were recorded for at least 20 min before cells were re-patched (in three cells LTP was followed up to 30 min). Analysis of EPSPs was focused on initial slope of EPSPs (3-5 ms from onset) to avoid contamination by polysynaptic EPSPs (Maccaferri and McBain, 1996; Lamsa et al., 2005). The high-frequency stimulation did not cause long-lasting changes in the resting membrane potential of the interneurons (Supplemental Fig. 1) (Ross and Soltesz, 2001).
Visualization of the cells (see Methods) revealed that LTP was elicited in 7 out of 14 interneurons whose axon targeted specifically the perisomatic domain of CA1 pyramidal cells. LTP was defined as potentiation of EPSP initial slope at least to 125% of baseline (tested with paired t-test) and specific to the pathway (significant difference to control pathway, tested with unpaired t-test). In cells with significant potentiation and pathway specificity, EPSP in the tetanized pathway increased to 156 ± 8% (P<0.005, n=7, 20 min after HFS) whereas EPSP in the control pathway did not change (102 ± 6%) (see Fig. 1A-C). Four of the interneurons with LTP were identified as axo-axonic cells, based on their axonal patterns showing radial bouton rows that follow axon initial segments of pyramidal cells (Somogyi et al., 1985). Two cells randomly selected were confirmed by electron microscopy to innervate axon initial segments and two were confirmed as expressing parvalbumin (PV) (Klausberger et al., 2003). The three other cells showing LTP and targeting pyramidal cells perisomatically were identified as basket cells. Immunohistochemical testing showed that these basket cells were all positive for PV and negative for cannabinoid receptor 1 (CB1R) (Fig. 1D,E; Supplemental table 1). One additional axo-axonic cell failed to show LTP. Average potentiation of EPSP in all eight PV+ perisomatic-targeting interneurons is shown in Figure 1C. The remaining six perisomatic-targeting cells which did not show LTP were negative for parvalbumin and positive for CB1R (Fig. 1F,G; Supplemental table 2). We conclude that perisomatic-targeting interneurons positive for either PV or CB1R show strict cell type-specific plasticity in the CA1 area. LTP was induced in 7 out of 8 identified PV+ perisomatic-targeting interneurons, but not in any of the identified CB1R+ basket cells (Fig. 1H).
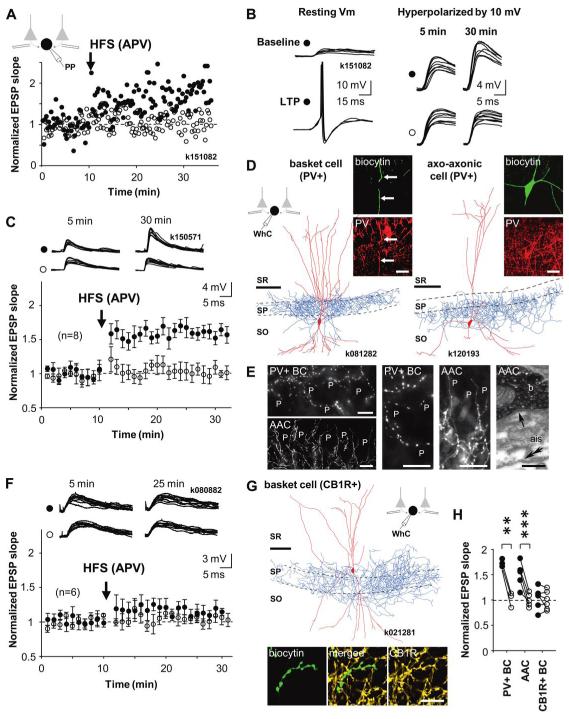
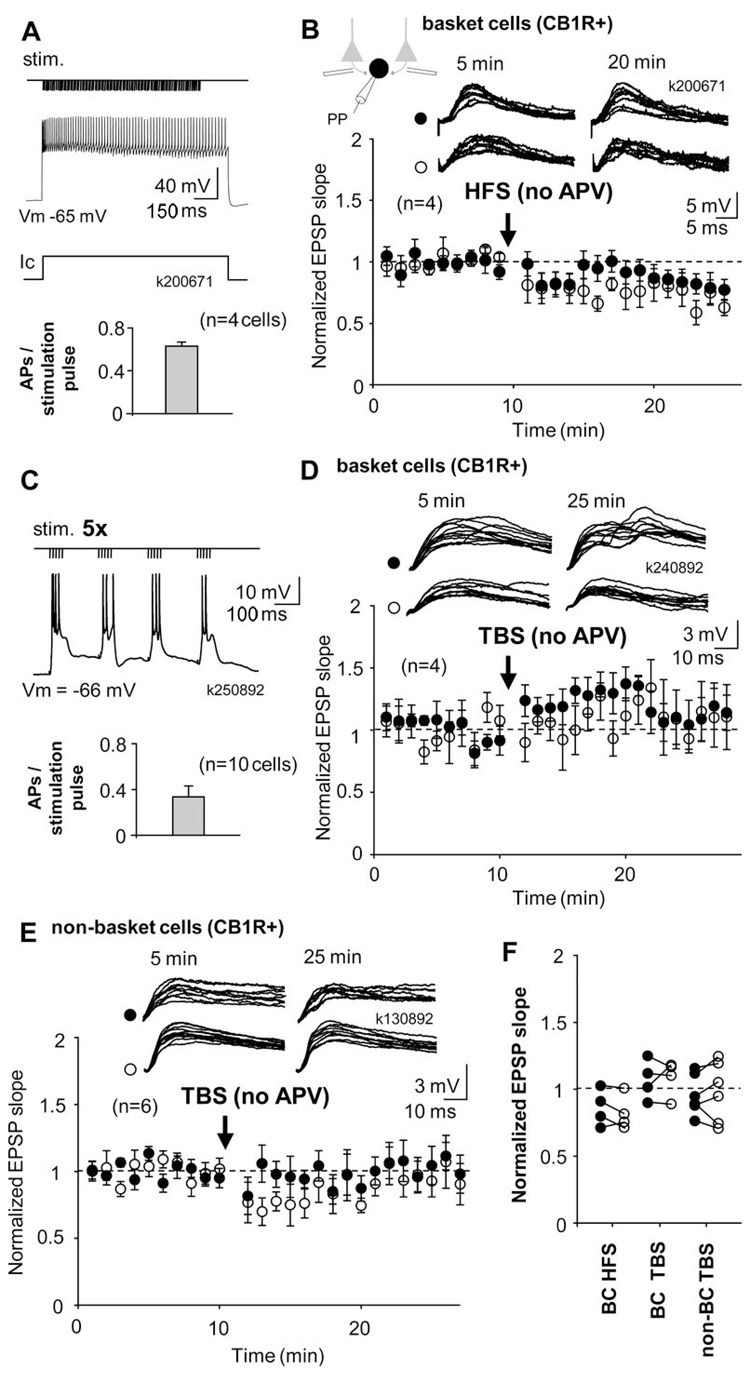
Figure 1.
Cell type-specific LTP in interneurons innervating the perisomatic domain of pyramidal cells. A-G) LTP is generated in PV+ basket cells and axo-axonic cells but not in basket cells expressing CB1R. Recordings were made in perforated patch; cells were re-patched in whole cell for post hoc identification using biocytin labelling. A) Pathway-specific LTP in a PV+ basket cell. Two glutamatergic afferent pathways were stimulated with electrodes in str.oriens/alveus. Following baseline period, high-frequency stimulation (HFS, 100Hz 1s × 2) was delivered to one pathway (solid symbols), whereas the other pathway served as control (open symbols). Schematic shows experimental design during perforated patch (PP) recording. Timing of HFS is indicated by an arrow. During HFS the postsynaptic cell was voltage-clamped to −70 mV. Potentiation of EPSP lasted at least 25 min and was restricted to the stimulated pathway. B) Consecutive EPSPs in the basket cell of (A) during baseline and 20 min following the HFS. Left, EPSP at resting membrane potential (resting Vm) triggered action potentials after LTP. To avoid action potentials, EPSPs were recorded during a hyperpolarizing step (−10 mV) throughout the experiment. Symbols indicate tetanized and control pathways as in (A). C) EPSP slope mean ± se in eight identified perisomatic-targeting PV+ interneurons. Data include three PV+ basket cells and five axo-axonic cells, symbols indicate tetanized and control pathway as in (A). Top, consecutive EPSPs in the two pathways during baseline and 20 min after the HFS. D) Digital visualization of a PV+ basket cell k081282 (left, dendrites red from two 70 μm thick sections, axon blue, from one section), and an axo-axonic cell k120193 (right, one section) recorded in whole cell (WhC). Images produced from confocal microscopic image stacks. Scale bar, 100 μm. Insets: immunofluorescence micrographs of the labelled cells demonstrating the expression of PV in the dendrites (indicated by arrow) and soma of the cells. Laser confocal microscope images; biocytin (green), PV (red), scale, 20 μm. (SR, str. radiatum; SP, str. pyramidale; SO, str. oriens). E) Distinct axonal patterns of a basket cell and an axo-axonic cell within the pyramidal cell layer. Left, epifluorescent micrographs of a PV+ basket cell (PV+ BC, k151082) showing undulating bouton laden axon collaterals, often running parallel with the pyramidal cell layer, amongst pyramidal cells (P), and an axo-axonic cell (AAC, k100871), showing their characteristic radial bouton bundles. The axo-axonic bouton rows follow axon initial segments (ais) of pyramidal cells (P) towards str. oriens. Scale, 20 μm. Middle, higher magnification epifluorescent micrographs of the same PV+ basket cell (PV+ BC, middle left) and axo-axonic cell (AAC, middle right). Scale, 20 μm. Right, electron micrograph showing a synapse (arrow) received by an ais from a bouton (b) of axo-axonic cell k100871. The bouton is identified by the electron opaque HRP endproduct and the ais by the membrane undercoating (double arrow). Scale, 0.25 μm. F) EPSPs in basket cells expressing CB1R do not show lasting plasticity. EPSP slope mean ± se in 6 basket cells tested for LTP and plotted as above. Top, EPSPs during baseline and 15 min after the HFS. G) Visualization of one CB1R+ basket cell by digital rendering of fluorescent images (two superimposed 70 μm thick sections). Scale, 100 μm. Lower panel: immunofluorescence micrographs of a biocytin-labelled (green) axon segment (CB1R, yellow, laser confocal image), scale, 10 μm. H) Comparison of baseline-normalized average EPSPs in all perisomatic-targeting cells 15-20 min after HFS relative to control pathways. Solid symbols indicate tetanized pathway, and open symbols show the control pathway. LTP is consistent in the PV+ interneuron types. Significance levels indicate a difference between pathways (**P<0.01, ***P<0.005, unpaired t-test).
LTP or LTD occurs in interneurons with distinct axonal target domains
In contrast to the perisomatic-targeting interneurons, dendrite-targeting PV+ or CB1R+ interneurons did not show LTP at all. Seven cells tested with HFS were identified as bistratified cells. This cell type is immunopositive for parvalbumin, somatostatin and NPY, and their axons innervate mainly pyramidal cell dendrites in str. radiatum and oriens (Halasy et al., 1996; Pawelzik et al., 2002; Klausberger et al., 2004). Remarkably, in 5 out of 7 bistratified cells, HFS induced LTD (reduction of the EPSP initial slope to at least 75% of baseline) whereas 2 cells showed no significant change in EPSP slope (Fig. 2A,B). LTD (63 ± 3%, P<0.005, n=5) in the bistratified cells was restricted to the stimulated pathway (control pathway 105 ± 3%). The systematic difference in plasticity between perisomatic- and dendrite-targeting PV+ cells reported here is unlikely to be explained by different postsynaptic membrane potential or action potential firing in these cells during afferent stimulation, because these measures were similar in the cell types (see Supplemental Fig. 1). Five interneurons, also studied with HFS, with axons mainly in the dendritic layers were immunopositive for CB1R and negative for PV (Ali, 2007) (Supplemental table 2). None of these CB1R+ non-basket cells showed a significant change in EPSP slope 15 min after the HFS, indicating an absence of LTP or LTD (Fig. 2C-E). Thus, LTD occurred specifically in dendrite-innervating PV+ bistratified cells under this induction protocol (Fig. 2F).
CB1R+ interneuron types with intact NMDAR-mediated transmission do not show LTP or LTD
Although CB1R+ basket cells did not demonstrate NMDAR-independent LTP, we asked whether they might show NMDAR-dependent LTP similar to pyramidal cells and some GABAergic interneurons in other hippocampal circuits (Kullmann and Lamsa, 2007). We performed further experiments in four additional post hoc identified CB1R+ basket cells in the absence of NMDAR blockers and depolarized the postsynaptic cell to 0 mV in current clamp during HFS. This was associated with intense firing of the postsynaptic cell (63.3 ± 3.7 action potentials evoked by a 100-pulse train, n=8 in 4 cells). This protocol, however, also failed to induce a lasting (≥15 min) change in the EPSPs (Fig. 3A,B).
Figure 3.
CB1R+ basket cells and non-basket cells with intact NMDAR-mediated transmission fail to show long-term plasticity. A) Schematic shows HFS protocol; tetanic stimulation (stim.) to one pathway is paired with depolarization of postsynaptic cell to 0 mV in current clamp (Ic). NMDARs are not blocked. Histogram shows that intense firing of postsynaptic action potentials was associated with presynaptic stimuli. B) Mean ± se of EPSP slope from four cells recorded in perforated patch and identified post hoc as CB1R+ basket cells. None of the cells showed significant lasting plasticity in the EPSP. Top, EPSPs in the two pathways during baseline and 10 min after HFS. C) Schematic shows theta-burst (TBS) stimulation protocol; trains of stimuli (100 Hz, 5 pulses) are delivered to one pathway at 5 Hz, while postsynaptic cell is at resting membrane potential (action potentials truncated). Histogram shows number of postsynaptic action potentials elicited by presynaptic stimuli. D) Perforated patch recording from four CB1R+ basket cells showed EPSPs without lasting plasticity. EPSP slope in thetabursted pathway was not different from baseline or from control pathway 15 min following the TBS. Top; consecutive EPSP traces from one experiment. E) Similar recordings show lack of long-term plasticity of EPSPs in CB1R+ non-basket cells (n=6). Cells were re-patched and identified as above. Top: EPSPs from an individual experiment. F) Baseline-normalized average EPSPs in the three types of experiments shown above. Solid symbols indicate HFS- or TBS -treated pathway, and open symbols show the control pathway. Data are taken 10-15 min following the HFS or TBS.
Next, we asked whether CB1R+ cells would show plasticity with a different stimulation protocol. In 10 further identified CB1R+ cells we used thetaburst stimulation (TBS) while the postsynaptic cell was in current clamp at resting membrane potential. Following baseline, high-frequency stimulation trains (100 Hz, 5 pulses) were delivered to one pathway at theta frequency (5 Hz, four cycles) while the second pathway served as control. The protocol was applied five times with 20 s interval (100 stimulation pulses altogether, Fig. 3C). The afferent stimulation induced depolarization above firing threshold in the cells (34.0 ± 8.8 postsynaptic action potentials by 100 stimulation pulses). Both pathways were followed at least 15 min following TBS. Four cells were identified as basket cells (Fig. 3D), and six cells as CB1R+ non-basket cells (Fig. 3E). Neither of these cell populations showed significant potentiation or depression (paired t-test) or difference between the pathways 15 min following the TBS (unpaired t-test). Averages of baseline-normalized EPSPs are shown in Fig, 3F.
Plasticity occurs in interneurons with CP-AMPARs
We next asked whether this cell type-specific plasticity we observed was correlate with the presence of CP-AMPA receptors in these cells (Lei and McBain, 2004; Isaac et al., 2007; Lamsa et al., 2007). We recorded EPSCs in whole cell mode from the re-patched cells (when access resistance allowed voltage clamp, see Methods). In addition, we recorded from a separate set of interneurons in whole cell only and subsequently identified their cell type (see Supplemental tables 1 and 2). Stimulation from str.oriens/alveus in all PV+ cells, including both control and tetanized pathways in re-patched cells, elicited strongly inward rectifying EPSCs (Fig. 4A), indicating a high percentage of CP-AMPARs in these synapses under baseline conditions as well as following plasticity (Jonas et al., 1994; Geiger et al., 1995; Lei and McBain, 2004; Lamsa et al., 2007). The rectification index (RI, see Methods) of EPSCs was consistently low in PV+ cell types (Catania et al., 1998). RI in axo-axonic cells was 0.09 ± 0.02 (n=13 stimulation pathways tested in 8 cells), basket cells 0.12 ± 0.03 (n=10 in 6 cells), and bistratified cells 0.11 ± 0.02 (n=22 in 12 cells). Although in most PV+ cells two stimulation pathways from str. oriens/alveus were tested, in some cells the control pathway was stimulated in str.radiatum. EPSCs evoked from str.radiatum showed similar strong inward rectification (RI=0.07 ± 0.05, n=6 pathways). In contrast, RI in CB1R+ basket cells (n=16 in 14 cells) and non-baskets (n=14 in 10 cells) was 0.72 ± 0.03 (n=30), reminiscent of that recorded in pyramidal cells (Supplemental figure 2). In only five out of the 30 pathways recorded in CB1R+ cells rectification was prominent (RI <0.5) (Fig. 4B). In some recordings, another stimulation was delivered from str.radiatum, which evoked EPSCs with RI=0.67 ± 0.07 (n=7 pathways).
Figure 4.
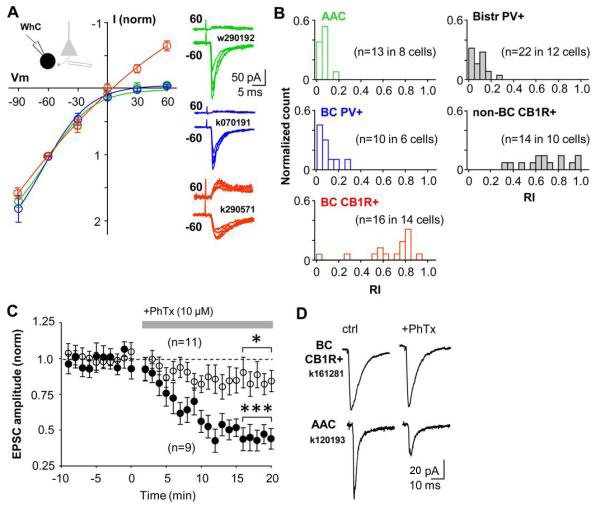
PV+ interneurons are excited via calcium permeable (CP-) AMPARs and CB1R-expressing interneurons via calcium impermeable (CI-) AMPARs in the CA1 area. A) I-V relations of AMPAR-mediated EPSCs are interneuron type-specific. PV+ basket cells and axo-axonic cells have highly inward rectifying EPSCs, which is a hallmark of CP-AMPARs. In contrast, EPSCs in CB1R+ basket cells show more linear current-voltage relation. EPSCs were evoked by stimulation in str.oriens/alveus. Mean ± se of normalized I-V relationship in the three perisomatic-targeting cell types illustrated in colours (green axo-axonic, blue PV+ basket cells and red CB1R+ basket cells). Right, EPSCs at +60 mV and −60 mV in three different cells (colour coding indicates cell type as above). B) Histograms showing EPSC rectification indices (RIs) in the five different interneuron types. RIs in PV+ interneuron types (axo-axonic, PV+ BC, PV+ bistr. cells) are on average 0.11, whereas in CB1R-expressing interneurons (BC-CB1R+ and non-BC CB1R+) RIs are on average 0.75. Colour coding of perisomatic-targeting interneuron types is as in (A). C) CP-AMPAR blocker philanthotoxin (PhTx) blocks excitatory input to PV+ interneuron types (solid symbols), but has a small effect on EPSCs in CB1R+ cells (n=11, P>0.05, open symbols). Data show mean ± se, a horizontal bar indicates timing of PhTx wash-in. PV+ cells include 3 basket, 3 axo-axonic and 3 bistratified cells. CB1R+ cells are basket cells. Significance level indicates difference to baseline (***P<0.005, paired t-test). D) Averaged EPSCs in one CB1R-expressing basket cell and in an axo-axonic cell during baseline and following wash-in of PhTx (20 min).
We next tested the effect of blocking CP-AMPARs on synaptic excitation of these interneuron types. EPSCs recorded in identified interneurons in whole-cell mode were evoked by stimulation in str.oriens/alveus and they were strongly suppressed following wash-in of CP-AMPAR blocker philanthotoxin-433 (PhTx, 10 μM) in all PV+ interneuron types (Fig. 4C,D). EPSC amplitude was reduced to 44 ± 6 % (P<0.005, paired t-test) by PhTx (n=9, includes 3 axo-axonic, 3 basket cells, 3 bistratified cells). In contrast, PhTx had a small effect on EPSCs in CB1R+ basket cells (in PhTx: 86 ± 9%, n=11 cells, P<0.05). As a reference, we confirmed that PhTx had no effect on synaptic excitation of identified CA1 pyramidal cells with linear EPSC IV-relation (Supplemental Fig. 2). Thus, CP-AMPAR blockade inhibits excitation of PV+ interneurons to a much stronger degree than of CB1R+ interneurons.
Disynaptic GABAergic transmission in CA1 pyramidal cells is strongly suppressed by CP-AMPAR blockers
Based on the strong effect of CP-AMPAR blockade on the excitation of PV+ interneurons, we tested the effect of PhTx on disynaptic inhibition in CA1 pyramidal cells. We recorded from the soma in whole cell mode and stimulated in str.oriens/alveus far (≥1 mm) from the recording site and close to the subiculum. GABAB and glutamate NMDA receptors were blocked with CGP55845 (1 μM) and DL-AP5 (100 μM), respectively. Cells were voltage clamped to the reversal of glutamatergic EPSCs (0 to +10 mV). We first measured maximum disynaptic IPSC (the disynaptic nature was verified at the end by applying NBQX 10 μM) for stimulation pathways. Next, stimuli with reducing intensities were applied to dissect the contribution of interneurons with CP-AMPARs at various stimulation strengths (see Methods). IPSCs were recorded in control conditions, and following wash-in of PhTx (10 μM). PhTx significantly reduced the amplitude of disynaptic IPSCs at each stimulation strength, but the inhibitory effect of PhTx was most pronounced in low amplitude IPSCs (Fig. 5A). When approximately 30% of the maximum IPSC was used, PhTx suppressed the disynaptic IPSC to 26 ± 3% of control (n=5 slices, P<0.005) (Fig. 5B), indicating a strong contribution of interneurons with CP-AMPARs to the IPSC (Jonas et al., 2004). In comparison, when the 100% IPSC was tested, PhTx reduced the disynaptic IPSC to 74 ± 4% (P<0.01). We confirmed that PhTx had no direct effect on GABAergic IPSCs (Supplemental Fig. 3).
Long-term potentiation of disynaptic GABAergic transmission involves interneurons with CP-AMPARs
Finally, we asked whether plasticity in the inputs to interneurons with CP-AMPARs could change disynaptic inhibition projected onto CA1 pyramidal cells. Given that LTP or LTD in interneurons with CP-AMPARs is induced without strong postsynaptic depolarization, and that repetitive afferent stimulation induces robust plasticity in synapses recruiting these cells, we hypothesised that the same afferent stimulation might be able to potentiate or depress disynaptic GABAergic transmission. We recorded IPSCs in whole cell mode from CA1 pyramidal cell soma clamped to the reversal of glutamatergic EPSCs as above, and stimulated at two different locations in str. oriens/alveus. One electrode was positioned as above close to subiculum, and another electrode close to the CA2 area. GABAB and glutamate NMDARs were blocked. Given that endocannabinoid receptor activation can mediate depression in certain GABAergic synapses onto pyramidal cells (Foldy et al., 2007; Heifets et al., 2008), and because this depression may also be induced by high-frequency electrical stimulation (Chevaleyre and Castillo, 2003; Lamsa et al., 2005), CB1Rs were blocked with AM-251 (5-10 μM). Stimulation intensity was set approximately to 30% of the maximum disynaptic IPSC amplitude in the pathways as above to maximize relative contribution of CP-AMPAR-containing interneurons in disynaptic IPSC (Supplemental Fig. 4). Following a baseline, the disynaptic pathway from the subicular site was stimulated with HFS whereas the untetanized pathway was used as control (Fig. 6A). Significant LTP (potentiation of averaged IPSC amplitude to at least 125% of baseline) was generated in 5 out of 8 slices, although potentiation varied in amplitude. Baseline-normalized average of IPSC amplitudes in all 8 slices showed potentiation in the tetanized pathway to 139 ± 8% (P<0.005, 25 min following the HFS), whereas the average of the control pathway did not change (89 ± 7%; Fig. 6B). Pathway-specificity of LTP was tested with unpaired t-test (P<0.005, Fig. 6C). Again, the disynaptic nature of IPSCs was verified by blockade of the IPSC with NBQX (10 μM) at the end of each experiment.
We repeated the same experiment in the presence of PhTx (10 μM) to block excitation of interneurons with CP-AMPARs. IPSC intensity was set up similarly approximately to 30% in the pathways (Supplemental Fig. 4) and HFS was delivered to the disynaptic pathway from the subicular site (Fig. 6D). Interestingly, HFS did not elicit lasting potentiation in any of the eight slices studied (Fig. 6E). Twenty min following the tetanus, IPSC amplitudes were 82 ± 5% in the tetanized pathway and 88 ± 7% in the untetanized control pathways (Fig. 6F). Although this moderate suppression of IPSC was significant (P<0.05) as compared to baseline, it was not pathway-specific. We confirmed that HFS does not change the amplitude of monosynaptic GABAergic IPSCs by reproducing the experiment above but in the continuous presence of NBQX (10 μM) and stimulating closer to the recording site in str. oriens (Fig. 6G). Taken together, the results demonstrate that LTP of disynaptic GABAergic transmission recorded in CA1 pyramidal cell somata involves interneurons with CP-AMPARs.
DISCUSSION
Here we have shown that excitatory connections to hippocampal interneurons express long-term plasticity in a cell type-dependent manner (Kullmann and Lamsa, 2007; Klausberger and Somogyi, 2008). Lasting plasticity in the GABAergic interneurons correlates with the expression of certain neurochemical markers and the axonal target domain of the interneurons. These results highlight the importance of carefully identifying interneurons in long-term plasticity studies. The two forms of plasticity are likely to modulate the synaptic strength from pyramidal cells to local GABAergic interneurons. Localization of LTP and LTD at separate and defined GABAergic microcircuits emphasizes the specified roles of distinct interneuron types in hippocampal function (Santhakumar and Soltesz, 2004; Freund and Katona, 2007; Klausberger and Somogyi, 2008).
Our results demonstrate that activity-induced plasticity takes place at excitatory synapses onto hippocampal PV+ interneurons, whereas afferents onto CB1R+ cells do not show plasticity. Although PV+ and CB1R+ interneurons provide parallel sources for perisomatic and dendritic innervation of pyramidal cells, PV+ neurons presumably play a major role in the synchronization of neuronal groups which process, transfer and store information during network oscillations (Buzsaki and Draguhn, 2004; Bartos et al., 2007; Fuchs et al., 2007; Mann and Paulsen, 2007; Tukker et al., 2007; Klausberger and Somogyi, 2008; Cardin et al., 2009; Sohal et al., 2009). CB1R+ interneurons may be less critical for synchronizing pyramidal cells, and they may modulate ensemble activities as a function of subcortical inputs (Freund and Katona, 2007). Activity-induced synaptic plasticity and modulatory fine-tuning plasticity appear to be located in separate and complementary GABAergic microcircuits provided by PV+ and CB1R+ cells, respectively (Freund and Katona, 2007). However, we can not fully exclude a possibility that synapses onto CB1R+ cells could undergo long-term plasticity under certain conditions. For example, permissive signal from an extrahippocampal locus might be needed to allow plasticity in these cells (Isaac et al., 2009; Varga V, 2009). In addition, afferent pathways emerging from a distinct population of glutamatergic hippocampal cells may behave differently. This, however, would not change the fact that in general the plasticity rules are very different in PV+ and CB1R+ cells.
PV+ cells show plasticity which is specific to the efferent target domain, such that perisomatic-targeting GABAergic circuits are potentiated and dendritic-targeting neurons are depressed. LTP increases the excitatory drive onto perisomatic-targeting inhibitory interneurons in a synapse-specific manner. The potentiated excitatory afferent synapse at the GABAergic cell has an increased contribution to its firing and consequently to the synchronization of its target cell assembly during network oscillation. In addition, depressed recruitment of dendritic-targeting bistratified cells would promote temporal integration of excitatory input to pyramidal cells from the CA3 area (Cossart et al., 2001). This in turn might increase the ability of CA1 cells to resonate with CA3 pyramidal cells, and could lower the threshold for LTP in CA3-CA1 synapses (Gustafsson et al., 1987), supporting rearrangement of temporal relationships within affected CA1 populations (Dragoi et al., 2003). However, because the inputs to different PV+ cell types likely arrive at distinct phases of oscillations in vivo (Klausberger and Somogyi, 2008), each interneuron type would possibly experience optimal plasticity-inducing conditions at different times (Kullmann and Lamsa, 2007).
Information processing requires the interactions within or between cell assemblies to be flexible, but the inputs to PV+ cells have long been considered poorly modifiable. Synchronised cell assembly formation during oscillations is dynamic and applies to particular representations and contexts. It is therefore expected that the synaptic strengths between pyramidal cells and interneurons also change in behaviorally relevant time scales (Csicsvari et al., 1998). The cell type-specific plasticity that we demonstrate here, and in particular, the contrasting effect of the same induction pattern leading to LTP or LTD in perisomatic- or dendrite-targeting PV+ cells, respectively, is a good candidate mechanism for maintaining the necessary flexibility in connection strengths during the formation or dissolution of cell assemblies.
What might be potential cellular mechanisms for LTP and LTD in PV+ cells? Plasticity was independent of NMDARs, and occurred at synapses with CP-AMPARs. Long-term plasticity in many interneurons depends on CP-AMPARs (Mahanty and Sah, 1998; Laezza et al., 1999; Kullmann and Lamsa, 2007; Lamsa et al., 2007; Oren et al., 2009). Both LTP and LTD in PV+ interneuron types were induced at membrane potentials below somatic firing threshold, consistent with previous reports on CP-AMPARs-mediated LTP and LTD (Laezza et al., 1999; Lamsa et al., 2007) and AMPAR subunit expression patterns in interneurons (Jonas et al., 1994; Geiger et al., 1995; Catania et al., 1998). In line with this, potentiation of disynaptic GABAergic transmission recorded in pyramidal cells required interneurons with CP-AMPARs, although this does not necessarily indicate an inductive role for these receptors in the LTP (Asrar et al., 2009). However, CP-AMPARs would provide temporally and spatially highly restricted calcium influx in aspiny postsynaptic interneuron dendrites (Goldberg and Yuste, 2005). Contributions of CP-AMPARs and other mechanisms including metabotropic glutamate receptors and voltage-gated calcium channels to LTP and LTD (Laezza et al., 1999; Alle et al., 2001; Perez et al., 2001; Lei and McBain, 2004; Lamsa et al., 2007; Galvan et al., 2008; Sarihi et al., 2008) remain to be dissected in PV+ interneurons. Properties may vary between distinct afferent pathways to the same cell (Toth and McBain, 1998). Most glutamatergic inputs to the three PV+ cell types reported here probably originate from CA1 and CA3 pyramidal cells, but the relative weight of these inputs is not known (Gulyas et al., 1999).
Synapses with CP-AMPARs provide the major pathway for long-term potentiation of disynaptic inhibition in the CA1 str. oriens/alveus, although potentiation level varied considerably between slices. This might be due to several factors. First, parallel depression of IPSCs in the dendritic domain of pyramidal cells might partially mask potentiation of IPSCs generated by perisomatic-targeting cells, although it is unclear whether LTD in bistratified cells results in reduced GABAergic transmission to pyramidal cell dendrites. However, because bistratified cells selectively target pyramidal cell dendrites, IPSCs from these interneuron types are much smaller at the level of the soma than IPSCs evoked by PV+ basket cells and axo-axonic cells (Maccaferri et al., 2000). In addition, number of bistratified cells is only a quarter of all PV+ cells in the CA1 stratum pyramidale (Baude et al., 2007). This might explain why in most experiments IPSC potentiation was seen and why net depression of IPSC was not systematically observed. Second, stimulation of excitatory fibres in some cases may not have recruited PV+ perisomatic-targeting cells or the excitatory input failed to induce plasticity in these cells. If plasticity in PV+ cells follows anti-Hebbian rule, strong depolarization during afferent stimulation would compromise or prevent LTP in some cells (Kullmann and Lamsa, 2007; Lamsa et al., 2007). Although LTP in oriens-lacunosum moleculare (O-LM) interneurons (Perez et al., 2001; Lamsa et al., 2007), which also express PV at low level (Klausberger et al., 2003), may contribute to the potentiation of disynaptic IPSC, their selective innervation of pyramidal cell distal dendritic domains elicits strongly attenuated IPSCs at the level of pyramidal cell soma (Maccaferri et al., 2000).
In conclusion, our findings reveal remarkable cell type-specific plasticity rules in the highly diverse hippocampal GABAergic interneuron population. We suggest that cell type-dependent plasticity in interneurons may not be restricted to the hippocampal CA1 area. Similar rules might occur in other brain areas with region- and afferent-specific variability. Because the interneuron types studied in this work are found in most cortical areas, their plasticity may also have widespread contributions to cortical networks outside the hippocampus (Buzsaki and Draguhn, 2004; Santhakumar and Soltesz, 2004; Yazaki-Sugiyama et al, 2009).
Supplementary Material
Acknowledgements
Supported by the Wellcome Trust, the UK Medical Research Council, and the DAAD German Academic Exchange Service. We are grateful to Tamas Bellak, Wendy Tynan, Kristina Detzner, and David Roberts for expert technical assistance. We thank Drs Colin Akerman, Marlene Bartos, Marco Capogna, Jozsef Csicsvari, Dimitri Kullmann, Peter Magill and Trevor Sharp for comments on the manuscript. We thank the following scientists for their generous gifts of antibodies: K. G. Baimbridge (antibody to parvalbumin), A. Buchan (to somatostatin), T. Gorcs (to VIP), A. Varro (to pro-CCK), M. Watanabe (to CB1 receptor, VGluT3) and S. El Mestikawy (to VGluT3). Antibodies 9303 and 55 raised against CCK and VIP, respectively, were provided by CURE/Digestive Diseases Research Center, Antibody/RIA Core (National Institutes of Health Grant DK41301).
REFERENCES
- Ali AB. Presynaptic Inhibition of GABAA receptor-mediated unitary IPSPs by cannabinoid receptors at synapses between CCK-positive interneurons in rat hippocampus. J Neurophysiol. 2007;98:861–869. doi: 10.1152/jn.00156.2007. [DOI] [PubMed] [Google Scholar]
- Alle H, Jonas P, Geiger JR. PTP and LTP at a hippocampal mossy fiber-interneuron synapse. Proc Natl Acad Sci U S A. 2001;98:14708–14713. doi: 10.1073/pnas.251610898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrar S, Zhou Z, Ren W, Jia Z. Ca permeable AMPA receptor induced long-term potentiation requires PI3/MAP kinases but not Ca/CaM-dependent kinase II. PLoS ONE. 2009;4:e4339. doi: 10.1371/journal.pone.0004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Baude A, Bleasdale C, Dalezios Y, Somogyi P, Klausberger T. Immunoreactivity for the GABAA receptor alpha1 subunit, somatostatin and Connexin36 distinguishes axoaxonic, basket, and bistratified interneurons of the rat hippocampus. Cereb Cortex. 2007;17:2094–2107. doi: 10.1093/cercor/bhl117. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Eidelberg E. Direct afferent excitation and long-term potentiation of hippocampal interneurons. J Neurophysiol. 1982;48:597–607. doi: 10.1152/jn.1982.48.3.597. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho TP, Buonomano DV. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input-output functions. Neuron. 2009;61:774–785. doi: 10.1016/j.neuron.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania MV, Bellomo M, Giuffrida R, Giuffrida R, Stella AM, Albanese V. AMPA receptor subunits are differentially expressed in parvalbumin- and calretinin-positive neurons of the rat hippocampus. Eur J Neurosci. 1998;10:3479–3490. doi: 10.1046/j.1460-9568.1998.00356.x. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Cowan AI, Stricker C, Reece LJ, Redman SJ. Long-term plasticity at excitatory synapses on aspinous interneurons in area CA1 lacks synaptic specificity. J Neurophysiol. 1998;79:13–20. doi: 10.1152/jn.1998.79.1.13. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Buzsaki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Harris KD, Buzsaki G. Place representation within hippocampal networks is modified by long-term potentiation. Neuron. 2003;39:843–853. doi: 10.1016/s0896-6273(03)00465-3. [DOI] [PubMed] [Google Scholar]
- Foldy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10:1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Galvan EJ, Calixto E, Barrionuevo G. Bidirectional Hebbian plasticity at hippocampal mossy fiber synapses on CA3 interneurons. J Neurosci. 2008;28:14042–14055. doi: 10.1523/JNEUROSCI.4848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R. Space matters: local and global dendritic Ca2+ compartmentalization in cortical interneurons. Trends Neurosci. 2005;28:158–167. doi: 10.1016/j.tins.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Wigstrom H, Abraham WC, Huang YY. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987;7:774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasy K, Buhl EH, Lorinczi Z, Tamas G, Somogyi P. Synaptic target selectivity and input of GABAergic basket and bistratified interneurons in the CA1 area of the rat hippocampus. Hippocampus. 1996;6:306–329. doi: 10.1002/(SICI)1098-1063(1996)6:3<306::AID-HIPO8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci U S A. 2008;105:10250–10255. doi: 10.1073/pnas.0711880105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Buchanan KA, Muller RU, Mellor JR. Hippocampal place cell firing patterns can induce long-term synaptic plasticity in vitro. J Neurosci. 2009;29:6840–6850. doi: 10.1523/JNEUROSCI.0731-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron Diversity series: Fast in, fast out--temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994;12:1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Kairiss EW, Abraham WC, Bilkey DK, Goddard GV. Field potential evidence for long-term potentiation of feed-forward inhibition in the rat dentate gyrus. Brain Res. 1987;401:87–94. doi: 10.1016/0006-8993(87)91167-x. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O’Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- Laezza F, Dingledine R. Voltage-controlled plasticity at GluR2-deficient synapses onto hippocampal interneurons. J Neurophysiol. 2004;92:3575–3581. doi: 10.1152/jn.00425.2004. [DOI] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science. 1999;285:1411–1414. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci. 2005;8:916–924. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe V, Morin F, Ratte S, Croce A, Conquet F, Lacaille JC. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J Physiol. 2004;555:125–135. doi: 10.1113/jphysiol.2003.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Two Loci of expression for long-term depression at hippocampal mossy fiber-interneuron synapses. J Neurosci. 2004;24:2112–2121. doi: 10.1523/JNEUROSCI.4645-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Zhang L, Shigemoto R, Somogyi P, Nusser Z. Cell type dependence and variability in the short-term plasticity of EPSCs in identified mouse hippocampal interneurones. J Physiol. 2002;542:193–210. doi: 10.1113/jphysiol.2002.020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci. 1996;16:5334–5343. doi: 10.1523/JNEUROSCI.16-17-05334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Toth K, McBain CJ. Target-specific expression of presynaptic mossy fiber plasticity. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol. 2000;524(Pt 1):91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- McBain CJ. Differential mechanisms of transmission and plasticity at mossy fiber synapses. Prog Brain Res. 2008;169:225–240. doi: 10.1016/S0079-6123(07)00013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron. 1997;18:295–305. doi: 10.1016/s0896-6273(00)80269-x. [DOI] [PubMed] [Google Scholar]
- Oren I, Nissen W, Kullmann DM, Somogyi P, Lamsa KP. Role of ionotropic glutamate receptors in long-term potentiation in rat hippocampal CA1 oriens-lacunosum moleculare interneurons. J Neurosci. 2009;29:939–950. doi: 10.1523/JNEUROSCI.3251-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelzik H, Hughes DI, Thomson AM. Physiological and morphological diversity of immunocytochemically defined parvalbumin- and cholecystokinin-positive interneurones in CA1 of the adult rat hippocampus. J Comp Neurol. 2002;443:346–367. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Lacaille JC. Long-term synaptic plasticity in hippocampal feedback inhibitory networks. Prog Brain Res. 2008;169:241–250. doi: 10.1016/S0079-6123(07)00014-3. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille JC. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci U S A. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ST, Soltesz I. Long-term plasticity in interneurons of the dentate gyrus. Proc Natl Acad Sci U S A. 2001;98:8874–8879. doi: 10.1073/pnas.141042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Soltesz I. Plasticity of interneuronal species diversity and parameter variance in neurological diseases. Trends Neurosci. 2004;27:504–510. doi: 10.1016/j.tins.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Sarihi A, Jiang B, Komaki A, Sohya K, Yanagawa Y, Tsumoto T. Metabotropic glutamate receptor type 5-dependent long-term potentiation of excitatory synapses on fast-spiking GABAergic neurons in mouse visual cortex. J Neurosci. 2008;28:1224–1235. doi: 10.1523/JNEUROSCI.4928-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Freund TF, Hodgson AJ, Somogyi J, Beroukas D, Chubb IW. Identified axo-axonic cells are immunoreactive for GABA in the hippocampus and visual cortex of the cat. Brain Res. 1985;332:143–149. doi: 10.1016/0006-8993(85)90397-x. [DOI] [PubMed] [Google Scholar]
- Toth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci. 1998;1:572–578. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27:8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V LA, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Kang S, Câteau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spikingGABAcircuits by visual experience. Nature. 2009;462:218–221. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.