Abstract
The granule cell layer of the cerebellum comprises the largest population of neurons in the vertebrate CNS. In amniotes, its precursors undergo a unique phase of transit amplification, regulated by Sonic hedgehog. They do so within a prominent but transient, secondary proliferative epithelium, the external germinal layer, which is formed by tangential migration of precursor cells from the rhombic lip. This behaviour is a hallmark of bird and mammal cerebellum development. Despite its significance for both development and disease, it is unclear whether an external germinal layer is a requirement for granule cell production or an expedient of transit amplification. Evidence for its existence in more basal vertebrates is contradictory. We therefore examined cerebellum development in the zebrafish, specifically in relation to the expression of the bHLH gene Atonal 1, which definitively characterises granule cell precursors. The expression of Atoh1a, b and c, in combination with patterns of proliferation and fate-maps, define precursor pools at the rhombic lip and cerebellar midline, but demonstrate that an external germinal layer is absent. Sonic hedgehog signalling is correspondingly absent in the zebrafish cerebellum. Sustained roofplate derived signals suggest that, in the absence of transit amplification, primary granule cell precursor pools are maintained throughout development. To determine whether this pattern is specific to zebrafish, or reflects a more general anamniote organisation we examined the expression of similar genes in the dogfish, Scylliorhinus canicula. We show that these anamniotes share a common groundplan of granule cell production that does not include an external germinal layer.
Keywords: granule cell, migration, Math1/Atonal1/Atoh1, Purkinje cell, cell proliferation, fate-map
INTRODUCTION
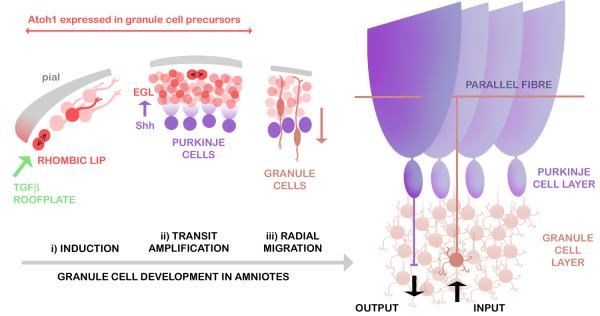
The cerebellum is a key component of the central nervous system of all jawed vertebrates (Nieuwenhuys et al., 1998). It has a well characterised role in the comparison of sensory and motor signals and as the site of proprioceptive integration (Sherrington, 1947). The cerebellum is characterised by a very simple, conserved neuronal circuit involving a few defined cell types (Fig.1). Of these, granule cells comprise not only the major class by number but also the majority of all cells in the CNS. Granule cell development in mammals and birds includes a unique phase of proliferation that bears the hallmarks of transit amplification: committed precursors (Wechsler-Reya and Scott, 1999; Klein et al., 2005) multiply by symmetrical divisions (Espinosa and Luo, 2008) within a prominent, but transient, sub-pial external germinal layer (EGL) (His, 1890; Schaper, 1897; Cajal, 1911) (Fig.1ii). The EGL is formed by migratory precursors derived from the upper or cerebellar rhombic lip (Ben-Arie et al., 1997; Wingate and Hatten, 1999): the neuroepithelial boundary of the roofplate of the fourth ventricle (Wingate, 2001).
FIGURE 1. Development of granule cells in relation to Purkinje cells in the amniote cerebellum.
Granule cell precursors (red) are initially induced at the rhombic lip by TGFβ signals (green) from the adjacent roofplate (i). Precursors migrate tangentially over the sub-pial cerebellar surface and divide again within the transient EGL (ii). Proliferation is regulated by Shh secreted from underlying Purkinje cells (purple). After their last cell division, postmitotic granule cells (brown) radially migrate into a layer below Purkinje cells (iii). In the mature circuit, glutamatergic granule cells receive inputs from precerebellar neurons and project T-shaped axons (parallel fibres) into an almost cell body-free (molecular) layer, where they synapse on the dendrites of GABAergic Purkinje cells. Purkinje cell outputs directly and indirectly regulate the activity of the vestibular system, thalamus and sub-cortical motor centres.
The EGL has become an important model of neuronal development, and is significant as the likely origin of the majority of childhood brain cancers (Kool et al., 2008). EGL proliferation is regulated by Sonic hedgehog (Shh) secreted by the underlying Purkinje cells (Dahmane and Ruiz-i-Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999)(Fig.1ii). Shh regulates both size and foliation of the cerebellum (Corrales et al., 2006) and mutation of its receptor, patched (Ptc), in granule cells results in medulloblastoma (Goodrich et al., 1997). Throughout proliferation, granule cell precursor identity is dependent on expression of the bHLH transcription factor Atonal1 (Atoh1(Ben-Arie et al., 1996; Ben-Arie et al., 1997), which is initially induced by TGFβ signalling at the rhombic lip (Alder et al., 1996; Alder et al., 1999) (Fig.1i) and only downregulated as postmitotic granule cells exit the EGL (Fig.1iii).
Vertebrate cerebellar structure differs in both the magnitude and arrangement of a stereotyped complement of cerebellar neurons (Meek, 1992a; Meek, 1992b) and glia (Bae et al., 2009). Notably, sharks and sturgeons have a nuclear granule cell organisation while an internal granule cell layer is seen in teleosts, amphibians and all amniotes (reptiles, birds and mammals) (Nieuwenhuys, 1967; Nieuwenhuys et al., 1998), suggesting these latter groups share a common pattern of development that includes an EGL (Fig.1). However, studies in the amphibian suggest that, while there is an external granule layer, it is non-proliferative (Gona, 1976). Moreover, evidence for a secondary proliferation layer in the cerebellum of fish is contradictory (Pouwels, 1978; Adolf et al., 2004; Candal et al., 2005; Mueller and Wulliman, 2005; Zupanc et al., 2005; Grandel et al., 2006; Rodriguez-Moldes et al., 2008; Volkmann et al., 2008; Kaslin et al., 2009).
What then is the relationship between cerebellar organisation and the presence of an EGL? We examined this question by assessing zebrafish and the shark for the presence of granule cell transit amplification, defined by 1) secondary proliferation 2) maintained Atoh1 expression 3) both at a distance to a source of TGFβ signalling and 4) responsive to Shh. The demonstrable absence by these criteria of an external germinal layer in either species implies that transit amplification in the cerebellum is an amniote evolutionary adaptation.
METHODS
Cloning of Pax6 and Atonal1 homologues
We constructed 3′ and 5′ RACE libraries (Clontech) from a st.28 embryonic catshark/spotted dogfish, Scylliorhinus canicula. Pax6 was cloned by PCR from its GenBank sequence. Atoh1 was cloned using degenerate, nested forward primers to the region encoding the conserved bHLH motif: atoF1: YTIAAYCAYGCITTYGAY and atoF2: AARTAYGARACIYTICARATG. Further 5′ sequence was retrieved using the reverse primer TGATTCGTAGGGCAATCTTTCACAT. Danio rerio Atoh1c (ENSDARG00000039602) was identified by screening the Ensembl database and cloned using specific forward (ATGCCCCATCCGGACACCCCTTTTGG) and reverse (CTATTTTACACCATTGTTCCTTTCCA) primers.
Histology
Juvenile zebrafish (1-8 days post hatching) and shark embryos (stages 28-33 (Farner, 1978)) were collected and fixed in paraformaldehyde (4% in PBS) and processed for in situ hybridisation using standard protocols, with riboprobes for: Atoh1a, Atoh1b Gdf7, Gfd6a, Atoh1c, Zic1, Shha, Shhb, Ptc1, N-myc (zebrafish) and Atoh1, Pax6, Shh, Ptc2 (shark). Embryos were transversely sectioned after embedding in methacrylate resin (zebrafish), paraffin (shark) or gelatin (shark). Wholemounts and paraffin sections were processed for immunohistochemistry with primary antibodies to phosphohistone H3 (1/100, Cell Signalling) or Zebrin II (1:100, a gift of Richard Hawkes, University of Calgary) and appropriate peroxidase-conjugated secondary antibodies, then counterstained with methyl-green (resin), cresyl violet or haemotoxylin/eosin (paraffin).
Acute fate-mapping in zebrafish with Kaede
Capped Kaede RNA was injected into zebrafish at the 1-cell stage (100ng). After 3-5 days fish were screened for (green) Kaede expression and spatially identified cells within the cerebellum of anaesthetised fish (0.01% Tricaine) were selectively illuminated by blue laser line (351nm/364nm). The distribution of photoconverted (red) cells was monitored by confocal microscopy for 16-48 hr (Nikon Eclipse 80i). Maps of photoconverted cells were compiled by tracing the area of red label directly from confocal micrographs (in Adobe Photoshop). The opacity of each trace was divided by the number of fate-maps and placed into separate layers on a standardised template.
RESULTS
In amniotes, granule cells are derived from proliferative Atoh1-positive (Ben-Arie et al., 1997) precursor cells at the rhombic lip and subsequently in the EGL. We cloned and contrasted previously undescribed homologues of Atoh1 in zebrafish and shark to provide a comprehensive picture of granule cell origins in these representative teleost and chondrychthyan species.
Atoh1 and proliferation zones in the developing zebrafish cerebellum
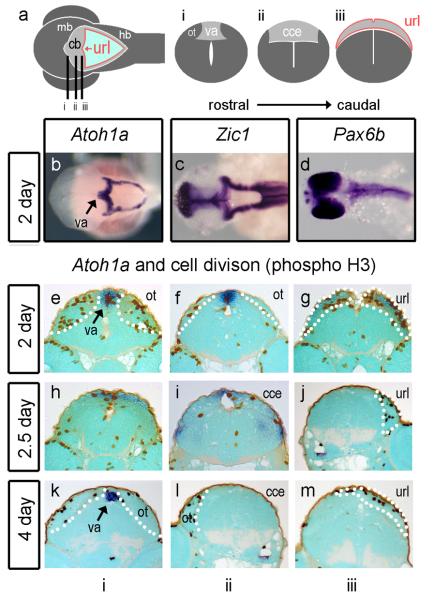
While its territory is allocated by patterning mechanisms similar to those in other vertebrates (Reifers et al., 1998), the teleost cerebellum has a distinct structure that comprises an upper rhombic lip, a main body or “corpus”, and a rostral apex known as the valvulus (Fig.2a) (Nieuwenhuys et al., 1998; Mueller and Wulliman, 2005; Bae et al., 2009). We examined the expression of Atoh1a and b in relation to these structures, using the expression of Phosphohistone H3 as a marker of cells undergoing proliferation. At 2 days both Atoh1a and b are expressed within both the upper rhombic lip adjacent to the cerebellum and lower rhombic lip of the hindbrain (Fig.2b: since expression of Atoh1a and b is identical within the cerebellum at all stages examined, only Atoh1a is shown in this and subsequent figures). Together, the lower and upper rhombic lip(s) form a continuous, diamond-shaped boundary at the edge of the roofplate of the fourth ventricle. The rhombic lip also expresses Zic1 (Fig.2c) as in chick and mouse but not Pax6a (not shown) or b (Fig.2d). Expression of Atoh1a/b also extends rostrally along the midline of the future cerebellar plate (corpus) into the presumptive valvulus, which comprises the apical, rostral pole of the dorsal cerebellum (Fig.2e). Proliferating cells are concentrated both here and within the Atoh1a-positive cerebellar midline (Fig.2f) and upper rhombic lip (Fig.2g). At 2.5 days, Atoh1a/b expression begins to be downregulated in all but the presumptive valvulus (Adolf et al., 2004) (Fig.2h-j). At 5 days, proliferation is largely absent from the now distinct main corpus of the cerebellum (Fig.2l), but maintained in the valvulus (Fig.2k) and the upper rhombic lip (which no longer expresses Atoh1a/b: Fig.2m).
FIGURE 2. Atoh1a and proliferation in the zebrafish.
Schematic dorsal view (a) of a 5-day embryonic zebrafish shows cerebellar and precerebellar territory in grey, the rhombic lip at the edge of the fourth ventricle in red and roofplate in light blue. The cerebellar or upper rhombic lip (url) is the portion that borders the cerebellum (cb) which lies between the dorsal lobes of the optic tectum (ot) of the midbrain (mb) and hindbrain (hb). Lines indicate the level of sections through i) the presumptive valvulus (va), ii) the corpus of the cerebellum (cce) and iii) url. At 2days (48 hr), before the corpus is defined, Atoh1a (blue) is expressed throughout the rhombic lip of both the cb and hb (b) in the same domain as Zic1. Pax6a (not shown) and b (c) are not expressed in the rhombic lip. In transverse section, proliferative (phosphohistone H3; brown), Atoh1-positive cells are seen at the va (e), flanked by dividing cells in the dorsal midbrain comprising the optic tectum (ot), at the cerebellar midline (f) and at the Atoh1a-positive url (g). At 2.5 days, proliferating cells are seen in the Atoh1a-positive presumptive valvulus (h) and in regions where Atoh1 is downregulated: the midline of the main corpus cerebellar (i) and the url (j). A similar pattern is observed at 4 days with infrequent division at the cb midline (k-m). Dotted white lines indicate the boundaries of cerebellar territory.
The Atoh1-positive precursor pool is not co-extensive with the granule cell layer
In amniotes, rhombic lip derived granule cell precursors migrate away from the roofplate and assemble in a transient Atoh1-positive EGL. Cells undergo a series of symmetric divisions (Espinosa and Luo, 2008) before migrating radially into the cerebellum. The spatial dimensions of the Atoh1-postive EGL thus define the extent of the future granule cell layer. We therefore examined the distribution of Atoh1a/b in relation to the distribution of granule cells to assess whether these orthologues define a candidate EGL.
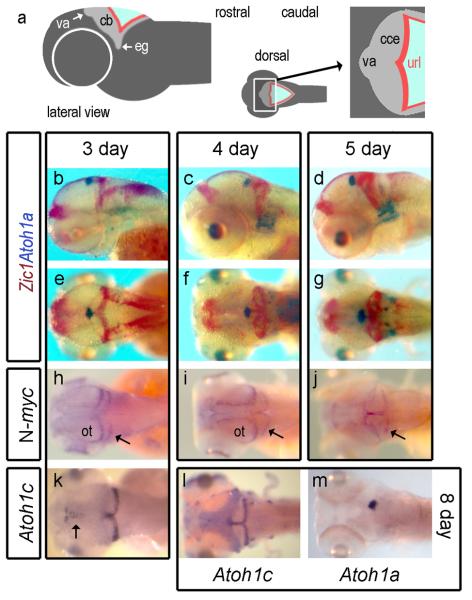
We compared Atoh1a/b expression with that of Zic1, a marker of both rhombic lip and its granule cell derivatives (Aruga et al., 1998) with respect to presumptive territories of the zebrafish cerebellum (Fig.3a) At 3 days, the expression domains of Atoh1a/b at the apical midline (presumptive valvulus) becomes spatially distinct to that of Zic1 (Fig.3b) and excluded from the corpus of the cerebellum as the granule cell layer, which also expresses Pax6a (not shown), emerges, At 4 days (Fig.3c) and 5 days (Fig.3d) Atoh1a/b expression remains restricted to the presumptive valvulus. In dorsal view at 3 days, Zic1 is expressed throughout the rhombic lip and abuts the domain of Atoh1a/b at the cerebellar midline (Fig.3e). The formation of a layer of Zic1-positive granule cells at 4 days (Fig.3f) to 5 days (Fig.3g) within the corpus of the cerebellum correlates with a down regulation of Zic1 within the hindbrain rhombic lip. At no point do the expression domains of Atoh1a/b (presumptive granule cell precursors) and Zic1 (definitive granule cells) overlap in a manner that suggest an external germinal layer..
FIGURE 3. Atoh1a and c in relation to granule cell formation in zebrafish.
Schematic lateral view of a zebrafish embryo (a) and expanded dorsal representation of the cerebellum showing the position of the va relative to the corpus of the cerebellum (cce) and url, whose lateral extensions comprise the granule cell eminences (eg). In b-g, Atoh1a (blue) is contrasted with Zic1 (red) in lateral view at 3 days (b), 4 days (c) and 5 days (d) and dorsal view at 3 days (e), 4 days (f) and 5 days (g). N-myc is expressed within caudal margin of the optic tectum (ot) and url at 3 days (h), expanding into the midline of both at 4 days (i) to include the va at 5 days (j). Atoh1c-positive cells (k) are found in the upper rhombic lip from 3 days onwards. At 8 days (l), Atoh1c expression is also seen in anterior lateral line neuromasts and occupies a complementary domain to valvular Atoh1a expression (m) in the cerebellar midline.
We also examined the expression of N-myc which characterizes proliferating cells within the mammalian EGL (Knoepfler et al., 2002) downstream of Shh signalling (Kenney et al., 2003; Hatton et al., 2006). As previously reported in the 3 day zebrafish (Loeb-Hennard et al., 2005), high levels of N-myc characterise the posterior margin of the tectum and upper rhombic lip (arrow, Fig.3h). As the cerebellar territory expands, N-myc expression is co-opted into the cerebellar midline (Fig.3i). By 5 days, N-myc is expressed in the valvulus, midline and cerebellar rhombic lip (Fig.3j). At no point during granule cell formation is N-myc expressed in a superficial layer within the cerebellum.
These patterns of proliferation and expression raise doubts as to the status of the reported external germinal layer in zebrafish (Mueller and Wulliman, 2005). Atoh1a/b marks only a subset of proliferating cells (in the presumptive valvulus). Proliferating cells at the upper rhombic lip which have been shown to generate granule cells (Volkmann et al., 2008) are, by contrast, Atoh1-negative. Neither the distribution of Atoh1 nor that of dividing cells is consistent with a superficial EGL that might give rise to definitive granule cells by radial migration.
One explanation is that there are further versions of Atoh1 within the zebrafish genome, one of which would identify an EGL that overlies the Zic1 domain. Accordingly, we searched available databases and identified and cloned an additional orthologue, Atoh1c. Its expression in the zebrafish cerebellum is initiated in the upper rhombic lip and cerebellar midline at 3 days (Fig.3k), similar to that of Pax6b (data not shown). Atoh1c persists in this domain to the latest stage examined (8 days: Fig.3l), as does the Atoh1a/b expression at the midline (Fig.3m), but does not spread over the surface of the cerebellum. The absence of a superficial layer of both cell proliferation and Atoh1expression in the developing cerebellum argues against the presence of an EGL.
The cerebellar midline gives rise to tangentially migrating cells
The domain of Atoh1c encompasses the upper rhombic lip, which gives rise to tangentially migrating cells (Koster and Fraser, 2006; Volkmann et al., 2008). However, the combined domains of Atoh1a/b and Atoh1c extend beyond the upper rhombic lip into the cerebellar midline and presumptive valvulus. Do these regions also contribute tangentially migrating derivatives to the cerebellum?
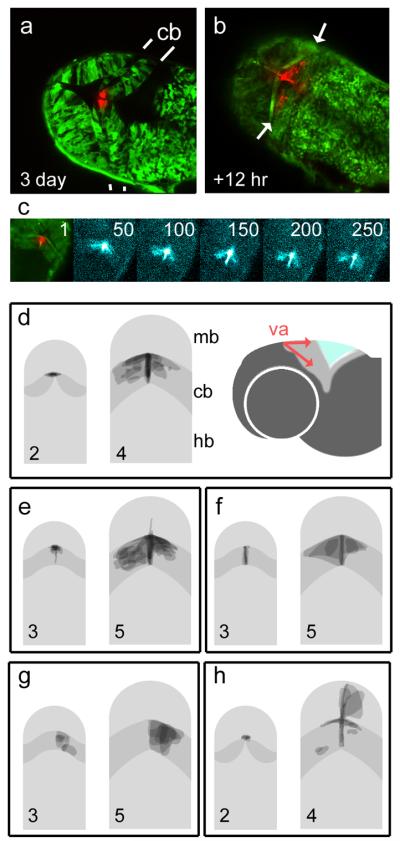
To investigate this possibility, we used the photoconvertible green protein, Kaede (Ando et al., 2002) to fate-map small clusters of cells by a spatially localised illumination with a blue laser. Red-labelled derivatives of this photoconverted cluster were examined 12 (Fig.4b) to 48 hr later. Photoconversion of the apical cerebellar midline (presumptive valvulus) always resulted in a bilateral label within the corpus of the cerebellum and a narrow trail of cells at the cerebellar midline (Fig.4a: 3 days). Time-lapse imaging of cell dispersal suggests an active, bilateral, tangential migration (Fig.4c and online supplementary movie file) with derivatives demarcating the range of migration within the first hour of filming. This bilateral distribution of cells can be seen in the superimposed maps of cell dispersal from the presumptive valvulus at 2 days (Fig.4d: n = 9) and 3 days (Fig.4e: n = 10). The cell movements comprising these lateral (dorsoventral) and midline streams from the valvulus are shown schematically in Figure 4d (right). Cerebellar midline caudal to the presumptive valvulus also gives rise to bilateral streams of cells (Fig.4f: n = 4). By contrast, clones of labeled cells within the corpus of the cerebellum are always restricted to one or other hemisphere (Fig.4g: n = 6), indicating that the cerebellar midline is a barrier to interhemispheric cell movement.
FIGURE 4. Fate-maps of midbrain and cerebellar precursor pools in zebrafish.
Kaede RNA was injected into embryos at the one-cell stage to produce green fluorescent fish. Optical sectioning with a laser scanning confocal microscope reveals the cerebellum in dorsal view with clearly demarcated rostral and caudal boundaries (indicated by white lines). Photoconversion of cells at the presumptive valvulus (red) of the cb at 72hr (a) gives rise to symmetrically dispersed label 12hr later (arrows in b) within the unconverted, green labeled cb. Sequence (c) of confocal projections (11 optical sections) showing the spread of photoconverted cells over time. Frame numbers represent 5 minute intervals. Frames 50-250 show photoconverted cells enhanced with a “thermal” colour look-up table. Although the spatial domain occupied by the label spans the width of the cerebellum, the accompanying time-lapse sequence of 270 frames (supplementary movie on-line) shows the continuous migration of midline derivatives replenishing the cerebellum. Figs d-h show superimposed fate-maps from a set of experiments, where cells were photoconverted at either 2 or 3 days and imaged after 48 hours. Pairs of images show that fate-maps (right side) grouped according to the location of labeled precursors (shown on left). The cerebellum is shown in dark grey. The valvulus of the cerebellum was labeled at 2 days (d) and 3 days producing comparable, bilateral streams of cells as seen in panels a,b, and c. To the right, a schematic lateral view shows direction of cell distribution from the va (red). Bilateral derivatives were seen after photoconversion of the cerebellar midline (f) but not the corpus of the cerebellum (g). Photoconversion of the caudal midline of the midbrain adjacent to the valvulus at 2 days (h), labeled derivatives in both the cerebellar and midbrain midline, as well as cells within the optic tectum. Rostral is to the top in all figures.
Together, timelapse movies and the symmetry and range of cell movement suggest that cell dispersal from the cerebellar midline is by active, tangential migration, as seen in the upper rhombic lip (Volkmann et al., 2008). This contrasts markedly with that of cells from the dorsal midline of the midbrain at 2 days, which always generates asymmetrically distributed clones, more suggestive of a passive clonal spread (Fig.4h: n = 5). In a manner reminiscent of chick isthmic development (Alexandre and Wassef, 2003), caudal midbrain precursors (at 2 days) also give rise to cells in the cerebellar midline. These cells do not give rise to tangential migrants, suggesting that the widespread derivatives seen in Figure 4f are generated only by midline cells derived from the cerebellum.
Shh and Gdf expression in the developing zebrafish cerebellum
Proliferative cells at the midline and presumptive valvulus express Atoh1 and give rise to migratory derivatives. This suggests that these cells might constitute an EGL. While at the rhombic lip the induction of granule cell precursors is regulated by TGFβ signalling from the roofplate, their transit amplification of granule cells in the EGL is maintained by Shh expression in underlying Purkinje cells (Dahmane and Ruiz-i-Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999). Shh signalling is an integral part of the signalling network that maintains the EGL and regulates cerebellar size in amniotes (Corrales et al., 2006). We therefore examined the expression of Atoh1 in relation to diffusible roofplate derived TGFβ signals, Gdf6a and Gdf7 (Davidson et al., 1999; Elsen et al., 2008), and both Shh homologues and their receptor, Ptc1, whose expression correlates with Shh signal transduction (Pearse et al., 2001).
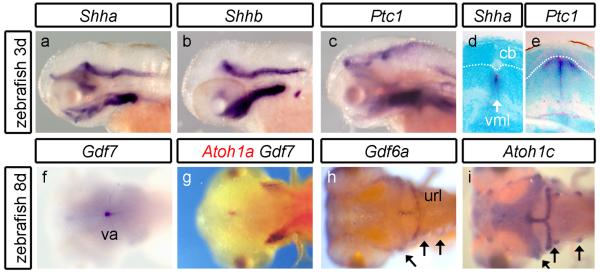
Neurally expressed Shh homologues, Shha (Fig.5a) and Shhb (Fig.5b), are excluded from the cerebellum at all stages examined (to 5 days). Ptc1 is also absent from the cerebellum (Fig.5c) suggesting that cerebellar neurons are not transducing Shh signals as the granule cell layer expands. Coronal sections confirm that Shha (Shhb not shown) and Ptc1 are expressed, respectively, within the hindbrain midline (Fig.5d) and within the hindbrain midline and in adjacent neuroepithelium (Fig.5e), but not the cerebellum. By contrast, Gdf7 and Gdf6a, which are expressed in the rhombic lip at earlier stages (not shown: (Elsen et al., 2008)), show a prolonged expression (to at least 8 days) in the valvulus (Fig.5f,g: Gdf7), cerebellar midline and upper rhombic lip (Fig.5h: Gdf6a). Curiously, the distinct spatial domains of Gdf7 and Gdf6a correlate with the perdurant expression domains of Atoh1a/b (Fig.5g) and Atoh1c (Fig.5i), respectively. For Gdf6a/Atoh1c this registration extends to their expression in a subset of anterior lateral line neuromasts (arrows in Fig.5h and i). Chromosome location gives no evidence of genetic linkage between these pairs of genes.
FIGURE 5. Lack of Shh signaling in the zebrafish cerebellum.
In lateral view of, neither Shha (a), Shhb (b) nor Ptc1 (c) are expressed in the cerebellum at 3 days development or at any stage through to 8 days (not shown). In transverse section, Shha (d) is expressed at the ventral midline (vml) of the hindbrain. Ptc1 (e) is expressed in both the vml and surrounding ventral tissue but not in the cb. Dorsal view at 8 days shows Gdf7 (f) expressed in the va. Double in situ hybridisation (g) shows that Gdf7 (blue) and Atoh1a (red) domains abut. In the CNS, Gdf6a (h) and Atoh1c (i) are expressed in the same spatial domain comprising the url and cerebellar midline. They are also coexpressed within a subset of anterior lateral line neuromasts (arrows).
Our analysis of zebrafish development suggests that the strategy for generating a granule cell layer deviates markedly from amniotes. A distinct Atoh1-positive, proliferative layer equivalent to an EGL is absent. Moreover, there is no Shh signalling in the cerebellum, while the coexpression of Gdf and Atoh1 into late stages suggests a prolonged dorsal induction of rhombic lip tissue. To determine whether this organisation is a secondarily derived teleost characteristic, or reflects a more widespread basal programme of cerebellum development, we examined the origins of granule cells in a chondrychthyan: the catshark/dogfish, Scylliorhinus canicula.
Cerebellar Atoh1 expression in the shark resembles that in zebrafish
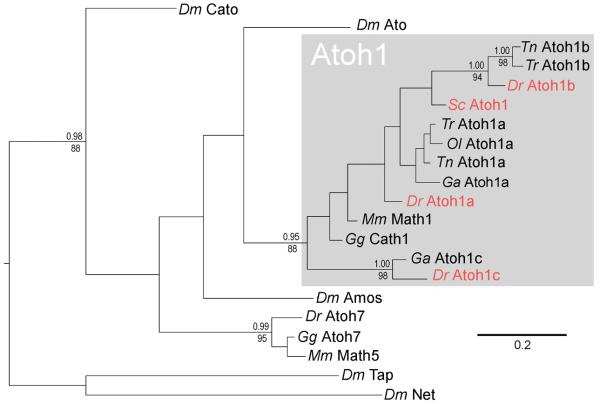
The chondrychthyan cerebellum displays distinctive morphology: granule cells are arranged into prominent midline eminences (Nieuwenhuys, 1967; Nieuwenhuys et al., 1998) which span the entire axis of a bag-like cerebellum. To investigate how this organisation arises we first screened an S.canicula cDNA library for Atoh1 by PCR amplification and discovered a single orthologue. The relationship between the conserved bHLH sequences of Atoh1 is shown in the phylogenetic tree in Figure 6. Shark Atoh1 is clearly orthologous to Atoh1 in other vertebrates. Of the euteleost Atoh paralogues, Atoh1a has been retained in all fish genomes sequenced to date. Atoh1b is only found in pufferfish (Tn and Tr) and zebrafish (Dr), and Atoh1c only in zebrafish and stickleback (Ga). Shark (Sc) Atoh1 predates the origin of these paralogues, which probably reside at the common ancestor of the euteloests, perhaps a result of a genome duplication followed by a subsequent gene duplication.
FIGURE 6. Phylogenetic relationships of vertebrate atonal genes.
The phylogenetic tree shown was derived by Bayesian analysis of the aligned bHLH region and is rooted with the Drosophila bHLH genes tap and net. A very similar topography was also recovered using maximum likelihood reconstruction. Support values above nodes represent Bayesian posterior probabilities and those below nodes represent bootstrap percentages of 1000 pseudoreplicates. Values are given for all nodes that were significantly supported in both analyses (pp > 0.95 and bs> 70). Genes shown in red are examined in this study. Dm – Drosophila melanogaster, Dr – Danio rerio, Ga - Gasterosteus aculeatus, Gg – Gallus gallus, Mm – Mus musculus, Ol - Oryzias latipes, Sc – Scyliorhinus canicula, Tn - Tetraodon nigroviridis, Tr – Takifugu rubripes.
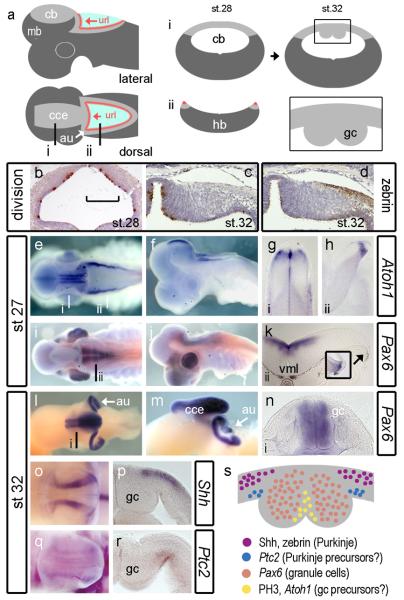
We focused our examination of shark development between stages (Farner, 1978) 27 and 32 (Fig.7a) when granule cell midline condensations become morphologically distinct in S.canicula (Rodriguez-Moldes et al., 2008). At st.28, phosphohistone H3 labelling reveals uniform proliferation rates throughout the ventricular surface of the presumptive cerebellum (Fig.7b). By st.32, highest rates of division are seen at the cerebellar midline (Fig.7c) and upper rhombic lip (not shown), medial to the midline granule cell condensation (shown schematically in Fig.7a). Zebrin immunolabel shows that Purkinje cells accumulate lateral (embryonic ventral) to granule cell eminences (Fig.7d).
FIGURE 7. Development of granule cells in the shark cerebellum.
Schematic dorsal and lateral views (a) of the shark cerebellum show the relationship of the cce to the url whose lateral extensions comprise the cerebellar auricles (au, white arrow). Levels of transverse sections through the cerebellum (i) and hindbrain are indicated (ii) on the dorsal plan. Schematic profiles for these sections are shown for a st.28 embryo (middle). At st.32, in schematic transverse section (right), the cerebellar midline shows prominent thickenings (boxed area) comprising accumulated postmitotic granule cells (gc) (Meek, 1992b; Nieuwenhuys et al., 1998; Rodriguez-Moldes et al., 2008). Cell proliferation (brown: phosphohistone H3-positive) in the neural tube at the level of the presumptive cerebellum at st.28 (b) and unilaterally at the dorsal midline st.32 (c). The medial extent of the Zebrin-positive domain of Purkinje cells coincides with the boundary of the midline eminences (d). At st.27, Atoh1 in wholemount dorsal (e) and lateral (f) view. Transverse sections indicated in a show Atoh1 adjacent to the cerebellar midline (g) and at the rhombic lip (h). By contrast, Pax6 in dorsal (i) and lateral (j) view is restricted to the ventral hindbrain and absent from the rhombic lip (k) until st.28 (inset). By st.32, dorsal (l) and lateral (m) views show that Pax6 is expressed in the cerebellar midline nuclei and auricles. Transverse section through the cerebellar midline shows Pax6 is restricted to granule cell bundles. At the same stages, Shh (o) is expressed in lateral sagittal stripes either side of granule cells, which in transverse section (p) is restricted to the pialward cells. In wholemount, faint Ptc2 expression (q) maps to that of Shh. In section, Ptc2 is expressed in the ventricular layer (r). These expression domains are summarised (s) in a schematic diagram a mid-cerebellar transverse section of the dorsal midline.
At.st.27, the expression of Atoh1 maps the spatial extent of the rhombic lip and domains adjacent to cerebellar midline (Fig.7e) and from st.27 onwards is never seen in a layer over the surface of the cerebellum. Atoh1 is excluded from the midline of the cerebellum midline itself (Fig.7g) and defines a discrete pool of rhombic lip precursors, which abuts the roofplate of the hindbrain (Fig.7h). We used the expression of Pax6 as a marker of definitive granule cells (Rodriguez-Moldes et al., 2008). As in zebrafish, at early stages Pax6 is expressed in ventral hindbrain (Fig.7i) and excluded from the cerebellum (Fig.7i, j) and hindbrain rhombic lip (Fig.7k), which only transiently expresses Pax6 at St.28 (Fig.7k, inset). We were unable to detect Atoh1 at later stages (after St.29), however at st.32, Pax6-positive granule cells have accumulated either side of the midline in cerebellum (Fig.7l) and in the upper rhombic lip or auricles (au in Fig.7l and m). The auricles and cerebellar midline are continuous and hence comprise a tightly folded linear aggregation of granule cells that extends throughout the extent of the corpus of the cerebellum (cce in Fig.7m). In cross-section (Fig.7n) the bilateral swellings either side of the cerebellar midline are uniformly Pax6-positive except for a thin superficial layer at the pial surface.
In contrast to the zebrafish, we detected Shh expression (at St.32) in the developing shark cerebellum (Fig.7o). Shh was restricted to two bands of expression lateral (embryonic ventral) to the granule cell eminences throughout the length of the corpus of the cerebellum (but not the upper rhombic lip). In section, Shh is restricted to pial, post-mitotic neurons (Fig.7p) that correspond to the location of the most medial Purkinje cell populations (see Fig.7d). We used expression of the Shh receptor, Ptc2 (Dahn et al., 2007) to determine which cells transduce Shh. In dorsal view, Ptc2 is weakly expressed in a similar domain to Shh (Fig.7q). In transverse section, Ptc2 is expressed in the ventricular layer, directly beneath the band of Shh-positive Purkinje cells, lateral to the granule cell eminence (Fig.7r). Although we cannot determine whether these are precursors of Shh-producing Purkinje cells themselves, precursors lateral to the granule cell precursor domain are candidates for producing a range of cerebellar GABAergic interneurons which include Purkinje cells.
These observations are summarised as a schematic diagram in Figure 7s. It seems likely that granule cell production is restricted to the region either side of the midline in a separate domain to Purkinje cells and other interneurons. Just lateral to the granule cell eminences, there is a close spatial registration of Shh in the Purkinje cell layer and Ptc2 in the underlying ventricular layer suggestive of Shh signalling pathway between neurons and precursors.
DISCUSSION
Our observations reveal a distinct pattern of granule cell specification and cell dispersal in zebrafish and the absence of a recognisable EGL. Comparison with the patterning of a shark reveals similarities in Atoh1 expression that diverge from the mouse and chick model. These results have implications for the zebrafish as an experimental model for cerebellum development (Bae et al., 2009). They also raise questions about the role of transit amplification and migration in generating diversity within the vertebrate CNS.
Zebrafish and shark share a similar plan of granule cell specification
Cerebellar granule cells are the most numerous neuron in the CNS and their generation is dependent on Atoh1. Its expression in the early rhombic lip of both hindbrain and cerebellum defines the precursors of the majority of neurons in both the cerebellar and precerebellar circuits (Machold and Fishell, 2005; Wang et al., 2005; Wingate, 2005). While a large number of genes are expressed specifically in developing granule cells (Millen et al., 1999), Atoh1 has a unique status in that it is essential for the production of an EGL (Ben-Arie et al., 1997). We have hence used the cerebellar expression of Atoh1 to define granule cell precursor identity.
In the zebrafish and shark, domains of Atoh1 expression define areas of intense cell proliferation. This includes the upper rhombic lip, which is a source of granule cells in the zebrafish (Volkmann et al., 2008) and migratory precursors of the EGL in both chick (Wingate and Hatten, 1999) and mouse (Ben-Arie et al., 1997; Machold and Fishell, 2005). In addition, in both zebrafish and sharks, Atoh1 is expressed continuously along a persistent embryonic cerebellar midline. Correspondingly, the Atoh1-positive midline appears to comprise a significant source of granule cells in both species. In sharks, granule cells form long, paramedian granule cell bundles or eminences. In zebrafish, the midline is a source of migratory derivatives, much the same as the upper rhombic lip. Although our Kaede fate labelling does not allow for a confirmation of the identity of these derivatives, it is a reasonable assumption that late embryonic granule cells could be derived from this Atoh1-positive midline precursor pool. By contrast, in both chick and mouse, the cerebellar midline disappears as the hemispheres fuse (Louvi et al., 2003; Sgaier et al., 2005) to generate an adult cerebellum with no discernable midline. Hemispheric fusion in the chick is associated with the integration of midbrain derivatives of the isthmic “node” into the cerebellar midline (Alexandre and Wassef, 2003). We see similar cell movements in zebrafish, however the role of these cells, which evidently neither promote midline fusion nor give rise to migratory derivatives, is unclear.
Importantly, we see no evidence in either shark or zebrafish of a transient, proliferative, Atoh1-positive superficial layer during cerebellum development. Rather, both species share a similar groundplan of cerebellar development (summarised in Fig.8a and b). While a zebrafish “EGL” has been previously identified by a long-term cumulative BrdU uptake (Mueller and Wulliman, 2005), time-lapse analysis of Kaede-mediated fate-maps indicates that recently born neurons migrate rapidly away from their proliferation zones (Fig.4c) making any other technique than acute proliferation assay (such as phosphohistone H3) misleading. Our results show that both zebrafish and shark have proliferation zones at the Atoh1-positive cerebellar midline and upper rhombic lip similar to those previously implied by observations in the trout (Pouwels, 1978; Candal et al., 2005), Medaka (Candal et al., 2005) and adult zebrafish (Zupanc et al., 2005; Grandel et al., 2006; Kaslin et al., 2009). Lack of an EGL in the zebrafish is complemented by an absence of Shh signalling in the cerebellum. However, Purkinje cells in the more basal shark express Shh. Since granule cell precursors are not the target of this signal, Shh signalling has an ancestral role in cerebellar development that is distinct from its amniote function of regulating granule cell proliferation.
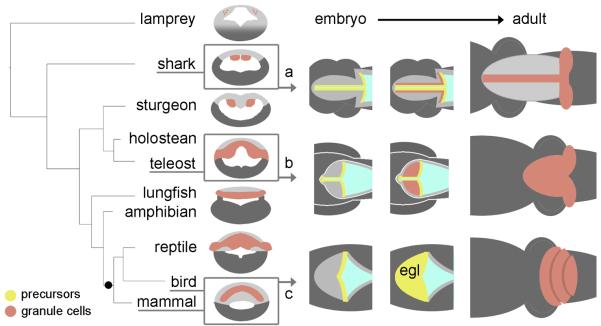
FIGURE 8. Summary of the evolutionary relationships between selected patterns of granule cell development in vertebrate cerebellum.
On the left, a simple phylogenetic tree of vertebrates is contrasted with the distribution of granule cells (brown) in adult cerebellum seen in schematic transverse section (Nieuwenhuys et al., 1998). All jawed vertebrates display a distinct cerebellum containing granule and Purkinje cells. The status of the cerebellum in the jawless lamprey (top) is less clear. To the right, the proposed developmental relationships between granule cell precursors (yellow) and definitive granule neurons are shown in schematic dorsal views for the shark (a), teleost (b) and bird or mammal (c). Our results suggest that an EGL is likely to have evolved as a developmental strategy after with the emergence of amniotes (black dot).
Our inability to detect late expression of Atoh1 in shark bears some similarity to the down-regulation of Atoh1 in the adult cerebellar stem cell niche of zebrafish (Kaslin et al., 2009). A continuity of granule cell production can be presumed in both instances raising the possibility that an Atoh1-independent mechanism for the maintenance of the granule cell precursor pool may be a feature of late cerebellar development in both these anamniotes.
Zebrafish granule cells derive from persistent primary proliferation rather than secondary proliferation
Atoh1 paralogues are segregated into two distinct spatial domains: Atoh1a/b in the presumptive valvulus) and Atoh1c (cerebellar midline and upper rhombic lip) correspond to expression of Gdf7 and Gdf6a, respectively. In mouse, Gdf7 is experimentally required for dorsal neural tube structures in mouse (Lee and Jessell, 1999) and correspondingly Atoh1 can be induced by TGFβ signals in vitro (Alder et al., 1996; Alder et al., 1999). It therefore seems likely that Gdf7 and Gdf6a expression maintains Atoh1a, b and c in the late embryonic zebrafish cerebellum; a hypothesis that could be tested by conditional downregulation of Gdf in late stage embryos. If this is the case, there is a mechanistic continuity between proliferation zones in the early and late embryo that suggests they comprise perdurant embryonic tissue rather than a secondary germinal zone. The absence of secondary proliferation in an EGL is supported by both the absence of Shh and the restricted cerebellar expression of N-myc to the Atoh1a/b/c domain. This indicates that N-myc can promote granule cell proliferation at the rhombic lip independent of Shh signalling (Kenney et al., 2003; Hatton et al., 2006).
Coexpression of Atoh1a/b and Gdf7 gives the presumptive valvulus a molecular identity that is distinct from the Atoh1c/Gdf6a domain. The mechanism of this unusual co-regulation of paired Atoh1 and Gdf homologues, which is also displayed in anterior neuromasts (Fig.5), is unclear. Since the valvulus is common to the cerebellum of all euteleosts (Nieuwenhuys et al., 1998), the molecular compartition of its midline may be a common feature of development. Correspondingly, while the presumptive valvulus does not constitute an EGL, its adult derivative maintains a stem cell niche characterized by a subset of granule cell markers (Kaslin et al., 2009). This unique pattern of apical proliferation is a distinguishing feature of the euteleost cerebellum. Furthermore, it is clear that variability in the size and shape of the cerebellum of bony fish principally reflects variability in the size of the valvulus (Schnitzlein and Faucette, 1969; Nieuwenhuys et al., 1998).
Proliferation, transit amplification and tangential migration as substrates for CNS evolution
The regional groundplan of the vertebrate CNS is highly conserved (Holland and Holland, 1999) with each region characterised by a stereotyped repertoire of neuronal types (Nieuwenhuys et al., 1998). Given such conservation, how does CNS specialisation emerge during development? For the cerebellum, our results suggest that small adjustments to migration and proliferation can result in major morphological differences, which in turn have well-characterised functional consequences (Meek, 1992a; Meek, 1992b). Thus in the shark cerebellum, the lack of migration of post-mitotic granule neurons away from the midline gives its cerebellum a hyper-elongated structure with granule cells in midline bundles (Fig.8a). In the teleost (Fig.8b), amphibian (Gona, 1976), chick and mouse (Fig.8c), tangential migration gives rise to a layer of granule cells. In chick and mouse, migratory rhombic lip derivatives also undergo a second wave of proliferation that results in the characteristic foliated cerebellum. However, the secondary proliferation is neither required for granule cell specification (in shark and zebrafish) nor necessary for a granule cell layer (zebrafish and frog), and thus the EGL is likely to be solely an adaptation for transit amplification. Furthermore, the absence of an EGL in sharks, teleosts (this study) and amphibians (Gona, 1976) suggests it is an amniote developmental adaptation.
Why did the EGL emerge in amniotes? The answer may lie in the geometry of proliferation. For a given rate of cell division, granule precursors arranged in a layer multiply at an exponential rate (proportional to area) compared to those within a linear rhombic lip (proportional to length). An EGL will therefore allow a threshold number of granule cells to be achieved more quickly. This might comprise a distinct advantage for shorter, definitive embryonic periods in land-adapted amniotes. Intriguingly, transit amplification is also found in the forebrain sub-ventricular zone: a transient, displaced proliferation zone that has similarly been proposed as a site of evolutionary adaptation in the mammalian cortex (Kriegstein et al., 2006; Abdel-Mannan et al., 2008). Transit amplification strategies may hence prove the major target for evolutionary adaptation in generating structural diversity in both the cerebellum and cortex.
In conclusion, our results show that the EGL is remarkable as one of the few, if not the only major embryonic structure of the vertebrate brain to be added relatively recently in evolution. We can speculate that its origins lie in cell-autonomous adaptation within the ancestral cerebellar rhombic lip prescursor that enabled migrating cells to both maintain Atoh1 expression and respond to Shh secreted by Purkinje cells. An intriguing question is whether these innovations occurred by a single gene regulatory step that can still be recognised as specific to the development of the amniote cerebellar rhombic lip. Identification of this adaptation may have important implications for understanding how transit amplification is initiated in normal development and subverted in tumorgenesis.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Laure Bally-Cuif for materials, Thomas Butts for the construction of the Atoh1 phylogenetic tree, Leah Toole for her thoughtful contributions to the development of this project, Jon Clarke for discussion of the manuscript and help with fate-mapping techniques and Andrew Lumsden for comments on earlier versions of this work. This research was supported by a Project Grant from the UK BBSRC and a Centre Grant from the UK MRC.
REFERENCES
- Abdel-Mannan O, Cheung AF, Molnar Z. Evolution of cortical neurogenesis. Brain Res Bull. 2008;75:398–404. doi: 10.1016/j.brainresbull.2007.10.047. [DOI] [PubMed] [Google Scholar]
- Adolf B, Bellipanni G, Huber V, Bally-Cuif L. atoh1.2 and beta3.1 are two new bHLH-encoding genes expressed in selective precursor cells of the zebrafish anterior hindbrain. Gene Expr Patterns. 2004;5:35–41. doi: 10.1016/j.modgep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. doi: 10.1016/s0896-6273(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Alder J, Lee KJ, Jessell TM, Hatten ME. Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat Neurosci. 1999;2:535–540. doi: 10.1038/9189. [DOI] [PubMed] [Google Scholar]
- Alexandre P, Wassef M. The isthmic organizer links anteroposterior and dorsoventral patterning in the mid/hindbrain by generating roof plate structures. Development. 2003;130:5331–5338. doi: 10.1242/dev.00756. [DOI] [PubMed] [Google Scholar]
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruga J, Minowa O, Yaginuma H, Kuno J, Nagai T, Noda T, Mikoshiba K. Mouse Zic1 is involved in cerebellar development. J Neurosci. 1998;18:284–293. doi: 10.1523/JNEUROSCI.18-01-00284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YK, Kani S, Shimizu T, Tanabe K, Nojima H, Kimura Y, Higashijima S, Hibi M. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Dev Biol. 2009;330:406–426. doi: 10.1016/j.ydbio.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, McCall AE, Berkman S, Eichele G, Bellen HJ, Zoghbi HY. Evolutionary conservation of sequence and expression of the bHLH protein Atonal suggests a conserved role in neurogenesis. Human Molecular Genetics. 1996;5:1207–1216. doi: 10.1093/hmg/5.9.1207. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Cajal SRy. Histologie du Système Nerveux de l'Hommme et des Vertébrés. A. Maloine; Paris: 1911. [Google Scholar]
- Candal E, Anadon R, Bourrat F, Rodriguez-Moldes I. Cell proliferation in the developing and adult hindbrain and midbrain of trout and medaka (teleosts): a segmental approach. Brain Res Dev Brain Res. 2005;160:157–175. doi: 10.1016/j.devbrainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Corrales JD, Blaess S, Mahoney EM, Joyner AL. The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development. 2006;133:1811–1821. doi: 10.1242/dev.02351. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Ruiz-i-Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Dahn RD, Davis MC, Pappano WN, Shubin NH. Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature. 2007;445:311–314. doi: 10.1038/nature05436. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Postlethwait JH, Yan YL, Beier DR, van Doren C, Foernzler D, Celeste AJ, Crosier KE, Crosier PS. Isolation of zebrafish gdf7 and comparative genetic mapping of genes belonging to the growth/differentiation factor 5, 6, 7 subgroup of the TGF-beta superfamily. Genome Res. 1999;9:121–129. [PubMed] [Google Scholar]
- Elsen GE, Choi LY, Millen KJ, Grinblat Y, Prince VE. Zic1 and Zic4 regulate zebrafish roof plate specification and hindbrain ventricle morphogenesis. Dev Biol. 2008;314:376–392. doi: 10.1016/j.ydbio.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Luo L. Timing neurogenesis and differentiation: insights from quantitative clonal analyses of cerebellar granule cells. J Neurosci. 2008;28:2301–2312. doi: 10.1523/JNEUROSCI.5157-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farner HP. [Embryonal development of the brain of the shark Scyliorhinus canicula (L.). I. Formation of the shape of the brain, the migration mode and phase and the structure of the diencephalon] J Hirnforsch. 1978;19:313–332. [PubMed] [Google Scholar]
- Gona AG. Autoradiographic studies of cerebellar histogenesis in the bullfrog tadpole during metamorphosis: the external granular layer. J Comp Neurol. 1976;165:77–87. doi: 10.1002/cne.901650107. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol. 2006;295:263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Hatton BA, Knoepfler PS, Kenney AM, Rowitch DH, de Alboran IM, Olson JM, Eisenman RN. N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006;66:8655–8661. doi: 10.1158/0008-5472.CAN-06-1621. [DOI] [PubMed] [Google Scholar]
- His W. Die entwickelung des menschlichen rautenhirns vom ende des ersten bis zum beginn des dritten monats. I. Verlängertes Mark. Abh Kön Sächs Ges d Wiss, Mat Phys Kl. 1890;29:1–74. [Google Scholar]
- Holland LZ, Holland ND. Chordate origins of the vertebrate central nervous system. Curr Opin Neurobiol. 1999;9:596–602. doi: 10.1016/S0959-4388(99)00003-3. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Ganz J, Geffarth M, Grandel H, Hans S, Brand M. Stem cells in the adult zebrafish cerebellum: initiation and maintenance of a novel stem cell niche. J Neurosci. 2009;29:6142–6153. doi: 10.1523/JNEUROSCI.0072-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- Klein C, Butt SJ, Machold RP, Johnson JE, Fishell G. Cerebellum- and forebrain-derived stem cells possess intrinsic regional character. Development. 2005;132:4497–4508. doi: 10.1242/dev.02037. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16:2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS, Caron HN, Cloos J, Mrsic A, Ylstra B, Grajkowska W, Hartmann W, Pietsch T, Ellison D, Clifford SC, Versteeg R. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS ONE. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster RW, Fraser SE. FGF signaling mediates regeneration of the differentiating cerebellum through repatterning of the anterior hindbrain and reinitiation of neuronal migration. J Neurosci. 2006;26:7293–7304. doi: 10.1523/JNEUROSCI.0095-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- Loeb-Hennard C, Kremmer E, Bally-Cuif L. Prominent transcription of zebrafish N-myc (nmyc1) in tectal and retinal growth zones during embryonic and early larval development. Gene Expr Patterns. 2005;5:341–347. doi: 10.1016/j.modgep.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Louvi A, Alexandre P, Metin C, Wurst W, Wassef M. The isthmic neuroepithelium is essential for cerebellar midline fusion. Development. 2003;130:5319–5330. doi: 10.1242/dev.00736. [DOI] [PubMed] [Google Scholar]
- Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Meek J. Why run parallel fibers parallel? Teleostean Purkinje cells as possible coincidence detectors, in a timing device subserving spatial coding of temporal differences. Neuroscience. 1992a;48:249–283. doi: 10.1016/0306-4522(92)90489-o. [DOI] [PubMed] [Google Scholar]
- Meek J. Comparative aspects of cerebellar organisation. European Journal of Morphology. 1992b;30:37–51. [PubMed] [Google Scholar]
- Millen KJ, Millonig JH, Wingate RJT, Alder JA, Hatten ME. Neurogenetics of the cerebellar system. J Child Neurol. 1999;14:574–581. doi: 10.1177/088307389901400905. [DOI] [PubMed] [Google Scholar]
- Mueller T, Wulliman MF. Atlas of Early Zebrafish Brain Development. Elsevier; Amsterdam: 2005. [Google Scholar]
- Nieuwenhuys R. Comparative anatomy of the cerebellum. In: Fox C, Snider R, editors. Progress in Brain Research. Elsevier; Amsterdam: 1967. pp. 1–93. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, ten Donkelaar HJ, Nicholson C, editors. The Central Nervous System of Vertebrates. Springer-Verlag; Berlin: 1998. [Google Scholar]
- Pearse RV, 2nd, Vogan KJ, Tabin CJ. Ptc1 and Ptc2 transcripts provide distinct readouts of Hedgehog signaling activity during chick embryogenesis. Dev Biol. 2001;239:15–29. doi: 10.1006/dbio.2001.0430. [DOI] [PubMed] [Google Scholar]
- Pouwels E. On the development of the cerebellum of the trout, Salmo gairdneri. V. Neuroglial cells and their development. Anat Embryol (Berl) 1978;153:67–83. doi: 10.1007/BF00569850. [DOI] [PubMed] [Google Scholar]
- Reifers F, Bohli H, Walsh EC, Crossley PH, Stainier DY, Brand M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–2395. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moldes I, Ferreiro-Galve S, Carrera I, Sueiro C, Candal E, Mazan S, Anadon R. Development of the cerebellar body in sharks: spatiotemporal relations of Pax6 expression, cell proliferation and differentiation. Neurosci Lett. 2008;432:105–110. doi: 10.1016/j.neulet.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Schaper A. Die frühsten differenzierungsvorgänge im centralnervensystem. Arch Entw mech Org. 1897;5:81–132. [Google Scholar]
- Schnitzlein HN, Faucette JR. General morphology of the fish cerebellum. In: Llinás R, editor. Neurobiology of Cerebellar Evolution and Development. American Medical Association; Chicago: 1969. pp. 77–106. [Google Scholar]
- Sgaier SK, Millet S, Villanueva MP, Berenshteyn F, Song C, Joyner AL. Morphogenetic and cellular movements that shape the mouse cerebellum; insights from genetic fate mapping. Neuron. 2005;45:27–40. doi: 10.1016/j.neuron.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. 6th Edition Cambridge University Press; Cambridge: 1947. [Google Scholar]
- Volkmann K, Rieger S, Babaryka A, Koster RW. The zebrafish cerebellar rhombic lip is spatially patterned in producing granule cell populations of different functional compartments. Dev Biol. 2008;313:167–180. doi: 10.1016/j.ydbio.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Wingate R. Math-Map(ic)s. Neuron. 2005;48:1–4. doi: 10.1016/j.neuron.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Wingate RJT. The rhombic lip and early cerebellar development. Curr Opin Neurobiol. 2001;11:82–88. doi: 10.1016/s0959-4388(00)00177-x. [DOI] [PubMed] [Google Scholar]
- Wingate RJT, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126:4395–4404. doi: 10.1242/dev.126.20.4395. [DOI] [PubMed] [Google Scholar]
- Zupanc GK, Hinsch K, Gage FH. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J Comp Neurol. 2005;488:290–319. doi: 10.1002/cne.20571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.