Abstract
The abundance and higher taxonomic composition of epizooic metazoan meiobenthic communities associated with mussel and tubeworm aggregations of hydrocarbon seeps at Green Canyon, Atwater Valley, and Alaminos Canyon in depths between 1400 and 2800 m were studied and compared to the infaunal community of non-seep sediments nearby. Epizooic meiofaunal abundances of associated meiobenthos living in tubeworm bushes and mussel beds at seeps were extremely low (usually <100 ind. 10 cm−2), similar to epizooic meiofauna at deep-sea hydrothermal vents, and the communities were composed primarily of nematodes, copepods, ostracods, and halacarids. In contrast, epizooic meiobenthic abundance is lower than previous studies have reported for infauna from seep sediments. Interestingly, non-seep sediments contained higher abundances and higher taxonomic diversity than epizooic seep communities, although in situ primary production is restricted to seeps.
Keywords: Gulf of Mexico, Meiobenthos, Meiofauna, Copepods, Nematodes, Cold seeps, Abundance, Vestimentifera, Bathymodiolus, Epizooic, Epibenthos
1. Introduction
The size class of meiofauna is generally defined as the portion of the community passing through a 1-mm sieve and being retained on a 32-μm sieve. This community comprises protists and metazoan animals that remain small even as adults (permanent meiofauna) and animals that temporarily belong to this size class during their larval/juvenile development (temporary meiofauna). As part of the sediment infauna, meiobenthos has been studied extensively worldwide from many different habitats, but less attention has been paid to the hard-substrate epibenthic or epizooic and epiphytal meiobenthos (Giere, 2009).
At cold seeps, a variety of geologically diverse, reducing habitats can be distinguished by the presence of microbial mats or macro/megafaunal communities (see Sibuet and Olu, 1998; Levin, 2005). Although some animals, such as thyrasid bivalves or siboglinids, inhabit the sediment with only the anterior part of their tubes extending above the sediment surface, siboglinid vestimentiferans, bathymodiolin and vesicomyid bivalves, and sponges can build large physical structures above the sediment surface to create habitat as foundation species for an associated macro- and meiofaunal community. In general, foundation species influence the abundance, composition, and structure of the associated community (Hacker and Gaines, 1997) and can provide food resources, living space, favorable settlement conditions, refuge from predators, and/or refuge from environmental stress (see Bruno and Bertness, 2001).
The Gulf of Mexico (GOM) was the site of the first discoveries of cold seeps in the 1980s (Paull et al., 1984; Kennicutt et al., 1985), and several ecological community studies have been carried out since then. Most studies have been completed at seeps from the upper slope, located shallower than 1000 m, but more recently some have included deeper sites from the lower slope (see Cordes et al., 2007; Roberts et al., 2007). Tubeworm bushes, composed mainly of mixed vestimentiferan populations of Lamellibrachia lymesi and Seepiophila jonesi, were studied from the upper Louisiana slope (Bergquist et al., 2003; Cordes et al., 2005). The tube surface area, taken as a measure of habitat size, increased the overall surface between 2.6- and 26-fold over the uncolonized seafloor (Bergquist et al., 2003). In deeper waters of the lower slope, studied vestimentiferan aggregations were composed primarily of Escarpia laminata (Brooks et al., 1990; Cordes et al., 2007). There are a number of foundation species of mussels on the lower slope, and beds may consist of single species such as Bathymodiolus brooksi (at Atwater Valley) and Bathymodiolus childressi (Mississippi Canyon), or mixed populations of B. brooksi and B. childressi (Alaminos Canyon) or B. brooksi and Bathymodiolus heckerae (Florida Escarpment) (Cordes et al., 2007).
Meiobenthic community studies at cold seeps are scarce and restricted mainly to assessments of abundance, biomass, and composition of higher taxa. They cover a wide geographical and depth range, from shallow-water sands at 10 m down to deep-sea muds at 5000 m. They include various types of hydrocarbon gas and oil seep (Montagna and Spies, 1985; Palmer et al., 1988; Shirayama and Ohta, 1990; Olu et al., 1997; Robinson et al., 2004; Soltwedel et al., 2005; Van Gaever et al., 2006; Sergeeva and Gulin, 2007; Sommer et al., 2007), gas, oil, and asphalt seeps (Montagna et al., 1987), gas hydrates (Sommer et al., 2007), and brine seeps (Powell et al., 1983, 1986), but exclusively describe the infaunal meiobenthos from sediments covered by bacterial mats, vesicomyid clams, siboglinid frenulates, and Sclerolinum in the periphery of mussel beds. Furthermore, some sediments with discharge of methane but devoid of any visible microbial mat or animals have also been studied. In the Gulf of Mexico, seep meiofauna studies were conducted for the shallow brine seep sand communities at East Flower Garden (Powell et al., 1983, 1986; Jensen, 1986) and the hydrocarbon seep bacterial mat communities at Alaminos Canyon (2200 m), Green Canyon (about 700 m), and Atwater Valley (about 2000 m) (Robinson et al., 2004). Epifaunal foraminiferan communities associated with tubeworm bushes on the upper slope were also examined in detail (Sen Gupta et al., 2007), but no study on the associated metazoan meiobenthos has been carried out so far.
This study examines the abundance and higher taxonomic composition of epizooic, permanent, metazoan meiobenthos associated with aggregations of tubeworms and mussels from three different locations: Green Canyon (GC), Atwater Valley (AV), and Alaminos Canyon (AC) in the Gulf of Mexico. In addition, non-seep sediment cores were taken in the vicinity of such aggregations at GC. The following questions were addressed: (1) Do abundance and higher taxonomic composition differ between geographical regions? (2) Do abundance and higher taxonomic composition differ between mussel and tubeworm aggregations? (3) Is the seep epizooic metazoan meiobenthic community similar to seep infauna or non-seep sediments? (4) Are there similarities in abundance and higher taxonomic composition of seep and hydrothermal vent communities associated with mussels and tubeworms?
2. Methods
2.1. Study area
The study was conducted at the three hydrocarbon seep locations: Green Canyon 852 (GC, depth 1400 m), Alaminos Canyon 818 (AC, depth 2800 m), and Atwater Valley 340 (AV, depth 2200 m) of the lower continental slope of the Gulf of Mexico (this issue). During two cruises in 2006 and 2007, a total of 13 samples were taken with the submersible DSV ALVIN (2006) and ROV JASON (2007). Five samples of each foundation group were collected at two different seep habitats: mussels, M-GC1, M-GC2, M-GC3, M-AV1, M-AC1 and tubeworms, T-GC1, T-GC2, T-GC3, T-AV1, T-AV2. Three samples of non-seep sediments were taken as controls (S-GC1, S-GC2, S-GC3) in close vicinity (<3-m distance) to seep megafauna communities (Table 1).
Table 1.
Sample information is given on geographical location, site, dive number (AD Alvin dive, JD Jason dive), latitude, longitude, depth, sample area (‘footprint’ of sediment surface above which the mussel pot or the bushmaster sampling device was placed; is equal to diameter of mussel pot and maximal diameter of bushmaster), surface area (total area of tubeworm tubes or mussel shells surfaces calculated per sample), surface area per sample area (total sample area per total surface area), volume of sediment (collected between mussels or tubeworms), and megafauna listed per species (% contributing to total megafauna).
| Sample | M-GC1 | M-GC2 | M-GC3 | M-AV1 | M-AC1 | T-GC1 | T-GC2 | T-GC3 | T-AV1 | T-AV2 | S-GC1 | S-GC2 | S-GC3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Green | Green | Green | Atwater | Alaminos | Green | Green | Green | Atwater | Atwater | Green | Green | Green |
| Canyon | Canyon | Canyon | Valley | Canyon | Canyon | Canyon | Canyon | Valley | Valley | Canyon | Canyon | Canyon | |
| Site | GC 852 | GC 852 | GC 852 | AT 340 | AC 818 | GC 852 | GC 852 | GC 852 | AT340 | AT 340 | GC 852 | GC 852 | GC852 |
| Dive number | AD 4186 | AD 4187 | JD 278 | JD 276 | AD 4192 | AD 4186 | AD 4187 | JD 273 | JD 277 | JD 270 | AD4177 | AD4177 | AD4177 |
| Latitude | 27°06.357 | 27°06.656 | 27°06.380 | 27°25.197 | 26°10.819 | 27°06.371 | 27°06.676 | 27°06.370 | 27°38.839 | 27°38.694 | 27°10.633 | 27°10.633 | 27°10.633 |
| Longitude | 91°09.974 | 91°09.937 | 91°09.953 | 88°21.853 | 94°37.380 | 91°09.968 | 91°09.932 | 91°09.967 | 88°22.429 | 88°21.843 | 91°16.608 | 91°16.608 | 91°16.608 |
| Depth (m) | 1410 | 1406 | 1408 | 2200 | 2744 | 1409 | 1406 | 1410 | 2175 | 2192 | 1450 | 1450 | 1450 |
| Sample area (cm2) | 531 | 531 | 531 | 531 | 531 | 2800 | 2800 | 2800 | 2800 | 2800 | 10.33 | 15.5 | 15.5 |
| Surface area (cm2) | 1670 | 1630 | 2140 | 2190 | 2900 | 15060 | 4980 | 8050 | 12740 | 16870 | 10.33 | 15.5 | 15.5 |
| Surface area per sample area | 3.15 | 3.07 | 4.03 | 4.12 | 5.46 | 5.38 | 1.78 | 2.88 | 4.55 | 6.03 | 1.00 | 1.00 | 1.00 |
| Sediment (ml) | No info | No info | 41.21 | 21 | 42 | No info | No info | 5.89 | 7500 | 16 | 41 | 107 | 88.5 |
| Megafauna | Mussels | Mussels | Mussels | Mussels | Mussels | Tubeworms | Tubeworms | Tubeworms | Tubeworms | Tubeworms | No | No | No |
|
Bathymodiolus brooksi (%) |
50 | 36.8 | 100 | 100 | 100 | ||||||||
| B. childressi (%) | 50 | 63.2 | |||||||||||
|
Lamellibrachia ssp. (%) |
71.4 | 85.3 | 80.8 | 5.5 | |||||||||
|
Escarpia laminata (%) |
28.6 | 14.7 | 19.2 | 94.5 | 100 |
2.2. Sample collections
Epifauna collections were carried out with the quantitative sampling devices Mussel Pot (a hydraulically actuated sampling pot lined with a net, 531 cm2 diameter of sample area; for further detail of the collection device see Cordes et al., this issue) for mussel aggregations and Bushmaster Jr. (a hydraulically actuated, custom-built sampling device lined with a net and having a 2800-cm2 diameter of sample area; for further details, see Urcuyo et al., 2003; Bergquist et al., 2003) for tubeworm aggregations. Infauna of non-seep sediment were collected with push cores (6.3 cm diameter, 30 cm length). Samples were separately put into isolated, previously cleaned plastic boxes on the basket of DSV ALVIN or ROV JASON, transported to the surface, and recovered on deck of the RV Atlantis in 2006 or NOAA ship Ron Brown in 2007. On board, the macro- and megafauna of Bushmaster and Mussel Pot samples were carefully rinsed with cold 32-μm-filtered seawater before we removed them from the samples in order to avoid loss of smaller fauna. Mussels and tubeworms of each collection were identified and counted (Table 1). The samples were sieved through a 1-mm sieve to separate macro- from meiofauna. Before sieving the samples through a 32-μm sieve, we measured the volume of sediment of the entire sample <1 mm. The meiofauna fraction was fixed in 4% buffered formalin. The larger size fractions were retained for complementary studies by collaborators (see Cordes et al., this issue).
In order to estimate the sediment depth distribution of meiobenthos in the push corer samples, we checked the fraction deeper than 5 cm carefully on board of the ship. Because one sample lacked any specimens, and two samples contained only a single nematode, we took the upper 5 cm of these samples, and fixed them in 4% buffered formalin without sieving. The push core sample S-GC1 was split into three parts along the entire length, and one part (52 ml) was used for the present study. The other two samples, S-GC2 and S-GC3, were split into half, and these parts (78 ml) were used for the present analyses.
2.3. Quantification of abundance
To extract meiofauna from the sediment, we used a density centrifugation technique with a medium consisting of a silicapolymer (Levasil®) mixed with kaolin (McIntyre and Warwick, 1984; Veit-Koehler et al., 2008). Except for sample T-AV1, all other samples were totally processed and the entire meiofauna community was counted and identified to higher taxon level. Sample T-AV1 was extremely large (7.5 l sediment including meiofauna after sieving through a 1-mm net); therefore, we carefully mixed the entire sample in a bucket, let it settle, randomly took a subsample of 217 ml, and estimated the total abundance from this subsample.
All taxa belonging to the permanent metazoan meiobenthos were considered in this study. We noticed the presence of crustacean nauplii but did not include them in further analyses because they could not be assigned to a specific higher crustacean taxon. We also recorded the protist meiobenthos, but did not include them in this study of permanent metazoan meiobenthos.
2.4. Data analyses
Total abundance of meiobenthos was standardized to 10-cm2 sample area and additionally to 10-cm2 surface area of mussel shells and tubeworm tubes. The surfaces of mussels and tube-worms were estimated for the main foundation species B. brooksi, B. childressi, B. heckerae, E. laminata, and Lamellibrachia spp. by measurements of lengths and widths for each individual in the collection (see Cordes et al., this issue, for methods). To test for significant differences in abundances among habitat types in the Green Canyon samples, data were square-root transformed and bootstrapping was used, as this is a well-proven method when working with a relatively low number of samples and high variances (10,000 resamplings each, t-test, 2-sided test, routine FTBOOT from the package Computer Intensive Statistics (Nemeschkal, 1999). Results were classically Bonferroni-corrected (p=α/n; α=0.05). To evaluate similarity and dissimilarity among all samples, a Bray–Curtis similarity matrix was generated. Abundance data from 10-cm2 sample area were square-root transformed, but were not standardized, to enable us to better recognize differences caused by total abundances, and similarity percentage (SIMPER) analysis, analysis of similarity (ANOSIM), and multi-dimensional scaling (MDS) ordination were performed using PRIMER v5 (Clarke and Gorley, 2001).
3. Results
3.1. Abundance
The total abundance of the permanent metazoan meiobenthos associated with mussel and tubeworm aggregations of most samples from three different locations at the northern Gulf of Mexico ranged from 1 to 8 1 individuals per 10-cm2 sample area. However, one tubeworm aggregate sample (T-AV1) from Atwater Valley (AV) revealed a total abundance between one and two orders of magnitude higher (447 individuals 10cm−2) than the nine other seep samples. Non-seep sediment control samples showed abundance values from 870 to 1523 individuals 10-cm−2 sample area (Table 2).
Table 2.
Meiobenthic abundance is shown as total abundance, individuals 10 cm−2 sample area, and ind. 10 cm−2 surface area for all 13 samples (5 mussel community samples, 5 tubeworm community samples, 3 non-seep sediment samples).
| Sample | M-GC1 | M-GC2 | M-GC3 | M-AV1 | M-AC1 | T-GC1 | T-GC2 | T-GC3 | T-AV1 | T-AV2 | S-GC1 | S-GC2 | S-GC3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total abundance | |||||||||||||
| Nematoda | 2 | 19 | 723 | 2513 | 3087 | 1005 | 181 | 1389 | 103618 | 1547 | 735 | 1089 | 1964 |
| Copepoda | 240 | 179 | 323 | 519 | 1229 | 759 | 64 | 153 | 20461 | 755 | 168 | 253 | 388 |
| Ostracoda | 0 | 11 | 14 | 22 | 3 | 3 | 0 | 1 | 1002 | 22 | 5 | 6 | 5 |
| Halacarida | 0 | 9 | 31 | 19 | 0 | 7 | 0 | 0 | 0 | 7 | 0 | 0 | 1 |
| Kinorhyncha | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Total | 242 | 218 | 1091 | 3073 | 4319 | 1774 | 245 | 1543 | 125081 | 2331 | 908 | 1349 | 2360 |
| ind. 10 cm−2 sample area | |||||||||||||
| Nematoda | 0.04 | 0.36 | 13.62 | 47.33 | 58.14 | 3.59 | 0.65 | 4.96 | 370 | 5.53 | 711.52 | 702.58 | 1267.10 |
| Copepoda | 4.52 | 3.37 | 6.08 | 9.77 | 23.15 | 2.71 | 0.23 | 0.55 | 73 | 2.70 | 162.63 | 163.23 | 250.32 |
| Ostracoda | 0.00 | 0.21 | 0.26 | 0.41 | 0.06 | 0.01 | 0.00 | 0.00 | 4 | 0.08 | 4.84 | 3.87 | 3.23 |
| Halacarida | 0.00 | 0.17 | 0.58 | 0.36 | 0.00 | 0.03 | 0.00 | 0.00 | 0 | 0.03 | 0.00 | 0.00 | 0.65 |
| Kinorhyncha | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0 | 0.00 | 0.00 | 0.65 | 1.29 |
| Total | 4.56 | 4.11 | 20.55 | 57.87 | 81.34 | 6.34 | 0.88 | 5.51 | 447 | 8.33 | 878.99 | 870.32 | 1522.58 |
| ind. 10 cm−2 surface area | |||||||||||||
| Total | 0.29 | 0.27 | 1.02 | 2.81 | 2.98 | 0.24 | 0.10 | 0.38 | 20 | 0.28 | 878.99 | 870.32 | 1522.58 |
Green Canyon (GC) was the only site where the number of samples was sufficient to compare statistically the abundances among mussel- and tubeworm-associated communities, and among the seep communities and adjacent non-seep sediments. We found no significant difference between mussel and tube-worm meiobenthos abundance (p=0.190), but significantly lower abundances at both seep communities than in non-seep sediments (both: p=0.003).
The mussel beds at AV, AC, and one sample from GC (M-GC3) were built exclusively by B. brooksi. In addition, B. childressi co-occurred in two GC samples, contributing with 50% and 63.2%, respectively, to the total mussel abundance. Also, the tubeworm aggregations of all collections were mixed populations of E. laminata and one or two species of Lamellibrachia (Table 1). As foundation species forming biogenic habitat, tubeworms and mussels considerably increase the surface area and thus the potential living space for meiobenthos. By estimating the actual surface of the foundation species, we found an increase of surface in both types of aggregations between 1.78- and 6.03-fold. The ratio of sample area to the surface area of tubes/shells was similar between the two biogenic habitat types, but was more variable in tubeworm bushes (1.78–6.03) than in mussel beds (3.07–5.46) (Table 1).
By assuming that the surface of foundation species was the actual living space of associated meiobenthos, we standardized the total abundance of this community to the surface area and calculated even lower densities, between only 1 and 3 individual per 10 cm−2. Again, one tubeworm sample (T-AV1) contained much greater densities of meiobenthos (20 individuals 10 cm−2) (Table 2). t-tests on abundance per surface area of GC samples revealed similar results as calculations per sample area, with similarly low abundances found in the seep habitat types (mussel and tubeworm: p=0.150; seep and non-seep both: p=0.003).
3.2. Taxonomic diversity
The seep metazoan meiobenthic communities were composed of the higher taxa Nematoda, Copepoda, Ostracoda, and Halacarida. In addition, nauplii larvae were found in seven out of ten samples with variable abundances but were excluded from analyses because it was impossible to assign them to a specific crustacean taxon. The protist phylum Foraminifera was also represented in all seep samples.
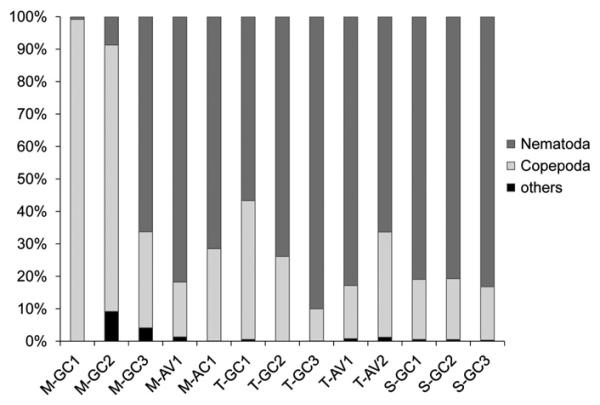
In all five tubeworm samples from the three different locations, the most prominent taxa were the nematodes, with relative abundances between 57% and 90%, followed by the copepods (10–43%). Ostracods and halacarids were relatively rare, often found with relative abundances below 1% and below 0.5%, respectively (Fig. 1).
Fig. 1.
Relative abundance (%) of taxa for meiobenthos (five mussel community samples, five tubeworm community samples, three non-seep sediment samples). Nematoda, Copepoda, and others (including Ostracoda, Halacarida, and Kinorhyncha) were present.
The relative distribution of higher taxa was more variable in mussel bed samples. In three samples (M-GC3, M-AV1, M-AC1), nematodes dominated (66–82%), followed by copepods (17–30%), and in two samples (M-GC1, M-GC2) copepods were most abundant (82% and 99%). Ostracods were present in 4 out of 5 samples, and halacarids in 3 out of 5 samples. In two of these more diverse communities, ostracods and halacarids together reached relative abundances of between 1% and 5%.
The non-seep control sediments collected in close vicinity to mussel and tubeworm aggregations at GC additionally harbored the taxon Kinorhyncha. The community was composed primarily of nematodes (80–81%), followed by copepods (16–19%), ostracods, halacarids, and kinorhynchs (all<1%). Remarkably, nauplii and foraminiferans were absent from these samples.
3.3. Community patterns
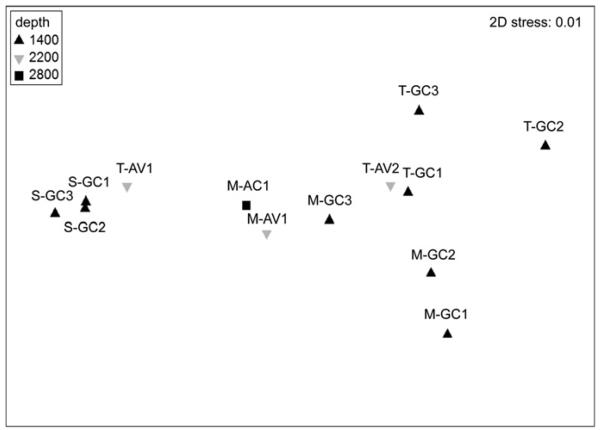
SIMPER and ANOSIM analyses did not demonstrate significant differences between mussel bed and tubeworm aggregation meiobenthic communities at the taxonomic level examined. There were also no significant differences among sites, despite the differences in depth (GC 1400 m, AV 2200 m, AC 2800 m) (Table 3). However, strong differences were detected between non-seep sediment communities and tubeworm- and mussel-associated communities (>74% Bray–Curtis dissimilarity), and these differences were significant in the ANOSIM (R=0.64; p=0.040 for tubeworm/sediment; R=0.81; p=0.020 for mussel/sediment). Multi-dimensional scaling (MDS) ordination revealed that metazoan meiobenthos from seep habitats and from adjacent non-seep sediments formed distinct groups, with the exception of sample T-AV1, which exhibited relatively high similarity to non-seep communities (Fig. 2).
Table 3.
Dissimilarity results (Diss. %) calculated by SIMPER, and ANOSIM results (R-statistics and possible significance level p) are shown for mussel compared to tubeworm communities, and mussel and tubeworm communities to non-seep sediment communities. Additionally, seep sites at different depths (1400, 2200, 2800 m) are compared with each other.
| Diss. (%) | R-Stat | p | |
|---|---|---|---|
| Mussel—tubeworms | 54 | 0.15 | 0.13 |
| Mussel—sediment | 74 | 0.81 | 0.02 |
| Tubeworm—sediment | 74 | 0.64 | 0.04 |
| Seep: 1400–2200 m | 55 | 0.25 | 0.13 |
| Seep: 1400–2800 m | 62 | 0.56 | 0.14 |
| Seep: 2200–2800 m | 35 | 0.56 | 1 |
Fig. 2.
Two-dimensional MDS configuration plot for 13 samples from five mussel community samples (M-GC1, M-GC2, M-GC3, M-AV1, M-AC1), five tubeworm community samples (T-GC1, T-GC2, T-GC3, T-AV1, T-AV2), and three non-seep sediment samples (S-GC1, S-GC2, S-GC3) from three different depths.
4. Discussion
The epizooic metazoan meiobenthic communities associated with tubeworm bushes and mussel beds at cold seeps in the Gulf of Mexico (GOM) can be characterized as a community composed of a limited number of higher taxa, including Nematoda, Copepoda, Ostracoda, and Halacarida, occurring in remarkably low abundances. These seep communities are similar to epizooic meiobenthic vent communities associated with bathymodiolin mussels or vestimentiferan tubeworms. However, communities associated with biogenic habitats differ from the infaunal communities studied from sands of shallow-water seeps and clays of deep-water seeps, which show much higher abundances compared to the epizooic meiobenthos from our studied sites.
Tubeworm aggregations and mussel beds are colonized not only by meiobenthos but also by a diverse and abundant macrobenthic community at the GOM cold seeps. In these same samples, mussel-associated macrofauna were present in densities between 235.5 and 1196.3 individuals per m2 (0.2 and 1.2 individuals per 10 cm2) and tubeworm-associated macrofauna were between 35.9 and 127.9 individuals per m2 (0.04 and 1.3 individuals per 10 cm2) (Cordes et al., this issue). In other samples from the upper slope, macrobenthic abundances calculated per sample area ranged from 209 to 9590 individuals per m2 (0.2–9 individuals per 10-cm2) (Bergquist et al., 2003), and abundances standardized to the tube surface vary from 4 to 233 individuals per m2 on the upper slope (Cordes et al., 2005), and 134–607 individuals per m2 on the lower slope (Cordes et al., 2007). Abundances per mussel shell surface from the Florida Escarpment, a different site in Atwater Valley, and Alaminos Canyon were between 160 and 4458 individuals per m2 (Cordes et al., 2007). It appears that the macro- and megafauna are relatively well represented in such aggregations, fueled by in situ primary production, whereas small meiobenthic animals are relatively scarce. Some shallow-water studies indicate that the interaction between macrofauna and meiofauna is negative for the smaller size class, because adult large animals are potentially predators and/or dislocate meiofauna by movement. In addition, the juvenile macrofauna, temporarily in the meiofauna-size class while growing up, can act as predators or competitors (Bell, 1980; Ólafsson, 2003). Also, a recent study at seeps on the Norwegian margin revealed a negative correlation between meio- and macrofaunal abundance, and predation pressure was speculated to be the underlying cause for this pattern (Van Gaever et al., 2009). However, whether the seep meiofauna community is regulated by such top-down or bottom-up processes remains to be tested.
Overall, the abundances and higher taxonomic composition of meiobenthos associated with tubeworm and mussel habitats from cold seeps in this study are quite similar to those at hydrothermal vents (Table 4). The epizooic communities of both environments are low in abundance (usually below 100 individuals per 10 cm2) and are mostly dominated by nematodes. In addition, communities with equal nematode to copepod distribution (East Pacific Rise, 9°50′N region, tubeworm aggregations, Gollner et al, 2007), copepod-dominated communities (this study; Juan de Fuca Ridge, Paralvinella aggregations, Tsurumi et al., 2003; East Pacific Rise 11°N region, mussel aggregations, Zekely et al., 2006; East Pacific Rise, 9°50′N region, tubeworm aggregations, Gollner et al., 2007), or foraminiferan-dominated communities (East Pacific Rise, 9150°N region, tubeworm aggregations, Gollner et al., 2007) have also been found. Similar to varying higher taxa proportions in mussel aggregations at GC of this study, the tubeworm aggregation at the East Pacific Rise vent site Riftia Field also exhibited a high variability (Gollner et al., 2007). This finding points to a patchy distribution, a common phenomenon, which other studies has been suggested to be related to the inhomogenous occurrence of food, predation, and/or displacement by larger animals (see Giere, 2009).
Table 4.
List of meiobenthic infaunal and epifaunal studies from vents and seeps, listed according to type of seep or vent, depth, sampling device, extraction/sieving technique, components of meiobenthos included in study (m metazoan permanent, p protist permanent, t temporary meiobenthos), habitat, abundance individuals 10 cm−2, and reference.
| Location | Type | Depth (m) | Sampling | Extraction/sieving | Fauna | Habitat | Abundance (10 cm−2) | Reference |
|---|---|---|---|---|---|---|---|---|
| Seep infauna | ||||||||
| Kattegat, North Sea | Gas | 10–12 | Corer | Sieving 45–500 μm | m+t | Reduced sediments | 650 | Jensen et al. (1992) |
| East Flower Garden Gulf of Mexico | Brine seep | 72 | Grab | Sieving >63 μm | m+t | Bac mats | 1–240 | Powell et al. (1983) |
| Isla Vista, Santa Barbara Channel | Oil/gas | 15 | Corer | Decantation | m(+p?)+t | Bac mats | 1360 | Montagna and Spies (1985) |
| Isla Vista, Santa Barbara Channel | Oil/gas | 18 | Corer | Decantation+sieving >63 μm | m+p+t | Fine sand sediment | 3550 | Montagna et al. (1987) |
| Oil/asphalt | 18 | Corer | Decantation+sieving >63 μm | m+p+t | Fines sand sediment | 2661 | ||
| Oil/gas | 19 | Corer | Decantation | m(+p?)+t | Bac mats | 2500 | Palmer et al. (1988) | |
| Hatsushima, Sagami Bay | Gas | 1100–1200 | Corer | Sieving >63 μm | m+p+t | Underneath calms | 371–414 | Shirayama and Ohta (1990) |
| Barbados prism | Gas | 5000 | Corer | No data | m(+p?)+t | Sediment center | 116 | Olu et al. (1997) |
| Gas | 5000 | Corer | No data | m(+p?)+t | Underneath clams | 6541–8438 | ||
| Gas | 5000 | Corer | No data | m(+p?)+t | Near clams | 845–1893 | ||
| Dnieper Canyon, Black Sea | Gas | 182–252 | Corer | Sieving 64 μm–1 mm | m+p+t | Bac mats | 2.39–52.50 | Sergeeva and Gulin (2007) |
| Hydrate Ridge, off Oregon | Gas hydrate | 800 | Corer | Centrifugation >32 μm | m+t | Bac mats | 623–965 | Sommer et al. (2007) |
| Gas hydrate | 800 | Corer | Centrifugation >32 μm | m+t | Underneath clams | 1021–1566 | ||
| Hå kon Mosby, SW Barents Sea slope | Gas | 1280 | Corer | Sieving 32–500 μm | m+p+t | Sediment center | 4471 | Soltwedel et al. (2005) |
| Gas | 1280 | Corer | Sieving 32–500 μm | m+p+t | In Siboglinidae | 2878–3899 | ||
| Gas | 1280 | Corer | Sieving 32–500 μm | m+p+t | bac mats | 3475 | ||
| Hå kon Mosby, SW Barents Sea slope | Gas | 1286–1288 | Corer | Centrifugation >32 μm | m+t | Sediment center | 513.2±38.4 | Van Gaever et al. (2006) |
| Gas | 1286–1288 | Corer | Centrifugation >32 μm | m+t | In Siboglinidae | 1741.3±577.8 | ||
| Gas | 1286–1288 | Corer | Centrifugation >32 μm | m+t | Bac mats | 11292.1±2256.2 | ||
| Hå kon Mosby, SW Barents Sea slope | Gas | 1250 | Corer | Centrifugation >32 μm | m+t | Grey mats | 1198±717 | Van Gaever et al. (2009) |
| Nyegga Area, Mid-Norwegian Margin | Gas | 730 | Corer | Centrifugation >32 μm | m+t | Reduced sediments | 333±69 | |
| Gas | 730 | Corer | Centrifugation >32 μm | m+t | In Siboglinidae | 7028±1279 | ||
| Storegga Slide, Mid-Norwegian Margin | Gas | 740 | Corer | Centrifugation >32 μm | m+t | In Siboglinidae | 41±22 | |
| Seep epifauna | ||||||||
| AC, AV, GC, Gulf of Mexico | Gas | 1400–2800 | Bushmaster | Centrifugation 32 μm–1 mm | m | Ass. Vestimentifera | 0.88–447 | This study |
| Gas | 1400–2800 | Mussel pot | Centrifugation 32 μm–1 mm | m | Ass. mussels | 4.11–81.34 | This study | |
| Vent infauna | ||||||||
| Guaymas, East Pacific Rise | Vent | 2000 | Corer (?) | Centrifugation >63 μm | m+t | Bac mats | 1–81 | Dinet et al. (1988) |
| Bay of Plenty, New Zealand | Vent | 4–12 | Corer | Sieving >50 μm | m+p | Bac mats | 1–241 | Kamenev et al. (1993) |
| Matupi Harbour, Papua New Guinea | Vent | 0–27 | Corer | Sieving >500 μm | m+p | Bac mats | 2–131 | Tarasov et al. (1999) |
| Aegean Sea, Mediterranean Sea | Vent | 10 | Corer | Elutriation >63 μm | m+p | Bac mats | 0–1075 | Thiermann et al. (1997) |
| Sulawesi, Indonesia | Vent | 3 | Corer | Centrifugation >30 μm | m+t | Sediments 10 cm off vent | 49±8 | Zeppilli and Danovaro (2009) |
| Vent | 3 | Corer | Centrifugation >30 μm | m+t | Sediments 1 m off vent | 652±3 | ||
| Vent epifauna | ||||||||
| Juan de Fuca Ridge | Vent | 2300 | Grab | Sieving >63 μm | m+p | Ass. Paralvinella | 14–87 | Tsurumi et al. (2003) |
| Mid Atlantic Ridge | Vent | 3492 | Mussel pot | Centrifugation >63 μm | m+p | Ass. mussels | 36–46 | Zekely et al. (2006) |
| N East Pacific Rise | Vent | 2480 | Mussel pot | Centrifugation >63 μm | m+p | Ass. mussels | 25–32 | |
| N and S East Pacific Rise | Vent | 2491–2690 | Mussel pot | Centrifugation >62 μm | m+p | Ass. mussels | 22–116 | Copley et al. (2007) |
| N East Pacific Rise | Vent | 2500 | Bushmaster | Centrifugation >63 μm | m+p | Ass. Vestimentifera | 1–976 | Gollner et al. (2007) |
While the present study describes the epizooic meiobenthos from cold seeps, all other meiobenthic seep studies concern the infauna inhabiting seep sediments (Table 4). They range from very shallow sites down to 5000-m depth, come from different geographic regions and a variety of seep types, mostly hydrocarbon gas or gas/oil seeps but also gas/oil/asphalt seeps, gas hydrates, or brine seeps. Most samples were taken from sites covered by bacterial mats or colonized by siboglinid tubeworms, or were obtained from underneath clam beds, but sometimes from sites devoid of any microbial or megafaunal community. In addition to different approaches in extraction techniques and size classes included in the meiofauna fraction, large variations also occur in studies in which only part of the meiobenthic community was analyzed. Some communities encompass the entire permanent (metazoan and protist) and temporary meiobenthos, and some include only parts. Overall, so far no trends in abundance according to depth, geographic regions, seep types, or habitat types are apparent. However, the available data set is rather limited.
Associated epizooic metazoan meiobenthos from seeps (1–81 individuals per 10 cm2) and vents (1–976 individuals per 10 cm2), as well as vent infauna from sediments (1–1075 individuals per 10 cm2), seem overall to be lower in abundance than infaunal meiobenthos from seeps (1–11292 individuals per 10 cm2) (Table 4). Low abundances of seep infauna were detected only in anoxic sediments of the Black Sea and in some samples from a brine seep at East Flower Garden Banks and at the Norwegian Margin (Powell et al., 1983; Sergeeva and Gulin, 2007; Van Gaever et al., 2009). All other infaunal abundances are at least above 100 individuals per 10 cm2 and most exceed 1000 individuals per 10 cm2 (Table 4). The vast majority of epizooic and infaunal vent and seep meiobenthic samples are dominated by nematodes, usually followed by copepods. Other dominant taxa include gnathostomulids and plathelminths in highly sulfidic brine seep samples (Powell et al., 1983) and rotifers in gas hydrate samples (Sommer et al., 2007).
Although in several meiobenthic studies of seeps the nearby non-seep deep-sea samples were found to be lower in abundance than the seep sediment samples (Olu et al., 1997; Robinson et al., 2004; Soltwedel et al., 2005; Van Gaever et al., 2006), our study could not confirm this trend. In general, the abundance of meiobenthos in the deep sea has been found to decrease with depth owing to a decrease in POM flux in addition to sedimentary factors such as calcium carbonate content and sorting (see Soltwedel, 2000). Ranges between 100 and 1000 individuals per 10 cm2 at shallower depths and between 10 and 100 individuals per 10 cm2 at deep sites are considered quite typical (see Giere, 2009). A very large data set from the GOM deep-sea meiobenthos, carried out at between 200- and 3000 m depth, indicated a range between 600 and 9500 individuals per 10 cm2 (Baguley et al., 2006). Calculated from the correlation between abundance and depth, approximately 2500 individuals per 10 cm2 are expected in about 1500-m depth (Baguley et al., 2006). This estimate is much higher than the actual abundances (870–1523 individuals per 10 cm2) in our comparable non-seep sediments at a similar depth of 1450 m. The more puzzling result of this study, however, was the remarkably low abundances at seep sites. The fact that meiobenthos associated with similar foundation species at vents is also low in abundance points to a commonality between seeps and vents, and is in sharp contrast to the high abundance of associated seep and vent macrobenthos. Since in situ primary production obviously fuels the large-sized community, it seems unlikely that meiobenthos is bottom-up controlled. Instead, the interactions with macrobenthos, such as high predation pressure and/or competition, are more likely to be underlying causes, but these hypotheses need to be tested. Also, detailed studies on the species richness and diversity patterns of these epizooic deep-sea communities are currently in progress and will help to elucidate the origin and evolution of seep meiobenthos.
Acknowledgments
This study was supported financially by Austrian Science Foundation Grants FWF P16774-B03 and P20190-B17 to M.B. and the Mineral Management Service Contract #1435-01-05-39187 to TDI-Brooks International. We thank Charles R. Fisher for collaborating with us, and for his continuous support and enthusiasm for including meiofauna in the larger framework of community ecology at seeps. We also thank the captains and crews of the RV Atlantis, NOAA ship Ron Brown, and the expedition leaders and crews of DSV ALVIN and ROV JASON for their expertise and valuable assistance.
References
- Baguley JG, Montagna PA, Hyde LJ, Kalke RD, Rowe GT. Metazoan meiofauna abundance in relation to environmental variables in the northern Gulf of Mexico deep sea. Deep-Sea Research Part I. 2006;53:1344–1362. [Google Scholar]
- Bell SS. Meiofauna–macrofauna interactions in a high salt marsh habitat. Ecological Monographs. 1980;50(4):487–505. [Google Scholar]
- Bergquist DC, Ward T, Cordes EE, McNelis T, Howlett S, Kosoff R, Hourdez S, Carney R, Fisher CR. Community structure of vestimentiferan-generated habitat islands from upper Louisiana slope cold seeps. Journal of Experimental Marine Biology and Ecology. 2003;289:197–222. [Google Scholar]
- Brooks JM, Wiesenburg DA, Roberts HH, Carney RS, MacDonald IR, Fisher CR, Guinasso NL, Sager WW, McDonald SJ, Burke RA, Aharon P, Bright TJ. Salt, seeps and symbiosis in the Gulf of Mexico. EOS. 1990;71:1772–1773. [Google Scholar]
- Bruno JF, Bertness MD. Habitat modification and facilitation in benthic marine communities. In: Bertness MD, Hay ME, Gaines SD, editors. Marine Community Ecology. Sinauer; Sunderland, MA, USA: 2001. pp. 201–218. [Google Scholar]
- Clarke KR, Gorley RN. PRIMER v5: user manual/tutorial. PRIMER-E, Plymouth Primer-E; Plymouth: 2001. [Google Scholar]
- Copley JTP, Flint HC, Ferrero TJ, Van Dover CL. Diversity of meiofauna and free-living nematodes in hydrothermal vent mussel beds on the northern and southern East Pacific Rise. Journal of the Marine Biological Association of the United Kingdom. 2007;87:1141–1152. [Google Scholar]
- Cordes EE, Hourdez S, Predmore BL, Redding ML, Fisher CR. Succession of hydrocarbon seep communities associated with the long-lived foundation species Lamellibrachia luymesi. Marine Ecology Progress Series. 2005;305:17–29. [Google Scholar]
- Cordes EE, Carneya SL, Hourdez S, Carney RS, Brooks JM, Fisher CR. Cold seeps of the deep Gulf of Mexico: community structure and biogeographic comparisons to Atlantic equatorial belt seep communities. Deep-Sea Research I. 2007;54:637–653. [Google Scholar]
- Cordes EE, Becker EL, Hourdez S, Fisher CR. Influence of foundation species, depth, and location on diversity and community composition at Gulf of Mexico lower- slope cold seeps. Deep Sea Research II. 〈doi:10.1016/j.dsr2.2010.05.010〉. [Google Scholar]
- Dinet A, Grassle F, Tunnicliffe V. Premières observations sur la meiofauna des sites hydrothermaux de la dorsale East-Pacifique (Guaymas, 21°N) et de l'Exlorer Ridge. Oceanologica Acta. 1988;85:7–14. [Google Scholar]
- Giere O. Meiobenthology: The Microscopic Motile Fauna of Aquatic Sediments. second ed Springer Verlag; Berlin and Heidelberg, Germany: 2009. p. 527. [Google Scholar]
- Gollner S, Zekely J, Govenar B, Le Bris N, Nemeschkal HL, Fisher CR, Bright M. Tubeworm-associated permanent meiobenthic communities from two chemically different hydrothermal vent sites on the East Pacific Rise. Marine Ecology Progress Series. 2007;337:39–49. [Google Scholar]
- Hacker SD, Gaines SD. Some implications of direct positive interactions for community species diversity. Ecology. 1997;78(7):1990–2003. [Google Scholar]
- Jensen P. Nematode fauna in the sulphide-rich brine seep and adjacent bottoms of the East Flower Garden NW, Gulf of Mexico: IV. Ecological aspects. Marine Biology. 1986;92:489–503. [Google Scholar]
- Jensen P, Aagaard I, Burke RA, Jr., Dando PR, Jorgensen NO, Kuijpers A, Laier T, O'Hara SCM, Schmaljohann R. “Bubbling reefs” in the Kattegat: submarine landscapes of carbonate-cemented rocks support a diverse ecosystem at methane seep. Marine Ecology Progress Series. 1992;83:103–112. [Google Scholar]
- Kamenev GM, Fadeev VI, Selin NI, Tarasov VG. Composition and distribution of macro- and meiobenthos around sub-littoral hydrothermal vents in the Bay of Plenty, New Zealand. New Zealand Journal of Marine and Freshwater Research. 1993;27:407–418. [Google Scholar]
- Kennicutt MC, Brooks JM, Bidigare RR, Fay RR, Wade TL, McDonald TJ. Vent-type taxa in a hydrocarbon seep region on the Louisiana slope. Nature. 1985;317:351–353. [Google Scholar]
- Levin LA. Ecology of cold seep sediments: interaction of fauna with flow, chemistry and microbes. Oceanography and Marine Biology: An Annual Review. 2005;43:1–46. [Google Scholar]
- McIntyre AD, Warwick RM. Meiofauna techniques. In: Holme NA, McIntyre AD, editors. Methods for the Study of Marine Benthos. second ed Blackwell Scientific Publications; Oxford, UK: 1984. pp. 217–244. [Google Scholar]
- Montagna PA, Spies RB. Meiofauna and chlorophyll associated with Beggiatoa mats of a natural submarine petroleum seep. Marine Environmental Research. 1985;46:231–242. [Google Scholar]
- Montagna PA, Bauer JE, Toal J, Hardin D, Spies RB. Temporal variability and the relationship between benthic and meiofaunal and microbial populations of a natural coastal petroleum seep. Journal of Marine Research. 1987;45:761–789. [Google Scholar]
- Nemeschkal HL. Morphometric correlation patterns of adult birds (Fringillidae: Passeriformes and Columbiformes) mirror the expression of developmental control genes. Evolution. 1999;53:899–918. doi: 10.1111/j.1558-5646.1999.tb05384.x. [DOI] [PubMed] [Google Scholar]
- Ólafsson E. Do macrofauna structure meiofauna assemblages in marine soft-bottoms? Vie Milieu. 2003;53:249–265. [Google Scholar]
- Olu K, Lance S, Sibuet M, Henry P, Fiala-Medioni A, Dinet A. Cold seep communities as indicators of fluid expulsion patterns through mud vulcanos seaward of the Barbados accretionary prism. Deep-Sea Research I. 1997;44:881–841. [Google Scholar]
- Palmer MA, Montagna PA, Spies RB, Hardin D. Meiofauna dispersal near natural petroleum seeps in the Santa Barbara Channel: a recolonization experiment. Chemical Pollution. 1988;4:179–189. [Google Scholar]
- Paull CK, Hecker B, Commeau R, Freeman-Lynde RP, Neuman C, Corso WP, Golubic S, Hokk JE, Sikes E, Curray J. Biological communities at the Florida escarpment resemble hydrothermal vent taxa. Science. 1984;226:965–967. doi: 10.1126/science.226.4677.965. [DOI] [PubMed] [Google Scholar]
- Powell EN, Bright TJ, Woods A, Gittings S. Meiofauna and the Thiobios in the East Flower Garden brine seep. Marine Biology. 1983;73:269–283. [Google Scholar]
- Powell EN, Bright TJ, Brooks JM. The effect of sulfide and an increased food supply on the meiofauna and macrofauna at the East Flower Garden brine seep. Helgoländer Meeresuntersuchungen. 1986;40:57–82. [Google Scholar]
- Roberts H, Carney R, Kupchik M, Fisher C, Nelson K, Becker E, Goehring S, Lessard-Pilon S, Telesnicki G, Bernard B, Brooks J, Bright M, Cordes E, Hourdez S, Hunt J, Jr., Shedd W, Boland G, Joye S, Samarkin V, Bernier M, Bowles M, MacDonald I, Niemann H, Peterson C, Potter J. Alvin explores the deep northern Gulf of Mexico slope. EOS Transactions of the American Geophysical Union. 2007;88(35):341–348. [Google Scholar]
- Robinson CA, Bernard JM, Levin LA, Mendoza GF, Blanks JK. Surficial hydrocarbon seep infauna from the Blake Ridge (Atlantic Ocean, 2150 m) and the Gulf of Mexico (690–2240 m) PSZN: Marine Ecology. 2004;25:313–336. [Google Scholar]
- Sen Gupta BK, Smith LE, Lobegeier MK. Attachment of Foraminifera to vestimentiferan tubeworms at cold seeps: refuge from seafloor hypoxia and sulfide toxicity. Marine Micropaleontology. 2007;62:1–6. [Google Scholar]
- Sergeeva NG, Gulin MB. Meiobenthos from an active methane seepage area in the NW Black Sea. Marine Ecology—An Evolutionary Perspective. 2007;28:152–159. [Google Scholar]
- Shirayama Y, Ohta S. Meiofauna in a cold-seep community off Hatsushima, Central Japan. Journal of the Oceanographic Society of Japan. 1990;46:118–124. [Google Scholar]
- Sibuet M, Olu K. Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive margins. Deep-Sea Research II. 1998;45:517–567. [Google Scholar]
- Soltwedel T. Metazoan meiobenthos along continental margins: a review. Progress in Oceanography. 2000;46:59–84. [Google Scholar]
- Soltwedel T, Portnova D, Kolar I, Mokievsky V, Schewe I. The small-sized benthic biota of the Håkon Mosby Mud Volcano (SW Barents Sea slope) Journal of Marine Systems. 2005;55:271–290. [Google Scholar]
- Sommer S, Gutzmann E, Pfannkuche O. Sediments hosting gas hydrates: oasis for metazoan meiofauna. Marine Ecology Progress Series. 2007;337:27–37. [Google Scholar]
- Tarasov VG, Gebruk AV, Shulkin VM, Kamenev GM, Fadeev VI, Koshmynin VN, Malakhov VV, Starynin DA, Obzhirov AI. Effect of shallow-water hydrothermal venting on the biota of Matupi Harbour (Rabaul Caldera, New Britain Island, Papua New Guinea) Continental Shelf Research. 1999;19:79–116. [Google Scholar]
- Thiermann F, Akoumianaki I, Hughes JA, Giere O. Benthic fauna of a shallow-water gaseohydrothermal vent area in the Aegean Sea (Milos, Greece) Marine Biology. 1997;128:149–159. [Google Scholar]
- Tsurumi M, de Graaf RC, Tunnicliffe V. Distributional and biological aspects of copepods at hydrothermal vents on the Juan de Fuca Ridge, north-east Pacific ocean. Journal of the Marine Biolological Association of the United Kingdom. 2003;83:469–477. [Google Scholar]
- Urcuyo IA, Massoth GJ, Julian D, Fisher CR. Habitat, growth and physiological ecology of a basaltic community of Ridgeia piscesae from the Juan de Fuca Ridge. Deep-Sea Research I. 2003;50:763–780. [Google Scholar]
- Van Gaever S, Moodley L, De Beer D, Vanreussel A. Meiobenthos at the Arctic Håkon Mosby Mud Volcano, with a parental-caring nematode thriving in sulphide-rich sediments. Marine Ecology Progress Series. 2006;321:143–155. [Google Scholar]
- Van Gaever S, Olu K, Deryke S, Vanreusel A. Metazoan meiofaunal communities at cold seeps along the Norwegian margin: influence of habitat heterogenity and evidence for connection with shallow-water habitats. Deep-Sea Research I. 2009;56:772–785. [Google Scholar]
- Veit-Koehler G, Laudien J, Knott J, Velez J, Sahade R. Meiobenthic colonization of soft sediments in arctic glacial Kongsfjorden (Svalbard) Journal of Experimental Marine Biology and Ecology. 2008;363:58–65. [Google Scholar]
- Zekely J, van Dover CL, Nemeschkal HL, Bright M. Hydrothermal vent meiobenthos associated with mytilid mussel aggregations from Mid-Atlantic Ridge and the East Pacific Rise. Deep-Sea Research I. 2006;53:1363–1378. [Google Scholar]
- Zeppilli D, Danovaro R. Meiofaunal diversity and assemblage structure in a shallow-water hydrothermal vent in the Pacific Ocean. Aquatic Biology. 2009;5:75–84. [Google Scholar]