Abstract
Anti-idiotypic antibodies (anti-ids) are part of natural immune responses with regulatory capacity. Their effect on an antigen-specific, so-called Ab1 antibody response, is dependent on 1) the original antigen, which they mirror, being Ab2 antibodies, and 2) their isotype. In the case of IgE-mediated allergy, natural anti-ids against allergen-specific IgE represent internal images of allergen molecules. A key biologic feature of allergens is that they can crosslink IgE, expressed by B-lymphocytes or passively bound via high affinity receptors to effector cells, which renders cellular activation. Therefore, the IgE cross linking capability of anti-ids determines whether they dampen or enhance immediate-type hypersensitivity. Correspondingly to classic antiallergen blocking IgG antibodies, anti-ids may also interact with inhibitory FcγRIIb receptors and, thereby, down-regulate TH2-type inflammation. Anti-ids and other B-cell epitope mimetics, like mimotopes and DARPins, represent antigen surrogates, which can be used for vaccination. Intriguingly, they may induce antibody responses without activating potentially proinflammatory, antiallergen T-lymphocytes. Taken together, collective evidence suggests that anti-ids, although representing immunologic classics, are a timeless concept in allergology.
Keywords: anti-idiotypes, allergy, vaccination, FcεRI, FcγRIIb, blocking
INTRODUCTION
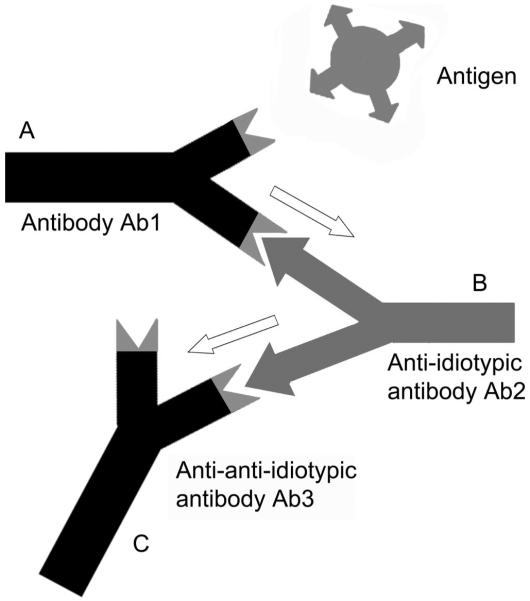
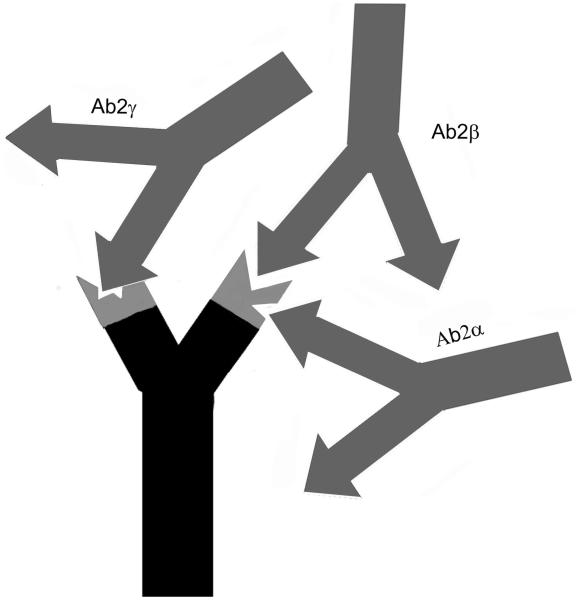
Besides a specific antigen binding site, antibody molecules possess antigenic determinants themselves. When these epitopes are located within the variable region of an antibody, they are designated idiotopes. Hence, each Fab arm of an antibody displays a set of idiotopes, representing epitopes for complementary antibody molecules (Fig. 1), so called anti-idiotypic antibodies (anti-ids). Anti-ids are part of regular immune responses and are thought to result in a web of interacting idiotypes. Classically, Jerne defined the order of an antibody response as follows: Antibody Ab1 is produced in response to an antigen and induces the production of anti-idiotypic Ab2, which can in turn stimulate the synthesis of an anti-(anti-idiotypic) antibody Ab3, and so forth (Fig. 1). Anti-idiotypic antibodies (Ab2) can be classified into several categories according to their fine specificity (Fig. 2): 1) conventional Ab2α antibodies recognize idiotopes of Ab1 outside of its antigen binding site, but still within the variable region; 2) internal image Ab2β antibodies recognize idiotopes directly within the antigen binding site of Ab1 and, therefore, mimic its original antigen epitope like “internal images”; 3) in case the idiotope recognized by Ab2 is not completely overlapping but close to the antigen binding site of Ab1, it may still be able to interfere with the antigen binding and is called Ab2γ. Consequently, binding of antigen by Ab1 is not affected by Ab2α, may be blocked by Ab2γ and is completely blocked by Ab2β.
FIGURE 1.
The anti-idiotypic network amplifies antigenic signals. (A) An antibody Ab1 is produced in response to a specific antigen. (B) With a defined idiotype, Ab1 induces the production of an anti-idiotypic antibody Ab2. This Ab2 may resemble the original antigen as an internal image. (C) Ab2 can stimulate the synthesis of an anti(anti-idiotypic) antibody Ab3 which principally is of the same specificity as Ab1.
FIGURE 2.
Categories of anti-idiotypic antibodies. Ab2α antibodies recognize idiotopes outside the antigen binding site, but still within the variable region of Ab1. Ab2γ recognizes idiotopes close to the antigen binding site of Ab1 and thus may interfere with the antigen binding. Internal image Ab2β binds directly within the antigen binding site.
ANTI-IDS IN EXPERIMENTAL STUDIES: THE BEGINNING
The first experiments regarding anti-ids were animal studies, which were performed to evaluate the effect of anti-ids either on an upcoming or ongoing immune response.
For instance, Cosenza and coworkers designed a mouse study where they used a monoclonal IgA antibody derived from the myeloma cell line TEPC 15, specific for the hapten phosphorylcholine.1 To generate an anti-idiotypic immune response, mice were immunized with the myeloma antibody Ab1 and sera were harvested. Parallel, they immunized another group of BALB/c mice with heat-killed pneumococci to induce a phosphorylcholine-specific Ab1 response. As a proof for the induction of specific antibodies, erythrocytes attached to either pneumococcal C polysaccharide or phosphorylcholine were incubated with the splenocytes from the latter group of mice, resulting in hemolytic plaques because of immune complex-mediated complement activation.2 Importantly, when spleen cells were preincubated with the anti-idiotypic serum generated upon immunization with myeloma IgA, plaque formation was specifically inhibited because of blockage of Ab1. This principle was later also shown for anti-ids directed against Ab1 specific for group A streptococcal antigen.3
However, anti-ids could not only inhibit effector responses, but also prevent de novo induction of specific immune responses in vivo. Naive BALB/c mice were administered the anti-idiotypic serum derived from immunization with TEPC myeloma IgA and subsequently intravenously immunized pneumococci. Antibody responses to phosphorylcholine but not to an irrelevant control allergen were completely blocked. Thus, anti-ids were discussed to be directed also against membrane immunoglobulins of naive antigen-specific B cells. Accordingly, it was hypothesized that membrane and secreted immunoglobulins share similar variable domains even when belonging to different isotypes.1,4 The key result of these studies was, however, that anti-ids act at the cellular level to specifically suppress antibody formation.
Hart and his group generated anti-ids by immunization of rabbits with p-azophenylarsonate-specific mouse antibodies. When naive mice were then injected with the anti-idiotypic rabbit serum and afterward challenged with KLH (keyhole limpet hemocyanin)-linked azophenylarsonate, de novo antibody formation was suppressed up to 97%.5 However, the suppression was not complete. This might have been because of the appearance of antibody responses directed against either the carrier protein KLH or the hapten (p-azophenylarsonate) as such. It seemed probable that the induced antiphenylarsonate antibodies (and their anti-ids), induced by carrier-hapten immunization, possessed idiotypes other than those elicited by either KLH or the hapten. Again, the conclusion of this study was that anti-ids acted suppressive at the cellular level via binding to immunoglobulins expressed by B-lymphocytes.
Furthermore, it became apparent that T cell receptors carry idiotypes similar to those of immunoglobulins, meaning that anti-ids could potentially regulate both, B-lymphocyte and T-lymphocyte function.6 The idiotypic relationship between hypervariable domains of T and B lymphocyte receptors has indeed been repeatedly documented.7-10 Furthermore, it has been shown that anti-ids can mediate cooperation between T and B lymphocytes (Fig. 3C). Furthermore, when B-lymphocytes expressing membrane Ab1 (mAb1) are targeted by Ab2, they internalize and process the anti-id (Fig. 3B) like the original antigen (Fig. 3A). Consequently, an anti-idiotypic peptide of Ab2 is presented in an MHC II context to T cells, again resulting in T cell-help. This would be an additional explanation for how B-lymphocytes recruit T cell help. In the early 1980s, also Blaser et al identified idiotypes on specific T-helper cells similar to the antigen-specific IgG. However, they dissected an antihapten from an anticarrier response and proposed that, in contrast to the above, especially an anticarrier anti-id might block T-helper cell function, rendering reduced antibody production.11
FIGURE 3.
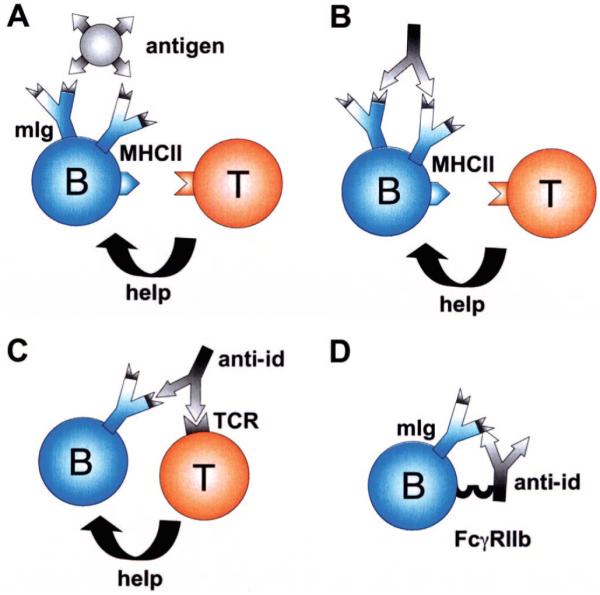
Mechanisms of anti-idiotypic antibodies on B (B) and T cell (T) interaction. (A): B cells recognize antigen by membrane-expressed immunoglobulins (mIg) and receive bystander help from T-helper cells. (B) Alternatively, an anti-id may bind to mIg of a B-cell. This leads to processing and MHCII display of an anti-idiotypic peptide rendering T cell activation and help. (C) Because of idiotypic similarities between B and T cell receptors, an anti-idiotypic antibody may also force cooperation between B and T cells by binding to both. (D) Inhibition of B cell proliferation is achieved by simultaneous binding of the anti-id to its corresponding idiotype on mIg and via its Fcγ domain to FcγRIIb on the B-cell.
Taken together, already at that timepoint, functional similarity between antigen and anti-id could be shown. There was mostly evidence that administration of anti-ids suppressed specific idiotype production.4,12 However, other studies13 suggested that under certain conditions anti-ids could enhance immune responses. For instance, administration of anti-ids of the IgG1 isotype raised in A/J mice against an antistreptococcal antigen A antibody (Ab1) could prime animals for a secondary boost to the antigen. Interestingly, anti-ids of the IgG2 isotype were rather associated with suppressive effects on naive, antigen-sensitive B cells.12 Therefore, besides antigen-specificity of the anti-idiotypic system, also the induced isotype seemed to contribute to the regulatory capacity of anti-ids.
NATURAL ANTI-IDs TO ALLERGEN-SPECIFIC IGE: QUALITATIVE AND QUANTITATIVE STUDIES
In allergy research, Malley et al reported the influence of anti-ids on primary and secondary IgE antibody responses to timothy grass (Phleum pratense) pollen extract.14 An anti-idiotypic antibody was produced to the grass pollen-specific IgE and was administered intraperitoneally followed by immunization of mice with timothy grass pollen extract. This anti-id profoundly prevented IgE production toward the allergen extract, and suppressed even up to 75% of the IgE response to a secondary boost. This suppression persisted for at least 35 days.14
Despite optimistic results such as these, the role of anti-idiotypic immune responses in humans induced during long-time immunotherapy could at that time only be postulated.
Bose and colleagues did the first quantifications of anti-ids in rye grass-allergic patients’ sera.15 Rye I-specific IgG anti-ids were purified from a ryegrass allergic patient who had been previously hyposensitized. In vitro, these IgG specifically blocked the binding of IgE and IgG to group I allergens of rye grass in sera of 20 individual allergics. This result pointed toward the recurrence of idiotypes and a constricted repertoire of human antibodies specific for an allergen. The authors discussed similar idiotopes of IgG and IgE within their antigen binding site. However, in this study it remained unclear whether the same anti-ids were able to inhibit antigen binding to both, IgE and IgG.
A functional distinction between paratope-associated idiotopes versus bystander idiotopes (ie, idiotopes located outside the antigen-binding site) of IgE and IgG was made by the group of Saint-Remy.16 They observed cross-reactivity of house dust mite-specific IgE and IgG, but only with respect to bystander idiotopes.17 They inhibited Ab1-Ab2 interactions with house dust mite allergens and thereby found that the majority of natural anti-ids in mite-allergic patients was of the Ab2β type, thus resembling internal images of the allergen. Still, the authors suggested the epitope-specificity of IgE and IgG to be different.
Interestingly, anti-ids specific for ragweed were also found in nonatopic individuals although to a much lesser extent than in allergic and immunotherapy-treated patients.18-20 The authors proposed that anti-ids detected in nonatopics probably represent a basal level of Ab1/Ab2 responsiveness because of the presence of allergen-specific IgG, but not IgE antibodies, in health.
Anti-id levels in untreated patients sensitive to grass pollen allergen Lol p I and in hyposensitized patients were compared with levels in allergic donors not sensitive to Lol p I, and nonallergic donors by Bose et al using sepharose purification. Via binding to radiolabeled, affinity-purified F(ab’)2 fragments of Lol p I monoclonal antibodies, the amounts of anti-ids and the ratio to allergen-specific antibodies were calculated.21 Again, anti-ids were observed to be present not only in allergic individuals but also in nonallergics, although the latter did not show any detectable IgG or IgE (Ab1) anti-Lol p I. Anti-id levels in allergics were shown to directly correlate with the level of pollen exposure or the hyposensitization status. In the majority of patients treated by immunotherapy an inverse relationship between serum levels of anti-Lol p I IgE and IgG antibodies (Ab1) and of anti-ids (Ab2) could be observed. An initial increase of specific idiotype (Ab1) levels was associated with a drop of the anti-id (Ab2) level. However, like during hyposensitization, the anti-id level later on increased toward a plateau. An increase of Ab2 was also reported by Castracane et al in a ragweed allergic human patient undergoing immunotherapy. They used an antiragweed specific F(ab’)2 fragment for coating in a solid phase assay.19,20 In line with earlier observations by Oudin and Cazenave, it had to be considered that a proportion of the raised anti-ids was most probably not specific to Lol p I, but could have also encountered similar idiotypes derived from different allergens.21,22 Hebert and colleagues measured elevated anti-id levels in sera of hyposensitized patients by the use of 3 different murine monoclonal anti-Lol p I antibodies.23 These murine antibodies were shown to share cross-reactive idiotypes with human anti-Lol p I IgE and IgG antibodies and therefore suitable in this study.24
THE FUNCTIONAL ROLE OF ANTI-IDS IN ALLERGY
Levels of anti-ids were generally found to be higher in healthy individuals than in untreated atopic patients. In the latter, levels can be elevated by immunotherapeutical treatment to the levels found in healthy volunteers.20,24
Valacer and colleagues inhibited IgE binding from allergic patients to ragweed antigen by administration of anti-ids purified from sera of nonallergic individuals. Strikingly, this effect could be achieved although no allergen-specific IgG or IgE could be detected in the sera of the healthy donors. The authors discussed that anti-ids in healthy persons could regulate the IgE response by suppressor mechanisms.25 Indeed, a dramatic suppressive effect of anti-ids on the IgE response was observed in experimental studies. A single anti-id directed against an idiotope of a murine antibody specific for a major epitope of grass pollen allergen Lol p IV down regulated the allergen-specific IgE response profoundly, whereas levels of other isotypes, for example, IgG1 and IgG2 were less affected.26
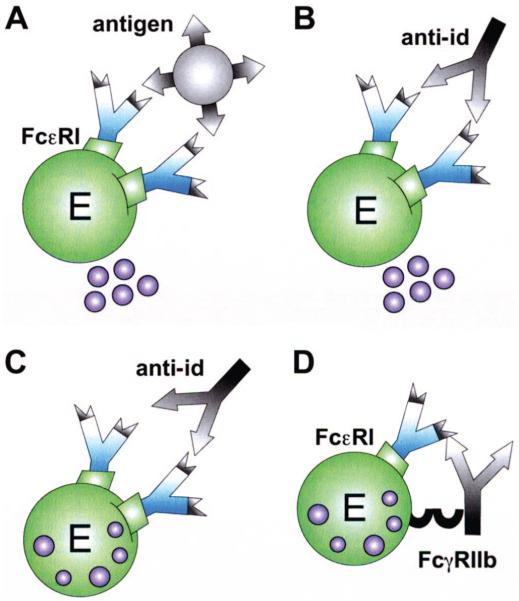
The enthusiasm about potential beneficial effects of anti-ids in the course of immunotherapy was dampened by the sudden awareness that anti-ids against allergen-specific IgE antibodies potentially could also target IgE bound to effector cells. This could lead to either mediator release by IgE-crosslinking (Fig. 4B) like it is the case with the original antigen (Fig. 4A), or to the inhibition of allergen-induced release (Fig. 4D). In fact, Geha et al demonstrated that passive cutaneous administration of an antitetanus toxin anti-id in a human patient elicited positive immediate-type skin reactions.27 In another study, passive cutaneous anaphylaxis experiments in rats were chosen to analyze the in vivo effects of anti-ids on mast cell bound IgE. Anti-ids were raised in syngeneic mice by immunization with dinitrophenol (DNP)-specific mouse monoclonal IgE. The anti-idiotypic serum induced a passive cutaneous anaphylactic reaction in skins of rats sensitized with the DNP-specific mouse IgE. In contrast, the same anti-id did not elicit a reaction in DNP-IgE sensitized rats when they simultaneously were passively administered grass pollen-specific IgE. Thus, a specific anti-id seemed to be more effective in histamine release when less IgE specificities were present in the sensitized individual. From this it was concluded that anti-idiotypic stimulation within a polyclonal response, like in human atopy, would rather inhibit mediator release (Fig. 4C). Furthermore, successful immunotherapy was considered to rely on continuous anti-id targeting of the mast cells rendering internalization and, therefore, depletion of cell bound specific IgE.28
FIGURE 4.
Mechanisms of anti-idiotypic antibodies on effector cells. Mediator release of effector cells (E) is triggered by cross-linkage of 2 receptor-bound IgE either via (A) recognition of the antigen or (B) the anti-id. (C) Effector cell inhibition is achieved by binding of the anti-id to its specific idiotype within a polyclonal response. (D) Cross-linkage of FcεRI and the inhibitory FcγRIIb receptor via an anti-idiotypic IgG antibody also inhibits degranulation.
The capability of human anti-idiotypic IgG purified from mite allergic patients to recognize cell bound IgE idiotypes of other donors, and to induce mediator release of basophils in the absence of allergen was demonstrated.29 Consequently, the possibility that degranulation in response to anti-ids could be a general event in allergy was proposed. However, the degranulating activity of anti-id preparations varied from one patient to the other. Several mechanisms were discussed: First, the precise specificity of anti-ids could play a role, because framework idiotopes could be private and, in contrast, paratope-associated idiotopes of antibodies were found to be more common among different individuals. Hence, the relative proportion of these 2 types of anti-ids could explain the varying degranulating activity of single anti-id preparations on basophils of different donors. Secondly, the authors discussed the repertoire of anti-ids to be dependent on the individually varying antiallergen IgE response, limiting their degranulation capacity when testing basophils of other donors.
Taken together, the impact of anti-ids in the regulation of IgE antibody responses to allergens has at that time not been revealed to the same extent as for infectious diseases or in malignancies.30-32 However, genetic engineering of antibodies opened novel methodological possibilities for precise investigation of the effects of anti-ids in the effector phase of type I allergy. As many studies previously were based on mouse monoclonal anti-ids, it was difficult to extrapolate the findings to the human setting. Therefore, the group around Shakib intended to engineer murine/human chimeric anti-ids and the corresponding antiallergen IgE,33 taking Derp1asa model allergen. Testing these chimerized IgE-anti-idiotypic antibodies on FcγRIIb transfected cells and purified basophils, they found an inhibition of the degranulation through the anti-ids. Interestingly, a novel mechanism of action was proposed, involving FcγRIIb-binding by the Fcγ domain of the anti-idiotypic IgG, and, consequently, cellular inhibition through the associated ITIM-motif of this receptor (Fig. 4D).34 In this context it is of interest that also allergen-specific IgG may recognize allergen already complexed with mIgE on B-lymphocytes or with IgE bound to the high affinity receptor. Upon simultaneous binding to FcγRIIb, allergen-specific IgG has been shown to down regulate not only effector cells, but also B-lymphocytes.35,36 Therefore, we postulate here that similarly anti-ids may exploit this mechanism and act on the B cell level, too (Fig. 3D).
Interestingly, anti-ids may not only dampen the allergic response after allergen immunotherapy, but may also have a protective function in offsprings of allergic mothers. In a mouse model, the maternally derived anti-id against allergen-specific IgE was shown to induce IgE suppression in the offspring which was long-lasting and dose-dependent.37 As molecular modeling ruled out an internal image function of the anti-ids, possibly also here the binding to FcγRIIb could contribute to the protective function.
ANTI-IDS PRIME AND ENHANCE ALLERGEN-SPECIFIC IMMUNITY
It had been suggested that anti-ids function differently in healthy, allergics, or hyposensitized patients. Bose proposed 1986 that anti-ids in allergics might function as “network antigens”, mimicking the allergen and therefore being able to accelerate an immune response.21 In accordance, an anti-idiotypic Ab2β antibody raised by a monoclonal anti-Lol p 1 antibody was able to successfully prime an allergen-specific antibody response, as a subsequent boost injection with the antigen Lol p I resulted in a further increase of specific IgE and IgG antibody levels.38 The administration of anti-ids augmented both, idiotype specific and total antibody responses against the allergen,38 a result that had been predicted before by studies outside the field of allergy.39-41 However, the exact mechanism for the enhancing effect of an anti-id resembling only a single epitope, remained unclear. One may speculate that upon the administration of Ab2 the induced Ab3 facilitates subsequent antigen presentation, leading to epitope spreading. Especially the IgM isotype was considered to potentiate the immune response against low doses of antigen via the idiotypic network. A further explanation could be that immune complexes formed after antigen immunization directly stimulate antigen-specific B cells.42
VACCINATION WITH ANTI-IDS TO CONTROL TOTAL IGE LEVELS
The immunotherapeutical potency of anti-ids for allergic patients was not only investigated with respect to the allergen-specific IgE response, but also regarding total IgE levels in allergics. It had been shown previously that natural anti-IgE isotype antibodies exist, which, depending on the epitope specificity of the Fcε domain, may down-regulate or enhance the effector function of IgE.43 Stadler et al aimed to generate nonanaphylactogenic IgG antibodies through active immunization with an anti-idiotypic antibody as an antigen surrogate. When they used a beneficial, nonanaphylactogenic anti-IgE antibody (termed BSW17) as a template, anti-ids could be generated from a combinatorial phage library displaying the Fab repertoire of a grass pollen allergic patient.44 According to the principle of molecular mimicry, these anti-ids resembled an epitope of the constant domain of IgE and actively induced BSW17-like anti-IgE specificities in mice.45 Therefore, as an alternative to passive anti-IgE therapy with, for example, omalizumab, vaccination with anti-idiotypic molecules for active induction of a protective anti-IgE response was envisaged by the authors.
VACCINATION WITH ANTI-IDS TO CONTROL ALLERGEN-SPECIFIC IGE LEVELS
In our own study, we generated anti-idiotypic Fab antibody fragments for IgE, which was specifically directed against timothy grass pollen allergen Phl p 5.46 The phage library containing the repertoire of a grass pollen sensitized individual, which is described above,47 was this time used for screening with allergen-specific IgE. Several high-binding Fab clones with mimicry potential to the allergen’s IgE epitope could be isolated. Upon immunization with clones in mice, antiallergen IgG could be induced via molecular mimicry. Furthermore, these anti-idiotypic Fabs resembled naturally occurring IgE epitopes of the allergen as observed by sequence analysis and molecular modeling.46 More recently, we applied one of the selected anti-idiotypic clones in a memory mouse model of acute allergic asthma to test whether it would be suitable for immunotherapy. According to a protocol by Mojtabavi et al48 acute allergic asthma was induced by injecting recombinant Phl p 5 intraperitoneally, followed by aerosol challenges with this allergen. Subsequently, groups of mice with acute asthma were vaccinated with 1) the anti-idiotypic Fab fragment, or 2) control antigen KLH before reinduction of acute asthma by an additional allergen aerosol challenge.
Whereas the IgE and IgG1 antibody levels of all groups remained unchanged during treatments, the extent of acute eosinophilic inflammation upon rechallenge with aerosolized allergen was much lower in the Fab-treated group compared with the untreated asthmatic and the nonasthmatic groups (data not shown). As the specific IgE levels were not affected, it is tempting to speculate that the reason for this profound anti-inflammatory property might be (similarly as with peptide mimotopes; see below) the absence of allergen-specific T cell epitopes in the anti-ids.49 Surface plasmon resonance studies indicated that high affinity polyclonal IgE may exhibit a binding behavior similar to monoclonal antibodies, indicating that each Phl p 5 allergen might only harbor a low number of IgE epitopes.50 Consequently, the anti-idiotypic strategy is expected to down-regulate IgE specificities being relevant and occurring frequently in the grass pollen allergic population.
DISTINCT TYPES OF ANTI-ID LIKE MOLECULES
Although antibodies are widely used for therapeutic applications, they have some potential drawbacks in large scale production including low expression yields and aggregation tendency. In this context, phage display technology does not only enable the selection of anti-ids, but also of peptide molecules, which structurally mimic B cell epitopes and therefore are called mimotopes.51 Several allergen mimotopes were already defined by other working groups and ourselves, like for the panallergen profilin,52 the major fish allergen parvalbumin,53 birch pollen allergens,54 grass pollen allergens,46,55 or house dust mite allergens.56 Mimotopes lack allergen-specific T-cell epitopes and have been demonstrated to induce blocking IgG antibodies without stimulation of allergen-specific T-helper cells.49,54 Furthermore, when therapeutically applied in a murine model of allergic asthma, they could, like reported here for an anti-idiotypic Fab fragment, prevent acute lung hypersensitivity and inflammation upon allergen rechallenge.57
Still, to achieve good quality of the immune response towards the relatively short peptide mimotopes, bystander T-cell help was needed. The immunogenic carrier for the mimotopes was in that case KLH providing bystander T-helper cell epitopes. Alternatively, tetanus toxoid (TT) could also be chosen as an immunostimulatory agent because of its promiscuous T-cell epitopes.58
Another possibility to enhance antigenic density is the attachment of linear peptides to tyrosine backbones. The so called multiple antigenic peptides (MAPs) can be synthesized in a straightforward manner and allow a dense display, most often as tetra- or octameric constructs.59 Taken up by antigen presenting cells (APC), they are processed to T-cell epitopes and can activate T-cells too. However, controversial studies have raised the question whether or not the addition of a promiscuous T-helper epitope to a MAP is needed for achieving a sufficient immune response upon MAP-vaccination.60
Another option to overcome the limitations of antibody libraries are designed ankyrin repeat proteins (DARPins). These DARPin libraries are a source of β-hairpin structures, which mimic naturally occurring repeat proteins, and play an important role in innate immunity61; reviewed in.62 In a study by Vogel et al, DARPin libraries were used to isolate ligands against the variable region of the nonanaphylactogenic anti-IgE antibody BSW17. Confirming the high specificity of the identified anti-idiotypic DARPins, they successfully prevented binding of BSW17 to IgE-sensitized rat basophils expressing human FcεRIα.63 Recent molecular studies indicated that hairpin scaffold libraries render mimetics, which closely mimic the crystal structure of a native protein.64 We are convinced that these novel developments will open up new avenues for anti-idiotypic strategies in allergy research.
SYNOPSIS
From several studies in the past it has emerged that natural anti-ids take part in the regulation of the immune response including immediate-type hypersensitivity. Obviously, each antibody response to an allergen is accompanied by the production of anti-idiotypic antibodies and anti-antibodies, together composing an idiotypic regulatory network. As predicted by Jerne, several studies could indeed show on the molecular level that anti-ids function as internal images of allergens. Thus, it may be hypothesized that anti-ids for allergen-specific IgE potentially nourish IgE memory in periods of allergen absence, like for instance outside the pollen season. In experimental studies, anti-ids have been able to specifically down-regulate, or vice versa, boost allergen-specific immune responses. This can on the one hand be because of the fact that they act on B- and T-lymphocyte antigen receptors in either a nonproductive (monovalent) manner or in a productive way by cross linking B cell receptors, or crosslinking B with T-lymphocytes, thereby forcing cellular crosstalk. Furthermore, anti-ids have been found in nonallergic individuals, pointing toward a protective regulatory mechanism.
Despite controversial in vitro results of the influence of anti-ids on the allergic response, levels of anti-ids usually get elevated during the course of specific immunotherapy (SIT). Therefore, anti-ids that mimic the relevant structural epitopes of an allergen are possibly attractive candidates for immunotherapy. For vaccination, mimotopes or DARPins represent novel anti-idiotypic alternatives. We propose that with anti-idiotypic tools, being synthetic or generated from the patient’s antibody repertoire, immunologic disorders like allergies could be manipulated.
Acknowledgments
This work was supported by the SFB grant F1808-B13 and the Hertha Firnberg stipend T283-B13, both of the Austrian Science Fund (FWF).
REFERENCES
- 1.Cosenza H, Kohler H. Specific inhibition of plaque formation to phosphorylcholine by antibody against antibody. Science. 1972;176:1027–1029. doi: 10.1126/science.176.4038.1027. [DOI] [PubMed] [Google Scholar]
- 2.Jerne NK, Nordin AA. Plaque formation in agar by single antibody-producing cells. Science. 1963;140:405. [PubMed] [Google Scholar]
- 3.Eichmann K, Falk I, Rajewsky K. Recognition of idiotypes in lymphocyte interactions. II. Antigen-independent cooperation between T and B lymphocytes that possess similar and complementary idiotypes. Eur J Immunol. 1978;8:853–857. doi: 10.1002/eji.1830081206. [DOI] [PubMed] [Google Scholar]
- 4.Cosenza H, Kohler H. Specific suppression of the antibody response by antibodies to receptors. Proc Natl Acad Sci U S A. 1972;69:2701–2705. doi: 10.1073/pnas.69.9.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart DA, Wang AL, Pawlak LL, Nisonoff A. Suppression of idiotypic specificities in adult mice by administration of antiidiotypic antibody. J Exp Med. 1972;135:1293–1300. doi: 10.1084/jem.135.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binz H, Wigzell H. Shared idiotypic determinants on B and T lymphocytes reactive against the same antigenic determinants. II. Determination of frequency and characteristics of idiotypic T and B lymphocytes in normal rats using direct visualization. J Exp Med. 1975;142:1218–1230. doi: 10.1084/jem.142.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach BA, Greene MI, Benacerraf B, Nisonoff A. Mechanisms of regulation of cell-mediated immunity. IV. Azobenzenearsonate-specific suppressor factor (s) bear cross-reactive idiotypic determinants the expression of which is linked to the heavy-chain allotype linkage group of genes. J Exp Med. 1979;149:1084–1098. doi: 10.1084/jem.149.5.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozes E, Haimovich J. Antigen specific T-cell helper factor cross reacts idiotypically with antibodies of the same specificity. Nature. 1979;278:56–57. doi: 10.1038/278056a0. [DOI] [PubMed] [Google Scholar]
- 9.Binz H, Wigzell H. Antigen-binding, idiotypic T-lymphocyte receptors. Contemp Top Immunobiol. 1977;7:113–177. doi: 10.1007/978-1-4684-3054-7_4. [DOI] [PubMed] [Google Scholar]
- 10.Rajewsky K, Eichmann K. Antigen receptors of T helper cells. Contemp Top Immunobiol. 1977;7:69–112. doi: 10.1007/978-1-4684-3054-7_3. [DOI] [PubMed] [Google Scholar]
- 11.Blaser K, Nakagawa T, de Weck AL. Suppression of anti-hapten IgE and IgG antibody responses by isologous anti-idiotypic antibodies against purified anti-carrier (ovalbumin) antibodies in BALB/c mice. J Immunol. 1981;126:1180–1184. [PubMed] [Google Scholar]
- 12.Eichmann K. Idiotype suppression. I. Influence of the dose and of the effector functions of anti-idiotypic antibody on the production of an idiotype. Eur J Immunol. 1974;4:296–302. doi: 10.1002/eji.1830040413. [DOI] [PubMed] [Google Scholar]
- 13.Eichmann K, Rajewsky K. Induction of T and B cell immunity by anti-idiotypic antibody. Eur J Immunol. 1975;5:661–666. doi: 10.1002/eji.1830051002. [DOI] [PubMed] [Google Scholar]
- 14.Malley A, Dresser DW. Anti-idiotype regulation of timothy grass pollen IgE antibody formation. I. In vivo induction of suppressor T cells. Immunology. 1982;46:653–659. [PMC free article] [PubMed] [Google Scholar]
- 15.Bose R, Marsh DG, Duchateau J, Sehon AH, Delespesse G. Demonstration of auto-anti-idiotypic antibody cross-reacting with public idiotypic determinants in the serum of rye-sensitive allergic patients. J Immunol. 1984;133:2474–2478. [PubMed] [Google Scholar]
- 16.Saint-Remy JM, Lebrun PM, Lebecque SJ, Masson PL. The human immune response against major allergens from house dust mite, Dermatophagoides pteronyssinus. II. Idiotypic cross-reactions of allergen-specific antibodies. Eur J Immunol. 1986;16:575–580. doi: 10.1002/eji.1830160520. [DOI] [PubMed] [Google Scholar]
- 17.Saint-Remy JM, Lebecque SJ, Lebrun PM, Jacquemin MG. Human immune response to allergens of house dust mite, Dermatophagoides pteronyssinus. IV. Occurrence of natural autologous anti-idiotypic antibodies. Eur J Immunol. 1988;18:83–87. doi: 10.1002/eji.1830180113. [DOI] [PubMed] [Google Scholar]
- 18.Castracane JM, Hall TJ, Tamir R, Rocklin RE. Anti-ragweed-specific anti-idiotypic antibodies in nonatopic and atopic subjects. Trans Assoc Am Physicians. 1985;98:123–130. [PubMed] [Google Scholar]
- 19.Castracane JM, Rocklin RE. Detection of human auto-anti-idiotypic antibodies (Ab2). I. Isolation and characterization of Ab2 in the serum of a ragweed immunotherapy-treated patient. Int Arch Allergy Appl Immunol. 1988;86:288–294. doi: 10.1159/000234586. [DOI] [PubMed] [Google Scholar]
- 20.Castracane JM, Rocklin RE. Detection of human auto-anti-idiotypic antibodies (Ab2). II. Generation of Ab2 in atopic patients undergoing allergen immunotherapy. Int Arch Allergy Appl Immunol. 1988;86:295–302. doi: 10.1159/000234587. [DOI] [PubMed] [Google Scholar]
- 21.Bose R, Marsh DG, Delespesse G. Anti-idiotypes to anti-Lolp I (Rye) antibodies in allergic and non-allergic individuals. Influence of immunotherapy. Clin Exp Immunol. 1986;66:231–240. [PMC free article] [PubMed] [Google Scholar]
- 22.Oudin J, Cazenave PA. Similar idiotypic specificities in immunoglobulin fractions with different antibody functions or even without detectable antibody function. Proc Natl Acad Sci U S A. 1971;68:2616–2620. doi: 10.1073/pnas.68.10.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mourad W, Hebert J. Human IgG and murine monoclonal antibodies share common idiotopes as determined by competitive binding to polystyrene and nitrocellulose-bound antigens. Immunol Invest. 1986;15:801–811. doi: 10.3109/08820138609036364. [DOI] [PubMed] [Google Scholar]
- 24.Hebert J, Bernier D, Mourad W. Detection of auto-anti-idiotypic antibodies to Lol p I (rye I) IgE antibodies in human sera by the use of murine idiotypes: levels in atopic and non-atopic subjects and effects of immunotherapy. Clin Exp Immunol. 1990;80:413–419. doi: 10.1111/j.1365-2249.1990.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valacer DJ, O’Reilly ME, Ilowite NT, Bonagura VR. Identification of anti-idiotypic antibodies in the sera of ryegrass-allergic and nonallergic individuals. J Allergy Clin Immunol. 1991;88:349–355. doi: 10.1016/0091-6749(91)90096-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhou EM, Kisil FT. Regulation of levels of serum antibodies to ryegrass pollen allergen Lol pIV by an internal image anti-idiotypic monoclonal antibody. Immunology. 1995;84:343–349. [PMC free article] [PubMed] [Google Scholar]
- 27.Geha RS. Elicitation of the prausnitz-kustner reaction by antiidiotypic antibodies. J Clin Invest. 1982;69:735–741. doi: 10.1172/JCI110511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wheeler AW, Deards MJ, Hickman BE, Spackman VM, Johansson SG. Reactivity of mast-cell-bound IgE idiotypes with anti-idiotypic antibody: mediator release or inhibition of antigen-induced mediator release? Int Arch Allergy Appl Immunol. 1990;91:192–197. doi: 10.1159/000235114. [DOI] [PubMed] [Google Scholar]
- 29.Weyer A, Le Mao J, Etievant M, David B, Guinnepain MT, Saint-Remy JM. Human auto-anti-idiotypic antibodies to mite-specific IgE can degranulate human basophils in vitro. Clin Exp Allergy. 1995;25:935–941. doi: 10.1111/j.1365-2222.1995.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhou EM, Dreesman GR, Kennedy RC. Anti-idiotypic antibodies: a new generation of vaccines against infectious agents. Microbiol Sci. 1987;4:36–40. [PubMed] [Google Scholar]
- 31.Oosterlaken TA, Harmsen M, Jhagjhoor-Singh SS, Ekstijn GL, Kraaijeveld CA, Snippe H. A protective monoclonal anti-idiotypic vaccine to lethal Semliki Forest virus infection in BALB/c mice. J Virol. 1991;65:98–102. doi: 10.1128/jvi.65.1.98-102.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su S, Ward MM, Apicella MA, Ward RE. A nontoxic, idiotope vaccine against gram-negative bacterial infections. J Immunol. 1992;148:234–238. [PubMed] [Google Scholar]
- 33.Furtado PB, McElveen JE, Gough L, Armour KL, Clark MR, Sewell HF, Shakib F. The production and characterisation of a chimaeric human IgE antibody, recognising the major mite allergen Der p 1, and its chimaeric human IgG1 anti-idiotype. Mol Pathol. 2002;55:315–324. doi: 10.1136/mp.55.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wigginton SJ, Furtado PB, Armour KL, Clark MR, Robins A, et al. An immunoglobulin E-reactive chimeric human immunoglobulin G1 anti-idiotype inhibits basophil degranulation through cross-linking of FcεRI with FcγRIIb. Clin Exp Allergy. 2008;38:313–319. doi: 10.1111/j.1365-2222.2007.02896.x. [DOI] [PubMed] [Google Scholar]
- 35.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, et al. FcγRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007;8:419–429. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 36.Ravetch JV, Nussenzweig M. Killing some to make way for others. Nat Immunol. 2007;8:337–339. doi: 10.1038/ni0407-337. [DOI] [PubMed] [Google Scholar]
- 37.Tanasa RI, Trad A, Lange H, Grotzinger J, Lemke H. Allergen IgE-isotype-specific suppression by maternally derived monoclonal anti-IgG-idiotype. Allergy. 2010;65:16–23. doi: 10.1111/j.1398-9995.2009.02104.x. [DOI] [PubMed] [Google Scholar]
- 38.Boutin Y, Hebert J. Modulation of immune response to Lol p I by pretreatment with anti-idiotypic antibody is not restricted to the idiotypic expression. Clin Exp Immunol. 1994;96:350–355. doi: 10.1111/j.1365-2249.1994.tb06566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sachs DH, El-Gamil M, Miller G. Genetic control of the immune response to staphylococcal nuclease. XI. Effects of in vivo administration of anti-idiotypic antibodies. Eur J Immunol. 1981;11:509–516. doi: 10.1002/eji.1830110613. [DOI] [PubMed] [Google Scholar]
- 40.Bluestone JA, Epstein SL, Ozato K, Sharrow SO, Sachs DH. Anti-idiotypes to monoclonal anti-H-2 antibodies. II. Expression of anti-H-2Kk idiotypes on antibodies induced by anti-idiotype or H-2Kk antigen. J Exp Med. 1981;154:1305–1318. doi: 10.1084/jem.154.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy RC, Adler-Storthz K, Henkel RD, Sanchez Y, Melnick JL, Dreesman GR. Immune response to hepatitis B surface antigen: enhancement by prior injection of antibodies to the idiotype. Science. 1983;221:853–855. doi: 10.1126/science.6603657. [DOI] [PubMed] [Google Scholar]
- 42.Heyman B. The immune complex: possible ways of regulating the antibody response. Immunol Today. 1990;11:310–313. doi: 10.1016/0167-5699(90)90126-t. [DOI] [PubMed] [Google Scholar]
- 43.Stadler BM, Rudolf MP, Zurcher AW, Miescher S, Vogel M. Anti-IgE in allergic sensitization. Immunol Cell Biol. 1996;74:195–200. doi: 10.1038/icb.1996.27. [DOI] [PubMed] [Google Scholar]
- 44.Stadler BM, Zurcher AW, Miescher S, Kricek F, Vogel M. Mimotope and anti-idiotypic vaccines to induce an anti-IgE response. Int Arch Allergy Immunol. 1999;118:119–121. doi: 10.1159/000024045. [DOI] [PubMed] [Google Scholar]
- 45.Vogel M, Lai L, Rudolf MP, Curcio-Vonlanthen V, Miescher S, Stadler BM. Cross reactive anti-tetanus and anti-melittin Fab fragments by phage display after tetanus toxoid immunisation. Hum Antibodies Hybridomas. 1996;7:11–20. [PubMed] [Google Scholar]
- 46.Hantusch B, Knittelfelder R, Wallmann J, Krieger S, Szalai K, et al. Internal images: human anti-idiotypic Fab antibodies mimic the IgE epitopes of grass pollen allergen Phl p 5a. Mol Immunol. 2006;43:2180–2187. doi: 10.1016/j.molimm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Vogel M, Miescher S, Biaggi C, Stadler BM. Human anti-IgE antibodies by repertoire cloning. Eur J Immunol. 1994;24:1200–1207. doi: 10.1002/eji.1830240529. [DOI] [PubMed] [Google Scholar]
- 48.Mojtabavi N, Dekan G, Stingl G, Epstein MM. Long-lived Th2 memory in experimental allergic asthma. J Immunol. 2002;169:4788–4796. doi: 10.4049/jimmunol.169.9.4788. [DOI] [PubMed] [Google Scholar]
- 49.Wallmann J, Proell M, Stepanoska T, Hantusch B, Pali-Scholl I, et al. A mimotope gene encoding the major IgE epitope of allergen Phl p 5 for epitope-specific immunization. Immunol Lett. 2009;122:68–75. doi: 10.1016/j.imlet.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hantusch B, Scholl I, Harwanegg C, Krieger S, Becker WM, et al. Affinity determinations of purified IgE and IgG antibodies against the major pollen allergens Phl p 5a and Bet v 1a: discrepancy between IgE and IgG binding strength. Immunol Lett. 2005;97:81–89. doi: 10.1016/j.imlet.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Geysen HM, Rodda SJ, Mason TJ. The delineation of peptides able to mimic assembled epitopes. Ciba Found Symp. 1986;119:130–149. doi: 10.1002/9780470513286.ch8. [DOI] [PubMed] [Google Scholar]
- 52.Leitner A, Vogel M, Radauer C, Breiteneder H, Stadler BM, et al. A mimotope defined by phage display inhibits IgE binding to the plant panallergen profilin. Eur J Immunol. 1998;28:2921–2927. doi: 10.1002/(SICI)1521-4141(199809)28:09<2921::AID-IMMU2921>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 53.Untersmayr E, Szalai K, Riemer AB, Hemmer W, Swoboda I, et al. Mimotopes identify conformational epitopes on parvalbumin, the major fish allergen. Mol Immunol. 2006;43:1454–1461. doi: 10.1016/j.molimm.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 54.Scholl I, Wiedermann U, Forster-Waldl E, Ganglberger E, Baier K, et al. Phage-displayed Bet mim 1, a mimotope of the major birch pollen allergen Bet v 1, induces B cell responses to the natural antigen using bystander T cell help. Clin Exp Allergy. 2002;32:1583–1588. doi: 10.1046/j.1365-2222.2002.01527.x. [DOI] [PubMed] [Google Scholar]
- 55.Hantusch B, Krieger S, Untersmayr E, Scholl I, Knittelfelder R, et al. Mapping of conformational IgE epitopes on Phl p 5a by using mimotopes from a phage display library. J Allergy Clin Immunol. 2004;114:1294–1300. doi: 10.1016/j.jaci.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 56.Szalai K, Fuhrmann J, Pavkov T, Scheidl M, Wallmann J, et al. Mimotopes identify conformational B-cell epitopes on the two major house dust mite allergens Derp1and Derp2. Mol Immunol. 2008;45:1308–1317. doi: 10.1016/j.molimm.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 57.Wallmann J, Epstein MM, Singh P, Brunner R, Szalai K, et al. Mimotope vaccination for therapy of allergic asthma: anti-inflammatory effects in a mouse model. Clin Exp Allergy. 2009 doi: 10.1111/j.1365-2222.2009.03392.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho PC, Mutch DA, Winkel KD, Saul AJ, Jones GL, Doran TJ, Rzepczyk CM. Identification of two promiscuous T cell epitopes from tetanus toxin. Eur J Immunol. 1990;20:477–483. doi: 10.1002/eji.1830200304. [DOI] [PubMed] [Google Scholar]
- 59.Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olszewska W, Obeid OE, Steward MW. Protection against measles virus-induced encephalitis by anti-mimotope antibodies: the role of antibody affinity. Virology. 2000;272:98–105. doi: 10.1006/viro.2000.0285. [DOI] [PubMed] [Google Scholar]
- 61.Forrer P, Stumpp MT, Binz HK, Pluckthun A. A novel strategy to design binding molecules harnessing the modular nature of repeat proteins. FEBS Lett. 2003;539:2–6. doi: 10.1016/s0014-5793(03)00177-7. [DOI] [PubMed] [Google Scholar]
- 62.Stumpp MT, Binz HK, Amstutz P. DARPins: a new generation of protein therapeutics. Drug Discov Today. 2008;13:695–701. doi: 10.1016/j.drudis.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 63.Vogel M, Keller-Gautschi E, Baumann MJ, Amstutz P, Ruf C, Kricek F, Stadler BM. Designed ankyrin repeat proteins as anti-idiotypic-binding molecules. Ann N Y Acad Sci. 2007;1109:9–18. doi: 10.1196/annals.1398.002. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura GR, Starovasnik MA, Reynolds ME, Lowman HB. A novel family of hairpin peptides that inhibit IgE activity by binding to the high-affinity IgE receptor. Biochemistry. 2001;40:9828–9835. doi: 10.1021/bi0109360. [DOI] [PubMed] [Google Scholar]