Abstract
Background:
Impairments in inhibitory function have been found in studies of cognition in schizophrenia. These have been linked to a failure to adequately maintain the task demands in working memory. As response inhibition is known to occur in both voluntary and involuntary processes, an important question is whether both aspects of response inhibition are specifically impaired in people with schizophrenia.
Methods:
The subjects were 33 patients presenting with a first episode of psychosis (27 with schizophrenia and 6 with schizoaffective disorder) and 24 healthy controls. We administered two motor response tasks; voluntary response inhibition was indexed by the stop signal task (Logan and Cowan, 1984) and involuntary response inhibition by the masked priming task (Eimer and Schlaghecken, 1998). We also administered neuropsychological measures of IQ and executive function to explore their associations with response inhibition.
Results:
Patients with schizophrenia compared to healthy controls showed significantly increased duration of the voluntary response inhibition process, as indexed by stop signal reaction time. In contrast, there were no group differences on the pattern of priming on the masked priming task, indicative of intact involuntary response inhibition. Neuropsychological measures revealed that voluntary response inhibition is not necessarily dependent on working memory.
Conclusions:
These data provide evidence for a specific impairment of voluntary response inhibition in schizophrenia.
Keywords: schizophrenia, inhibition, stop signal task, masked priming, working memory
A fundamental problem in schizophrenia research is to determine which of the many cognitive deficits apparent on neuropsychological testing reflect abnormalities in specific cognitive processes rather than general performance decrements (see Joyce and Huddy, 2004; MacDonald and Carter, 2002). This is important for the future identification of dysfunctional neural processes and their genetic underpinnings as aetiological factors in the development of the disorder (Gottesman and Gould, 2003).
Patients with schizophrenia show pronounced deficits in executive function at all stages of the illness (e.g. Hutton et al. 1998; Pantelis et al. 1997; see also Elvevag and Goldberg, 2000; Neuchterlein et al. 2004 for reviews). The term executive function encompasses several more discrete cognitive functions, notably working memory and response inhibition, which are thought to interact to optimise performance under changing or novel conditions. It is well established that patients with schizophrenia have impaired working memory which holds true when the study design or the data analysis controls for general performance decrements (see Barch, 2006). More recently, the working memory deficit has taken on added importance in also understanding inhibitory task deficits in schizophrenia. In the the Stroop task, the apparent inhibitory effects have been attributed to a failure of context processing (Barch et al., 2004); working memory impairments have also been linked to performance on the antisaccade task (Hutton et al. 2002, Hutton et al. 2004) and the Wisconsin Card Sorting Task (Hartman et al. 2003; Li, 2004). These studies have highlighted the importance of maintaining complex task demands in working memory whilst performing tasks thought to index the inhibitory dimension of executive function.
The stop signal task provides a means to assess the specificity of inhibitory deficits in schizophrenia as inhibitory performance is clearly operationalised as a volitional act to prevent responding. This is a choice reaction time task in which the goal is to perform a speeded response at the onset of a GO stimulus, which is typically a directional arrow. On a minority of trials the procedure is interrupted by a signal to stop after the onset of the GO stimulus and the subject must attempt to prevent any response. Logan and Cowan (1984) have described a ‘race’ model of stopping based on the assumption that two processes are operating – a GO process initiating action and a STOP process preventing the action – and that success or failure to stop is determined by whether the GO or STOP process completes first. Within this model the speed of the STOP process may be determined and this is commonly taken as a good indicator of stopping proficiency. The speed of the STOP process can be estimated by manipulating the delay between the onset of the GO stimulus and the STOP signal – termed the stop signal delay (SSD). One method for manipulating SSD is to actively move the STOP signal closer or further from the GO stimulus, according to the subject's performance. If the subject fails to stop the delay is decreased on the subsequent trial and vice versa for successful stops. By employing this ‘dynamic tracking’ method, performance is intentionally stabilised at a critical criterion set at 50%, when it can be assumed that the STOP and GO processes are completing at about the same time, i.e. the race between GO and STOP processes is tied. The stop signal reaction time (SSRT) is then estimated by subtracting the SSD at this point from the GO reaction time (Band et al. 2003). Thus the SSRT is never directly observed; it can only be measured via manipulation of the subject's stopping proficiency so that stopping occurs at chance levels.
Relatively few studies have examined stopping performance in schizophrenia and the findings have been inconsistent. Rubia et al. (2001), using a simplified version of the stop signal task in an fMRI paradigm, found no performance decrements in patients with schizophrenia. Badcock et al. (2002), using a version of the task that featured a series of fixed stop signal delays, found that patients with schizophrenia failed to trigger an inhibitory response as often as controls but the stopping times of triggered responses were the same as controls. A second study using this method found the opposite pattern with intact triggering of responses and slower stopping times (Enticott et al. 2007). Only one study has used the more accurate dynamic tracking method (Bellgrove et al. 2006) and this was in adolescent-onset schizophrenia. No differences were found between controls and the whole group of patients but SSRT was slower in a subgroup with the undifferentiated subtype of schizophrenia, and then only in left handed responses. These variable findings contrast with those from other clinical groups tested on the stop signal task. For example, in a review of the area, Aron and Poldrack (2005) report consistent evidence for abnormal performance in attention deficit hyperactivity disorder (ADHD).
The stop signal task indexes voluntary motor inhibition via systematic manipulation of overt auditory cues - the subject is explicitly instructed to inhibit responding. Motor inhibition that occurs at an unconscious level has also been described by Eimer and Schlaghecken (2003) using a masked priming task. Like the stop-signal task, the masked priming task measures choice reaction times of a left or right response to directional arrows. However response inhibition is demonstrated via covert priming instead of overt action inhibition. Thus there is no stop signal tone and the subject responds to all stimuli, with both prime and target GO stimuli being directional arrows. A mask immediately follows this prime preventing any conscious processing of the direction of the arrow. This is important because although the primes are intended to produce partial response activation, the absence of conscious processing prevents an overt response. On different trials, the prime and GO arrows are either compatible or incompatible with each other and are presented with differing intervening delays. Compared with incompatible trials, performance benefits are found on compatible trials for a 0 ms delay between prime and target, the positive compatibility effect, but performance costs are found when the delay between prime and target is 96 ms or longer; the negative compatibility effect (NCE). To elucidate this effect, Eimer and Schlaghecken (1998) recorded the lateralised readiness potential (LRP) during responses. They found that the masked prime partially activated the corresponding response as predicted, but crucially this was followed by an inhibition of the response. These data suggest that a self-inhibition process automatically counters the covert response activation of the prime. When the delay is short this process does not have time to operate leading to speeded responses to the target when prime and target are in the same direction; but when the delay is longer, this process does have time to operate leading to performance costs on compatible trials.
There have been no studies of unconscious response inhibition of this type in schizophrenia. However, it is becoming increasingly apparent that preconscious information processing can be disrupted in schizophrenia when other methods are used. Initial event related potential (ERP) findings in both auditory (Shelley et al. 1991) and visual modalities (Butler et al. 2001) have been well replicated. Other findings indicate impairment in the preconscious inhibition of irrelevant sensory information as exemplified by P50 gating (Adler et al. 1982) and prepulse inhibition paradigms (Braff et al. 1978).
In the present study we sought to further clarify the nature of inhibitory function in schizophrenia by administering procedurally similar tasks of inhibition, stop signal and masked priming, in a group of adults with verified schizophrenia or schizoaffective disorder following a first episode of psychosis. This enabled a direct comparison between the inhibitory control of the same manual response to a directional arrow occurring at the voluntary conscious level and at the involuntary unconscious level. Other neuropsychological measures of executive functioning were additionally obtained to provide novel data describing the relationship between both measures of response inhibition and other established indices of executive function – most importantly working memory.
Method
Participants
Thirty three patients were recruited as part of a prospective, longitudinal study of first episode psychosis in West London. Patients eligible were screened using the WHO Psychosis Screen (Jablensky et al. 1992) and were recruited if aged between 16 and 50 years, presenting with a psychotic illness for the first time and had received no more than 12 weeks of antipsychotic medication. The diagnosis was ascertained using a structured interview, the diagnostic module of the Diagnostic Interview for Psychosis (DIP, Jablensky et al. 2000), which includes items from the Operational Criteria Checklist for Psychosis (OPCRIT, McGuffin et al. 1991) and the World Health Organization Schedules for Clinical Assessment in Neuropsychiatry (SCAN, Wing et al. 1990). Patients with mental and behavioural disorders due to psychoactive substance abuse or organic disorders were excluded from the study. As part of the longitudinal study all participants are routinely contacted one year later, at which time the diagnosis is reviewed. Twenty six patients agreed to undergo a repeat diagnostic interview. The diagnostic outcome of the remaining 7 patients was established by two psychiatrists (TREB and EMJ) using the OPCRIT checklist to compile information from the responsible psychiatrists and community psychiatric nurses and the clinical notes. The final DSM IV diagnoses at follow-up were schizophrenia in 27 patients and schizoaffective disorder in 6 patients.
These patients were compared to 24 healthy volunteers recruited from the same catchment area as patients by advertising in local colleges and hospitals. Exclusion criteria were a history of psychiatric illness in themselves or their first-degree relatives, previous head injury or other neurological illness or endocrine disorder affecting brain function, such as epilepsy and thyroid disease, and drug or alcohol abuse. Permission to conduct the study was obtained from Merton, Sutton and Wandsworth, Riverside, and Ealing Research Ethics Committees. All participants gave written informed consent and were paid an honorarium for their time.
Following recruitment, cognitive assessments were performed a mean of 13 (SD 17) days after clinical assessment. All patients were being prescribed antipsychotic medication at the time of testing: 31 patients were receiving second-generation (20 olanzapine, 9 risperidone, 2 amisulpiride) and two were receiving a first-generation antipsychotic (haloperidol).
Stop Signal Task
The subjects were seated approximately 80cm in front of the monitor with responses made on a custom-made button box. Subject's used the index and middle finger of their dominant hand. Subjects were first familiarised with the simple left/right discrimination task by performing sixteen trials with no stop signal tone. Each trial began with a centrally presented pale blue circle for 500ms, after this period the black directional arrow would appear within the circle, and the subjects' task was to make a left/right button press consistent with the arrow. The circle and arrow would remain on the screen until a response was made, or for 1000ms duration in the absence of a response. Incorrect responses were indicated to subjects by the word “WRONG!” presented in the centre of the screen for 2000ms. Following each trial a period of 1500ms elapsed before presentation of the next trial.
Following the practice trials, subjects performed stop signal trials that were identical to the practice trials in all respects, except on 25% of trials a stop signal would be presented; the stop signal was a 100 ms, 300 Hz tone. Subjects were instructed to withhold responding whenever they heard the stop signal tone, while still responding as fast as possible to the arrow. They were told that stopping and responding as fast as possible were equally important.
A tracking algorithm was used that adapted the response rating by continuously altering the delay between the target arrow and the stop signal (termed the stop signal delay [SSD]). There were four interleaved staircases, each of which started at different SSD on their first presentation - 100ms, 200ms, 400ms and 500ms. In a trial where a stop signal was presented and the subject failed to stop, the delay of that particular staircase was increased by 50ms. Where the subject successfully stopped, the delay was decreased by 50ms. As a result, subjects successfully inhibit on approximately 50% of stop trials. The majority of participants completed five blocks of 64 trials, with two control and four patients completing four blocks. Following each block, subjects were shown a graph depicting their response trial for each block and the number of errors made. They were once again encouraged to respond as quickly as possible whilst avoiding errors. The response time of no signal trials was used to derive an estimate of the average response time. Stop signal reaction time (SSRT) was determined by calculating the difference between the median response time and the average SSD. The average SSD was computed as a grand average of the average of the last 10 movements of each staircase. The standard deviation of response times and the probability of responding on stop signal trials were also determined for each subject.
Masked Motor Priming Task
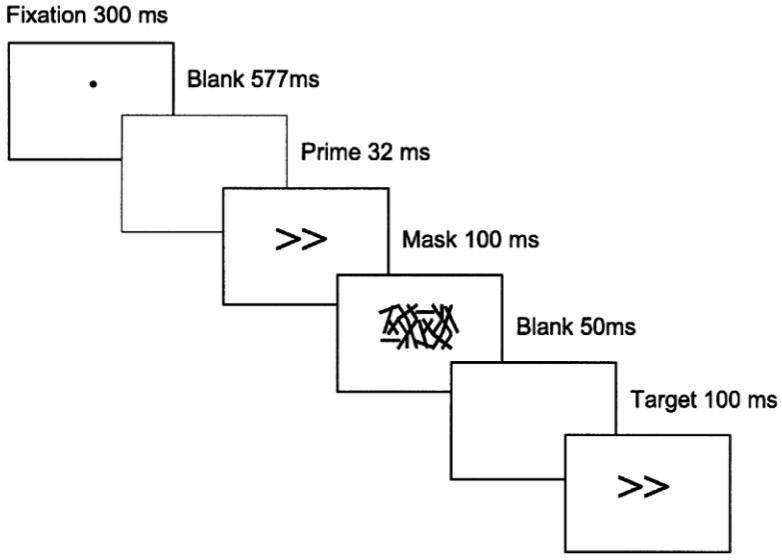
The trial sequence is depicted on figure 1. Each trial started with a fixation dot at the centre of the screen for 300ms. The screen was then cleared for 577ms, followed by the prime stimulus presented at fixation for 32ms, and then the mask for 100ms. Stimuli were left and right pointing double arrows (<<, >>). Masks were constructed from a 6 × 5 matrix randomly filled with overlapping lines of differing length and orientation. A new random mask was constructed on each trial. Stimuli were black on a white background. In the 0 SOA (stimulus onset asynchrony) condition, the target was presented concurrently with the mask, offset was above or below by 150 pixels. In contrast, in the 150 SOA condition, the mask remained on the screen for 100ms, followed by the blank screen then the target, again offset either above or below the mask. In both conditions the response period was 1000ms following target presentation. A period of 1700ms elapsed before presentation of the next trial for the 150ms condition and 1850ms for the 0ms condition. Subjects used the index and middle finger of their dominant hand and completed one block of ten practice trials and three blocks of fifteen experimental trials. Following each block, subjects were shown a graph depicting their response trial for each block and the number of errors made. Subjects were encouraged to respond as fast as possible whilst avoiding errors. Subjects' median response times and errors were recorded.
Figure 1.
Experimental trial sequence for the masked priming task for the 150ms condition.
Neuropsychological Assessments
Premorbid IQ was assessed using the Wechsler Test of Adult Reading (Wechsler 2001). Current IQ was estimated from the four subtest form of the Wechsler Adult Intelligence Scale III (Wechsler 1999), validated for use in schizophrenia (Blyler et al. 2000). Executive and memory tests were taken from the Cambridge Automated Neuropsychological Test Battery (CANTAB) as follows:
Spatial span (Owen, Downes, Sahakian, Polkey and Robbins 1990)
This measures the ability to remember the order of sequences of squares presented on the screen in increasing number.
Spatial working memory (Owen et al. 1990)
Patients are required to ‘open’ sets of boxes, varying between 3 and 8 in number, to find tokens. Errors are recorded when boxes in which tokens have been found are re-opened.
Tower of London planning (Owen et al. 1990)
Subjects were instructed to move coloured ‘balls’ in an arrangement displayed on the screen to match a goal arrangement.
Attentional set shifting (Owen et al. 1991)
Subjects are required to learn a series of visual discriminations along two dimensions. The subject is guided through various learning stages, until the critical extra dimensional shift stage (EDS) is reached where the previously irrelevant dimension becomes relevant, thus assessing ability to inhibit the previously reinforced dimension. This task may be taken as an analogue of the WCST.
Statistical Methods
Comparison between patient and control groups were analysed with separate ANOVAs, t-tests and the Chi Squared test and Spearman's or Pearson's correlation coefficient where appropriate, using SPSS version 13.0.
Results
Table 1 shows both demographic and neuropsychological data.
Table 1.
Demographic characteristics and neuropsychological performance of the patient and control groups. Differences in neuropsychological test performance are shown with premorbid IQ (WTAR) as a covariate.
| Measure | Patients Mean (SD) |
Controls Mean (SD) |
Statistic | df | p | |
|---|---|---|---|---|---|---|
| Sex Ratio (m/f) | 21/12 | 9/15 | χ2 = 3.8 | 1 | < 0.1 | |
| Age | 23.6 (6.4) | 26.0 (5.7) | t = 1.4 | 55 | NS. | |
| Premorbid IQ (WTAR) |
91.2 (11.5) | 98.9 (7.4) | t = 2.9 | 55 | 0.006 | |
| Current IQ (WAIS III) |
83.6 (13.3) | 99.7 (12.7) | t = 4.5 | 55 | <0.001 | |
| Spatial Span | 5.4 (1.2) | 6.4 (1.1) | Group, F = 7.8 WTAR, F < 1 |
55 | 0.007 NS. |
|
| Spatial Working Memory (Errors) |
26.7 (11.8) | 19.5 (15.9) | Group, F = 3.4 WTAR, F < 1 |
54 | 0.070 NS. |
|
| Attentional Set Shifting: | ||||||
| Pass/Fail | 16/16 | 19/5 | χ2 = 5.0 | 1 | 0.026 | |
| EDS errors | 17.0 (11.7) | 10.5 (9.5) | Group, F = 1.7 WTAR, F = 5.6 |
53 | NS. 0.022 |
|
| Tower of London (No. of perfect solutions) |
6.6 (2.5) | 8.4 (1.6) | Group, F = 8.6 WTAR, F < 1 |
54 | 0.005 NS. |
|
Neuropsychological measures
The patients showed lower current and premorbid IQ score than the control group. In addition, separate paired sample t tests for each group revealed a significant fall in current IQ from premorbid levels for the patient group (t(32) = 3.6 , p = 0.001) that was not present in controls (t(23) = 0.36). For this reason, to control for the group difference in expected intellectual function, premorbid IQ indexed by the WTAR was included as a covariate in subsequent analysis. In these analyses, patients performed significantly worse on spatial span and Tower of London perfect solutions. In addition, significantly more patients failed the extra dimensional stage of the attentional set shifting task.
Stop Signal Task
Table 2 shows stop signal performance for patients and healthy control subjects. Error rates on the GO task were very low in both groups, although patients did make more left/right discrimination errors. The probability of inhibition was very close to 50% in both groups, with no significant difference in their overall probability of inhibition. There was a trend for slower GO responses in the patient group. To control for the influence of generalised slowing or IQ effects on SSRT, an analysis of covariance was carried out with premorbid IQ and GO RT as covariates. This analysis revealed a robust group effect on SSRT (F(4,57) = 9.2, p <0.01, partial η2 = 0.15), with patients showing significantly longer SSRT in comparison to controls, with none of the other factors significantly contributing to SSRT.
Table 2.
Performance characteristics of the patient and control group on the stop signal task. Differences in performance are shown with premorbid IQ (WTAR) as a covariate. Medians scores for SSRT are given in square brackets.
| Patients Mean (SD) |
Controls Mean (SD) |
Statistic | df | P | |
|---|---|---|---|---|---|
| Go RT (ms) | 531 (125) | 471 (82) | Group F = 3.4 WTAR F = 0.0 |
55 | 0.070 NS. |
| SSRT* (ms) | 234 (93) [227] |
158 (39) [161] |
Group F = 9.2 WTAR F = 1.9 |
55 | 0.002 NS. |
| Probability of Inhibition (%) | 50.0 (8.9) | 51. (4.6) | Group F = 0.0 WTAR F = 1.4 |
55 | NS. NS. |
| Go task errors (%) | 2.3 (1.9) | 0.8 (1.0) | Group F = 7.3 WTAR F = 3.6 |
55 | 0.009 NS. |
Masked Priming Task
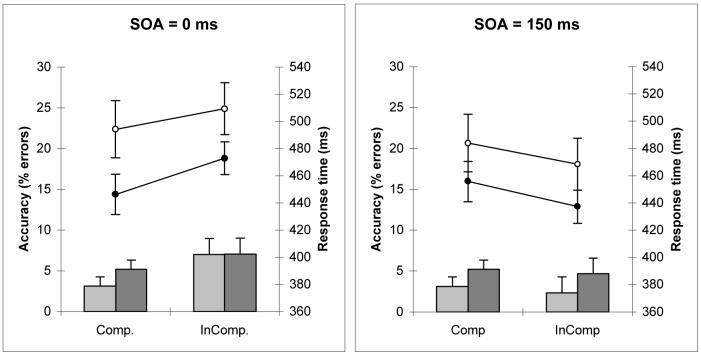
Data for three control participants was corrupted therefore the following analysis is for 21 of the control participants. Response times and accuracy data are given in Figure 2a and 2b. Four-way mixed ANOVAs were conducted for both mean response times and accuracy, with SOA and compatibility as within group factors, patients/controls as the between group factor and premorbid IQ as a covariate. The analysis revealed no significant effects for any factor for either response times or accuracy. Subsequent analysis was therefore performed to establish the presence of compatibility and SOA effects for the patient and control groups separately. These analyses revealed a robust SOA × compatibility effect for both patients (F(1,23) = 12.4 p < 0.01 partial η2 = 0.28) and controls (F(1,23) = 14.9 p < 0.01 partial η2 = 0.42). Further analysis of simple main effects indicated that responses were faster for compatible trials at the short SOA for patients (F(32) = 5.6 p < 0.05) and controls (F(20) = 11.5 p < 0.01) . In contrast, at the long SOA, responses were faster for incompatible trials for patients (F(32) = 11.2 p < 0.01) and controls (F(20) = 9.6 p < 0.01). Pearson's correlations between SSRT and NCE were not significant (controls: r=0.01; patients: r=0.05).
Figure 2a and 2b.
Response time (ms) for the patient group (white circles) and control group (black circles) on the right axes and accuracy (% errors) for patients (dark bars) and controls (light bars) on the left axes. Figure 1a is for 0 ms SOA trials and figure 1b is for 150 ms SOA trials.
Relationships between NCE, SSRT, neuropsychological and clinical variables
There were no significant correlations between NCE, SSRT and age in controls or patients. In the patients, neither NCE nor SSRT showed a significant correlation with age at onset of psychosis or length of illness. To explore the relationship between NCE, SSRT and neuropsychological measures, correlation coefficients were calculated for SSRT and NCE with current IQ, planning performance, spatial span, spatial working memory errors and errors on the extra-dimensional attentional set shift stage (EDS) for those subjects who reached this stage of the task (19 controls, 16 patients). As multiple test adjustments were not used for these analysis they are interpreted as exploratory (see Bender and Lange, 2001). In controls, there were no significant correlations between either NCE or SSRT and any of the neuropsychological measures. In patients, there was a significant correlation between SSRT and EDS errors (r=0.38, p < 0.05). The distribution of EDS errors is non-linear because of ceiling error scores if the subjects fail this stage. Repeating the correlation analysis using Spearman's ρ gave the same result for this measure (patients: ρ=0.40, p<0.05). All other correlations were not significant.
Discussion
One of the main findings of this study was that patients with schizophrenia were impaired at inhibiting a planned act. Using the dynamic tracking method to match the probability of inhibition in groups of patients and healthy controls, prolonged stop signal reaction times (SSRT) were evident in patients even when group differences in GO reaction times and IQ were taken into account. Previous studies using a method of fixed stop signal delays have been inconsistent in their findings (Badcock et al. 2002; Enticott et al., 2007) and a study of adolescent onset psychosis using the dynamic tracking method found slower stopping proficiency only in a subgroup of patients (Bellgrove et al. 2006). Our findings clarify these different results by showing that the stopping process, having been initiated, was significantly slower in a representative group of patients who were adults very early in the course of their Illness.
In the same patients, the finding of abnormally prolonged SSRT contrasted with the normal pattern of effects on the masked priming task. This was strikingly demonstrated by a specific pattern of facilitation of reaction time to compatible prime target pairs (the positive compatibility effect) when the delay between the prime and the target was very short, and inhibition of reaction time to compatible pairs (the negative compatibility effect, NCE) at a longer delay. The NCE is considered to reflect a self-inhibition process generated automatically from within the motor effector system to counter the external response activating effect of priming stimuli (see Eimer and Schlaghecken, 2003). Thus the results of our study indicate that patients with schizophrenia show intact automatic inhibition but impaired voluntary inhibition of activated motor responses.
Our findings also suggest that the locus of motor inhibition abnormalities in schizophrenia lies in executive function, i.e. in those processes exerting attentional control on performance. Functional neuroimaging studies find that tasks requiring high attentional control, such as working memory, conflict monitoring and response inhibition, activate a network of prefrontal areas involving dorsolateral prefrontal, anterior cingulate and inferior frontal cortex (Duncan and Owen, 2000) and that the right inferior frontal cortex (IFC) is specifically involved in the inhibitory component of executive processes (Aron et al. 2004). Aron et al. (2003 a) showed that the amount of right IFC damage correlated with SSRT, and Chambers et al. (2006) found that transcranial magnetic stimulation of the right IFC caused slowing of SSRT in healthy subjects. The implication of these findings for our study is that the right IFC is dysfunctional in schizophrenia, a suggestion compatible with a recent neuroimaging study of response inhibition in schizophrenia (Kaladjian et al. 2007). Since a large number of studies have already shown that dorsolateral prefrontal and cingulate cortex function is impaired in this disorder, our results suggest that all three nodes of the frontal network shown by Duncan and Owen (2000) to be active during executive tasks are abnormal in schizophrenia.
In contrast, studies have found that the NCE is abolished in the striatal disorders Huntington's disease (Aron et al. 2003 b) and Parkinson's disease (Seiss and Praamstra, 2004). Sumner et al. (2007) found that the NCE was also abolished in a patient with a small focal lesion of the SMA, but not in patients with larger frontal lesions excluding the SMA. These findings suggest that the SMA fronto-striatal output pathway mediating the NCE is intact in schizophrenia.
Our findings are compatible with other studies of schizophrenia revealing intact priming effects in memory (Kazes et al. 1999; Perry et al. 2000; Barch et al. 1996). Of particular relevance are studies of the effect of masking on response times in a digit-matching task which found intact subliminal but impaired conscious processing of the same visual material in schizophrenia (Dehaene et al. 2003; Del Cul et al. 2006). However our results are at odds with findings in schizophrenia of attenuated pre-pulse inhibition (PPI), a measure thought to reflect subliminal inhibitory processing (Braff et al. 1978 and see Turetsky et al. 2007). An increasing number of studies have shown that PPI is under some degree of attentional control at medium to long prepulse to pulse intervals (i.e., around 60ms) (Dawson et al.1993; Dawson et al. 2000; Hazlett et al. 1998; Kedzior and Martin-Iverson, 2007; Neumann, 2007). In addition, an fMRI study found specific activation of right IFC by the PPI procedure as well as activation of striatum and thalamus (Kumari et al. 2003). One explanation is that the NCE and PPI reflect different inhibitory processes, with the NCE being unconscious and mediated by an SMA-striato-thalamic output system, and PPI being more attention-demanding and mediated by striato-thalamic outputs emanating from right IFC, an area already known to be involved in the inhibition of voluntary responses. Other findings of a lack of correlation in the same patients between PPI and another index of inhibition known to be impaired in schizophrenia, P50 suppression (Braff et al., 2007), support the view that there are several forms of inhibition mediated by separate neural processes differentially affected in this disorder.
When we explored the association between stop signal and other executive tasks, we found a significant correlation between SSRT and the number of errors at the extra-dimensional shift stage (EDS) of the attentional set shifting task. There are a number of distinct cognitive operations contributing to successful EDS performance, most notably inhibition of a pre-potent response set and switching attention. Aron et al. (2004), in a study of patients with frontal cortex lesions (Aron et al. 2003 a), found a significant and specific positive correlation between the degree of damage to right IFC and the residual switch cost, a measure of the effect of the previous response set on current performance, thus supporting the role of this area in mediating inhibition when switching between responses. Our finding of a link between SSRT and attentional set shifting errors in schizophrenia patients implicates a common higher-order inhibitory impairment governing poor performance on both tasks, possibly due to right IFC dysfunction.
We found no correlation between SSRT and working memory errors in patients or controls. Clark et al. (2007), using the same tasks as us, found significant correlations between SSRT and spatial working memory errors in groups of patients with adult ADHD and right IFC damage. They argued that the basis of their correlations lies in the inhibitory requirements of both tasks mediated by the right IFC. The lack of correlation between SSRT and working memory in our patients, together with strong evidence for an impairment in the ability to manipulate information in working memory being a reflection of dorsolateral prefrontal cortex dysfunction in schizophrenia (see Barch 2006), suggests that increased working memory errors and prolonged SSRT reflect independent impairments mediated by separate dysfunctional frontal areas in this disorder. Aside from this issue of specific cortical regions driving distinct cognitive abnormalities in schizophrenia, the current study provides evidence for a wider distinction between impaired cortical function (demonstrated by prolonged SSRT) and intact subcortical function (demonstrated by normal NCE). This suggestion chimes with the conclusion of a recent study which found impaired rapid reward learning but intact learning over a longer time period (Waltz et al., 2007). Waltz et al (2007) argue that this demonstrates a dissociation between cortical and subcortically mediated cognitive functions since the prefrontal cortex mediates rapid learning of reinforcement contingencies, while the striatum plays a key role in gradual learning of stimulus response pairings
Our patients were all being prescribed atypical antipsychotic medications that antagonise dopamine receptors. Whether this medication influenced performance on these tasks is unclear. Existing evidence predicts that antipsychotic medication should not impair the stopping process. Models of striatal dopamine modulation of learning show that increased dopamine release facilitates appetitive learning (GO) via the direct output pathway whereas dopamine under-activity facilitates suppression of responding following negative reinforcement (NO GO) via the indirect output pathway (Frank et al., 2004). Relevant to stop signal performance, Aron et al (2007) have shown that the stopping process is mediated by a hyperdirect pathway from right IFC to subthalamic nucleus which ‘bypasses’ the striatum and this proposed mechanism is compatible with animal models of stop signal performance which suggest that dopamine is involved in the GO but not the STOP process (Eagle et al. 2007). There have been no studies addressing the contribution of dopaminergic mechanisms to the NCE. We therefore cannot rule out a possible normalising effect of medication on striatal output pathways mediating the intact negative compatibility effect and studies of unmedicated patients are required to clarify this.
In summary, we have found evidence for impaired conscious and preserved unconscious inhibition of activated motor responses in schizophrenia. The observation that task performance and response times were largely equivalent between patients and controls, and the lack of association between SSRT and IQ or spatial working memory impairment in the schizophrenia group, suggest that this is a specific effect and not due to either a generalised cognitive abnormality or a working memory deficit. These findings further suggest that unconscious inhibitory motor processing is intact in schizophrenia and does not contribute to the slowing of activated volitional motor inhibitory responses. Finally, on that basis of previous work, these findings implicate an abnormality of right inferior frontal cortex function in schizophrenia, thus adding to the evidence that executive impairments in this disorder are mediated by a dysfunctional frontal network, involving dorsolateral prefrontal, cingulate and inferior frontal cortices, which is normally activated during performance of tasks requiring executive function or attentional control.
Acknowledgements
This study was supported by Wellcome Trust programme grant 064607. We are grateful to the consultants and nurses of West London and South West London and St George's Mental Health NHS Trusts for greatly facilitating the study and to Isobel Harrison and Stan Mutsatsa for patient assessments. We thank Martin Eimer and Friederike Schlaghecken for initial assistance with the masked prime paradigm.
Footnotes
Portions of this study were presented at The International Congress on Schizophrenia Research, Savannah, USA, April 2005.
Contributor Information
Vyv C Huddy, Division of Neuroscience and Mental Health, Imperial College London.
Adam R Aron, Department of Psychology, University of California San Diego.
Masuma Harrison, Division of Neuroscience and Mental Health, Imperial College London.
Thomas RE Barnes, Division of Neuroscience and Mental Health, Imperial College London.
Trevor W Robbins, Department of Experimental Psychology, University of Cambridge.
Eileen M Joyce, Institute of Neurology, University College London.
Reference List
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003 a;6:1329. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, Sahakian BJ, Robbins TW. Inhibition of subliminally primed responses is mediated by the caudate and thalamus: evidence from functional MRI and Huntington's disease. Brain. 2003 b;126:713–723. doi: 10.1093/brain/awg067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1285–1291. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badcock JC, Michie PT, Johnson L, Combrinck J. Acts of control in schizophrenia: dissociating the components of inhibition. Psychological Medicine. 2002;32:287–297. doi: 10.1017/s0033291701005128. [DOI] [PubMed] [Google Scholar]
- Band GPH, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychologica. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Barch BM. What can research on schizophrenia tell us about the cognitive neuroscience of working memory? Neuroscience. 2006;139:73–84. doi: 10.1016/j.neuroscience.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Barch DM, Cohen JD, Servan-Schreiber D, Steingard S, Cohen JD, Steinhauer SS, van Kammen DP. Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. Journal of Abnormal Psychology. 1996;105:592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Cohen JD. Factors influencing stroop performance in schizophrenia. Neuropsychology. 2004;18:477–483. doi: 10.1037/0894-4105.18.3.477. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Chambers CD, Vance A, Hall N, Karamitsios M, Bradshaw JL. Lateralized deficit of response inhibition in early-onset schizophrenia. Psychological Medicine. 2006;36:495–505. doi: 10.1017/S0033291705006409. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing-when and how? Journal of Clinical Epidemiology. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the 'WAIS-III for use with patients with schizophrenia. Schizophrenia Research. 2000;46:209–215. doi: 10.1016/s0920-9964(00)00017-7. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Pre-stimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA, Swerdlow NR. Prepulse inhibition and P50 suppression are both deficient but not correlated in schizophrenia patients. Biological Psychiatry. 2007;61:1204–1207. doi: 10.1016/j.biopsych.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Swartz SG, Greenstein VC, et al. Dysfunction of Early-Stage Visual Processing in Schizophrenia. American Journal of Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, et al. Executive “brake failure” following deactivation of human frontal lobe. Journal of Cognitive Neuroscience. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, Sahakian BJ. Association between response inhibition and working memory in adult ADHD: a link to right frontal cortex pathology? Biological Psychiatry. 2007;61:1395–1401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Hazlett EA, Filion DL, Nuechterlein KH, Schell AM. Attention and Schizophrenia - Impaired Modulation of the Startle Reflex. Journal of Abnormal Psychology. 1993;102:633–641. doi: 10.1037//0021-843x.102.4.633. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Hazlett EA, Nuechterlein KH, Filion DL. On the clinical and cognitive meaning of impaired sensorimotor gating in schizophrenia. Psychiatry Research. 2000;96:187–197. doi: 10.1016/s0165-1781(00)00208-0. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Artiges E, Naccache L, Martelli C, Viard A, Schurhoff F, et al. Conscious and subliminal conflicts in normal subjects and patients with schizophrenia: The role of the anterior cingulate. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13722–13727. doi: 10.1073/pnas.2235214100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cul A, Dehaene S, Leboyer M. Preserved subliminal processing and impaired conscious access in schizophrenia. Archives of General Psychiatry. 2006;63:1313–1323. doi: 10.1001/archpsyc.63.12.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft MR, Goodchild HL, Robbins TWR. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology. 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Eimer M, Schlaghecken F. Effects of masked stimuli on motor activation: Behavioral and electrophysiological evidence. Journal of Experimental Psychology-Human Perception and Performance. 1998;24:1737–1747. doi: 10.1037//0096-1523.24.6.1737. [DOI] [PubMed] [Google Scholar]
- Eimer M, Schlaghecken F. Response facilitation and inhibition in subliminal priming. Biological Psychology. 2003;64:7–26. doi: 10.1016/s0301-0511(03)00100-5. [DOI] [PubMed] [Google Scholar]
- Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Critical Review Neurobiology. 2000;14:1–21. [PubMed] [Google Scholar]
- Enticott PG, Ogloff JR, Bradshaw JL. Response inhibition and impulsivity in schizophrenia. Psychiatry Research. 2007;157:251–4. doi: 10.1016/j.psychres.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'Reilly RC. By carrot or by stick: Cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hartman M, Steketee MC, Silva S, Lanning K, Andersson C. Wisconsin Card Sorting Test performance in schizophrenia: the role of working memory. Schizophrenia Research. 2003;63:201–217. doi: 10.1016/s0920-9964(02)00353-5. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Haznedar MM, Singer MB, Germans MK, Schnur DB, et al. Prefrontal cortex glucose metabolism and startle eyeblink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology. 1998;35:186–198. [PubMed] [Google Scholar]
- Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TRE, Joyce EM. Executive function in first-episode schizophrenia. Psychological Medicine. 1998;28:463–473. doi: 10.1017/s0033291797006041. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Joyce EM, Barnes TRE, Kennard C. Saccadic distractibility in first-episode schizophrenia. Neuropsychologia. 2002;40:1729–1736. doi: 10.1016/s0028-3932(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Huddy V, Barnes TRE, Robbins TW, Crawford TJ, Kennard C, et al. The relationship between antisaccades, smooth pursuit, and executive dysfunction in first-episode schizophrenia. Biological Psychiatry. 2004;56:553–559. doi: 10.1016/j.biopsych.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, et al. Schizophrenia - Manifestations, Incidence and Course in Different Cultures - A World-Health-Organization 10-Country Study. Psychological Medicine. 1992:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- Jablensky A, McGrath J, Herrman H, Castle D, Gureje O, Evans M, et al. Psychotic disorders in urban areas: an overview of the Study on Low Prevalence Disorders. Australian and New Zealand Journal of Psychiatry. 2000;34:221–236. doi: 10.1080/j.1440-1614.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- Joyce E, Huddy V. Defining the cognitive impairment in schizophrenia. Psychological Medicine. 2004;34:1151–1155. doi: 10.1017/s0033291704003472. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Grimault S, Anton LC, Mazzola-Pomietto P. Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophrenia Research. 2007;97:184–193. doi: 10.1016/j.schres.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kazes M, Berthet L, Danion JM, Amado I, Willard D, Robert P, Poirier MF. Impairment of consciously controlled use of memory in schizophrenia. Neuropsychology. 1999;13:54–61. doi: 10.1037//0894-4105.13.1.54. [DOI] [PubMed] [Google Scholar]
- Kedzior KK, Martin-Iverson MT. Attention-dependent reduction in prepulse inhibition of the startle reflex in cannabis users and schizophrenia patients - A pilot study. European Journal of Pharmacology. 2007;560:176–182. doi: 10.1016/j.ejphar.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Geyer MA, ffytche D, Soni W, Mitterschiffthaler MT, et al. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Research-Neuroimaging. 2003;122:99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Li CSR. Do schizophrenia patients make more perseverative than non-perseverative errors on the Wisconsin Card Sorting Test? A meta-analytic study. Psychiatry Research. 2004;129:179–190. doi: 10.1016/j.psychres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Logan G, Cowan WB. On the ability to inhibit throught and action: a theory of an act of control. Psychological Review. 1984;91:295–327. [Google Scholar]
- MacDonald AW, Carter CS. Cognitive experimental approaches to investigating impaired cognition in schizophrenia: A paradigm shift. Journal of Clinical and Experimental Neuropsychology. 2002;24:873–882. doi: 10.1076/jcen.24.7.873.8386. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Farmer A, Harvey I. A Polydiagnostic Application of Operational Criteria in Studies of Psychotic Illness - Development and Reliability of the Opcrit System. Archives of General Psychiatry. 1991;48:764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Research. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Neumann DL. Prepulse inhibition of the startle blink reflex is modulated during a memory task requiring prepulses to be encoded for later report. International Journal of Psychophysiology. 2007;63:55–63. doi: 10.1016/j.ijpsycho.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and Spatial Working Memory Following Frontal-Lobe Lesions in Man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-Dimensional Versus Intra-Dimensional Set Shifting Performance Following Frontal-Lobe Excisions, Temporal-Lobe Excisions Or Amygdalo-Hippocampectomy in Man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barnes TRE, Nelson HE, Tanner S, Weatherley L, Owen AM, et al. Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain. 1997;120:1823–1843. doi: 10.1093/brain/120.10.1823. [DOI] [PubMed] [Google Scholar]
- Perry W, Light GA, Davis H, Braff DL. Schizophrenia patients demonstrate a dissociation on declarative and non-declarative memory tests. Schizophrenia Research. 2000;46:167–174. doi: 10.1016/s0920-9964(99)00229-7. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, et al. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophrenia Research. 2001;52:47–55. doi: 10.1016/s0920-9964(00)00173-0. [DOI] [PubMed] [Google Scholar]
- Seiss E, Praamstra P. The basal ganglia and inhibitory mechanisms in response selection: evidence from subliminal priming of motor responses in Parkinson's disease. Brain. 2004;127:330–339. doi: 10.1093/brain/awh043. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, Mcconaghy N. Mismatch negativity - an index of a preattentive processing deficit in schizophrenia. Biological Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- Sumner P, Nachev P, Morris P, Peters AM, Jackson SR, Kennard C, Husain M. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron. 2007;54:697–711. doi: 10.1016/j.neuron.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Johnson SC, Kohler C, Gur RE. The profile of auditory information processing deficits in schizophrenia. Schizophrenia Bulletin. 2007;33:413. doi: 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BJ, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biological Psychiatry. 2007;62:756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third Edition London: Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. London: Psychological Corporation; 2001. [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. Scan - Schedules for Clinical-Assessment in Neuropsychiatry. Archives of General Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]