Abstract
The left angular gyrus (AG) is reliably activated across a wide range of semantic tasks, and is also a consistently reported component of the so-called default network that it is deactivated during all goal-directed tasks. We show here that there is only partial overlap between the semantic system and the default network in left AG and the overlap defines a reliable functional landmark that can be used to segregate functional subdivisions within AG. In 94 healthy human subjects, we collected fMRI data during fixation and eight goal directed tasks that involved semantic matching, perceptual matching or speech production in response to familiar or unfamilair stimuli presented in either verbal (letters) or nonverbal (pictures) formats. Our results segregated three different left AG regions that were all activated by semantic relative to perceptual matching: (i) a mid-region (mAG) that overlapped with the default network because it was deactivated during all tasks relative to fixation; (ii) a dorso-mesial region (dAG) that was more activated by all tasks relative to fixation; and (iii) a ventro-lateral region (vAG) that was only activated above fixation during semantic matching. By examining the effects of task and stimuli in each AG subdivision, we propose that mAG is involved in semantic associations irrespective of the presence or absence of a stimulus; dAG is involved in searching for semantics in all visual stimuli, and vAG is involved in the conceptual identification of visual inputs. Our findings provide a framework for reporting and interpreting AG activations with greater definition.
Keywords: functional MRI, language, semantic system, default network, angular gyrus, words and pictures
Introduction
In this paper, we used fMRI to characterise the different spatial patterns of activation and deactivation in the left angular gyrus (AG) when systematically varying semantic, perceptual and speech processing in a large group of 94 healthy subjects. The AG is a posterior part of the inferior parietal lobule corresponding to Brodmann’s area (BA) 39 or area PG of von Economo and Koskinas (see (Triarhou, 2007)). Its role in reading comprehension was first recorded by Dejerine (Dejerine, 1891) and popularized by the seminal work of Geschwind (Geschwind, 1965, 1970). Early functional imaging studies demonstrated AG activation during semantic processing of auditory (Demonet et al., 1992) and visual (Vandenberghe et al., 1996) stimuli and these findings have been replicated with high consistency and reliability across multiple studies with different semantic tasks and stimuli (see meta-analysis reviews in (Cabeza and Nyberg, 2000; Vigneau et al., 2006; Binder et al., 2009)). For instance, Binder et al. (2009) found that the most consistent semantic activation across 120 functional neuroimaging studies was located within the left AG (Binder et al., 2009). They described AG as a heteromodal association area.
In addition to being associated with semantic processing, the left AG has also been identified as part of the so-called “default network” (Raichle et al., 2001) that is deactivated during goal-directed tasks as compared to rest or any passive baselines. This default or resting-state network has frequently been described as the reduction of activity in specific brain regions when subjects are engaged in effortful tasks (Shulman et al., 1997; Gusnard and Raichle, 2001; Mazoyer et al., 2001; Raichle et al., 2001), or engage in self-relevant internal thoughts about past and future events (Buckner et al., 2008; Andrews-Hanna et al., 2010a; Andrews-Hanna et al., 2010b). The task-independent deactivation in left AG is remarkably reliable (Shehzad et al., 2009) and consistent across different tasks, paradigms, subjects and studies, see recent meta-analysis reviews (Buckner et al., 2008; Laird et al., 2009; Smith et al., 2009; Spreng et al., 2009; Biswal et al., 2010).
To explain why left AG is part of both the default and the semantic networks, Binder and colleagues (Binder et al., 1999; McKiernan et al., 2003; McKiernan et al., 2006; Binder et al., 2009) proposed that task-unrelated thoughts during conscious passive states are essentially semantic because they involve activation and manipulation of acquired knowledge about the world that is interrupted during effortful tasks so that competition between exogenous and endogenous attentional and executive resources is reduced. This explanation highlights a common source of processing during semantic tasks and task-unrelated thoughts. However, AG is a large area with different anatomical (e.g. (Eidelberg and Galaburda, 1984; Ebeling and Steinmetz, 1995; Rushworth et al., 2006; Caspers et al., 2008; Kiriyama et al., 2009)) and functional subdivisions (e.g. (Seghier and Price, 2009; Vandenberghe and Gillebert, 2009; Andrews-Hanna et al., 2010b; Brownsett and Wise, 2010; Sharp et al., 2010; Uddin et al., 2010)). Here we use a range of different tasks and stimuli, including fixation, to systematically subdivide semantic activations in AG according to their response properties and to establish how the identified AG subdivisions overlap with the default network.
Methods
Subjects
Ninety-eight healthy subjects (aged 31.6±16.7 years, 52 females, 46 males) gave written informed consent to participate in this study. According to the Edinburgh handedness questionnaire (Oldfield 1971): 58 were right-handed and 40 were either left-handed or ambidextrous. All subjects were native English speakers, had normal or corrected-to-normal vision, and had no history of neurological or psychiatric disorders. Four subjects were excluded after data acquisition because of low performance on the semantic matching task (accuracy < 75%). The data from the remaining 94 subjects (aged 30.8±15.8 years, 50 females, 44 males) were included in our group analyses (see below). The inclusion of a large heterogeneous sample of subjects who differed in their handedness, age and gender allows our findings to be generalised across different populations as well as giving us the opportunity to explicitly investigate the influence of these demographic variables on brain activity in different regions. Importantly, because of the well-known relationship between language laterality and handedness (e.g. (Pujol et al., 1999; Knecht et al., 2000; Szaflarski et al., 2002)), we aimed to test whether any effect in left AG would be significant when atypical data from left handers were included or excluded.
The study was approved by the National Hospital for Neurology and Institute of Neurology Joint Ethics Committee.
Experimental design
Our participants were engaged in 8 goal directed tasks as well as fixation. The semantic network was identified by comparing semantic decisions on semantic stimuli (pictures of objects or their written names) to perceptual decisions on non-semantic stimuli (meaningless pictures of nonobjects or greek letter strings) (e.g. (Vandenberghe et al., 1996; Josse et al., 2008)). The default network was identified as that which was deactivated during the non-semantic stimuli relative to fixation (e.g. (Shulman et al., 1997; Laird et al., 2009)). The non-semantic stimuli were meaningless and unfamiliar, and thus a semantic search for a recognisable entity will not be successful. The remaining four conditions involved the presentation of the same four sets of stimuli with a different task. For nonverbal semantic stimuli (pictures of objects), participants named aloud the objects in the pictures; for verbal semantic stimuli (written object names), participants read aloud the object names; for non-semantic stimuli (pictures of non-objects and greek letters), participants said “1,2,3”; see (Josse et al., 2008; Josse et al., 2009) for further details.
This factorial experimental design allowed us to investigate how the following factors influence the direction and height of activation in different subdivisions within the left AG:
Stimuli: By including semantic and non-semantic stimuli we were able to identify which parts of the AG were involved in semantic processing and the correspondence between these parts and the default network (which was expected to be deactivated for all conditions relative to fixation). The inclusion of different types of non-semantic stimuli was also important for assessing the impact of perceptual processing in AG because the pictures of nonojects were physically bigger than the greek letters (maximum visual angle was 7.3°×8.5° for pictures and 4.9°×1.2° for words). In addition, the perceptual decision task required attention to the visual stimulus whereas saying “1,2,3” was unrelated to the visual stimulus.
Semantic versus production task: By comparing activation for semantic decisions and speech production, we can dissociate different types of semantic processing. Semantic matching involves a search for semantic features that are shared across two stimuli, and short term memory to maintain these features while a decision is made. In contrast, naming and reading involve the retrieval of a unique conceptual representation that can be used to access the corresponding sounds of the associated words.
Verbal versus non-verbal stimuli: The key distinction here is that verbal semantic stimuli (i.e. the written object names) are more strongly linked to their sounds than the nonverbal stimuli (i.e. the pictures of objects), see (Glaser and Glaser, 1989). Therefore, during semantic decisions, we expected more phonological activation when the stimuli are written words than pictures of objects. Conversely, the demands on semantic activation are higher during picture naming than reading aloud because, in the absence of non-semantic links between orthography and phonology, objects need to be conceptually identified before they can be named. The comparison of semantic processing in response to pictures and written words (Vandenberghe et al., 1996; Van Doren et al., 2010) also allows us to make inferences concerning possible access to a common semantic system, see (Binder et al., 2009). For example, access to the semantic system from phonology was expected to be stronger during written word processing than picture processing. Conversely, accessing the semantic system directly from visual inputs was expected to be stronger during picture processing than written word processing.
Experimental procedures
There were four separate scanning runs or sessions. In 2 sessions, the participants made semantic and perceptual decisions, interleaved with blocks of fixation. In the other 2 sessions, the participants performed the 4 speech production tasks interleaved with blocks of fixation. The order of conditions was counterbalanced within and across session. Each session consisted of 24 blocks of stimuli of the same type/condition with an additional 12 blocks of fixation that were presented every two stimulus blocks. Each stimulus block lasted 18 seconds and consisted of 4 trials during which 3 stimuli were simultaneously presented on the screen for 4.32 seconds, followed by 180ms of fixation. Every two stimulus blocks, fixation continued for 14.4 seconds.
All stimuli were presented in triads with one item (picture or letter string) above and two items below in the same format as the item above. During semantic and perceptual decisions, the item above acted as a target that was semantically or physically related to one of the items below. In the speech production conditions, there was no semantic or perceptual relationship between any of the three items. Prior to each stimulus block, a brief instruction was presented on the screen for 3.6 seconds to indicate what sort of response would be necessary:
“Match pictures” cued a finger press response to indicate whether the target picture was semantically related to the picture on the lower-left or lower-right (e.g. is “truck” or “ship” most closely related to “anchor”),
“Match words” cued the same semantic task as “Match pictures” but with written object names rather than pictures,
“Same pictures” cued a finger press response to indicate whether the target picture was perceptually identical to the picture on the lower-left or lower-right.
“Same symbols” cued the same perceptual task as “Same pictures” except with meaningless greek letter strings rather than pictures of meaningless objects.
“Name” indicated that the participant should name each of the 3 objects in the pictures aloud.
“Read” indicated that the participant should read aloud each of the 3 words.
“1,2,3 Pictures” indicated that the participant should say “1,2,3” while looking at each of the 3 pictures of meaningless nonobjects.
“1,2,3 Symbols” indicated that the participant should say “1,2,3” while looking at each of the 3 strings of greek letters.
To ensure that the task was understood correctly, all subjects undertook a short training session before entering the scanner with a different set of words and pictures. Stimulus presentation in the scanner was via a video projector, a front-projection screen and a system of mirrors fastened to the MRI head coil. Additional details about the stimulus selection can be found in (Josse et al., 2008; Josse et al., 2009). Responses during the matching task were recorded using a button box held under one hand throughout the experiment. Subjects who responded with the right hand (n=57) indicated the lower-left stimulus with their first finger and the lower-right stimulus with their middle finger (to avoid spatial conflict). Likewise, subjects who responded with their left hand (n=37) indicated the lower-left stimulus with their middle finger and the lower-right stimulus with their first finger. The hand of response was held constant in both the activation (semantic decision) and control (perceptual decision) conditions. Therefore it had no effect on activation for semantic versus perceptual decisions. It was included as a variable because the data from adult participants will also be used in a study of stroke patients, some of whom are no longer able to use one of their hands due to hemiparesis. Therefore, we scanned four groups of adult healthy controls: right handed responding with their right hand, right handed responding with their left hand, left handed responding with their right hand and left handed responding with their left hand. In contrast, the data from the younger participants (<18 years) will also be used in a study of developmental dyslexia who do not have hemiparesis. Therefore, all the younger participants were right handed and responded with their right hand. The influence of age, gender, handedness and hand of response on all our results was carefully evaluated, see results section.
MRI acquisition
Experiments were performed on a 1.5T Siemens system (Siemens Medical Systems, Erlangen, Germany). Functional imaging consisted of an EPI GRE sequence (repetition time/echo time/flip angle = 3600ms/50ms/90°, field of view = 192mm, matrix = 64×64, 40 axial slices, 2mm thick with 1mm gap). Functional scanning was always preceded by 14.4s of dummy scans to insure steady-state tissue magnetization.
fMRI Data analysis
Data processing and statistical analyses were performed with the Statistical Parametric Mapping SPM5 software package (Wellcome Trust Centre for Neuroimaging, London UK, http://www.fil.ion.ucl.ac.uk/spm/). All functional volumes were spatially realigned, un-warped, normalized to MNI space using the unified normalisation-segmentation procedure of SPM5, and smoothed with an isotropic 6-mm full-width at half-maximum Gaussian kernel, with resulting voxels size of 2×2×2 mm3. Time-series from each voxel were high-pass filtered (1/128 Hz cut-off) to remove low-frequency noise and signal drift. The pre-processed functional volumes of each subject were then submitted to a fixed-effects analysis, using the general linear model at each voxel. Each stimulus onset was modelled as an event using condition-specific ‘stick-functions’ having a duration of 4.32 sec per trial and a stimulus onset interval of 4.5 sec. These were convolved with a canonical hemodynamic response function thus providing regressors for the linear model. The contrast images for each of the 8 conditions (correct trials only) compared to fixation were then entered into a second-level analysis (i.e. random-effects analysis) to enable inferences at the group level.
Our second level analyses systematically explored the direction (activation or deactivation) and amplitude of the signal change, reflected here by the weighted-beta values (Poline, 2003).
The semantic system was identified by comparing semantic decisions on semantic stimuli to perceptual decisions on non-semantic stimuli (at p<0.05 FWE-corrected).
The default network was identified as the main effect of fixation relative to all unfamiliar (non-semantic) stimuli irrespective of task or modality (i.e. deactivation in 4 conditions at p<0.05 FWE-corrected). We excluded the 4 conditions that used familiar stimuli because they may involve direct or indirect semantic access that can bias the overlap with the semantic network. One alternative method to identify the default network is to use a data-driven approach such as independent component analysis (ICA) (McKeown et al., 1998) to segregate the different resting-state networks (e.g. (Greicius and Menon, 2004; Damoiseaux et al., 2006)). This can be achieved by running ICA on the fixation epochs (i.e. interleaved resting periods) that are present in our block paradigm (see procedure in (Fair et al., 2007)). However, we opted for defining the default network as a deactivation relative to fixation because (i) the number of fixation datapoints in our paradigm is relatively small (4 datapoints per fixation epoch) and this may not be sufficient to guarantee a robust ICA analysis, (ii) we aimed to define both networks (semantic and default) using the same methodology, in this case by hypothesis-driven “cognitive subtraction” between different conditions, and (iii) many previous studies have defined the default network as deactivation relative to a fixation (rest) condition (see review in (Laird et al., 2009)) and found robust and reliable results (Shulman et al., 1997; Raichle et al., 2001).
Overlap of Semantic system and Default network was identified by using the inclusive masking option in SPM to identify activation that was common for (i) semantic – perceptual decisions with (ii) fixation – unfamiliar stimuli. We also visualised the overlap between the two systems on a 3D cortex mesh that is available in SPM8.
Divergence of Semantic system from Default network in AG was identified by using the exclusive masking option in SPM to identify where activation was significant (p<0.05 FWE-corrected) for semantic – perceptual decisions but not significant (p<0.001 uncorrected) for fixation – unfamiliar stimuli (and vice-versa).
Functional specialization within AG voxels that were more activated for semantic than perceptual decisions was investigated further by contrasting (a) semantic decisions versus naming; (b) perceptual decisions versus saying 1,2,3; (c) semantic versus nonsemantic stimuli; (d) pictures of objects versus written words during semantic decisions and naming; (e) nonobjects versus greek letters, during perceptual decisions and saying 1,2,3 and (f) the interactions between stimuli and task. See Experimental design section (above) for full details of the type of processing tapped by each of these manipulations. Critically, we also compared each condition to fixation to dissociate voxels that were activated or deactivated (e.g. see Box 2 in Gusnard and Raichle, 2001).
In addition, we assessed the influence of age, gender, handedness, hand of response used for matching conditions and/or the in-scanner semantic decision times on brain activation across our 94 subjects. To do that, we applied correlation analyses (Pearson correlation coefficient) on continuous variables (age and reaction times) or two-sample t-tests on dichotomic variables (gender, handedness and hand of response).
Statistical thresholds
The main effects of interest (semantic system and default network) are reported at p<0.05 FWE-corrected for multiple comparisons across the whole brain in the group analysis. This threshold is lowered to describe any effects of task and stimuli in each AG region at p<0.001 uncorrected for the group analysis.
Localisation of AG
Anatomically, AG can be seen as the continuation of the superior/middle temporal gyri into the inferior parietal lobe with a medial boundary defined by the intraparietal sulcus. Its anterior boundary with the supramarginal gyrus and its posterior boundary with the superior occipital lobe are not well defined. It is usually considered to correspond with Brodmann’s area BA39 or von Economo and Koskinas’s area PG (also defined as area 69 in the unified nomenclature of (Triarhou, 2007)). Recent cytoarchitectonic studies (e.g. (Caspers et al., 2006; Caspers et al., 2008)) have suggested that AG extends to areas PGa (rostral, gravity centre at [−46 −65 +44]) and PGp (caudal, gravity centre at [−43 −78 35]). Functionally, left AG has shown huge variability across functional neuroimaging studies. For instance, Vigneau et al. (2006) assessed an average coordinates over 27 foci at [−45 −68 +26] with a large standard deviation of about 14.1 mm (see Table 4 in (Vigneau et al., 2006)). Binder et al. (2009) showed a wide distribution of activated peaks across 120 functional studies in the parietal regions (see Figure 2 in (Binder et al., 2009)).
Thus, to ensure that we included activity in the entire left AG, we reported all significant foci that were located within a mask that extended from −20mm to −60mm in the x-direction, from −54mm to −82mm in the y-direction, and from +16mm to +54mm in the z-direction (e.g. (Rushworth et al., 2006)). Finally, we also compared our activated patterns to the probabilistic cytoarchitectonic maps that are available in the MNI-space within the Anatomy Toolbox in SPM8 (Eickhoff et al., 2005) (for a similar procedure see (Wu et al., 2009)).
Results
In-scanner behaviour
All subjects performed the tasks with high accuracy (see Table 1 for more details). Response times varied from 0.95 sec to 2.6 sec for semantic decisions on familiar stimuli and from 0.68 sec to 2.24 sec for perceptual decisions on unfamiliar stimuli (see average values in Table 1).
Table 1.
summary of in-scanner behavioural responses: mean (±SD) of accuracy (in [%]) and reaction times (in [sec]) during all conditions over our 94 subjects.
| task | matching | production | ||
|---|---|---|---|---|
| modality stimuli |
words | pictures | words | pictures |
| familiar | 93.0±4.3 % 1.70±0.27 sec |
91.0±5.4 % 1.75±0.28 sec |
99.5±1.7% --- |
89.0±9.8% --- |
| Unfamiliar | 97.5±4.2 % 1.09±0.20 sec |
99.0±3.2 % 1.13±0.27 sec |
99.9±1% --- |
99.9±1 % --- |
fMRI Activation
The semantic system
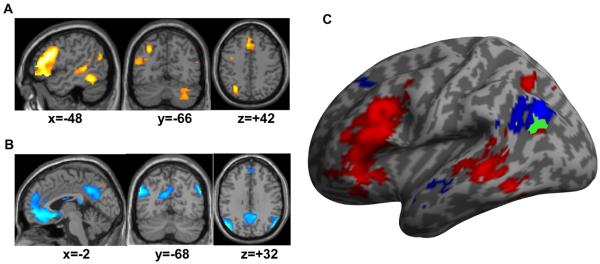
Semantic relative to perceptual matching activated a strongly left-lateralised set of regions (Figure 1A) including left AG, inferior and middle frontal regions, middle and superior temporal regions, precentral cortex, occipito-temporal cortex, and the supplementary motor area. In the right hemisphere, activation was only significant in the cerebellum. Left AG activation extended from dorso-mesial (junction between BA 39 and 7) to ventro-lateral (junction between BA 39 and 19), see coronal view in Figure 1A and list of peaks in Table 2. There was no significant effect of the hand of response used in the matching tasks on activation for semantic relative to perceptual decisions.
Figure 1.
main effect from the group analysis (at p<0.05 FWE-corrected) over our 94 subjects for (A) semantic matching relative to perceptual matching (semantic system), and for (B) deactivations relative to fixation over all non-semantic stimuli (default network). All significant effects are shown in red-to-yellow (A) or blue-to-white (B) colour coding and projected on an individual T1-weighted image. (C) projection of the semantic system (red) and the default network (blue) on a 3D cortical mesh of the left hemisphere. The common voxels between the two systems are shown in green (only present within the left angular gyrus).
Table 2.
list of the local group peaks (MNI coordinates and Z scores) within the left angular gyrus. In bold: significant at p<0.05 FWE-corrected, n.s.: not significant at p<0.001 uncorrected.
| Coordinates x, y, z |
Semantic network Z-score |
Default network Z-score |
|---|---|---|
| −30, −66, 42 (dAG) | 7.6 | n.s. |
| −48, −68, 28 (mAG) | 5.7 | 7.9 |
| −48, −68, 20 (vAG) −34, −64, 24 |
7.0
5.8 |
3.8
3.1 |
| −42, −76, 36 | n.s | 8.3 |
| −50, −70, 32 | n.s | 8.4 |
| −58, −60, 26 | n.s. | 7.9 |
| −60, −56, 24 | n.s. | 7.5 |
The default network
The default network, as defined by deactivation relative to fixation during all unfamiliar stimuli is illustrated in Figure 1B. It includes the typical set of regions reported by other studies (e.g. (Shulman et al., 1997; Raichle et al., 2001)) including bilateral inferior parietal regions (of which AG is a part), medial prefrontal cortex, precuneus and posterior cingulate cortex. The local peaks detected in the left angular gyrus are listed in Table 2.
Overlap between the Semantic system and Default network in left AG
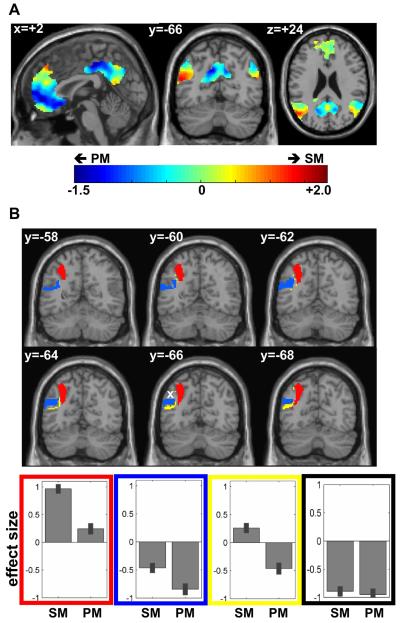
Consistent with prior reports, (e.g. (Binder et al., 1999; Binder et al., 2009)), we found that semantic activation and the default network overlapped in left AG (Figure 1C). It was also interesting to note that the semantic system did not overlap with the default network in any area other than left AG (see Figure 1C). Moreover, we found that, within anatomically defined left AG, the default network only intersected one part of the extensive dorsal-mesial to ventral-lateral AG activation observed for semantic relative to perceptual decisions (e.g. Figures 1C and 2A). Thus, parts of left AG were activated by semantics but were not part of the default network.
Figure 2.
(A) activation amplitude (effect size) during semantic matching minus perceptual matching within the default network (masked inclusively). Red = semantic more than perceptual matching, Green = similar amplitudes during both matching tasks, Blue = semantic less than perceptual matching. The only voxels that showed higher activation within the default network for semantic matching were located in the left angular gyrus. (B) the sign of the effect size for the semantic system within the left angular gyrus is shown on six coronal slices (from y = −58mm to y = −68mm). Although all voxels were significantly more activated during semantic matching than perceptual matching, their response can be characterised into three main patterns (see bar graphs): activated by both matching conditions relative to fixation (red), deactivated by both matching condition relative to fixation (blue), or activated during semantic matching but deactivated during perceptual matching relative to fixation (yellow). The effect size of a voxel within the default network that is not part of the semantic system (indicated by a white cross) is shown on a bar graph in black. SM = semantic matching, PM = perceptual matching.
Functional subdivisions in left AG
The intersection between the two networks (maximum overlap at [x=−48, y=−68, z=+28], see Table 2) marks a reliable functional landmark that segregates semantic activation in left AG into three functional subdivisions (see Figure 2B): a dorsal and mesial part (dAG) at approximately z= +40mm (which is above the overlap), a ventral part (vAG) at approximately z= +20mm (which is below the overlap), and a middle (mAG) at approximately z=+30mm (which corresponds to the location of the overlap).
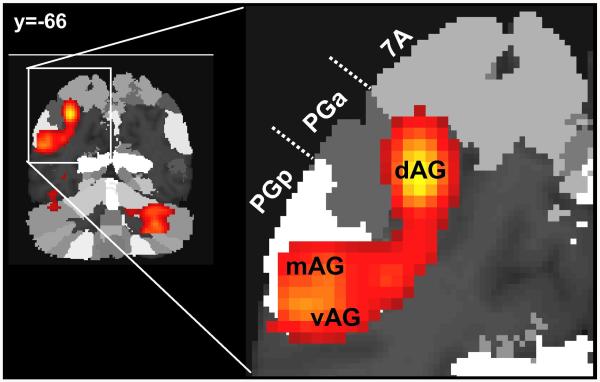
Comparison of these functional subdivisions to the cytoartechtonic regions PGa and PGp (see Figure 3) shows that dAG was closer to the centre of gravity of area PGa and extends anteriorly to area hIP3 (overlap with this area = 14%) and dorsally to area 7A (overlap with this area = 7%), whereas mAG and vAG were mainly located in PGp (overlap with this area = 74%). Note that the AG activity of the default network also extents over the two cytoartechtonic regions PGp (overlap with this area = 47%) and PGa (overlap with this area = 25%).
Figure 3.
The extent of the semantic activation in the left AG overlapped on the the probabilistic cytoarchitectonic (coronal view at y = −66, with a zoom on the left AG). PGp (white), PGa (dark gray) and 7A (light gray) represent the cytoarchitectonic regions from the Anatomy Toolbox.
Functional specialization in vAG, dAG and mAG
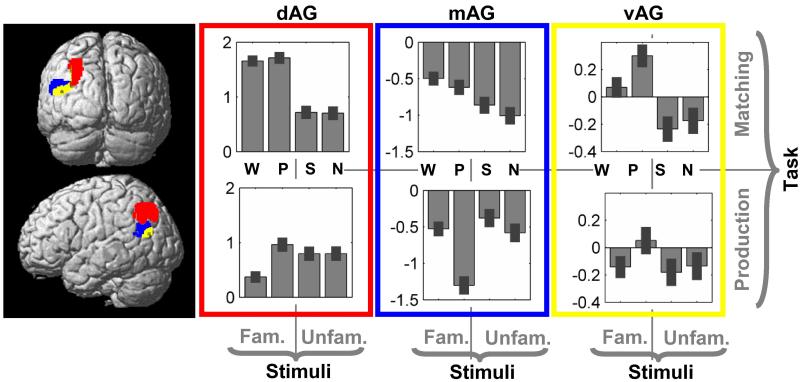
All three AG subdivisions were more activated for semantic decisions on familiar stimuli than perceptual decisions on unfamiliar stimuli. Moreover, there was no evidence, in any of the subdivisions, that processing was influenced by the demands on perceptual processing (p>0.001 for perceptual matching versus saying “1,2,3”) or the intensity or size of the visual stimulus (p>0.001 for nonobjects versus greek letters). Differences in the response properties of the three AG subdivisions are illustrated in Figures 2 and 4 and summarised below.
Figure 4.
illustration of the signal level (effect size in bar graphs) in the three subdivisions vAG (in yellow), mAG (in blue), and dAG (in red) for all tasks and stimuli: W = words, P = pictures, S = string of Greek letters, N = non-objects, Fam = familiar, Unfam = unfamiliar.
The defining differences were in relation to fixation (see Figure 2B). Specifically, mAG activation was below fixation for both semantic (Z=−5.2) and non-semantic stimuli (Z=−7.8), dAG, activation was above fixation for both semantic (Z=8.9) and nonsemantic stimuli (Z=6.4); and vAG activation was nonsignificantly above fixation during semantic decisions but below fixation for non-semantic stimuli (Z=−4.3). These patterns remained significant and robust even after excluding left handed subjects (see detailed results on data from right-handed subjects only in Figures S1 and S2 of the Supplementary Material).
Other task and stimulus effects revealed the following (see Figure 4): In mAG, activation was higher for semantic decisions than picture naming (Z=5.6); reading aloud than picture naming (Z=5.5) and articulation (say “1,2,3”) than perceptual decisions (Z=3.3). In dAG, activation was higher for semantic decisions than picture naming (Z=5.3) and picture naming than reading aloud (Z=5.3). In vAG, activation was higher for semantic decisions on pictures than words (Z=6.7).
Other factors influencing activation in vAG, dAG and mAG
For each subdivision and condition, we investigated whether semantic activation correlated, across our 94 subjects, with age, gender, handedness and/or the in-scanner semantic decision times. In mAG, deactivation during semantic decisions on pictures was stronger in subjects with faster reaction times (p=0.03). This was not observed in vAG or dAG (p>0.1). In vAG, males showed higher semantic activation than females (t= 3.2, p=0.002). This gender effect was not observed in mAG or dAG (p>0.1). There were no other significant correlations, but in mAG, there was a nonsignificant trend for deactivation to be stronger in older than younger subjects (p=0.06).
Discussion
This study investigated the spatial overlap between the neural systems associated with semantic processing and the default network. We found that the intersection of the semantic system with the default network defines a functional landmark that segregates three different functional subdivisions in AG that we refer to as mAG (at the point of overlap), vAG (ventral to the overlap) and dAG (dorsal to the overlap). By comparing the effect of task and stimuli in each AG subdivision, our results also contribute towards an understanding of the range of functions that AG is involved in. Our work should provide a useful framework for predicting and interpreting the location of AG activation in past and future studies.
Previous anatomical and functional studies have subdivided the inferior parietal lobe in different ways (Eidelberg and Galaburda, 1984; Ebeling and Steinmetz, 1995; Caspers et al., 2006; Rushworth et al., 2006; Caspers et al., 2008; Kiriyama et al., 2009; Seghier and Price, 2009; Vandenberghe and Gillebert, 2009; Andrews-Hanna et al., 2010b; Brownsett and Wise, 2010; Sharp et al., 2010; Uddin et al., 2010). Our results provide further anatomical and functional differentiation of semantic activations in AG that enable us to demonstrate where and how semantic activation in AG overlaps with the default network.
Overlap of Semantic system and Default network
The default network intersected the middle of the AG (mAG) area that was activated by semantic relative to perceptual decisions. Within mAG, activation was proportional to the level of semantic associations with most activation when semantic associations were allowed to occur continuously and randomly during fixation; high when the task focused on semantic associations between 3 concepts; and least during perceptual decisions and naming that focused attention on perceptual processing and name retrieval respectively. It is however worth noting that the exact nature of the processing involved during fixation (or rest) is poorly defined (Shulman et al., 1997; Mason et al., 2007; Smith et al., 2009) and thus may not be uniquely attributed to semantic processing. For example, it is also involved in episodic memory (Mazoyer et al., 2001; Anticevic et al., 2010; Yang et al., 2010) and self-relevant internal cognitive processes (Andrews-Hanna et al., 2010a). Critically, the response in mAG was not dependent on visual inputs as shown recently by (Brownsett and Wise, 2010) who reported activation at [−50 −68 +26] for both written and spoken narratives and by (Obleser et al., 2007) who associated activation at [−50 −60 +34] with a semantic resource for speech comprehension.
The lower mAG response for picture naming relative to all the other tasks is consistent with picture naming being the most demanding task (e.g. (McKiernan et al., 2003; Greicius and Menon, 2004; McKiernan et al., 2006; Kelly et al., 2008; Esposito et al., 2009; Pyka et al., 2009)). In particular, we note that higher mAG activation for reading relative to picture naming might reflect unconstrained semantic associations that occur in parallel to the direct links from orthography and phonology (e.g. (Strain et al., 1995)) or post articulation because reading is faster than picture naming (Fraisse, 1969; Potter and Faulconer, 1975) and our inter-stimulus onset was held constant. Alternatively, increased mAG activation during reading than picture naming might reflect increased demands on top-down modulation that predicts the visual input (Carreiras et al., 2009). Future studies, using other high-temporal resolution techniques such as magnetoencephalography, could evaluate these hypotheses by mapping the timing of the responses (see examples in (Service et al., 2007; Cornelissen et al., 2009; Vartiainen et al., 2009)) during reading aloud and determining whether mAG activation occurred prior to completion of visual processing, in parallel with speech production or after speech production was complete.
Semantic activation in AG that did not overlap with Default network
Activation in vAG and dAG differed from that in mAG in two distinct ways. First, vAG and dAG activation was higher for semantic decisions than fixation which suggests these regions are more involved in stimulus driven semantics than amodal semantic associations. Second, vAG and dAG activation was higher for picture naming than for reading aloud whereas mAG activation was higher for reading aloud than picture naming. This suggests that vAG and dAG may be more involved in the conceptual identification of visual input, which is essential for picture naming but not reading (Glaser and Glaser, 1989).
There were also differences in the response properties of vAG and dAG. The first was that dAG was activated (versus fixation) by non-semantic as well as semantic stimuli whereas vAG was only activated above fixation during semantic decisions. The second was that vAG was more activated when semantic decisions were made on pictures than words. We suggest below that the dAG is involved in searching for a semantic representation and vAG is associated with later stages of conceptual identification.
The observation that dAG was activated during perceptual as well as semantic decisions is unlikely to be related to visual attention or eye movements because dAG activation did not differ for perceptual decisions (that require a visual comparison of three stimuli) and saying “1,2,3” (that involves minimal attention to the stimuli). It is also unlikely to reflect visual or perceptual processing per se because dAG activation was not differentially activated by (a) perceptual decisions and saying “1,2,3” or (b) nonobjects and greek letters. We therefore propose that dAG activation in response to non-semantic (unfamiliar) stimuli might reflect a search for semantics even though this search will be implicit (not task related) and unsuccessful during the non-semantic conditions. Our proposal is in line with a previous report that suggested dAG (at [−44 −76 44] and [−56 −60 36]) is part of a bottom-up support network when stimulus meaning is being retrieved (Whitney et al., 2009).
In vAG, the response profile was more specific to later stages of conceptual identification. In this area, activation was higher than fixation during semantic decisions but below fixation for perceptual decisions as reported previously (Binder et al., 1999; McKiernan et al., 2003; McKiernan et al., 2006; Binder et al., 2009). Higher vAG activation for pictures than words suggests responses increase when conceptual identification is accessed directly from visual inputs (Viswanathan and Childers, 2003). In contrast, higher mAG activation for words than pictures reflects the fact that conceptual identification of words can occur indirectly after accessing phonology.
In summary, we are proposing that mAG is involved in semantic associations irrespective of the presence or absence of a stimulus; dAG is involved in searching for semantics in all visual stimuli, and vAG is involved in the later stages of conceptual identification from visual inputs.
At this greater spatial definition of the left AG subdivisions, future work can investigate how the different AG parts interact with other core semantic regions including the pars orbitalis in the inferior frontal gyrus (Bookheimer, 2002; Binder et al., 2009; Price, 2010) and the anterior ventral temporal cortex (Price, 2010; Visser et al., 2010). Understanding how different parts of AG interact with other regions can also help to refine previous language models; for instance, several recent studies have suggested that the left AG provides top-down “semantic constraints” during language comprehension (see Price, 2010), a role that we can now potentially attribute to mAG/vAG rather than dAG.
Conclusions
Our findings are in line with previous literature that highlighted the need for a better characterisation of the spatial heterogeneity in left AG. Here, we have addressed the issue by providing higher spatial precision in the left AG subdivisions along with some of the corresponding functional characteristics. Our results should therefore help to interpret the diverse set of left AG activations that have been identified in different contexts including the default network (Buckner et al., 2008; Laird et al., 2009), the semantic system (Binder et al., 2009; Price, 2010), the reading system (Price and Mechelli, 2005), the attention system (Corbetta and Shulman, 1998; Cabeza et al., 2008), the number processing system (Dehaene et al., 1998), and the autobiographical memory system (Svoboda et al., 2006). The left AG subdivisions that we have identified should also be useful for explaining why the effects of left AG damage have inconsistent consequences on a range of skills including speech comprehension, speech production, finger agnosia, spatial disorientation, acalculia and agraphia (e.g. (Luria, 1970; Hart and Gordon, 1990; Ardila et al., 2000; Jefferies and Lambon Ralph, 2006; Corbett et al., 2009)). Future studies are required to look at the timing of activations in these three AG subdivisions and their connectivity, and to further compare the AG subdivisions identified here with AG areas associated with resting-state networks other than the default network (e.g. see (Damoiseaux et al., 2006; van den Heuvel et al., 2008; Smith et al., 2009)).
Supplementary Material
Acknowledgments
This work was funded by the Welcome Trust and the James S. MacDonnell Foundation (conducted as part of the Brain Network Recovery Group initiative). We would like to thank our three radiographers (Amanda Brennan, Janice Glensman and David Bradbury) as well as Clare Shakeshaft, Laura Stewart and Tom Schofield for their help with fMRI data collection, Caroline Ellis, Goulven Josse and Ferath Kherif for their help with data analysis, and Hwee Ling Lee and Sue Ramsden for their valuable help setting up the fMRI database.
REFERENCES
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010a;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron. 2010b;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A, Concha M, Rosselli M. Angular gyrus syndrome revisited: Acalculia, finger agnosia, right-left disorientation and semantic aphasia. Aphasiology. 2000;14:743–754. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Biswal BB, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annual Review in Neurosciences. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Brownsett SLE, Wise RJS. The contribution of the parietal lobes to speaking and writing. Cereb Cortex. 2010;20:517–523. doi: 10.1093/cercor/bhp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, Price CJ. An anatomical signature for literacy. Nature. 2009;461:983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eichkhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212:481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Ehsan S, Lambon Ralph MA. Different impairments of semantic cognition in semantic dementia and semantic aphasia: evidence from the non-verbal domain. Brain. 2009;132:2593–2608. doi: 10.1093/brain/awp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond B Biol Sci. 1998;353:1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen PL, Kringelbach ML, Ellis AW, Whitney C, Holliday IE, Hansen PC. Activation of the left inferior frontal gyrus in the first 200 ms of reading: evidence from magnetoencephalography (MEG) PloS One. 2009;4:e5359. doi: 10.1371/journal.pone.0005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Dehaene-Lambertz G, Cohen L. Abstract representations of numbers in the animal and human brain. Trends Neurosci. 1998;21:355–361. doi: 10.1016/s0166-2236(98)01263-6. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Sur un cas de cecite verbale avec agraphie, suivi d’autopsie. CR Societe de Biologie. 1891;43:197–201. [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Ebeling G, Steinmetz H. Anatomy of the parietal lobe: mapping the individual pattern. Acta Neurochir. 1995;136:8–11. doi: 10.1007/BF01411428. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Galaburda AM. Inferior parietal lobule. Divergent architectonic asymmetries in the human brain. Arch Neurol. 1984;41:843–852. doi: 10.1001/archneur.1984.04050190049013. [DOI] [PubMed] [Google Scholar]
- Esposito F, Aragri A, Latorre V, Popolizio T, Scarabino T, Cirillo S, Marciano E, Tedeschi G, Di Salle F. Does the default-mode functional connectivity of the brain correlate with working-memory performances? Arch Ital Biol. 2009;147:11–20. [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisse P. Why is naming longer than reading? Acta Psychologica. 1969;30:96–103. [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970;170:940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Glaser WR, Glaser MO. Context effects in stroop-like word and picture processing. J Exp Psychol Gen. 1989;118:13–42. doi: 10.1037//0096-3445.118.1.13. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hart JJ, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27:226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Josse G, Seghier ML, Kherif F, Price CJ. Explaining function with anatomy: language lateralization and corpus callosum size. J Neurosci. 2008;28:14132–14139. doi: 10.1523/JNEUROSCI.4383-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse G, Kherif F, Flandin G, Seghier ML, Price CJ. Predicting language lateralization from gray matter. J Neurosci. 2009;29:13516–13523. doi: 10.1523/JNEUROSCI.1680-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kiriyama I, Miki H, Kikuchi K, Ohue S, Matsuda S, Mochizuki T. Topographic analysis of the inferior parietal lobule in high-resolution 3D MR imaging. Am J Neuroradiol. 2009;30:520–524. doi: 10.3174/ajnr.A1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein E, Henningsen H. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria AR. Traumatic Aphasia: Its Syndromes, Psychology and Treatment. Walter De Gruyter; The Netherlands: 1970. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J, Wise RJS, Alex Dresner M, Scott SK. Functional integration across brain regions improves speech perception under adverse listening conditions. J Neurosci. 2007;27:2283–2289. doi: 10.1523/JNEUROSCI.4663-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poline JB. Contrasts and classical inference. In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD, editors. Human Brain Function. Academic Press; London: 2003. [Google Scholar]
- Potter MC, Faulconer BA. Time to understand pictures and words. Nature. 1975;253:437–438. doi: 10.1038/253437a0. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mechelli A. Reading and reading disturbance. Curr Opin Neurobiol. 2005;15:231–238. doi: 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Pyka M, Beckmann CF, Schöning S, Hauke S, Heider D, Kugel H, Arolt V, Konrad C. Impact of working memory load on FMRI resting state pattern in subsequent resting phases. PLoS One. 2009;4:e7198. doi: 10.1371/journal.pone.0007198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen-Berg H. Connection patterns distinguish regions of human parietal cortex. Cereb Cortex. 2006;16:1418–1430. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Price CJ. Dissociating functional brain networks by decoding the between-subject variability. Neuroimage. 2009;45:349–359. doi: 10.1016/j.neuroimage.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service E, Helenius P, Maury S, Salmelin R. Localization of syntactic and semantic brain responses using magnetoencephalography. J Cogn Neurosci. 2007;19:1193–1205. doi: 10.1162/jocn.2007.19.7.1193. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Awad M, Warren JE, Wise RJS, Vigliocco G, Scott SK. The neural response to changing semantic and perceptual complexity during language processing. Hum Brain Mapp. 2010;31:365–377. doi: 10.1002/hbm.20871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Strain E, Patterson K, Seidenberg MS. Semantic effects in single-word naming. J Exp Psychol Learn Mem Cogn. 1995;21:1140–1154. doi: 10.1037//0278-7393.21.5.1140. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Triarhou LC. A proposed number system for the 107 cortical areas of Economo and Koskinas, and Brodmann area correlations. Stereotact Funct Neurosurg. 2007;85:204–215. doi: 10.1159/000103259. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Hulshoff Pol H. Normalized cut group clustering of resting-state fMRI data. PLoS ONE. 2008;3:e2001. doi: 10.1371/journal.pone.0002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doren L, Dupont P, De Grauwe S, Peeters R, Vandenberghe R. The amodal system for conscious word and picture identification in the absence of a semantic task. NeuroImage. 2010;49:3295–3307. doi: 10.1016/j.neuroimage.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Gillebert CR. Parcellation of parietal cortex: convergence between lesion-symptom mapping and mapping of the intact functioning brain. Behav Brain Res. 2009;199:171–182. doi: 10.1016/j.bbr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Vartiainen J, Parviainen T, Salmelin R. Spatiotemporal convergence of semantic processing in reading and speech perception. J Neurosci. 2009;29:9271–9280. doi: 10.1523/JNEUROSCI.5860-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci. 2010;22:1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Childers TL. An enquiry into the process of categorization of pictures and words. Percept Mot Skills. 2003;96:267–287. doi: 10.2466/pms.2003.96.1.267. [DOI] [PubMed] [Google Scholar]
- Whitney C, Grossman M, Kircher TT. The influence of multiple primes on bottom-up and top-down regulation during meaning retrieval: evidence for 2 distinct neural networks. Cereb Cortex. 2009;19:2548–2560. doi: 10.1093/cercor/bhp007. [DOI] [PubMed] [Google Scholar]
- Wu SS, Chang TT, Majid A, Caspers S, Eickhoff SB, Menon V. Functional heterogeneity of inferior parietal cortex during mathematical cognition assessed with cytoarchitectonic probability maps. Cereb Cortex. 2009;19:2930–2945. doi: 10.1093/cercor/bhp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weng X, Zang Y, Xu M, Xu X. Sustained activity within the default mode network during an implicit memory task. Cortex. 2010;46:354–366. doi: 10.1016/j.cortex.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.