Abstract
When neurons exit the cell cycle after their terminal mitosis, they detach from the apical surface of the neuroepithelium. Despite the fact that this detachment is crucial for further neurogenesis and neuronal migration, the underlying mechanisms are still not understood. Here, taking advantage of the genetics and imaging possibilities of the zebrafish retina as a model system, we show by knock down experiments that the guidance molecule Slit1b as well as its receptor Robo3 are required for apical retraction of retinal ganglion cells (RGCs). In contrast, N-cadherin seems to be responsible for maintenance of apical attachment as expression of dominant-negative N-cadherin causes RGCs to lose apical attachments prematurely and rescues retraction in slit1b morphants. These results suggest that Slit-Robo signaling downregulates N-cadherin activity to allow apical retraction in newly generated RGCs.
Keywords: zebrafish, retinal ganglion cell, slit/robo, cadherin, apical retraction
Introduction
Upon neurogenesis in the vertebrate CNS, progenitors undergo divisions at the apical surface of the neuroepithelium, which in the final round of division gives rise to postmitotic neurons (Gotz and Huttner, 2005). These newly generated neurons often first adopt a neuroepithelial morphology but then quickly detach their process from the apical surface and migrate towards the basal side of the neuroepithelium (Nadarajah et al., 2001; Miyata et al., 2004). Recent studies in the mouse cortex disagree on whether integrin function (Loulier et al., 2009) or adherens junctions (Cappello et al., 2006; Imai et al., 2006; Loulier et al., 2009) regulate retraction of apical processes. It is also not known whether extrinsic signals regulate this event.
In a study focused on axon initiation in transgenically labeled ath5:gapGFP RGCs, Zolessi et al. (2006) made the unexpected observation that slit1b morphants showed inhibition of apical retraction, and used this finding to show that axonogenesis, which happened on schedule, therefore, did not depend on apical retraction. Zolessi et al, (2006), however did not investigate the mechanism of retraction. Slit proteins act as repulsive guidance ligands for axonal growth cones expressing Robo receptors and it was reported that activated Robo or addition of purified Slit can inhibit N-cadherin mediated cell adhesion in chick retinal cell culture (Rhee et al., 2002; Rhee et al., 2007). In Drosophila, Slit-Robo signaling negatively regulates cadherin-mediated adhesion on cardioblasts to enable proper lumen formation (Medioni et al., 2008; Santiago-Martinez et al., 2008). These results raised the possibility that a similar mechanism mediates the retraction of apical processes in the neuroepithelium. We find that Slit1B or Robo3 downregulation in transgenically labeled RGCs leads to inhibition of apical retraction. Expression of dominant-negative N-cadherin in RGCs results in the opposite phenotype – premature apical detachment. N-cadherin dominant negative overexpression rescues apical retraction in slit1b morphants. These results are consistent with a model in which Slit/Robo signaling downregulates N-cadherin based adhesion allowing apical retraction.
Materials and Methods
Animals
Wildtype and transgenic zebrafish were bred and kept at 26.5°C. Embryos obtained from natural mating were raised at 28.5°C in 0.003% phenylthiourea to prevent pigment formation. Transgenic lines [Tg(ath5:gapGFP) and Tg(ath5:gapRFP)] were from Zolessi et al. (2006).
Constructs and Antibodies
Constructs used for embryo injection: ath5:gapGFP (Zolessi et al., 2006), hsp70:mCherryCAAX and hsp70:ncadhΔEC-mCherry. pDONR-ncadhΔEC was subcloned from pEGFP-N1-ncadhΔEC, a gift from Dr James Jontes (Jontes et al., 2004), using primers to add att sites at the 5′ and 3′ ends of the n-cadherin sequence. hsp70:mCherryCAAX and hsp70:ncadhΔEC-mCherry constructs were made using the Tol2kit as published (Kwan et al., 2007). Entry clones, except pDONR-ncadhΔEC, were kind gifts from Dr Kate Lewis and Dr Chi-bin Chien.
Primary antibodies used were diluted in blocking solution as follows: Zn5, 1:500 (Zebrafish International Resource Center), anti-N-cadherin antibody, 1:200 (Abcam, ab12221), anti-GFP, 1:200 (Roche 11814460001), and anti-GFP-FITC conjugated 1:500 (Abcam, ab6662). Secondary antibodies used: donkey or goat anti-mouse IgG conjugated to Alexa 488, 594 or 647 fluorophores (Invitrogen), diluted as 1:1000 in blocking solution.
Image analyses
Optical sections of whole-mount embryos were obtained with a confocal microscope (SP2; Leica) at approximately 0.6μm intervals, as described previously (Poggi et al., 2005). Images were analyzed using Volocity software (Improvision). Using Zn5 (or ath5:gapGFP, in the case of Figure 3) as a marker for the differentiated ganglion cells, a differentiated RGC with retracted apical process had both its soma and apical process located within the labeled ganglion cell layer, while a RGC with an unretracted apical process was defined as one with a cell body was positive cell body that retained an apical process outside of the ganglion cell layer. Counting was performed manually.
Figure 3.
Expression of heat-shock induces dominant-negative N-cadherin leads to premature apical processes retraction in RGC progenitors. (A) Confocal images of the apical region (AP) of the retina of a 36hpf Tg(ath5:gapGFP) embryo stained with anti-N-cadherin and anti-GFP antibodies. N-cadherin (red) expression is detected in the apical processes of Ath5:gapGFP+ cells (arrowheads). Confocal images of the retinas of Tg(ath5:gapGFP) embryos injected with hsp70:mCherryCAAX (B,C) or hsp70:ncadhΔEC-mCherry (D,E) at 36hpf. Boxed regions in B and D are enlarged in C and E, respectively. Arrowheads in C indicate the unretracted apical process of an Ath5:gapGFP positive cell expressing Hsp70:mCherryCAAX. Arrowheads in E indicate Ath5:gapGFP positive cells expressing Hsp70:NcadhΔEC-mCherry with retracted apical processes. Scale bars: 20μm. (F) Percentage of mCherry and Ath5:gapGFP double-positive cells with unretracted apical processes at 36hpf (n=128 for Hsp70:mCherryCAAX expressing cells; n=110 for Hsp70:NcadhΔEC-mCherry expressing cells). Error bars indicate standard errors (***p<0.001).
DNA, RNA and MO injections
Plasmid DNA encoding the fluorescent proteins were injected into the cytoplasm of one-cell stage zebrafish embryos. ath5:gapGFP construct was injected together with meganuclease I-SceI at a concentration of 10ng/μl (Zolessi et al. 2006). To improve integration and expression of the transgenes, all the Tol2 constructs were injected with the transposase RNA in a 1:1 ratio (Kwan et al., 2007). RNA and morpholinos (MOs; Genetools) were injected into the yolk of one- to four-cell stage embryos. MOs used were as follows: standard control MO (5′-CCTCTTACCTCAGTTACAATTTATA- 3′), 0.2ng of anti-slit1b, translation blocking (5′-GCTCGGTGTCCGGCATCTCCAAAAG-3′, Zolessi et al., 2006), 2ng of anti-robo2, translation blocking (5′-GTAAAAGGTGTGTTAAAGGACCCAT-3′), and a combination of 1ng of anti-robo3var1, translation blocking (5′-CCCTAAAAGCGCTACAATCCACCTG-3′) and 1ng of anti-robo3var2, translation blocking (5′-TCTTTATCAGGAACGCAGCATCTC-3′, Challa et al., 2005; Devine and Key, 2008).
Blastomere transplantation
Embryos were dechorionated with Pronase (30mg/ml; Sigma). Blastomeres from transgenic donor embryos, Tg(ath5:gapRFP), injected with H2B-YFP mRNA and morpholinos were transplanted into the animal poles of unlabeled host blastulas using a glass micropipette.
Heat Shock Experiments
Embryos injected with the heat-shock promoter driven constructs were raised at 28.5°C until 20 or 24hpf. The embryos were then incubated at room temperature for 30-60 minutes, transferred to a tube of pre-warmed medium and heat-shocked at 37°C for one hour.
RNA synthesis
pCS2FA-transposase was a gift from Dr Brian Link. The transposase RNA was transcribed using the mMessage machine in vitro transcription kit (Ambion) from the SP6 promoter of pCS2FA-transposase, after linearization with NotI. The mRNA was subsequently purified using RNeasy RNA purification kit (Qiagen).
In situ hybridization
slit1b and robo3 anti-sense probes were generated by digesting pCR2.1-TOPO-slit1b-2C and pCR2.1-TOPO-robo3 with BamH1 and HindIII, respectively, then transcribing with T7 RNA polymerase. pCR2.1-TOPO-slit1b-2C and pCR2.1-TOPO-robo3 are kind gifts from Dr Chi-bin Chien. Whole mount in situ hybridization of slit1b mRNA was performed on wild-type embryos as previously described (Shimamura et al., 1994), hybridized embryos were subsequently sectioned for image acquisition. In situ hybridization for robo3 mRNA was performed on 20μm cryosections as previously described (Butler et al., 2001).
Statistical analysis
The Mann-Whitney U test was used to compare the percentage of Ath5:gapGFP expressing cells with unretracted apical processes in WT and slit1b morphants per retina, using InStat software (GraphPad). Binomial test was used in all other experiments to assess statistical significance.
Results
Slit1b and Robo3 regulate apical process retraction of RGCs
To validate and extend the findings of Zolessi et al. (2206) on the role of Slit1b in apical retraction, embryos were injected with ath5:gapGFP with or without slit1b morpholino and fixed at 48 hours post fertilization (hpf). As Ath5:gapGFP is expressed by both the differentiated RGCs and their progenitors, Zn5, which labels the axon and soma of RGCs in zebrafish, was used as a definitive marker for the differentiated ganglion cells (Schmitt and Dowling, 1996). An Ath5:gapGFP+ RGC was judged to have an unretracted apical process if its cell body was in the RGC layer and positive for Zn5 yet it retained an apical process that extended outside of the ganglion cell layer (arrowheads in Fig. 1E and F). At 48hpf, only few RGCs in control retinas had unretracted apical processes, while a significant number of RGCs in the slit1b morphant retina retained unretracted apical processes (Fig. 1). The reason that the RGC layer is thinner in slit1b morphants is probably because many RGC somas have trouble migrating basally when still attached apically (Zolessi et al., 2006). To check that this phenotype is not a result of a general developmental delay, we compared the relative timing of apical retraction to another RGC developmental event – axonogenesis using time-lapse analysis of Ath5:gapGFP expressing RGCs starting at 35hpf. We found that only 31.6±10.6% of normal RGCs sent out axons before retracting their apical processes (n=19), but in slit1b morphants, although axonogenesis was not premature, 68.4±9.1% of RGCs in extended an axon while retaining an apical process (n=26). This significant difference (p<0.01), indicates that apical retraction is inhibited compared to axonogenesis by the knocking down Slit1b.
Figure 1.
Apical processes retraction of RGCs is delayed in slit1b morphants. Analysis was performed on ath5:gapGFP DNA injected embryos at 48hpf. Extended-focus confocal images of the retinas of a control embryo (A) or a slit1b morphant (D). Boxed regions in A and D are enlarged in B and E, respectively. Arrowheads point to the retracted apical processes in the control retina (B) and unretracted apical processes in the slit1b morphant retina (E). Zn5 (magenta) labels the ganglion cell soma and axon. C and F, single optical section images of the boxed regions in A and D, respectively, showing that the Ath5:gapGFP+ cells express Zn5 and are thus RGCs (arrowheads). Scale bars: 20μm. (G) Percentage of Ath5:gapGFP positive RGCs (defined as cells that are Zn5 positive) with unretracted apical processes at 48hpf. White bar indicates the average value from 12 control retinas and black bar indicates the average value from 7 slit1b morphant retina, only retinas with more than 10 GFP and Zn5 double-positive cells are included in the quantification. Error bars indicate SEM (**p<0.01).
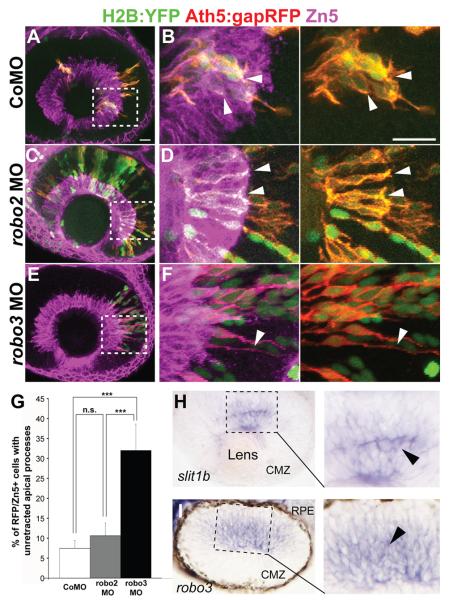
Robo proteins are receptors for Slit ligands. Therefore, we wondered whether newborn RGCs express Robo, which could mediate the Slit1b regulated apical retraction. Among the four zebrafish robo genes, only robo2 and robo3 mRNA are detected in the developing retina (Lee et al., 2001). In zebrafish, two forms of robo3 exist -- robo3v1 and robo3v2 (Challa et al., 2005). Therefore, to test if Robo2 or 3 in RGCs is involved in apical process retraction, we injected Tg(ath5:gapRFP) embryos with control, robo2, or a combination of robo3v1 and robo3v2 morpholinos and H2B-YFP mRNA as a nuclear marker of donor cells. Blastomeres from injected embryos were then transplanted into uninjected wildtype hosts of the same stage. We found that Ath5:gapRFP positive cells from control and robo2 morpholino injected embryos retracted their apical processes normally, while Ath5:gapRFP positive cells from robo3 morphants retained apical processes (Fig. 2B, D and F). Thus, robo3 knockdown, but not robo2 knockdown, phenocopies slit1b morphants, suggesting that Robo3 is the receptor downstream of Slit1b during apical retraction.
Figure 2.
Knockdown of robo3 but not robo2 inhibits apical process retraction. Confocal images from wildtype hosts transplanted with blastomeres from Tg(ath5:gapRFP) embryos injected with control morpholino (A,B), robo2 morpholino (C,D) or a combination of robo3v1 and v2 morpholinos (E,F) at 48hpf. Zn5 (magenta) labels the ganglion cell layer. Boxed regions in A, C and E are enlarged in B, D, and F. Arrowheads in B and D indicate the retracted apical processes of transplanted Ath5:gapRFP+ RGCs from control or robo2 morpholinos injected embryos; arrowheads in F indicate the unretracted apical process of a transplanted RGC from a robo3v1 and v2 double-morphant. Scale bars: 20μm. (G) Percentage of Ath5:gapRFP and Zn5 double-positive cells with unretracted apical processes at 48hpf (n=187 cells from control morpholino injected embryos; n= 94 cells from robo2 morphants; n=50 cells from robo3 morphants). Error bars indicate standard errors (***p<0.001). (H, I) A retinal section from a 38hpf whole-mount in situ hybridized embryo, showing that slit1b and robo3 mRNAs are expressed in the developing zebrafish retina. (I) In situ hybridization image from a similarly staged retinal section showing that robo3 mRNA is expressed in subsets of cells in the developing retina. Note that pigments were allowed to form and the retinal pigmented epithelium (RPE) is thus visible. Abbreviation, CMZ, ciliary marginal zone.
An important question is why Slit1b and Robo3 do not cause neuroepithelial cells to detach prior to their final division. RGCs of the embryonic zebrafish retina go through their final S-phase between 30 and 40hpf (Hu and Easter, 1999) and finish mitosis a few hours later (Poggi et al., 2005). Using in situ hybridization at a variety of timepoints between 24 and 40hpf, we first detected a clear signal for both slit1b and robo3 mRNA in scattered cells at about 36-38hpf (Fig 2H and I). Thus, the timing of expression of this receptor-ligand pair may provide part of the answer to this question.
Expression of dominant-negative N-cadherin leads to premature apical process retraction
Immunohistochemistry reveals that N-cadherin is expressed strongly in the developing zebrafish retina (Liu et al., 2001), consistent with a possible role in apical retraction. As N-cadherin is expressed in the apical processes of Ath5:gapGFP positive RGC progenitors (Fig. 3A), we investigated whether it is involved in apical process attachment using a dominant-negative construct lacking the extracellular N-cadherin domain (Nieman et al., 1999; Jontes et al., 2004). To bypass the severe defects that result from disrupting N-cadherin in early development (Pujic and Malicki, 2001), we controlled the expression of the dominant-negative protein by a heat-shock inducible promoter. Expression was induced by a one-hour heat-shock at 37°C at 20hpf in Tg(ath5:gapGFP) embryos either injected with hsp70:ncadhΔEC-mCherry or a control construct (hsp70:mCherryCAAX). The injected embryos were fixed at 36hpf. As most RGCs have just exited cell cycle at 36hpf, we speculated that most of the newly born RGCs should still retain apical processes (Hu and Easter, 1999). As expected, the majority of the Ath5:gapGFP+ cells expressing hsp70:mCherryCAAX had unretracted processes attached to the apical surface. However, Ath5:gapGFP+ cells expressing the dominant-negative N-cadherin protein had fewer unretracted apical process (Fig. 3D). Thus N-cadherin function appears to be essential for the attachment of newborn RGC apical processes.
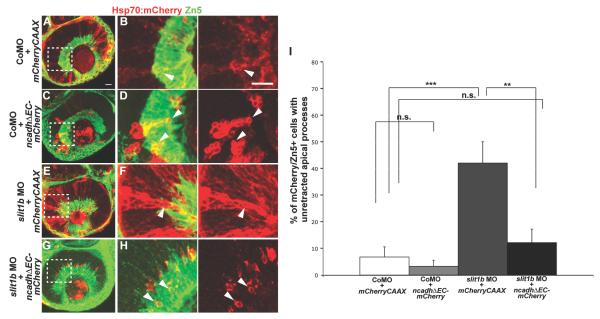
Dominant-negative N-cadherin rescues the retraction phenotype in slit1b morphants
As we found thatA possible explanation for the fact that blocking Slit1b/Robo3 signaling inhibits apical retraction whereas interfering with N-cadherin function leads to precocious retraction in developing RGCs. One possible explanation for these opposing phenotypes is that Slit/Robo signaling normally down-regulates N-cadherin mediated apical adhesion. If this was the case, the dominant-negative N-cadherin phenotype should be epistatic to the Slit1b phenotype. To test this notion, embryos were co-injected with control morpholino and hsp70:mCherryCAAX or hsp70:ncadhΔEC-mCherry, or with slit1b morpholino and hsp70:mCherryCAAX or hsp70:ncadhΔEC-mCherry. Embryos were then heat-shocked at 24hpf and fixed at 48hpf. Knockdown of Slit1b was confirmed to inhibit apical retraction, as shown by a significantly higher proportion of Hsp70:mCherryCAAX RGCs with unretracted apical processes in slit1b morphants compared to those in the control morphants (Fig. 4A-F). The Hsp70:NcadhΔEC-mCherry expressing RGCs, however, retracted their apical processes efficiently in these morphants (Fig. 4G), showing that blocking N-cadherin function in the morphant cells rescues apical retraction. These results suggest a model in which Slit-Robo signaling in newborn RGCs downregulates N-cadherin mediated attachment at the apically located adherens junction allowing apical retraction upon cell cycle exit.
Figure 4.
Expression of dominant-negative N-cadherin rescues apical retraction in slit1b morphant RGCs. Confocal images from control morpholino injected embryos expressing Hsp70:mCherryCAAX (A,B) or Hsp70:NcadhΔEC-mCherry (C,D); confocal images from slit1b morphants expressing Hsp70:mCherryCAAX (E,F) or Hsp70:NcadhΔEC-mCherry (G,H) at 48hpf. Zn5 (pseudo-colored in green) labels the ganglion cells. Boxed regions in A, C, E and G are enlarged in B, D, F and H. Arrowheads in F indicate the unretracted apical process of an Hsp70:mCherryCAAX expressing RGC (defined as cells that are Zn5+) in a slit1b morphant; arrowheads in B, F and H indicate mCherry positive RGCs with retracted apical processes. Scale bars: 20μm. (I) Percentage of mCherry positive RGCs with unretracted apical processes at 48hpf (n=44 Hsp70:mCherryCAAX expressing cells from control morpholino injected embryos; n=61 Hsp70:NcadhΔEC-mCherry expressing cells from control morpholino injected embryos; n=38 Hsp70:mCherryCAAX expressing cells from slit1b morphants; n=41 Hsp70:NcadhΔEC-mCherry expressing cells from slit1b morphants). Error bars indicate standard errors (**p<0.01; ***p<0.001).
Discussion
In this report, we define a putative molecular pathway underlying the retraction of apical processes in newborn RGCs. We first show that knocking down Slit1b inhibits apical process retraction, corroborating previous data (Zolessi et al., 2006). We identified Robo3 as the likely receptor for Slit1b in this process. As work in other systems has shown that Slit/Robo signaling is capable of modulating cadherin function (Rhee et al., 2002; Medioni et al., 2008; Santiago-Martinez et al., 2008), we next investigated whether N-cadherin, which is known to be part of the adherens junctions at the apical complex in neuroepithelial cells, is also involved in the process of RGC apical retraction. We found that the expression of a dominant negative N-cadherin in RGCs leads to premature detachment of their apical processes and rescues the apical detachment phenotype in slit1b morphants.
Our results are thus consistent with the possibility that N-cadherin is downregulated by Slit-Robo signaling, leading to the loss of apical adhesion. To establish further details of this molecular pathway, it will be necessary to investigate the links between Slit/Robo signaling and N-cadherin. Epithelial/mesenchyme transition has been found to be mediated by Rab5 dependent endocytosis of E-cadherin (Fujita et al., 2002; Palacios et al, 2005; Emery and Knoblich 2006), and E-cadherin internalization can be induced by soluble factors (Kamei et al., 1999; Ulrich et al., 2005; Bryant et al., 2007). Preliminary experiments with endocytosis inhibitors and the expression of dominant-negative Rab5, however, failed to interfere with apical retraction of RGCs in our system (data not shown), suggesting that other effectors might be at play. Likely candidates include those discovered by Rhee et al. (2002, 2007), in cell culture systems, who found that Slit activation of Robo leads to the phosphorylation of β-catenin by Abl; this causes a decrease in the binding affinity of β-catenin for N-cadherin and reduces N-cadherin mediated adhesion by severing its link to the cytoskeleton.
Transient disruption of integrin functions by injection of blocking antibody in mouse ventricular zone leads to detachment of apical processes from the ventricular surface (Loulier et al., 2009); this suggests that integrin signaling could also play a role in the apical attachment of neuroepithelial cells in the developing mouse cortex. Our results, though more consistent with a role for adherens junctions in apical attachment as suggested by Capello et al., (2006) and Imai et al., (2006), do not rule out the possibility that both cadherins and integrins are involved in the attachments of apical processes, and that neither of them alone is sufficient. Given the expression of Slits, Robos and Cadherin in different developing tissues (Bitzur et al., 1994; Challa et al., 2001; Lee et al., 2001; Hutson et al., 2003), we propose that Slit/Robo signaling and cadherin downregulation may be a common mechanism of apical processes retraction.
Precocious detachment affects the proliferation potential (Cappello et al., 2006; Imai et al., 2006), and failure to retract would inhibit the temporally orchestrated migration to distant sites, such as the cerebral cortex. Obviously a key issue for future research is what regulates the timing of this signaling. One attractive possibility in the retina is that Robo3 is only expressed when cells have completed their final mitosis. Our in situ results on the timing and localized expression of robo3 mRNA in subsets of retinal cells are consistent with this idea. Interestingly, Slit1b expression is also turned on just at this stage, so, even if retinal cells prematurely express Robo3 they cannot detach until Slit1b is expressed and vice versa. We hope this work will stimulate further research into these relatively unexplored questions of developmental cell biology.
Acknowledgements
This project was supported by a Wellcome Trust Programme Grant (WAH) and Croucher Foundation Scholarship (GKWW) during her PhD work and several of the “data not shown” can be found in her 2011 dissertation “Apical Process Retraction upon Neurogensis in the Zebrafish Retina” filed in the University Library at Cambridge University. We would like to thank Flavio Zolessi and Lucia Poggi for sharing the morpholinos, ath5 constructs and transgenic fish lines. We are grateful for the kind gifts of the pEGFP-N1-ncadhΔEC by Assistant Professor James Jontes (Ohio State University, USA), the pCS2FA-transposase by Associate Professor Brian Link (Medical College of Wisconsin, USA), and the different Tol2 entry clones and other constructs by Associate Professor Katharine Lewis (Syracuse University, USA) and Professor Chi-bin Chien (University of Utah, USA). We would also like to thank Jeremy Skepper for support with confocal microscopy, Adrian McNabb, Kirsty Scott and Tomasz Dyl for taking care of our animal stocks, and Patricia Jusuf, Owen Randlett, Helen Lynn and Georgina Stooke-Vaughan for technical assistance, and Michalis Agathocleous for statistical advice.
References
- Bitzur S, Kam Z, Geiger B. Structure and distribution of N-cadherin in developing zebrafish embryos: morphogenetic effects of ectopic overexpression. Dev Dyn. 1994;201:121–136. doi: 10.1002/aja.1002010204. [DOI] [PubMed] [Google Scholar]
- Butler K, Zorn AM, Gurdon JB. Nonradioactive in situ hybridization to Xenopus tissue sections. Methods. 2001;23:303–12. doi: 10.1006/meth.2000.1142. [DOI] [PubMed] [Google Scholar]
- Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Brauninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB, Brakebusch C, Gotz M. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- Challa AK, Beattie CE, Seeger MA. Identification and characterization of roundabout orthologs in zebrafish. Mech Dev. 2001;101:249–253. doi: 10.1016/s0925-4773(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Challa AK, McWhorter ML, Wang C, Seeger MA, Beattie CE. Robo3 isoforms have distinct roles during zebrafish development. Mech Dev. 2005;122:1073–1086. doi: 10.1016/j.mod.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Hu M, Easter SS. Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Dev Biol. 1999;207:309–321. doi: 10.1006/dbio.1998.9031. [DOI] [PubMed] [Google Scholar]
- Hutson LD, Jurynec MJ, Yeo SY, Okamoto H, Chien CB. Two divergent slit1 genes in zebrafish. Dev Dyn. 2003;228:358–369. doi: 10.1002/dvdy.10386. [DOI] [PubMed] [Google Scholar]
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–1744. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- Jontes JD, Emond MR, Smith SJ. In vivo trafficking and targeting of N-cadherin to nascent presynaptic terminals. J Neurosci. 2004;24:9027–9034. doi: 10.1523/JNEUROSCI.5399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Ray R, Chien CB. Cloning and expression of three zebrafish roundabout homologs suggest roles in axon guidance and cell migration. Dev Dyn. 2001;221:216–230. doi: 10.1002/dvdy.1136. [DOI] [PubMed] [Google Scholar]
- Liu Q, Babb SG, Novince ZM, Doedens AL, Marrs J, Raymond PA. Differential expression of cadherin-2 and cadherin-4 in the developing and adult zebrafish visual system. Vis Neurosci. 2001;18:923–933. [PubMed] [Google Scholar]
- Loulier K, Lathia JD, Marthiens V, Relucio J, Mughal MR, Tang SC, Coksaygan T, Hall PE, Chigurupati S, Patton B, Colognato H, Rao MS, Mattson MP, Haydar TF, Ffrench-Constant C. beta1 integrin maintains integrity of the embryonic neocortical stem cell niche. PLoS Biol. 2009;7:e1000176. doi: 10.1371/journal.pbio.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medioni C, Astier M, Zmojdzian M, Jagla K, Semeriva M. Genetic control of cell morphogenesis during Drosophila melanogaster cardiac tube formation. J Cell Biol. 2008;182:249–261. doi: 10.1083/jcb.200801100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Nieman MT, Kim JB, Johnson KR, Wheelock MJ. Mechanism of extracellular domain-deleted dominant negative cadherins. J Cell Sci. 1999;112(Pt 10):1621–1632. doi: 10.1242/jcs.112.10.1621. [DOI] [PubMed] [Google Scholar]
- Poggi L, Vitorino M, Masai I, Harris WA. Influences on neural lineage and mode of division in the zebrafish retina in vivo. J Cell Biol. 2005;171:991–999. doi: 10.1083/jcb.200509098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujic Z, Malicki J. Mutation of the zebrafish glass onion locus causes early cell-nonautonomous loss of neuroepithelial integrity followed by severe neuronal patterning defects in the retina. Dev Biol. 2001;234:454–469. doi: 10.1006/dbio.2001.0251. [DOI] [PubMed] [Google Scholar]
- Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J. Cables links Robo-bound Abl kinase to N-cadherin-bound beta-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat Cell Biol. 2007;9:883–892. doi: 10.1038/ncb1614. [DOI] [PubMed] [Google Scholar]
- Rhee J, Mahfooz NS, Arregui C, Lilien J, Balsamo J, VanBerkum MF. Activation of the repulsive receptor Roundabout inhibits N-cadherin-mediated cell adhesion. Nat Cell Biol. 2002;4:798–805. doi: 10.1038/ncb858. [DOI] [PubMed] [Google Scholar]
- Santiago-Martinez E, Soplop NH, Patel R, Kramer SG. Repulsion by Slit and Roundabout prevents Shotgun/E-cadherin-mediated cell adhesion during Drosophila heart tube lumen formation. J Cell Biol. 2008;182:241–248. doi: 10.1083/jcb.200804120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K, Hirano S, McMahon AP, Takeichi M. Wnt-1-dependent regulation of local E-cadherin and alpha N-catenin expression in the embryonic mouse brain. Development. 1994;120:2225–2234. doi: 10.1242/dev.120.8.2225. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Comparison of topographical patterns of ganglion and photoreceptor cell differentiation in the retina of the zebrafish, Danio rerio. J Comp Neurol. 1996;371:222–234. doi: 10.1002/(SICI)1096-9861(19960722)371:2<222::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Zolessi FR, Poggi L, Wilkinson CJ, Chien CB, Harris WA. Polarization and orientation of retinal ganglion cells in vivo. Neural Develop. 2006;1:2. doi: 10.1186/1749-8104-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]