Summary/abstract
The quantification of malaria transmission for the stratification of malaria risk has long been a concern for epidemiologists. During the era of the global malaria eradication programme, measurements of malaria endemicity were institutionalized by their incorporation into rules that defined action points for the activities of malaria control programmes. Here we use this review to revisit the historical development of these indices and their contemporary relevance. This is at a time when many malaria endemic countries are scaling-up their malaria control activities and reconsidering their prospects for elimination. These considerations are also important to an international community that has been recently challenged to revaluate the prospects for malaria eradication.
Keywords: global malaria eradication programme, control, elimination, eradication, intervention, malaria atlas project
Malariologists have always sought to grade the malaria transmission continuum, from intense transmission to interrupted transmission, for conceptual, communication and ultimately control purposes. The global malaria eradication programme (GMEP) coordinated by the World Health Organization (WHO) is important in this history as it focussed the research of malariologists and the operational activities of malaria control programmes for a quarter of a century (c. 1950-1975).1-11 This paper reviews a central aspect of its activities; the institutionalization of the criteria for the measurement of malaria and their use in its control, elimination and proposed eradication (Box 1).2, 12-15 The indices selected during the GMEP and their critical values were not only used to define transitions between specific activities of malaria control programmes, but also established a common nomenclature for describing malaria risk that survives today. The metrics and thresholds used achieved a de facto consensus through their widespread application, but were never universally accepted among malaria specialists.
Box 1 - the nomenclature of disease control, elimination and eradication.
The terms control, elimination and eradication need defining from the outset of this review. There has been evolution and argument on the definition of these terms during the last century3, 131 and their uncritical use today testifies to a continuing confusion.132, 133 Control is the deliberate reduction of disease incidence to a locally acceptable and manageable level; control must therefore be sustained to maintain the reduction.131, 134 Elimination is the deliberate reduction of infection incidence to zero in a delimited geographical area; intervention is therefore required to stop re-establishment.131, 134 Eradication is the permanent global reduction of infection incidence to zero through deliberate efforts; interventions are thus no longer required.131, 134 The term extinction is not appropriately used in an epidemiological context, as it requires the destruction of the pathogen in nature and the laboratory; equally hard to achieve as to prove.131, 134 When describing previous work, it is not always possible to use these terms precisely; certification of “eradication” for a country is a non-sequitur for example; it should be certification of elimination. In these cases the terms are enclosed with inverted commas to highlight the ambiguity.
The collective memory of these measurements and decision-rules, their rationale and experience of their operational performance has waned. Reflection on these considerations is timely because new initiatives have been established to map the global geographical limits of Plasmodium falciparum malaria and its endemicity within this range.16-18 In addition, this is also at a time when the political commitment to malaria interventions has been reinvigorated: many malaria endemic countries are taking their control activities to scale19-21 and others are reconsidering the prospects for malaria elimination.22-25 Most recently the Bill and Melinda Gates foundation (http://www.gatesfoundation.org) issued a challenge to the malaria community to reconsider the prospects for malaria eradication. The aim of this review is to evaluate what malaria metrics should now be measured, mapped and monitored for prioritizing, planning and practising malaria control and where possible, its elimination.
To help understand the evolution of the GMEP rubrics and provide the relevant context, the historical development of the methods used to measure malaria endemicity is first explained. Those indices adopted by the GMEP and how they were used to guide the malaria control and “eradication” activities globally are then explored. Theoretical, empirical and control developments since the GMEP, that might suggest modification of these rubrics, are subsequently outlined. The reforms to these criteria indicated by this review are further documented with emphasis on where further clarification is needed.
Endemicity and early malariometry
The very etymology of endemic (in the population), versus epidemic (upon the population) shows the early recognition that the level of a disease and therefore its character vary between populations and places. The first method used to quantify malaria endemicity (and thus the first method of malariometry) was introduced in India in 1848 and involved determining the spleen rate, the proportion of a sampled population with palpable enlargement of the spleen26 found during a malaria survey (an investigation of selected age-groups of a randomly sampled population in order to assess the degree of malarial endemicity in a location). The term rate has unfortunately always been used in the context of malaria surveys despite the metric measured being a prevalence. Thus from the very beginning of malariometry attention was focussed on the clinical manifestations of malaria infection in the human population.
How to partition the malaria prevalence estimates obtained by surveys in an epidemiologically meaningful manner was subject to active and prolonged debate that is not examined here in detail. It took over 100 years to reach a consensus which characterised prevalence values from spleen rate surveys as follows: holoendemic >75%, hyperendemic 51-75%, mesoendemic 11-50% and hypoendemic <10, when measured in the 2-9 year old age-group.12 Shortly after this “consensus”, however, it was suggested that examining the peripheral blood for asexual malaria parasites during malaria surveys to define a parasite rate (PR) - historically always by microscopy, was preferred due to its increased specificity.13 This was despite the process being more invasive for participants, more logistically demanding and resulting in a more seasonally variable measure.27 Identical names for the endemic levels and their divisions were suggested with the only difference being that the measurement of holoendemic malaria, was recommended to be restricted to the one-year age group.13 These endemicity classes were put forward as a working hypothesis from expert opinion synthesis of empirical data.12, 27 Many malaria specialists remained unconvinced of their utility. George Macdonald (1903-1967) was characteristically vociferous: “The first two of these names have come into common use and may well be adopted in a colloquial form, while the last two have not received any general acceptance and do not deserve it”.28 Macdonald preferred the stable and unstable classification of malaria endemicity that he derived from a deeper mathematical understanding of the entomological determinants of malaria transmission.29
The stable-unstable classification was facilitated by the abstraction of the malaria life cycle between Anopheles and humans into a dynamic model of transmission by Sir Ronald Ross (1857-1932).30-33 Macdonald, in the light of a better understanding of mosquito population biology, elaborated on the opaque writings of Ross and expressed clearly the minimal set of parameters for a simple malaria transmission model (see Supplementary Information S1).34 Importantly, Macdonald then considered the implications of these modelled relationships for characterising malaria endemicity29 and showed that the stability of malaria was determined by the average number of feeds that a mosquito takes on man during its life; the stability index (formally = a / -loge p, see Supplementary Information S1 for definitions). This vector-based index differentiated stable malaria (insensitive to natural and man-made perturbations, with values >2.5) from unstable (very sensitive to climate and very amenable to control, with values <0.5).29 Intermediate stability was designated between these extremes. Macdonald considered this distinction fundamental and stated that “other classifications should be subordinate to this”.29 This method has the virtue of being able to classify malaria endemicity entirely from information on the bionomics of locally dominant Anopheles vectors.2 Like other vector-based malaria metrics35-38 it is rarely implemented and although the stable-unstable concept survives, it is used less precisely than Macdonald would have condoned. The reasons for the relative paucity of vector-based indices are the technical complexity of obtaining entomological-based metrics, ethical concerns related to exposing humans to malaria infection and measurement error issues.37-40
Regardless of the theoretical diversity, host-based assessments of malaria prevalence through malaria surveys dominate the formal and informal contemporary literature17 and are hence considered here in detail.
The statistics of prevalence
The prevalence of any condition is measured from a sample of a reasonably homogeneous population so that its precision should depend on the sample size and the amount of the disease.41-43 It is clear from an innate understanding of probability that the confidence we can place in an estimate of prevalence will decrease as the numbers sampled become smaller and/or as the disease becomes rarer. This is trivial to express mathematically and detailed guidance on the statistical sampling in malaria surveys44-46 was outlined for malaria control personnel as part of the mentoring activities of the GMEP.1-11 It is also simple to appreciate that as the reliability of malariometric surveys diminishes with prevalence, so the population sampled must increase as malaria becomes less frequent, if a specified level of confidence in an estimate is to be maintained. Since obtaining blood from people is not without cost (personal and programmatic) there comes a point where measuring prevalence is not optimal. This was defined operationally during the GMEP: “As soon, however, as the general volume of malaria has been reduced to any considerable extent, the indices furnished by malariometric surveys are no longer sensitive enough to measure further progress ... Analysis of evaluation data from eradication programmes as well as closer observations in the field have shown that the point at which malariometric surveys cease to be sufficiently sensitive is reached when parasite rates have dropped to a level of between 1% and 3%”.47 Therefore, when malaria prevalence dropped below these levels, alternative measures were required. These measures have usually been obtained by looking at the rate of clinical malaria in a population; its clinical incidence. Before examining in detail the structured operation of the GMEP, methods for measuring malaria incidence in populations are examined briefly.
Measuring incidence
The measurement of malaria incidence requires every suspected malaria case to be diagnosed through a comprehensive surveillance system comprising passive case detection (examination of those suspected, usually febrile cases presenting routinely to any point of the health service), supplemented by active case detection (examination of fever cases sought through domiciliary visits at regular intervals).15, 42 The results are usually expressed as an annual parasite incidence (API) per 1000 of the entire population of the administrative areas for which it is representative. During the GMEP the API was only deemed valid if the annual blood examination rate (ABER), the proportion of the target population examined, exceeded ten percent.1, 2, 5 The other metric often presented in this surveillance trio is the slide positivity rate (SPR); the percentage of examined slides found positive. These surveillance indices are of course related as follows: API = (ABER * SPR) / 10.48 The division by ten is necessary because API is expressed per 1000 and the other terms per 100. Interestingly, the formal definition of an area endemic for malaria is incidence-based and refers to “a constant measurable incidence both of cases and of natural transmission in an area over a succession of years”.27 To be consistent with the malaria endemicity stratification rubrics reviewed thus far, however, it would be more consistent to define this as a constant measurable prevalence.
The GMEP plan of intervention
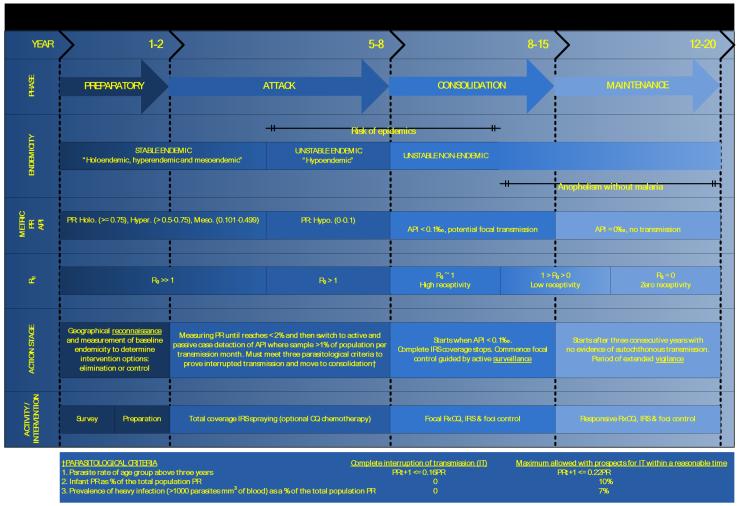
The measurement of the PR and the API had critical roles in the GMEP and together helped define the transitions between the four phases of intervention: preparatory, attack, consolidation and maintenance.1-3, 5 We have attempted to reconcile the measurements and transition points used in the GMEP with the epidemiological stratifications outlined above (Figure 1). It is worth noting that parasite specificity in relation to these indices was not routinely defined in the GMEP literature, as the goal was the eradication of all species of Plasmodia.
Figure 1.
Malaria endemicity, its stratification and time-lines for action phases of the global malaria eradication programme (GMEP). The schematic derives details of the phase, approximate timings, action stage and activity of the GMEP1-3, 5 and integrates these with malaria endemicity classifications defined by the host prevalence12, 13, vector stability indices29 and the basic reproductive number (see Supplementary Information SI).42 The year row presents an estimate of the duration of each phase (optimistic scenario-pessimistic scenario). PR: parasite rate, PfPR: Plasmodium falciparum parasite rate, Ro: basic reproductive number, API: annual parasite incidence, IRS: indoor residual spraying, RxCQ: radical treatment with chloroquine, IT: interruption of transmission.
The main objective of the GMEP was to complete the interruption of transmission of malaria through a time-limited geographically comprehensive indoor residual spraying (IRS, intra-domiciliary spraying of an insecticide with residual action against Anopheles, many species of which rest inside human structures) campaign of sufficient duration to eliminate the parasite reservoir, while minimizing the possibility of the development of insecticide resistance (usually suggested as 3-4 years).1-3, 5
Malaria PR surveys were recommended during the first preparatory phase to evaluate if the endemic level precluded the prospect of IRS-based cessation of transmission in this 3-4 year time-frame: to a good approximation this was synonymous with the holoendemic areas of Africa49, although exact prevalence levels for defining this important division seem to have been avoided.1-3, 5 If “eradication” was deemed feasible, complete geographical reconnaissance of the domiciles to be sprayed was undertaken and planning for IRS implementation initiated. Usually within 1-2 years, the IRS-based attack phase commenced and the declining parasite rate was used to monitor the progress of IRS and ultimately to verify the interruption of transmission.14
The attack phase was usually estimated to take 3-4 years; 1-1.5 years to the interruption of transmission and 1-1.5 and 2-2.5 for the disappearance of the P. falciparum and P. vivax parasite reservoir in humans respectively. Comprehensive surveillance was initiated when the PR fell below 2%, but in reality this was often initiated two years after the onset of the attack phase, on the assumption of success in interrupting transmission, since it took control programmes time to establish the necessary surveillance infrastructure. An imminent move to the consolidation phase was indicated when PR <= 2% and the three other parasitological criteria were met1-3, 5 (Figure 1). The criteria that were necessary to confirm the interruption of transmission were suggested by Macdonald14 and later endorsed and augmented by the W.H.O.50 as (i) the PR in populations greater than three years of age decreased by 78% per annum (a ratio of 1:0.22 per annum); (ii) that the PR in those born after the onset of the programme was 0% and that (iii) the prevalence of heavy infection (defined as > 1000 parasites per mm3 of blood) was 0%. Less strict criteria were necessary for the expectation of the interruption of transmission in a “reasonable” time (Figure 1).50
When the API was <0.1‰, the consolidation phase started and comprehensive IRS was stopped51. Originally this value was set higher (API <= 0.5‰)52, but this was reduced when experience showed that national malaria “eradication” programmes often overestimated the comprehensiveness of their surveillance, so that outbreaks were common after the cessation of IRS at this API level. The consolidation phase maintained a targeted control component, guided by active case detection to eliminate residual foci in human reservoirs and the remaining “islands” of transmission in the environment. During the GMEP, suspected malaria cases were treated presumptively (without awaiting the result of microscopic diagnosis of malaria infection) and radically (with an effective drug or drug combination for sufficient time, that eliminated all blood- and liver-stage parasites such that there was no possibility of relapse) on microscopic confirmation of infection. The duration of the consolidation phase was highly variable, depending as much on the longevity of the political commitment, as on the general epidemiological heterogeneity of the local malaria context.1-11 Movement to the maintenance phase was initiated after three years without autochthonous (local) transmission.53 To prove this required a very high level of surveillance with documentary evidence that every new case discovered could be classified as: (i) imported, a malaria case whose infection origin could be traced by a travel history to an area of transmission, outside the country in which it was diagnosed); (ii) relapsing, renewed clinical symptoms and/or parasitaemia resulting from an original infection due to the survival of hypnozoites (thus specific to P. vivax and P. ovale) for a period longer than the normal periodicity of paroxysms); induced, a malaria case resulting from any form of parenteral inoculation; or (iv) introduced, a first generation case of locally transmitted malaria following an imported case.27, 53 This was somewhat ambiguously extended to include an assessment of the likelihood of maintaining “eradication” in the later stages of the GMEP.51
The maintenance phase involved the introduction of vigilance measures by the public health service, not the specialized malaria “eradication” teams. These required assessments of both the vulnerability (a measure of the probability of reintroduction based on its proximity to other malarious areas and the flux of infected humans or Anopheles between the two) and receptivity (its natural endemic level) of an area, which together defined its malariogenic potential. We do not comment on the feasibility of maintaining elimination as this has been comprehensively reviewed elsewhere3, 54, but emphasize that two broad types of failures were identified during the GMEP; obviously those where the endemic level of malaria was such that transmission was never interrupted55-58, but more importantly for those nations reconsidering elimination, areas where transmission was interrupted but could not be maintained largely because the health care infrastructure was unable to support the required level of vigilance.3, 54, 59, 60 It is also worth noting that guidance on how to measure the concepts of vulnerability and susceptibility was never explicit, despite the central importance of these concepts.
The eventual certification of malaria eradication by the WHO had its own standards50, 52, 61, 62 and those reaching certification status were entered into the “Official register of areas where malaria eradication has been achieved” following inspection by a WHO delegation. A general pessimism surrounds many reviews of the GMEP7-11, 54, but it should be remembered that twenty four countries eliminated malaria, partly as a result of its activities and were entered into the register. The first of these was northern Venezuela (June 1961) and the last Singapore (November 1982).5, 23 Furthermore, despite the replacement of the GMEP with the global malaria control strategy63, many nations now continue to pursue the goal of elimination.22-25 The United Arab Emirates was the latest nation to receive official WHO certification of malaria eradication in 2007.64
What is important to understand, for those countries scaling-up their control activities and/or now targeting elimination, is do these metrics and action points remain feasible, plausible and useful? To explore this further and before looking at the modern context of malaria control, it is sensible to, as so many have done in the past: examine the predictions of mathematical models. Since these considerations are equation rich and of most interest to the malaria specialist, they have been annexed in supplemental information S1. It is also important to further clarify that P. vivax has biological, clinical and epidemiological differences that make it more difficult to measure, model and control.65-67 We do not review these factors in detail, as this has been done comprehensively elsewhere65-67, but note that in contrast to the GMEP, all further considerations are specific to P. falciparum.
The revised context
The main purpose of this review is to re-examine the historical bench-marks for standard malaria metrics employed by the GMEP for their contemporary relevance in a new era of malaria control and elimination. It is stressed that the aim of this exercise is not to be dogmatic, but to provide an evidence-based schematic that is informative for the modern day mapping of malaria for its control and facilitates a structured discussion of areas where further information is needed. To increase the relevance of these suggestions, the operational differences experienced by contemporary malaria control programmes, compared with those functioning during the GMEP are first considered.
Despite the-long term vision of the Millennium Development Goals68, the usual planning horizon of national malaria control programmes, regional offices of the WHO and international development agencies is constrained by the durability of funding commitments. It is thus pragmatic to conceive of five-year cycles during which nations can have staged impacts along the path towards their longer-term visions.
In contrast to the GMEP, a diversity of interventions are now used and the majority of malaria endemic nations have already developed comprehensive intervention plans (http://www.theglobalfund.org). It is assumed that all areas endemic for malaria would therefore wish to implement a combined set of geographically comprehensive interventions69 and that predominant (but not exclusively70, 71) among these, will be the free distribution of ITNs72-75, IRS with new insecticides76-78, prompt and effective radical treatment with ACTs76, 79 and intermittent presumptive therapy (IPT, a curative dose of an effective antimalarial drug given at fixed times regardless of infection in high risk groups such as pregnant women and infants) in high risk groups, also probably with ACTs.80, 81 In urban environments, environmental management and larval control might also be highly cost-effective.37, 82-84 While it is clear that each of these interventions will also substantially affect transmission, the combination of their impacts and how to optimize them is largely unknown. How can we use the evidence available to evaluate optimally control choices and their potential impact during the typical funding cycle? It is also worth noting that the testing of the performance of any modelled expectations of the efficacy of intervention combinations is complicated by the scaling-up of malaria control. Classic intervention trials are not ethical within the context of universal ITN coverage. Everyone is susceptible to clinical malaria, albeit with different levels of risk, but transmission is reduced when ITNs are used by those who carry asymptomatic malaria. ITN distribution programs should aim to cover all the population with ITNs, not just the most vulnerable, and to achieve the highest possible levels of ownership and use.85 The impact on intervention suites will now need to be assessed (and modelled) through carefully designed “surveillance trials”, where the incremental effects of additional intervention modes over universal bed-net coverage are tested.
Finally, in contrast to the time of the GMEP, the current status of global malaria surveillance is a cause of very real concern.86-89 Very few contemporary studies have tested the fidelity of national case surveillance activities86, 90 and this has obvious but unpredictable operational ramifications. Conversely, the last five years has seen a significant increase in the use of PR in national indicator surveys65, 91-93 that provide very intimate epidemiological detail for specific countries. A further challenge when establishing revised criteria, therefore, is to recognize and consider the problems and trends in malariometry during formulation.
A revised scheme
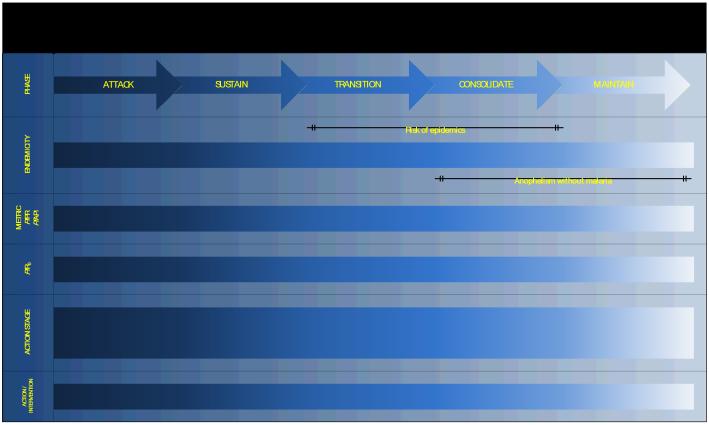
Having reviewed much of the relevant empirical and theoretical evidence, refinements in the way that malaria risk is measured and mapped are now suggested, along with some practical considerations (Figure 2). For heuristic reasons, these changes are related to classic endemic divisions, in the same way as we have done for the GMEP phases (Figure 1). A deliberate effort has been made to diminish the clear divisions between the now five stages of intervention: attack, sustain, transition, consolidate and maintain. We have also chosen not to make guesses about the duration of intervention phases, as the modelling framework to support these speculations requires further work. We rather view them pragmatically as steps of five year funding cycles that are implemented sequentially over one to two decades to achieve a long-term goal. This extended time-frame is informed by the experience of the GMEP, which showed that the time required to move from the attack to the maintenance phase (Figure 1) was highly variable between countries1-11 and in the order of decades, even when starting from low endemicity.94-102 How to monitor the steady progress through these steps is outlined (Figure 2).
Figure 2.
Malaria endemicity, its stratification and the mapping criteria of the Malaria Atlas Project (MAP). PfPR: Plasmodium falciparum parasite rate, PfAPI: Plasmodium falciparum annual parasite incidence, PfRo: basic reproductive number Plasmodium falciparum (see Supplementary Information SI), RM: Ross-Macdonald, IRS: indoor residual spraying, RxACT: radical treatment with artemisinin combination therapy; ITN: insecticide treated net, IRS: indoor residual spraying, IPT: intermittent presumptive therapy.
The attack phase commences in all areas where PfPR >= 40%; a threshold from which theory predicts it is unlikely to eliminate malaria with ITNs alone (see Supplementary Information SI). In other areas, the attack phase may commence with a combination of interventions applied nearly universally in a population. Reductions in clinical malaria are expected following high coverage, but a drop in the incidence of clinical malaria is not sufficient indication that malaria transmission has been reduced sufficiently. Practical and theoretical considerations, as well as experience suggest that the progress of the attack phase should continue to be measured by repeated PfPR based malaria surveys, as was done during the GMEP. This also resonates with the trend for incorporating PR data collection in national indicator surveys.65, 91-93 Since the GMEP era, however, further work has revealed a range of factors that can affect the precision with which PfPR is measured, including the performance of microscopists103, 104 and the timing of the PR measurement during the course of an infection.105 These measurement error issues remain important, but as rapid diagnostic tests (RDTs) become increasingly widespread106, 107, the logistical ease of malaria surveys increases. It should be anticipated that even with continuous, combined interventions deployed effectively at scale, some very high transmission areas may be very difficult to move to an endemic level that would indicate a transition to the sustain phase (PfPR < 40%).
In contrast to the GMEP, the interventions activities continue on movement from the attack to the sustain phase. Impact continues to be measured with PfPR malaria surveys until <= 5%. We have raised the level of a shift to surveillance from the 2-3% level of the GMEP since, as theory has shown, the information provided for control is minimal (all levels below PfPR 10% are of incredibly low transmission) and the error and thus numbers required for reliable surveys is maximal. As the PfPR falls below the 10% level, considerable effort should be invested in improving the rigour and depth of active and passive case surveillance.
When PfPR <= 5%, the transition phase is reached. In addition to malaria surveys, complementary measurements of PfAPI, over a series of three years, should also have been shown to have kept PfAPI below 1‰ p.a. The pairing of these metrics at these levels is justified by the results of the largest ever simultaneous review of the global PfAPI and PfPR data18. This stage represents an important transition conceptually and operationally, where active and passive surveillance for clinical malaria becomes the dominant measurement tool and is central to informing intensified control activity, targeted at the last foci of transmission.
The movement to the consolidation phase is signalled when PfAPI < 0.1‰ pa and is informed by historical experience of the GMEP.51 It may be prudent to add an additional requirement to sustain this level over a period of years, to ensure there have been no lapses in surveillance.
The movement to the maintenance phase is heralded when PfAPI = 0‰. The rigour with which nations should conduct these final surveillance and audit stages should exceed those suggested during the GMEP as they can be less sanguine about the prospects for global eradication and the risks of reintroduction of malaria vectors and imported malaria has increased and will persist for the foreseeable future.108-110 It should also be noted that, in the past, confirming the genesis of any P. falciparum cases found during the maintenance phase and any subsequent outbreaks after a country had been declared malaria free, was achieved through standard epidemiological investigation and serology.111 Today, whether these cases represent unique clusters of local transmission can now be established unequivocally by genotyping.112
Discussion
In a time of renewed political and national commitment to malaria control there is much that we still do not know. These uncertainties have been highlighted and those of particular concern are elaborated upon here.
Modelling the potential impact of existing suites of interventions and how these may be combined optimally is an obvious need. This would enable a further control related stratification of endemicity above the PfPR = 40% level. When considering only this conservative modelling framework, a very much larger proportion of the world than is traditionally considered may be suitable for elimination.1-11 Recent companion work shows that nearly one billion (42%) of the 2.37 billion people at risk of malaria in 2007 live in areas of unstable transmission (PfAPI < 0.1‰ pa) from which elimination is feasible.18 Significant mapping and modelling work is required to investigate the prospects for control and elimination in areas of stable transmission.
The time for interventions to achieve their desired impact once they are deployed is also an area of considerable uncertainty and is an extension of this modelling at the high transmission end of the spectrum. Even without this caveat, we are mindful here of the advice of Paul F. Russell (1894-1983) that “Time more than money and continuity more than perfection - these must be the mottoes guiding malaria control in the tropics”113 and reiterate that decades of work are required to succeed in elimination, even from a relatively modest baseline endemicty.94-102 There is room to be sanguine with regard to elimination in principle, but this optimism needs to be tempered by the realities of practice.1-11
In addition, the mathematics of low transmission P. falciparum malaria is not adequately addressed using these classical and deterministic modelling frameworks. Although these concerns were not completely ignored historically114-117, and more recent contributions have been made118, the stochastic modelling of low transmission malaria for the purposes of optimizing elimination merits attention. As infection becomes rare, it may be more efficient to replace campaign-style malaria control by focusing attention on people who have clinical malaria and on their neighbours who may be infected asymptomatically.71 The critical process is to find and eliminate the largely invisible reservoir of parasites in humans. Understanding transmission in these environments is essential and demands models that deal with rare infections in individuals in an explicitly spatial context.
The heterogeneity in transmission, necessary in the newer malaria transmission models (see Supplementary Information S1) to more realistically describe the PfPR-PfEIR and PfPR-PfRo relationship119, 120, is likely to be an important and related factor in malaria control121 and its elimination. Whether it turns out to be a help or hindrance will be determined largely by whether the sources of heterogeneity can be targeted equitably. A large remaining research agenda is identifying the human and/or vector based contributions to this transmission heterogeneity.119, 120
Quantifying vulnerability and receptivity was never done rigorously during the GMEP (although both are amenable through the modelling framework (see Supplementary Information S1) and is fundamental to those nations and/or regions wishing to maintain malaria free status. Useful guidelines on aspects of these activities have been released122, 123 and while they represent thorough canonical reviews, they have yet to fully embrace some of the reconnaissance opportunities offered by the democratization of high spatial resolution satellite imagery on modern 3D cartographic systems (e.g. http://earth.google.com), or the increasing ability to quantify and evaluate the impact of human transport systems.108-110 Moreover, serological measures are now available that can more reliably parameterize the history of individual infections and thus can be of considerable help, not only in verifying the interruption of transmission, especially when age-stratified124-127, as was first demonstrated in Mauritius128, but also in establishing the provenance of the natural parasite reservoir versus imported cases.129 It is also interesting to note that if these serological histories could be inferred from the blood samples collected with RDTs in national indicator cluster surveys, then the epidemiological information content of these investigations would be transformed radically. In addition, the added value of geo-positioning of clusters with hand-held GPS systems cannot be understated.
A malaria stratification of PfPR guided by the modelled feasibility of control and elimination is a primary research goal for the Malaria Atlas Project (http://www.map.ox.ac.uk). This complements considerable efforts to map the global limits of malaria and its endemicity within this range.16-18 Together they may provide a platform, not only to map extensively the likely impact of interventions, but the successive five year steps required to realize national plans. It will soon once again be possible to document what proportion of the global population is at risk of P. falciparum malaria, and the totals who reside under each intervention stage and thus to audit the cost and time required to move these populations towards sustainable control, and, where feasible, elimination.
We conclude with an important caveat, reinforced during the GMEP that despite metrics being useful guides, those implementing interventions should include flexibility and local knowledge in the application of any decision criteria: “In the study of malaria problems and in the formulation of control programmes, action based on generalisations is likely to be followed by the most disastrous consequences. It has been well said that the most hazardous of human tendencies is the drawing of general conclusions from limited experience, and in no instance it is more applicable than in the planning of malaria control measures”.130 There is still much to do in terms of assembling the relevant data16-18, addressing these with theory and thus helping to refine these generalisations at regional, national and sub-national levels.
Supplementary Material
Acknowledgements
We would like to thank Aafje Rietveld for helping answer questions regarding the WHO certification of malaria eradication. We also thank David J. Bradley, Carlos A. Guerra, Ellis F. McKenzie, G. Dennis Shanks and Andrew J. Tatem for commenting on the manuscript. SIH is funded by a Senior Research Fellowship from the Wellcome Trust (#079091). RWS is a Wellcome Trust Principal Research Fellow (#079080). They both acknowledge the support of the Kenyan Medical Research Institute (KEMRI). This paper is published with the permission of the director of KEMRI. This work forms part of the output of the Malaria Atlas Project (MAP, http://www.map.ox.ac.uk) principally funded by the Wellcome Trust, U.K.
Funding: SIH is funded by a Senior Research Fellowship from the Wellcome Trust (#079091). RWS is a Wellcome Trust Principal Research Fellow (#079080). The Wellcome Trust has no intellectual or editorial input into the content of this review.
Footnotes
Conflicts of interest: we declare that we have no conflicts of interest.
Search strategy and selection criteria: Information for this review was identified through PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez), the ISI Web of Science (http://wok.mimas.ac.uk), the WHO library (http://dosei.who.int; please note that all WHO manuscripts cited are archived in digital format at this extremely useful site), suggestions of reviewers (formal and informal), and the bibliographies of the resulting articles. We used the following Boolean search statement: “malaria” AND (“eradication” OR “elimination”), “malaria” AND (“control” OR “campaign”), “malaria” AND (“survey” OR “metric”). Articles in English, French and Spanish were selected and no date restrictions were applied.
References
- 1.Black RH. Manual of epidemiology and epidemiological services in malaria programmes. World Health Organization; Geneva: 1968. [Google Scholar]
- 2.Pampana E. A textbook of malaria eradication. Second ed. Oxford University Press; London: 1969. [Google Scholar]
- 3.Yekutiel P. Eradication of infectious diseases: a critical study. In: Klingberg MA, editor. 2 Contributions to epidemiology and biostatistics. Karger; Basel, Switzerland: 1980. p. 164. [Google Scholar]
- 4.Bruce-Chwatt LJ. Lessons learned from applied field research activities in Africa during the malaria eradication era. Bull World Health Organ. 1984;62:19–29. [PMC free article] [PubMed] [Google Scholar]
- 5.Nájera JA. Malaria control, achievements, problems and strategies. World Health Organization; Geneva: 1999. WHO/MAL/99.1087. [PubMed] [Google Scholar]
- 6.Nájera JA. Epidemiology in the strategies for malaria control. Parassitologia. 2000;42:9–24. [PubMed] [Google Scholar]
- 7.Scholtens RG, Kaiser RL, Langmuir AD. An epidemiologic examination of the strategy of malaria eradication. Int J Epidemiol. 1972;1:15–24. doi: 10.1093/ije/1.1.15. [DOI] [PubMed] [Google Scholar]
- 8.Gabaldon A. Global eradication of malaria: changes of strategy and future outlook. Am J Trop Med Hyg. 1969;18:641–56. doi: 10.4269/ajtmh.1969.18.641. [DOI] [PubMed] [Google Scholar]
- 9.Lepes T. Present status of the global malaria eradication programme and prospects for the future. J Trop Med Hyg. 1974;77:47–53. [PubMed] [Google Scholar]
- 10.Gramiccia G, Beales PF. The recent history of malaria control and eradication. In: Wernsdorfer WH, McGregor I, editors. Malaria: principles and practice of malariology. Churchill Livingstone; Edinburgh: 1988. pp. 1335–78. [Google Scholar]
- 11.Spielman A, Kitron U, Pollack RJ. Time limitation and the role of research in the worldwide attempt to eradicate malaria. J Med Ent. 1993;30:6–19. doi: 10.1093/jmedent/30.1.6. [DOI] [PubMed] [Google Scholar]
- 12.W.H.O. Report on the malaria conference in equatorial Africa. World Health Organization; Geneva: 1951. Held under the Joint Auspices of the World Health Organization and of the Commission for technical co-operation in Africa south of the Sahara. Kampala, Uganda, 27 November - 9 December 1950. [PubMed] [Google Scholar]
- 13.Metselaar D, Van Thiel PH. Classification of malaria. Trop Geographical Med. 1959;11:157–61. [Google Scholar]
- 14.Macdonald G, Goeckel GW. The malaria parasite rate and interruption of transmission. Bull World Health Organ. 1964;31:365–77. [PMC free article] [PubMed] [Google Scholar]
- 15.Pull JH. Malaria surveillance methods, their uses and limitations. Am J Trop Med Hyg. 1972;21:651–7. doi: 10.4269/ajtmh.1972.21.651. [DOI] [PubMed] [Google Scholar]
- 16.Hay SI, Snow RW. The Malaria Atlas Project: developing global maps of malaria risk. PLoS Med. 2006;3:e473. doi: 10.1371/journal.pmed.0030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerra CA, Hay SI, Lucioparedes LS, et al. Assembling a global database of malaria parasite prevalence for the Malaria Atlas Project. Malaria J. 2007;6:17. doi: 10.1186/1475-2875-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerra CA, Gikandi PW, Tatem AJ, et al. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 2007 doi: 10.1371/journal.pmed.0050038. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feachem RG, Sabot OJ. Global malaria control in the 21st century: a historic but fleeting opportunity. Journal of the American Medical Association. 2007;297:2281–4. doi: 10.1001/jama.297.20.2281. [DOI] [PubMed] [Google Scholar]
- 20.G.F.T.A.M. The Global Fund. Who we are what we do. The Global Fund to Fight AIDS, Tuberculosis and Malaria (GFTAM); Geneva: 2007. [Google Scholar]
- 21.G.F.T.A.M. An evolving partnership: The Global Fund and civil society in the fight against AIDS, tuberculosis and malaria. The Global Fund to Fight AIDS, Tuberculosis and Malaria (GFTAM); Geneva: 2007. [Google Scholar]
- 22.W.H.O. Regional Office for Europe . Regional strategy: from malaria control to elimination in the WHO European Region 2006-2015. World Health Organization Regional Office for Europe; Copenhagen, Denmark: 2006. WHO-EUR/06/5061322. [Google Scholar]
- 23.W.H.O. Informal consultation on malaria elimination: setting up the WHO agenda. World Health Organization; Geneva: 2006. WHO/HTM/MAL/2006.1114. [Google Scholar]
- 24.W.H.O. P.A.H.O. Regional strategic plan for malaria in the Americas 2006-2010. Pan American Health Organization, Regional Office for the Americas; Washington, D.C: 2006. [Google Scholar]
- 25.W.H.O. Regional Office for the Eastern Mediterranean . Strategic plan for malaria control and elimination in the WHO Eastern Mediterranean Region 2006-2010. World Health Organization Regional Office for the Eastern Mediterranean; Cairo: 2007. WHO-EM/MAL/340/E. [Google Scholar]
- 26.Dempster TE. Notes on the application of the test of organic disease of the spleen, as an easy and certain method of detecting malarious localities in hot climates, Agra. 1848 reprint in Rec. Malar. Surv, India, 1930, 1, 69. [Google Scholar]
- 27.W.H.O. World Health Organization; Geneva: 1963. Terminology of malaria and of malaria eradication. Report of a drafting committee. [Google Scholar]
- 28.Macdonald G. The epidemiology and control of malaria. Oxford University Press; London: 1957. Local features of malaria; pp. 63–99. [Google Scholar]
- 29.Macdonald G. The analysis of equilibrium in malaria. Trop Dis Bull. 1952;49:813–1129. [PubMed] [Google Scholar]
- 30.Ross R. The prevention of malaria. John Murray; London: 1911. [Google Scholar]
- 31.Ross R. An application of the theory of probabilities to the study of a priori pathometry. Part I. Proc R Soc Lond Ser A. 1916;92:204–30. [Google Scholar]
- 32.Ross R, Hudson HP. An application of the theory of probabilities to the study of a priori pathometry. Part II. Proc R Soc Lond Ser A. 1917;93:212–25. [Google Scholar]
- 33.Ross R, Hudson HP. An application of the theory of probabilities to the study of a priori pathometry. Part III. Proc R Soc Lond Ser A. 1917;93:225–40. [Google Scholar]
- 34.Macdonald G. The epidemiology and control of malaria. Oxford University Press; London: 1957. [Google Scholar]
- 35.Garrett-Jones C. Prognosis for interruption of malaria transmission through assessment of the mosquito’s vectorial capacity. Nature. 1964;204:1173–5. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]
- 36.Garrett-Jones C. The human blood index of malaria vectors in relation to epidemiological assessment. Bull World Health Organ. 1964;30:241–61. [PMC free article] [PubMed] [Google Scholar]
- 37.Hay SI, Guerra CA, Tatem AJ, et al. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hay SI, Rogers DJ, Toomer JF, et al. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review. Trans Roy Soc Trop Med Hyg. 2000;94:113–27. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drakeley C, Schellenberg D, Kihonda J, et al. An estimation of the entomological inoculation rate for Ifakara: a semi-urban area in a region of intense malaria transmission in Tanzania. Trop Med Int Health. 2003;8:767–74. doi: 10.1046/j.1365-3156.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 40.Dye C. Vectorial capacity: must we measure all its components? Parasitol Today. 1986;2:203–9. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- 41.Jovani R, Tella JL. Parasite prevalence and sample size: misconceptions and solutions. Trends Parasitol. 2006;22:214–8. doi: 10.1016/j.pt.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Molineaux L, Muir DA, Spencer HC, et al. The epidemiology of malaria and its measurement. In: Wernsdorfer WH, McGregor I, editors. Malaria: principles and practice of malariology. Churchill Livingstone; Edinburgh: 1988. pp. 999–1089. [Google Scholar]
- 43.Gregory RD, Blackburn TM. Parasite prevalence and host sample size. Parasitol Today. 1991;7:316–8. doi: 10.1016/0169-4758(91)90269-t. [DOI] [PubMed] [Google Scholar]
- 44.Swaroop S. Statistical considerations and methodology in malaria eradication. Part I. Statistical considerations. World Health Organization; Geneva: 1959. WHO/Mal/240. [Google Scholar]
- 45.Swaroop S. Statistical considerations and methodology in malaria eradication. Part II. Statistical methodology. World Health Organization; Geneva: 1959. WHO/Mal/240. [Google Scholar]
- 46.Swaroop S, Gilroy AB, Uemura K. Statistical methods in malaria eradication. World Health Organization; Geneva: 1966. [PubMed] [Google Scholar]
- 47.Yekutiel P. Problems of epidemiology in malaria eradication. Bull World Health Organ. 1960;22:669–83. [PMC free article] [PubMed] [Google Scholar]
- 48.Ray AP, Beljaev AE. Epidemiological surveillance: a tool for assessment of malaria and its control. J Commun Dis. 1984;16:197–207. [PubMed] [Google Scholar]
- 49.W.H.O. World Health Organization; Geneva: 1974. Malaria control in countries where time-limited eradication is impracticable at present. Report of a W.H.O. interregional conference. [PubMed] [Google Scholar]
- 50.W.H.O. W.H.O. expert committee on malaria: twelfth report. World Health Organization; Geneva: 1966. [PubMed] [Google Scholar]
- 51.W.H.O. W.H.O. expert committee on malaria: tenth report. World Health Organization; Geneva: 1964. [PubMed] [Google Scholar]
- 52.W.H.O. W.H.O. expert committee on malaria: eighth report. World Health Organization; Geneva: 1961. [PubMed] [Google Scholar]
- 53.W.H.O. W.H.O. expert committee on malaria: sixth report. World Health Organization; Geneva: 1957. [PubMed] [Google Scholar]
- 54.W.H.O. Re-examination of the global strategy of malaria eradication. A report by the Director-General to the 22nd World Health Assembly, May 30, 1969. Official Records of the World Health Organization. 1969;176:106–26. [Google Scholar]
- 55.Draper CC, Smith A. Malaria in the Pare area of Tanganyika. Part II. Effects of three years’ spraying of huts with dieldrin. Trans Roy Soc Trop Med Hyg. 1960;54:342–57. doi: 10.1016/0035-9203(60)90115-2. [DOI] [PubMed] [Google Scholar]
- 56.Foll CV, Pant CP, Lietaert PE. A large-scale field trial with Dichlorvos as a residual fumigant insecticide in northern Nigeria. Bull World Health Organ. 1965;32:531–50. [PMC free article] [PubMed] [Google Scholar]
- 57.Molineaux L, Gramiccia G. The Garki Project Research on the epidemiology and control of malaria in the Sudan savanna of West Africa. World Health Organization; Geneva: 1980. Chapter 5. Parasitology; pp. 109–72. [Google Scholar]
- 58.Smith A, Draper CC. Malaria in the Taveta area of Kenya and Tanganyika. Part II. Results after three and a half years’ treatment of huts with dieldrin. East Afr Med J. 1959;36:629–43. [PubMed] [Google Scholar]
- 59.Sharma VP. Re-emergence of malaria in India. Indian Journal of Medical Research. 1996;103:26–45. [PubMed] [Google Scholar]
- 60.Mouchet J, Laventure S, Blanchy S, et al. The reconquest of the Madagascar highlands by malaria. Bull Soc Pathol Exot. 1997;90:162–8. [PubMed] [Google Scholar]
- 61.W.H.O. W.H.O. expert committee on malaria: thirteenth report. World Health Organization; Geneva: 1967. [Google Scholar]
- 62.W.H.O. W.H.O. expert committee on malaria: sixteenth report. World Health Organization; Geneva: 1974. [PubMed] [Google Scholar]
- 63.W.H.O. A global strategy for malaria control. World Health Organization; Geneva: 1993. [PubMed] [Google Scholar]
- 64.W.H.O. United Arab Emirates certified malaria-free. Wkly Epidemiol Rec. 2007;4:25–32. [PubMed] [Google Scholar]
- 65.Brooker S, Leslie T, Kolaczinski K, et al. Spatial epidemiology of Plasmodium vivax, Afghanistan. Emerg Infect Dis. 2006;12:1600–2. doi: 10.3201/eid1210.060051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sattabongkot J, Tsuboi T, Zollner GE, et al. Plasmodium vivax transmission: chances for control? Trends Parasitol. 2004;20:192–8. doi: 10.1016/j.pt.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Rosenberg R. Plasmodium vivax in Africa: hidden in plain sight? Trends Parasitol. 2007;23:193–6. doi: 10.1016/j.pt.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Sachs JD, McArthur JW. The Millennium Project: a plan for meeting the millennium development goals. Lancet. 2005;365:347–53. doi: 10.1016/S0140-6736(05)17791-5. [DOI] [PubMed] [Google Scholar]
- 69.Barat LM. Four malaria success stories: how malaria burden was successfully reduced in Brazil, Eritrea, India, and Vietnam. Am J Trop Med Hyg. 2006;74:12–6. [PubMed] [Google Scholar]
- 70.Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco-epidemiological settings with environmental management: a systematic review. Lancet Infectious Diseases. 2005;5:695–708. doi: 10.1016/S1473-3099(05)70268-1. [DOI] [PubMed] [Google Scholar]
- 71.Macauley C. Aggressive active case detection: a malaria control strategy based on the Brazilian model. Social Science and Medicine. 2005;60:563–73. doi: 10.1016/j.socscimed.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 72.Nyarango PM, Gebremeskel T, Mebrahtu G, et al. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malaria J. 2006;5:33. doi: 10.1186/1475-2875-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Noor AM, Amin AA, Akhwale WS, et al. Increasing coverage and decreasing inequity in insecticide-treated bed net use among rural Kenyan children. PLoS Med. 2007;4:e255. doi: 10.1371/journal.pmed.0040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill J, Lines J, Rowland M. Insecticide-treated nets. Adv Parasitol. 2006;61:77–128. doi: 10.1016/S0065-308X(05)61003-2. [DOI] [PubMed] [Google Scholar]
- 75.Fegan GW, Noor AM, Akhwale WS, et al. Effect of expanded insecticide-treated bednet coverage on child survival in rural Kenya: a longitudinal study. Lancet. 2007;370:1035–9. doi: 10.1016/S0140-6736(07)61477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barnes KI, Durrheim DN, Little F, et al. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med. 2005;2:e330. doi: 10.1371/journal.pmed.0020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coleman M, Sharp B, Seocharan I, et al. Developing an evidence-based decision support system for rational insecticide choice in the control of African malaria vectors. J Med Ent. 2006;43:663–8. doi: 10.1603/0022-2585(2006)43[663:daedss]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 78.Hemingway J, Beaty BJ, Rowland M, et al. The Innovative Vector Control Consortium: improved control of mosquito-borne diseases. Trends Parasitol. 2006;22:308–12. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Sutherland CJ, Ord R, Dunyo S, et al. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2005;2:e92. doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Meara WP, Breman JG, McKenzie FE. The promise and potential challenges of intermittent preventive treatment for malaria in infants (IPTi) Malaria J. 2005;4:33. doi: 10.1186/1475-2875-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White NJ. Intermittent presumptive treatment for malaria. A better understanding of the pharmacodynamics will guide more rational policymaking. PLoS Med. 2005;2:e3. doi: 10.1371/journal.pmed.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walker K, Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Med Vet Ent. 2007;21:2–21. doi: 10.1111/j.1365-2915.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- 83.Keiser J, Utzinger J, Caldas de Castro M, et al. Urbanization in sub-saharan Africa and implication for malaria control. Am J Trop Med Hyg. 2004;71:118–27. [PubMed] [Google Scholar]
- 84.Robert V, MacIntyre K, Keating J, et al. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg. 2003;68:169–76. [PubMed] [Google Scholar]
- 85.Killeen GF, Smith TA, Ferguson HM, et al. Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide-treated nets. PLoS Med. 2007;4:e229. doi: 10.1371/journal.pmed.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erhart A, Thang ND, Xa NX, et al. Accuracy of the health information system on malaria surveillance in Vietnam. Trans Roy Soc Trop Med Hyg. 2007;101:216–25. doi: 10.1016/j.trstmh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Gething PW, Noor AM, Gikandi PW, et al. Improving imperfect data from health management information systems in Africa using space-time geostatistics. PLoS Med. 2006;3:e271. doi: 10.1371/journal.pmed.0030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma VP. Battling the malaria iceberg with chloroquine in India. Malaria J. 2007;6:105. doi: 10.1186/1475-2875-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Snow RW, Guerra CA, Noor AM, et al. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chilundo B, Sundby J, Aanestad M. Analysing the quality of routine malaria data in Mozambique. Malaria J. 2004;3:3. doi: 10.1186/1475-2875-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eliades MJ, Wolkon A, Morgah K, et al. Burden of malaria at community level in children less than 5 years of age in Togo. Am J Trop Med Hyg. 2006;75:622–9. [PubMed] [Google Scholar]
- 92.Sintasath DM, Ghebremeskel T, Lynch M, et al. Malaria prevalence and associated risk factors in Eritrea. Am J Trop Med Hyg. 2005;72:682–7. [PubMed] [Google Scholar]
- 93.Kolaczinski J, Graham K, Fahim A, et al. Malaria control in Afghanistan: progress and challenges. Lancet. 2005;365:1506–12. doi: 10.1016/S0140-6736(05)66423-9. [DOI] [PubMed] [Google Scholar]
- 94.Benzerroug EH. The malaria eradication programme in Algeria: present situation. Trans Roy Soc Trop Med Hyg. 1990;84:347. doi: 10.1016/0035-9203(90)90310-b. [DOI] [PubMed] [Google Scholar]
- 95.de Zulueta J, Muir DA. Malaria eradication in the Near East. Trans Roy Soc Trop Med Hyg. 1972;66:679–96. doi: 10.1016/0035-9203(72)90082-x. [DOI] [PubMed] [Google Scholar]
- 96.Bruce-Chwatt LJ. Malaria eradication in Portugal. Trans Roy Soc Trop Med Hyg. 1977;71:232–40. doi: 10.1016/0035-9203(77)90014-1. [DOI] [PubMed] [Google Scholar]
- 97.Logan JA. The Sardinian project: an experiment in the eradication of an indigenous malarious vector. The American Journal of Hygiene Monographic Series No. 20. American Journal of Hygiene; Baltimore: 1953. [Google Scholar]
- 98.Goh KT. Eradication of malaria from Singapore. Singapore Med J. 1983;24:255–68. [PubMed] [Google Scholar]
- 99.Ciuca AM. Le paludisme en Roumanie de 1949 a 1955. Bull World Health Organ. 1956;15:725–51. [PMC free article] [PubMed] [Google Scholar]
- 100.Taiwan Provincial Malaria Research Institute. WHO Malaria Team in Taiwan Malaria control and eradication in Taiwan: progress report, May 1952 50 June 1957. Bull World Health Organ. 1958;19:595–620. [PMC free article] [PubMed] [Google Scholar]
- 101.Simic C. Malaria in Yugoslavia. Bull World Health Organ. 1956;15:753–66. [PMC free article] [PubMed] [Google Scholar]
- 102.Colbourne MJ. Prospects for malaria eradication with special reference to the Western Pacific. Trans Roy Soc Trop Med Hyg. 1962;55:179–93. doi: 10.1016/0035-9203(62)90153-0. [DOI] [PubMed] [Google Scholar]
- 103.McKenzie FE, Sirichaisinthop J, Miller RS, et al. Dependence of malaria detection and species diagnosis by microscopy on parasite density. Am J Trop Med Hyg. 2003;69:372–6. [PMC free article] [PubMed] [Google Scholar]
- 104.Zurovac D, Midia B, Ochola SA, et al. Microscopy and outpatient malaria case management among older children and adults in Kenya. Trop Med Int Health. 2006;11:432–40. doi: 10.1111/j.1365-3156.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- 105.O’Meara WP, Collins WE, McKenzie FE. Parasite prevalence: a static measure of dynamic infections. Am J Trop Med Hyg. 2007;77:246–9. [PMC free article] [PubMed] [Google Scholar]
- 106.Bell D, Peeling RW. Evaluation of rapid diagnostic tests: malaria. Nat Rev Microbiol. 2006;4:S34–S8. doi: 10.1038/nrmicro1524. [DOI] [PubMed] [Google Scholar]
- 107.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tatem AJ, Hay SI, Rogers DJ. Global traffic and disease vector dispersal. Proc Natl Acad Sci USA. 2006;103:6242–7. doi: 10.1073/pnas.0508391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tatem AJ, Rogers DJ, Hay SI. Global transport networks and infectious disease spread. Adv Parasitol. 2006;62:293–343. doi: 10.1016/S0065-308X(05)62009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tatem AJ, Rogers DJ, Hay SI. Estimating the malaria risk of African mosquito movement by air travel. Malaria J. 2006;5:57. doi: 10.1186/1475-2875-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maldonado YA, Nahlen BL, Roberto RR, et al. Transmission of Plasmodium vivax malaria in San Diego County, California, 1986. Am J Trop Med Hyg. 1990;42:3–9. doi: 10.4269/ajtmh.1990.42.3. [DOI] [PubMed] [Google Scholar]
- 112.Singh B. Molecular methods for diagnosis and epidemiological studies of parasitic infections. Int J Parasitol. 1997;27:1135–45. doi: 10.1016/s0020-7519(97)00111-2. [DOI] [PubMed] [Google Scholar]
- 113.Russell PF. Epidemiology of malaria in the Philippines. American Journal of Public Health. 1936;26:1–7. doi: 10.2105/ajph.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moskovskij SD. The dynamics of malaria eradication. World Health Organization; Geneva: 1964. WHO/MAL/436. [Google Scholar]
- 115.Moskovskij SD. A further contribution to the theory of malaria eradication. Bull World Health Organ. 1967;36:992–6. [PMC free article] [PubMed] [Google Scholar]
- 116.Sergiev PG. Epidemiology of disapearing malaria. I. The epidemiology of malaria in the late stages of eradictaion in countries with temperate and sub-tropical climates. World Health Organization; Geneva: 1960. WHO/MAL/252. [Google Scholar]
- 117.Sergiev PG. Epidemiology of disapearing malaria. II. The importance of asymptomatic parasite carriers in malaria eradication. World Health Organization; Geneva: 1960. WHO/MAL/253. [Google Scholar]
- 118.Gu WD, Mbogo CM, Githure JI, et al. Low recovery rates stabilize malaria endemicity in areas of low transmission in coastal Kenya. Acta Trop. 2003;86:71–81. doi: 10.1016/s0001-706x(03)00020-2. [DOI] [PubMed] [Google Scholar]
- 119.Smith DL, Dushoff J, Snow RW, et al. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–5. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith DL, McKenzie FE, Snow RW, et al. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 2007;5:e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Woolhouse ME, Dye C, Etard JF, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94:338–42. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.W.H.O. Regional Office for the Eastern Mediterranean . Guidelines on the elimination of residual foci of malaria transmission. World Health Organization Regional Office for the Eastern Mediterranean; Cairo: 2007. (EMRO Technical Publications Series, 33). [Google Scholar]
- 123.W.H.O. Regional Office for the Eastern Mediterranean . Guidelines on prevention of the reintroduction of malaria. World Health Organization Regional Office for the Eastern Mediterranean; Cairo: 2007. (EMRO Technical Publications Series, 34). [Google Scholar]
- 124.Drakeley C, Sutherland C, Bouserna JT, et al. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol. 2006;22:424–30. doi: 10.1016/j.pt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 125.Drakeley CJ, Corran PH, Coleman PG, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–13. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Snow RW, Molyneux CS, Warn PA, et al. Infant parasite rates and immunoglobulin M seroprevalence as a measure of exposure to Plasmodium falciparum during a randomized controlled trial of insecticide-treated bed nets on the Kenyan coast. Am J Trop Med Hyg. 1996;55:144–9. [PubMed] [Google Scholar]
- 127.Webster HK, Gingrich JB, Wongsrichanalai C, et al. Circumsporozoite antibody as a serologic marker of Plasmodium falciparum transmission. Am J Trop Med Hyg. 1992;47:489–97. doi: 10.4269/ajtmh.1992.47.489. [DOI] [PubMed] [Google Scholar]
- 128.Bruce-Chwatt LJ, Draper CC, Konfortion P. Seroepidemiological evidence of eradication of malaria from Mauritius. Lancet. 1973;2:547–51. doi: 10.1016/s0140-6736(73)92361-1. [DOI] [PubMed] [Google Scholar]
- 129.Alves J, Roque AL, Cravo P, et al. Epidemiological characterization of Plasmodium falciparum in the Republic of Cabo Verde: implications for potential large-scale re-emergence of malaria. Malaria J. 2006;5:32. doi: 10.1186/1475-2875-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Covell G. Lectures on Malaria. 4th edition Republic of India; Delhi: 1949. [Google Scholar]
- 131.Dowdle WR, Hopkins DR. The eradication of infectious diseases. John Wiley and Sons; New York: 1998. [Google Scholar]
- 132.Molyneux DH, Hopkins DR, Zagaria N. Disease eradication, elimination and control: the need for accurate and consistent usage. Trends Parasitol. 2004;20:347–51. doi: 10.1016/j.pt.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 133.Miller M, Barrett S, Henderson DA. Chapter 62. Control and eradication. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al., editors. Disease control priorities in developing countries. 2nd Edition Oxford University Press; New York: 2006. pp. 1163–76. [Google Scholar]
- 134.Dowdle WR. The principles of disease elimination and eradication. Bull World Health Organ. 1998;76:22–5. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.