Abstract
Background
Malnutrition is common in the developing world and associated with disease and mortality. Because malnutrition frequently occurs among children in the community as well as those with acute illness, and because anthropometric indicators of nutritional status are continuous variables that preclude a single definition of malnutrition, malnutrition-attributable fractions of admissions and deaths cannot be calculated by simply enumerating individual children.
Objective
We determined the malnutrition-attributable fractions among children admitted to a rural district hospital in Kenya, among inpatient deaths and among children with the major causes of severe disease.
Design
We analyzed data from children between 6 and 60 mo of age, comprising 13 307 admissions, 674 deaths, 3068 admissions with severe disease, and 562 community controls by logistic regression, using anthropometric z scores as the independent variable and admission or death as the outcome, to calculate the probability of admission as a result of “true malnutrition” for individual cases. Probabilities were averaged to calculate attributable fractions.
Results
Z scores < -3 were insensitive for malnutrition-attributable deaths and admissions, and no single threshold was both specific and sensitive. The overall malnutrition-attributable fraction for in-hospital deaths was 51% (95% CI: 42%, 61%) with midupper arm circumference. Similar malnutrition-attributable fractions were seen for the major causes of severe disease (severe malaria, gastroenteritis, lower respiratory tract infection, HIV, and invasive bacterial disease).
Conclusions
Despite global improvements, malnutrition still underlies half of the inpatient morbidity and mortality rates among children in rural Kenya. This contribution is underestimated by using conventional clinical definitions of severe malnutrition.
INTRODUCTION
Malnutrition is a significant public health problem in developing countries. It is a strong risk factor for admission to hospital and death (1). Accurate estimates of the burden of malnutrition on the community are needed to help policy makers plan interventions (2). Cohort studies to measure baseline anthropometry and then subsequent mortality were conducted in Uganda (3), Sudan (4), Guinea-Bissau (5), and Zaire (6). Some of those studies identified lower than expected death rates and were conducted without systematic demographic surveillance, limiting their utility in calculating the fraction of all deaths or admissions in the community that might be attributable to malnutrition.
However, data from case-control studies can be used in logistic regression models to calculate attributable fractions, which indicate the percentage of all cases linked to the risk factor studied. This approach was widely used to calculate malaria-attributable fractions (7, 8) and was applied to mortality data in conjunction with anthropometric cross-sectional surveys of the community in sub-Saharan Africa and Asia acquired >20 y ago (9-12). Those studies found high estimates of malnutrition-attributable death, ranging from 30% to 50% of childhood deaths. However, recent global improvements in nutrition have been seen (13), and this is thought to have reduced the estimated global attributable fractions to 14-19% of all childhood deaths (2).
We apply the logistic regression approach to indicate the risk of death or hospital admission secondary to malnutrition, using a recent data set collected in a rural hospital in Kenya. We examine the contribution malnutrition makes to all admissions and inpatient death and to the following clinical presentations: severe malaria, lower respiratory tract infection (LRTI), gastroenteritis, and invasive bacterial disease.
SUBJECTS AND METHODS
Location
The Kenya Medical Research Institute Centre for Geographic Medicine Research (Coast) is located at Kilifi District Hospital, Kenya. The district is among the poorest in Kenya. Two of the locations served by the hospital ranked in the bottom 5 of 210 locations surveyed nationally, based on household expenditure (http://www.cbs.go.ke/surveys/poverty/pdf/KenyaPovAtlasIIfinal2cl.pdf). Government-employed clinical officers admitted children from the hospital outpatient department to the pediatric ward where research was conducted. The hospital serves ≈240 000 people, who mainly belong to the Mijikenda group and are rural farmers. Approval for this study was given by the Kenyan Medical Research Institute National Ethics Committee, and procedures were in accordance with the Helsinki Declaration of 1975 as revised in 1983.
Clinical and laboratory methods
From 1 August 1998 to 30 July 2002 we prospectively collected standardized clinical, anthropometric, and laboratory data on all children admitted to the pediatric wards [described in detail elsewhere (14)]. Midupper arm circumference (MUAC) was measured from 1 October 1999. Children were weighed on admission with the use of an electronic scale (Weylux; H Fereday and Sons, London, United Kingdom). In children <2 y old, length was measured with the use of a measuring board of standard design. Height was measured by a wall-mounted scale for children >2 y old. Weight-for-age z score (WAZ), height-for-age z score (HAZ), weigh-for-height z score (WHZ), and MUAC-for-age z score were calculated with the use of the current World Health Organization reference standards as supplied in the “igrowup” STATA package (Stata Corp, College Station, TX). Children with kwashiorkor [defined as bilateral pedal edema with typical skin and hair changes (15)] were considered to have severe malnutrition, and WAZ and WHZ were not calculated. Data from children between 6 mo and 5 y of age were analyzed in this study.
Complete blood counts were performed with the use of an automated counter (Beckman Coulter, Buckinghamshire, United Kingdom). Severe anemia was defined as hemoglobin < 50 g/L. Thick and thin blood smear was stained with 10% Giemsa and examined at ×1000 magnification for asexual forms of Plasmodium falciparum, and results were expressed per microliter with the use of the automated red and white cell indexes.
Blood was aerobically cultured for pathogenic bacteria (BACTEC; Becton Dickinson and Co, Franklin Lakes, NJ) as previously described (14). Lumbar puncture was guided by a clinical protocol (16). Meningitis was defined as a positive cerebrospinal fluid (CSF) culture, a positive CSF latex agglutination test, bacteria seen on CSF Gram stain, or a CSF leukocyte count > 50 cells/μL. HIV infection was determined at the end of the study with the use of stored plasma samples. These samples were tested without the immediate availability of any personal identifiers, but results were subsequently linked to clinical data identified by a serial number only. Samples were systematically collected for all admissions with an invasive bacterial infection and all admissions after 1 October 1999 with the use of enzyme-linked immunoabsorbent assay. Polymerase chain reaction was used to confirm HIV infection in children < 18 mo of age.
Severe disease was defined as coma (Blantyre coma score < 3), respiratory distress (deep breathing), severe anemia (blood hemoglobin concentration < 50 g/L), prostration, or ≥2 seizures in 24 h. These features were used to define severe malaria (17), but they can be used to define severe illness of any cause (18, 19). Within this group, LRTI was defined by the clinician’s diagnosis, gastroenteritis was defined by ≥3 loose stools in 24 h, meningitis and bacteremia were defined by laboratory studies (14, 16), and severe malaria required >2500 parasites/μL blood with one of the features of severe disease given above.
Clinical management
Children were managed on the 35-bed general pediatric ward and 6-bed high-dependency ward. Medical care was provided according to a standardized protocol, with research clinicians providing 24-h coverage for high-dependency and general ward areas (14). Children whose condition was diagnosed as severe malnutrition received stabilization and rehabilitation care according to current guidelines from the World Health Organization (15).
Community survey data
An age- and location-matched community control was found for each child admitted with bacteremia. They were recruited within 1 week of presentation of the case of bacteremia. Children were examined for kwashiorkor, weighed, and measured. Z scores for height, weight, and MUAC were calculated as for admitted children. The distribution of ages and locations are given for controls and admitted children in Table 1.
TABLE 1.
Distribution of ages and sublocations of children for admissions and controls1
| Controls | Admissions | |
|---|---|---|
|
| ||
| n (%) | n (%) | |
| Age (mo) | ||
| 6-12 | 98 (21) | 3215 (24) |
| 12-24 | 172 (38) | 4448 (33) |
| 24-36 | 108 (24) | 2958 (22) |
| 36-48 | 46 (10) | 1638 (12) |
| 48-60 | 32 (7) | 1048 (8) |
| Sublocation | ||
| Roka | 66 (14) | 1403 (11) |
| Tezo | 152 (33) | 4910 (37) |
| Takaungu | 113 (25) | 2088 (16) |
| Junju | 53 (12) | 912 (7) |
| Mtwapa | 43 (9) | 739 (6) |
| Ngerenya | 23 (5) | 604 (5) |
| Ganze | 8 (2) | 368 (3) |
| Kauma | 10 (2) | 309 (2) |
| Sokoke | 24 (5) | 622 (5) |
| Chonyi | 56 (12) | 963 (7) |
The comparability between controls and admissions for age distribution and location can be seen. Sublocations with >2% of children are given. Data on age were missing for 5 controls.
Analysis
The numbers of observations in each category are shown in Table 2. The (predominantly negative) z scores were made positive, and positive z scores set to 0. A logistic regression model was then applied, using log(p) = a + bxτ where p is the probability of admission or death, x is the z score,and τ is the power function of the z score which maximizes the likelihood estimation. Allowing the power function (τ) to vary is necessary because the relation between risk and the linear z score may not fit a logistic regression model. If it does not, the logistic regression model would constrain children with moderate malnutrition to be ascribed a risk proportionate to children with severe malnutrition. Allowing a power function means that the model could indicate a slight increase in risk in moderate malnutrition, with an abrupt increase in risk with severe malnutrition, if that was a better fit for the data.
TABLE 2.
Malnutrition-attributable fractions estimated by midupper arm circumference (MUAC), weight-for-age z score (WAZ), and weight-for-height z score (WHZ)1
| Endpoint | Observations2 | MUAC | WAZ | WHZ |
|---|---|---|---|---|
|
| ||||
| n | % (95% CI) | % (95% CI) | % (95% CI) | |
| Admissions | ||||
| Age < 24 mo | 7985 | 29 (15, 41) | 13 (8, 39) | 39.9 (29, 48) |
| Age > 24 mo | 5322 | 23.4 (18, 36) | 13.5 (9, 29) | 43.3 (31, 55) |
| All | 13 307 | 26.5 (17, 38) | 11.3 (8, 26) | 40.8 (33, 48) |
| Death | ||||
| Age < 24 mo | 472 | 57.4 (45, 70) | 39.5 (24, 55) | 49.2 (35, 66) |
| Age > 24 mo | 202 | 42.3 (28, 57) | 17.5 (10, 31) | 25.3 (9, 65) |
| All | 674 | 51.4 (42, 61) | 27.5 (18, 40) | 50.4 (36, 67) |
| Severe disease | ||||
| Bacteremia | 312 | 51.8 (37, 71) | 25.3 (17, 36) | 59.1 (45, 73) |
| Gastroenteritis | 556 | 61.2 (43, 77) | 39.8 (29, 74) | 68.9 (60, 78) |
| LRTI3 | 1012 | 40.1 (17, 70) | 47.6 (12, 70) | 59.4 (44, 74) |
| Meningitis | 86 | 35.4 (20, 88) | 29.7 (15, 52) | 44.7 (12, 76) |
| Malaria | 2420 | 54.7 (42, 63) | 43.1 (19, 57) | 58.8 (53, 65) |
| HIV + | 211 | 63 (34, 81) | 26 (17, 73) | 59.1 (46, 72) |
| HIV + and gastroenteritis | 214 | 66.7 (38, 81) | 61.1 (22, 84) | 74.3 (60, 86) |
| HIV + and LRTI | 451 | 46.3 (11, 78) | 21.1 (5, 62) | 62.5 (38, 84) |
| HIV + and malaria | 1132 | 54.3 (39, 64) | 41.1 (19, 57) | 63.7 (56, 71) |
For each endpoint, the attributable fractions among different subgroups are shown, as calculated using 3 different markers of nutritional status (MUAC, WAZ, and WHZ). LRTI, lower respiratory tract infection.
The numbers of observations indicate the number of cases used in the model to calculate the attributable fraction (ie, not including the controls).
Defined by the clinician’s diagnosis.
The logistic model for risk of admission compared community controls with all admissions. The logistic model for risk of death compared deaths with inpatient survivors. The sensitivity, specificity, and positive predictive values of various age-specific threshold values for parasite density were estimated by calculating the attributable fraction for malnutrition. Children with kwashiorkor were not used in analysis of the sensitivity and specificity of z score thresholds, but they were assumed to have malnutrition when calculating attributable fractions. CIs were estimated by bootstrapping, using 5000 iterations.
RESULTS
Thirteen thousand, three hundred seven children aged between 6 mo and 5 y were admitted 1 August 1998 through 30 July 2002. Outcome data were missing for 2 children. Six hundred seventy-four children died (5%). One thousand seventy-six (8%) children had bilateral pretibial or generalized edema, of whom 190 died (17.7%). These children were considered to have kwashiorkor (ie, definite malnutrition) in attributable fraction studies but were not used in the models to derive sensitivity and specificity of anthropometric measures. The controls appear well matched to admissions for age and geographical distribution (Table 1). Because MUAC was not initially collected, complete and systematic anthropometry data were available for 10 761 (69%) admissions and 461 out of 562 (82%) controls, and analysis of the sensitivity and specificity of the different anthropometric markers was restricted to this subset.
Sensitivity and specificity
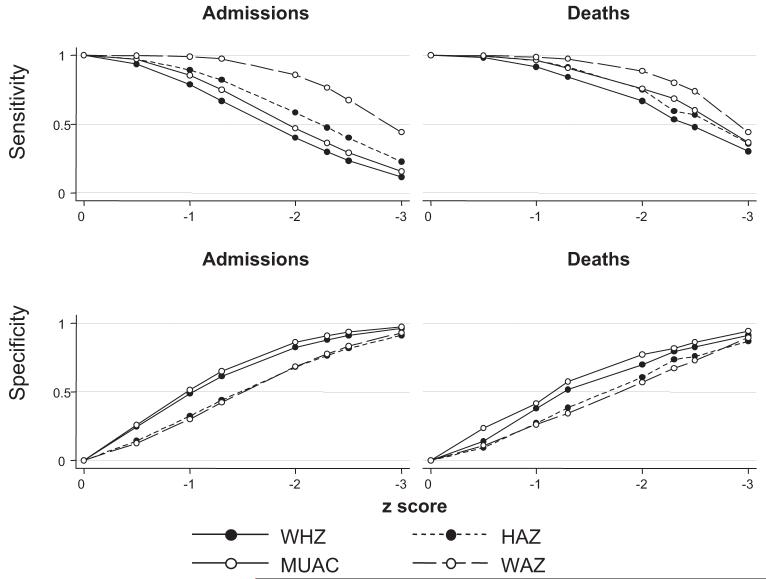
The sensitivities and specificities for MUAC-for-age z score, WHZ, HAZ, and WAZ are shown by z score in Figure 1. For these variables, a z score of ≤-3 was an insensitive definition for malnutrition-associated admissions and deaths. The sensitivities for admission ranged from 14% to 61%. For malnutrition-attributable deaths, the sensitivities ranged from 32% to 52% (ie, using z score < -3 would have excluded 48-68% of the deaths attributable to malnutrition).
FIGURE 1.
The sensitivity and specificity of z score thresholds for diagnosis of malnutrition-attributable admission to hospital or death are shown for height-for-age z score (HAZ), weight-for age z score (WAZ), weight-for-height z score (WHZ), and midupper arm circumference (MUAC)-for-age z score. n = 12 207 for deaths and controls; n = 11 254 for admissions and controls.
Although definitions based on WAZ seemed to be more sensitive, the specificity was lower: at z score > -3, the specificity was 89% (95% CI: 86%, 92%), compared with 94% (95% CI: 93%, 95%) for MUAC-for-age z score. At similar z score thresholds, WAZ and HAZ were less specific than MUAC-for-age z score and WHZ for both malnutrition admissions and malnutrition deaths. At a z score of <-2, WAZ and HAZ had specificities of 58% (95% CI: 55%, 61%) and 61% (95% CI: 57%, 64%), whereas MUAC-for-age z score and WHZ had specificities of 75% (95% CI: 72%, 78%) and 71% (95% CI: 67%, 74%), respectively.
Attributable fractions
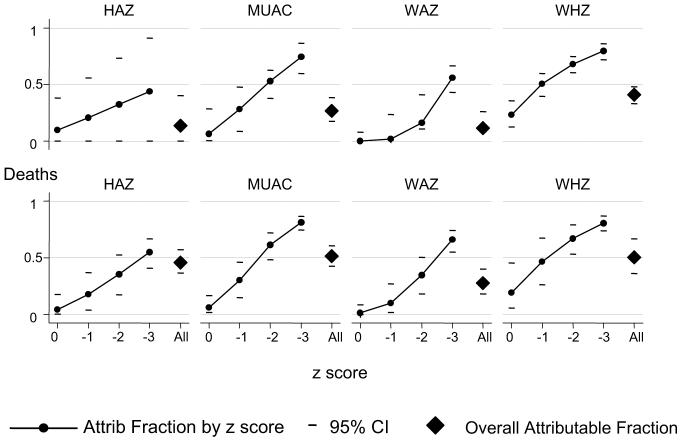
Overall (including children with kwashiorkor), the attributable fractions for malnutrition were 11-41% for admissions, and 28-51% for deaths (Figure 2). The malnutrition-attributable fractions for deaths, but not for admissions, appeared to be lower among older children (Table 2).
FIGURE 2.
The malnutrition-attributable (Attrib) fractions for admissions and deaths are shown according to z score and for all admissions. A separate graph shows the attributable fractions for each analysis, according to height-for-age z score (HAZ), weight-for age z score (WAZ), weight-for-height z score (WHZ), and midupper arm circumference (MUAC)-for-age z score. n = 12 207 for deaths and controls; n = 11 254 for admissions and controls.
Children with z scores between 0 and -1 contributed little to the attributable fractions; estimates based on HAZ, MUAC-for-age z score, or WAZ ranged from 0% to 10% for admissions and from 1% to 6% for deaths. Estimations based on WHZ were higher, at 23% (95% CI: 13%, 35%) for admissions and 19% (95% CI: 6%, 40%) for deaths (data not tabulated; Figure 2).
The attributable fractions increased with lower z scores but did not reach 100% even for the group with z score < -3, with attributable fractions ranging from 44% to 80% for admissions and from 55% to 81% for deaths. Markers of wasting (MUAC, WHZ) had high specificity at z score < -3, but markers of stunting (HAZ) did not (data not tabulated; Figure 2).
Subgroup analysis by concomitant diagnoses
The effect of concomitant comorbidity was studied on a well-characterized subgroup of children who had signs or symptoms of severe disease on admission, for whom full clinical data were available. This subgroup included 3068 of the original 13 307 admissions. The CIs for estimates based on HAZ were wider than those based on MUAC-for-age z score, WAZ, and WHZ, and so estimates for malnutrition-attributable fractions among severely unwell children in Table 2 are based on MUAC-for-age z score, WAZ, and WHZ. Estimates of attributable fractions among severe admissions were higher than the estimates for all admissions and were comparable to the attributable fractions estimated for malnutrition-associated deaths (Table 2). Although there is a tendency for gastroenteritis and severe malaria to have higher malnutrition-attributable fractions than LRTI, bacteremia, and meningitis, the CIs are wider than these differences.
HIV
Systematic HIV testing was done for all children with severe disease admitted between 1 October 1999 and 30 July 2002. During this period, an HIV status was available for 1755 of 1918 admissions, and full anthropometry was available for 1343 of these children. Two hundred eleven (15.7%) were positive. The contribution of HIV was assessed by examining the malnutrition-attributable fractions among children with HIV infection and comparing these results with children known to be HIV uninfected (Table 2). There were too few HIV-uninfected children with bacteremia or meningitis to allow subgroup analysis. However, the malnutrition-attributable fractions among HIV-uninfected children with gastroenteritis, LRTI, and malaria were similar to the fractions among HIV-infected children.
DISCUSSION
The malnutrition-attributable fractions vary depending on the anthropometric marker used, but the CIs overlap. We consider the most reliable marker to be MUAC, for reasons described below, which gave an overall malnutrition-attributable fraction for in-hospital deaths of 51% (95% CI: 42%, 61%). HAZ measures a different form of malnutrition [stunting rather than wasting (20)]. The CIs obtained with HAZ were wide (except for death) and so were not used for further analysis. Previous data confirm that acute wasting is more strongly linked with acute disease and death than is stunting (12, 21). Weight on admission is potentially confounded, because a common feature of acute malaria and other febrile illnesses is dehydration (22). This might cause an apparent increase in the attributable fraction, because dehydration would then cause an apparent increase in the WAZ and WHZ. However, MUAC is unlikely to vary markedly with dehydration, and so we focus on this estimate.
The cases we use for calculating attributable fractions are inpatient admissions and inpatient deaths. Most children with acute illnesses are treated in the community without hospital referral, and the majority of deaths occur without hospital admission (23). Because socioeconomic status influences both the likelihood of admission during an acute illness and nutritional intake, the attributable fractions presented here may be an underestimate. To address this would require longitudinal follow-up of a large number of children recruited in the community, so as to avoid the bias inherent in hospital admission.
Furthermore, malnutrition and infection may have a circular relation in causality (24), and episodes of malaria may precede growth faltering (25). However, in considering the acute event, it seems more likely that the direction of causality is from malnutrition to the acutely acquired infection precipitating hospital admission, irrespective of the original causality of the child’s malnutrition. An important exception is HIV, which causes both infection and malnutrition. However, in subgroup analyses we obtained similar results in HIV-positive and -negative children, suggesting that confounding from chronic HIV did not explain the high malnutrition-attributable fractions we found.
Our attributable fractions are comparable to those found with data acquired 20 y ago, ranging from 30-60% of deaths in Senegal (12) to 55% in Asia and Tanzania (9). Those other studies also found variation in estimates according to the anthropometric measurement used in analysis. It is striking that our data are comparable to those obtained 20 y, because a recent analysis of pooled global data suggest the situation is improving. Only 14-19% of deaths were attributed to malnutrition, having fallen from 35% only a few years previously (2). This may reflect global improvements in nutrition (13). However, nutrition does not appear to be improving in East Africa since 1980, at least as indicated by markers of stunting (13). Although our attributable fractions are calculated primarily from markers of wasting rather than stunting, the international trend over time in both measures is probably similar. Our data, from a poor, rural community in Kenya, probably reflect the situation where improvements in nutrition have not occurred. The global improvements in nutrition may be encouraging, but substantial inequalities probably underlie the summary figures.
We fit a power function to the z scores used in our study. This is important, because a logistic model would otherwise constrain the risk at a z score of -1 to be proportionate to the risk at a z score of -3. However, it is biologically plausible that z scores at -3 might be strongly associated with death, but z scores at -1 only weakly so or not at all. Using a power function allows the line linking risk to z score to vary in shape according to the data. Without this, the attributable fractions estimated would probably be accurate at low z scores but overestimated at intermediate z scores. However, despite using a power function, there were high malnutrition-attributable fractions among children dying with mild and moderate wasting.
Children presenting to outpatient departments may be more likely to be admitted if they have obvious signs of malnutrition. This could increase the malnutrition-attributable fraction even without a biological link between disease and malnutrition. However, because the malnutrition-attributable fractions for death and severe disease (calculated from admissions only) are considerably higher than those for all admissions, it seems unlikely that the high attributable fractions relate to unnecessary admissions.
Our data suggest that malnutrition is still a major contributor for the major causes of childhood death in rural Kenya, despite recent global improvements in health. Most of the direct causes for admission that were attributable to malnutrition were infectious diseases, which were then recorded as the primary diagnoses by clinicians. The mechanisms by which malnutrition predisposes to infection were recently reviewed (24). It is possible that associated micronutrient deficiencies were important, but no biochemical data on these are available to us.
Targeted interventions exist to reduce death from malaria with insecticide-treated nets (26) or intermittent presumptive treatment (27), pneumococcal vaccine to prevent LRTI (28), and oral rehydration solution (29) and rotavirus vaccine (30) to prevent death from gastroenteritis. However, these data suggest that malnutrition is a common predisposing factor, and interventions that reduce malnutrition will reduce deaths from all these causes.
Acknowledgments
We thank Kevin Marsh for helpful comments made in drafting the manuscript.
Footnotes
This paper is published with the permission of the director of the Kenya Medical Research Institute (KEMRI).
Supported by KEMRI and the Wellcome Trust. JB, CRN, and PB are supported by the Wellcome Trust.
This is a free access article, distributed under terms that permit unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited. http://www.nutrition.org/publications/guidelines-and-policies/license/.
REFERENCES
- 1.Briend A, Dykewicz C, Graven K, Mazumder RN, Wojtyniak B, Bennish M. Usefulness of nutritional indices and classifications in predicting death of malnourished children. Br Med J (Clin Res Ed) 1986;293:373–5. doi: 10.1136/bmj.293.6543.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 3.Vella V, Tomkins A, Borghesi A, Migliori GB, Ndiku J, Adriko BC. Anthropometry and childhood mortality in northwest and southwest Uganda. Am J Public Health. 1993;83:1616–8. doi: 10.2105/ajph.83.11.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fawzi WW, Herrera MG, Spiegelman DL, el Amin A, Nestel P, Mohamed KA. A prospective study of malnutrition in relation to child mortality in the Sudan. Am J Clin Nutr. 1997;65:1062–9. doi: 10.1093/ajcn/65.4.1062. [DOI] [PubMed] [Google Scholar]
- 5.Smedman L, Sterky G, Mellander L, Wall S. Anthropometry and subsequent mortality in groups of children aged 6-59 months in Guinea-Bissau. Am J Clin Nutr. 1987;46:369–73. doi: 10.1093/ajcn/46.2.369. [DOI] [PubMed] [Google Scholar]
- 6.Group TKS. Anthropometric assessment of young children’s nutritional status as an indicator of subsequent risk of dying. J Trop Pediatr. 1983;29:69–75. doi: 10.1093/tropej/29.2.69. [DOI] [PubMed] [Google Scholar]
- 7.Rogers WO, Atuguba F, Oduro AR, Hodgson A, Koram KA. Clinical case definitions and malaria vaccine efficacy. J Infect Dis. 2006;193:467–73. doi: 10.1086/499314. [DOI] [PubMed] [Google Scholar]
- 8.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191:1932–9. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelletier DL, Frongillo EA, Jr, Habicht JP. Epidemiologic evidence for a potentiating effect of malnutrition on child mortality. Am J Public Health. 1993;83:1130–3. doi: 10.2105/ajph.83.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelletier DL, Frongillo EA, Jr, Schroeder DG, Habicht JP. The effects of malnutrition on child mortality in developing countries. Bull World Health Organ. 1995;73:443–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Pelletier DL, Frongillo EA, Jr, Schroeder DG, Habicht JP. A methodology for estimating the contribution of malnutrition to child mortality in developing countries. J Nutr. 1994;124(supp):2106S–22S. doi: 10.1093/jn/124.suppl_10.2106S. [DOI] [PubMed] [Google Scholar]
- 12.Garenne M, Maire B, Fontaine O, Briend A. Distributions of mortality risk attributable to low nutritional status in Niakhar, Senegal. J Nutr. 2006;136:2893–900. doi: 10.1093/jn/136.11.2893. [DOI] [PubMed] [Google Scholar]
- 13.de Onis M, Frongillo EA, Blossner M. Is malnutrition declining? An analysis of changes in levels of child malnutrition since 1980. Bull World Health Organ. 2000;78:1222–33. [PMC free article] [PubMed] [Google Scholar]
- 14.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 15.WHO . Pocket book of hospital care for children. Geneva, Switzerland: WHO; 2005. [Google Scholar]
- 16.Berkley JA, Versteeg AC, Mwangi I, Lowe BS, Newton CR. Indicators of acute bacterial meningitis in children at a rural Kenyan district hospital. Pediatrics. 2004;114:e713–9. doi: 10.1542/peds.2004-0007. [DOI] [PubMed] [Google Scholar]
- 17.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 18.Bejon P, Berkley JA, Mwangi T, et al. Defining childhood severe falciparum malaria for intervention studies. PLoS Med. 2007;4:e251. doi: 10.1371/journal.pmed.0040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwer S, Newton CR, Berkley JA. Over-diagnosis and co-morbidity of severe malaria in African children: a guide for clinicians. Am J Trop Med Hyg. 2007;77:6–13. [PMC free article] [PubMed] [Google Scholar]
- 20.Waterlow JC, Buzina R, Keller W, Lane JM, Nichaman MZ, Tanner JM. The presentation and use of height and weight data for comparing the nutritional status of groups of children under the age of 10 years. Bull World Health Organ. 1977;55:489–98. [PMC free article] [PubMed] [Google Scholar]
- 21.Waterlow JC. Protein-energy malnutrition: the nature and extent of the problem. Clin Nutr. 1997;16(suppl 1):3–9. doi: 10.1016/s0261-5614(97)80043-x. [DOI] [PubMed] [Google Scholar]
- 22.Maitland K, Pamba A, Newton CR, Levin M. Response to volume resuscitation in children with severe malaria. Pediatr Crit Care Med. 2003;4:426–31. doi: 10.1097/01.PCC.0000090293.32810.4E. [DOI] [PubMed] [Google Scholar]
- 23.Snow RW, Schellenberg JR, Forster D, Mung’ala VO, Marsh K. Factors influencing admission to hospital during terminal childhood illnesses in Kenya. Int J Epidemiol. 1994;23:1013–9. doi: 10.1093/ije/23.5.1013. [DOI] [PubMed] [Google Scholar]
- 24.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyakeriga AM, Troye-Blomberg M, Chemtai AK, Marsh K, Williams TN. Malaria and nutritional status in children living on the coast of Kenya. Am J Clin Nutr. 2004;80:1604–10. doi: 10.1093/ajcn/80.6.1604. [DOI] [PubMed] [Google Scholar]
- 26.Lengeler C. Insecticide-treated bednets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;(2) doi: 10.1002/14651858.CD000363. CD000363. [DOI] [PubMed] [Google Scholar]
- 27.Schellenberg D, Cisse B, Menendez C. The IPTi Consortium: research for policy and action. Trends Parasitol. 2006;22:296–300. doi: 10.1016/j.pt.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Greenwood BM, Weber MW, Mulholland K. Childhood pneumonia–preventing the world’s biggest killer of children. Bull World Health Organ. 2007;85:502–3. doi: 10.2471/BLT.07.044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartling L, Bellemare S, Wiebe N, Russell K, Klassen TP, Craig W. Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD004390.pub2. CD004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunliffe N, Nakagomi O. Introduction of rotavirus vaccines in developing countries: remaining challenges. Ann Trop Paediatr. 2007;27:157–67. doi: 10.1179/146532807X220262. [DOI] [PubMed] [Google Scholar]