Abstract
Objective
The association between quantity of fruit and vegetable intake and risk of type 2 diabetes (T2D) is not clear, and the relationship with variety of intake is unknown. The current study examined the association of both quantity and variety of fruit and vegetable intake and risk of T2D.
Research Design and Methods
We examined the 11-year incidence of T2D in relation to quantity and variety of fruit, vegetables and combined fruit and vegetable intake in a case-cohort study of 3,704 participants (n=653 T2D cases) nested within the European Prospective Investigation into Cancer and Nutrition-Norfolk Study, who completed 7-day prospective food diaries. Variety of intake was derived from the total number of different items consumed in a one week period. Multivariable Prentice-weighted Cox regression was used to estimate hazard ratios (HR) and 95% CI.
Results
Greater quantity of combined fruit and vegetable intake was associated with 21% lower hazard of T2D (HR 0.79; 0.62-1.00) comparing extreme tertiles, in adjusted analyses including variety. Separately, quantity of vegetable intake (HR 0.76; 0.60-0.97), but not fruit, was inversely associated with T2D in adjusted analysis. Greater variety in fruit (HR 0.70; 0.53-0.91), vegetable (HR 0.77; 0.61-0.98), and combined fruit and vegetable (HR 0.61; 0.48-0.78) intake was associated with a lower hazard of T2D, independent of known confounders and quantity of intake comparing extreme tertiles.
Conclusions
These findings suggest that a diet characterised by greater quantity of vegetable and greater variety of both fruit and vegetable intake is associated with a reduced risk of T2D.
INTRODUCTION
Type 2 diabetes (T2D) is one of the most common noncommunicable diseases worldwide and is a leading cause of premature mortality [1], morbidity [2], and health care expenditure [3]. Lifestyle intervention trials that include dietary changes have been shown to be effective in preventing the development of diabetes [4]. However, it is still largely unknown which aspects of the diet confer this beneficial effect.
A World Health Organization (WHO) expert consultation recommended a minimum intake of 400 grams or five portions (based on an average portion weighing 80 g) of combined fruit and vegetables (F&V) per day for the prevention of several major noncommunicable diseases, including type 2 diabetes [5]. In addition, the five-a-day programme in the UK and similar programmes in other countries (e.g. the US [6]) recommend consuming a variety of different F&V, thereby ensuring adequate intake of micronutrients, dietary fibre, and a multitude of other important bioactive compounds [7]. Yet, the specific role of variety in F&V intake and T2D has not been examined. Additionally, as studies have generally used a food frequency questionnaire (FFQ) to assess F&V intake, which is suitable for ranking individuals according to their relative but not absolute intake [8], there is also an absence of research on the importance of meeting the five-a-day quantity recommendation for F&V intake. The use of a prospective food diary offers a more precise measure of dietary intake and can overcome some of the limitations of the FFQ [9], but so far no studies have employed this dietary assessment method in relation to T2D risk.
In order to develop effective dietary public health strategies for T2D prevention, it is essential to clarify the contribution of both quantity and variety of F&V intake to T2D risk. The aim of this study was therefore to evaluate the association between the quantity and variety of fruit, vegetables, and combined F&V consumption, as assessed using a prospective 7-day food diary, and incident T2D.
RESEARCH DESIGN AND METHODS
Study population
The Norfolk component of the European Prospective Investigation of Cancer (EPIC-Norfolk) study recruited 25,639 men and women aged 40-79 years at baseline in 1993-97. The EPIC-Norfolk study was initiated to investigate the relationship between diet and cancer, but has since broadened its scope to include a range of chronic diseases, including T2D. The recruitment procedures, collection of questionnaire data, and anthropometric and dietary measures have been described in detail elsewhere [10, 11]. In brief, participants residing in Norfolk, England, were recruited from age-sex registers of general practices and attended a baseline health check. Follow-up of participants constituted a postal questionnaire at 18 months, a second health check in 1998-2000 and a further postal questionnaire in 2002-04.
From the 25,639 participants in EPIC-Norfolk at baseline, we ascertained incident cases of T2D (n=892) and selected a random subcohort of 4,000 participants. This subcohort was representative of the entire EPIC-Norfolk cohort in terms of age, BMI, education level, physical activity level, smoking status and total energy intake (data not shown). Among the subcohort there were 143 individuals who developed incident T2D during follow-up. Of the 4,749 participants, we excluded those with unknown diabetes status (n=1) or prevalent diabetes at baseline (n=121), those with fewer than 7 days of diary data (n=435) or those who did not return a diary (n=15), or those with missing information on potential confounding variables (n=73). Participants with prevalent myocardial infarction, stroke or cancer were also excluded (n=400). The final sample for analysis consisted of 653 incident T2D cases and a subcohort of 3,166 individuals (including 115 incident T2D cases). All volunteers gave written informed consent and the study was approved by the Norwich district ethics committee.
Case ascertainment
We ascertained incident T2D cases by self-report of doctor-diagnosed diabetes from three follow-up health and lifestyle questionnaires, i.e. answering ‘yes’ to “Has a doctor ever told you that you have diabetes?” or diabetes medication that was self-reported or brought to the second health check. In addition, external sources of information through record linkage included listing of any EPIC-Norfolk participant in the general practice diabetes register, local hospital diabetes register, hospital admissions data with screening for any diabetes-related admissions among study participants, and Office of National Statistics mortality data with coding for diabetes. Participants who gave a self-report of history of diabetes that could not be confirmed against any other sources of ascertainment were not considered as a confirmed case of T2D. Follow-up was censored at the date of diagnosis of T2D, the 31st of July 2006, or the date of death, whichever came first.
Assessment of diet and lifestyle variables
At the baseline medical examination, participants were instructed by trained interviewers on how to complete the 7-day food diary [11, 12]. The food diary consisted of 45 colour pages containing food portion photographs and detailed instructions on how to record and describe preparation methods and quantities of foods eaten at main meals, snacks and between meals. Completed diaries were returned by post to the co-ordinating centre at the University of Cambridge. The food diary has been validated with weighed food records, 24-hour urine collections and blood biomarkers [13].
Intake of fruits and vegetables (including tinned and dried) was calculated from food diary data to give average daily quantity of intake for each participant. In order to precisely quantify the actual intake of fruits and vegetables consumed alone or from dishes (in accordance with the UK five-a-day public health guidelines) [14], all recorded foods and dishes were disaggregated into their component parts. Composite dishes containing fruits and/or vegetables included home made and shop bought desserts, vegetable bakes, stews, pies, and soups for example. The fruit and vegetable quantity and type was derived for the composite dishes by using recipes from McCance & Widdowson as previously described [12] and by using ingredients listed on the packages of products and ready-made meals. Potatoes were not included as a vegetable since they differ from vegetables regarding energy and carbohydrate content and are frequently used as a substitute for cereals [15]. F&V juices were also not included as they differ from their source of origin in terms of food matrix and fibre content, and as such may be dissimilarly associated with diabetes [16]. Variety of fruit, vegetables, and combined F&V intake was derived by calculating the total number of different items consumed at least once in a one week period, irrespective of quantity of intake. The groupings of items included 58 different fruit items (range 0-58), 59 different vegetable items (range 0-59) and hence a total of 117 different F&V items consumed over a week-long period as recorded in the 7-day food diary.
At recruitment, participants completed a detailed health and lifestyle questionnaire. Participants self-reported their education level (low, O level, A level, degree), occupational social class (manual, non-manual), smoking status (current, former, never), and baseline history (yes, no) of myocardial infarction, stroke, and cancer. Area deprivation was assessed from residential postcodes using the Townsend Deprivation Index, which provides a material measure of deprivation and disadvantage based on unemployment, car-ownership, home ownership and household overcrowding. A higher Townsend Index score indicates greater deprivation [17]. A validated four point physical activity index was derived incorporating occupational and leisure-time components of physical activity [18]. Trained nurses measured height, weight and waist circumference following standardised protocols [10]. Body mass index (BMI) was calculated as weight divided by height (kg/m2). Venous blood samples were taken by trained study nurses. Haemoglobin A1c (HbA1c) was measured halfway through the baseline health check (1995-97) and was available in approximately half of the EPIC-Norfolk cohort. HbA1c was measured using high-performance liquid chromatography on a Bio-Rad Diamat (Bio-Rad, Hercules, Richmond, California), on a sample of EDTA-anticoagulated blood.
Statistical analysis
Baseline characteristics were summarised by tertiles of F&V quantity and F&V variety among the subcohort participants, using means with SDs, medians with interquartile ranges (IQR), or frequencies (where appropriate).
Multivariable Prentice-weighted Cox regression [19] was used to estimate the associations between quantity and variety of fruit, vegetables and F&V intake combined and hazard of T2D, with intake defined as tertiles (with the lowest tertile as the reference category). To check the proportional hazards assumption of the models, interactions between quantity and variety of fruit, vegetables and F&V intake combined, with current age (i.e. the underlying timescale) were tested. The proportional hazards assumption was not violated for quantity and variety of fruit, vegetables or F&V intake combined (all p-values ≥0.32). Hazard ratios (HR) and 95% confidence intervals (95% CI) were estimated using the following modelling strategy. Age was used as the underlying timescale in all models. Model 1 was adjusted for sex. In Model 2, we additionally adjusted for BMI (continuous), waist circumference (continuous), education level (low, O level, A level, degree), Townsend Deprivation Index (continuous), occupational social class (manual, non-manual), physical activity level (inactive, moderately inactive, moderately active, active), smoking status (current, former, never), family history of diabetes (yes, no), total energy intake (continuous), and season of diary completion (December, January, February = winter; March, April, May = spring; June, July, August = summer; September; October, November = autumn). In model 3, in order to estimate the association between quantity of F&V consumption and hazard of T2D independent of the effect of variety, we additionally adjusted for variety of F&V intake and vice versa for the analysis of variety in intake. We examined multicolinearity in model 3 using the variance inflation factor (VIF).
In sensitivity analyses, the association between F&V quantity and F&V variety and the hazard of T2D was also investigated by including other potentially confounding variables in model 3, including hypertension (yes, no), dairy intake (continuous), total fibre intake (continuous), red and processed meat intake (continuous), and percentage energy from carbohydrate (continuous), protein (continuous), fat (continuous), and alcohol intake (continuous). Analyses were also repeated after additionally excluding participants who: 1) developed T2D within the first two years of follow-up (n=26); 2) had a baseline HbA1c level ≥6.5% (n=15) in the subsample with HbA1c data available (n=1,333), and 3) were in the top and bottom 1% of the ratio of energy intake to energy expenditure. Multiplicative interaction terms were added to model 3 for quantity and variety of combined F&V intake to examine effect modification by sex, age (<60 years, ≥60 years), BMI (normal weight: <25 kg/m2, overweight/obese: ≥25 kg/m2), and smoking status (never smoker, ever smoker) by using the Wald test. Additionally, spline regression was used to demonstrate the continuous association between quantity and variety of combined F&V intake and the HR (95% CI) of T2D with knots placed at quartiles of the distribution [20].
All statistical analyses were performed using Stata/SE 11.1 (Stata-Corp, College Station, Texas, USA). Statistical significance was set at P<0.05.
RESULTS
The median (interquartile range) duration of follow-up was 10.9 (9.8-11.8) years. The median quantity of combined F&V intake was 3.7 (2.5-5.0) portions per day and the mean (standard deviation) variety of combined F&V intake was 11.7 (3.9) items/week. Fewer than 26% of study participants reported consuming at least five portions of F&V per day. There was nearly a 3-fold difference in quantity and in excess of a 2-fold difference in variety of F&V consumption between the highest versus lowest tertile of F&V intake (Table 1). Participants who consumed higher quantities and a greater variety of F&V had more favourable lifestyle, anthropometric, and dietary profiles. Baseline characteristics by tertiles of quantity and variety for fruit intake and vegetable intake separately showed similar results (data not shown). The Pearson correlation coefficient between quantity of fruit and quantity of vegetable intake was 0.29, and between variety of fruit and variety of vegetable intake it was 0.30. Quantity of combined F&V intake was strongly positively correlated with variety of combined F&V intake (0.60).
Table 1.
Descriptive characteristics at baseline by fruit and vegetable quantity and variety tertiles in 3,166 subcohort participants in the EPIC-Norfolk Study
| Tertiles of F&V quantity | Tertiles of F&V variety | |||||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) quantity of intake (p/d) or mean (±SD) variety of intake (n/week) |
Low 2.1 (1.6-2.5) |
Medium 3.7 (3.3-4.0) |
High 5.7 (5.0-6.8) |
P value |
Low 8.0 ± 1.8 |
Medium 12.0 ± 0.8 |
High 16.3 ± 2.3 |
P value |
| N | 1,055 | 1,056 | 1,055 | 1,265 | 927 | 974 | ||
| Age at recruitment (years) | 58.5 (9.8) | 59.1 (9.2) | 59.8 (9.0) | 0.002 | 58.8 (9.7) | 59.3 (9.4) | 59.3 (8.9) | 0.24 |
| Men, No. (%) | 523 (49.6) | 437 (41.4) | 408 (38.7) | <0.001 | 637 (50.4) | 393 (42.4) | 338 (34.7) | <0.001 |
| Education level, No. (%) | <0.001 | <0.001 | ||||||
| Low | 450 (42.7) | 351 (33.2) | 345 (32.7) | 525 (41.5) | 325 (35.1) | 296 (30.4) | ||
| O level | 97 (9.2) | 118 (11.2) | 103 (9.8) | 123 (9.7) | 98 (10.6) | 97 (10.0) | ||
| A level | 411 (39.0) | 443 (42.0) | 426 (40.4) | 498 (39.4) | 369 (39.8) | 413 (42.4) | ||
| Degree | 97 (9.2) | 144 (13.6) | 181 (17.2) | 119 (9.4) | 135 (14.6) | 168 (17.3) | ||
| Occupational social class, No. (%) | <0.001 | <0.001 | ||||||
| Manual | 476 (46.2) | 432 (41.6) | 355 (33.9) | 584 (47.2) | 352 (38.6) | 327 (33.9) | ||
| Non-manual | 555 (53.8) | 606 (58.4) | 692 (66.1) | 653 (52.8) | 561 (61.5) | 639 (66.2) | ||
| Townsend Deprivation Index | −2.0 (2.2) | −2.2 (2.1) | −2.2 (2.1) | 0.30 | −1.9 (2.3) | −2.2 (2.1) | −2.3 (2.0) | 0.003 |
| BMI (Kg/m2) | 26.2 (3.7) | 26.1 (3.7) | 26.2 (3.9) | 0.52 | 26.4 (3.8) | 26.1 (3.8) | 26.0 (3.7) | 0.002 |
| Waist circumference (cm) | 88.4 (11.9) | 87.3 (12.2) | 86.8 (12.3) | <0.001 | 88.9 (12.2) | 87.2 (11.8) | 85.9 (12.3) | <0.001 |
| Physical activity level, No. (%) | <0.001 | 0.001 | ||||||
| Inactive | 358 (33.9) | 279 (26.4) | 270 (25.6) | 409 (32.3) | 264 (28.5) | 234 (24.0) | ||
| Moderately inactive | 278 (26.4) | 319 (30.2) | 329 (31.2) | 335 (26.5) | 288 (31.1) | 303 (31.1) | ||
| Moderately active | 207 (19.6) | 255 (24.2) | 254 (24.1) | 273 (21.6) | 199 (21.5) | 244 (25.1) | ||
| Active | 212 (20.1) | 203 (19.2) | 202 (19.2) | 248 (19.6) | 176 (19.0) | 193 (19.8) | ||
| Smoking status, No. (%) | <0.001 | <0.001 | ||||||
| Current smoker | 191 (18.1) | 89 (8.4) | 71 (6.7) | 201 (15.9) | 84 (9.1) | 66 (6.8) | ||
| Former smoker | 425 (40.3) | 446 (42.2) | 436 (41.3) | 539 (42.6) | 374 (40.4) | 394 (40.5) | ||
| Never smoker | 439 (41.6) | 521 (49.3) | 548 (51.9) | 525 (41.5) | 469 (50.6) | 514 (52.8) | ||
| Total energy intake (Kcal/d) | 1867 (1559- 2271) |
1918 (1628- 2279) |
1932 (1641- 2274) |
0.006 | 1882 (1562- 2285) |
1904 (1621- 2294) |
1929 (1657- 2242) |
0.04 |
| Alcohol intake (%total energy) | 1.7 (0.0-6.4) | 2.1 (0.0-5.6) | 2.1 (0.0-6.0) | 0.48 | 1.6 (0.0-5.6) | 1.9 (0.0-5.8) | 2.5 (0.5-6.4) | <0.001 |
| Variety in F&V intake (no. items/wk) | 8.8 (2.9) | 11.9 (3.0) | 14.3 (3.6) | <0.001 | - | - | - | - |
| Variety in fruit intake (no. items/wk) | 2.3 (1.5) | 3.9 (1.5) | 5.3 (2.0) | <0.001 | - | - | - | - |
| Variety in vegetable intake (no. items/wk) | 6.5 (2.4) | 8.0 (2.6) | 9.1 (2.7) | <0.001 | - | - | - | |
| Quantity of F&V intake (portions/d) | - | - | - | - | 2.5 (1.8 to 3.5) | 3.7 (2.9 to 4.8) | 5.0 (4.0 to 6.3) | <0.001 |
| Quantity of fruit intake (portions/d) | - | - | - | - | 1.1 (0.5 to 1.9) | 1.9 (1.1 to 2.8) | 2.7 (1.8 to 3.7) | <0.001 |
| Quantity of vegetable intake (portions/d) | - | - | - | - | 1.3 (0.9 to 1.7) | 1.8 (1.4 to 2.3) | 2.3 (1.8 to 2.9) | <0.001 |
Data are means ± SD, medians (IQR), and % for categorical variables.
P values are from the test for trend for continuous variables and the χ2 test for categorical variables.
As shown in Table 2, quantity of fruit, vegetables, and combined F&V intake were all inversely associated with incident T2D (Model 1). Further adjustment did not appreciably alter the HRs (Model 2). After additionally adjusting for the effects of variety of intake, an inverse association with quantity of vegetable intake remained, but the associations for quantity of fruit and quantity of combined F&V intake with T2D were attenuated, such that fruit was no longer associated and F&V intake was borderline inversely associated with T2D. Further adjustment for hypertension, dairy intake, total fibre intake and percentage energy from carbohydrate, protein, fat, alcohol intake, and red and processed meat intake did not change our results (data not shown). Those meeting the recommendation to consume at least five portions of F&V per day did not differ from those not meeting this recommendation in hazard of T2D, excluding and including variety of intake (HR: 0.85, 0.70-1.02, and HR: 0.98, 0.80-1.21, respectively). As shown in Table 3, greater variety in fruit, vegetables, and combined F&V intake was inversely associated with incident T2D in adjusted analyses and also when accounting for quantity of intake. The relative reduction in the hazard of T2D with every additional two item increase in F&V variety per week was 8% (HR: 0.92, 0.87-0.97). The mean estimated VIF was <1.9 (<1.6 for both F&V quantity and F&V variety) indicating that colinearity of the variables included in model 3 was low.
Table 2.
Hazard ratios (95% CI) of incident diabetes for quantity of fruit, vegetables, and fruit and vegetable intake in the EPIC-Norfolk Study
| Tertiles of quantity of fruit intake |
||||
|---|---|---|---|---|
| Low | Medium | High | P for trend | |
| Cases/total (n) | 261/1,269 | 193/1,214 | 199/1,221 | |
| Median (IQR) intake (portions/d) | 0.6 (0.3-0.9) | 1.8 (1.5-2.1) | 3.4 (2.9-4.4) | |
| Model 1 | 1 (Reference) | 0.72 (0.59-0.86) | 0.77 (0.64-0.93) | 0.004 |
| Model 2 | 1 (Reference) | 0.72 (0.59-0.87) | 0.75 (0.61-0.91) | 0.003 |
| Model 3 | 1 (Reference) | 0.81 (0.65-1.00) | 0.91 (0.71-1.16) | 0.46 |
|
| ||||
|
Tertiles of quantity of vegetable intake
|
||||
| Cases/total (n) | 245/1,260 | 229/1,236 | 179/1,208 | |
| Median (IQR) intake (portions/d) | 1.1 (0.8-1.3) | 1.7 (1.6-1.9) | 2.6 (2.3-3.1) | |
| Model 1 | 1 (Reference) | 0.91 (0.76-1.06) | 0.72 (0.59-0.87) | 0.001 |
| Model 2 | 1 (Reference) | 0.88 (0.73-1.06) | 0.72 (0.58-0.87) | 0.001 |
| Model 3 | 1 (Reference) | 0.91 (0.74-1.11) | 0.76 (0.60-0.97) | 0.03 |
|
| ||||
|
Tertiles of quantity of F&V intake
|
||||
| Cases/total (n) | 268/1,277 | 188/1,206 | 197/1,221 | |
| Median (IQR) intake (portions/d) | 2.1 (1.6-2.5) | 3.7 (3.3-4.0) | 5.7 (5.0-6.8) | |
| Model 1 | 1 (Reference) | 0.70 (0.58-0.84) | 0.72 (0.60-0.87) | <0.001 |
| Model 2 | 1 (Reference) | 0.68 (0.56-0.82) | 0.68 (0.56-0.83) | <0.001 |
| Model 3 | 1 (Reference) | 0.73 (0.60-0.90) | 0.79 (0.62-1.00) | 0.04 |
Data are HRs (and 95% CI) estimated using Prentice-weighted Cox regression, with age as the underlying time scale variable. Adjustment for covariates was performed using multivariable Prentice-weighted Cox proportional analyses.
Model 1 was adjusted for: sex.
Model 2 as model 1 plus: BMI, waist circumference, education level, Townsend Deprivation Index, occupational social class, smoking status, physical activity, family history of diabetes, energy intake, and season.
Model 3 as model 2 plus: fruit variety for fruit quantity, or vegetable variety for vegetable quantity or F&V variety for F&V quantity.
Table 3.
Hazard ratios (95% confidence interval) of incident diabetes for variety of fruit, vegetables, and fruit and vegetable intake in the EPIC-Norfolk Study
| Tertiles of variety of fruit intake |
||||
|---|---|---|---|---|
| Low | Medium | High | P for trend | |
| Cases/total (n) | 355/1,744 | 197/1,247 | 101/713 | |
| Mean intake (no. items/wk) | 2.0 ± 1.0 | 4.4 ± 0.5 | 6.9 ± 1.2 | |
| Model 1 | 1 (Reference) | 0.74 (0.62-0.88) | 0.67 (0.54-0.84) | <0.001 |
| Model 2 | 1 (Reference) | 0.72 (0.60-0.86) | 0.71 (0.56-0.89) | <0.001 |
| Model 3 | 1 (Reference) | 0.72 (0.59-0.87) | 0.70 (0.53-0.91) | 0.002 |
|
| ||||
|
Tertiles of variety of vegetable intake
|
||||
| Cases/total (n) | 348/1,759 | 172/1,004 | 133/941 | |
| Mean intake (no. items/wk) | 5.5 ± 1.4 | 8.5 ± 0.5 | 11.4 ± 1.5 | |
| Model 1 | 1 (Reference) | 0.85 (0.71-1.02) | 0.68 (0.56-0.84) | <0.001 |
| Model 2 | 1 (Reference) | 0.85 (0.70-1.03) | 0.73 (0.59-0.89) | 0.002 |
| Model 3 | 1 (Reference) | 0.87 (0.72-1.07) | 0.77 (0.61-0.98) | 0.03 |
|
| ||||
|
Tertiles of variety of F&V intake
|
||||
| Cases/total (n) | 321/1,530 | 193/1,084 | 139/1,090 | |
| Mean intake (no. of items) | 8.0 ± 1.8 | 12.0 ± 0.8 | 16.3 ± 2.3 | |
| Model 1 | 1 (Reference) | 0.83 (0.69-0.99) | 0.57 (0.47-0.70) | <0.001 |
| Model 2 | 1 (Reference) | 0.88 (0.73-1.06) | 0.60 (0.49-0.74) | <0.001 |
| Model 3 | 1 (Reference) | 0.88 (0.73-1.07) | 0.61 (0.48-0.78) | <0.001 |
Data are HRs (and 95% CI) estimated using Prentice-weighted Cox regression, with age as the underlying time scale variable. Adjustment for covariates was performed using multivariable Prentice-weighted Cox proportional analyses.
Model 1 was adjusted for: sex.
Model 2 as model 1 plus: BMI, waist circumference, education level, Townsend Deprivation Index, occupational social class, smoking status, physical activity, family history of diabetes, energy intake, and season
Model 3 as model 2 plus: fruit quantity for fruit variety, or vegetable quantity for vegetable variety or F&V quantity for F&V variety.
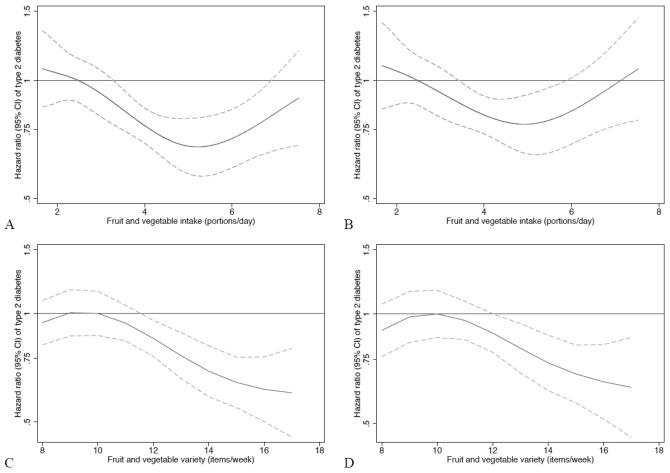
Figure 1 shows the continuous association between quantity and variety of F&V intake and T2D. As shown, the HR for diabetes decreased between an intake of approximately 3.5 to 7.0 portions/day (Figure A) and this association was largely unchanged after accounting for the effects of variety of F&V (Figure B). For variety, consuming ≥12 different F&V items/week was associated with a decreased HR of T2D (Figure C) and this association was largely unaffected after accounting for the effects of quantity of F&V intake (Figure D). The percentage of participants achieving a quantity of >3.5 portions/d was 53.2%, and the percentage achieving a variety of >12 different F&V items/week was 40.3%.
Figure 1.
The upper percentile of the first tertile for quantity and variety of F&V intake was used as the reference category. Figure A represents the association between quantity of F&V intake and HR (95% CI) of T2D adjusted for sex, BMI, waist circumference, education level, Townsend Deprivation Index, occupational social class, physical activity level, smoking status, family history of diabetes, total energy intake, and season. Figure B as Figure A but additionally adjusted for variety of F&V intake. Figure C represents the association between variety of F&V intake and HR (95% CI) of T2D adjusted for sex, BMI, waist circumference, education level, Townsend Deprivation Index, occupational social class, physical activity level, smoking status, family history of diabetes, total energy intake, and season. Figure D as Figure C but additionally adjusted for quantity of F&V intake.
Results were unaffected in sensitivity analyses after excluding participants who: 1) developed T2D within the first two years of follow-up; 2) had a baseline HbA1c level of ≥6.5% in the subsample with HbA1c data, and 3) were in the top and bottom 1% of energy intake to energy expenditure (data not shown). We found no evidence of interaction between either quantity or variety in F&V intake with sex, age, BMI, or smoking status and incident T2D (P >0.10).
CONCLUSIONS
In this prospective study of nearly 4,000 men and women with dietary information from prospective 7-day food diaries, we observed that greater quantity of vegetable intake and greater variety of both vegetable and fruit intake may independently be beneficial for reducing the risk of T2D. After accounting for potential confounding factors and the effects of quantity of intake, each different additional two item per week increase in variety of F&V intake was associated with an 8% reduction in the incidence of T2D.
Previous epidemiological studies have reported inconsistent findings for an association between quantity of F&V intake and risk of T2D. Two separate meta-analyses have reported no overall association between fruit, vegetables and combined F&V intake and T2D risk, although there was significant heterogeneity of association between the included studies [21, 22]. The meta-analysis by Carter et al. [21] did however find a significant inverse association between green leafy vegetable intake and risk of T2D (HR for highest vs. lowest intake group: 0.86; 0.77-0.97). Although low heterogeneity in F&V consumption may be one explanation for the null findings in some study populations, our current results suggest that it may also be due to differences in the assessment methods used for measuring F&V intake. Most epidemiological studies have used an FFQ to assess quantity of F&V intake. Whilst FFQs can be used to rank individuals according to their relative intake [8], they are less suitable for the assessment of absolute intake [11, 23], which they tend to overestimate. For example, in the EPIC-Norfolk study, mean consumption of F&V was much higher when assessed by FFQ (6.5 portions/d) than by a food diary (3.8 portions/d) [11]. For this reason, FFQs are not ideal for examining adherence to, or for informing, public health guidelines. Furthermore, FFQs are based on perceptions of habitual intake, whereas food diaries are based on self-report of foods and amounts actually consumed in real-time [8]. Additionally, because FFQs contain only a limited list of pre-coded food items which tend to be grouped together, unlike the food diary which is open-ended, they may not be as suitable as food diaries for assessing variety of food intake. Despite the fact that variety in F&V intake has been advocated by many national and international bodies [5-7], no studies that we are aware of have explored associations between variety in intake and risk of T2D. Our current findings suggest that quantity (at least 3.5 portions of F&V per day) and variety (at least 12 different F&V items per week) in F&V intake are both inversely and independently associated with T2D. However, only about half, and 40% of the participants reported meeting these thresholds for quantity and variety of intake, respectively.
There are several unique strengths of our study including the large sample size, prospective study design, use of a 7-day prospective food diary with disaggregated F&V data, thorough assessment of new cases of T2D with self-report information supplemented by external sources, and comprehensive information on covariates, thus minimising sources of bias and confounding. Another strength of our study was that we had HbA1c data available on a subsample of participants, and were thus able to demonstrate that our findings were unlikely to have been influenced by the presence of previously undiagnosed cases of T2D at baseline. However, several potential limitations of our study merit discussion. Firstly, because of the observational nature of the study, we cannot exclude the possibility of residual confounding or confounding by unmeasured factors. Secondly, we used baseline dietary consumption data to characterise individuals and did not take into account possible misclassification with respect to changes in consumption patterns over time. However, as this type of misclassification is likely to be non-differential, the effect would be to attenuate the observed HRs towards the null, suggesting that the true associations between quantity and variety in F&V intake may be stronger than reported in the current study. We were also not able to adjust for lifestyle factors (e.g. smoking and physical activity) that may have changed during follow-up. Finally, our population is predominantly of European-Caucasian origin (99.1%) and middle aged. Thus, the generalisability of our findings to other populations may be limited. Nevertheless, in comparison with the general population of England, EPIC-Norfolk participants are comparable with respect to characteristics including anthropometry, blood pressure and lipids [10].
The biological mechanisms for the inverse associations of F&V intake on T2D risk are not clear. Our findings suggest that F&V may be inversely associated with T2D through two distinct but complementary pathways. A plausible biological mechanism to explain the beneficial effect of quantity of F&V intake on T2D is via the low energy and high fibre content of F&V, and as such their ability to reduce the overall energy content of the diet. It has previously been demonstrated that those who consume the highest quantity of F&V, in comparison with low consumers, have a lower risk of weight gain [24, 25], a major risk factor for T2D [26]. A decreased risk of T2D with increasing quantities of vegetable intake in particular may be explained by the fact that vegetables are generally consumed with other foods as part of a meal and therefore may displace or buffer the harmful effects of deleterious foods from the diet, such as energy dense foods or foods that increase the risk of T2D. Alternatively, higher consumption of specific vegetables, particularly green leafy vegetables, might reduce the risk of T2D due to the presence of relatively high concentrations of potentially beneficial bioactive compounds [21]. The biological mechanisms for the inverse associations of variety of F&V intake on T2D are not clear but may be attributable to individual or combined effects of the many different bioactive phytochemicals contained in F&V (e.g. vitamin C and carotenoids [27, 28]). Thus, consumption of a wide variety of F&V will increase the likelihood of consuming these phytochemicals. As the current study was not designed to examine mechanisms of association, future studies will be required to investigate this further.
In conclusion, using the prospective 7-day food diary to assess F&V intake, we found that greater variety of both fruits and vegetables is associated with a reduced risk of T2D, while for quantity, only greater vegetable, but not fruit intake, was associated with a reduced risk. These findings support current public health recommendations encouraging consumption of F&V as part of a balanced diet, and place particular emphasis on the important and independent role that both quantity and variety in F&V intake may play in helping to prevent the development of T2D.
Acknowledgements
This study was supported by grants from the Medical Research Council, the Food Standards Agency, Cancer Research UK and the British Heart Foundation. The sponsors did not participate in the design or conduct of this study; in the collection, management, analysis, or interpretation of data; in the writing of the manuscript; or in the preparation, review, approval, or decision to submit this manuscript for publication.
AJC had full access to all the data in the study and takes responsibility for the accuracy of the data analysis. NGF, NJW, and KTK are the guarantors of this work and, as such, had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. NJW and KTK obtained funding. NJW, KTK and RNL acquired the data. NJW, NGF and AJC conceived and designed the study. AJC, NGF, and SJS analysed and interpreted the data. AJC drafted the manuscript, and all authors critically revised the manuscript for important intellectual content and have approved the final version.
The authors thank the EPIC-Norfolk participants and the EPIC-Norfolk team for their contributions. We also thank Amit Bhaniani, Department of Public Health and Primary Care, University of Cambridge, for help with data, and Anna Rickard and Soren Brage, MRC Epidemiology Unit, Institute of Metabolic Science, Cambridge, for helpful discussion in earlier stages of this work.
The study was approved by the Norwich district research ethics committee and informed consent was given by all participants.
Footnotes
No potential conflicts of interest relevant to this article were reported.
Publisher's Disclaimer: “This is an author-created, uncopyedited electronic version of an article accepted for publication in Diabetes Care. The American Diabetes Association (ADA), publisher of Diabetes Care, is not responsible for any errors or omissions in this version of the manuscript or any version derived from it by third parties. The definitive publisher-authenticated version will be available in a future issue of Diabetes Care in print and online at http://care.diabetesjournals.org.”
REFERENCES
- [1].Alwan A. Global status report on noncommunicable diseases 2010: Description of the global burden of NCDs, their risk factors and determinants. World Health Organization; Geneva: 2011. [Google Scholar]
- [2].Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993 Jun 10;328(23):1676–85. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- [3].Jonsson B. Revealing the cost of Type II diabetes in Europe. Diabetologia. 2002 Jul;45(7):S5–12. doi: 10.1007/s00125-002-0858-x. [DOI] [PubMed] [Google Scholar]
- [4].Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. Bmj. 2007 Feb 10;334(7588):299. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].WHO/FAO . Diet, nutrition and the prevention of chronic diseases: report of a joint FAO/WHO Expert Consultation. World Health Organization; Geneva: 2003. (WHO Technical Report Series, NO. 916). Report No.: Technical Report Series; 916. [PubMed] [Google Scholar]
- [6].U.S. Department of Agriculture. U.S. Department of Health and Human Services . Dietary Guidelines for Americans, 2010. 7th ed Government Printing Office; Washington, DC: Dec, 2010. [Google Scholar]
- [7].National Health Service [Last accessed 1st July 2011];5 A Day. Available: http://www.nhs.uk/livewell/5aday/pages/5adayhome.aspx/ [cited 2011 1st July 2011] Available from: http://www.nhs.uk/livewell/5aday/pages/5adayhome.aspx/
- [8].Willett WC. Nutritional Epidemiology. 2nd ed Oxford University Press; New York: 1998. [Google Scholar]
- [9].Bingham SA, Luben R, Welch A, Wareham N, Khaw KT, Day N. Are imprecise methods obscuring a relation between fat and breast cancer? Lancet. 2003 Jul 19;362(9379):212–4. doi: 10.1016/S0140-6736(03)13913-X. [DOI] [PubMed] [Google Scholar]
- [10].Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999 Jul;80(Suppl 1):95–103. [PubMed] [Google Scholar]
- [11].Bingham SA, Welch AA, McTaggart A, Mulligan AA, Runswick SA, Luben R, et al. Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public Health Nutr. 2001 Jun;4(3):847–58. doi: 10.1079/phn2000102. [DOI] [PubMed] [Google Scholar]
- [12].Welch AA, McTaggart A, Mulligan AA, Luben R, Walker N, Khaw KT, et al. DINER (Data Into Nutrients for Epidemiological Research) - a new data-entry program for nutritional analysis in the EPIC-Norfolk cohort and the 7-day diary method. Public Health Nutr. 2001 Dec;4(6):1253–65. doi: 10.1079/phn2001196. [DOI] [PubMed] [Google Scholar]
- [13].Bingham SA, Gill C, Welch A, Cassidy A, Runswick SA, Oakes S, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol. 1997;26(Suppl 1):S137–51. doi: 10.1093/ije/26.suppl_1.s137. [DOI] [PubMed] [Google Scholar]
- [14].National Health Service [Last accessed 20th October 2011];5 A Day: what counts? Available: http://www.nhs.uk/Livewell/5ADAY/Pages/Whatcounts.aspx. [cited; Available from:
- [15].Agudo A, Slimani N, Ocke MC, Naska A, Miller AB, Kroke A, et al. Consumption of vegetables, fruit and other plant foods in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts from 10 European countries. Public Health Nutr. 2002 Dec;5(6B):1179–96. doi: 10.1079/PHN2002398. [DOI] [PubMed] [Google Scholar]
- [16].Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008 Jul;31(7):1311–7. doi: 10.2337/dc08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. Croom Helm; London: 1988. [Google Scholar]
- [18].Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003 Jun;6(4):407–13. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- [19].Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999 Dec;52(12):1165–72. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- [20].Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995 Jul;6(4):356–65. doi: 10.1097/00001648-199507000-00005. Jul. [DOI] [PubMed] [Google Scholar]
- [21].Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. Bmj. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hamer M, Chida Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta-analysis. J Hypertens. 2007 Dec;25(12):2361–9. doi: 10.1097/HJH.0b013e3282efc214. [DOI] [PubMed] [Google Scholar]
- [23].Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993 Jul;93(7):790–6. doi: 10.1016/0002-8223(93)91754-e. Jul. [DOI] [PubMed] [Google Scholar]
- [24].Buijsse B, Feskens EJ, Schulze MB, Forouhi NG, Wareham NJ, Sharp S, et al. Fruit and vegetable intakes and subsequent changes in body weight in European populations: results from the project on Diet, Obesity, and Genes (DiOGenes) Am J Clin Nutr. 2009 Jul;90(1):202–9. doi: 10.3945/ajcn.2008.27394. [DOI] [PubMed] [Google Scholar]
- [25].Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. New England Journal of Medicine. 2011 Jun 23;364(25):2392–404. doi: 10.1056/NEJMoa1014296. 2011 Jun 23 J Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001 Sep 13;345(11):790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- [27].Harding AH, Wareham NJ, Bingham SA, Khaw K, Luben R, Welch A, et al. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: the European prospective investigation of cancer- -Norfolk prospective study. Arch Intern Med. 2008 Jul 28;168(14):1493–9. doi: 10.1001/archinte.168.14.1493. [DOI] [PubMed] [Google Scholar]
- [28].Hozawa A, Jacobs DR, Jr., Steffes MW, Gross MD, Steffen LM, Lee DH. Associations of serum carotenoid concentrations with the development of diabetes and with insulin concentration: interaction with smoking: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2006 May 15;163(10):929–37. doi: 10.1093/aje/kwj136. [DOI] [PubMed] [Google Scholar]