Abstract
Signalling between the epithelium and stromal cells is crucial for growth, differentiation and repair of the epithelium. While the retinoblastoma protein is known to regulate the growth of keratinocytes, in a cell-autonomous fashion, here we describe a function of Rb in the stromal compartment. We find that Rb depletion in fibroblasts leads to inhibition of differentiation and enhanced proliferation of the epithelium. Analysis of conditioned medium identified that keratinocyte growth factor (KGF) levels were elevated following Rb depletion. These findings were also observed with organotypic co-cultures. Treatment of keratinocytes with KGF inhibited differentiation and enhanced keratinocyte proliferation, whilst reduction of KGF levels in Rb-depleted fibroblasts was able to restore expression of differentiation markers. Our findings suggest a crucial role for dermal fibroblasts in regulating the differentiation and proliferation of keratinocytes, and we demonstrate a role for stromal Rb in this cross-talk.
Introduction
The epidermis is part of the body’s primary defence, building a barrier against pathogens and environmental influences (Eckert et al., 1997). Epidermal development is a tightly controlled interplay between differentiation and proliferation of keratinocytes (Fuchs and Green, 1980), and cross-talk between stromal and epithelial cells is essential to regulate development and homeostasis of the epithelium (Maas-Szabowski et al., 2001) as well as during wound healing (Desmouliere et al., 2004).
Interleukin-1alpha (IL1A) (Chedid et al., 1994) and Interleukin-1beta (IL1B) (Maas-Szabowski et al., 1999) are produced by keratinocytes and act as paracrine inducers of Keratinocyte Growth Factor (KGF), which is solely produced by stromal fibroblasts (Maas-Szabowski et al., 2000), and in a double paracrine loop acts upon keratinocytes to further induce IL1A and IL1B expression. KGF was identified as a soluble factor essential for the regulation of epidermal development (Andreadis et al., 2001; Maas-Szabowski et al., 2001) and has been shown to accelerate the healing process as KGF is dramatically upregulated following wounding. Organotypic cultures, which are an in vitro skin equivalent, when treated with KGF revealed that KGF delays the expression of differentiation markers as well as increasing proliferation of keratinocytes in the epidermis (Andreadis et al., 2001; Lotti et al., 2007). In contrast, KGF treatment enhances keratinocyte differentiation in isolated keratinocytes in monolayer cultures, whilst also enhancing proliferation (Lotti et al., 2007). These conflicting views regarding the role of KGF in controlling epithelial differentiation require further investigation.
The Retinoblastoma protein (Rb) is a well-known tumour suppressor due to its essential role in cell cycle control and differentiation (Nguyen et al., 2004; Pickard et al., 2010). Rb controls cell cycle progression from G1 into S phase in a cell autonomous manner (Hatakeyama and Weinberg, 1995). In addition to these actions, Rb has been implicated in the control of differentiation and cell survival of neural cells through non-cell autonomous mechanisms in chimeric mouse models (Lipinski et al., 2001). Homozygous knockout of Rb in mice leads to embryonic lethality, which is largely due to the lack of placental development. Chimeric mice developing wild type Rb placentas go to term, indicating that Rb has a fundamental cell non-autonomous role in controlling differentiation of mouse embryonic tissue (de Bruin et al., 2003). Therefore we hypothesised that Rb may have a role in the stromal control of epithelium homeostasis.
In this paper we show that Rb plays a major role in the cross-talk between stromal fibroblasts and the epithelium and that Rb in stromal fibroblasts is essential for differentiation and proliferation of keratinocytes. This is due to its regulation of KGF expression, which inhibits differentiation of keratinocytes therefore leading to incorrect maturation of the epidermis.
Results
Interleukin 1 alpha and beta induce Rb inactivation
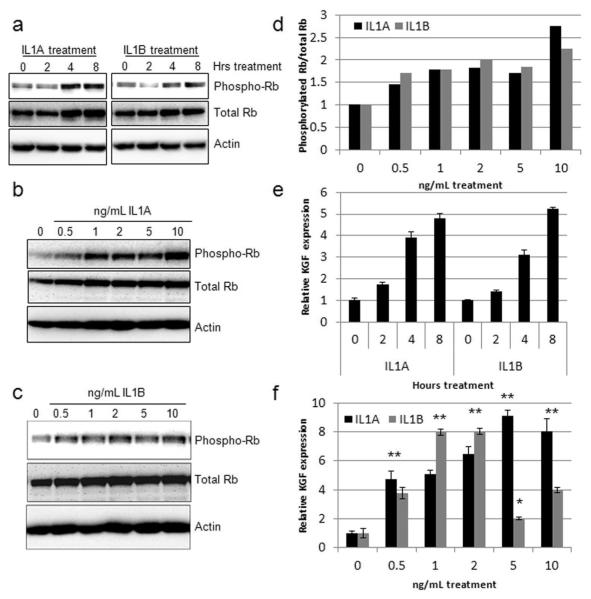
Work by Maas-Szabowski et al. proposed that KGF production and release by stromal fibroblasts is stimulated by IL1A and IL1B release from the keratinocytes (Maas-Szabowski et al., 2000). Primary human foreskin fibroblasts (HFFs) were treated with IL1A and IL1B and Western blot analysis revealed phosphorylation and hence inactivation of Rb at early time points (Figure 1A) which was maintained for at least 24 hours at various concentrations (Figures 1B and 1C). IL1A and IL1B induced Rb phosphorylation in a dose dependent manner and while there was a modest increase in total levels of Rb following treatment for 24 hours, densitometry readings confirmed that the ratio of phosphorylated to total Rb protein increased after treatment with IL1A and IL1B (Figure 1D). Similar results were obtained in triplicate analyses. Quantitative Real-Time PCR identified a significant induction of KGF expression upon treatment with either IL1A or IL1B at both early (Figure 1E) and late time points (Figure 1F). This data suggested that Rb function is inactivated upon IL1 treatment and it was hypothesised that Rb may regulate KGF expression.
Figure 1. IL1A/B induce Rb phosphorylation in HFFs.
A) Western blot analysis of Rb phosphorylation over a short time course of 10 ng/mL IL1A/B treatment. B and C) Induction of Rb phosphorylation following 24 hours treatment with various concentrations of IL1A and IL1B, respectively. Quantification of Rb phosphorylation is shown in D. E) Real time PCR analysis of KGF expression levels following IL1A/B treatment, for the indicated lengths of time. F) Induction of KGF expression by various concentrations of IL1A/B after 24h treatment at various concentrations. * p<0.05 and ** p<0.01 in a Students T-test compared to untreated samples.
Rb expression in fibroblasts is required to control proliferation and differentiation of epithelial cells
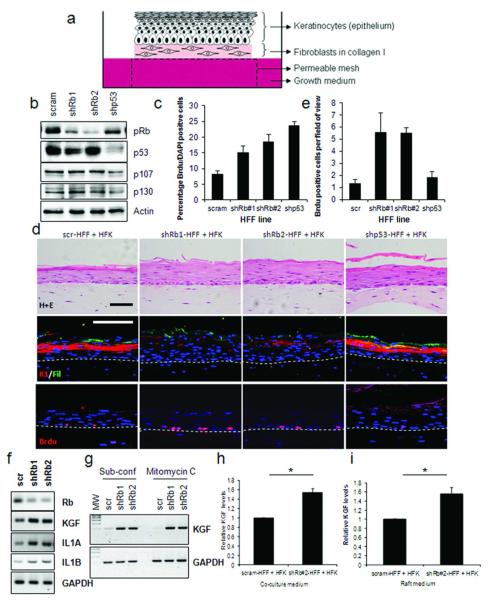
It has been identified that Rb can function through both cell-autonomous and non-cell autonomous mechanisms (Lipinski et al., 2001). In order to evaluate the cell non-autonomous functions of Rb, we used three-dimensional organotypic cultures, which utilise fibroblasts to support the growth of an over-lying epithelial layer (Figure 2A). Rb levels were reduced in primary human foreskin fibroblasts (HFFs) using retroviral transfection of shRNA directed against the coding (shRb#1) and the 3-prime untranslated region (shRb#2) of the Rb transcript. Western blot analysis showed reduction of Rb protein expression in the stable HFF lines, while the other Rb family members p107 or p130 were unaffected (Figure 2B). Depletion of Rb caused increased proliferation in HFFs compared to controls as did depletion of p53, which is known to cause increased proliferation of fibroblasts (Berns et al., 2004) (Figure 2C). The HFF lines were incorporated into a collagen and used to feed primary human foreskin keratinocytes (HFKs) in 3D organotypic cultures for 14 days. Haematoxylin and eosin (H+E) staining showed that the gross morphology of the epithelium was not dramatically altered when cultured with Rb-depleted HFFs (Figure 2D, upper panels). However, staining for the early differentiation marker keratin-1 and the late differentiation marker filaggrin showed their expression was greatly reduced in epithelium cultured with Rb-depleted HFFs indicating inhibition of differentiation (Figure 2D). Proliferation within these cultures was assessed by pulsing the organotypic cultures with Brdu and a marked increase in proliferation was observed in the epithelium cultured with the Rb-depleted HFFs compared to scramble control or p53 knockdown HFFs (Figure 2D, and quantified in Figure 2E). Similar findings were observed using Rb-depleted fibroblasts derived from adult oral tissue (human oral fibroblasts, HOFs) with either oral or foreskin epithelial cells resulted in similar phenotypes, with both differentiation and proliferation modulated (Supplemental Figure 1). Therefore, depletion of Rb in stromal fibroblasts caused an increase in proliferation of keratinocytes in the epithelium while there was no such cell non-autonomous effect of p53 depletion in stromal fibroblasts.
Figure 2. Rb-depletion in fibroblasts disrupts differentiation and proliferation of neighbouring epithelium.
A) Schematic of organotypic cultures comprised of epithelial cells seeded on top of modified fibroblasts. B) Western blots confirming knockdown of Rb and p53 in HFFs compared to scrambled shRNA controls (scram). C) Proliferation in fibroblasts assessed by Brdu incorporation (See materials and methods). D) Immuno-fluorescent detection of differentiation markers and Brdu incorporation in organotypic cultures indicate disruption of differentiation and enhanced proliferation in epithelium cultured with Rb-depleted fibroblasts. Proliferation is quantified in E). Scale bars represent 100 μM. F) KGF, IL1A and IL1B expression in Rb-depleted fibroblasts detected by RT-PCR. G) KGF expression in sub-confluent and mitomycin-C treated fibroblasts. H) ELISA detection of KGF secretion in monolayer co-cultures and I) organotypic cultures.
As IL1A and IL1B treatment of fibroblasts leads to inactivation of the Rb protein we next examined the expression of KGF in Rb depleted fibroblasts by RT-PCR, KGF along with IL1A and IL1B were found to be elevated in Rb-depleted fibroblasts (Figure 2F), it was also established that the elevated KGF expression was not due to increased proliferation as inhibition of proliferation, by mitomycin C treatment, did not alter KGF levels in Rb depleted fibroblasts (Figure 2G). ELISA detection of KGF in media from monolayer co cultures of fibroblasts and keratinocytes as well as media from organotypic cultures showed elevated secretion of KGF from cultures containing Rb-depleted fibroblasts (Figure 3H and I). To further assess the role of Rb in regulating KGF expression, Rb-depleted fibroblasts were treated with IL1A and IL1B in order to determine whether the IL1-induction of KGF was still functional in these cells. Real-time PCR confirmed that in Rb-depleted fibroblasts IL1A/B induced KGF expression to levels greater than those in control cells, suggesting that Rb acts as a suppressor of KGF expression and in the absence of Rb there is deregulated induction of KGF (Supplemental Figure 2A).
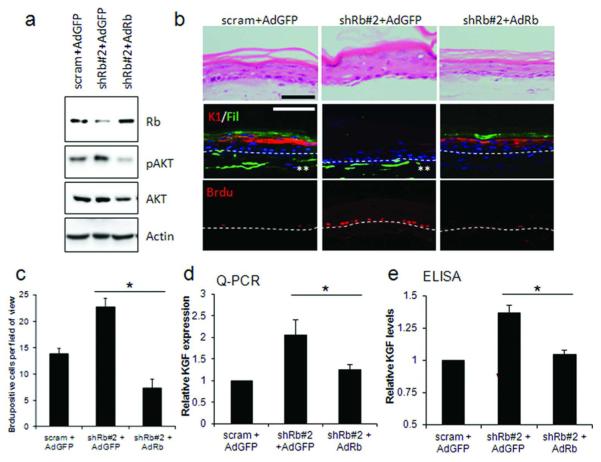
Figure 3. Rescue of Rb-depletion by adenoviral re-expression restores differentiation and proliferation defects.
A) Restoration of Rb protein levels by adenoviral overexpression in shRb#2-fibroblasts. Re-expression of Rb also reduced active AKT levels. B) Immuno-fluorescent detection of keratin-1, filaggrin and Brdu identified that re-expression of Rb restored differentiation and reduced proliferation in the epithelium of organotypic cultures. Brdu incorporation is quantified in C). Scale bars represent 100 μM. D) Q-PCR and E) ELISA detection of KGF following re-expression of Rb. * represents p<0.05 in a Students T-test. ** indicates GFP expression in fibroblasts.
To further ensure that the loss of differentiation and the enhancement of proliferation were indeed mediated by Rb and not off target effects, the levels of Rb were restored by adenoviral expression of Rb in shRb#2 HFFs (shRNA targeting the 3′-UTR of Rb). Western blotting confirmed both knockdown and restoration of Rb levels in HFFs (Figure 3A) and also restoration of AKT activation, a function of Rb previously observed in the epithelium (Menges et al., 2006). Organotypic cultures from these Rb restored HFFs showed rescue of differentiation and reduced proliferation in the epithelium which was comparable to the controls (Figures 3B and C), and in addition, the levels of KGF expression and secretion were also restored to control levels (Figure 3D and E).
Rb inactivation in fibroblasts disrupts differentiation of epithelial cells and regulates KGF expression
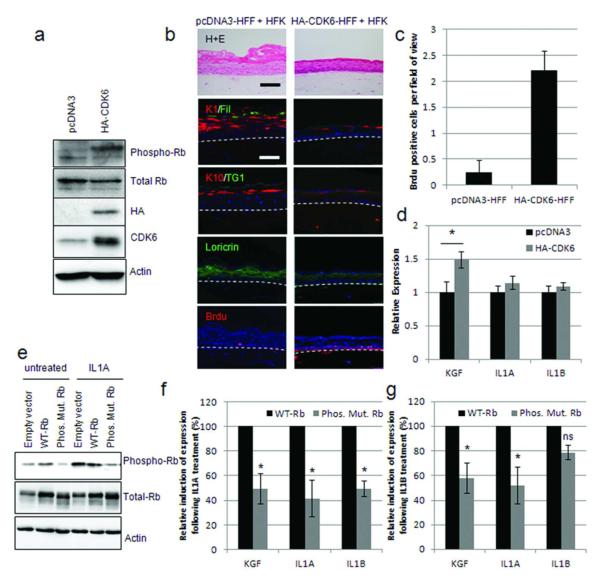
In order to recapitulate the inactivation of Rb in HFFs observed on IL1A and IL1B treatment, cyclin-dependent kinase 6 (CDK6) was expressed in HFFs. CDK6 has previously been shown to phosphorylate Rb when expressed on its own (Fiaschi-Taesch et al., 2009), and western blotting demonstrated that Rb was phosphorylated following CDK6 expression (Figure 4A). These fibroblasts were incorporated into organotypic cultures with HFKs, and the differentiation of the epithelium assessed by immuno-fluorescence. Similar to Rb-depleted fibroblasts, CDK6 expressing fibroblasts inhibited the differentiation of the epithelium as well as promoting proliferation (Figure 4B and C). Interestingly, results from three independently generated lines, showed that expression of CDK6 induced KGF, but did not significantly alter IL1A or IL1B expression (Figure 4D), further implicating Rb in the regulation of KGF expression. To further establish whether the phosphorylation of Rb is required for the induction of KGF by IL1A and IL1B, we utilized a mutated Rb construct where potential cdc2 phosphorylation sites are mutated to unphosphorylatable amino acids (Phos. Mut. Rb). This plasmid or one encoding wild-type Rb was transfected into fibroblasts before treating with IL1A. It was not possible to generate stable cell lines with either wild-type Rb (WT-Rb) or the phosphorylation mutant, therefore transient transfection was conducted for 24 hours before IL1A treatment. Equivalent expression of WT-Rb and the mutant was confirmed by western blot (Figure 4E). As previously observed with similar Rb mutant proteins (Barrientes et al., 2000) the phosphorylation mutant ran at a slightly smaller size due to the absence of phosphorylation (Figure 4E). Following IL1A 24 hour treatment WT-Rb was phosphorylated whereas the mutant was not and IL1A treatment resulted in the induction of KGF expression in WT-Rb expressing cells comparable to that observed in control cells (3.5-5.8 fold induction across three experiments). In Phos.Mut.Rb expressing cells the induction of KGF, as well as IL1A/B expression, was significantly reduced (Figure 4F). Similar results were obtained with IL1B treatment (Figure 4G). This suggests that in the cascade of IL1-mediated signalling, Rb must be inactivated, by phosphorylation, in order for KGF to be expressed. Whilst the phosphorylation mutant did not completely abolish IL1A induction of KGF, this may represent a measure of the transfection efficiency in fibroblasts (70% transfection efficiency).
Figure 4. Rb inactivation enhances KGF expression.
A) HA-tagged CDK6 expression in HFFs confirmed by Western blotting. B) Organotypic cultures grown with HA-CDK6 expressing fibroblasts were stained for differentiation markers and Brdu-incorporation which is quantified in C). D) Q-PCR analysis of KGF, IL1A and IL1B in HA-CDK6 expressing HFFs. * p<0.05 in a Students T-test. E) Western blot detection of wild-type Rb (WT-Rb) and a non-phosphorylatable mutant (Phos.Mut.Rb) expressed in HFFs. F) Q-PCR assessment of the induction of KGF, IL1A and IL1B expression following 10 ng/mL IL1A (F), or IL1B (G) treatment of cells described in E. Induction of KGF/IL1A and IL1B expression in WT-Rb HFFs was assigned as 100%, results are from triplicate experiments. * represents p<0.05 in a Students T-test compared to WT-Rb samples.
KGF inhibits differentiation of primary human keratinocytes
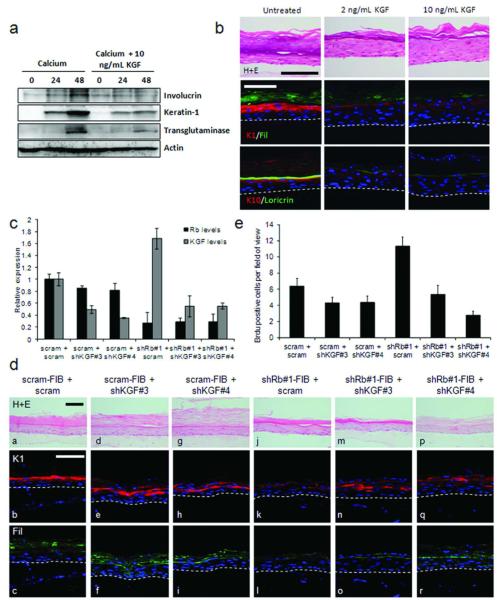
We next investigated the effects of KGF treatment on keratinocyte differentiation, as its function described in the literature remains unclear. Monolayer and organotypic cultures were treated with KGF. Monolayers of HFKs were treated with 1.5 mM calcium to induce differentiation in the presence or absence of 10 ng/mL KGF, and Western blotting of early (involucrin and keratin-1) and late (transglutaminase-1) differentiation markers indicated that KGF reduced the levels of differentiation markers (Figure 5A). KGF treatment of organotypic cultures resulted in a thicker undifferentiated epithelium that lacked clear stratification, probably due to increased proliferation in the presence of KGF (Supplemental Figure 2B) Expression of early and late differentiation makers were reduced in cultures treated with KGF (Figure 5B), with the early markers only being expressed at low levels in the upper epithelium. This suggested that KGF may be the responsible factor for the loss of epithelial differentiation when cultured with Rb-depleted fibroblasts. To directly assess whether increased KGF expression was the causal factor for the loss of differentiation and increased proliferation in Rb knockdown cultures, KGF levels were reduced by shRNA in Rb-depleted HFFs and controls (Figure 5C and Supplemental Figure 2C). Surprisingly, knockdown of KGF in control HFFs did not dramatically alter the differentiation of the epithelium (Figure 5D compare panels a-c, with d-f and g-i), but resulted in a small but reproducible reduction in proliferation (Figure 5E). However, in organotypic cultures with Rb-depleted HFFs there was restoration of the differentiation markers keratin-1 and filaggrin when KGF levels were depleted (Figure 5D comparing panels m-o and p-r to j-l). Together this suggests that elevated levels of KGF in Rb-depleted HFFs mediate the loss of differentiation and enhanced proliferation observed within the epithelium.
Figure 5. KGF inhibits differentiation of epithelial cells.
A) Addition of KGF to keratinocyte cultures inhibited calcium induced differentiation as assessed by Western blotting of involucrin, keratin-1 and transglutaminase levels. B) Organotypic cultures grown in the presence of 2 or 10 ng/mL KGF showed disrupted differentiation. Representative cultures from triplicate analyses are shown. C) Knockdown of KGF levels in Rb-depleted fibroblasts was assessed by Q-PCR. D) Organotypic cultures using fibroblasts in C showed that KGF depletion did not significantly alter differentiation in control cultures (compare a-c with d-f and g-i), however in cultures grown with Rb-depleted fibroblasts, KGF depletion restored epithelial differentiation (compare j-l with m-o and p-r). E) Brdu incorporation in the epithelium of cultures shown in D. Scale bars represent 100 μM.
Discussion
As the epidermis is an essential part of the body’s defence it is important that tissue regeneration and maintenance are tightly controlled processes. Keratinocytes are the primary cell type constituting the epidermis and their gradual differentiation results in a mature epidermis (Eckert et al., 1997; Fuchs and Green, 1980). Keratinocytes rely on constant cross-talk with the stromal compartment to ensure correct maturation of the epidermis. It is known that IL1A and IL1B, produced by keratinocytes, induce KGF in stromal fibroblasts (Chedid et al., 1994; Maas-Szabowski and Fusenig, 1996). Our results suggest that stromal Rb plays a vital role in the cross-talk between keratinocytes and stromal fibroblasts. Our results implicate Rb as a regulator of IL1 signalling in stromal fibroblasts, which in turn acts to control expression of KGF, and therefore growth of the epithelium.
Under our experimental conditions Rb phosphorylation was observed as early as 4 hours following IL1 treatment of HFFs, which was concomitant with a strong induction of KGF expression (Figure 1). KGF has been shown to be induced following 2 hours IL1A/B treatment (Chedid et al., 1994), however these experiments used the lung fibroblast cell line M426, and the cells were treated in the absence of serum, which may account for this difference. In fibroblasts depleted of Rb, we observed elevated KGF expression, which is further increased when these cells are treated with IL1A or IL1B, suggesting that a normally suppressive pathway has been compromised. Similarly in cells transfected with an Rb mutant which cannot be phosphorylated/inactivated the induction of KGF is stunted. Regulation of KGF expression by Rb is supported by evidence from microarray data comparing mouse embryonic fibroblasts from Rb null mice to litter mate controls which also identify significantly increased expression of KGF with Rb-depletion (GEO Dataset GDS3099 (Liu et al., 2009)).
In the literature, there are conflicting views as to whether KGF promotes or inhibits differentiation (Andreadis et al., 2001; Lotti et al., 2007). The differing results may be due to the model systems used, where one study used organotypic cultures (Andreadis et al., 2001), the other used monolayer culture (Lotti et al., 2007). Our findings show that following KGF treatment of keratinocytes or their co-culture with Rb-depleted fibroblasts, acts to disrupt the differentiation process, with KGF-depletion in Rb knockdown fibroblasts restoring differentiation in the epithelium. The absence of an effect when KGF levels are depleted in normal fibroblasts is an interesting finding, these results replicate observations in KGF knockout mice where the epithelium differentiates normally (Guo et al., 1996) and suggests that KGF is not required to form a mature epithelium. It is likely that related factors, such as FGF-10, play the important role in the formation and maintenance of the epithelium, as indicated by poor proliferation and differentiation in the epithelium of the knockout mice (Suzuki et al., 2000). Our results further imply that IL1A and IL1B originating from the epithelium ultimately act to disrupt the differentiation process and enhance proliferation by driving expression of KGF in the stroma. IL1A and IL1B are also known to upregulate IL-6 and IL-8 expression in fibroblasts (Maas-Szabowski et al., 1999), both of which have been shown to enhance proliferation of the epithelium (Tuschil et al., 1992; Yoshizaki et al., 1990).
Our data describe a role for Rb in the stromal compartment and this adds to a growing body of evidence indicating the importance of the stromal compartment in epithelial differentiation and tumourigenesis. Rb itself has been implicated in the control of apoptosis and differentiation of neural cells in a cell non-autonomous manner and the results presented show a cell non-autonomous function of Rb in controlling the growth and maturation of the stratified epithelium.
Materials and Methods
Cell culture
Human oral and foreskin keratinocytes and oral fibroblasts were isolated as previously described (McKeown et al., 2003; Pickard et al., 2010). Human foreskin fibroblasts were purchased from Cascade Biologics. Fibroblasts were maintained in sub-confluent cultures in DMEM supplemented with 10% FBS. Sub-confluent fibroblasts were treated with the indicated reagents for the indicated times, before harvesting cells for protein (urea buffer: 8 M Urea, 50 mM Tris-HCl pH7.5, supplemented with protease and phosphatase inhibitors (Roche) and 0.1% beta-mercaptoethanol) and RNA (Trizol (Invitrogen)). Oral and foreskin keratinocytes were grown in Epilife supplemented with human keratinocyte growth supplement (Invitrogen). Co-culture experiments were conducted in E-medium supplemented with 10% foetal clone I (Hyclone) (McCance et al., 1988) in a 3:1 ratio of keratinocytes:fibroblasts. For ELISA and proliferation experiments, co-culture samples were analysed 48 hours after co-culture. Organotypic cultures were grown as previously described (McCance et al., 1988), for 14 days, before sectioning and H+E staining using standard procedures. To differentiate keratinocyte monolayers cells were grown to confluence and then serum was withdrawn before adding 1.5 mM CaCl2 for the indicated times. Mitomycin C treatments were at a final concentration of 2 μg/mL for 2 hours, following treatment fresh medium was added, and harvested after 24 hours.
Proliferation
Monolayer cultures were pulsed with 10 μM Brdu for 15 minutes before fixing in 4% PFA. Organotypic cultures were pulsed with 20 μM Brdu for 16 hours prior to fixing in 4% PFA. Proliferation was assessed by immuno-fluorescent detection of Brdu following antigen retrieval in citrate buffer (DAKO). At least 2000 cells were counted for monolayer cultures and 10 fields for organotypic cultures, selected randomly.
Generation of stable knockdown fibroblasts and immortalised keratinocytes
Knockdown fibroblasts were generated by retroviral transduction of shRNA using the phoenix system. Vectors with shRNA targeting Rb and p53 have been previously described (Incassati et al., 2006; Pickard et al., 2010), and cells were selected using 1.25 μg/mL puromycin for 3 days. Experiments with knockdown of both Rb and KGF, shRNAs targeting Rb were shuttled into pSuper-retro-neo and pSuper-retro-hygro, cells were selected in 400 μg/mL G418 or 20 μg/mL hygromycin for 2-4 days before transducing with shRNAs targeting KGF (Origene (TR312988, puromycin selection, molecules 3 and 4)). Ad-GFP and Ad-Rb, previously described (Pickard et al., 2010), were used to infect fibroblasts (MOI of 50). pcDNA3 and HA-CDK6 (Addgene, Plasmid 1868) fibroblast lines were generated by transfecting HFFs with 5 μg plasmid, using a 3:1 ratio of PEI:DNA, and selection with 400 μg/mL G418 for 2 days, 24 hours post-transfection. To generate WT-Rb and Phos.Mut.Rb expressing fibroblasts, cells were transiently transfected with 1 μg of pSG5 Rb-(previously described (Nead et al., 1998)) or pEF1-unphosphorylatable Rb, encoding full length Rb protein but containing the following mutations T252A, T353R, S608A, S612A, S788A, S794A, S795A, S807A and S811E as previously reported (Chang et al., 1995; Knudsen and Wang, 1997) and allowed to express for 24 hours before IL1A or IL1B treatment.
Antibodies
The following antibodies were used for Western blotting: Cell signaling: phospho-Rb (#9308), total AKT (#9272), phospho-AKT(Ser473) (#9271). BD Biosciences Rb (#554136), p130 (#610261). Santa Cruz: p107 (sc-318) p53 (sc-126), transglutaminase 1 (sc-18127), HA (Santa Cruz, sc-805), CDK6 (sc-177). Sigma-Aldrich: beta-actin (AC74, 1:10,000), involucrin (I9018). K1 (Covance, PRB-149R, 1:2000). Immuno-fluorescent detection of protein expression in organotypic cultures was conducted as previously described (Pickard et al., 2010). Antibodies for immuno-fluorescence, without antigen retrieval: K1 (as above) and filaggrin (Neomarkers, MS-449-P1). For immuno-fluorescence with antigen retrieval: loricrin (Covance, AF62, 1:2000), Santa Cruz: K13 (sc-57003), Brdu (sc-32323, 1:50), K10 (Biodesign international, M44101M). Antibody dilution 1:1000 unless stated.
Semi-quantitative RT-PCR, real time PCR and ELISA
RT-PCR was conducted using 1μg RNA isolated using Trizol (Invitrogen). cDNA was generated using random oligos and MLV-reverse transcriptase (Invitrogen). cDNA was diluted 1:10 in dH2O prior to PCR. PCR conditions were 1.5 mM MgCl2, 0.2 μM primers, 0.2 mM dNTPs and 0.1U Taq (Promega). Real-time PCR was conducted using cDNA generated as above, and conducted using Sybr green (Roche), results were quantified against standard curves for each primer set and normalised to GAPDH (fibroblasts) or RPLPO (keratinocytes). Primers for this study are described in Supplemental table 1. KGF ELISA (R&D systems) was conducted according to the manufacturer’s protocol, ELISA results were normalised to cell number.
Supplementary Material
Acknowledgements
We would like to thank: Dr Chris Irwin, Queen’s University Belfast for oral fibroblasts and keratinocytes, Professor Tony Kouzarides, Cambridge, for kindly providing pEF1-non-phosphorylatable Rb and Ken Arthur for sectioning organotypic cultures. Funding for this work was supported by NIDCR: ROI 15935 and Wellcome Trust: 082840/Z/07/Z
Abbreviations
- KGF
keratinocyte growth factor
- IL1A
interleukin 1 alpha
- IL1B
interleukin 1 beta
- HFF
human foreskin fibroblasts
- HFK
human foreskin keratinocytes
- Rb
retinoblastoma protein
- K1
keratin 1
- Fil
filaggrin
Footnotes
Conflict of interest The authors state no conflict of interest.
References
- Andreadis ST, Hamoen KE, Yarmush ML, Morgan JR. Keratinocyte growth factor induces hyperproliferation and delays differentiation in a skin equivalent model system. Faseb J. 2001;15:898–906. doi: 10.1096/fj.00-0324com. [DOI] [PubMed] [Google Scholar]
- Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M, et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature. 2004;428:431–7. doi: 10.1038/nature02371. [DOI] [PubMed] [Google Scholar]
- Chang MW, Barr E, Seltzer J, Jiang YQ, Nabel GJ, Nabel EG, et al. Cytostatic gene therapy for vascular proliferative disorders with a constitutively active form of the retinoblastoma gene product. Science (New York, NY. 1995;267:518–22. doi: 10.1126/science.7824950. [DOI] [PubMed] [Google Scholar]
- Chedid M, Rubin JS, Csaky KG, Aaronson SA. Regulation of keratinocyte growth factor gene expression by interleukin 1. The Journal of biological chemistry. 1994;269:10753–7. [PubMed] [Google Scholar]
- de Bruin A, Wu L, Saavedra HI, Wilson P, Yang Y, Rosol TJ, et al. Rb function in extraembryonic lineages suppresses apoptosis in the CNS of Rb-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6546–51. doi: 10.1073/pnas.1031853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. The International journal of developmental biology. 2004;48:509–17. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiological reviews. 1997;77:397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, et al. Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes. 2009;58:882–93. doi: 10.2337/db08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–42. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Fuchs E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes & development. 1996;10:165–75. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M, Weinberg RA. The role of RB in cell cycle control. Progress in cell cycle research. 1995;1:9–19. doi: 10.1007/978-1-4615-1809-9_2. [DOI] [PubMed] [Google Scholar]
- Incassati A, Patel D, McCance DJ. Induction of tetraploidy through loss of p53 and upregulation of Plk1 by human papillomavirus type-16 E6. Oncogene. 2006;25:2444–51. doi: 10.1038/sj.onc.1209276. [DOI] [PubMed] [Google Scholar]
- Knudsen ES, Wang JY. Dual mechanisms for the inhibition of E2F binding to RB by cyclin-dependent kinase-mediated RB phosphorylation. Molecular and cellular biology. 1997;17:5771–83. doi: 10.1128/mcb.17.10.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski MM, Macleod KF, Williams BO, Mullaney TL, Crowley D, Jacks T. Cell autonomous and non-cell-autonomous functions of the Rb tumor suppressor in developing central nervous system. The EMBO journal. 2001;20:3402–13. doi: 10.1093/emboj/20.13.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Knabb JR, Spike BT, Macleod KF. Elevated poly-(ADP-ribose)-polymerase activity sensitizes retinoblastoma-deficient cells to DNA damage-induced necrosis. Mol Cancer Res. 2009;7:1099–109. doi: 10.1158/1541-7786.MCR-08-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti LV, Rotolo S, Francescangeli F, Frati L, Torrisi MR, Marchese C. AKT and MAPK signaling in KGF-treated and UVB-exposed human epidermal cells. Journal of cellular physiology. 2007;212:633–42. doi: 10.1002/jcp.21056. [DOI] [PubMed] [Google Scholar]
- Maas-Szabowski N, Fusenig NE. Interleukin-1-induced growth factor expression in postmitotic and resting fibroblasts. The Journal of investigative dermatology. 1996;107:849–55. doi: 10.1111/1523-1747.ep12331158. [DOI] [PubMed] [Google Scholar]
- Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. Journal of cell science. 1999;112(Pt 12):1843–53. doi: 10.1242/jcs.112.12.1843. [DOI] [PubMed] [Google Scholar]
- Maas-Szabowski N, Stark HJ, Fusenig NE. Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. The Journal of investigative dermatology. 2000;114:1075–84. doi: 10.1046/j.1523-1747.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- Maas-Szabowski N, Szabowski A, Stark HJ, Andrecht S, Kolbus A, Schorpp-Kistner M, et al. Organotypic cocultures with genetically modified mouse fibroblasts as a tool to dissect molecular mechanisms regulating keratinocyte growth and differentiation. The Journal of investigative dermatology. 2001;116:816–20. doi: 10.1046/j.1523-1747.2001.01349.x. [DOI] [PubMed] [Google Scholar]
- McCance DJ, Kopan R, Fuchs E, Laimins LA. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:7169–73. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown ST, Hyland PL, Locke M, Mackenzie IC, Irwin CR. Keratinocyte growth factor and scatter factor expression by regionally defined oral fibroblasts. European journal of oral sciences. 2003;111:42–50. doi: 10.1034/j.1600-0722.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- Menges CW, Baglia LA, Lapoint R, McCance DJ. Human papillomavirus type 16 E7 up-regulates AKT activity through the retinoblastoma protein. Cancer research. 2006;66:5555–9. doi: 10.1158/0008-5472.CAN-06-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nead MA, Baglia LA, Antinore MJ, Ludlow JW, McCance DJ. Rb binds c-Jun and activates transcription. The EMBO journal. 1998;17:2342–52. doi: 10.1093/emboj/17.8.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Baglia LA, Huang SM, Baker CM, McCance DJ. Acetylation regulates the differentiation-specific functions of the retinoblastoma protein. The EMBO journal. 2004;23:1609–18. doi: 10.1038/sj.emboj.7600176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard A, Wong PP, McCance DJ. Acetylation of Rb by PCAF is required for nuclear localization and keratinocyte differentiation. Journal of cell science. 2010;123:3718–26. doi: 10.1242/jcs.068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Yamanishi K, Mori O, Kamikawa M, Andersen B, Kato S, et al. Defective terminal differentiation and hypoplasia of the epidermis in mice lacking the Fgf10 gene. FEBS letters. 2000;481:53–6. doi: 10.1016/s0014-5793(00)01968-2. [DOI] [PubMed] [Google Scholar]
- Tuschil A, Lam C, Haslberger A, Lindley I. Interleukin-8 stimulates calcium transients and promotes epidermal cell proliferation. The Journal of investigative dermatology. 1992;99:294–8. doi: 10.1111/1523-1747.ep12616634. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K, Nishimoto N, Matsumoto K, Tagoh H, Taga T, Deguchi Y, et al. Interleukin 6 and expression of its receptor on epidermal keratinocytes. Cytokine. 1990;2:381–7. doi: 10.1016/1043-4666(90)90069-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.