Abstract
Pseudomonas aeruginosa chronically infects patients with cystic fibrosis and is associated with greater morbidity. There has been limited progress on the clinical development of new antibiotics with novel modes of action. This review addresses some of the latest research developments on the exploitation of candidate adjuvant therapeutic agents that may act alongside conventional antibiotics as an alternative therapeutic strategy. After considering key mechanisms this opportunistic pathogen employs to control virulence, the progress of various strategies including the inhibition of quorum sensing, efflux pumps and lectins, as well as the use of iron chelators, bacteriophages, immunisation and immunotherapy is reviewed. Both, therapeutic approaches in early development and clinical phase are discussed.
Introduction
Concern regarding the impact of antibiotic resistance has led the European Commission to develop an action plan against the rising threat – priority is given to mitigating the development of antibiotic resistance through appropriate use, prevention of infection and developing effective antimicrobials[1]. In a paired surveillance report by the European Centre of Disease Prevention and Control, reporting on resistance trends of the top seven bacterial pathogens of importance to human health, Pseudomonas aeruginosa is detailed as being of particular concern due to its ubiquity and intrinsic tolerance to many antibiotics[2]. Many patients have increased susceptibility to P. aeruginosa infections including those with chronic lung conditions such as cystic fibrosis (CF), bronchiectasis and chronic obstructive pulmonary disease (COPD)[3]; individuals who are immunosuppressed, have indwelling catheters and those receiving intensive care[4].
Of those patients with CF, those with chronic pulmonary infection with Pseudomonas aeruginosa suffer a more rapid deterioration in lung function, greater morbidity and a shorter life expectancy[5]. Repeated prolonged courses of broad-spectrum antibiotics lead to the selection of increasing antibiotic tolerant and resistant strains[6]. Although infection with P. aeruginosa may be eradicated if treatment is commenced early[7], no antibiotics are able to eradicate an established chronic P. aeruginosa infection and there are no such agents on the horizon [8]. A new paradigm for the management of chronic pulmonary infection with P. aeruginosa in CF is clearly needed. Antibiotic adjuvants, agents that act alongside a co-administered antibiotic, potentiating its action, may offer a future strategy. We recently published a Cochrane review and found that few such interventions had been assessed in rigorous clinical trials and so could not recommend their use [9]. There are however numerous of these novel strategies under development some of which are reaching clinical trials. This review will evaluate the opportunities these strategies may present and consider when such therapeutic approaches may be available for our patients.
Pseudomonas aeruginosa and the CF lung
P. aeruginosa has two modes of growth: as motile planktonic cells adept at colonising new sites, and as a biofilm, enabling communities of organisms to protect themselves from host immune and antibiotic attack. In contrast to the situation with chronic infection, isolates of P. aeruginosa in previously uninfected individuals appear to be almost fully susceptible to first line antibiotics [10]. However, many characteristics of strains responsible for early P. aeruginosa infection, such as pyocyanin and protease production, appear not to be predictive of persistence compared to strains that are successfully eradicated[11]. Instead a combination of host-pathogen factors may be important in the conversion to chronic infection rather than bacterial factors in isolation, in contrast to the situation with non-CF bronchiectasis.
The biofilm mode of growth is associated with a mucoid phenotype of P. aeruginosa [12]. Individuals with CF who are chronically infected with mucoid organisms have a more rapid decline in clinical status compared to those with non-mucoid P. aeruginosa who in turn decline more rapidly than those without infection [16].
There is significant phenotypic variation between P. aeruginosa strains found to infect the CF lung both within[17] and between[18] individuals. Rapid mutations may occur in subsets of bacteria within individuals that provide adaptation for chronic infection and are linked to the development of antibiotic resistance[18]. The innate antibacterial tolerance of P. aeruginosa provides significant therapeutic challenges but this, accompanied by its modest nutritional demands and ability to use both aerobic and anaerobic metabolism, makes it a versatile opportunistic pathogen. The biofilm mode of growth provides a significant challenge for therapy as even when antibiotics are able to penetrate the biofilm, the combination of oxygen limitation and low bacterial metabolic activity result in limited bacterial killing[19] with a core of inactive ‘persister’ cells that are uniquely tolerant to antibiotics but can re-populate the biofilm once administration of an antibiotic ceases[20].
In the CF lung, a lack of functioning cystic fibrosis transmembrane regulator (CFTR) in the apical membrane of the respiratory epithelial cell results in an environment that is favourable to P. aeruginosa. Physical effects of thick secretions, dehydrated epithelial surfaces and accompanying mucus plugging allow the organism to establish a colony. There is an on-going debate regarding whether the CFTR mutation is itself pro-inflammatory or whether the excessive inflammation is secondary to bacterial infection. The defect of CFTR itself may promote infection with P. aeruginosa either by increasing adherence[21] or reducing clearance of the organism[21, 22]. P. aeruginosa is ubiquitous in the environment and as the CF lung epitomizes the ideal niche for the organism to become pathogenic[4], this organism exerts a significant burden on the well-being of patients with CF.
Recent advances in antibiotic therapies
New antibiotic formulations have been developed over recent years and some are within the developmental pipeline. Tobramycin inhalation solution (TIS) has been introduced for the long-term management of chronic P. aeruginosa infection, with a Cochrane review suggesting some benefit from TIS in terms of lung function and pulmonary exacerbation rate but which raised concern regarding an increase in antibiotic resistance[23]. A recent registry study examining data from the Cystic Fibrosis Foundation’s Patient Registry has suggested that TIS use is associated with reduced mortality [24]. TIS has also been demonstrated to be effective in delaying re-infection in those with early P. aeruginosa infection[25]. A dry powder formulation of tobramycin for inhalation has been developed that does not require a nebuliser and so appears to be more convenient, but as effective as TIS[26, 27]. It is suggested this may improve adherence to treatment. Aztreonam lysine has been recently approved for inhalation for those with P. aeruginosa infection the benefit of which appears to be good sputum penetration, delayed time to next exacerbation and improved lung function[13].
Antibiotics in development include liposomal preparations of amikacin and ciprofloxacin[14] and the first trial of a levofloxcacin inhalation solution which has recently reported with favourable results[15]. A new family of peptidomimetics has generated excitement as early in vitro and animal models suggests potency but also specificity for P. aeruginosa[107].
Anti-virulence strategies
Much of the organism’s capacity for virulence, antibiotic resistance and evasion of the host immune system is controlled by complex chemical signalling mechanisms. These mechanisms offer the potential for exploitation as therapeutic targets in the development of novel antibacterial agents (Table 1).
Table 1. Virulence approaches by Pseudomonas aeruginosa, their role in pathogenicity and opportunities for intervention.
| Virulence approaches | Mechanism of action/activity | Therapeutic strategies |
|---|---|---|
| Alginate biofilm* | Biofilm formation[28] | Alginate lyase[29] |
| Quorum sensing*[30] | Coordination of virulence factor production |
Quorum sensing inhibitors |
| Immune modulation[31] | ||
| Phenotypic transfer and variability | Resistance acquisition[12] Environmental adaptation |
Anti-sense inhibitors[32] |
| Pili* | Adhesion[33] | Immunisation |
| Twitching motility[33] | ||
| Biofilm formation[33] | ||
| Horizontal gene transfer[34] (natural transformation) |
||
| RND efflux pumps*[35] | Antibiotic removal[36] | Efflux pump inhibitors |
| QS molecule release | ||
| Lectin* | Cell aggregation proteins[37] | Lectin binding site competitive |
| Ciliary dysregulation[38] | inhibition | |
| Flagella | Motility[33] | Immunisation[39] |
| - flagellin | Immune induction | |
| Immune modulation*[31] | AHL/AQ signal | Quorum sensing inhibition |
| Rhamnolipid*[40] | Biosurfactants – diffusible nutrition |
Quorum sensing inhibition |
| Swarming/motility | ||
| PMN necrotic killing | ||
| Iron sequestration* | Pyoverdin | Quorum sensing inhibition |
| Pyochelin | Iron metabolism | |
| PQS[41] | inhibition/chelation[42] | |
| Enzymes | Quorum sensing inhibition | |
| invasion-mediating* | Elastases | P-lactamase inhibitors |
| Phospholipase | ||
| Lecithinase | ||
| (alkaline protease) | ||
| antibiotic-modifying | P-lactamases | |
| Cephalosporinase | ||
| Aminoglycoside-modifying enzymes | ||
| Toxins | Lipopolysaccharide (endotoxin) | Immunotherapy & Immunisation[43] |
| Exotoxin A | Antibody to secretion system | |
| Exoenzyme S | apparatus[44] | |
| Host-cardiovascular effects[45] | AHL-mediated vasodilatation – increasing blood flow for nutrient delivery |
Quorum sensing inhibition |
(denotes Quorum sensing regulated factor)
Quorum sensing inhibition
P. aeruginosa regulates much of its virulence via quorum sensing (QS). QS is a mechanism by which individual bacteria communicate with each other through the production and detection of small signal molecules. The concentration of signal molecules within their environment increases in line with the number of bacteria within the colony producing that signal. Eventually this concentration reaches a threshold and activates virulence gene expression within the bacterial community in a coordinated manner[46]. These signals may also be perceived by other bacterial species, resulting in competition for the same niche[30] and even eukaryotic host cells resulting in cross-kingdom signalling[31, 45].
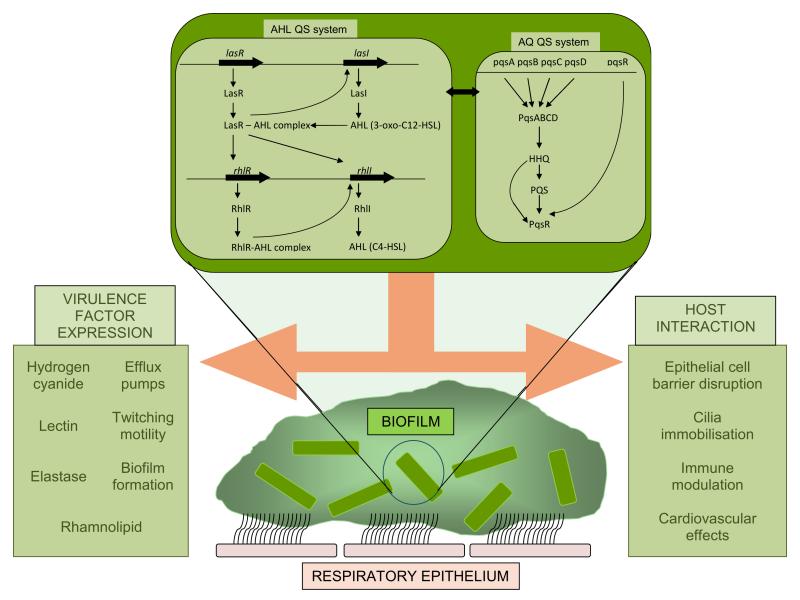
P. aeruginosa uses at least three QS signalling pathways (Figure 1). The Las and Rhl pathways utilise the N-acylhomoserine lactones (AHLs) N-(3-oxo-dodecanoyl)-L-homoserine lactone (3OC12-HSL) and N-butanoyl-L-homoserine lactone (C4-HSL) respectively[47]. The alkylquinoline (AQ) pathway uses 2-heptyl-3-hydroxy-4-quinolone, also known as the Pseudomonas Quinolone Signal (PQS), and its biosynthetic precursor 2-heptyl-4-quinolone (HHQ)[48]. These QS molecules are released into the environment by free diffusion (C4-HSL)[49], via efflux pumps (3OC12-HSL)[49] and embedded within micro-vesicles (PQS)[50]. Once the signal molecule concentration inside cells reaches a threshold it binds to its cognate transcriptional factor; 3OC12-HSL to LasR, C4-HSL to RhlR and PQS/HHQ to PqsR. The subsequent activation results in the induction of expression of multiple virulence genes and the upregulation of the signal biosynthetic genes resulting in the production of more signal (autoinduction)[51].
Figure 1. Quorum Sensing pathways of Pseudomonas aeruginosa.
P. aeruginosa uses AHLs and AQs mediated quorum sensing systems to control the production of virulence factors and the interaction with the host. The balance between these signalling mechanisms is also a key determinant in biofilm formation.
QS regulates the production of multiple virulence products (table 1) that includes rhamnolipid (inhibiting the function of host polymorphonuclear leukocytes (PMNs)). Wild type biofilms are resistant to tobramycin and the action of PMNs however QS-deficient biofilms are significantly more sensitive to the action of tobramycin and PMNs[52]. While QS has a significant role in the formation of antibiotic resistant P. aeruginosa biofilms with QS-negative mutants forming abnormal, flat and biocide-sensitive biofilms[53], the addition of PQS substantially enhances biofilm development[54]. The pivotal role of QS in the formation of biofilms is demonstrated in a mouse foreign body model of infection whereby silicone implants were infected with wild type and QS-deficient bacteria. The QS-deficient bacteria were rapidly cleared compared to the wild type which were only cleared after treatment with a QS inhibitor[55].
QS, pivotal for the regulation of virulence in P. aeruginosa, is therefore a prime therapeutic target. Quorum sensing inhibition (or ‘Quorum quenching’) of AHL signalling pathways can be achieved at different levels including the interference of signal generation, the degradation of signal molecules, preventing their accumulation, and the antagonism of the signals mode of action[56]. In the case of signal degradation, a number of enzymes of bacterial origin, capable of degrading AHL molecules and attenuating bacterial virulence gene expression have been identified[56].
QS signal molecules have been identified in the sputa of patients with CF and P. aeruginosa infection[57, 58]. Many potential QS inhibitors (QSI) which show similarities to the signal molecules have been identified by high-throughput technologies. Quorum quenching activity has been found in a number of foods including chamomile, carrot and garlic but also in algae. Some of the most potent candidates, such as algal furanones, are toxic to man but provide the chemical basis for the development non-toxic QSIs[59, 60].
Garlic (allium sativum) is one of the more potent naturally occurring QSI’s. Garlic extracts have been shown to increase the susceptibility of P. aeruginosa biofilms to antibiotics in vitro and promote clearance of P. aeruginosa in a chronic mouse infection model[61]. However direct extrapolation from an animal model to man is not possible because doses administered to mice in this model were considerably greater than those that could be tolerated by humans[61]. In a small pilot randomised controlled trial of a commercial garlic formulation in adults and children with CF and chronic P. aeruginosa infection, there was a non-significant improvement in clinical parameters and it was possible to detect QS molecules in the plasma and sputa of these patients indicating QS activity[62]. Hence the current challenge is to identify effective strategies to translate QSIs from natural sources to use in the clinic.
Commonly used antibiotics including azithromycin, ceftazidime and ciprofloxacin have been shown to have a negative impact on QS-dependent virulence factor production[63]. Interestingly, azithromycin acts as a QSI at concentrations below its minimum inhibitory concentration (MIC)[63, 64]. A murine chronic infection model has demonstrated significantly more clearance in those treated with azithromycin compared to control[64].
Lectin inhibitors
Lectins are outer membrane proteins which recognise sugar residues and allow bacterial cells to cross-link, aggregate and so form the architecture of the biofilm[37, 65]. Lectins may also interfere with normal ciliary beating in the human airway[38] and form another barrier to host-mediated clearance of the organism.
The two specific lectins, LecA and LecB have fucose-specific and galactose-specific binding sites and so may be blocked by competitive inhibitors (fucose and galactose moieties respectively). Studies performed in vitro show that these inhibitors either on their own or accompanied by an antibiotic, facilitate dissolution of biofilms or prevent their formation [65-67].
A small randomised trial in CF patients, without a control group, demonstrated a positive trend towards improvement in those that received fucose/galactose inhalation treatment[68]. Patients with chronic P. aeruginosa infection were recruited during an infective exacerbation and randomised to receive either inhalation of sugars alone or inhalation accompanied by intravenous antibiotics. Both groups demonstrated a significant reduction in sputum P. aeruginosa’s colony forming units and TNF-α levels. More recently, multivalent dendrimers with these sugars attached to them have shown higher affinities than monovalent fucose, showing potential as therapeutic agents[69].
Iron chelation
Iron metabolism in the respiratory tract is complex[70] and only available as free iron in minute quantities in the healthy lung as it is normally bound by ferritin and transferrin. However compared to non-CF sputa, the sputa of patients with CF is a rich iron source, and correlates with chronic P. aeruginosa infection[71]. The acquisition of iron is essential for the survival of this bacterium. It sequesters iron from its environment predominantly, but not exclusively[72] using the siderophores pyoverdin and pyochelin[73]. The human innate immune system has developed to recognise and block biofilm development through the action of lactoferrin, at sub-bacteriocidal concentrations[74]. Lactoferrin chelates iron prompting the bacteria to increase motility, rather than form a biofilm. However, in the CF airway the affinity of these siderophores for iron is higher than that of serum proteins and so the protein-bound iron may be sequestered directly by the action of pyoverdin [75] or liberated by proteolytic cleavage[76] see Lamont IL et al. [77] for a detailed review.
Gallium and desferrioxamine are both in clinical use for non-bacteriological indications. However their iron chelation activity is of interest as this may interfere with bacterial iron metabolism. Gallium-gentamicin liposomal co-encapsulation preparations have been shown to enhance in vitro activity of gentamicin against CF clinical isolates of P. aeruginosa[42]. A pharmacokinetic and safety study in patients with CF is underway (Clinicaltrial.gov identifier NCT01093521). Combinations of administration of desferrioxamine and tobramycin have been shown in vitro to significantly reduce biomass of preformed biofilms and prevent the formation of biofilm on CF epithelial cells[78].
Anti-resistance strategies
Efflux pumps
Efflux pumps allow the organism to regulate their internal environment by removing toxic substances, including antibiotics[35], metabolites and quorum sensing signal molecules[49]. They are also implicated in host invasion. Elements of multi-drug resistance are attributed to five families of efflux pumps of which P. aeruginosa has many of interest within the RND family (resistance nodulation division) which are implicated in resistance to many antibiotics including ciprofloxacin, ceftazidime and tobramycin[79].
Some of the efflux pumps expressed by P. aeruginosa are only active under specific growth conditions, such as those encountered in biofilms[80]. Indeed, quorum sensing is partly dependent upon efflux, as the signal molecule 3OC12-HSL is not diffusible across the cell membrane and requires active transport involving efflux pumps[49]. Findings in clinical strains have been inconsistent as over-expression of certain efflux pumps increases antibiotic resistance[81], but can also be associated with reduced virulence[82]. This may in part be related to the effects upon QS whereby increased efflux activity may increase the transport of efflux pump-dependent QS molecules (pro-virulent) but other QS molecules may be exported from within a cell preventing the concentration of these molecules reaching a quorum (and so inhibit virulence). Interestingly, these attenuated strains over-expressing efflux pumps formed better biofilms[82]. It would appear that antibiotic resistance and virulence may have competing costs for the organism and that intervention at the level of the efflux pump may have varied consequences.
The use of efflux pump inhibitors (EPI’s) have revealed that intrinsic antibiotic resistance may be overcome, acquired resistance may be reversed, and the emergence of new resistant strains to a co-administered antibiotic may be reduced[83]. Indeed there are EPIs which can ameliorate fluoroquinolone resistance in clinical strains[36]. A screen for EPI candidates revealed Phe-Arg-β-naphthylamide (PAβN) to act as an EPI [76] however progression along the drug development pipeline was halted due to phototoxicity [84]. While existing drugs, such as selective serotonin reuptake inhibitors, in the case of Staphylococcus aureus, appear to have EPI activity, an agent that acts upon P. aeruginosa has not been published [84]). Mpex Pharmaceuticals are currently developing an EPI agent in partnership with Glaxo-Smithkline due to the potential of this type of treatment in the clinic. Mpex have also been developing levofloxacin inhalation solution, the efficacy of which they have shown is increased 8-fold in the presence of a candidate EPI [85] and so it is conceivable that the product of the EPI development would be co-administered with this.
Agents that reduce the effect of efflux pumps may be useful therapeutically and result in a more effective action of antibiotics. However such an approach is likely to be complex given the opposing actions of virulence, growth and resistance[82] and the variety of efflux pumps hosted by P. aeruginosa[35].
Genetic ‘Inhibitors’ of resistance mechanisms
Anti-sense or antigene strategies have been proposed as inhibitors of resistance mechanisms at the nucleic acid level, targeting DNA and mRNA to prevent transcription and/or translation of specific genes[32]. By doing so the expression of the antibiotic resistance gene is blocked and the gene product conferring resistance is not produced.
Such an approach has been successful in vitro in reverting resistant strains to sensitive phenotypes. Delivery of the antisense agent to the site of infection is a challenge that may be overcome by linking the antisense molecule to a cell-permeabilizing peptide (CPP). This has been achieved with E. coli[86], however when considering the impermeability of P. aeruginosa this may be quite a different undertaking. Although at a prospecting stage, a delivery strategy using a bacteriophage vector[32] or a conjugate of an agent linked to a delivery peptide, as has recently been described in the investigation of activity of such an agent upon antibiotic resistant Gram negative and Gram positive bacterial species may be possible[87].
Bacteriophages
Bacteriophages are naturally-occurring viruses that infect bacterial cells, in many cases causing lysis of the bacterium. They are present in all environments and we are continually exposed to them. The advantages of using bacteriophages to kill bacteria are that only a specific bacterium will be killed, the viruses replicate at the site of the infection and few side effects have been described[88].
Studies using P. aeruginosa in vitro bacterial biofilms have been encouraging, demonstrating the ability of the phages to penetrate the biofilm and kill the bacteria[89]. In a murine model of intraperitoneal P. aeruginosa infection, a non-replicating phage yielded a 70% survival rate at day seven compared to a 20% survival at day two in untreated mice[90].
Bacteriophage therapy has been used routinely in Tblisi, Georgia although little peer-reviewed data are available[91]. A recent human clinical trial of a bacteriophage treatment of refractory P. aeruginosa-related chronic otitis externa has further demonstrated potential benefit[92].
This randomised double-blind placebo-controlled trial using a six phage strain preparation recruited patients with previously chronic and unresponsive otitis externa. These patients were selected as their infections were confirmed to exhibit in vitro bacterial sensitivity to the phage preparation. Significant reductions in patient and physician evaluation scores were observed in all domains for the treated group which was directly correlated with a significant decrease in P. aeruginosa counts. The mean duration of bacteriophages replication was 23 days in the treated group and in those patients that completely cleared their P. aeruginosa infection, no bacteriophages could be isolated thereafter. A randomised clinical trial assessing the efficacy of bacteriophage therapy for venous leg ulcers has recently completed and results are awaited[93].
There are multiple challenges with translating the promising in vitro studies with bacteriophages to widespread clinical use. While the recent clinical trials indicate a move of regulatory authorities to consider and approve such studies, gaining regulatory authority for widespread clinical use will be difficult. There are also significant technical challenges with phage selection, purification, storage and sterility control[94]. Difficulties associated with phage therapy however include their specificity for individual bacterial strains, preventing therefore the use of a single phage to treat infections involving multiple strains. In the chronic otitis media study, of those that were screened, 86.2% were sensitive to the six-phage mix. Phage virulence and dose, as well as the challenges posed by the immune system of the mammalian host must also be considered for this kind of treatment[89]. These are of particular relevance to pulmonary infection in cystic fibrosis. Possible hazards must be considered when evaluating the potential therapeutic use of bacteriophages, like the possibility of the infected organism acquiring virulence traits from the phage, as has been demonstrated recently[95].
Endolysins
Bacteriophages produce endolysins to exert their hydrolase action on the peptidoglycan cell wall resulting in the lysis of the organism. The potential of endolysins in the treatment of infection has been tested in animal disease models and found to successfully clear Streptococcus pneumoniae from colonised mucosal surfaces[96].
Whilst translation of this may be less complicated in Gram positive bacteria, the challenge of delivering an endolysin, or so-called ‘enzybiotic’ through the less permeable outer membrane of Gram negatives is more complex[97]. However in combination with an agent administered to penetrate the outer membrane, such an approach could be possible, yet very early in development[98].
Immunisation & Immunotherapy
Prevention of primary infection by immunisation is an ambitious aim considering the diversity of mechanisms used by the organism to cause disease and the variability with which they are expressed. The use of exotoxin A toxoid and lipopolysaccharide as antigens have shown reduced mortality in murine models[43]. Other strategies involving immunogenic bacterial proteins, including flagellin, the highly immunogenic protein that comprises the flagellum, are currently in development. While there have been some clinical trials of vaccine preparations published suggesting a positive effect, few are of high quality in terms of randomisation and design. Three of these trials were included in a Cochrane review involving 996 patients with a follow-up of between two and twelve years where the authors concluded that vaccination cannot currently be recommended[99].
Whilst immunisation strategies continue to be developed, immunotherapy also offers a similar approach to increase the efficacy of conventional antibiotics by artificially stimulating the immune system. Immunotherapy studies are largely marred by similar criticisms of either no or poor randomisation and non-contemporaneous controls. However the authors of the currently available studies suggest that Immunoglobulin Y (IgY) prophylaxis could increase the time to first infection, reduce the number of infection events, reduce the time to established chronic infection and delay the conversion to a mucoid strain[100]. These results have prompted its licensing in Sweden[99] and its designation as an orphan drug. A Phase III double blind randomised controlled trial of 180 patients is underway (Clinicaltrial.gov identifier NCT01455675) recruiting those with P. aeruginosa infection with the primary outcome measure being time from start of treatment to the first isolation of P. aeruginosa from sputum culture or throat swab. The completion of this study is expected in December 2014. A phase IIa open pilot trial of a monoclonal anti-lipopolysaccharide IgM antibody in those with ventilator-associated pneumonia suggested that such a therapy is safe and suggested a degree of efficacy in the small number of patients treated[102].
Antibodies have also been used against specific elements of the type III secretion apparatus of P. aeruginosa. These have ameliorated infection in a mouse pneumonia model with a subsequent reduction in mortality and bacterial load[103]. Prelimary reports of a Phase I/II study in patients with CF suggested a dose-dependent reduction in sputum inflammatory markers although the full report is awaited[44] as are results from a Phase I/II study of patients with ventilator associated pneumonia.
Innate immunity supplementation
A component of the airway response to bacteria is the generation of hypothiocyanite (OSCN−), which is bactericidal. However, the generation of this is defective in patients with CF[104]. Preliminary data suggest an impressive reduction in bacterial growth in vitro and in a murine model of infection with a nebulised OSCN/lactoferrin preparation[105]. This preparation (Meveol) has recently been granted Orphan Drug status.
Summary
P. aeruginosa exploits multiple mechanisms to elude the host immune response and the action of conventional antibiotics. Frequent and prolonged courses of broad spectrum intravenous antibiotics encourage the emergence of resistance.
Conventional antibiotics, such as azithromycin, may have some efflux and quorum sensing inhibitory effects which may invigorate work to develop antibiotics of existing classes that have additional effects[106]. However the longevity of such an approach is likely to be limited as the organism evolves to overcome such an effect. With the exception of a new generation of peptidomimetic antibiotics early in the discovery process[107], new antibiotics exploiting novel mechanisms of action do not appear to be in the pipeline. Consequently, the targets discussed above may act as useful adjuvants, increasing the effectiveness of antibiotics at our disposal. The optimal timing for the use of these agents remains uncertain, however it is likely that some approaches may be more suited to prophylaxis, while others may prolong the opportunity for eradication of early infection or may enable a treatment strategy for chronic infection. It will however be some time before such treatments reach the bedside. The novel nature of each of these approaches suggests that clinical application of these therapies may be slow and progress limited by a lack of experience of similar approaches with which to satisfy regulatory bodies. Furthermore, the limited markets for specific CF-applied therapies mean that biotechnology companies may limit interest in strategies without wider application. Nevertheless, novel approaches are required to limit P. aeruginosa’s resistance to antibiotics and the host immune system so that treatment of this versatile organism may be successful.
Acknowledgements
MNH is funded by a Wellcome Trust Clinical Research Training Fellowship (WT092295MA)
References
- 1.European Commission & Directorate General for Health and Consumers Communication from the Commission to the European Parliament and the Council – Action plan against the rising threats from Antimicrobial Resistance. 2011 Contract No.: COM (2011) 748.
- 2.European Antimicrobial Resistance Surveillance Network (EARS-Net) Surveillance report - Antimicrobial resistance surveillance in Europe 2010. European Centre of Disease Prevention and Control; 2011. Available from: http://ecdc.europa.eu/en/publications/Publications/1111_SUR_AMR_data.pdf.pdf. [Google Scholar]
- 3.Garcia-Vidal C, Almagro P, Romaní V, Rodríguez-Carballeira M, Cuchi E, Canales L, Blasco D, Heredia JL, Garau J. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective study. European Respiratory Journal. 2009;34(5):1072–8. doi: 10.1183/09031936.00003309. [DOI] [PubMed] [Google Scholar]
- 4.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171(11):1209–23. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 6.Mouton JW, den Hollander JG, Horrevorts AM. Emergence of antibiotic resistance amongst Pseudomonas aeruginosa isolates from patients with cystic fibrosis. J Antimicrob Chemother. 1993;31(6):919–26. doi: 10.1093/jac/31.6.919. [DOI] [PubMed] [Google Scholar]
- 7.Langton-Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane database of systematic reviews (Online) 2009;(4):CD004197. doi: 10.1002/14651858.CD004197.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Talbot G, Bradley J, Edwards JJ, Gilbert D, Scheld M, Bartlett J. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42(5):657–68. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 9.Hurley MN, Forrester DL, Smyth AR. Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis. Cochrane Database Syst Rev. 2010;(10):CD008037. doi: 10.1002/14651858.CD008037.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald D, Cuthbertson L, Doherty C, Campana S, Ravenni N, Taccetti G, Govan JRW. Early Pseudomonas aeruginosa infection in individuals with cystic fibrosis: is susceptibility testing justified? Journal of Antimicrobial Chemotherapy. 2010;65(11):2373–5. doi: 10.1093/jac/dkq342. [DOI] [PubMed] [Google Scholar]
- 11.Tramper-Stranders GA, van der Ent CK, Molin S, Yang L, Hansen SK, Rau MH, Ciofu O, Johansen HK, Wolfs TFW. Initial Pseudomonas aeruginosa infection in patients with cystic fibrosis: characteristics of eradicated and persistent isolates. Clinical Microbiology and Infection. 2011 doi: 10.1111/j.1469-0691.2011.03627.x. Online First DOI:10.1111/j.469-0691.2011.03627.x. [DOI] [PubMed] [Google Scholar]
- 12.Drenkard E, Ausubel F. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;416(6882):740–3. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- 13.McCoy KS, Quittner AL, Oermann CM, et al. Inhaled Aztreonam Lysine for Chronic Airway Pseudomonas aeruginosa in Cystic Fibrosis. Am J Resp Crit Care Med. 2008;178:921–928. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heijerman H, Westerman E, Conway S, et al. Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: A European consensus. J Cyst Fibros. 2009;8:295–315. doi: 10.1016/j.jcf.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Geller D, Flume P, Staab D, et al. Levofloxacin Inhalation Solution (MP-376) in Patients with Cystic Fibrosis with Pseudomonas aeruginosa. Am J Resp Crit Care Med. 2011;183:1510–1516. doi: 10.1164/rccm.201008-1293OC. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Kosorok MR, Farrell PM, Laxova A, West SEH, Green CG, Collins J, Rock MJ, Splaingard ML. Longitudinal Development of Mucoid Pseudomonas aeruginosa Infection and Lung Disease Progression in Children With Cystic Fibrosis. JAMA. 2005;293(5):581–8. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 17.Fothergill JL, Mowat E, Ledson MJ, Walshaw MJ, Winstanley C. Fluctuations in phenotypes and genotypes within populations of Pseudomonas aeruginosa in the cystic fibrosis lung during pulmonary exacerbations. J Med Microbiol. 2010;59(Pt 4):472–81. doi: 10.1099/jmm.0.015875-0. [DOI] [PubMed] [Google Scholar]
- 18.Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High Frequency of Hypermutable Pseudomonas aeruginosa in Cystic Fibrosis Lung Infection. Science. 2000;288(5469):1251–3. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 19.Walters MC, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of Antibiotic Penetration, Oxygen Limitation, and Low Metabolic Activity to Tolerance of Pseudomonas aeruginosa Biofilms to Ciprofloxacin and Tobramycin. Antimicrobial Agents and Chemotherapy. 2003;47(1):317–23. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004;230(1):13–8. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 21.Zar H, Saiman L, Quittell L, Prince A. Binding of Pseudomonas aeruginosa to respiratory epithelial cells from patients with various mutations in the cystic fibrosis transmembrane regulator. J Pediatr. 1995;126(2):230–3. doi: 10.1016/s0022-3476(95)70549-x. [DOI] [PubMed] [Google Scholar]
- 22.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci U S A. 1997;94(22):12088–93. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan G, Singh M, Dwan K. Inhaled antibiotics for long-term therapy in cystic fibrosis. Cochrane Database Syst Rev. 2011;3:CD001021. doi: 10.1002/14651858.CD001021.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Sawicki GS, Signorovitch JE, Zhang J, Latremouille-Viau D, von Wartburg M, Wu EQ, Shi L. Reduced mortality in cystic fibrosis patients treated with tobramycin inhalation solution. Pediatr Pulmonol. 2012;47(1):44–52. doi: 10.1002/ppul.21521. [DOI] [PubMed] [Google Scholar]
- 25.Ratjen F, Munck A, Kho P, Angyalosi G. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax. 2010;65(4):286–91. doi: 10.1136/thx.2009.121657. [DOI] [PubMed] [Google Scholar]
- 26.Konstan MW, Flume PA, Kappler M, Chiron R, Higgins M, Brockhaus F, Zhang J, Angyalosi G, He E, Geller DE. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J Cyst Fibros. 2011;10(1):54–61. doi: 10.1016/j.jcf.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konstan MW, Flume P, Brockhous F, Angyalosi G, He E, Geller D. Safety and efficacy of tobramycin inhalation powder (TIPTM) in treating cystic fibrosis patients infected with Pseudomonas aeruginosa (Pa) Journal of Cystic Fibrosis. 2010;9(S1):S22. [Google Scholar]
- 28.Hentzer M, Teitzel G, Balzer G, Heydorn A, Molin S, Givskov M, Parsek M. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol. 2001;183(18):5395–401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alkawash MA, Soothill JS, Schiller NL. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. APMIS. 2006;114(2):131–8. doi: 10.1111/j.1600-0463.2006.apm_356.x. [DOI] [PubMed] [Google Scholar]
- 30.Riedel K, Hentzer M, Geisenberger O, Huber B, Steidle A, Wu H, Hoiby N, Givskov M, Molin S, Eberl L. N-Acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology. 2001;147(12):3249–62. doi: 10.1099/00221287-147-12-3249. [DOI] [PubMed] [Google Scholar]
- 31.Telford G, Wheeler D, Williams P, Tomkins PT, Appleby P, Sewell H, Stewart GSAB, Bycroft BW, Pritchard DI. The Pseudomonas aeruginosa Quorum-Sensing Signal Molecule N-(3-Oxododecanoyl)-L-Homoserine Lactone Has Immunomodulatory Activity. Infect Immun. 1998;66(1):36–42. doi: 10.1128/iai.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodford N, Wareham DW, on behalf of the UK Antibacterial Antisense Study Group Tackling antibiotic resistance: a dose of common antisense? J Antimicrob Chemother. 2009;63(2):225–9. doi: 10.1093/jac/dkn467. [DOI] [PubMed] [Google Scholar]
- 33.Barken K, Pamp S, Yang L, Gjermansen M, Bertrand J, Klausen M, Givskov M, Whitchurch C, Engel J, Tolker-Nielsen T. Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environ Microbiol. 2008;10(9):2331–43. doi: 10.1111/j.1462-2920.2008.01658.x. [DOI] [PubMed] [Google Scholar]
- 34.van Schaik EJ, Giltner CL, Audette GF, Keizer DW, Bautista DL, Slupsky CM, Sykes BD, Irvin RT. DNA Binding: a Novel Function of Pseudomonas aeruginosa Type IV Pili. J Bacteriol. 2005;187(4):1455–64. doi: 10.1128/JB.187.4.1455-1464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alibert-Franco S, Pradines B, Mahamoud A, Davin-Regli A, Pagès J. Efflux mechanism, an attractive target to combat multidrug resistant Plasmodium falciparum and Pseudomonas aeruginosa. Curr Med Chem. 2009;16(3):301–17. doi: 10.2174/092986709787002619. [DOI] [PubMed] [Google Scholar]
- 36.Coban A, Ekinci B, Durupinar B. A multidrug efflux pump inhibitor reduces fluoroquinolone resistance in Pseudomonas aeruginosa isolates. Chemotherapy. 2004;50(1):22–6. doi: 10.1159/000077280. [DOI] [PubMed] [Google Scholar]
- 37.Tielker D, Hacker S, Loris R, Strathmann M, Wingender J, Wilhelm S, Rosenau F, Jaeger K. Pseudomonas aeruginosa lectin LecB is located in the outer membrane and is involved in biofilm formation. Microbiology. 2005;151(Pt 5):1313–23. doi: 10.1099/mic.0.27701-0. [DOI] [PubMed] [Google Scholar]
- 38.Adam E, Mitchell B, Schumacher D, Grant G, Schumacher U. Pseudomonas aeruginosa II lectin stops human ciliary beating: therapeutic implications of fucose. Am J Respir Crit Care Med. 1997;155(6):2102–4. doi: 10.1164/ajrccm.155.6.9196121. [DOI] [PubMed] [Google Scholar]
- 39.Doring G, Meisner C, Stern M. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proceedings of the National Academy of Sciences. 2007;104(26):11020–5. doi: 10.1073/pnas.0702403104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen P, Bjarnsholt T, Phipps R, Rasmussen T, Calum H, Christoffersen L, Moser C, Williams P, Pressler T, Givskov M, Høiby N. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007;153(Pt 5):1329–38. doi: 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- 41.Diggle S, Matthijs S, Wright V, Fletcher M, Chhabra S, Lamont I, Kong X, Hider R, Cornelis P, Cámara M, Williams P. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol. 2007;14(1):87–96. doi: 10.1016/j.chembiol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Halwani M, Yebio B, Suntres ZE, Alipour M, Azghani AO, Omri A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J Antimicrob Chemother. 2008;62(6):1291–7. doi: 10.1093/jac/dkn422. [DOI] [PubMed] [Google Scholar]
- 43.Manafi A, Kohanteb J, Mehrabani D, Japoni A, Amini M, Naghmachi M, Zaghi A, Khalili N. Active immunization using exotoxin A confers protection against Pseudomonas aeruginosa infection in a mouse burn model. BMC Microbiol. 2009;9:23. doi: 10.1186/1471-2180-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milla CE, Accorso FJ, Chmiel j, McCoy K, Billings J, Atkinson JJ, Clancy JP, Liou t, Acton J, Lynch SV, Slusher NA, Burns J, Mayer-Hamblett N, Harris JK, Patel R, Tremblay T, Parli T. Modulating Pseudomonas aeruginosa chronic inflammation with the anti-PcrV antibody KB001: Results of a pilot Clinical and Pharmacodynamic study in subjects with Cystic Fibrosis. Am J Respir Crit Care Med. 2010;181:A1845. [Google Scholar]
- 45.Gardiner SM, Chhabra SR, Harty C, Williams P, Pritchard DI, Bycroft BW, Bennett T. Haemodynamic effects of the bacterial quorum sensing signal molecule, N-(3-oxododecanoyl)-L-homoserine lactone, in conscious, normal and endotoxaemic rats. Br J Pharmacol. 2001;133(7):1047–54. doi: 10.1038/sj.bjp.0704174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuqua C, Winans SC, Greenberg EP. Census and consensus in bacterial ecosystems The LuxR-LuxI Family of Quorum-Sensing Transcriptional Regulators. Annual Review of Microbiology. 1996;50(1):727–51. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 47.Williams P, Winzer K, Chan WC, Cámara M. Look who’s talking: communication and quorum sensing in the bacterial world. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1483):1119–34. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diggle SP, Cornelis P, Williams P, Camara M. 4-Quinolone signalling in Pseudomonas aeruginosa: Old molecules, new perspectives. International Journal of Medical Microbiology. 2006;296(2-3):83–91. doi: 10.1016/j.ijmm.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 49.Pearson J, Van Delden C, Iglewski B. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol. 1999;181(4):1203–10. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437(7057):422–5. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 51.Williams P, Cámara M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Current Opinion in Microbiology. 2009;12(2):182–91. doi: 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Bjarnsholt T, Jensen P, Burmølle M, Hentzer M, Haagensen J, Hougen H, Calum H, Madsen K, Moser C, Molin S, Høiby N, Givskov M. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology. 2005;151(Pt 2):373–83. doi: 10.1099/mic.0.27463-0. [DOI] [PubMed] [Google Scholar]
- 53.Davies D, Parsek M, Pearson J, Iglewski B, Costerton J, Greenberg E. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280(5361):295–8. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 54.Diggle S, Winzer K, Chhabra S, Worrall K, Cámara M, Williams P. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol Microbiol. 2003;50(1):29–43. doi: 10.1046/j.1365-2958.2003.03672.x. [DOI] [PubMed] [Google Scholar]
- 55.Christensen L, Moser C, Jensen P, Rasmussen T, Christophersen L, Kjelleberg S, Kumar N, Høiby N, Givskov M, Bjarnsholt T. Impact of Pseudomonas aeruginosa quorum sensing on biofilm persistence in an in vivo intraperitoneal foreign-body infection model. Microbiology. 2007;153(Pt 7):2312–20. doi: 10.1099/mic.0.2007/006122-0. [DOI] [PubMed] [Google Scholar]
- 56.Dong Y, Wang L, Zhang L. Quorum-quenching microbial infections: mechanisms and implications. Philos Trans R Soc Lond B Biol Sci. 2007;362(1483):1201–11. doi: 10.1098/rstb.2007.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chambers CE, Visser MB, Schwab U, Sokol PA. Identification of N-acylhomoserine lactones in mucopurulent respiratory secretions from cystic fibrosis patients. FEMS Microbiol Lett. 2005;244(2):297–304. doi: 10.1016/j.femsle.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 58.Middleton B, Rodgers H, Cámara M, Knox A, Williams P, Hardman A. Direct detection of N-acylhomoserine lactones in cystic fibrosis sputum. FEMS Microbiol Lett. 2002;207(1):1–7. doi: 10.1111/j.1574-6968.2002.tb11019.x. [DOI] [PubMed] [Google Scholar]
- 59.Fulghesu L, Giallorenzo C, Savoia D. Evaluation of different compounds as quorum sensing inhibitors in Pseudomonas aeruginosa. J Chemother. 2007;19(4):388–91. doi: 10.1179/joc.2007.19.4.388. [DOI] [PubMed] [Google Scholar]
- 60.Smith K, Bu Y, Suga H. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem Biol. 2003;10(6):563–71. doi: 10.1016/s1074-5521(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 61.Bjarnsholt T, Jensen P, Rasmussen T, Christophersen L, Calum H, Hentzer M, Hougen H, Rygaard J, Moser C, Eberl L, Høiby N, Givskov M. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology. 2005;151(Pt 12):3873–80. doi: 10.1099/mic.0.27955-0. [DOI] [PubMed] [Google Scholar]
- 62.Smyth AR, Cifelli PM, Ortori CA, Righetti K, Lewis S, Erskine P, Holland ED, Givskov M, Williams P, Camara M, Barrett DA, Knox A. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis--a pilot randomized controlled trial. Pediatr Pulmonol. 2010;45(4):356–62. doi: 10.1002/ppul.21193. [DOI] [PubMed] [Google Scholar]
- 63.Skindersoe M, Alhede M, Phipps R, Yang L, Jensen P, Rasmussen T, Bjarnsholt T, Tolker-Nielsen T, Høiby N, Givskov M. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52(10):3648–63. doi: 10.1128/AAC.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffmann N, Lee B, Hentzer M, Rasmussen T, Song Z, Johansen H, Givskov M, Høiby N. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(-/-) mice. Antimicrob Agents Chemother. 2007;51(10):3677–87. doi: 10.1128/AAC.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diggle S, Stacey R, Dodd C, Cámara M, Williams P, Winzer K. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ Microbiol. 2006;8(6):1095–104. doi: 10.1111/j.1462-2920.2006.001001.x. [DOI] [PubMed] [Google Scholar]
- 66.Kadam RU, Bergmann M, Hurley M, Garg D, Cacciarini M, Swiderska MA, Nativi C, Sattler M, Smyth AR, Williams P, Camara M, Stocker A, Darbre T, Reymond JL. A Glycopeptide Dendrimer Inhibitor of the Galactose-Specific Lectin LecA and of Pseudomonas aeruginosa Biofilms. Angew Chem Int Ed Engl. 2011;50(45):10631–5. doi: 10.1002/anie.201104342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johansson E, Crusz S, Kolomiets E, Buts L, Kadam R, Cacciarini M, Bartels K, Diggle S, Cámara M, Williams P, Loris R, Nativi C, Rosenau F, Jaeger K, Darbre T, Reymond J. Inhibition and dispersion of Pseudomonas aeruginosa biofilms by glycopeptide dendrimers targeting the fucose-specific lectin LecB. Chem Biol. 2008;15(12):1249–57. doi: 10.1016/j.chembiol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Hauber H, Schulz M, Pforte A, Mack D, Zabel P, Schumacher U. Inhalation with fucose and galactose for treatment of Pseudomonas aeruginosa in cystic fibrosis patients. Int J Med Sci. 2008;5(6):371–6. doi: 10.7150/ijms.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kolomiets ESM, Kadam RU, Johansson EMV, Jaeger KE, Darbre T, Reymond JL. Glycopeptide Dendrimers with High Affinity for the Fucose-Binding Lectin LecB from Pseudomonas aeruginosa. ChemMedChem. 2009;4:562–9. doi: 10.1002/cmdc.200800380. [DOI] [PubMed] [Google Scholar]
- 70.Mateos F, Brock JH, Pérez-Arellano JL. Iron metabolism in the lower respiratory tract. Thorax. 1998;53(7):594–600. doi: 10.1136/thx.53.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reid DW, Carroll V, O’May C, Champion A, Kirov SM. Increased airway iron as a potential factor in the persistence of Pseudomonas aeruginosa infection in cystic fibrosis. European Respiratory Journal. 2007;30(2):286–92. doi: 10.1183/09031936.00154006. [DOI] [PubMed] [Google Scholar]
- 72.Martin LW, Reid DW, Sharples KJ, Lamont IL. Pseudomonas siderophores in the sputum of patients with cystic fibrosis. BioMetals. 2011;24(6):1059–67. doi: 10.1007/s10534-011-9464-z. [DOI] [PubMed] [Google Scholar]
- 73.Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64(2):518–23. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417(6888):552–5. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- 75.Xiao R, Kisaalita WS. Iron acquisition from transferrin and lactoferrin by Pseudomonas aeruginosa pyoverdin. Microbiology. 1997;143(7):2509–15. doi: 10.1099/00221287-143-7-2509. [DOI] [PubMed] [Google Scholar]
- 76.Britigan BE, Hayek MB, Doebbeling BN, Fick RB. Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infection and Immunity. 1993;61(12):5049–55. doi: 10.1128/iai.61.12.5049-5055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lamont I, Konings A, Reid D. Iron acquisition by Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. BioMetals. 2009;22(1):53–60. doi: 10.1007/s10534-008-9197-9. [DOI] [PubMed] [Google Scholar]
- 78.Moreau-Marquis S, O’Toole GA, Stanton BA. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. American journal of respiratory cell and molecular biology. 2009;41(3):305–13. doi: 10.1165/rcmb.2008-0299OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Piddock L. Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol. 2006;4(8):629–36. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L, Mah T. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol. 2008;190(13):4447–52. doi: 10.1128/JB.01655-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hocquet D, Roussel-Delvallez M, Cavallo J-D, Plesiat P. MexAB-OprM- and MexXY-Overproducing Mutants Are Very Prevalent among Clinical Strains of Pseudomonas aeruginosa with Reduced Susceptibility to Ticarcillin. Antimicrob Agents Chemother. 2007;51(4):1582–3. doi: 10.1128/AAC.01334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanchez P, Linares JF, Ruiz-Diez B, Campanario E, Navas A, Baquero F, Martinez JL. Fitness of in vitro selected Pseudomonas aeruginosanalB and nfxB multidrug resistant mutants. J Antimicrob Chemother. 2002;50(5):657–64. doi: 10.1093/jac/dkf185. [DOI] [PubMed] [Google Scholar]
- 83.Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, Blais J, Cho D, Chamberland S, Renau T, Leger R, Hecker S, Watkins W, Hoshino K, Ishida H, Lee VJ. Identification and Characterization of Inhibitors of Multidrug Resistance Efflux Pumps in Pseudomonas aeruginosa: Novel Agents for Combination Therapy. Antimicrob Agents Chemother. 2001;45(1):105–16. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piddock LJV. Clinically Relevant Chromosomally Encoded Multidrug Resistance Efflux Pumps in Bacteria. Clinical Microbiology Reviews. 2006;19(2):382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Renau TE, Léger R, Yen R, She MW, Flamme EM, Sangalang J, Gannon CL, Chamberland S, Lomovskaya O, Lee VJ. Peptidomimetics of Efflux Pump Inhibitors Potentiate the Activity of Levofloxacin in Pseudomonas aeruginosa. Bioorganic & Medicinal Chemistry Letters. 2002;12(5):763–6. doi: 10.1016/s0960-894x(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 86.Tan X-X, Actor JK, Chen Y. Peptide Nucleic Acid Antisense Oligomer as a Therapeutic Strategy against Bacterial Infection: Proof of Principle Using Mouse Intraperitoneal Infection. Antimicrob Agents Chemother. 2005;49(8):3203–7. doi: 10.1128/AAC.49.8.3203-3207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wesolowski D, Tae HS, Gandotra N, Llopis P, Shen N, Altman S. Basic peptide-morpholino oligomer conjugate that is very effective in killing bacteria by gene-specific and nonspecific modes. Proc Natl Acad Sci U S A. 2011;108(40):16582–7. doi: 10.1073/pnas.1112561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sulakvelidze A, Alavidze Z, Morris JG., Jr Bacteriophage Therapy. Antimicrob Agents Chemother. 2001;45(3):649–59. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donlan RM. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends in Microbiology. 2009;17(2):66–72. doi: 10.1016/j.tim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 90.Hagens S, Habel A, von Ahsen U, von Gabain A, Blasi U. Therapy of Experimental Pseudomonas Infections with a Nonreplicating Genetically Modified Phage. Antimicrob Agents Chemother. 2004;48(10):3817–22. doi: 10.1128/AAC.48.10.3817-3822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kutateladze M, Adamia R. Phage therapy experience at the Eliava Institute. Med Mal Infect. 2008;38(8):426–30. doi: 10.1016/j.medmal.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 92.Wright A, Hawkins C, Änggard E, Harper D. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa. Current Opnion in Otolaryngology. 2009;34(4):349–57. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 93.Wolcott R. [Accessed 30.11.11];A Prospective, Randomized, Double-Blind Controlled Study of WPP-201 for the Safety and Efficacy of Treatment of Venous Leg Ulcers ( NCT00663091) ClinicalTrialsgov
- 94.Skurnik M, Pajunen M, Kiljunen S. Biotechnological challenges of phage therapy. Biotechnology Letters. 2007;29(7):995–1003. doi: 10.1007/s10529-007-9346-1. [DOI] [PubMed] [Google Scholar]
- 95.Fancello L, Desnues C, Raoult D, Rolain JM. Bacteriophages and diffusion of genes encoding antimicrobial resistance in cystic fibrosis sputum microbiota. Journal of Antimicrobial Chemotherapy. 2011;66(11):2448–54. doi: 10.1093/jac/dkr315. [DOI] [PubMed] [Google Scholar]
- 96.Loeffler JM, Nelson D, Fischetti VA. Rapid Killing of Streptococcus pneumoniae with a Bacteriophage Cell Wall Hydrolase. Science. 2001;294(5549):2170–2. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- 97.Borysowski J, Weber-Dabrowska B, Gorski A. Bacteriophage Endolysins as a Novel Class of Antibacterial Agents. Experimental Biology and Medicine. 2006;231(4):366–77. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- 98.Briers Y, Walmagh M, Lavigne R. Use of bacteriophage endolysin EL188 and outer membrane permeabilizers against Pseudomonas aeruginosa. Journal of applied microbiology. 2011;110(3):778–85. doi: 10.1111/j.1365-2672.2010.04931.x. [DOI] [PubMed] [Google Scholar]
- 99.Johansen HK, Gotzsche PC. Vaccines for preventing infection with Pseudomonas aeruginosa in cystic fibrosis. Cochrane Database Syst Rev. 2008;(4):CD001399. doi: 10.1002/14651858.CD001399.pub2. [DOI] [PubMed] [Google Scholar]
- 100.Nilsson E, Larsson A, Olesen HV, Wejaker PE, Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatr Pulmonol. 2008;43(9):892–9. doi: 10.1002/ppul.20875. [DOI] [PubMed] [Google Scholar]
- 101.Kollberg HNE, Jobannesson M, Wejker PE, Larsson A. Anti-Pseudomonas IgY is now licensed for Prophylaxis and Treatment of CF Patients in Sweden. Journal of Cystic Fibrosis. 2005;4(Supplement 1):S28–S. [Google Scholar]
- 102.Lu Q, Rouby J-J, Laterre P-F, Eggimann P, Dugard A, Giamarellos-Bourboulis EJ, Mercier E, Garbino J, Luyt C-E, Chastre J, Georgescu-Kyburz V, Rudolf MP, Gafner V, Lazar H, Koch H, Perez A, Krämer SD, Tamm M. Pharmacokinetics and safety of panobacumab: specific adjunctive immunotherapy in critical patients with nosocomial Pseudomonas aeruginosa O11 pneumonia. Journal of Antimicrobial Chemotherapy. 2011;66(5):1110–6. doi: 10.1093/jac/dkr046. [DOI] [PubMed] [Google Scholar]
- 103.Baer M, Sawa T, Flynn P, Luehrsen K, Martinez D, Wiener-Kronish JP, Yarranton G, Bebbington C. An engineered human antibody fab fragment specific for Pseudomonas aeruginosa PcrV antigen has potent antibacterial activity. Infection and immunity. 2009;77(3):1083–90. doi: 10.1128/IAI.00815-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moskwa P, Lorentzen D, Excoffon KJDA, Zabner J, McCray PB, Jr., Nauseef WM, Dupuy C, Banfi B. A Novel Host Defense System of Airways Is Defective in Cystic Fibrosis. Am J Respir Crit Care Med. 2007;175(2):174–83. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Georgi E, Le Guellec S, Vecellio L, Fichant E, Stordeur P, Bordeau P, Perraudin J. Feasibility study of OSCN- and lactoferrin (Meveol) nebulization for cystic fibrosis patients. Journal of Cystic Fibrosis. 2011;10(Suppl 1.):S18. [Google Scholar]
- 106.Mahamoud A, Chevalier J, Alibert-Franco S, Kern WV, Pages J-M. Antibiotic efflux pumps in Gram-negative bacteria: the inhibitor response strategy. J Antimicrob Chemother. 2007;59(6):1223–9. doi: 10.1093/jac/dkl493. [DOI] [PubMed] [Google Scholar]
- 107.Srinivas N, Jetter P, Ueberbacher BJ, Werneburg M, Zerbe K, Steinmann J, Van der Meijden B, Bernardini F, Lederer A, Dias RLA, Misson PE, Henze H, Zumbrunn J, Gombert FO, Obrecht D, Hunziker P, Schauer S, Ziegler U, Käch A, Eberl L, Riedel K, DeMarco SJ, Robinson JA. Peptidomimetic Antibiotics Target Outer-Membrane Biogenesis in Pseudomonas aeruginosa. Science. 2010;327(5968):1010–3. doi: 10.1126/science.1182749. [DOI] [PubMed] [Google Scholar]