Abstract
Resistant starch (RS) reaches the large intestine largely intact, where it is fermented by the gut microbiota, resulting in the production of short-chain fatty acids (SCFAs) that have beneficial effects on the human body. Bifidobacteria are a major species widely used in the probiotic field, and are increased in the gut by RS, indicating their importance in RS metabolism in the intestine. Bifidobacteria have a genetic advantage in starch metabolism as they possess a significant number of starch-degrading enzymes and extraordinary three RS-degrading enzymes, allowing them to utilize RS. However, to date, only three species of RS-degrading bifidobacteria have been reported as single isolates B. adolescentis, B. choerinum, and B. pseudolongum. In this review, we describe recent studies on RS utilization by Bifidobacterium, based on their biochemical characteristics and genetic findings. This review provides a crucial understanding of how bifidobacteria survive in specific niches with abundant RS such as the human gut.
Keywords: Resistant starch, Human gut bacteria, Bifidobacteria, Amylases, Short-chain fatty acids
Introduction
Non-digestible carbohydrates (NDCs) are one of the main food sources in the human diet and generally provide the principal energy source for the microbiota that inhabits the human large intestine. NDCs include plant cell wall polysaccharides composed of the resistant starches (RS), cellulose, xylan, arabinoxylan, and pectin. NDCs are resistant to human digestive enzymes upon ingestion, and are therefore incompletely digested in the gastrointestinal tract (GIT) and reach the large intestine largely intact (Fu et al., 2019). NDCs in the gut can be fermented by commensal gut microbiota, resulting in the production of short-chain fatty acids (SCFAs) (Ashaolu et al., 2021), which are the key signaling molecules between the host and the gut microbiota (Raigond et al., 2015; Silva et al., 2020). In addition, SCFAs exert several physiological health benefits including colonocyte and gut health, inflammation regulation, mineral absorption, weight loss, glycemic response, and satiety (Alexander et al., 2019).
RS is a major fermentable carbohydrate source with maximal contribution in SCFA production by gut microbiota (Den Besten et al., 2013). Although some starch-utilizing gut bacteria, including members of Bacteroides and various Bacillota (Firmicutes), have been reported (Cerqueira et al., 2020), our understanding of RS utilization by human gut bacteria remains limited. It is suggested that RS metabolism in the human gut occurs by the concerted action of primary degraders that destroy insoluble starch granules resistant to digestive enzymes and secondary scavengers that consume enzyme-accessible products (destroy granules and their hydrolyzed products). Ruminococcus bromii and Bifidobacterium adolescentis are widely regarded as primary RS degraders in the human gut, and several secondary scavengers belonging to Bacteroidota (Bacteroidetes) and Bacillota (e.g., Bacteroides thetaiotaomicron and Eubacterium rectale) have been studied (Dobranowski and Stintzi, 2021).
Although only two species have been reported as the primary RS degraders in the human gut, several other species are likely to exist. Therefore, active research is underway to identify new potential microbiota involved in direct RS structural degradation. Studies on RS metabolism in primary RS degraders could support this possibility. In this review, we describe recent studies on RS degradation by Bifidobacterium, a primary RS degrader, including their biochemical and genetic analysis.
Resistant starch
Starch is one of the most produced food sources in the world, as it is the most important dietary component and a common source of carbohydrates consumed in the human diet. As a staple food, most starch is ingested in a cooked and gelatinized form, easily degraded into glucose by human digestive enzymes, and finally absorbed as an energy source for the human body (Salyers et al., 1983). Starch comprises amylose and amylopectin and is stored in an insoluble, rigid, and tightly packed manner owing to its cracks, pores, and crystalline structure (Imberty et al., 1991). Starch exists in most plants as granules of various sizes (Ellis et al., 1998; Singh et al., 2003), and typical raw starch granules are resistant to human digestive enzymes. Raw or uncooked starch is rarely degraded during passage through the stomach, duodenum, and small intestine and reaches the large intestine in an intact form. This type of nondigestible starch is called resistant starch (RS), and is defined as starch that cannot be attacked and degraded by mammalian α-glucosidic hydrolyzing enzymes in the small intestine but reaches the large intestine where it is fermented by the gut microbiota (Englyst and Cummings, 1985). Englyst et al. (1992) proposed a starch classification based on its digestive rate. Based on the results of in vitro digestion, starches can be divided into three types: rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS). In addition, RS is currently classified into five types (Table 1) based on its resistance to physical inaccessibility (RS1), native granular structure (RS2), gelatinization and retrogradation (heating and cooling) (RS3), chemical modification (RS4), and amylose–lipid complex (RS5).
Table 1.
Types of resistant starch (RS)
| RS type | Description | Example | References |
|---|---|---|---|
| RS1 | Physically inaccessible starch | Coarsely ground or whole-kernel grains | Englyst et al. (1992) |
| RS2 | Granular starch with the B- or C-polymorph | High amylose corn starch, raw potato, raw banana starch | Englyst et al. (1992) |
| RS3 | Retrograded starch | Cooked and cooled starch foods | Woo and Seib (2002) |
| RS4 | Chemically modified starches | Cross-linked starch and octenyl succinate starch | Han and BeMiller (2007) |
| RS5 | Amylose–lipid complex | Stearic acid-complexed high-amylose starch | Seneviratne and Biliaderis (1991) |
The intake of RS has recently been reported to promote the abundance and diversity of gut microbiota, which has positive effects on human health, particularly gut health (DeMartino and Cockburn, 2020). Numerous studies have shown that fermentation of RS by the gut microbiota can confer health benefits, including reduction of insulin resistance (Robertson et al., 2005), infectious diarrhea (Niderman-Meyer et al., 2010; Ramakrishna et al., 2000), colorectal cancer (Le Leu et al., 2009; Young et al., 2005), among others (Walker et al., 2011; Wu et al., 2011).
Human gut bacteria and short chain fatty acids (SCFAs)
Recent studies have reported that the correlation between the gut microbiota and the host affects intra-intestinal diseases (inflammatory bowel disease, irritable bowel syndrome, coeliac diseases, colorectal cancer, and Clostridioides difficile infection), and extra-intestinal diseases (obesity, type II diabetes, metabolic syndrome, allergy, and asthma) (Valdés et al., 2015). Gut microbiota produce secondary metabolites using carbohydrates in the large intestine as a carbon source. One of the primary advantages of the gut microbiota is that they contain various carbohydrate-active enzymes that are absent in the human genome. The 1013–14 microorganisms present in the human gut contribute a significant portion of their genomes for carbohydrate degradation and uptake, indicating the importance of carbohydrate utilization by gut microbiota (Wardman et al., 2022). Among the various types of carbohydrates consumed by humans, NDCs reach the large intestine and are assimilated by gut bacteria to produce metabolites, especially SCFAs, that link host nutrition to intestinal homeostasis maintenance. SCFAs are the end products of NDCs fermentation by anaerobic gut microbiota and are important fuels for intestinal epithelial cells (IEC). SCFAs regulate IEC function through various mechanisms that modulate their proliferation, differentiation, and subpopulations, such as enteroendocrine cells, leading to improved intestinal motility, intestinal barrier functions, and host metabolism (Martin-Gallausiaux et al., 2021). In addition, the accumulation of SCFAs in the gut lowers pH and reduces harmful metabolites, providing growth advantages to other commensal gut bacteria (Walker et al., 2011) and positively altering the abundance and diversity of the gut microbiota (Phillips et al., 1995; Walker et al., 2005). The abundance of SCFAs can be enhanced through metabolic cross-feeding of the gut microbiota (Flint et al., 2007).
SCFAs are saturated aliphatic organic acids composed of two to six carbons. The gut microbiota mainly produce acetate, propionate, and butyrate among SCFAs (Martin-Gallausiaux et al., 2021). Recent studies have indicated that SCFAs, especially butyrate, have important gut and immunomodulatory functions (Martin-Gallausiaux et al., 2021). Butyrate has been reported to improve colonic health, prevent colon cancer, provide energy sources to IEC, and inhibit the proliferation of malignant cells (Gonçalves and Martel, 2013). The production of SCFAs, especially butyrate, depends on carbohydrate fermentation by the gut microbiota, which is positively affected by the intake of significant levels of RS (Champ et al., 2003). Among the dietary fibers in the human diet, only RS fermentation in the human gut produces high amount of butyrate (Raigond et al., 2015). In bacteria, acetate can be produced directly from acetyl-CoA or via the Wood-Ljungdahl pathway using formate (Den Besten et al., 2013) (Fig. 1). Propionate is formed from phosphoenolpyruvate via the succinate decarboxylation or acrylate pathway. Butyrate is produced by the enzyme butyrate kinase from two molecules of acetyl CoA or by the enzyme butyryl CoA:acetate-CoA-transferase with the utilization of exogenously derived acetate. Pyruvate, one of the main SCFAs, is typically synthesized from glucose through glycolysis, indicating the importance of RS as a glucose source in reaching the large intestine.
Fig. 1.
Schematic overview of general short chain fatty acids (SCFAs) production in bacteria. In the bacterial glycolysis pathway, carbohydrate sources are converted to pyruvate, which is the main precursor for the biosynthesis of SCFAs such as acetate, propionate, and butyrate
RS degradation by the human gut bacteria
Ze et al. (2012) investigated the starch degradation and utilization abilities of four dominant amylolytic bacteria in the human gut, Bacteroides thetaiotaomicron, Eubacterium rectale, R. bromii, and B. adolescentis. To date, the starch-degrading enzyme systems from human gut symbionts have been reported in these strains (Fig. 2). Starch degradation followed a similar strategy in these strains, which specialized in the cell enveloped-associated multi-protein system. The systems are composed of the following proteins: cell enveloped-multi-protein includes starch binding domain-like modules (SBDs), protein(s) anchored to the cell surface that capture starch molecules using SBDs, and other enzyme(s) on the cell surface that degrade the starch molecules.
Fig. 2.
Simple model of starch utilization system in primary amylolytic bacteria in the human gut. Starch utilization systems in a Bacteroides thetaiotaomicron, b Eubacterium rectale, and RS degradation system in c Ruminococcus bromii. Starch degradation is initiated by capturing starch molecules by cell-envelope protein(s)/enzyme(s) having carbohydrate binding domains (CBMs). Other enzymes(s) on the cell surface act on the small saccharides generated from starch molecules. Next, small saccharides are transported into the cytoplasm by appropriate transporters (i.e. ABC)
Bac. thetaiotaomicron (Fig. 2a) consists of a starch utilization system (Sus) composed of several cell surface proteins related to starch degradation (Martens et al., 2009). Glycan moves through the surface capsular polysaccharide layer and is captured by the outer membrane protein SusD, which directly binds to starch. Glycans bound to the cell surface are broken down into small oligosaccharides by the outer membrane enzyme SusG and transported into the cytoplasm through SusC. Small oligosaccharides are degraded into mono- or disaccharides by periplasmic enzymes SusA and SusB. Hydrolyzed saccharides induce signals for transcriptional regulators that activate polysaccharide utilization genes and are transported to the cytoplasm.
In E. rectale (Fig. 2b), two glycosyl hydrolase (GH) family 13 enzymes and three ABC transporter solute-binding proteins have been reported to be involved in starch degradation (Cockburn et al., 2015). These cell surface enzymes and proteins interact to capture and break down starch and transport hydrolyzed products to the cytoplasm. EUR_21100, a cell surface enzyme on the peptidoglycan layer, has carbohydrate-binding modules (CBM26 and CBM41) located at the N-terminus that capture and degrade starch to produce maltooligosaccharides. The maltooligosaccharides are degraded by the membrane enzyme EUR_01860 to produce glucose and maltose, and these molecules are transported into the cell through the solute-binding proteins EUR_01240, EUR_01830, and EUR_31480 for metabolic processes.
Although Bac. thetaiotaomicron and E. rectale can degrade soluble starch, they do not have significant ability to degrade granular starches or RS pretreated by boiling (Ze et al., 2012). Ze et al. (2012) first described the degradation of RS in the human gut in the presence of two RS degraders, R. bromii and B. adolescentis. Follow-up studies reported the RS degradation system of human gut-originated R. bromii L2-63 through genomic analysis and protein–protein interaction studies of cohesin and dockerin (Fig. 2c) (Mukhopadhya et al., 2018; Ze et al., 2015). When R. bromii L2-63 was cultured using starch as a carbon source, eight major extracellular amylases (designated as Amy 1, 2, 4, 5, 9, 10, 12, and 16) belonging to GH family 13 with signal peptides (SP) were identified through genomic analysis. Among them, Amy 4, 9, 10, 12, and 16 contained dockerin, and the cohesin module (Sca 1; scaffolding protein 1) was also present in Amy 4. In addition, other individual proteins, but no enzyme, four cohesin modules (Sca 2, 3, 4, and 5), and seven dockerin were present in R. bromii L2-63. The binding action of five cohesins and 12 dockerins was investigated using a microarray approach, and the dockerins of Amy 4 and Amy 9 were predicted to be anchored on the cell surface by binding to the membrane protein Sca2. Moreover, cell-free complexes between Amy (4, 9, 10, 12, and 16) and Sca 3 or 4 may have been formed. Amy 4, 9, 10, and 12 could be integrated into Sca5 and attached to the cell surface. These enzyme complexes, called amylosomes, can effectively attach to RS through carbohydrate-binding modules (CBMs) and degrade RS. This cohesin-dockerin interaction is often found in cellulose-degrading Ruminococcus and several types of cellulolytic bacteria (Fontes and Gilbert, 2010). Like other multi-enzyme complexes for glycan degradation, such as cellulosomes, a model of cell-enveloped multi-protein systems for starch degradation highlights the activities of multiple genes that can elaborate on their individual and isolated functions. However, cohesin-dockerin interaction in starch-degrading bacteria was first reported in R. bromi.
When intact RS reaches the large intestine, it encounters several gut microorganisms containing RS-degrading enzymes. Because of the complex structure of RS, primary-degrading bacteria such as R. bromii and B. adolescentis first degrade the semi-crystalline RS structure, which partially degrade RS making it accessible for enzymatic degradation, followed by the other starch-degrading bacteria, including Bac. thetaiotaomicron and E. rectale, To date, strains belonging to only two species (R. bromii and B. adolescentis) have been reported as the primary RS degraders in the human gut. In particular, R. bromii has attracted attention as a keystone species in RS metabolism due to its significant abundance in the human gut. In addition to R. bromii, B. adolescentis has only recently been studied as a primary RS degrader, despite its importance as a powerful probiotic belonging to the Bifidobacterium genus that provides positive health benefits to the host. Previous studies have reported that RS ingestion is closely related to an increased number of bifidobacteria in the gut (Brown et al., 1997; Centanni et al., 2018; Silvi et al., 1999; Sybille et al., 2013). However, the genetic basis for its adaptation to the human gut, including the mechanisms of RS metabolism, has only recently been studied.
Genetic advantage in starch utilization by Bifidobacterium
Since Tissier first isolated Bacillus bifidus communis (renamed Lactobacillus bifidus, next Bifidobacterium genus) from the feces of breastfeeding infants in 1899, many bifidobacterial strains have been isolated from various ecological niches, such as sewage, insect gut, oral cavity, water kefir, and many mammals (Klijn et al., 2005; Laureys et al., 2016; Ventura et al., 2007). Advances in genetic research tools led to a detailed analysis of the bifidobacteria genome in 2008 (Sela et al., 2008), and 3,199 whole-genome assemblies from 103 bifidobacterial species have been reported to date.
Milani et al. (2014, 2015). sequenced the genomes from the type strains of each of the 47 representative (sub)species across the Bifidobacterium genus and assessed the overall genetic characteristics of the representative bifidobacterial group using genomic information, such as the corresponding pan-genome, core genome, and variome In total, Bifidobacterium-specific Clusters of Orthologous Genes (BifCOGs) represented 18,181 pan-genomic (pan BifCOGs) and 551 bifidobacterial core genomic (core BifCOGs) coding sequences. In particular, the carbohydrate metabolism family is the most abundantly representative functional class of pan-BifCOGs. More than 15.3% of the coding sequences are involved in carbohydrate metabolism and transport. However, only 5.5% of the core BifCOGs are involved in carbohydrate degradation and assimilation, outnumbering those found in many other gut microbiota. The average abundance of the carbohydrate metabolism functional family summarized in the metagenomic datasets of the Human Microbiome Project (HMP) was 8.0%, which is 5.7% lower than that of pan BifCOGs (13.7%). They suggested that a strong selective pressure for the acquisition and retention of accessory (novel) genes for carbohydrate metabolism in bifidobacteria exists in the particular ecological niche with abundant carbohydrates in which they reside and survive.
Duranti et al. (2014) confirmed that starch-like carbohydrates supported the growth of B. adolescentis 22 L, and suggested a nutrient absorption strategy targeting dietary and plant-derived glycans, in particular, starch and starch hydrolysates, through the analysis of the genome of B. adolescentis 22 L. They suggested that at least nine starch-hydrolyzing enzymes including α-amylase, amylopullulanase, glycogen/starch phosphorylase, phosphoglucomutase, and 4-α-glucanotransferase participate in starch metabolism. In addition, a gene cluster predicted to function as a type IVa pilus, which has not been detected previously in bifidobacteria, has been found in the genome of B. adolescentis 22 L. Transcriptomic analysis revealed that this gene cluster is upregulated when B. adolescentis 22 L is cultured on starch compared to when it is cultured on glucose. Type IVa pili are essential and conserved host colonization factors that are involved in various cellular processes, such as motility, adherence, conjugation, and DNA uptake, suggesting that the starch substrate in the gut somehow provides B. adolescentis 22 L with an advantage in gut colonization and host-microbe interactions.
Liu et al. (2015) reported that bifidobacteria have a genetic advantage in starch degradation. They compared the GH profile of a pooled-bifidobacterial genome (PBG) with that of a representative human gut microbiome (RM) to determine the potential substrate preference of bifidobacteria. The results indicated that the genome of bifidobacteria encoded more GH genes or gene products that degrade starch than those of other gut bacteria, suggesting a genetic advantage of this bacterial species for the utilization of starch and starch hydrolysates (e.g., maltooligosaccharides). In the PBG, the highest percentages of GHs were predicted to target plant cell wall polysaccharides (43%), but only 15% of GHs contained SP, suggesting that bifidobacteria have a relative weakness in the utilization of complex carbohydrates such as plant cell wall polysaccharides. However, the number of GHs required for degrading starch and starch hydrolysates was higher than that of RM (27% vs. 11%). They analyzed B. longum subsp. longum BBMN68 isolated from the feces of centenarians as a model strain. BBMN68 contained GH genes encoding α-amylase (BBMN68_650, BBMN68_1257), α-glucosidase (BBMN68_1428 and BBMN68_1261), and α-1, 6-glucosidase (BBMN68_1600 and BBMN68_1430) responsible for starch degradation, as well as other related GH genes encoding sucrose phosphorylase (BBMN68_1267), 4-α-glucanotransferase (BBMN68_1259 and BBMN68_1607), and pullulanase-like glycosidases (BBMN68_732, BBMN68_749, BBMN68_1610, and BBMN68_1127). The transcription of these genes in the predicted starch pathway revealed that α-glucosidase (BBMN68_1261), α-1, 6-glucosidase (BBMN68_1600 and BBMN68_1430), α-amylase (BBMN68_650), and two components of ABC-type sugar transporters (BBMN68_1403 and BBMN68_1670) were upregulated.
However, genetic strategies involved in RS utilization by Bifidobacterium at the protein level have not been sufficiently explored, even though this commensal gut bacterium favors the utilization of this nutritional carbohydrate polymer. Therefore, the molecular mechanisms underlying RS degradation by Bifidobacterium can be understood by studying the enzymes involved in RS degradation by Bifidobacterium.
RS-degrading Bifidobacterium species as a single isolate
Bifidobacterium is a primary RS degrader that generates enzyme-accessible substrates by destroying the structure of RS granules and is an important species for the nutrition of other gut bacteria. Therefore, the isolation and culture of primary degrading strains could potentially facilitate the development of related probiotics to achieve a balanced microbiota in the gut. However, only a few studies have described a single isolate of Bifidobacterium that degrades non-gelatinized RS granules and only three Bifidobacterium species (B. adolecentis, B. choerinum, and B. pseudolongum) have been reported with confirmed RS utilization (Table 2). Therefore, research is at an initial stage to explain RS degradation by Bifidobacterium.
Table 2.
Reported single isolate of RS-degrading Bifidobacterium species
| Species | Strain | Origin | RS utilization | References |
|---|---|---|---|---|
| B. adolescentis | L2-32 | Infant feces in 1996 | 43.8%a | Ze et al. (2012) |
| P2P3 | Adult feces in 2017 | 63.3%a | Jung et al. (2019) | |
| DSM 20087 | Bovine rumen before 1990 | 9.8%a | Jung et al. (2019) | |
| DSM 24849 | Adult feces in 2011 | 47.3%a | Jung et al. (2019) | |
| B. choerinum | FMB-1 | Bovine rumen in 2017 | 58.3%a | Jung et al. (2018b) |
| B. pseudolongum | FMB-2 | Bovine rumen in 2017 | 47.1%a | Jung et al. (2018b) |
| St6, St10, St12 | Mouse ceca in 2018 | 77%b | Centanni et al. (2018) |
a Total carbon method and b total starch assay kit (Megazyme) were applied to measure RS utilization
The first reported single isolate of RS-degrading Bifidobacterium was B. adolescentis. Ze et al. (2012) first reported B. adolecentis L2-32 and R. bromii L2-63 as primary degraders of RS in the human gut (isolated from the fecal samples of healthy infants and children, respectively). They reported that B. aoldescentis L2-32 could utilize non-gelatinized granules of RS type 2 (high amylose corn starch; HACS) at 26.6% [Ingredion, Hi-Maize (HM) 240], 53.3% (HM 958), and 42.8% (Sigma, S4180), and type 3 (retrograded HACS) at 36.9% [Ingregion, Novelose (NL) 330]. B. adolescentis L2-32 can grow on starch hydrolysates, such as glucose, maltose, maltotriose, maltotetraose, pullan, and panose, but not on isomaltose and fructose.
Jung et al. (2018b) cultured the bovine rumen fluid from Korean cattle in vitro (Hanwoo; Bos taurus coreanae) in the presence of raw granules (RS type 2; S4180) and investigated the changes in the bacterial microbiome. Interestingly, they observed aggregation of starch granules and adhesion of bacterial cells during cultivation, with slow granule degradation in the culture over time. The initial major bacterial taxa in the rumen fluid were Succiniclasticum sp. (ca. 45%), and the proportion of Streptococcus sp. significantly increased (approximately 80%, probably due to the influence of the basal rumen medium) as the culture continued, followed by dominance by Clostridum sp., Prevotella sp., Dialister sp., and Lachnospiraceae family. However, Lactobacillus sp. drastically increased up to 90% in the presence of RS, and significant Bifidobacterium sp. was continuously observed in the remaining proportion. Further, they applied the rumen fluid-RS culture with Bifidobacterium spp. and Lactobacillus spp. to a solid medium containing raw starch and stained the grown colonies with iodine. They isolated two RS-degrading Bifidobacterium species (B. choerinum FMB-1 and B. pseudolongum FMB-2) with high RS-degrading activities of almost 60% and 50%, respectively. In addition, co-culture experiments showed that the human gut-originated Levilactobacillus brevis ATCC 14869T could grow using reducing sugars generated from RS granules by B. choerinum FMB-1.
Centanni et al. (2018) supplemented the diet of rats with RS (HM 1043) and analyzed the relative abundance of Bifidobacteriaceae, Lachnospiraceae, and Bacteroidaceae using the cecal microbiome. The initial proportions of microbiota were 5%, 20%, and 17%, respectively, but Bifidobacteriaceae increased to 30% after 48 h of RS ingestion and to 40% after 144 h. The bifidobacterial bloom in the rat cecum under these conditions mostly contained B. animalis and some B. pseudolongum. The two species were isolated from the cecum of RS-fed rats and further analyzed. B. animalis could not degrade RS to any extent, whereas B. pseudolongum had a high RS degrading activity. Moreover, B. animalis had shorter growth times than B. pseudolongum when maltose was the sole carbon source. Thus, they suggested that B. pseudolongum has the characteristics of a keystone species in the rat cecal microbiota owing to its high ability to degrade RS, although it was present in a relatively low abundance.
Jung et al. (2019) cultured human fecal microbiota with RS type 2 (S4180) and found that RS granules aggregated and then degraded slowly, similar to the bovine rumen microbiota. They hypothesized that cell adhesion to the granules was strongly related to granule aggregation and degradation, thus they isolated cells attached to the granules by using a series of enriched cultures and washing/separation of RS granules (Fig. 3). The conditions for gut bacteria isolation were as follows: medium, modified chopped meat containing 15% bovine rumen fluid (CMR); air, 85% N2, 10% CO2, and 5% H2 gas, and cultivation at 37 °C with 30 rotation/min. They were able to isolate and grow various obligate anaerobic gut bacteria, including Eubacterium, Bacteroides, Collinsella, and Clostridium from the supernatant. Interestingly, only B. adolescentis was isolated from the granule aggregates, and B. adolescentis strain P2P3 was able to utilize up to 64% of HACS granules without gelatinization, and other commercial RS type 2 (HM 260), type 3 (HM 958), and type 4 [Ingredion, Versafibe (VF) 1490 and 2470). However, B. choerinum FMB-1 could not utilize HM 260, NL 330, and VF 2470, suggesting that the utilization of RS-degrading Bifidobacterium differs according to RS type. In a co-culture, human gut-originated Bac. thetaiotaomicron ATCC 29148T was able to grow sustainably using enzyme-accessible sugars released from RS granules by B. adolescentis P2P3. In addition, B. adolescentis P2P3 stimulated the secretion of Th1 type cytokines from mouse macrophages in vitro, which was not observed in other B. adolescentis strains (DSM20083T, 20086, and 20087).
Fig. 3.
Isolation procedure of RS-degrading human gut bacteria. (1) The subculture is repeated several times to enhance the concentration of RS-degrading bacteria. (2) Subculture vials are stored until the RS granules settle. (3) The supernatant is mixed to sterilized RS granules and wash away the remaining cells attached to the RS granules. (4) The precipitated RS granules are washed with PBS buffer to obtain pure RS granule-attached cells through re-precipitation. (5) After culturing by spreading on a solid medium containing raw starch granules as the sole carbon source, colonies with a transparent periphery are selected
RS-degrading enzymes in Bifidobacterium
Centanni et al. (2018) performed transcriptional analysis of B. pseudolongum (isolated from RS-fed rats) in the presence of RS and confirmed increased expression levels of several enzymes including type I pullulanase, α-amylase, glycogen debranching enzyme, maltose-1-phosphate maltosyltransferase, and 4-α-glucanotransferase. They suggested that type I pullulanase (AH67_RS04855) could be an important gene for RS hydrolysis because strains of this species produce an extracellular pullulanase with hydrolysis products such as glucose, maltose, maltotriose, and pannose (Ryan et al., 2006). All of the sequenced B. pseudolongum had type I pullulanase, and this gene encodes a 1745 amino acid multi-domain protein including SP, catalytic, and carbohydrate-binding domains (CBM) 25, 41, and 48. In addition, they reported that this pullulanase gene is positioned downstream of three genes related to aromatic acid biosynthesis in B. pseudolongum, and a partial pullulanase gene is present at this location in B. animalis (in reported strains IM386, ATCC 27672, and MCC0499).
The pullulanase (PulP) gene has also been identified in B. adolescentis P2P3, which possesses strong RS-degrading activity. Kim et al. (2021) reported that PulP possesses an α-amylase domain at the N-terminus and a type I pullulanase domain at the C-terminus, with one CBM25 and two CBM41 between them. They investigated the specific properties of PulP and truncated mutants, in which each of the two catalytic domains and/or CBMs were eliminated. When only one catalytic domain was present, only the catalytic activity of the corresponding wild-type enzyme (α-amylase or type-I pullulanase) was found. In addition, the removal of CBMs resulted in a loss of enzyme activity and binding affinity to the substrate compared to the wild-type counterpart. However, intact PulP produced very small amounts of hydrolyzed products (maltooligosaccharides, mainly maltotriose) from the raw starch granules, and could not structurally degrade the raw starch granules. This result suggests that type I pullulanase, found in RS-degrading B. pseudolongum and B. adolescentis, may not be a key enzyme for RS degradation.
Jung et al. (2019) reported that RS utilization differed between strains, even in the same B. adolecentis species. They compared RS utilization of B. adolecentis strains (P2P3, DSM 20083T, 20086, 20087, 24849, and L2-32), and observed that the strains P2P3, DSM 20087, DSM 24849, and L2-32 could utilize RS granules (63.3%, 9.8%, 47.3%, and 43.8%, respectively), whereas DSM 20083T and DSM 20086 did not degrade RS. In addition, strains that degrade RS tended to form granular aggregates and adhered to them.
Crittenden et al. (2001) studied the adhesion of 19 bifidobacteria strains to native starch granules, and suggested that highly adherent strains were able to hydrolyze starch granules. However, not all hydrolytic strains had adhesion ability, indicating that starch adhesion is not essential for their degradation. In addition, they suggested that adhesion might be mediated by cell surface protein(s) specific for α-1,4-linked glucose sugars, because adhesion was inhibited by maltooligosaccharides, amylose, and soluble starch.
Combining these results, Jung et al. (2020b) applied a comparative genomic approach to investigate the key enzyme(s) for RS degradation in Bifidobacterium. First, they analyzed the genomes of two RS-degrading isolates, B. adolescentis P2P3 and B. choerinum FMB-1 (Jung et al., 2020a, 2018a) (Table 3). A total of 19 starch-active proteins expected to participate in starch metabolism were found in B. adolescentis and 14 were found in B. choerinum FMB-1. These enzymes included glycogen phosphorylase, 4-α-glucanotransferase, glycogen debranching enzyme, α-amylase, α-1,4-glucan branching enzyme, glycosidase, pullulanase, starch-binding protein, α-1,4-glucan-maltose-1-phosphate, maltosyltransferase, and α-glucosidase. A non-RS-degrading strain, B. adolescentis DSM20083T, also contained these enzymes, but four proteins/enzymes annotated as three starch-binding proteins (WP_124917489.1, WP_124917356.1, and WP_124917357.1) and one α-amylase (WP_124917409.1) were only present in the RS-degrading strain, B. adolescentis P2P3 and B. choerinum FMB-1. Moreover, they performed in silico and enzymatic analyses and reported that three starch-binding proteins containing an α-amylase catalytic domain belonging to the GH 13_28 subfamily, showed strong α-amylase activity to degrade the structure of RS granules, and reducing sugars (enzyme-accessible sugars) were released during degradation.
Table 3.
List of α-glucan active proteins detected in RS-degrading Bifidobacterium species
| No | Protein function | Protein_ID/Cover/Identity | ||
|---|---|---|---|---|
| B. adolescentis P2P3 | B. choerinum FMB-1 | B. adoelscentis DSM 20083 | ||
| 1 | Glycogen/starch/α-glucan phosphorylase | WP_117838111.1 | WP_024540347.1/99%/80% | WP_011742583.1/100%/98% |
| 2 | 4-α-Glucanotransferase | WP_124917072.1 | WP_024540981.1/99%/67% | WP_041777242.1/100%/99% |
| 3 | Glycogen debranching enzyme GlgX | WP_033499301.1 | – | BAF39092.1/82%/87% |
| 4 | α-Amylase | WP_124917091.1 | – | WP_050731445.1/100%/99% |
| 5 | 1,4-α-Glucan branching enzyme | WP_003809562.1 | WP_099721766.1/98%/81% | WP_011743089.1/100%/99% |
| 6 | Glycogen debranching enzyme GlgX | WP_011743102.1 | WP_024541280.1/97%/84% | WP_003809530.1/99%/100% |
| 7 | Glycosidase | WP_021913761.1 | - | BAF39488.1/65%/99% |
| 8 | Type I pullulanase | WP_033499429.1 | WP_099721362.1/89%/62% | WP_011743123.1/100%/80% |
| 9 | α-Amylase | WP_070122448.1 | – | WP_011743335.1/100%/99% |
| 10 | Starch-binding protein | WP_124917489.1 | WP_099720817.1/97%/49% | – |
| 11 | Starch-binding protein | WP_124917356.1 | WP_099720815.1/99%/50% | – |
| 12 | Starch-binding protein | WP_124917357.1 | WP_099720816.1/87%/52% | – |
| 13 | α-1,4-Glucan-maltose-1-phosphate maltosyltransferase | WP_124917371.1 | WP_099720854.1/92%/52% | WP_011743724.1/100%/98% |
| 14 | Pullulan hydrolase type III | WP_124917408.1 | WP_099720922.1/89%/60% | BAF40340.1/82%/84% |
| 15 | α-Amylase | WP_124917409.1 | WP_099720923.1/97%/63% | – |
| 16 | 4-α-Glucanotransferase | WP_039775972.1 | WP_099721692.1/99%/66% | WP_011743858.1/100%/99% |
| 17 | α-Amylase | WP_003811282.1 | WP_099720926.1/99%/67% | WP_003811282.1/100%/100% |
| 18 | α-Glucosidase | WP_046999836.1 | WP_099720933.1/100%/75% | WP_011743867.1/100%/97% |
| 19 | α-Glucosidase | WP_124917425.1 | – | WP_011743891.1/100%/99% |
The cover and identity represent values compared to B. adolescentis P2P3
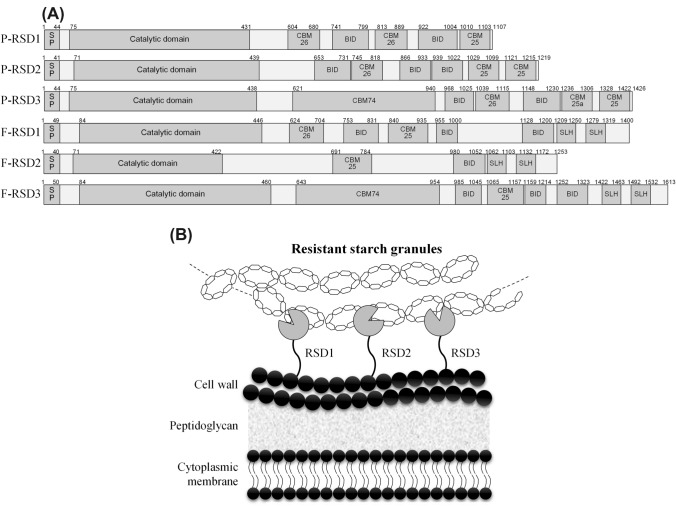
Among them, RS granule degradation was highest in RSD2. These three RS-degrading α-amylases (RSD1, 2, and 3) were similar in domain structure, including SP, α-amylase catalytic domain, multiple CBMs (25, 26, and 74), and other domains predicted as cell wall-anchoring domains [bacterial immunoglobulin-like domain (BID) and additional S-layer homology domain (SLH)] (Fig. 4a). All RSDs have CBM25 and/or CBM26, which contain important residues (His, Trp, Asn, Asp, Tyr, and Gln) at key positions for granular binding, and RSD 3 additionally has a recently reported CBM74 that occurs widely in bacteria and has a binding ability to starch granules. Therefore, they suggested that RSDs with an SP cell-wall anchoring domain may result in extracellular expression followed by cell wall anchoring and capture of RS granules using their specific CBMs (25, 26, and 74) (Fig. 4b). Thus, these mechanisms could explain the granular adhesion of RS-degrading bifidobacteria.
Fig. 4.
Three RS-degrading α-amylases (RSDs) found in bifidobacteria. a Multi-domain structures of RSDs in B. adolescentis P2P3 (P-RSD) and B. choerinum FMB-1 (R-RSD). SP; signal peptide, CBM; carbohydrate binding domain, and BID; bacterial Ig-like domain, SLH; S-layer homology domain. The numbers represent the start and end positions of the domain amino acids. b Proposed depiction of cell-anchored three RSDs. The three RSDs released out of the cells are anchored on the cell surface. The RS granules are captured by CBM, which is then hydrolyzed by the enzymatic catalytic region
Recently, Milani et al. (2015) reported that various Bifidobacterium species (B. actinocoloniiforme, B. asteroides, B. bohemicum, B. bombi, B. coryneforme, and B. indicum) isolated from bumblebees and honeybees have very limited groups of GH13 enzymes, but a large set of GH3 and GH45 enzymes are predicted to participate in the hydrolysis of plant-derived carbohydrates, such as cellodextrin, (abarino)galactan, and (arabino)xylan. Therefore, it can be inferred that bifidobacteria possess key enzymes for RS degradation to adapt to specific ecological niches, such as the human gut, which is abundant in RS. The three RS-degrading enzymes of bifidobacteria show only the activity of typical α-amylases but can destroy the structure of insoluble RS granules. In addition, partially hydrolyzed starch and maltooligosaccharides (enzyme-accessible substrates) can act as supplemental feeding substrates for other gut microbiota members.
Future aspect
As the primary RS degraders, bifidobacteria initiate RS metabolism by degrading the structure of RS granules via three specific degrading enzymes, allowing other gut bacteria and their enzymes to participate in starch degradation. In this review, three Bifidobacterium species (B. adolescentis, B. choerinum, and B. pseudolongum) that have been reported to utilize RS were discussed. However, Bifidobacterium species has been reported in various environments including the intestinal environment, and 117 species including 18 subspecies have been reported at the time of writing. The genetic and physiological analysis of Bifidobacterium, including studies of RS-degrading enzymes, will be of great help in the future discovery of other species that utilize RS, and in understanding the physiology and ecology according to their habitats and diets. These studies will further improve the utilization of Bifidobacteriaum as useful probiotics for effective utilization of RS in the gut. Moreover, these significant findings shed light on the metabolic fate of RS in the gut, a main source of SCFAs, which exerts numerous health benefits in humans. Although further studies are required to understand how different types of RS affect gut microbiota, the current studies on RS-utilizing gut microbiota could provide new avenues to bioengineer more potent probiotics and prebiotics related to RS for added benefits for gut health.
Acknowledgements
This work was supported by the Korean government, Ministry of Science and ICT [National Research Foundation of Korea (No. 2021R1A4A1023437)], and the Ministry of Environment [National Institute of Biological Resources (No. NIBR202203111)].
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dong-Hyun Jung, Email: dhjung529@gmail.com.
Cheon-Seok Park, Email: cspark@khu.ac.kr.
References
- Alexander C, Swanson KS, Fahey GC, Jr, Garleb KA. Perspective: physiologic importance of short-chain fatty acids from nondigestible carbohydrate fermentation. Advances in Nutrition. 2019;10:576–589. doi: 10.1093/advances/nmz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashaolu T, Ashaolu J, Adeyeye S. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: a critical review. Journal of Applied Microbiology. 2021;130:677–687. doi: 10.1111/jam.14843. [DOI] [PubMed] [Google Scholar]
- Brown I, Warhurst M, Arcot J, Playne M, Illman RJ, Topping DL. Fecal numbers of bifidobacteria are higher in pigs fed Bifidobacterium longum with a high amylose cornstarch than with a low amylose cornstarch. The Journal of Nutrition. 1997;127:1822–1827. doi: 10.1093/jn/127.9.1822. [DOI] [PubMed] [Google Scholar]
- Centanni M, Lawley B, Butts CA, Roy NC, Lee J, Kelly WJ, Tannock GW. Bifidobacterium pseudolongum in the ceca of rats fed Hi-Maize starch has characteristics of a keystone species in bifidobacterial blooms. Applied and Environmental Microbiology. 2018;84:e00547–e618. doi: 10.1128/AEM.00547-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira FM, Photenhauer AL, Pollet RM, Brown HA, Koropatkin NM. Starch digestion by gut bacteria: crowdsourcing for carbs. Trends in Microbiology. 2020;28:95–108. doi: 10.1016/j.tim.2019.09.004. [DOI] [PubMed] [Google Scholar]
- Champ M, Langkilde A-M, Brouns F, Kettlitz B, Collet YLB. Advances in dietary fibre characterisation 1 Definition of dietary fibre, physiological relevance, health benefits and analytical aspects. Nutrition Research Reviews. 2003;16:71–82. doi: 10.1079/NRR200254. [DOI] [PubMed] [Google Scholar]
- Cockburn DW, Orlovsky NI, Foley MH, Kwiatkowski KJ, Bahr CM, Maynard M, Demeler B, Koropatkin NM. Molecular details of a starch utilization pathway in the human gut symbiont Eubacterium rectale. Molecular Microbiology. 2015;95:209–230. doi: 10.1111/mmi.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden R, Laitila A, Forssell P, Mättö J, Saarela M, Mattila-Sandholm T, Myllärinen P. Adhesion of bifidobacteria to granular starch and its implications in probiotic technologies. Applied and Environmental Microbiology. 2001;67:3469–3475. doi: 10.1128/AEM.67.8.3469-3475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino P, Cockburn DW. Resistant starch: impact on the gut microbiome and health. Current Opinion in Biotechnology. 2020;61:66–71. doi: 10.1016/j.copbio.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Den Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobranowski PA, Stintzi A. Resistant starch, microbiome, and precision modulation. Gut Microbes. 2021;13:1926842. doi: 10.1080/19490976.2021.1926842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranti S, Turroni F, Lugli GA, Milani C, Viappiani A, Mangifesta M, Gioiosa L, Palanza P, van Sinderen D, Ventura M. Genomic characterization and transcriptional studies of the starch-utilizing strain Bifidobacterium adolescentis 22L. Applied and Environmental Microbiology. 2014;80:6080–6090. doi: 10.1128/AEM.01993-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RP, Cochrane MP, Dale MFB, Duffus CM, Lynn A, Morrison IM, Prentice RDM, Swanston JS, Tiller SA. Starch production and industrial use. Journal of the Science of Food and Agriculture. 1998;77:289–311. doi: 10.1002/(SICI)1097-0010(199807)77:3<289::AID-JSFA38>3.0.CO;2-D. [DOI] [Google Scholar]
- Englyst HN, Cummings JH. Digestion of the polysaccharides of some cereal foods in the human small intestine. The American Journal of Clinical Nutrition. 1985;42:778–787. doi: 10.1093/ajcn/42.5.778. [DOI] [PubMed] [Google Scholar]
- Englyst HN, Kingman S, Cummings J. Classification and measurement of nutritionally important starch fractions. European Journal of Clinical Nutrition. 1992;46:S33–50. [PubMed] [Google Scholar]
- Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environmental Microbiology. 2007;9:1101–1111. doi: 10.1111/j.1462-2920.2007.01281.x. [DOI] [PubMed] [Google Scholar]
- Fontes CM, Gilbert HJ. Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annual Review of Biochemistry. 2010;79:655–681. doi: 10.1146/annurev-biochem-091208-085603. [DOI] [PubMed] [Google Scholar]
- Fu X, Liu Z, Zhu C, Mou H, Kong Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Critical Reviews in Food Science and Nutrition. 2019;59:S130–S152. doi: 10.1080/10408398.2018.1542587. [DOI] [PubMed] [Google Scholar]
- Gonçalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Current Drug Metabolism. 2013;14:994–1008. doi: 10.2174/1389200211314090006. [DOI] [PubMed] [Google Scholar]
- Han J-A, BeMiller JN. Preparation and physical characteristics of slowly digesting modified food starches. Carbohydrate Polymers. 2007;67:366–374. doi: 10.1016/j.carbpol.2006.06.011. [DOI] [Google Scholar]
- Imberty A, Buléon A, Tran V, Péerez S. Recent advances in knowledge of starch structure. Starch-Stärke. 1991;43:375–384. doi: 10.1002/star.19910431002. [DOI] [Google Scholar]
- Jung D-H, Chung W-H, Seo D-H, Kim Y-J, Nam Y-D, Park C-S. Complete genome sequence of Bifidobacterium adolescentis P2P3, a human gut bacterium possessing strong resistant starch-degrading activity. 3 Biotech. 2020;10:1–9. doi: 10.1007/s13205-019-1978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D-H, Chung W-H, Seo D-H, Nam Y-D, Yoon S, Park C-S. Complete genome sequence of Bifidobacterium choerinum FMB-1, a resistant starch-degrading bacterium. Journal of Biotechnology. 2018;274:28–32. doi: 10.1016/j.jbiotec.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Jung D-H, Seo D-H, Kim G-Y, Nam Y-D, Song E-J, Yoon S, Park C-S. The effect of resistant starch (RS) on the bovine rumen microflora and isolation of RS-degrading bacteria. Applied Microbiology and Biotechnology. 2018;102:4927–4936. doi: 10.1007/s00253-018-8971-z. [DOI] [PubMed] [Google Scholar]
- Jung D-H, Seo D-H, Kim Y-J, Chung W-H, Nam Y-D, Park C-S. The presence of resistant starch-degrading amylases in Bifidobacterium adolescentis of the human gut. International Journal of Biological Macromolecules. 2020;161:389–397. doi: 10.1016/j.ijbiomac.2020.05.235. [DOI] [PubMed] [Google Scholar]
- Jung D-H, Kim G-Y, Kim I-Y, Seo D-H, Nam Y-D, Kang H, Song Y, Park C-S. Bifidobacterium adolescentis P2P3, a human gut bacterium having strong non-gelatinized resistant starch-degrading activity. Journal of Microbiology and Biotechnology. 2019;29:1904–1915. doi: 10.4014/jmb.1909.09010. [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Kim H, Kim Y-J, Jung D-H, Seo D-H, Jung J-H, Park C-S. Enzymatic analysis of truncation mutants of a type II pullulanase from Bifidobacterium adolescentis P2P3, a resistant starch-degrading gut bacterium. International Journal of Biological Macromolecules. 2021;193:1340–1349. doi: 10.1016/j.ijbiomac.2021.10.193. [DOI] [PubMed] [Google Scholar]
- Klijn A, Mercenier A, Arigoni F. Lessons from the genomes of bifidobacteria. FEMS Microbiology Reviews. 2005;29:491–509. doi: 10.1016/j.fmrre.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Laureys D, Cnockaert M, De Vuyst L, Vandamme P. Bifidobacterium aquikefiri sp. Nov., isolated from water kefir. International Journal of Systematic and Evolutionary Microbiology. 2016;66:1281–1286. doi: 10.1099/ijsem.0.000877. [DOI] [PubMed] [Google Scholar]
- Le Leu RK, Hu Y, Brown IL, Young GP. Effect of high amylose maize starches on colonic fermentation and apoptotic response to DNA-damage in the colon of rats. Nutrition & Metabolism. 2009;6:11. doi: 10.1186/1743-7075-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ren F, Zhao L, Jiang L, Hao Y, Jin J, Zhang M, Guo H, Lei X, Sun E. Starch and starch hydrolysates are favorable carbon sources for Bifidobacteria in the human gut. BMC Microbiology. 2015;15:54. doi: 10.1186/s12866-015-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Koropatkin NM, Smith TJ, Gordon JI. Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. Journal of Biological Chemistry. 2009;284:24673–24677. doi: 10.1074/jbc.R109.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proceedings of the Nutrition Society. 2021;80:37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- Milani C, Lugli GA, Duranti S, Turroni F, Bottacini F, Mangifesta M, Sanchez B, Viappiani A, Mancabelli L, Taminiau B. Genome encyclopaedia of type strains of the genus Bifidobacterium. Applied and Environmental Microbiology. 2014;80:6290–6302. doi: 10.1128/AEM.02308-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani C, Lugli GA, Duranti S, Turroni F, Mancabelli L, Ferrario C, Mangifesta M, Hevia A, Viappiani A, Scholz M. Bifidobacteria exhibit social behavior through carbohydrate resource sharing in the gut. Scientific Reports. 2015;5:15782. doi: 10.1038/srep15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhya I, Moraïs S, Laverde-Gomez J, Sheridan PO, Walker AW, Kelly W, Klieve AV, Ouwerkerk D, Duncan SH, Louis P. Sporulation capability and amylosome conservation among diverse human colonic and rumen isolates of the keystone starch-degrader Ruminococcus bromii. Environmental Microbiology. 2018;20:324–336. doi: 10.1111/1462-2920.14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niderman-Meyer O, Zeidman T, Shimoni E, Kashi Y. Mechanisms involved in governing adherence of Vibrio cholerae to granular starch. Applied and Environmental Microbiology. 2010;76:1034–1043. doi: 10.1128/AEM.01533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J, Muir JG, Birkett A, Lu ZX, Jones GP, O'Dea K, Young GP. Effect of resistant starch on fecal bulk and fermentation-dependent events in humans. The American Journal of Clinical Nutrition. 1995;62:121–130. doi: 10.1093/ajcn/62.1.121. [DOI] [PubMed] [Google Scholar]
- Raigond P, Ezekiel R, Raigond B. Resistant starch in food: a review. Journal of the Science of Food and Agriculture. 2015;95:1968–1978. doi: 10.1002/jsfa.6966. [DOI] [PubMed] [Google Scholar]
- Ramakrishna B, Venkataraman S, Srinivasan P, Dash P, Young GP, Binder HJ. Amylase-resistant starch plus oral rehydration solution for cholera. New England Journal of Medicine. 2000;342:308–313. doi: 10.1056/NEJM200002033420502. [DOI] [PubMed] [Google Scholar]
- Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism–. The American Journal of Clinical Nutrition. 2005;82:559–567. doi: 10.1093/ajcn/82.3.559. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Fitzgerald GF, van Sinderen D. Screening for and identification of starch-, amylopectin-, and pullulan-degrading activities in bifidobacterial strains. Applied and Environmental Microbiology. 2006;72:5289–5296. doi: 10.1128/AEM.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers AA, Leedle J. Carbohydrate metabolism in the human colon. 1st edn, pp 129-144. Human intestinal microflora in health and disease. Hentges D (ed). Elsevier Academic Press, NY, USA (1983).
- Sela D, Chapman J, Adeuya A, Kim J, Chen F, Whitehead T, Lapidus A, Rokhsar D, Lebrilla C, German J. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proceedings of the National Academy of Sciences. 105: 18964-18969 (2008) [DOI] [PMC free article] [PubMed]
- Seneviratne H, Biliaderis C. Action of α-amylases on amylose-lipid complex superstructures. Journal of Cereal Science. 1991;13:129–143. doi: 10.1016/S0733-5210(09)80030-1. [DOI] [Google Scholar]
- Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Frontiers in Endocrinology. 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvi S, Rumney C, Cresci A, Rowland I. Resistant starch modifies gut microflora and microbial metabolism in human flora-associated rats inoculated with faeces from Italian and UK donors. Journal of Applied Microbiology. 1999;86:521–530. doi: 10.1046/j.1365-2672.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Singh N, Singh J, Kaur L, Sodhi NS, Gill BS. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chemistry. 2003;81:219–231. doi: 10.1016/S0308-8146(02)00416-8. [DOI] [Google Scholar]
- Sybille T, June Z, Michael K, Roy M, Maria LM. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiology Ecology. 2013;83:299–309. doi: 10.1111/j.1574-6941.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- Valdés L, Cuervo A, Salazar N, Ruas-Madiedo P, Gueimonde M, González SJF. The relationship between phenolic compounds from diet and microbiota: impact on human health. Food & Function. 2015;6:2424–2439. doi: 10.1039/C5FO00322A. [DOI] [PubMed] [Google Scholar]
- Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, van Sinderen D. Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiology and Molecular Biology Reviews. 2007;71:495–548. doi: 10.1128/MMBR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AW, Duncan SH, Leitch ECM, Child MW, Flint HJ. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Applied and Environmental Microbiology. 2005;71:3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. The ISME Journal. 2011;5:220. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman JF, Bains RK, Rahfeld P, Withers SG. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nature Reviews Microbiology. 2022;20:542–556. doi: 10.1038/s41579-022-00712-1. [DOI] [PubMed] [Google Scholar]
- Woo K, Seib P. Cross-linked resistant starch: Preparation and properties. Cereal Chemistry. 2002;79:819–825. doi: 10.1094/CCHEM.2002.79.6.819. [DOI] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GP, Hu Y, Le Leu RK, Nyskohus L. Dietary fibre and colorectal cancer: a model for environment–gene interactions. Molecular Nutrition & Food Research. 2005;49:571–584. doi: 10.1002/mnfr.200500026. [DOI] [PubMed] [Google Scholar]
- Ze X, Ben David Y, Laverde-Gomez JA, Dassa B, Sheridan PO, Duncan SH, Louis P, Henrissat B, Juge N, Koropatkin NM. Unique organization of extracellular amylases into amylosomes in the resistant starch-utilizing human colonic Firmicutes bacterium Ruminococcus bromii. Mbio. 2015;6:e01058–e1115. doi: 10.1128/mBio.01058-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. The ISME Journal. 2012;6:1535–1543. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]