Abstract
The regulatory protein ToxT directly activates the transcription of virulence factors in Vibrio cholerae, including cholera toxin (CT) and the toxin-coregulated pilus (TCP). Specific environmental signals stimulate virulence factor expression by inducing the transcription of toxT. We demonstrate that transcriptional activation by the ToxT protein is also modulated by environmental signals. ToxT expressed from an inducible promoter activated high-level expression of CT and TCP in V. cholerae at 30°C, but expression of CT and TCP was significantly decreased or abolished by the addition of 0.4% bile to the medium and/or an increase of the temperature to 37°C. Also, expression of six ToxT-dependent TnphoA fusions was modulated by temperature and bile. Measurement of ToxT-dependent transcription of genes encoding CT and TCP by ctxAp- and tcpAp-luciferase fusions confirmed that negative regulation by 37°C or bile occurs at the transcriptional level in V. cholerae. Interestingly, ToxT-dependent transcription of these same promoters in Salmonella typhimurium was relatively insensitive to regulation by temperature or bile. These data are consistent with ToxT transcriptional activity being modulated by environmental signals in V. cholerae and demonstrate an additional level of complexity governing the expression of virulence factors in this pathogen. We propose that negative regulation of ToxT-dependent transcription by environmental signals prevents the incorrect temporal and spatial expression of virulence factors during cholera pathogenesis.
The often-fatal human diarrheal disease cholera is caused by the bacterium Vibrio cholerae. This organism expresses a number of virulence factors which allow it to colonize the human intestine and cause disease. Expression of the two major virulence factors cholera toxin (CT) and the toxin-coregulated pilus (TCP), as well as that of a number of other virulence factors, is regulated by environmental stimuli resulting in little to no expression outside the host but high levels of expression within the host intestine. Laboratory conditions which stimulate V. cholerae virulence factor expression have been elucidated and include temperature, osmolarity, pH, CO2, amino acids, and bile (for a review, see reference 28). However, the true in vivo signals which influence CT and TCP expression are still not known.
Coordinate expression of CT, TCP, and other virulence factors is controlled by a transmembrane protein, ToxR (23). ToxR, along with another transmembrane transcriptional activator, TcpP (12), activates expression of toxT in response to specific laboratory conditions (13). ToxT is an AraC-like regulatory protein that directly activates transcription of several virulence genes, including the ctx and tcp genes, which encode CT and TCP, respectively (4, 14). Differential expression of virulence factors in different biotypes of V. cholerae has been shown to be due to differential toxT expression (3). Moreover, expression of ToxT from an inducible promoter in V. cholerae strains containing mutations in toxR or tcpP leads to high-level expression of CT and TCP even under noninducing laboratory conditions (4, 12), whereas there is no expression of either of these factors in a V. cholerae strain lacking toxT (2). These data have been incorporated into a cascade model for virulence where inducing environmental signals within the host stimulate ToxR and TcpP to activate transcription of toxT, whose product then activates virulence factor expression in a constitutive manner (28).
We demonstrate that ToxT-dependent transcriptional activation of virulence factors is also regulated by environmental signals, indicating environmental modulation of ToxT transcriptional activity. Our results illuminate an additional level of environmental control over virulence factor expression in V. cholerae. We suggest that negative regulation of ToxT transcriptional activity prevents incorrect temporal and spatial expression of virulence factors.
MATERIALS AND METHODS
Bacterial strains.
Escherichia coli DH5α (11) was used for cloning experiments, and strain SM10λpir (22) was used to transfer plasmids to V. cholerae by conjugation. All V. cholerae strains used in this study are isogenic with the classical Ogawa strain O395 (20), and Salmonella typhimurium strains are isogenic with strain 14028 (American Type Culture Collection). The ΔtoxR1 mutation was introduced into the chromosome of V. cholerae strains by use of plasmid pMD60, as previously described for KKV61 (O395 ΔtoxR1 [17]). This method was used to introduce ΔtoxR1 mutations into strains KP1.25, KP9.62, KP3.51, KP3.44, KP8.11, KP2.16, KP5.51, and KP8.56 (24) and VJ740 (2), forming strains KKV356 to 363 and KKV365, respectively. The ΔtcpP mutation from O395N1 ΔtcpP (12) was amplified by PCR with specific oligonucleotides, cloned into the vector pCVD442 (5) to form pKEK164, and then recombined into the chromosome of VJ740 (2) by a similar method to form strain KKV489. Construction of S. typhimurium strains bearing chromosomal promoter-lacZYA fusions integrated into the putPA locus has been described previously (6, 16, 19).
Construction of plasmids expressing ToxT.
Construction of pKEK87, a translational fusion of toxT under control of the PBAD promoter, has already been described (18). The same PCR-derived toxT fragment used to construct pKEK87 was also ligated into pUC118 (33) that had been digested with SmaI and XbaI, to form pKEK162, and into pmalc (maltose-binding protein [MBP] fusion vector; New England Biolabs) that had been digested with StuI and XbaI, to form pKEK156; these plasmids express ToxT and an MBP-ToxT fusion protein, respectively, from an isopropyl-β-d-thiogalactoside (IPTG)-inducible Plac promoter. To express MBP and MBP-ToxT from the Plac promoter of pUC118 or the PBAD promoter of pBAD24 (10), PCR was performed on pmalc and pKEK156 with specific oligonucleotides, and then the PCR fragments were inserted into the SmaI site of pUC118 or the NcoI site of pBAD24 made blunt ended with the Klenow fragment of DNA polymerase, to form translational fusions to the initiating methionine codon of malE. This resulted in plasmids pKEK168 and pKEK169, which express MBP and MBP-ToxT from the Plac promoter of pUC118, respectively, and pKEK159 and pKEK160, which express these proteins from the PBAD promoter of pBAD24.
Construction of ctxA and tcpA promoter transcriptional fusions.
Specific oligonucleotides were used to amplify the ctxA and tcpA promoter regions by PCR with V. cholerae O395 chromosomal DNA; the ctxA promoter fragment extended from nucleotide −494 to +6 with respect to the start site of transcription (23), and the tcpA promoter fragment extended from nucleotide −468 to +78 with respect to the start site of transcription (1). These promoter fragments were ligated into the lacZ transcriptional fusion plasmid pRS551 (27) and integrated into the S. typhimurium chromosome as described previously (19) to form strains KK201 and KK207, respectively.
To form transcriptional fusions to the firefly luciferase luc gene, the luc gene was first amplified by PCR with specific oligonucleotides from plasmid pGPLO1 (8) and ligated into the EcoRI and BamHI sites of the vector pWSK30 (34) to form pKEK172. The ctxA and tcpA promoter fragments described above were then ligated into pKEK172 to form transcriptional luc fusions, and finally, PCR-amplified internal ∼500-bp sequences of ′ctxA′ and ′tcpA′ were ligated into these plasmids downstream of luc to facilitate a double-recombination event. This resulted in the formation of the ΔctxA::luc plasmid pKEK170 (resulting in luc insertion into a deletion which removes coding sequence for amino acids 1 to 216 of CtxA) and the ΔtcpA::luc plasmid pKEK177 (resulting in luc insertion into a deletion which removes coding sequence for amino acids 2 to 50 of TcpA). The ΔctxA::luc and ΔtcpA::luc insertion-deletions were subsequently cloned into pCVD442 (5), forming plasmids pKEK171 and pKEK178, respectively, and integrated into the chromosome of V. cholerae KKV365 (ΔtoxR1 ΔtoxT) as described previously (17).
Growth conditions.
V. cholerae strains containing plasmids expressing ToxT were first grown for 6 h to overnight in a roller drum in 1 ml of Luria broth (LB) containing 50 μg of ampicillin per ml and 100 μg of streptomycin per ml in 11-mm-diameter culture tubes at 37°C. Cultures were diluted 1:100 in 0.15 M NaCl, and then 10 μl was used to inoculate 5 ml of LB containing 50 μg of ampicillin per ml and 100 μg of streptomycin per ml in 16-mm-diameter culture tubes; media additionally contained 0.3 mM IPTG or 0.05% arabinose, as required for Plac or PBAD promoter induction, and 0.4% sodium choleate (bile; Sigma) as indicated. Cultures were grown overnight in a roller drum at either 30 or 37°C.
Enzyme assays.
β-Galactosidase and alkaline phosphatase (PhoA) assays were performed as described previously (21, 31). Luciferase assays were performed by first sonicating cultures grown under the conditions indicated and then diluting in luciferase buffer and measuring relative light units in a Berthold Lumat luminometer model LB9507 as described previously (8) with assay reagents from Promega Corp.
Detection of protein expression.
CT was measured in culture supernatants by ganglioside M1 enzyme-linked immunosorbent assay (GM1-ELISA) with polyclonal rabbit serum directed against purified B subunit of CT (a kind gift of J. Mekalanos) as described previously (30). TcpA and MBP were measured in whole-cell lysates by Western analysis with rabbit polyclonal antisera against TcpA (a kind gift of J. Mekalanos) and MBP (New England Biolabs), utilizing an alkaline phosphatase detection kit (Bio-Rad).
RESULTS
Temperature and bile modulate ToxT-dependent expression of CT and TCP.
According to the current cascade model of V. cholerae virulence, environmental regulation of virulence factor expression is primarily due to environmental regulation of ToxR-dependent transcription of toxT (28). This model thus predicts that ToxT expressed from a ToxR-independent promoter should activate expression of virulence factors regardless of environmental conditions. Gupta and Chowdhury (9) demonstrated that the addition of 0.4% bile to the growth medium of V. cholerae decreased transcription of the ctxA and tcpA genes under inducing laboratory conditions. We were intrigued by their results because the negative effect of bile on virulence factor expression appeared to be independent of ToxR. Therefore, experiments were conducted to determine if environmental sensing of bile was ToxT dependent.
To determine whether ToxT-dependent expression of CT and TCP is modulated by the presence of bile, we first introduced the plasmid pKEK162, which expresses ToxT from an IPTG-inducible Plac promoter, into the wild-type V. cholerae strain O395. This strain was grown under normal laboratory inducing conditions for virulence factor expression, i.e., growth in LB at 30°C; the wild-type strain containing the vector alone produces high levels of CT and TCP under these conditions (data not shown) (3).
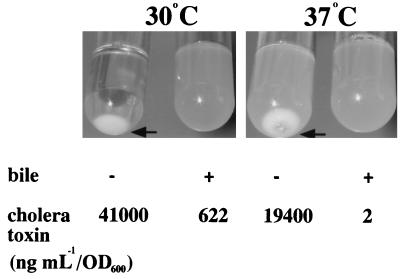
When the wild-type strain carrying pKEK162 was grown at 30°C in the presence of IPTG, the cells agglutinated due to the production of high levels of TCP, resulting in complete clearing of the supernatant (Fig. 1, arrow). If, however, 0.4% bile is added to the medium prior to growth, no agglutination occurs, which is suggestive of lower levels of TCP expression; control experiments indicated that the addition of 0.4% bile to the supernatant after agglutination occurred did not solubilize bacterial aggregates (data not shown). The level of CT present in the culture supernatants was measured by GM1-ELISA. The wild-type strain expressing increased levels of ToxT in the absence of bile produced 41,000 ng ml−1 per unit of optical density at 600 nm (OD600 unit), whereas in the presence of bile, it produced only 622 ng ml−1/OD600 unit. Control experiments indicated that 0.4% bile does not interfere with detection of CT in culture supernatants by GM1-ELISA (data not shown).
FIG. 1.
ToxT-dependent expression of CT and TCP is modulated by temperature and bile in a V. cholerae wild-type strain. V. cholerae O395 carrying plasmid pKEK162, which expresses ToxT from the Plac promoter, was grown as described in Materials and Methods in LB containing 0.3 mM IPTG in the absence (−) or presence (+) of 0.4% bile at 30 or 37°C. Arrows indicate agglutinated cells resulting from TCP expression. CT levels in culture supernatants were determined as described in Materials and Methods.
When the wild-type strain carrying pKEK162 was grown at 37°C in the presence of IPTG (normally noninducing conditions for the production of virulence factors [3]), the cells weakly agglutinated, probably due to the production of lower levels of TCP in this strain at 37°C than at 30°C (Fig. 1, arrow). Under the same growth conditions, a wild-type strain carrying the vector alone exhibits no agglutination (data not shown). The addition of 0.4% bile to the growth medium prevented agglutination of the wild-type strain expressing ToxT at 37°C also, again suggesting lower levels of TCP expression. Measurement of CT revealed that this strain produces 19,400 ng ml−1/OD600 unit in the absence of bile but only 2 ng ml−1/OD600 unit in the presence of bile. These results demonstrate that both temperature and bile appear to modulate ToxT-dependent expression of the two major virulence factors CT and TCP in a wild-type V. cholerae strain.
Temperature and bile modulate expression of ToxT-dependent TnphoA fusions in the absence of ToxR.
To determine if ToxR played a role in environmental sensing of bile and temperature, we introduced a nonpolar toxR chromosomal deletion mutation (ΔtoxR1) into V. cholerae strains containing TnphoA fusions to eight genes which were originally identified as ToxR-activated genes (tag) (24). Included were TnphoA fusions to ctxA, tcpI, and tagA, which were subsequently shown to be directly activated by ToxT (4). The ToxT expression plasmid pKEK162 was introduced into these strains, and they were grown in the presence of IPTG at 30 or 37°C in the presence or absence of 0.4% bile. Alkaline phosphatase activity resulting from expression of the TnphoA fusions was then measured.
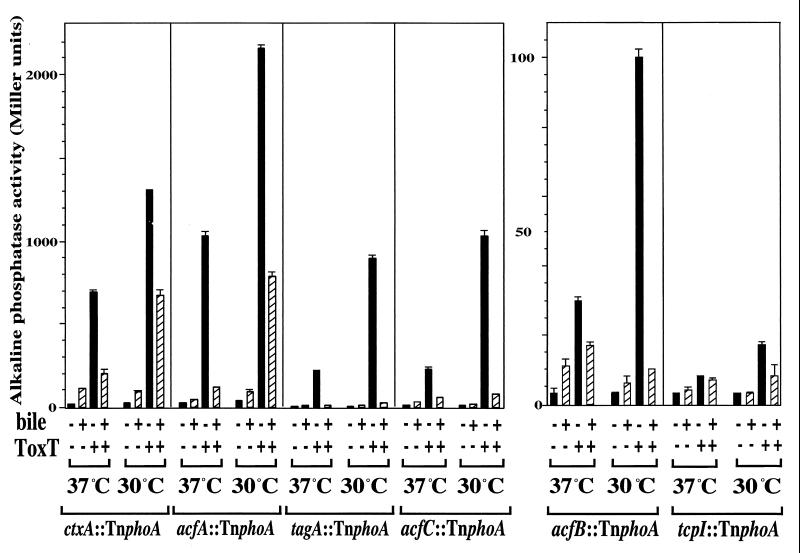
In the strains containing the vector alone, little to no alkaline phosphatase activity was observed, whereas the introduction of the plasmid expressing ToxT led to increases in alkaline phosphatase activity at 30°C for six of the eight TnphoA fusions tested (Fig. 2). These results confirm that expression of ctxA-, tcpI-, and tagA-TnphoA fusions is ToxT dependent and demonstrate that expression of acfA-, acfB-, and acfC-TnphoA fusions is also ToxT dependent (acfB and acfC are probably transcribed together [1]). Neither the acfD-TnphoA fusion nor another tag-TnphoA fusion (from strain KP2.16 [24]) was expressed in a ΔtoxR strain carrying pKEK162, indicating that expression of these fusions is ToxT independent (data not shown).
FIG. 2.
ToxT-dependent TnphoA fusions are modulated by temperature and bile in the absence of ToxR. V. cholerae strains containing a ΔtoxR1 mutation and TnphoA fusions to ToxT-dependent genes and carrying either plasmid pKEK162, which expresses ToxT from the Plac promoter (ToxT +), or the vector pUC118 (ToxT −) were grown as described in Materials and Methods in LB containing 0.3 mM IPTG in the absence (black bars) or presence (hatched bars) of 0.4% bile at 30 or 37°C. The V. cholerae strains used were KKV356 (ctx::TnphoA), KK357 (acfA::TnphoA), KKV358 (acfB::TnphoA), KKV359 (acfC::TnphoA), KKV362 (tcpI::TnphoA), and KKV363 (tagA::TnphoA). Alkaline phosphatase activities are the averages and standard deviations from three samples (note differences in scale).
When the ToxT-dependent TnphoA fusion strains expressing ToxT were grown at 37°C instead of 30°C, there was a decrease in alkaline phosphatase activity in all six TnphoA fusions (Fig. 2). At both temperatures, when 0.4% bile was added to the growth medium, there was a decrease and in many cases complete elimination of ToxT-dependent expression of all six TnphoA fusions. We obtained similar results when ToxT was expressed from an arabinose-inducible promoter in these TnphoA fusion strains (data not shown), consistent with temperature and bile modulation of ToxT-dependent virulence factor expression, even in the absence of ToxR.
ToxT expression from Plac is not affected by temperature or bile.
Because temperature and the presence of bile influence ToxT-dependent virulence factor expression and this effect is independent of ToxR, we wished to exclude the possibility that these environmental signals influence expression of ToxT from the inducible plasmid. Although we expressed toxT from both IPTG- and arabinose-inducible promoters and obtained similar results, we considered that ToxT levels were being modulated, resulting in apparent modulation of ToxT-dependent gene expression.
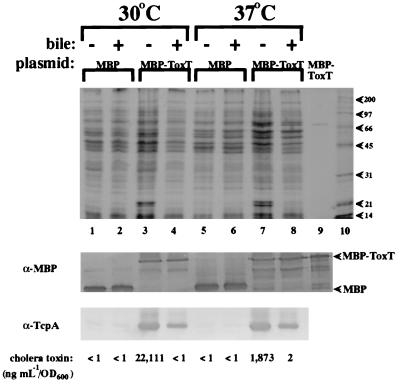
To directly measure ToxT expression from the Plac promoter of pUC118, we constructed pKEK169, which expresses an MBP-ToxT fusion protein from the Plac promoter. The control vector, pKEK168, expresses MBP from this same promoter. V. cholerae KKV365 (ΔtoxR1 ΔtoxT) carrying pKEK168 and pKEK169 was grown in the presence of IPTG at both 30 and 37°C and in the presence and absence of 0.4% bile (this strain grew at the same rate at each temperature regardless of the presence or absence of 0.4% bile). Whole-cell lysates of these cultures were matched by cell density, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then subjected to Western analysis with rabbit polyclonal MBP antiserum (Fig. 3). Similar amounts of MBP and MBP-ToxT are expressed from the Plac promoter, at both 37 and 30°C and in both the presence and absence of 0.4% bile, indicating that neither temperature nor bile affects IPTG-induced Plac promoter expression.
FIG. 3.
Expression of MBP-ToxT from Plac is not affected by temperature or bile. V. cholerae KKV365 (ΔtoxR1 ΔtoxT) carrying either plasmid pKEK169, which expresses MBP-ToxT from the Plac promoter (lanes 3, 4, 7, and 8), or pKEK168, which expresses MBP from the Plac promoter (lanes 1, 2, 5, and 6), was grown as described in Materials and Methods in LB containing 0.3 mM IPTG in the absence (lanes 1, 3, 5, and 7) or presence (lanes 2, 4, 6, and 8) of 0.4% bile at 30°C (lanes 1 to 4) or 37°C (lanes 5 to 8). Whole-cell lysates were matched by OD600, separated on a sodium dodecyl sulfate–12% polyacrylamide gel, and then stained with Coomassie blue (upper panel). Lane 9, partially purified MBP-ToxT; lane 10, molecular weight standards (weights are in thousands). The samples in lanes 1 to 9 were subjected to Western analysis (see Materials and Methods) with rabbit polyclonal antiserum against MBP (α-MBP; middle panel); MBP-ToxT and MBP are indicated by arrowheads. The samples in lanes 1 to 8 were also subjected to Western analysis with rabbit polyclonal antiserum against TcpA (α-TcpA, bottom panel). CT in culture supernatants corresponding to the samples in lanes 1 to 8 was quantitated as described in Materials and Methods.
CT was measured in the same culture supernatants of KKV365 used for the MBP Western analysis (Fig. 3). MBP fused to the amino terminus of ToxT does not appear to adversely affect ToxT activity, as similar amounts of CT (and TcpA) were detected when either ToxT or MBP-ToxT was expressed from Plac in this strain (data not shown). The highest level of CT was seen when MBP-ToxT was expressed at 30°C in the absence of bile (22,111 ng ml−1/OD600 unit), while an increase to 37°C resulted in a 12-fold decrease in CT (1,873 ng ml−1/OD600 unit). The addition of 0.4% bile to the medium abolished CT expression at both temperatures. The whole-cell lysates were also subjected to Western analysis with rabbit polyclonal TcpA antiserum, which recognizes the major structural subunit of TCP (Fig. 3). High levels of TcpA could be detected in those cultures expressing MBP-ToxT in the absence of bile at both 37 and 30°C, although no temperature effect on TcpA expression was observed (as was also visible in the Coomassie blue-stained gel). Less TcpA was detectable in those cultures expressing MBP-ToxT in the presence of 0.4% bile. No CT or TcpA was detectable in cultures expressing only MBP. Similar results were obtained when MBP-ToxT was expressed from an arabinose-inducible promoter in this same strain (pKEK160) (data not shown).
Because TcpP, like ToxR, is involved in the regulation of virulence factors in response to environmental stimuli, experiments were performed to determine if TcpP was involved in ToxT-dependent modulation by environmental signals. MBP-ToxT or ToxT was expressed from Plac in strain KKV489 (ΔtcpP ΔtoxT), and results similar to those shown above for strain KKV365 were obtained (data not shown). These results confirm that ToxT-dependent virulence factors are modulated by environmental signals even when levels of ToxT protein remain constant and that ToxT-dependent modulation is independent of ToxR and TcpP.
ToxT-dependent transcription of ctxA and tcpA is modulated by temperature and bile in V. cholerae.
In order to measure ToxT-dependent transcription of the structural genes encoding CT and TCP, ctxA and tcpA promoter transcriptional fusions to the firefly luciferase gene (luc) were constructed. These fusions (ΔctxA::luc and ΔtcpA::luc) were then recombined into the chromosome of V. cholerae KKV365 (ΔtoxR1 ΔtoxT), the same strain used to analyze CT and TCP expression (see above). The resulting strains, KKV523 (ΔctxA::luc ΔtoxR1 ΔtoxT) and KKV515 (ΔtcpA::luc ΔtoxR1 ΔtoxT), were transformed with pKEK162 (expressing ToxT). Transcription was measured by measuring luciferase activity after growth of these strains at 30 or 37°C and in the presence or absence of 0.4% bile.
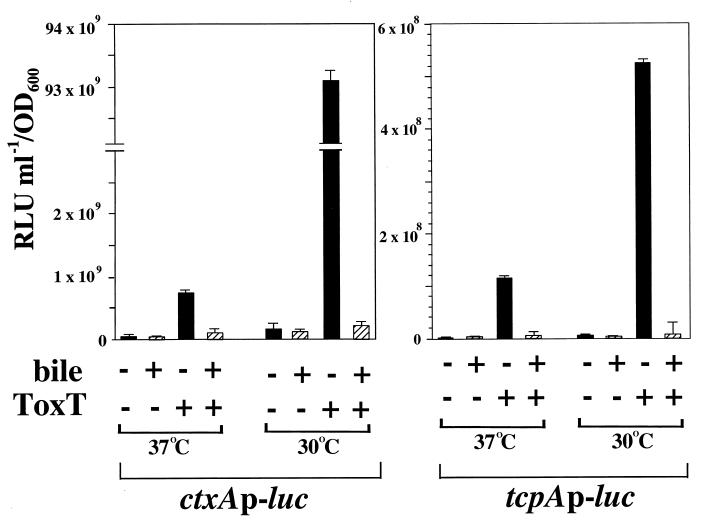
When the ctxA promoter-luciferase fusion strain KKV523 carrying the inducible ToxT plasmid pKEK162 was grown at 30°C in the presence of IPTG, high levels of ctxA transcription were observed, i.e., a 72-fold increase over the level of ctxA transcription in this strain carrying the vector alone (Fig. 4). As predicted from CT levels observed in this strain, ToxT-dependent transcription of ctxA at 37°C is significantly less than that at 30°C, representing a 13-fold decrease. The addition of 0.4% bile to the growth medium causes further decreases in ToxT-dependent ctxA transcription at both temperatures, i.e., a 51-fold decrease at 30°C and a 6-fold decrease at 37°C. In control experiments, 0.4% bile was added to those stationary-phase cultures which had grown in the absence of bile, and no difference in levels of luciferase activity was seen, indicating that 0.4% bile does not affect luciferase activity (data not shown).
FIG. 4.
ToxT-dependent transcription of ctxA and tcpA is modulated by temperature and bile in V. cholerae. ΔtoxR1 ΔtoxT V. cholerae strains containing chromosomal ctxAp-luc (KKV523) and tcpAp-luc (KKV515) transcriptional fusions and carrying either plasmid pKEK162, which expresses ToxT from the Plac promoter (ToxT +), or the vector pUC118 (ToxT −) were grown as described in Materials and Methods in LB containing 0.3 mM IPTG in the absence (black bars) or presence (hatched bars) of 0.4% bile at 30 or 37°C. Cultures were assayed for luciferase activity as described in Materials and Methods. Results are the averages and standard deviations from three samples. RLU, relative light units.
Expression of ToxT from the IPTG-inducible promoter in the tcpA promoter-luciferase fusion strain KKV515 results in high levels of tcpA transcription at 30°C, i.e., a 144-fold increase over the level of tcpA transcription in this strain carrying the vector alone (Fig. 4). Increasing the growth temperature to 37°C decreased ToxT-dependent transcription fivefold. The addition of 0.4% bile caused significant decreases in ToxT-dependent tcpA transcription at both temperatures, i.e., a 90-fold decrease at 30°C and a 32-fold decrease at 37°C. We obtained similar results with these two luc fusion strains when MBP-ToxT was expressed from Plac (pKEK169) or when ToxT was expressed from PBAD (pKEK87), consistent with temperature and bile modulating ToxT transcriptional activity. In control experiments, transcription of the ToxT-independent promoter of the V. cholerae flagellin gene flaA (17) was not modulated by temperature (37 versus 30°C), but the presence of 0.4% bile induced flaA transcription fourfold (data not shown), demonstrating that bile and temperature do not exert pleiotropic negative effects on transcription in general.
ToxT-dependent transcription of ctxA and tcpA is relatively insensitive to temperature and bile in S. typhimurium.
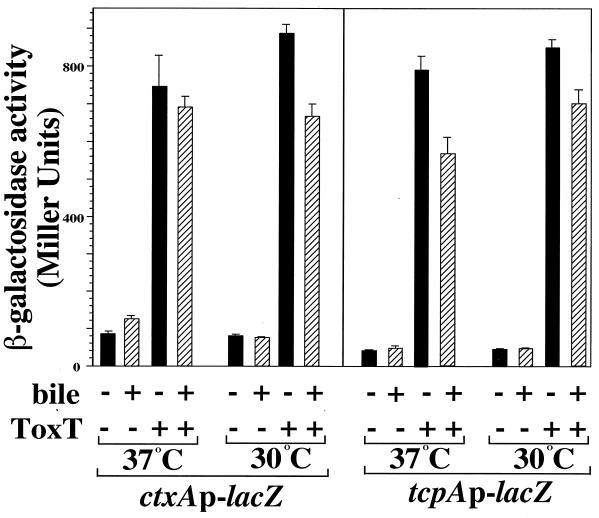
In order to determine if ToxT-dependent transcription was modulated by temperature and bile in a heterologous host, we constructed chromosomal ctxAp-lacZ and tcpAp-lacZ transcriptional fusions in S. typhimurium and transformed the resulting strains, KK201 and KK207, with pKEK162 (expressing ToxT). Transcription was measured by measuring β-galactosidase activity after growth of these strains at 30 or 37°C and in the presence or absence of 0.4% bile.
High-level transcription of both the ctxAp-lacZ and tcpAp-lacZ fusions was dependent on expression of ToxT (Fig. 5), but ToxT-dependent transcription of both promoters exhibited only a slight decrease (1.2- and 1.1-fold, respectively) at 37°C compared to that seen at 30°C. Transcription of both promoters was only slightly decreased by the addition of bile to the medium at either temperature (1.1- to 1.4-fold decreases in transcription). We obtained similar results with these two S. typhimurium lacZ fusion strains when MBP-ToxT was expressed from Plac (pKEK169) or when ToxT was expressed from PBAD (pKEK87). These results indicate that ToxT is not inherently more active at 30 than at 37°C, and together with the transcription data above, they indicate V. cholerae-specific environmental modulation of ToxT-dependent transcription, at least under the conditions tested.
FIG. 5.
ToxT-dependent transcription of ctxA and tcpA is relatively insensitive to temperature and bile in S. typhimurium. S. typhimurium strains containing chromosomal ctxAp-lacZ (KK201) and tcpAp-lacZ (KK207) transcriptional fusions and carrying either plasmid pKEK162, which expresses ToxT from the Plac promoter (ToxT +), or the vector pUC118 (ToxT −) were grown as described in Materials and Methods (with streptomycin omitted from the medium) in LB containing 0.3 mM IPTG in the absence (black bars) or presence (hatched bars) of 0.4% bile at 30 or 37°C. Cultures were assayed for β-galactosidase as described in Materials and Methods. Results are the averages and standard deviations from three samples.
DISCUSSION
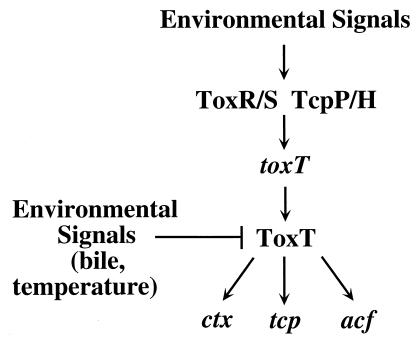
According to the current cascade model of V. cholerae virulence, inducing environmental conditions are sensed by the ToxR-ToxS and TcpP-TcpH regulatory systems, which respond by activating transcription of toxT (3, 4, 12). Although additional regulatory proteins (e.g., TcpI and Crp [26, 29]) affect virulence gene expression by unknown mechanisms, toxT transcription appears to be the primary event associated with high-level expression of virulence factors (3). The regulatory protein ToxT directly activates transcription of the structural genes for the two major virulence factors, CT and TCP, as well as other factors involved in pathogenesis. In the present study, we demonstrate that transcriptional activation by the ToxT protein is also modulated by environmental signals, thus revealing an additional level of complexity of environmental control over V. cholerae pathogenesis (Fig. 6).
FIG. 6.
Model of environmental regulation of the cascade controlling V. cholerae virulence. It has previously been shown that environmental signals modulate transcription of toxT by the ToxR-ToxS and TcpP-TcpH regulatory systems (3, 12); the present study demonstrates that environmental signals (temperature and bile) negatively regulate transcriptional activation of virulence factors (ctx, tcp, and acf) by the ToxT protein. Environmental regulation of V. cholerae virulence thus influences multiple levels of this regulatory cascade.
We have shown that ToxT-dependent transcription in V. cholerae can be significantly reduced or eliminated by an increase from 30 to 37°C and/or the presence of bile. Interestingly, ToxT-dependent transcriptional activation in S. typhimurium was relatively insensitive to temperature and the same concentration of bile, indicating that ToxT is not inherently more active at 30 than at 37°C and indicating V. cholerae-specific environmental regulation of ToxT-dependent transcription. We predict that ToxT activity in V. cholerae is regulated by direct interaction with either a small molecule or another protein, similar to other transcriptional activators in the AraC family, with which it has homology. Modulation of transcriptional activity of AraC occurs when the protein binds arabinose in its amino-terminal domain, thus changing its conformation, altering its DNA-binding activity, and resulting in activation of transcription (7). The MBP-ToxT fusion protein is regulated by temperature and bile in a manner similar to that of the native-length ToxT, which may argue against interaction with a protein that might be sterically hindered by the MBP protein fused to the ToxT amino terminus. Because bile can enter the cytoplasm of enteric pathogens (32), it is possible that ToxT interacts directly with bile and becomes transcriptionally inactive; however, temperature regulation of ToxT activity would require interaction with some cytoplasmic messenger of temperature, given the inherent ToxT temperature insensitivity seen in S. typhimurium.
We imagine that the signals which stimulate toxT transcription within the host may not occur at the appropriate niche for V. cholerae to successfully establish infection, and thus negative control over ToxT activity is necessary to prevent incorrect temporal and spatial virulence gene expression. Our preliminary data indicate that transcription of toxT is stimulated by 0.4% bile at 37°C (25a), the same conditions that we have shown repress ToxT-dependent transcriptional activation. We hypothesize that within the lumen of the intestine, toxT transcription may be induced by the presence of bile or other signals, yet the bacteria must first penetrate the mucus lining before they colonize the epithelial cell surface. Premature expression of TCP by ToxT would immobilize the organisms in an inappropriate location where the bacteria are liable to be swept away by peristalsis. Also, prolonged expression of TCP would prevent dissemination of the organisms after colonization of the intestinal epithelia; negative regulation of ToxT activity provides a mechanism for the bacteria to facilitate exit from the host by a cessation of TCP expression.
Bile is likely used as an important environmental signal by V. cholerae to establish a successful infection. Gupta and Chowdhury (9) demonstrated that bile increases the V. cholerae swarm size in motility agar, indicative of increased motility and/or chemotaxis. We predict that the presence of bile within the intestinal lumen both prevents ToxT transcriptional activation of virulence factors and increases motility and/or chemotaxis to drive the bacteria into the mucus lining. The bile concentration used in these studies (0.4%) is at the low end of estimated concentrations of bile in the intestines of healthy individuals (0.2 to 2% for individual bile salts [15]). Presumably the concentration of bile would decrease at the intestinal cell surface where V. cholerae normally colonizes, reducing motility and allowing ToxT transcription of virulence factors.
The temperature modulation of ToxT activity is surprising considering that maximal activity in V. cholerae is at 30°C, not 37°C as is certainly found in the human intestine. Notably, ToxT transcriptional activity was decreased but not abolished at the higher temperature, which still allows virulence factor expression at 37°C. There may be unidentified relevant environmental stimuli which increase ToxT transcriptional activity at 37°C in vitro. The classical V. cholerae biotype used in these studies exhibits optimal virulence factor expression at 30°C under laboratory conditions, but the El Tor biotype expresses virulence factors optimally at 37°C (3). ToxT expressed from pKEK162 (derived from a classical strain) demonstrated optimal activity at 30°C even in an El Tor strain (25a); it will be interesting to determine if the temperature optimum of ToxT derived from an El Tor strain is 37°C.
Certain host factors are known to affect the incidence and severity of cholera, but the mechanisms underlying any increase in susceptibility are not understood. One condition which predisposes humans to cholera is chronic malnutrition (25), and it has been hypothesized that this may lead to immune deficiencies which increase susceptibility. Bile, which aids in the digestion of fatty foods, is stored within the gall bladder and is released in response to food intake (15); bile concentrations in the intestine are thus lower during fasting periods. We suggest that low concentrations of bile in the intestine in response to malnutrition may also predispose individuals to cholera, due to less-inhibitory effects on ToxT transcriptional activity at the intestinal cell surface.
ACKNOWLEDGMENTS
We thank Victor DiRita, John Gunn, and John Mekalanos for providing strains and materials, Raynia McGee for purifying MBP-ToxT, and John Gunn for making constructive comments on the manuscript.
This work was supported by an institutional new faculty award of the Howard Hughes Medical Institute to K.E.K.
REFERENCES
- 1.Brown R C, Taylor R K. Organization of tcp, acf, and toxT genes within a ToxT-dependent operon. Mol Microbiol. 1995;16:425–439. doi: 10.1111/j.1365-2958.1995.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 2.Champion G A, Neely M N, Brennan M A, DiRita V J. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. doi: 10.1046/j.1365-2958.1997.2191585.x. [DOI] [PubMed] [Google Scholar]
- 3.DiRita V J, Neely M, Taylor R K, Bruss P M. Differential expression of the ToxR regulon in classical and El Tor biotypes of Vibrio cholerae is due to biotype-specific control over toxT expression. Proc Natl Acad Sci USA. 1996;93:7991–7995. doi: 10.1073/pnas.93.15.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiRita V J, Parsot C, Jander G, Mekalanos J J. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott T. A method for constructing single-copy lac fusions in Salmonella typhimurium and its application to the hemA-prfA operon. J Bacteriol. 1992;174:245–253. doi: 10.1128/jb.174.1.245-253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallegos M T, Schleif R, Bairoch A, Hofmann K, Ramos J L. AraC/XylS family of transcriptional regulators. Microbiol Mol Biol Rev. 1997;61:393–410. doi: 10.1128/mmbr.61.4.393-410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunn J S, Miller S I. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 1996;178:6857–6864. doi: 10.1128/jb.178.23.6857-6864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Chowdhury R. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect Immun. 1997;65:1131–1134. doi: 10.1128/iai.65.3.1131-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:577–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 12.Hase C C, Mekalanos J J. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 1998;95:730–734. doi: 10.1073/pnas.95.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins D E, DiRita V J. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol Microbiol. 1994;14:17–29. doi: 10.1111/j.1365-2958.1994.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 14.Higgins D E, Nazareno E, DiRita V J. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J Bacteriol. 1992;174:6974–6980. doi: 10.1128/jb.174.21.6974-6980.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann A F. Bile secretion and the enterohepatic circulation of bile acids. In: Feldman M, Scharschmidt B F, Sleisenger M H, editors. Gastrointestinal and liver disease. W. B. Philadelphia, Pa: Saunders Co.; 1998. pp. 937–948. [Google Scholar]
- 16.Ikeda T P, Shauger A E, Kustu S. Salmonella typhimurium apparently perceives external nitrogen limitation as internal glutamine limitation. J Mol Biol. 1996;259:589–607. doi: 10.1006/jmbi.1996.0342. [DOI] [PubMed] [Google Scholar]
- 17.Klose K E, Mekalanos J J. Differential regulation of multiple flagellins in Vibrio cholerae. J Bacteriol. 1998;180:303–316. doi: 10.1128/jb.180.2.303-316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klose K E, Mekalanos J J. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol Microbiol. 1998;28:501–520. doi: 10.1046/j.1365-2958.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 19.Klose K E, Mekalanos J J. Simultaneous prevention of glutamine synthesis and high-affinity transport attenuates Salmonella typhimurium virulence. Infect Immun. 1997;65:587–596. doi: 10.1128/iai.65.2.587-596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekalanos J J, Collier R J, Romig W R. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979;254:5855–5861. [PubMed] [Google Scholar]
- 21.Miller J H. A short course in bacterial genetics. 2nd ed. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 22.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller V L, Taylor R K, Mekalanos J J. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell. 1987;48:271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 24.Peterson K M, Mekalanos J J. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun. 1988;56:2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson S H. Host susceptibility. In: Wachsmuth I K, Blake P A, Olsvik O, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: American Society for Microbiology; 1994. pp. 273–289. [Google Scholar]
- 25a.Schuhmacher, D. A., and K. E. Klose. Unpublished results.
- 26.Shaw C E, Peterson K M, Mekalanos J J, Taylor R K. Genetic studies of Vibrio cholerae TCP pilus biogenesis. Adv Res Cholera Relat Diarrheas. 1990;7:51–58. [Google Scholar]
- 27.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 28.Skorupski K, Taylor R K. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol. 1997;25:1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 29.Skorupski K, Taylor R K. Cyclic AMP-CRP negatively regulates the coordinate expression of cholera toxin and TCP in Vibrio cholerae. Proc Natl Acad Sci USA. 1997;94:265–270. doi: 10.1073/pnas.94.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svennerholm A M, Holmgren J. Identification of the Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr Microbiol. 1978;1:19–23. [Google Scholar]
- 31.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thanassi D G, Cheng L W, Nikaido H. Active efflux of bile salts by Escherichia coli. J Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 34.Wang R F, Kushner S. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]