Abstract
We have investigated the expression and pharmacology of endothelin (ET) receptors in human aortocoronary saphenous vein grafts.
Subtype-selective ligands were used to autoradiographically identify ETA ([125I]-PD151242) and ETB ([125I]-BQ3020) receptors. In graft saphenous vein ETA receptors predominated in the media, with few ETB receptors identified. Neither subtype was detected in the thickened neointima.
The ratio of medial ETA:ETB receptors was 75% : 25% in both graft and control saphenous vein.
ET-1 contracted control (EC50 2.9 nM) and graft (EC50 4.5 nM) saphenous vein more potently than diseased coronary artery (EC50 25.5 nM).
In all three blood vessels ET-1 was 100 times more potent than ET-3 and three times more potent than sarafotoxin 6b (S6b). Little or no response was obtained in any vessel with the ETB agonist sarafotoxin 6c (S6c).
The ETA antagonist PD156707 (100 nM) blocked ET-1 responses in all three vessels with pKb values of approximately 8.0.
For individual graft veins the EC50 value for ET-1 and ‘age' of graft in years showed a significant negative correlation.
In conclusion there is no alteration in ET receptor expression in the media of saphenous veins grafted into the coronary circulation compared to control veins. ETA receptors predominantly mediate the vasoconstrictor response to ET-1 in graft vein, with no apparent up-regulation of ETB receptors. The sensitivity of the graft vein to ET-1 increased with graft ‘age', suggesting that these vessels may be particularly vulnerable to the increased plasma ET levels that are detected in patients with cardiovascular disease.
Keywords: Endothelin, endothelin receptors, ETA, ETB, human saphenous vein graft, intimal proliferation, vasospasm, vein graft disease
Introduction
The saphenous vein is the most widely used graft vessel employed to bypass atherosclerotic blood vessels of the heart and thus relieve the symptoms of coronary artery disease. Perioperative aortocoronary vein graft spasm is a serious complication. It results in hypoperfusion of the vein and damage to the endothelium, thus contributing to graft failure (Roubos et al., 1995). Post-operative graft spasm is more rare, but can be fatal (Victor et al., 1981). In a small proportion of patients the vein grafts close completely following surgery. This occurs either within days of surgery, when the cause is frequently the development of occluding mural thrombi (Lie et al., 1977; Bourassa et al., 1982), or more usually, closure occurs over a period of 5–10 years (Lie et al., 1977; Atkinson et al., 1985). The fibrointimal proliferative nature of this late vein graft disease closely resembles that of atherosclerotic coronary artery disease (Bulkley & Hutchins, 1977; Campeau et al., 1983; Atkinson et al., 1985). Arterial grafts are less susceptible to long term failure (Rossiter et al., 1974; Ivert et al., 1988), however the requirement to bypass multiple vessels of the heart, in many patients, precludes the exclusive use of arterial preparations. Antiplatelet drugs can be of some benefit, particularly in preventing early vein graft stenosis (Chesebro et al., 1982; Fuster & Chesebro, 1986; Goldman et al., 1988). However, to improve the long term patency of the autogenous saphenous vein for coronary artery bypass surgery novel drug therapies must be developed.
Recent studies have shown that expression of the peptide endothelin-1 (ET-1) is increased in human failed vein grafts (Brody et al., 1996; Masood et al., 1996). ET-1 is a potent vasoconstrictor of human blood vessels (Maguire & Davenport, 1995) including saphenous vein (Costello et al., 1990; Lüscher et al., 1990; Bax et al., 1993; Akar et al., 1994; White et al., 1994) and is mitogenic or co-mitogenic for vascular smooth muscle cells (see Battistini et al., 1993). Endothelin (ET) may therefore be involved in the initiation and/or development of both vein graft spasm and stenosis following surgery. We have therefore determined which of the ET receptor subtypes are expressed in the blocked vein graft and which are responsible for the vasoconstrictor actions of the ET peptides in this diseased tissue and compared this to non-diseased saphenous vein harvested for grafting. We have previously shown that the media of human saphenous vein expresses mainly ETA receptors but also has a small population of ETB receptors that comprise less than 25% of total (Davenport et al., 1995). We were particularly interested to investigate whether or not the expression of ETB receptors is augmented in late graft disease as it has been suggested that upregulation of this subtype may occur in coronary artery disease (Bacon et al., 1996).
Preliminary data were presented to the British Pharmacological Society (Maquire & Davenport, 1997) and the Fifth International Conference on Endothelin (Maguire & Davenport, 1998).
Methods
Tissue collection
Graft saphenous veins and atherosclerotic coronary arteries were obtained from eight male patients (41–61 years old) transplanted for ischaemic heart disease. All patients received coronary artery bypass grafts (CABG) 2–13 years prior to cardiac transplantation with a mean ‘age' of saphenous vein grafts of 6.5±3.4 years (n=8). Coronary arteries were additionally obtained from 12 patients (11 male, 1 female; 42–59 years) transplanted for ischaemic heart disease or cardiomyopathies. Drug therapies included diuretics, anti-arrhythmics, angiotensin converting enzyme inhibitors, anti-coagulants, nitrates, beta blockers, calcium channel blockers and aspirin. Non-diseased ‘control' saphenous veins were obtained from 26 patients (25 male, 1 female; 35–75 years) undergoing CABG surgery. Veins were collected following surgical distension.
Receptor autoradiography
ET receptor subtypes were detected autoradiographically as previously described (Davenport et al., 1988). Briefly, 10 μm cryostat-cut sections of saphenous vein graft and saphenous vein were thaw mounted on gelatine coated slides and pre-incubated in HEPES buffer (HEPES 50 mM containing MgCl2 5 mM and 0.3% w/v BSA, pH 7.4) for 30 min at room temperature (23°C). To visualize the ET receptor subtypes present in these tissues the wash buffer was replaced with HEPES buffer containing 0.1 nM [125I]-ET-1, to label both receptor subtypes, 0.1 nM [125I]-PD151242 (Davenport et al., 1994) to label ETA receptors or 0.3 nM [125I]-BQ3020 (Molenaar et al., 1992) to identify ETB receptors. The concentration of each ligand used was calculated to bind to approximately 30% of the respective receptor populations identified, according to the dissociation constant of each radioligand. Non-specific binding was defined by the inclusion of 1 μM of the corresponding unlabelled peptide. After a 2 h incubation period the sections were washed in ice-cold Tris buffer (50 mM, pH 7.4) and apposed to radiation sensitive film (Hyperfilm βmax, Amersham Pharmacia Biotech., Buckinghamshire, U.K.) for 5 days. Data were analysed by computer-assisted image analysis (Quantimet 920, Cambridge Instruments) to determine relative optical densities (OD) (mean±s.e.mean) for each receptor subtype.
In vitro pharmacology
Ring preparations (4 mm) were cut from lengths of saphenous vein graft, saphenous vein or atherosclerotic coronary artery. We were primarily interested in the medial smooth muscle ET receptors that mediate vasoconstriction and whether, or not, these were altered in disease. The endothelium was therefore removed, by gently rubbing the luminal surface with a metal seeker (verified histologically).
Blood vessel rings were transferred to 5 ml organ baths (Linton Instrumentation, Diss, Norfolk, U.K.) containing oxygenated Krebs solution (37°C), set up for isometric force recordings (F30 isometric force transducers, Hugo Sachs Electronik, March-Hugstetten, Germany) and allowed to equilibrate for 1 h. Responses were obtained to KCl (50 mM) at increasing levels of basal tension until no further increase in KCl response was observed. The rings were then allowed to re-equilibrate for 30 min. Cumulative concentration-response curves were constructed to ET-1, ET-3, sarafotoxin 6b (S6b), sarafotoxin 6c (S6c) and 5-hydroxytryptamine (5-HT), one curve per preparation. Experiments were terminated by the addition of KCl (50 mM) to determine the maximum possible contractile response in each case. Agonist responses were expressed as a percentage of this KCl maximum (%KCl). For the antagonist experiments ET-1 curves were determined in the absence (control) and presence of the ETA-selective, non-peptide PD156707 (100 nM) (Doherty et al., 1995) which was added to the bathing medium 30 min prior to the ET-1. Values of EC50 were determined for each experiment from the graphs of agonist concentration (log10) plotted against response (%KCl). Data for each agonist were pooled from all experiments and finally expressed as the geometric mean with 95% confidence intervals (CI). The potency (estimated pKb) of PD156707 was determined from the Gaddum-Schild equation assuming a regression slope of unity.
Mean EC50 values were compared using the Mann-Whitney U-test, all other data (expressed as arithmetic mean±s.e.mean) were compared using Student's two-tailed t-test. The significance level was set at 95% (P<0.05).
Materials
ET-1, ET-3, sarafotoxin 6b and sarafotoxin 6c were purchased from the Peptide Institute Inc. (Osaka, Japan), prepared as 10−4 M stock solutions in 0.1% acetic acid and kept at −20°C until required. BQ3020 ([Ala11,15]-Ac-ET-1(6–21) (Molenaar et al., 1992) and PD151242 ((N-[(hexahydro-1-azepinyl)carbonyl])L-Leu(1-Me)-D-Trp-D-Tyr) (Davenport et al., 1994) were synthesized by solid phase t-Boc chemistry. PD156707 (Doherty et al., 1995) was a gift from Park-Davis Pharmaceutical Research, Annarbor, MI, U.S.A. PD156707 was prepared as a 10−2 M solution in DMSO and stored at −20°C. Radiolabelled chemicals (specific activity 2000 Ci mmol−1) were from Amersham Pharmacia Biotech. (Buckinghamshire, U.K.). All other chemicals and reagents were from Sigma-Aldrich Co. Ltd. (Dorset, U.K.) and were of analar grade or better.
Krebs solution was of the following composition (mM): NaCl, 89; NaHCO3, 45; KCl, 5; MgSO4, 0.5; NaH2PO4, 1; D-glucose, 10; CaCl2, 2.25; EDTA, 40 μM; glutamic acid, 5 mM; fumaric acid 5 mM; sodium pyruvate, 5 mM (pH 7.4).
Results
Distribution of ET receptor subtypes in saphenous vein grafts
[125I]-PD151242 identified a high density of ETA receptors localized to the medial layer of the saphenous vein graft sections. Binding was also observed to vasa vasorum in the surrounding adventitia. These same regions expressed much lower levels of ETB receptors, which were detected with [125I]-BQ3020 (Figure 1). Neither receptor subtype was detected over the thickened intimal smooth muscle layer. At this resolution it was not possible to detect binding of either ligand to the single layer of endothelial cells lining the vessel lumen.
Figure 1.

Colour-coded images of the distribution of ET receptors in transverse sections of two adjacent retrieved aortocoronary saphenous vein grafts. (A) Localization of specific ETA binding with [125I]-PD151242 (0.1 nM) and (B) specific ETB binding with [125I]-BQ3020 (0.3 nM). These images were computer generated by digitally subtracting the autoradiographic image of the non-specific binding, defined by inclusion of the respective unlabelled peptide (1 μM), from the total binding image for each radioligand. 125I standards were co-exposed with the tissue sections and receptor density was colour-coded by interpolation from the resulting standards curve. (C) A section stained with haematoxylin and eosin. The graft on the left has a thickened intimal layer whilst the graft on the right is completely occluded.
Relative densities of ET receptor subtypes in diseased and control saphenous veins
Measurements of medial optical density (OD) were taken from the developed autoradiograms of sections of control saphenous vein and diseased saphenous vein grafts. In control saphenous vein the mean OD value for specific binding of [125I]-PD151242 was 0.43±0.06 (n=7) and for [125I]-BQ3020 was 0.13±0.02 (n=7) giving a relative density of ETA receptors to ETB receptors of 77 : 23%. In diseased saphenous vein grafts the OD values were respectively 0.14±0.01 (n=6) and 0.05±0.02 (n=6) giving a ratio of ETA:ETB of 74 : 26%.
Contractile responses in diseased saphenous vein graft and coronary artery compared with control saphenous vein
Immediately prior to construction of the agonist concentration response curves the basal resting tension for saphenous vein grafts (1.57±0.26 g weight, n=8) was significantly greater than that for control saphenous vein (0.87±0.17 g weight, n=16) (Student's two-tailed t-test, P<0.05), but not different than that for diseased coronary arteries obtained from the same explanted hearts (1.75±0.44 g weight, n=7).
Maximum responses to KCl (50 mM) were comparable in saphenous vein graft (2.97±0.54 g weight, n=8) and atherosclerotic coronary artery (2.76±0.32 g weight, n=7). The response to KCl in control saphenous vein tended to be lower (1.96±0.43 g weight, n=16), although this did not reach statistical significance.
ET-1 was a more potent constrictor than 5-HT in all three blood vessels (Figure 2). The sensitivity of the saphenous vein graft to ET-1 was not different to that of the control saphenous vein, however, ten times more ET-1 was required to contract the atherosclerotic coronary artery than either of the saphenous vein preparations (Table 1). There was no difference in the maximum response to ET-1 in the control and graft saphenous veins expressed as a percentage of the response to KCl (50 mM), but these two were significantly greater than that for ET-1 in diseased coronary artery (Student's two-tailed t-test, P<0.05). (Table 1, Figure 2). Similar data were obtained with 5-HT. EC50 values and maximum responses in saphenous vein graft and control were not different but higher concentrations of this agonist were required to contract the coronary artery and the maximum response in this artery was significantly depressed compared to the vein preparations (Student's two-tailed t-test, P<0.05) (Figure 2).
Figure 2.

Cumulative concentration-response curves to (a) ET-1 and (b) 5-HT in saphenous vein graft (SVgraft), control saphenous vein (SV) and atherosclerotic coronary artery (CA). Agonist responses are expressed as a percentage of the maximal contraction to KCl (50 mM) and are the means±s.e.mean (n=3–15).
Table 1.
.Potency of ET receptor agonists in in vitro preparations of saphenous vein (SV) graft, control saphenous vein and atherosclerotic coronary artery (CA)

Characterization of vasoconstrictor ET receptors
The relative potencies of the ET peptides and related compounds (Table 1) were used to determine the receptor subtypes responsible for vasoconstriction in atherosclerotic saphenous vein grafts and coronary arteries compared to non-diseased saphenous veins. In each of the three vessels ET-1 was more potent than ET-3. ET-1 was effective in the nanomolar range whilst the concentration-response curves to ET-3 were incomplete at 700 nM. Where tested, the non-selective endothelin agonist S6b was approximately four times less potent than ET-1 but elicited the same maximum response as ET-1. The responses obtained with the ETB-selective agonist S6c were more variable. No response to S6c was obtained in any of the diseased coronary artery preparations investigated. In the saphenous vein graft and control saphenous vein responses to S6c were observed in 65 and 30% of vessels respectively. However where responses to S6c were elicited, although potent, the maximum response was less than 25% of that to ET-1 in the same vessel (Figure 3).
Figure 3.

Cumulative concentration-response curves to ET receptor agonists in (a) saphenous vein graft, (b) control saphenous vein and (c) atherosclerotic coronary artery. Agonist responses are expressed as a percentage of the maximal contraction to KCl (50 mM) and are the means±s.e.mean (n=3–15).
To further investigate the vasoconstrictor ET receptors in these blood vessels we determined the ability of an ETA-selective antagonist, PD156707, to block the response to ET-1. In the presence of 100 nM PD156707 the concentration response curves to ET-1 were displaced to the right in saphenous vein graft, control saphenous vein and control coronary arteries which were obtained from patients other than those from whom the saphenous vein grafts were obtained and whom were diagnosed with either ischaemic heart disease or cardiomyopathy (Figure 4). The pKb values for PD156707 were 8.05±0.23 (n=6), 8.77±0.29 (n=5) and 7.94±0.13 (n=7) respectively, which are comparable to values reported for antagonism by this compound of ETA receptors in animal vasculature (Reynolds et al., 1995). Importantly there was no evidence that any part of the ET-1 response was resistant to this antagonist in either the diseased or control tissues.
Figure 4.

Antagonism of ET-1 by 100 nM PD156707 in (a) saphenous vein graft, (b) control saphenous vein and (c) control coronary artery. ET-1 responses are expressed as a percentage of the response to KCl (50 mM) and are the means±s.e.mean (n=5–7).
Potency of ET-1 with graft ‘age'
The potency of ET-1 determined in each of the eight graft vein specimens was correlated (Pearson) against the number of years since bypass surgery (i.e. graft age). A negative correlation (r=−0.82, P<0.02) was obtained with the sensitivity of saphenous vein graft increasing with the age of the graft (Figure 5).
Figure 5.
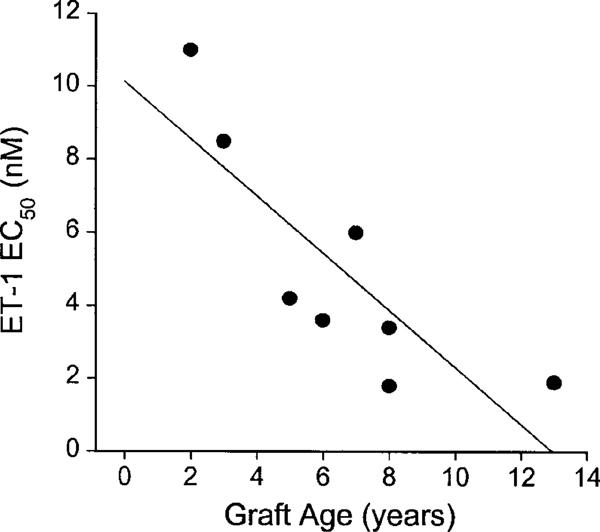
Influence of graft age (years) on sensitivity of grafts from individual patients (n=8) to ET-1 (EC50 nM). A significant correlation (Pearson; r=−0.82; P<0.02) was obtained.
Discussion
Autoradiographical visualization of ET receptor subtypes in sections of human saphenous vein graft graphically demonstrated the presence of ET receptors over the medial smooth muscle layer and the absence (or very low expression) of receptors over the thickened intimal smooth muscle layer. In the media there was no change in the relative density of the two receptor subtypes in diseased graft saphenous vein compared to control vessels. This was consistent with findings in the atherosclerotic human coronary artery (Bacon et al., 1996; Russell et al., 1997) which showed no evidence for up-regulation of smooth muscle ETB receptors in disease. In a rabbit model, both ETB mRNA and S6c-mediated contractile responses were actually reduced in saphenous vein grafts compared to native veins (Eguchi et al., 1997). Our data were from grafts that had failed after a number of years, however, alterations in receptor expression in the earlier stages of the disease process may have contributed to earlier graft failure. Such temporal receptor alterations have been noted in the arteries of rats in which proliferation has been initiated by balloon catheterization. An increase in receptor message and protein was observed over a few days and returned towards normal levels after a matter of weeks (Wang et al., 1996; Viswanathan et al., 1997).
Whilst we found no difference in the ratio of the two subtypes in control and graft saphenous vein this did not preclude functional alterations in agonist efficacy. However, characterization of the ET receptors responsible for vasoconstriction in saphenous vein graft clearly showed that the ET peptides mediate their effects through the ETA receptor. There was, therefore, no change in agonist activity profile observed in graft saphenous vein compared to the control saphenous vein. Thus, as determined by the autoradiographical data, no up-regulation of the ETB constrictor response was observed. The agonist data was confirmed using an ETA receptor antagonist, PD156707, which we had previously shown to exhibit high selectivity and affinity for human ETA receptors (Maguire et al., 1997). PD156707 blocked the response to ET-1 in all three blood vessels with the estimated pKb values in the range expected for the antagonism of the ETA subtype.
We did not find ET receptors on proliferated smooth muscle cells of the intima of either saphenous vein graft or atherosclerotic coronary artery (Bacon et al., 1996). If migrating and proliferating medial smooth muscle cells, under the influence of locally derived factors, form the thickened intimal layer then apparently down regulation of ET receptor expression has occurred. In some investigations, ETA receptor antagonists have been shown to reduce or prevent vascular smooth muscle cell proliferation both in vitro (Alberts et al., 1994; Kanse et al., 1995; Zamora et al., 1996) and in vivo (Ferrer et al., 1995; Chen et al., 1997). Thus proliferation is driven by activation of the ETA receptor. Why smooth muscle cells, which are thought to have undergone this process, should then lose the receptors that are responsible is not clear. One possibility is that released ET-1, which is increased in proliferating smooth muscle cells both in culture (Haug et al., 1996) or in tissue from atherosclerotic human blood vessels (Zeiher et al., 1995; Bacon et al., 1996; Brody et al., 1996) may bind to adjacent receptors in the intima and effectively mask their presence. However this is unlikely as in the media of mammary arteries from hypertensives high, homogenous levels of ETA receptor mRNA were identified, but in the thickened intima levels of mRNA were very low (Hasegawa et al., 1994). An alternative hypothesis is that as these cells change from the contractile phenotype to the synthetic phenotype (Campbell et al., 1988) receptors involved in vasoconstriction become redundant and are down-regulated. Indeed this has been demonstrated in rabbit aortic vascular smooth muscle cells that express ETA receptors. Subcultures of these smooth muscle cells show an alteration in phenotype which changes from contractile to synthetic and this is associated with a 10 fold reduction in the density of [125I]-ET-1 binding sites (Seradeil-Le Gal et al., 1991).
We found a tendency for the contractile response to a maximum concentration of KCl (50 mM) to be greater in the diseased saphenous vein and coronary artery than in the control saphenous vein. Adaptive changes would be expected in the wall structure and dynamics of saphenous vein that is grafted into the higher pressure arterial system. However it is clear from histological analyses that this does not represent true morphological arterialization, rather fibrotic thickening of the media combined with smooth muscle proliferation and extracellular connective tissue deposition of the intima (Bulkley & Hutchins, 1977; Lie et al., 1977; Atkinson et al., 1985). In contrast there did not appear to be any alteration in the maximum response to either ET-1 or 5-HT in diseased compared to non-diseased saphenous vein. This might be expected, at least for ET-1, as we have shown that the proliferated smooth muscle cells of the thickened intima in saphenous vein grafts do not have detectable ET receptors and therefore would not contribute to vasoconstriction. A similar observation has previously been made by O'Neil and colleagues (1994) who reported no significant difference between the maximum responses to ET-1 (as %KCl) in short (<1 year) and long (8–11 years) term saphenous vein grafts and control saphenous veins harvested following surgical distension. It was only when ET-1 responses were compared in control saphenous veins harvested before distension that a significant reduction in ET-1 maximum response was observed in graft vessels. In the present investigation distended veins were used as the control, as it is in this condition that they are grafted into the coronary circulation. The maximum for ET-1 and 5-HT were significantly lower in coronary artery compared to both of the saphenous vein groups. This may be due to differences in receptor density or coupling efficiency for transmitters in human arteries compared to veins.
We found that the control saphenous vein was more sensitive than the diseased coronary artery to both ET-1 and 5-HT and that this sensitivity was retained by the thickened saphenous vein graft. Thus saphenous veins grafted into the coronary vasculature are particularly vulnerable to the constricting effects of circulating and locally released vasoconstrictor mediators. Increased concentrations of ET have been measured not only in the plasma of patients with coronary artery disease (Yasuda et al., 1990; Lerman et al., 1991), but also within atheromatous plaques (Zeiher et al., 1995; Bacon et al., 1996). Additionally there are further transient increases of plasma ET-1 during cardiopulmonary bypass (Hynynen et al., 1992; Rammos et al., 1996). Increased ET-1 levels may result in direct constriction of the venous smooth muscle, or may potentiate the constricting effects of other mediators such as 5-HT (Chester et al., 1992). Interestingly we found that the sensitivity of the vein to ET-1 positively correlated with the time that had lapsed since grafting.
Our findings that the ETA receptor is responsible for the profound vasoconstrictor responses of the aortocoronary saphenous vein graft to ET-1 both at the time of operation and in the late stages of disease has implications for the use of antagonists selective for this subtype in the management of patients. At present, during the harvesting and preparation of the saphenous vein, directly acting vasodilators such as papaverine, sodium nitroprusside and nifedipine are used to prevent or reduce spasm and thus maintain endothelial integrity (LoGerfo et al., 1984; Roubos et al., 1995). If ET does contribute substantially to spasm then these drugs may not be completely effective. Calcium channel antagonists such as nifedipine are only partially effective in blocking vasoconstrictor effects of ET-1 (Stork & Cocks, 1994) as this agonist mobilizes intracellular calcium stores in addition to stimulating extracellular calcium influx (Ushio-Fukai et al., 1995). It has also been suggested that, in vitro, contractions to ET-1 are not fully reversed by sodium nitroprusside in vein grafts, whereas they are in arterial graft vessels such as mammary artery and gastroepiploic artery (Uydes-Dogan et al., 1996). ETA antagonism might, therefore, prove more effective for the prevention of perioperative graft vasospasm than currently available therapies. In the long term, the vein grafts are particularly vulnerable to the increased local production of ET-1 in coronary artery disease. This may lead to constriction of the graft that, if superimposed on a thickened intima or atherosclerotic plaque, will reduce the already narrowed lumen, further compromising blood flow to the myocardium. If in addition to blocking the unwanted vasoconstrictor effect of ET-1, the efficacy of ETA antagonists to prevent intimal hyperplasia of human blood vessels can also be demonstrated, then this class of compounds may also become invaluable in increasing the long term patency of the saphenous vein graft.
Acknowledgments
We would like to thank Rhoda Kuc for her excellent technical assistance. We are grateful to Jean Chadderton and the theatre and consultant staff of Papworth Hospital, Cambridgeshire, U.K. Supported by grants from the British Heart Foundation, Isaac Newton Trust and Royal Society.
Abbreviations
- BSA
bovine serum albumin
- CABG
coronary artery bypass graft
- ET
endothelin
- 5-HT
5-hydroxytryptamine
- OD
optical density
- S6b
sarafotoxin 6b
- S6c
sarafotoxin 6c
References
- AKAR F., UYDES B.S., AYRANCIOGLU K., YENER A., ASLAMACI S., ARSAN M., TÖRÜNER A., KANZIK I. Endothelial function of human gastroepiploic artery in comparison with saphenous vein. Cardiovasc. Res. 1994;28:500–504. doi: 10.1093/cvr/28.4.500. [DOI] [PubMed] [Google Scholar]
- ALBERTS G.F., PEIFLEY K.A., JOHNS A., KLEHA J.F., WINKLES J.A. Constitutive endothelin-1 overexpression promotes smooth muscle cell proliferation via an external autocrine loop. J. Biol. Chem. 1994;269:10112–10118. [PubMed] [Google Scholar]
- ATKINSON J.B., FORMAN M.B., VAUGHN W.K., ROBINOWITZ M., MCALLISTER H.A., VIRMANI R. Morphologic changes in long term saphenous vein bypass grafts. Chest. 1985;88:341–348. doi: 10.1378/chest.88.3.341. [DOI] [PubMed] [Google Scholar]
- BACON C.R., CARY N.R.B., DAVENPORT A.P. Endothelin peptide and receptors in human atherosclerotic coronary artery and aorta. Circ. Res. 1996;79:794–801. doi: 10.1161/01.res.79.4.794. [DOI] [PubMed] [Google Scholar]
- BATTISTINI B., CHAILLER P., D'ORLÉANS-JUSTE P., BRIÈRE N., SIROIS P. Growth regulatory properties of endothelins. Peptides. 1993;14:385–399. doi: 10.1016/0196-9781(93)90057-n. [DOI] [PubMed] [Google Scholar]
- BAX W.A., BOS E., SAXENA P.R. Heterogeneity of endothelin/sarafotoxin receptors mediating contraction of the human isolated saphenous vein. Eur. J. Pharmacol. 1993;239:267–268. doi: 10.1016/0014-2999(93)91010-k. [DOI] [PubMed] [Google Scholar]
- BOURASSA M.G., CAMPEAU L., LESPÉRANCE J., GRONDIN C.M. Changes in grafts and coronary arteries after saphenous vein aortocoronary bypass surgery: results at repeat angiography. Circulation. 1982;65 Suppl II:90–97. doi: 10.1161/01.cir.65.7.90. [DOI] [PubMed] [Google Scholar]
- BRODY J.I, , CAPUZZI D.M., FINK G.B. In situ endothelin in coronary artery disease. Angiology. 1996;47:1027–1032. doi: 10.1177/000331979604701101. [DOI] [PubMed] [Google Scholar]
- BULKLEY B.H., HUTCHINS G.M. Accelerated ‘atherosclerosis': A morphologic study of 97 saphenous vein coronary artery bypass grafts. Circulation. 1977;55:163–169. doi: 10.1161/01.cir.55.1.163. [DOI] [PubMed] [Google Scholar]
- CAMPBELL G.R., CAMPBELL J.H., MANDERSON J.A., HORRIGAN S., RENNICK R.E. Arterial smooth muscle. A multifunctional mesenchymal cell. Arch. Pathol. Lab. Med. 1988;112:977–986. [PubMed] [Google Scholar]
- CAMPEAU L., ENJALBERT M., LESPÉRANCE J., VAISLIC C., GRONDIN C.M., BOURASSA M.G. Atherosclerosis and late closure of aortocoronary saphenous vein grafts: sequential angiographic studies at 2 weeks, 1 year, 5 to 7 years, and 10 to 12 years after surgery. Circulation. 1983;68:1–7. [PubMed] [Google Scholar]
- CHEN S.J., CHEN Y.F., OPGENORTH T.J., WESSALE J.L., MENG Q.C., DURAND J., DICARLO V.S., OPARIL S. The orally active nonpeptide endothelin-A receptor antagonist A-127722 prevents and reverses hypoxia-induced pulmonary hypertension and pulmonary vascular remodelling in Sprague-Dawley rats. J. Cardiovasc. 1997;29:713–725. doi: 10.1097/00005344-199706000-00003. [DOI] [PubMed] [Google Scholar]
- CHESEBRO J.H., CLEMENTS I.P., FUSTER V., ELVEBACK L.R., SMITH H.C., BARDSLEY W.T., FRYE R.L., HOLMES D.R., VLIETSTRA R.E., PLUTH J.R., WALLACE R.B., PUGA F.J., ORSZULAK T.A., PIEHLER J.M., SCHAFF H.V., DANIELSON G.K. A platelet-inhibitor-drug trial in coronary-artery bypass operations: Benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency. N. Engl. J. Med. 1982;307:73–78. doi: 10.1056/NEJM198207083070201. [DOI] [PubMed] [Google Scholar]
- CHESTER A.H., O'NEIL G.S., ALLEN S.P., LUU T.N., TADJKARIMI S., YACOUB M.H. Effect of endothelin on normal and diseased human coronary arteries. Eur. J. Clin. Invest. 1992;22:210–213. doi: 10.1111/j.1365-2362.1992.tb01828.x. [DOI] [PubMed] [Google Scholar]
- COSTELLO K.B., STEWART D.J., BAFFOUR R. Endothelin is a potent constrictor of human vessels used in coronary revascularization surgery. Eur. J. Pharmacol. 1990;186:311–314. doi: 10.1016/0014-2999(90)90450-k. [DOI] [PubMed] [Google Scholar]
- DAVENPORT A.P., BERESFORD I.J.M., HALL M.D., HILL R.G., HUGHES J.Quantitative autoradiography Molecular Neuroanatomy 1988IIIAmsterdam: Elsevier; 121–145.ed. van Leeuwen, F.W., Buijs, R.M., Pool, C.W., Pach, O. pp [Google Scholar]
- DAVENPORT A.P., KUC R.E., FITZGERALD F., MAGUIRE J.J., BERRYMAN K., DOHERTY A.M. [125I] PD151242: a selective radioligand for human ETA receptors. Br. J. Pharmacol. 1994;111:4–6. doi: 10.1111/j.1476-5381.1994.tb14015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT A.P., O'REILLY G., KUC R.E. Endothelin ETA and ETB mRNA and receptors expressed by smooth muscle in the human vasculature: majority of the ETA sub-type. Br. J. Pharmacol. 1995;114:1110–1116. doi: 10.1111/j.1476-5381.1995.tb13322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOHERTY A.M., PATT W.C., EDMUNDS J.J., BERRYMAN K.A., REISDORPH B.R., PLUMMER M.S., SHAHRIPOUR A., LEE C., CHENG X.-M., WALKER D.M., HALEEN S.J., KEISER J.A., FLYNN M.A., WELCH K.M., HALLAK H., TAYLOR D.G., REYNOLDS E.E. Discovery of a novel series of orally active non-peptide endothelin-A (ETA) receptor-selective antagonists. J. Med. Chem. 1995;38:1259–1263. doi: 10.1021/jm00008a002. [DOI] [PubMed] [Google Scholar]
- EGUCHI D., NISHIMURA J., KOBAYASHI S., KOMORI K., SUGIMACHI K., KANAIDE H. Down-regulation of endothelin B receptors in autogenous saphenous veins grafted into the arterial circulation. Cardiovasc. Res. 1997;35:360–367. doi: 10.1016/s0008-6363(97)00103-x. [DOI] [PubMed] [Google Scholar]
- FERRER P., VALENTINE M., JENKINSWEST T., WEBER H., GOLLER N.L., DURHAM S.K., MOLLOY C.J., MORELAND S. Orally-active endothelin antagonist BMS-182874 suppresses neointimal development in balloon-injured rat carotid arteries. J. Cardiovasc. Pharmacol. 1995;26:908–915. doi: 10.1097/00005344-199512000-00009. [DOI] [PubMed] [Google Scholar]
- FUSTER V., CHESEBRO J.H. Role of platelets and platelet inhibitors in aortocoronary vein-graft disease. Circulation. 1986;73:227–232. doi: 10.1161/01.cir.73.2.227. [DOI] [PubMed] [Google Scholar]
- GOLDMAN S., COPELAND J., MORITZ T., HENDERSON W., ZADINA K., OVITT T., DOHERTY J., READ R., CHESLER E., SAKO Y., LANCASTER L., EMERY R., SHARMA G.V.R.K., JOSA M., PACOLD I., MONTOYA A., PARIKH D., SETHI G., HOLT J., KIRKLIN J., SHABETAI R., MOORES W., ALDRIDGE J., MASUD Z., DEMOTS H., FLOTEN S., HAAKENSON C., HARKER L.A. Improvement in early saphenous vein graft patency after coronary artery bypass surgery with antiplatelet therapy: Results of a Veterans Administration Cooperative Study. Circulation. 1988;77:1324–1332. doi: 10.1161/01.cir.77.6.1324. [DOI] [PubMed] [Google Scholar]
- HASEGAWA K., FUJIWARA H., DOYAMA K., INADA T., OHTANI S., FUJIWARA T., HOSODA K., NAKAO K., SASAYAMA S. Endothelin-1-selective receptor in the arterial intima of patients with hypertension. Hypertension. 1994;23:288–293. doi: 10.1161/01.hyp.23.3.288. [DOI] [PubMed] [Google Scholar]
- HAUG C., VOISARD R., LENICH A., BAUR R., HÖHER M., OSTERHUES H., HANNEKUM A., VOGEL U., MATTFELDT T., HOMBACH V., GRÜNERT A. Increased endothelin release by cultured human smooth muscle cells from atherosclerotic coronary arteries. Cardiovasc. Res. 1996;31:807–813. doi: 10.1016/0008-6363(96)00012-0. [DOI] [PubMed] [Google Scholar]
- HYNYNEN M., SAIJONMAA O., TIKKANEN I., HEINONEN J., FYHRQUIST F. Increased plasma endothelin immunoreactivity during cardiopulmonary bypass: A preliminary observation. J. Thoracic Cardiovasc. Surg. 1992;103:1024–1025. [PubMed] [Google Scholar]
- IVERT T., HUTTUNEN K., LANDOU C., BJÖRK V.O. Angiographic studies of internal mammary artery grafts 11 years after coronary artery bypass grafting. J. Thoracic Cardiovasc. Surg. 1988;96:1–12. [PubMed] [Google Scholar]
- KANSE S.M., WIJELATH E., KANTHOU C., NEWMAN P., KAKKAR V.V. The proliferative responsiveness of human vascular smooth-muscle cells to endothelin correlates with endothelin receptor density. Lab. Invest. 1995;72:376–382. [PubMed] [Google Scholar]
- LERMAN A., EDWARDS B.S., HALLETT J.W., HEUBLEIN D.M., SANDBERG S.M., BURNETT J.C., JR Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. New. Engl. J. Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- LIE J.T., LAWRIE G.M., MORRIS G.C. Aortocoronary bypass saphenous vein graft atherosclerosis. Am. J. Cardiology. 1977;40:907–914. doi: 10.1016/0002-9149(77)90041-8. [DOI] [PubMed] [Google Scholar]
- LOGERFO F.W., HAUDENSCHILD C.D., QUIST W.C. A clinical technique for prevention of spasm and preservation of endothelium in saphenous vein grafts. Arch. Surg. 1984;119:1212–1214. doi: 10.1001/archsurg.1984.01390220086020. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F., YANG Z., TSCHUDI M., VON SEGESSER L., STULZ P., BOULANGER C., SIBENMANN R., TURINA M., BÜHLER F.R. Interaction between endothelin-1 and endothelium-derived relaxing factor in human arteries and veins. Circ. Res. 1990;66:1088–1094. doi: 10.1161/01.res.66.4.1088. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., DAVENPORT A.P. ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br. J. Pharmacol. 1995;115:191–197. doi: 10.1111/j.1476-5381.1995.tb16338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGUIRE J.J., DAVENPORT A.P. Endothelin (ET) receptor pharmacology in human saphenous vein grafts. The Pharmacologist. 1997;39:78. [Google Scholar]
- MAGUIRE J.J., DAVENPORT A.P. PD156707: A potent antagonist of endothelin-1 in human diseased coronary arteries and vein grafts. J. Cardiovasc. Pharmacol. 1998;31 Suppl 1:S239–S240. doi: 10.1097/00005344-199800001-00067. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., DAVENPORT A.P. Affinity and selectivity of PD156707, a novel nonpeptide endothelin antagonist, for human ETA and ETB receptors. J. Pharmacol. Exp. Therap. 1997;280:1102–1108. [PubMed] [Google Scholar]
- MASOOD I., PORTER K.E., LONDON N.M.J., PRINGLE J.H. Endothelin-1 expression in vein graft stenosis. J. Vasc. Surg. 1996;24:901–902. doi: 10.1016/s0741-5214(96)70030-7. [DOI] [PubMed] [Google Scholar]
- MOLENAAR P., KUC R.E, DAVENPORT A.P. Characterization of two new ETB selective radioligands, [125I]-BQ3020 and [125I]-[Ala1,3,11,15]ET-1 in human heart. Br. J. Pharmacol. 1992;107:637–639. doi: 10.1111/j.1476-5381.1992.tb14498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'NEIL G.S., CHESTER A.D., SCHYNS C.J., TADJKARIMI S., BORLAND J.A.A., YACOUB M.H. Effect of surgical preparation and arterialization on vasomotion of human saphenous vein. J. Thoracic Cardiovasc. Surg. 1994;107:699–706. [PubMed] [Google Scholar]
- RAMMOS K.ST., KOULLIAS G.J., HATZIBOUGIAS J.D., ARGYRAKIS N.P., PANAGOPOULOS P.G. Plasma endothelin-1 levels in adult patients undergoing coronary revascularization. Cardiovasc. Surg. 1996;4:808–812. doi: 10.1016/s0967-2109(96)00036-1. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E.E., KEISER J.A., HALEEN S.J., WALKER D.M., OLSZEWSKI B., SCHROEDER R.L., TAYLOR D.G., HWANG O., WELCH K.M., FLYNN M.A., THOMPSON D.M., EDMUNDS J.J., BERRYMAN K.A., PLUMMER M., CHENG X.-M., PATT W.C., DOHERTY A.M. Pharmacological characterization of PD156707, an orally active ETA receptor antagonist. J. Pharmacol. Exp. Ther. 1995;273:1410–1417. [PubMed] [Google Scholar]
- ROSSITER S.J., BRODY W.R., KOSEK J.C., LIPTON M.J., ANGELL W.W. Internal mammary artery versus autogenous vein for coronary bypass graft. Circulation. 1974;50:1236–1243. doi: 10.1161/01.cir.50.6.1236. [DOI] [PubMed] [Google Scholar]
- ROUBOS N., ROSENFELDT F.L., RICHARDS S.M., CONYERS R.A.J., DAVIS B.B. Improved preservation of saphenous vein grafts by the use of glyceryl trinitrate-verapamil solution during harvesting. Circulation. 1995;92 Suppl II:31–36. doi: 10.1161/01.cir.92.9.31. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., SKEPPER J.N., DAVENPORT A.P. Detection of endothelin receptors in human coronary artery vascular smooth muscle cells but not endothelial cells by using electron microscope autoradiography. J. Cardiovasc. Pharmacol. 1997;29:820–826. doi: 10.1097/00005344-199706000-00017. [DOI] [PubMed] [Google Scholar]
- SERRADEIL-LE GAL C., HERBERT J.M., GARCIA C., BOUTIN M., MAFFRAND J.P. Importance of the phenotypic state of vascular smooth muscle cells on the binding and the mitogenic activity of endothelin. Peptides. 1991;12:575–579. doi: 10.1016/0196-9781(91)90104-w. [DOI] [PubMed] [Google Scholar]
- STORK A.P., COCKS T.M. Pharmacological reactivity of human epicardial coronary arteries: phasic and tonic responses to vasoconstrictor agents differentiated by nifedipine. Br. J. Pharmacol. 1994;113:1093–1098. doi: 10.1111/j.1476-5381.1994.tb17108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USHIO-FUKAI M., NISHIMURA J., KOBAYASHI S., KANAIDE H. Endothelin-1 and endothelin-3 regulate differently vasoconstrictor responses of smooth muscle of the porcine coronary artery. Br. J. Pharmacol. 1995;114:171–179. doi: 10.1111/j.1476-5381.1995.tb14922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UYDES-DOGAN B.S., NEBIGIL M., S-ALSAMACI M.D., ONUK E., KANZIK L., AKBAR F. The comparison of vascular reactivities of arterial and venous grafts to vasodilators: management of graft spasm. Int. J. Cardiol. 1996;53:137–145. doi: 10.1016/0167-5273(95)02533-2. [DOI] [PubMed] [Google Scholar]
- VICTOR M.F., KIMBIRIS D., ISKANDRIAN A.S., MINTZ G.S., BEMIS C.E., PROCACCI P.M., SEGAL B.L. Spasm of a saphenous vein bypass graft. Chest. 1981;80:413–415. doi: 10.1378/chest.80.4.413. [DOI] [PubMed] [Google Scholar]
- VISWANATHAN M., DE OLIVEIRA A.M., JOHREN O, SAAVEDRA J.M. Increased endothelin ET(A) receptor expression in rat carotid arteries after balloon injury. Peptides. 1997;18:247–255. doi: 10.1016/s0196-9781(96)00285-9. [DOI] [PubMed] [Google Scholar]
- WANG X., DOUGLAS S.A., LOUDEN C., VICKERY-CLARK L.M., FEUERSTEIN G.Z., OLSTEIN E.H. Expression of endothelin-1, endothelin-3, endothelin-converting enzyme, and endothelin-A and endothelin-B receptor mRNA after angioplasty-induced neointimal formation in the rat. Circ. Res. 1996;78:322–328. doi: 10.1161/01.res.78.2.322. [DOI] [PubMed] [Google Scholar]
- WHITE D.G., GARRATT H., MUNDIN J.W., SUMNER M.J., VALLANCE P.J., WATTS I.S. Human saphenous vein contains both endothelin ETA and ETB contractile receptors. Eur. J. Pharmacol. 1994;257:307–310. doi: 10.1016/0014-2999(94)90144-9. [DOI] [PubMed] [Google Scholar]
- YASUDA M., KOHNO M., TAHARA A., ITIGANE H., TODA I., AKIOKA K., TERAGAKI M., OKU H., TAKEUCHI K., TAKEDA T. Circulating immunoreactive endothelin in ischaemic heart disease. Am. Heart J. 1990;119:801–806. doi: 10.1016/s0002-8703(05)80315-1. [DOI] [PubMed] [Google Scholar]
- ZAMORA M.R., STELZNER T.J., WEBB S., PANOS R.J., RUFF L.J., DEMPSEY E.C. Overexpression of endothelin-1 and enhanced growth of pulmonary artery smooth muscle cells from fawn-hooded rats. Am. J. Physiol. 1996;14:L101–L109. doi: 10.1152/ajplung.1996.270.1.L101. [DOI] [PubMed] [Google Scholar]
- ZEIHER A.M., GOEBEL H., SCHÄCHINGER V., IHLING C. Tissue endothelin-1 immunoreactivity in the active coronary atherosclerotic plaque. Circulation. 1995;91:941–947. doi: 10.1161/01.cir.91.4.941. [DOI] [PubMed] [Google Scholar]


