Abstract
To study the involvement of cytokines and their corresponding autoantibodies (Aabs) in inflammatory mechanisms in patients with lower respiratory tract infections, blood samples were taken from patients at the time of admission to the hospital and before treatment. Cell-released capturing enzyme-linked immunosorbent assay was used to measure the levels of gamma interferon (IFN-γ) and interleukin-4 (IL-4) produced spontaneously by peripheral mononuclear cells (PMNC). ELISA was used to measure Aabs to these cytokines in sera. The levels of both cytokines were inversely related to the levels of their corresponding Aabs. While a high level of IFN-γ was observed together with a low level of anti-IFN-γ Aab, decreased IL-4 levels were observed with increased levels of Aabs to IL-4. Immunoglobulins were purified, digested to obtain Fab fragments, and tested for specificity and cross-reactivity. The Aabs and their Fab fragments were tested in cytokine biological assays and showed neutralizing effects. Our data demonstrated increased levels of the proinflammatory cytokine IFN-γ and decreased release of the anti-inflammatory cytokine IL-4 during early presentation of lower respiratory tract infection. The levels of these cytokines were inversely related to the levels of their corresponding Aabs that exhibited regulatory effects on the cytokine biological function in vitro.
Respiratory tract infections are the most common infectious disorders. A significant proportion of all infectious respiratory tract diseases are acute infections of the lower respiratory tract, characterized by fever and other respiratory symptoms. A diverse variety of microorganisms can cause these infections, but bacteria are the most common (13). Antigen-specific activation of T cells can effectively control, be irrelevant to, or even exacerbate infection by an infectious agent. The resultant effect of T-cell activation depends upon the subsets of T cells activated and the cytokines produced (15). Normally, alveolar macrophages are the main cells that respond to bacteria reaching lower airways, but if the microbial inoculum is too high or too virulent, these cells recruit polymorphonuclear neutrophils into alveoli from vascular compartments through secretion of certain cytokines such as interleukin-8 (IL-8) and tumor necrosis factor alpha (TNF-α) (14).
Unlike endocrine hormones, the majority of cytokines normally act locally in a paracrine or even autocrine fashion and rarely persist in the circulation system, but nonlymphoid cells can be triggered by bacterial products to release cytokines which may detected in the bloodstream often to the detriment of the host (20). After production, cytokines are targeted by different regulators at different stages: at the gene activation stage, during secretion, and in circulation through binding to soluble receptors and autoantibodies (Aabs), as well as at the level of cytokine-target cell interaction. Any hereditary or acquired disturbances in these complex regulatory processes may contribute to the pathophysiology of many infectious inflammatory diseases (1, 4, 8).
The recent demonstration of Aabs to cytokines in healthy individuals, as well as in patients with inflammatory disorders, suggests that anticytokine antibodies may be involved in physiological and disease processes (3). Antibodies to TNF-β, TNF-α, gamma interferon (IFN-γ), and IL-4 have been reported in sera of AIDS patients (17). Also, Aabs to cytokines were reported in sera of healthy individuals, which suggests further complexities in the way that cytokine function is regulated in vivo (21). In the present study, the induction of the proinflammatory cytokine IFN-γ and the anti-inflammatory cytokine IL-4 and their Aabs were examined at the time of admission to the hospital and before the start of treatment. An inverse correlation between the levels of both cytokines and their Aabs is presented.
MATERIALS AND METHODS
Patients.
Venous blood samples were taken from patients coming to the outpatient department for acute infectious diseases at Huddinge Hospital, Stockholm, Sweden, during the autumn of 1996, September to December. The patients included in this study had a diagnosis of a bacterial pneumonia with an acute onset. There were 19 patients collectively, 10 women and 9 men, with a mean age of 56.7 years (range, 34 to 85). All patients had acute onset of fever and chills, most with cough, some with pleuritic chest pain, and some with shortness of breath. The length of time the patient had these symptoms before going to the hospital ranged from a few hours up to 2 weeks, with an average of 3 days. Seven patients had other, underlying diseases for which they received treatment (i.e., epilepsy, angina pectoris, diabetes mellitis and prostatic cancer, goiter, cardiac atrial flutter and hypertonia, an autoimmune disorder suspected to be rheumatoid arthritis, and chronic bronchitis). On admission to the hospital, 18 of the patients had a pulmonary infiltrate on chest X-ray, consistent with acute pneumonia. One patient had a pleural effusion but no pneumonic infiltrate. Blood chemistry showed elevated C-reactive protein levels in all patients, a mean level of 173 g/liter (range, 21 to 260), and a mean leukocytosis of 13.2 × 109/liter (range, 6.7 to 25.0). An etiologic agent was isolated in two cases, one with pneumococcus in nasopharyngeal culture and one patient with a positive serology result for Mycoplasma pneumoniae. In the acute stage, most patients were treated with penicillin intravenously. Patients with suspected atypical etiology were given erythromycin. There were no treatment failures. Fifteen colleagues from our department were tested the same way and considered healthy controls.
Preparation of PMNC.
Peripheral blood mononuclear cells (PMNC) were obtained by density gradient centrifugation on lymphoprep (Nyegaard, Oslo, Norway). The cells at interphase were collected, washed three times with Dulbecco’s modified Eagle medium (Gibco BRL, Paisley, United Kingdom) containing antibiotics, washed once with phosphate-buffered saline (PBS), and resuspended in complete culture medium. The cells were counted by phase-contrast microscopy and adjusted to a final concentration of 106 cells per ml in medium with 50 IU of penicillin per ml, 50 μg of streptomycin per ml, 1% essential amino acids (10 ml/liter), and 5% heat-inactivated fetal calf serum (Gibco). Cell viability measured by trypan blue exclusion always exceeded 95%.
Detection of IFN-γ and IL-4.
To detect cellular production of IFN-γ and IL-4, a cell-released capturing enzyme-linked immunosorbent assay (ELISA) was introduced as described previously (2). The assay is based on capturing the cytokine at the time of release from the cells by a specific capturing monoclonal antibody (MAb). In order to detect the secreted cytokine in this assay, enzyme immunoassay or radioimmunoassay flat-bottom, high-binding plates (Costar) were coated with 100 μl of anti-IFN-γ or anti-IL-4 MAbs (Mabtech, Stockholm, Sweden) diluted 1:200 in carbonate bicarbonate buffer (pH 9.6) at 4°C overnight. After three washes with PBS, the wells were blocked with 100 μl per well of 2% bovine serum albumin (BSA) for 90 min in room temperature. After repeated washings with PBS, suspensions of peripheral blood lymphocytes were applied in 200 μl to obtain a final concentration of 2 × 105 cells per well. This cell number was selected after performing titration experiments to attain the optimal cell density for the assay. Cultures either were not stimulated or received phytohemagglutinin (Difco, Detroit, Mich.) at a final concentration of 0.1 μg/ml. After 48 h of incubation at 37°C in a humidified atmosphere of 5% CO2, cells were removed by flicking the plate, followed by repeated washings in PBS. To detect any captured IFN-γ or IL-4, biotinylated detecting MAb (Mabtech) diluted 1:2,000 in PBS containing 0.5% Tween 20 (PBST) and 2% BSA (PBSAT) was added to the wells. After 1 h of incubation at 37°C and 10 washes, 100 μl of avidin-biotin alkaline phosphatase complex (ABC-AP; Vector Laboratories, Burlingame, Calif.) diluted 1:100 in PBS was added for 30 min. Unbound ABC-AP was removed by five consecutive washes with PBS, and 100 μl of freshly prepared enzyme substrate solution was added to each well. Absorbance was measured after 15 min of incubation in the dark in a 405 Multiscan photometer (mcc/340; Labsystem, Helsinki, Finland). Standard curves were plotted, and IL-4 and IFN-γ concentrations in samples were determined by interpolation from the standard curve.
Detection of Aabs to IFN-γ and IL-4.
Anticytokine Aabs in sera from the 19 patients were detected by ELISA. Flat-bottom 96-well polystyrene plates (Polysorp F96; Nunc, Glostrup, Denmark) were coated with 0.1 μg of either recombinant human IFN-γ or IL-4 per ml of carbonate bicarbonate buffer (pH 9.6) overnight at room temperature. The next day, the cells were washed twice with PBST and saturated with PBS containing 5% BSA for 1 h at 37°C. After the cells were washed, sera were dispensed in the wells in 100-μl amounts at a 1:200 dilution in PBSAT. After 1 h of incubation at 37°C and five washes, 100 μl of biotinylated goat anti-human immunoglobulin M (IgM) and IgG (Sigma, St. Louis, Mo.) diluted in 1:2,000 in PBSAT were added. After another hour of incubation at 37°C and five washes, 100 μl of ABC-AP (Vector Laboratories) diluted 1:100 in PBS was added for 30 min. Unbound ABC-AP was removed by five consecutive washes with PBS, and 100 μl of freshly prepared enzyme substrate solution was added to each well. Absorbance was measured in a 405 Multiscan photometer (mcc/340; Labsystem). To detect possible non-cytokine-specific reactions due to impurities of the cytokine antigen or to polyclonal B-cell activation, the sera were tested by ELISA with ovalbumin as the antigen on solid phase; ELISA was run according to the same protocol and in parallel with the anti-cytokine Aab assay. The absorbance values obtained when testing the sera against ovalbumin were very low and were deducted from the absorbance value obtained by testing the sera against the cytokines, the resulting absorbances being used for quantification of the anticytokine Aab levels. In order to relatively quantify the Aabs, standard curves for anti-IFN-γ and anti-IL-4 (MabTech) were obtained simultaneously by incubating different known concentrations of each antibody for 60 min at room temperature in wells precoated with the corresponding cytokine. The procedure for developing the plate was continued as described above, and the absorbances measured from the standard concentration of the antibodies were used to plot the standard curves, using computer software. Thereafter, the absorbances obtained from the specimens, which correspond to Aab levels, were automatically converted to nanograms per milliliter by the computer from the standard curve.
To further control the specificity of the Aabs, Fab fragments of purified serum immunoglobulins were examined in parallel experiments and showed significant binding. Moreover, preabsorption of the sera with a recombinant cytokine (IFN-γ for example) inhibited the binding effects to this particular cytokine but not to IL-4 and vice versa.
Purification of immunoglobulins and preparation of Fab fragments.
Immunoglobulins were purified from sera as described previously (12). All sera were diluted 1:10 in PBS, and then 7.5 ml of a 36% Na2SO4 solution was added slowly with stirring. After incubation for 1 h at room temperature, the suspension was centrifuged for 20 min at 7,155 × g. The precipitate was redissolved in 20 ml of 0.12 M NaCl and reprecipitated with 15 ml of Na2SO4 solution. After 1 h, the suspension was recentrifuged for 20 min at 7,155 × g. The pellet was redissolved and dialyzed against phosphate buffer (10 mM phosphate, pH 7.6). Following dialysis, the retentate was passed through a column of DEAE-cellulose. The concentration of the emerging IgG was measured with a spectrophotometer. Depending on the concentration of the purified IgG, crystalline papain diluted 1:100 in 10 ml of PBS (10 mM phosphate [pH 7.3], 0.15 M NaCl) with 1 mM EDTA and 25 mM mercaptoethanol was added. The Fab fragments were further purified by ion-exchange chromatography with DEAE-cellulose. Furthermore, the digested fragments were run through an affinity column of insolubilized protein A to get rid off any other soluble factors that might contaminate the fragments.
Specificity and cross-reactivity.
The purified Fab fragments of the 19 sera were tested again by ELISA (described above). To exclude any cross-reactivity to various cytokines, preabsorption of the Fab fragments with the particular cytokines (IL-4 or IFN-γ) was performed and showed inhibition of the blocking effects of the Aabs in the biological assay for that particular cytokine but not in the biological assay for the other cytokine.
Biological assay for IL-4.
The proliferation assay was used to evaluate the effect of IL-4 on proliferation of PMNC in vitro by measuring [3H]thymidine incorporation in response to IL-4. Briefly, aliquots of 200 μl containing 2 × 106 PMNC per ml were applied into round-bottomed microtiter plates (Nunc). Separate cultures received either no stimulation or 20 ng of recombinant IL-4 in the presence or absence of different dilutions (1:10, 1:100, and 1:1,000) of sera pooled from patients 3 and 5. After 48 h of incubation, the cells were pulsed with [methyl-3H]thymidine (10 μl, 100 μCi/ml) (Amersham, Little Chalfont, United Kingdom) for another 12 h. After the cells were harvested, the [methyl-3H]thymidine uptake was measured by standard liquid scintillation counting techniques with a beta-counter.
Statistics.
The unpaired Student t test was used.
RESULTS
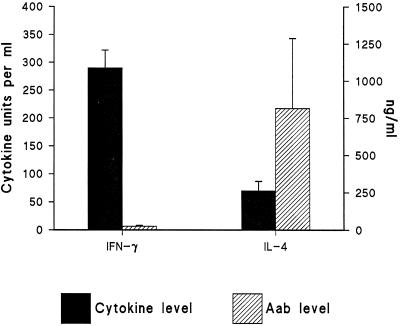
We selected the cytokines (IFN-γ and IL-4) in view of their potential immunoregulatory roles and their ability to induce a wide range of effects on many cells. Regardless of the different etiologies of infection, all patients had a history of acute onset of a lower respiratory tract infection, with fever and chills, most with cough. In all patients, the level of IFN-γ secreted was high compared to that of IL-4 of the same patients in the acute phase of the disease (Fig. 1).
FIG. 1.
IFN-γ and IL-4 levels and the levels of their corresponding Aabs. The left y axis shows the IFN-γ and IL-4 levels (in units per milliliter). The right y axis shows the anti-cytokine IgG Aab levels in sera of patients with lower respiratory tract infections (for relative quantification, see Materials and Methods). The data represent whole IgG binding. All samples were assayed in triplicate, and the mean ± standard deviation for each subject was first determined. Then, the means and standard deviations for all patients were calculated and are shown in the figure.
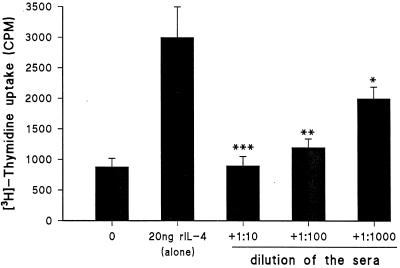
In all 19 patients included in this study, anti-IL-4 Aabs of IgG isotype showed marked increase in the acute phase of the disease (Fig. 1), as characterization of human anticytokine Aabs revealed that they are polyclonal in nature and belong almost exclusively to the IgG class (4, 19). Aabs to IFN-γ were almost undetectable. Anti-IL-4 Aabs showed high specificity to their corresponding cytokines. This specificity was demonstrated by the ligand binding to the Fab fragments of the immunoglobulins, combined with saturation analysis; i.e., Fab fragments of sera from patients 3 and 5 were preincubated with IL-4 overnight and tested again (Table 1). The effect of Fab fragments on the biological function of IL-4 was clearly shown when we tested the effect of IL-4 on PMNC proliferation with the addition of sera pooled from patient 3 and patient 5 compared to healthy controls (Fig. 2). There was dose-dependent reduction of proliferation when sera from pneumonia patients were added to the cells (for the P values, see the legend to Fig. 2). No detectable effects were found from the control sera. The profile of anti-IL-4 and anti-IFN-γ Aabs showed inverse correlation to the level of cytokine production; i.e., low levels of Aabs were associated with high levels of IFN-γ produced, whereas high levels of Aabs to IL-4 were accompanied by low levels of IL-4 produced (Fig. 1). Specimens from healthy controls showed no measurable levels of anticytokine Aabs.
TABLE 1.
Specificity of the anti-IL-4 Aabsa
| Fab fragment | % of binding
|
|
|---|---|---|
| Patient 3 | Patient 5 | |
| Bound to IL-4 | 100 | 100 |
| Preabsorbed with IL-4 | 2 | 7 |
| Preabsorbed with IFN-γ | 95 | 93 |
The original binding of the Fab fragment purified from plasma to a particular cytokine in the ELISA system is shown. 100% indicates the actual binding of the Fab fragment to the cytokine before preabsorption. The effects of preabsorption of the Fab fragment with either IL-4 or IFN-γ on original binding were then calculated. Fab fragments were prepared from two patients.
FIG. 2.
Neutralizing effects of the anti-IL-4 Aabs. The effects of different dilutions of sera pooled from patients 3 and 5 on IL-4-induced PMNC proliferation are shown. The means ± standard deviations for data obtained from seven sera examined separately are shown. The asterisks indicate statistical significance (∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.05) from the T-cell proliferation values before and after the addition of sera. rIL-4, recombinant IL-4.
DISCUSSION
Clinically important cytokines function systemically as pleiotropic hormones with overlapping effects on many cell types. All engage in a complex network of agonists and antagonists via specific receptors. The series of events involved in the inflammatory response in the lung have been thought to occur sequentially. As recently reviewed, a pathogen arriving in the lower airways is coated with alveolar lining fluid and phagocytosed by alveolar macrophages. Cytokines such as IL-1 and TNF-α that mediate the inflammatory response are released. However, cell-mediated immunity also plays an important role in pulmonary defense against certain pathogens, including viruses and intracellular parasites that can survive within resident macrophages (13). It has been postulated that cell-mediated immune responses in the lung are compartmentalized, in the sense that antigens in the alveoli that are ingested by macrophages will not be presented and therefore will not serve as stimulants for cell-mediated immune function. However, if the antigen is recognized by dendritic cells or other antigen-presenting cells, cell-mediated immune function can be stimulated (13). The increased levels of circulating cytokines such as IFN-γ and IL-4 in acute lower respiratory tract infections may contribute significantly to disease manifestations, and specific therapeutic intervention with the cytokine or cytokine inhibitors such as Aabs may have the most relevance clinically. In our study, we were able to detect circulating free IFN-γ and IL-4 despite potential serum Aabs. We actually measured the levels of cytokine Aabs in serum and correlated them to the number of IFN-γ- and IL-4-secreting cells rather than to the levels of cytokines in serum. This possibility was considered because circulating Aabs to cytokines might occur as complexes with their respective cytokines and vice versa and the various levels of either Aabs or cytokines may depend on complex formation and not strictly reflect the level of production. It is possible that the various levels of Aabs depend on complex formation and not production.
IFN-γ is of major importance since it is produced by Th1-like cells (16) and released very early upon T-cell activation (7). It not only up regulates the inflammatory response but also induces production of other cytokines that have a major role in the early phase of infection, like TNF-α and IL-1 (6). On the other hand, IL-4 is produced by Th2 clones (18). It counteracts effects of IFN-γ and is also known to down regulate the production of IFN-γ (5, 23). The type of invasive pathogen dictates the preferential differentiation of Th0 cells into the Th1 and Th2 subsets and consequently the kind of cytokine produced and the consequences that follow. Cytokine response in patients with pneumonia caused by Chlamydia and Mycoplasma spp. showed increased levels of IL-6, TNF-α, and IFN-γ during infection (11). In another study, serum cytokine levels in patients with legionella pneumonia showed relative predominance of Th1 type of cytokines such as IFN-γ and IL-12 during the acute phase and diminished thereafter during convalescence (22). In a recent study of Aabs to IFN-γ, TNF-α, IL-10, and IL-4 in patients with multiple sclerosis, aseptic meningitis, and stroke, we found increased levels of Aabs to the four cytokines in both cerebrospinal fluid and plasma samples from patients with multiple sclerosis or aseptic meningitis, whereas in cerebrospinal fluid samples from stroke patients, only Aabs to IL-4 and IL-10 were detected. These data showed for the first time the presence of anticytokine Aabs in neurological autoimmune and nonautoimmune diseases (9). In the current study, blood samples were taken from patients an average of 3 days after onset of symptoms, high levels of IFN-γ were demonstrated in all patients in contrast to low levels of IL-4 at the same time. IL-4 is known as a potent anti-inflammatory cytokine, down regulating IFN-γ production by IL-2-activated NK cells. As shown above, anti-IL-4 Aab was found to neutralize the cytokine in vitro; high levels of this Aab in the sera of these patients may be an additional factor for the sequence of infection when they neutralize IL-4 in vivo, thus inhibiting the biological function as an anti-inflammatory cytokine. Low levels of anti-IFN-γ Aab are not necessarily a contributing factor in disease development but rather the result of increased consumption of Aab during active inflammation with increased local or systemic production of the cytokine. Thus, anti-cytokine Aabs may be the result of a leaky B-cell response triggered by immunoinflammatory processes.
As treatment of many infectious, neoplastic, autoimmune, and traumatic diseases is aimed at modifying endogenously produced cytokines, studies of natural regulators of the cytokine network are becoming increasingly important. To improve management of many diseases, more research on how these Aabs act is needed.
ACKNOWLEDGMENTS
This study was supported financially by the Swedish Medical Research Council, Swedish Society for Medicine, Swedish Association of Neurologically Disabled, and County Council of Östergötland.
REFERENCES
- 1.Arend W P. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- 2.Bakhiet M, Özenci V, Withagen C, Mustafa M, Fredrikson S, Link H. A new cell enzyme-linked immunosorbent assay demonstrates gamma interferon suppression by beta interferon in multiple sclerosis. Clin Diagn Lab Immunol. 1999;6:415–419. doi: 10.1128/cdli.6.3.415-419.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendtzen K, Hansen M B, Ross C, Poulsen L K, Svenson M. Cytokines and autoantibodies to cytokines. Stem Cells. 1995;13:206–222. doi: 10.1002/stem.5530130303. [DOI] [PubMed] [Google Scholar]
- 4.Bendtzen K, Svenson M, Jonsson V, Hippe E. Autoantibodies to cytokines: friends or foes. Immunol Today. 1990;11:167–169. doi: 10.1016/0167-5699(90)90068-k. [DOI] [PubMed] [Google Scholar]
- 5.Chretien I, Pene J, Briere F, de Waal Malefijt R, Rousset F, De Vries J E. Regulation of human IgE synthesis. 1. Human IgE synthesis in vitro is determined by the reciprocal antagonistic effects of interleukin-4 and interferon-gamma. Eur J Immunol. 1990;20:243–251. doi: 10.1002/eji.1830200203. [DOI] [PubMed] [Google Scholar]
- 6.Collart M A, Belin D, Vassalli J D, de Kossodo S, Vassalli P. Gamma-interferon enhances macrophage transcription of the tumor necrosis factor/cachetin, interleukin-1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986;164:2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabtree G R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello C A, Wollf S M. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 9.Elkarim R A, Mustafa M, Kivisakk P, Link H, Bakhiet M. Cytokine autoantibodies in multiple sclerosis, aseptic meningitis and stroke. Eur J Clin Invest. 1998;28:295–299. doi: 10.1046/j.1365-2362.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 10.Janeway C A J, Traveres P, editors. Immunobiology: the immune system in health and disease. 2nd ed. New York, N.Y: Garland Publishing; 1996. pp. 12–23. [Google Scholar]
- 11.Kragsbjerg P, Vikerfors T, Holmberg H. Cytokine responses in patients with pneumonia caused by Chlamydia or Mycoplasma. Respiration. 1998;65:299–303. doi: 10.1159/000029280. [DOI] [PubMed] [Google Scholar]
- 12.Mage M G. Preparation of Fab fragments from IgGs of different animal species. Methods Enzymol. 1980;70:143–150. doi: 10.1016/s0076-6879(80)70045-9. [DOI] [PubMed] [Google Scholar]
- 13.Mandell G L, et al., editors. Principles and practice of infectious diseases. 4th ed. Edinburgh, United Kingdom: Churchill Livingstone, Ltd.; 1995. pp. 619–632. [Google Scholar]
- 14.Montan C, Torres A. Lung inflammatory response in pneumonia. Monaldi Arch Chest Dis. 1998;53:56–63. [PubMed] [Google Scholar]
- 15.Mustafa A S, Al-Attiyah R J, Nath I, Chugh T D. T-cell subsets and cytokines interplay in infectious diseases, 169–179. S. Basel, Switzerland: Karger; 1996. [Google Scholar]
- 16.Olsson T. Cytokines in neuroinflammatory diseases: role of myelin autoreactive T cell production of interferon-gamma. J Neuroimmunol. 1992;40:211–218. doi: 10.1016/0165-5728(92)90135-8. [DOI] [PubMed] [Google Scholar]
- 17.Pederson M, Permin H, Bindslev-Jensen C, Bendtzen K, Norn S. HIV antigen-induced release of histamine from basophils from HIV infected patients. Mechanism and relation to disease progression and immunodeficiencies. Allergy. 1991;46:206–212. doi: 10.1111/j.1398-9995.1991.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 18.Powrie F, Coffman R L. Cytokine regulation of T-cell function: potential for therapeutic intervention. Trends Pharmacol Sci. 1993;14:164–168. doi: 10.1016/0165-6147(93)90202-u. [DOI] [PubMed] [Google Scholar]
- 19.Pozzetto B, Mogensen K E, Tovey M G, Gresser I. Characteristics of autoantibodies to human interferon in a patient with varicella-zoster disease. J Infect Dis. 1984;150:707–713. doi: 10.1093/infdis/150.5.707. [DOI] [PubMed] [Google Scholar]
- 20.Roitt I M, editor. Roitt’s essential immunology. 9th ed. Oxford, United Kingdom: Blackwell Scientific; 1997. pp. 141–150. [Google Scholar]
- 21.Svenson M, Poulsen L K, Fomsgaard A, Bendtzen K. IgG autoantibodies against interleukin-1 alpha in sera of normal individuals. Scand J Immunol. 1989;29:489–492. doi: 10.1111/j.1365-3083.1989.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 22.Tateda K, Matsumoto T, Ishii Y, Furuya N, Ohno A, Miyazaki S, Yamaguchi K. Serum cytokines in patients with Legionella pneumonia: relative predominance of Th1-type cytokines. Clin Diagn Lab Immunol. 1998;5:401–403. doi: 10.1128/cdli.5.3.401-403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vercelli D, Jabara N H, Lauener R P, Geha R S. IL-4 inhibits the synthesis of IFN-γ and induces the synthesis of IgE in human mixed lymphocyte cultures. J Immunol. 1990;144:570–573. [PubMed] [Google Scholar]