Abstract
Embryo-uterine interactions leading to the attachment reaction is followed by stromal cell proliferation and differentiation into decidual cells (decidualization) at the sites of blastocyst apposition. In rodents, decidualization is also induced by application of an artificial stimulus (intraluminal oil infusion) in a pseudopregnant uterus, or to one that has been appropriately prepared by exogenous progesterone (P4) and estrogen. The process of decidualization is under the control of these steroids in the presence of blastocysts or deciduogenic stimuli. Although it is well known that estrogen is required for the induction of progesterone receptors in the uterus, the functional importance of estrogen in the process of decidualization is poorly understood. To better understand the role of estrogenic actions in decidualization, we used wild-type and estrogen receptor-α knock-out (ERKO) mice for induction of decidualization employing a defined steroid hormonal treatment schedule. Our results demonstrate that P4 alone induces decidualization in ovariectomized wild-type or ERKO mice in response to intraluminal oil infusion in the absence of estrogen. A combined treatment of either estradiol-17β (E2) or its catecholmetabolite 4-hydroxyestradiol-17β (4-OH-E2) with P4 does not potentiate the decidual response produced by P4 treatment alone in either ovariectomized wild-type or ERKO mice. The induction of decidual response was associated with up-regulation of decidual cell marker genes, such as progesterone receptor, metallothionein-1, and cyclooxygenase-2. The results suggest that the stromal cell sensitivity to decidualization is critically dependent on P4-regulated events, and estrogenic induction of progesterone receptor via classical nuclear ER-α is not critical for this process.
The establishment of successful pregnancy requires precise coordination between the receptive uterus and activated state of the blastocyst. The importance of ovarian estrogen and progesterone (P4) in these processes has been known for many years (1–4). The attachment of blastocyst trophectoderm with the uterine luminal epithelium occurs at 2200–2300 h on day 4 (day 1 = vaginal plug) in the mouse and is followed by stromal cell proliferation and differentiation into decidual cells at the sites of blastocyst apposition. In rodents, decidual cell response can also be elicited by intraluminal oil infusion into a pseudopregnant uterus during the receptive period or to a uterus, which has been appropriately prepared by exogenous estrogen and P4 (4–7). The process of decidualization is considered to be mediated by cell-specific events in a compartmentalized manner. For example, the de-epitheliated uterine horn is incapable of inducing decidual cell reaction (8).
Normally, estrogen is required for the induction of progesterone receptors (PR) in the uterus. However, the nature of interactions between the two ovarian steroids that trigger the decidualization process remains undefined. The down-regulation of the nuclear estrogen receptor-α (ER-α) (9–11) and very low levels of ER-β (12) in the deciduum suggest that the participation of nuclear ERs is limited in decidualization. In contrast, decidualization is associated with the up-regulation of PR (11). Further, P4 is an absolute requirement for sustained decidualization in the rodent (reviewed in Ref. 4) because this response is prevented by blocking P4 actions with neutralizing antibody or administration of antiprogestins, RU-486 or ZK299 (11, 13, 14). Paradoxically, continued P4 treatment preceded by estrogen priming and accompanied by a “prenidatory” estrogen injection has been shown to be essential for elicitation of decidualization in the mouse uterus by intraluminal oil infusion, although estrogen is not an absolute requirement for decidualization in P4-treated uterus by a traumatic stimulus (15, 16). This differential responsiveness of the uterus to decidual cell response under two different experimental conditions is not clearly understood, but these observations suggest that an up-regulation of uterine PR necessary for oil-induced response requires estrogenic effects (13), whereas this up-regulation for trauma-induced response does not require estrogenic effects. Nonetheless, P4 effects via PR are primary requirements for decidualization, and this is consistent with the observation of failure of decidualization in PR knock-out (PRKO) mice (17).
We have recently demonstrated that certain effects of a catecholestrogen 4-OH-E2 in the uterus is independent of ER-α (18). Because estrogen is considered as an inducer of PR, we examined whether estrogenic effects mediated by either primary estrogen or catecholestrogen are essential for the induction of PR with respect to decidualization in ERKO mice. We demonstrate herein that decidual response induced by intraluminal oil infusion is elicited by P4 alone in ovariectomized ERKO mice in the absence of any estrogenic treatments.
Materials and Methods
Animals
Litter-mate wild-type and homozygous mutant mice (129/J/C57BL/6J) for estrogen receptor-α (ERKO) were housed in the animal care facility at the University of Kansas Medical Center in accordance with NIH standards for the care and use of experimental animals. Adult wild-type and ERKO mice were ovariectomized and rested for one week before any treatments were initiated.
Treatment schedules for decidualization
The protocol for the induction of decidualization (deciduoma) has previously been described (7, 19). To examine whether P4 alone can induce deciduoma, ovariectomized wild-type or ERKO mice were injected with P4 (1 mg) for 3 days (days 1–3). The induction of deciduoma was initiated by infusing sesame oil (25 µl) in one uterine horn on day 4, whereas the contralateral horn served as a control. Mice were maintained on daily injection of P4 (1 mg) from days 4–7. They were killed on day 8 and uterine wet weights of the infused and noninfused (control) horns were recorded. To examine decidual response in the presence of estrogens and P4, ovariectomized wild-type or ERKO mice were treated as follows: E2 (100 ng) or 4-OH-E2 (100 ng) for 3 days (days 1–3), no treatments on days 4 and 5, P4 (1 mg) plus E2 (10 ng) or 4-OH-E2 (10 ng) on days 6 and 7, and P4 (1 mg) on days 8–11. Intraluminal oil injection was made on day 8. Mice were killed on day 12 and uterine wet weights of the infused and noninfused (control) horns were recorded. The fold increases in uterine weights were used as an index of decidualization. After recording wet weights, uteri were frozen and stored at −70 C. Decidual cell reaction was confirmed by histological examination and by examining the expression of decidual cell marker genes.
Hybridization probes
The subcloning and vectors for mouse cyclooxygenase-2 (COX-2), PR, and metallothionein-1 (MT-1) complementary DNAs have been previously described (7, 20–22). A 347-bp complementary DNA fragment (nt 175–521) for mouse ER-β (23) was derived by RT-PCR using mouse ovary RNA sample. The fragment was subcloned in pCR-Script (SK)+ vector and the sequencing was performed by dideoxy method to confirm the nucleotide sequence. For hybridization, antisense 32P-labeled cRNA probes were generated using SP6 polymerase. For in situ hybridization, sense and antisense 35S-labeled cRNA probes were generated using appropriate RNA polymerases. The probes had specific activities of 2 × 109 dpm/µg.
In situ hybridization
In situ hybridization was performed as previously described (18, 24, 25). In brief, frozen sectioned were mounted onto poly-l-lysine coated slides and fixed in 4% paraformaldehyde in PBS for 15 min at 4 C. Sections were prehybridized followed by hybridization with 35S-labeled specific cRNA probes for 4 h at 45 C. After hybridization and washing, the sections were incubated with RNase-A (20 µg/ml) at 37 C for 20 min. RNase-A resistant hybrids were detected by autoradiography using Kodak NB-2 liquid emulsion (Eastman Kodak Co.). The slides were poststained with hematoxylin and eosin. The reddish brown grains indicate the sites of messenger RNA (mRNA) accumulation. This hue is the result of lateral light scattering from the eosin staining under dark-field microscopy. Sectioned hybridized with the sense probes served as negative controls. Computer-assisted image analysis (OPTIMA II program) of grain densities under darkfield of five different regions on each section showing positive signals was used to quantitate autoradio-graphic signals.
Results
Effects of progesterone and estrogens on decidualization in wild-type and ERKO mice
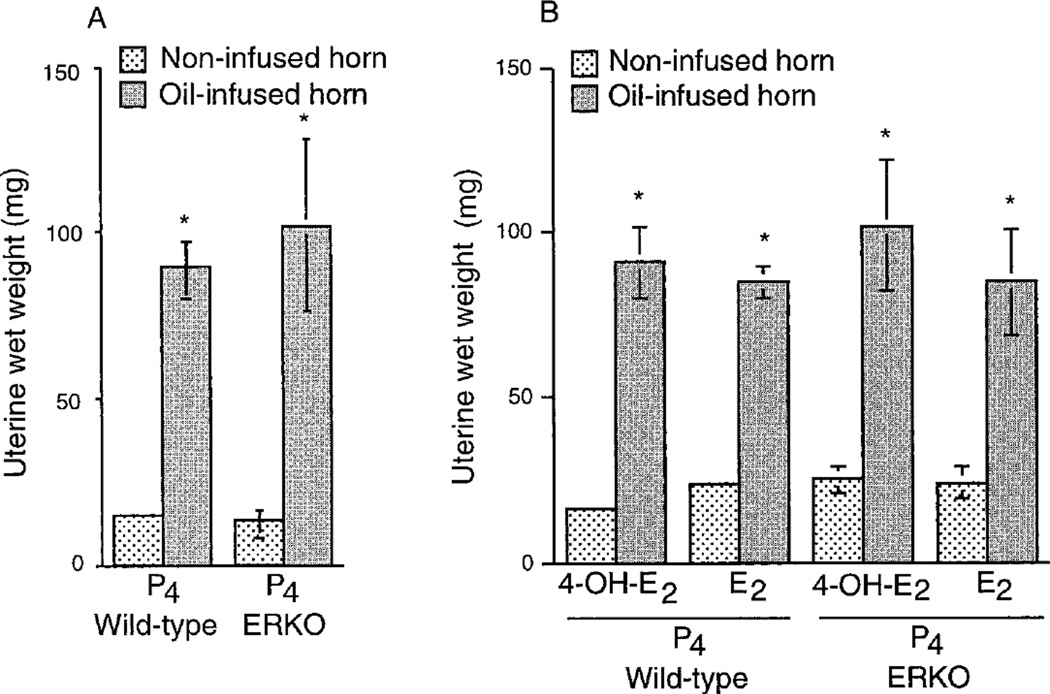
The decidual cell response was examined in the ovariectomized wild-type and ERKO mice using an established protocol for experimentally induced decidualization as described in Materials and Methods. Differences in uterine wet weights between the infused and noninfused horns indicated the extent of decidualization. As shown in Fig. 1A, P4 injections alone produced a significant induction of decidual response in both wild-type and ERKO mice. The combined injections of either E2 or 4-OH-E2 with P4 also showed a similar induction of decidualization in these mice (Fig. 1B) and no apparent potentiating effects of these estrogens were observed (Fig. 1, A and B). Regardless of the hormone treatments, both wild-type and ERKO mice showed 5- to 6-fold increases in uterine weights in the infused horns compared with noninfused horns (Fig. 1, A and B).
Fig. 1.
Effects of P4 and/or estrogens on decidual response. Ovariectomized wild-type or ERKO mice were treated with P4 (A) or P4 plus E2 or P4 plus 4OH-E2 (B), which sensitize the uterus for optimal decidualization. Intraluminal infusion of oil was made in one horn on the appropriate day of the hormone treatment, whereas the contralateral noninfused horn served as a control. Mice were killed 4 days later after induction of decidualization as described in Materials and Methods. These experiments were repeated three times with 3–4 mice in each group. Induction of decidualization was determined by the increases in wet weights of the infused horns as opposed to the noninfused horns. Results are mean ± sem. Values are statistically different (P < 0.05, Student’s t test) between the infused and the noninfused horns in each group. *, Bars marked with an asterisk are not significantly different from each other (P > 0.05).
Expression of ER-β mRNAs in ovaries and in infused and noninfused uteri of wild-type and ERKO mice
Although very low levels of ER-β mRNA are present in the mouse uterus (12, 26), it is not known whether this receptor is up-regulated in the ERKO uterus as a compensatory mechanism. This prompted us to examine the ER-β expression in the ERKO mouse uterus during decidualization. The expression of ER-β mRNA was examined by in situ hybridization in infused and noninfused uteri of wild-type and ERKO mice. Wild-type and ERKO mouse ovaries served as positive controls. While the expression of ER-β mRNA was clearly detected in ovarian follicles of both wild-type and ERKO mice, its expression was below the limit of detection in infused or noninfused uterine horns of both wild-type and ERKO mice (data not shown).
Expression of PR mRNAs in infused and noninfused uteri of wild-type and ERKO mice
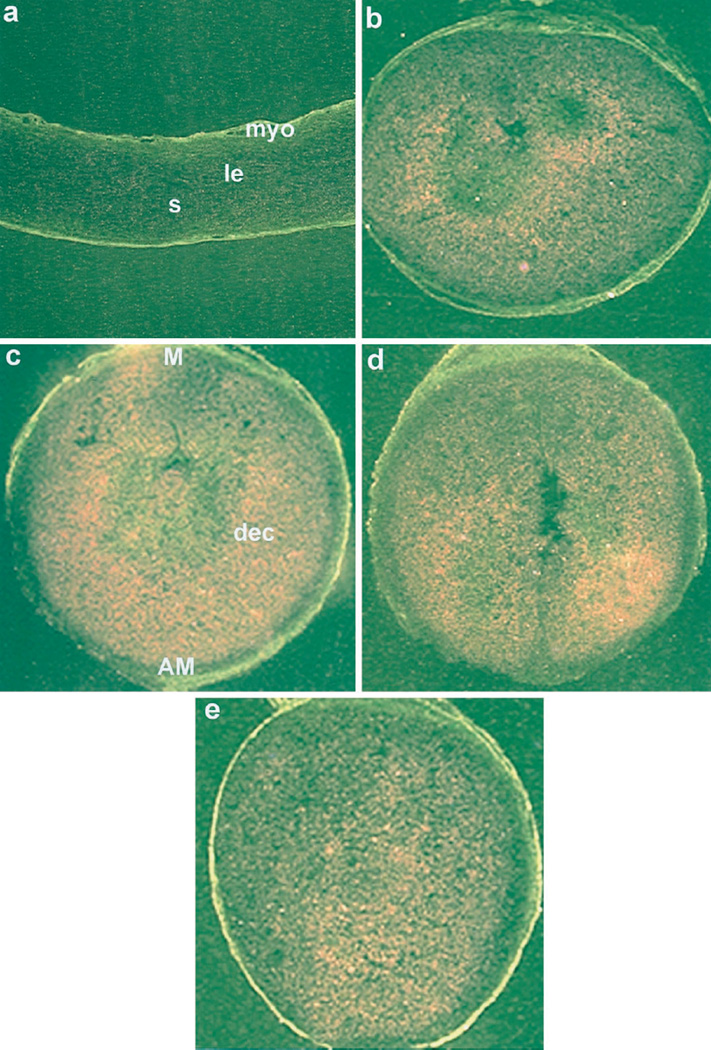
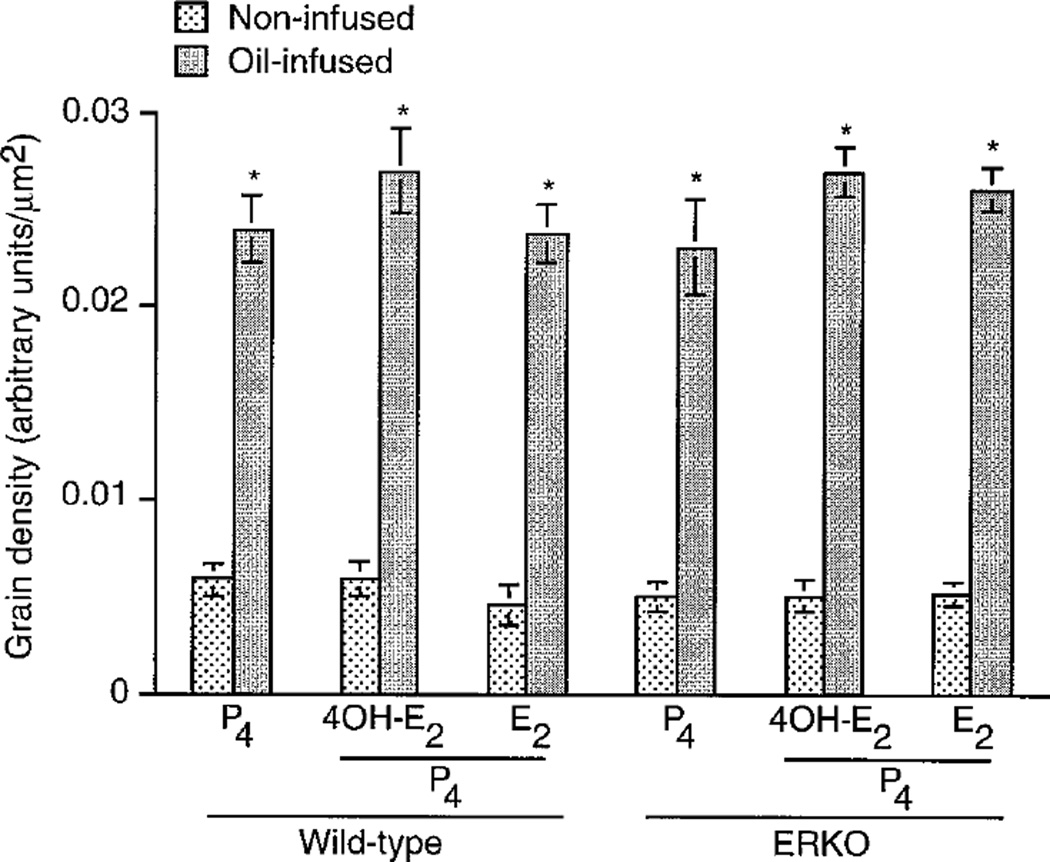
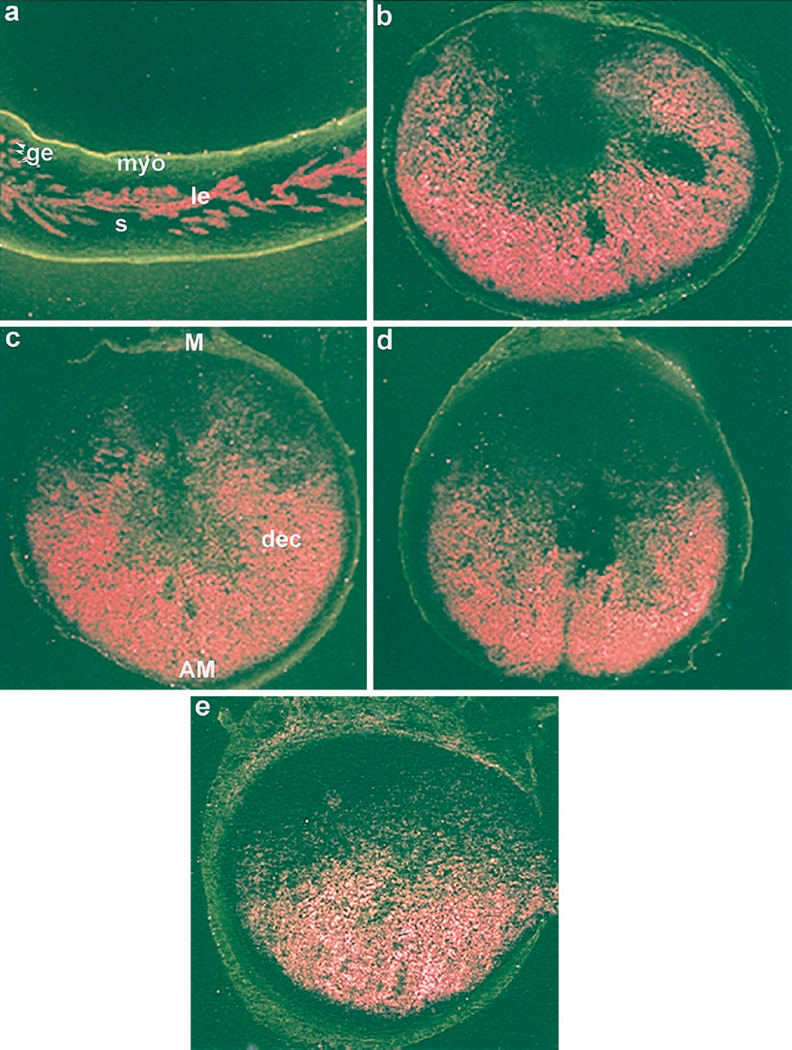
Because up-regulation of PR is associated with decidualization (11), we examined the expression of PR mRNA in experimentally induced deciduoma in wild-type and ERKO mice. Consistent with the previous report (27), low basal levels of PR mRNA were detected in uteri of noninfused horns of both wild-type (data not shown) and ERKO mice irrespective of hormone treatments (Fig. 2a). However, the levels of PR mRNA accumulation were higher in decidualizing stromal cells in oil-infused uterine horns of both the wild-type (Fig. 2e) and ERKO (Fig. 2, b–d) mice irrespective of hormone treatments. The quantitation of autoradiographic signals by image analysis (OPTIMA II program) showed significant accumulation of PR mRNA (4-to 5-fold), in the decidualizing stroma compared with that of the noninfused uterine stroma in both wild-type and ERKO mice under each hormonal condition examined (Fig. 3). However, increases in mRNA accumulation in the decidualizing stroma were not significantly different among the treatment groups (Fig. 3).
Fig. 2.
In situ hybridization of PR mRNA in wild-type and ERKO uteri. Induction of decidualization followed the protocol as described in the legend to Fig. 1. Darkfield photomicrographs of representative uterine sections of non-infused horn treated with P4 (a) and infused horn treated with P4 plus E2 (b) or P4 plus 4-OH-E2 (c) or P4 (d) from ERKO mice, as well as infused horn from wild-type mice treated with P4 (e) are shown. Magnifications are shown at 20×. le, Luminal epithelium; s, stroma; myo, myometrium; AM, antimesometrial pole; M, mesometrial pole; dec, decidual cells. These experiments were repeated three times with three mice in each group.
Fig. 3.
Quantitation of PR mRNA signals in the decidualized uteri of wild-type and ERKO mice treated with different hormones. Experimental designs are same as described in the legend to Fig. 2. The quantitation of hybridization signals was achieved by computer-assisted analysis (OPTIMA II program) of reddish brown grain densities under the darkfield microscopy. The grain densities of five different regions on each section showing positive signals were computed separately from four to five mice in each group. The grain density was used as an index for mRNA accumulation. Results are mean ± sem. Values are statistically different (P < 0.05, Student’s t test) between the infused and the noninfused horns in each group. *, Bars marked with an asterisk are not significantly different from each other (P > 0.05).
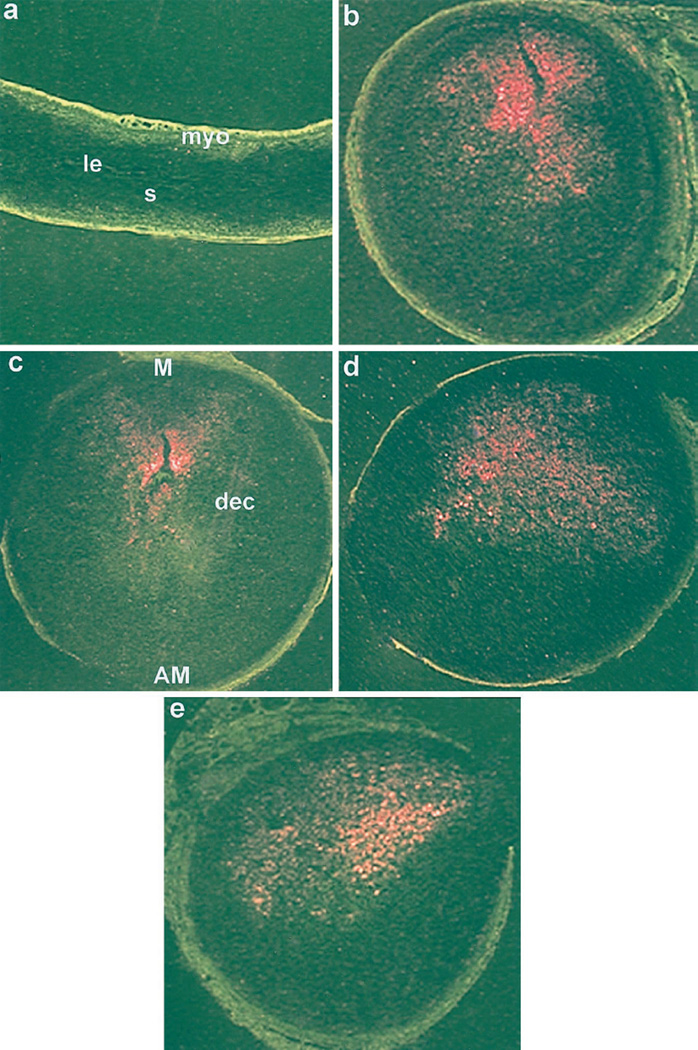
Expression of cyclooxygenase-2 (COX-2) and MT-1 mRNAs in infused and noninfused uteri of wild-type and ERKO mice
Because COX-2 expression at the mesometrial stroma and MT-1 expression at the antimesometrial stroma are considered decidual cell-specific marker genes (7, 21, 28), we examined the expression of COX-2 and MT-1 mRNAs in the infused and noninfused uterine horns of both wild-type and ERKO mice by in situ hybridization (Figs. 4 and 5). These genes were expressed correctly without showing any apparent differences in the mRNA levels (data not shown) in the decidualizing stroma in both wild-type and ERKO mice regardless of the hormone treatments (Figs. 4, b–e, and 5, b–e). In contrast, whereas COX-2 mRNA was undetectable (Fig. 4a), MT-1 mRNA was detected only in the epithelium of the noninfused horns (Fig. 5a). These results further support that decidualization occurs in both the wild-type and ERKO mice by P4 alone in the absence of estrogens.
Fig. 4.
In situ hybridization of COX-2 mRNA in wild-type and ERKO uteri. Induction of decidualization followed the protocol as described in the legend to Fig. 1. Darkfield photomicrographs of representative uterine sections of non-infused horn treated with P4 (a) and infused horn with P4 plus E2 (b) or P4 plus 4-OH-E2 (c) or P4(d) from ERKO mice, and infused horn from wild-type mice treated with P4 (e) are shown. Magnifications are shown at 20×. le, Luminal epithelium; s, stroma; myo, myometrium; AM, antimesometrial pole; M, mesometrial pole; dec, decidual cells. These experiments were repeated two times with three mice in each group.
Fig. 5.
In situ hybridization of MT-1 mRNA in wild-type and ERKO uteri. Induction of decidualization followed the protocol as described in the legend to Fig. 1. Dark-field photomicrographs of representative uterine sections of noninfused horn treated with P4 (a) and infused horn treated with P4 plus E2 (b) or P4 plus 4-OH-E2 (c) or P4 (d) from ERKO mice, and infused horn from wild-type mice treated with P4 (e) are shown. Magnifications are shown at 20×. le, Luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium; AM, antimesometrial pole; M, mesometrial pole; dec, decidual cells. These experiments were repeated two times with three mice in each group.
Discussion
Although the uterus is a major target for estrogen and P4 effects, and although the critical biological processes during implantation/decidualization are primarily dependent upon temporal and cell-specific interactions mediated by these steroids, the molecular mechanisms involved in these processes are not clearly understood. During normal pregnancy, estrogen and P4 are essential for blastocyst implantation and subsequent decidualization. Although estrogen is a requirement for the initial attachment reaction, the participation of this steroid in subsequent decidualization process in not clearly understood. In this regard, ERKO mice provide a valuable model to examine the effects estrogen mediated via ER-α in the process of decidualization. We examined decidual response in the ovariectomized wild-type and ERKO mice using an established protocol for experimentally induced decidualization as described previously by us (7). Our data clearly demonstrate that P4 alone can induce deciduoma in oil-infused uterine horns in both the wild-type and ERKO mice (Fig. 1, A and B). Furthermore, the estrogenic influence is not required to potentiate this response. These results suggest that priming with P4 alone is enough to obtain full complement of stromal cell decidualization in response to an artificial stimulus in the mouse uterus.
The induction of deciduoma in ERKO mice in the absence of estrogens strongly suggests that the involvement of ER-α is not essential in this process. We have recently demonstrated that although E2 is ineffective in inducing an estrogen-responsive gene, LF, in uteri of ovariectomized ERKO mice, 4-OH-E2 was effective in this response. The induction of LF by 4-OH-E2 was not altered by either E2 or by an antiestrogen (18). These results suggested that 4-OH-E2 mediated effects on LF gene expression do not involve classical ERs. Thus, the existence of a novel nuclear or a membrane receptor for 4-OH-E2 is speculated (29–32). The relative binding studies with in vitro translated proteins have shown that the ER-β is able to bind E2 or 4-OH-E2 with an affinity similar to that of ER-α (23, 33). The dramatic down-regulation of the nuclear ER-α (9) and very low levels of ER-β in the infused (deciduoma) or noninfused uterine horns in both the wild-type and ERKO mice (data not shown) suggest limited participation, if any, of nuclear ERs in decidualization. This is consistent with the observation that P4, but not estrogen, is an absolute requirement for sustained decidualization in rodents (4, 17). However, it is to be noted that estrogen is essential for the initiation of implantation in the P4-primed uterus (4).
Because estrogen actions are important for the induction of PR, it is not clear how PR is induced in the ERKO uterus and mediates P4 action. It is possible that PR is up-regulated in the uterus by yet an unidentified mechanism at the onset of decidualization in the absence of estrogen functions. Indeed, induction of deciduoma was associated with PR upregulation in infused horns of both wild-type and ERKO mice irrespective of the hormone treatments (Figs. 2 and 3). These results again suggest that initiation and/or progression of decidualization, but not ER-α, are perhaps the primary inducers of PR during this process. This is consistent with the observations of neutralization of PR functions and deciduoma formation by anti-P4 antibody, antiprogestins or deletion of PR gene (11, 13, 14, 17). Alternatively, stromal cell sensitivity to a decidual stimulus could be initiated by basal levels of uterine PR and minimal occupancy of PR could be sufficient for decidualization.
COX-2-derived PGs are essential for implantation and decidualization (7). Because COX-2 is expressed in the decidua in a cell-specific manner and is induced by application of a deciduogenic stimulus, it serves as a marker for decidual response (7, 28). Analysis of COX-2 mRNA expression in infused uterine horns of the both wild-type and ERKO mice demonstrated that this gene was correctly expressed at the mesometrial pole, confirming deciduoma formation (Fig. 4, b–e). Thus, COX-2 expression is directly associated with decidualization and is independent of ER-α. Heightened expression of MT-1 in the decidua or deciduoma at the antimesometrial pole have previously been reported (21). Thus, an elevated expression of MT-1 mRNA in the oil-infused uterine horns in the present investigation is consistent with the previous reports (Fig. 5).
Collectively, the results of this investigation present an important concept that estrogen induction of PR in mediating P4 effects via classical ER-α is not essential for the initiation and progression of decidual cell response in the mouse.
Acknowledgments
We thank Dr. H. Lim for her critical reading and input in preparation of the manuscript.
Footnotes
This research was supported in part by grants from the NIEHS (ES-07814 to Sa.K.D), the NICHD (HD-12304 and HD-29968 to Su.K.D), the NICHD (HD-35114 to B.C.P), and the core support from NICHD Center Grants (HD-02528 and HD-33994).
References
- 1.Psychoyos A. Endocrine control of egg implantation. In: Greep RO, Astwood EG, Geiger SR, editors. Handbook of Physiology. Washington, DC: American Physiological Society; 1973. pp. 187–215. [Google Scholar]
- 2.Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the mouse receptive uterus. Proc Natl Acad Sci USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huet-Hudson YM, Andrews GK, Dey SK. Cell-type-specific localization of c-Myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125:1683–1690. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- 4.Dey SK. Implantation. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive Endocrinology, Surgery, and Technology. New York: Lippincott-Raven; 1996. pp. 421–434. [Google Scholar]
- 5.De-Feo VJ. Decidualization. In: Wynn RM, editor. Cellular Biology of the uterus. New York: Appleton-Century-Crofts; 1967. pp. 191–205. [Google Scholar]
- 6.Wordinger RJ, Jackson FL, Morrill A. Implantation, deciduoma formation and live births in mast cell-deficient mice (W/Wv) J Reprod Fertil. 1986;77:471–476. doi: 10.1530/jrf.0.0770471. [DOI] [PubMed] [Google Scholar]
- 7.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 8.Lejeune B, Van-Hoeck J, Leroy F. Transmitter role of the luminal uterine epithelium in the induction of decidualization in rats. J Reprod Fertil. 1981;61:235–240. doi: 10.1530/jrf.0.0610235. [DOI] [PubMed] [Google Scholar]
- 9.Das N, Wang J, Dey SK. Uterine preparation for implantation in the mouse is associated with coordinate expression of estrogen-responsive finger protein and estrogen receptor. Mol Reprod Dev. 1996;46:499–506. doi: 10.1002/(SICI)1098-2795(199704)46:4<499::AID-MRD8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Villee CA, Armstrong EG, Jr, Talley DJ, Hoshiai H. Decidual cell function: role of steroid hormones and their receptors. In: Glasser SR, Bullock DW, editors. Cellular and Molecular Aspects of implantation. New York: Plenum; 1981. pp. 241–252. [Google Scholar]
- 11.Parandoosh Z, Crombie DL, Tetzke TA, Hayes JS, Heap RB, Wang MW. Progesterone and oestrogen receptors in the decidualized mouse uterus and effects of different types of anti-progesterone treatment. J Reprod Fertil. 1995;105:215–220. doi: 10.1530/jrf.0.1050215. [DOI] [PubMed] [Google Scholar]
- 12.Das SK, Lim H, Paria BC, Dey SK. Cyclin D3 in the mouse uterus is associated with the decidualization process during early pregnancy. J Mol Endocrinol. 1999;22:91–101. doi: 10.1677/jme.0.0220091. [DOI] [PubMed] [Google Scholar]
- 13.Crombie DL, Mukherjee R, McDonnell DP, Hayes JS, Wang MW. Creatine kinase activity as an indicator of unopposed estrogen action in the mouse uterus associated with anti-progesterone treatment. J Steroid Biochem Mol Biol. 1994;49:123–129. doi: 10.1016/0960-0760(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Funk C, Glasser SR, Mullholland J. Progesterone regulation of heparin-binding epidermal growth factor-like growth factor gen expression during sensitization and decidualization in the rat uterus: effects of antiprogestin, ZK 98,299. Endocrinology. 1994;135:1256–1263. doi: 10.1210/endo.135.3.8070371. [DOI] [PubMed] [Google Scholar]
- 15.Finn CA. The implantation reaction. In: Wynn RM, editor. Biology of the Uterus. New York: Plenum; 1977. pp. 245–308. [Google Scholar]
- 16.Finn CA. Endocrine control of endometrial sensitivity during the induction of the decidual cell reaction in the mouse. J Endocrinol. 1966;36:239–248. doi: 10.1677/joe.0.0360239. [DOI] [PubMed] [Google Scholar]
- 17.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 18.Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB. Estrogenic responses in estrogen receptor-α deficient mice reveal a novel estrogen signaling pathway. Proc Natl Acad Sci USA. 1997;94:12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wordinger RJ, Jackson FL, Morill A. Implantation, deciduoma formation and live births in mast cell-deficient mice (W/Wv) J Reprod Fertil. 1986;77:471–476. doi: 10.1530/jrf.0.0770471. [DOI] [PubMed] [Google Scholar]
- 20.DeWitt DL, Meade EA. Serum and glucocorticoid regulation of gene transcription and expression of the prostaglandin H synthase-1 and prostaglandin H synthase-2 isozymes. Arch Biochem Biophys. 1993;306:94–102. doi: 10.1006/abbi.1993.1485. [DOI] [PubMed] [Google Scholar]
- 21.Das SK, Tan J, Johnson DC, Dey SK. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology. 1998;139:2905–2915. doi: 10.1210/endo.139.6.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De SK, McMaster MT, Dey SK, Andrews GK. Cell specific metallothionein gene expression in mouse decidua and placentae. Development. 1989;107:611–621. doi: 10.1242/dev.107.3.611. [DOI] [PubMed] [Google Scholar]
- 23.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 24.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 25.Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 26.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 27.Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty I, Das SK, Wang J, Dey SK. Developmental expression of the cyclooxygenase-I and cyclooxygenase-2 genes in the periimplantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol. 1996;16:107–122. doi: 10.1677/jme.0.0160107. [DOI] [PubMed] [Google Scholar]
- 29.Szego CM. Cytostructural correlates of hormone action: new common ground in receptor-mediated signal propagation for steroid and peptide antagonists. Endocrine. 1994;2:1079–1093. [Google Scholar]
- 30.Schaeffer JM, Stevens S, Smith RG, Hsueh AJW. Binding of 2-hydroxyestradiol to rat anterior pituitary cell membranes. J Biol Chem. 1980;255:9838–9843. [PubMed] [Google Scholar]
- 31.Enmark E, Gustafsson JA. Orphan nuclear receptors—the first eight years. Mol Endocrinol. 1996;10:1293–1307. doi: 10.1210/mend.10.11.8923456. [DOI] [PubMed] [Google Scholar]
- 32.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 33.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]