Abstract
The occurrence, diversity, and pathogenicity of Vibrio spp. were investigated in two estuaries along the Italian Adriatic coast. Vibrio alginolyticus was the predominant species, followed by Vibrio parahaemolyticus, non-O1 Vibrio cholerae, and Vibrio vulnificus. By using a biochemical fingerprinting method, all isolates were grouped into nine phenotypes with similarity levels of 75 to 97.5%. The production of toxins capable of causing cytoskeleton-dependent changes was detected in a large number of Vibrio strains. These findings indicate a significant presence of potentially pathogenic Vibrio strains along the Adriatic coast.
Estuarine and freshwater environments both represent the critical reservoirs of Vibrio species (6, 12, 21). The prevalence of pathogenic vibrios appears to be influenced by the physicochemical features of the environment (9, 22), whereas factors influencing the production and the activity of their toxins remain to be defined. It is known that a high bacterial density is correlated with coastal eutrophication, and it has been suggested that Vibrio spp. are among the major causative agents of acute diarrheal diseases (8, 10, 14, 18, 19, 23).
To evaluate the health risk associated with the presence of potentially pathogenic vibrios, we investigated the virulence of Vibrio strains isolated during warm weather months (April to October 1995) from two estuaries (Metauro and Foglia rivers) along the Northwest Adriatic coast. All isolates were also phenotyped with a high-resolution biochemical fingerprinting system to evaluate the intraspecies diversity.
Procedures and sampling sites, as well as in vitro assays to evaluate the virulence of isolated strains, were described in an earlier parallel study on Aeromonas occurrence in the same coastal area (13).
Bacteriological analysis.
Enrichment for potentially pathogenic Vibrio species and viable bacterial counting were performed by the membrane filtration technique. Water volumes of 0.1, 1, 10, and 100 ml (for counting) and 500 ml (for enrichment) were filtered through 0.45-μm-pore-size filters (Millipore). All filters except those used for the enrichment were placed on thiosulfate-citrate-bile-sucrose (TCBS; Oxoid) agar plates with 2% NaCl and incubated at 37°C for 16 to 18 h. The number of viable Vibrio isolates was estimated as CFU 100 ml of water−1. The mean number of bacteria in each water sample was estimated according to the method of Bolinches et al. (5). The primary and secondary enrichments into alkaline-peptone-water (APW) for Vibrio cholerae detection were performed as suggested previously (3, 14).
Vibrio identification and typing.
Vibrio strains were identified by colony shape and pigmentation on TCBS, Gram staining, cytochrome-oxidase and catalase activities, motility, and susceptibility to O/129 vibriostatic agent (10- and 150-μg disks; Oxoid). Only oxidase-positive, gram-negative, vibriostatic susceptible colonies were selected for biochemical tests according to the classical procedures. The API 20E and ID32 system (bioMerieux) and the additional test recently suggested by Alsina and Blanch (1, 2) were used for Vibrio identification at the species level. Further, biovars of Vibrio cholerae strains were defined by using O1 polyvalent antisera (57142; Sanofi Diagnostic Pasteur). Moreover, V. parahaemolyticus strains were tested on Wagatsuma agar for their hemolytic activity (Kanagawa phenomenon [20]). All Vibrio strains were further typed by using the Phene Plate (PhP) system (17) specifically developed for typing Vibrio species (15). V. alginolyticus COD.18 and V. parahaemolyticus COD.66 (kindly provided by D. Ottaviani, IZS, Ancona, Italy) were included as reference strains.
A Spearman rank correlation, a Wilcoxon matched-pair signed-ranks nonparametric test, and χ2 analyses were used for statistical analyses.
Assays for supernatant and bacterial cytotoxicity and for bacterial adhesion.
Vibrio spp. were inoculated in brain heart infusion broth supplemented with 0.5% NaCl and grown in 25-ml flasks incubated at 37°C for 18 h with agitation at 150 rpm. For the supernatant cytotoxicity assay, cell-free filtrates were prepared by centrifugation (3,000 rpm) at 4°C for 30 min, with subsequent filtration of the supernatants through a 0.45-μm-pore-size filter (Millipore). The filtrates were either refrigerated prior to use or stored at −80°C. For both the adhesion and cytotoxicity assays, exponentially growing bacteria were washed three times in phosphate-buffered saline and resuspended in serum-free Dulbecco modified Eagle medium (DMEM).
For the supernatant cytotoxicity assay, Vibrio strains found to be stable in the biochemical tests were selected. CHO cell monolayers were maintained in DMEM containing 10% fetal calf serum. Serial doubling dilutions of filtrates starting from 1:2 were tested for 24 h as previously described (13).
Bacterial adhesion and cytotoxicity were tested on HEp-2 cells growing in DMEM containing 10% fetal calf serum. Bacteria were added to HEp-2 cells at a multiplicity of infection of 100 in serum-free DMEM and incubated for 1 h 30 min at 37°C. Cells were then washed three times in serum-free DMEM to remove nonadherent bacteria, fixed in methanol, and stained with May-Grunwald-Giemsa. When assessed by light microscopy (LM), a sample was considered cytotoxic when at least 50% of cultured cells rounded up, whereas adhesive capacity was expressed as the percentage of cells with more than 10 bacteria on the cell surface. Samples for fluorescence and scanning electron microscopy (SEM) were prepared as previously described (13).
Occurrence of Vibrio spp.
Counts of viable vibrios, grown on TCBS medium, ranged from 102 CFU 100 ml−1 (April) to 105 CFU 100 ml−1 (August) from Metauro estuary and up to ca. 106 CFU 100 ml−1 (August) from Foglia estuary. Table 1 shows the occurrence of Vibrio species in both sampling sites isolated by using TCBS or APW medium. The frequency of all the isolated species did not differ significantly between the two sites (χ2 = 0.534; P = 0.766), except for V. vulnificus, which occurred only in Foglia estuary. APW medium used as a secondary enrichment allowed a good recovery of non-O1 V. cholerae compared to TCBS medium.
TABLE 1.
Vibrio CFU and Vibrio species isolated on TCBS medium or APW medium from the Metauro and Foglia estuaries between April and October 1995
| Month | Vibrio CFU (100 ml−1)
|
No. of isolates
|
Origin (medium) | No. (%) of strains
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
V. alginolyticus
|
V. parahaemolyticus
|
V. cholerae non-O1
|
V. vulnificus
|
||||||||||
| Metauro | Foglia | Metauro | Foglia | Metauro | Foglia | Metauro | Foglia | Metauro | Foglia | Metauro | Foglia | ||
| April | 5.0 × 102 | 3.0 × 103 | 3 | 4 | TCBS | 2 (67) | 2 (50) | ||||||
| APW | 1 | 1 | 1 | ||||||||||
| May | 1.0 × 102 | 3.0 × 103 | 4 | 7 | TCBS | 2 (50) | 4 (57) | 1 (14) | |||||
| APW | 2 | 2 | |||||||||||
| June | 2.5 × 103 | 5.0 × 104 | 5 | 11 | TCBS | 5 (100) | 9 (82) | 1 (9) | |||||
| APW | 1 | ||||||||||||
| July | 1.1 × 1014 | 1.0 × 106 | 6 | 8 | TCBS | 2 (33) | 1 (13) | 1 (13) | |||||
| APW | 1 | 2 | 2 | 2 | 1 | 2 | |||||||
| August | 1.0 × 106 | 3.0 × 107 | 12 | 11 | TCBS | 3 (25) | 4 (36) | 2 (17) | 1 (9) | ||||
| APW | 5 | 2 | 4 | 2 | |||||||||
| September | 3.0 × 105 | 2.0 × 106 | 5 | 5 | TCBS | 4 (80) | 2 (40) | 2 (40) | |||||
| APW | 1 | 1 | |||||||||||
| October | 1.5 × 105 | 1.0 × 106 | 8 | 14 | TCBS | 4 (50) | 6 (43) | 2 (25) | 1 (7) | ||||
| APW | 2 | 7 | |||||||||||
| Total | 43 | 60 | 34 (79) | 42 (70) | 6 (14) | 10 (17) | 3 (7) | 6 (10) | 2 (3) | ||||
Ecology and diversity of Vibrio strains.
The Wilcoxon matched-pair signed-ranks nonparametric test computed on abiotic data (N total, phosphate, dissolved oxygen, temperature, salinity, and pH) measured monthly in both sampling sites did not show significant differences, except for the N total concentration, which was significantly higher in the Foglia than in the Metauro estuary (Fig. 1; P = 0.0425). This finding coincides with a significantly higher number of Vibrio strains recovered from the Foglia estuary compared to the Metauro estuary and with a similar trend (Fig. 1; P = 0.018). We observed also a positive correlation between the Vibrio occurrence (Fig. 1b and d) and temperature (Fig. 1a and c) throughout the study (ρ = 0.559, P = 0.038, Spearman coefficients). These results are in agreement with recently reported data concerning the Southern Italian coast (7).
FIG. 1.
Viable Vibrio spp. log10 of CFU 100 ml−1 (○), temperature (▴), and per mille salinity NaCl (□) measured monthly in the Metauro (a) and Foglia (c) estuaries. Viable Vibrio spp. log10 CFU 100 ml−1 (○), nutrient concentrations as total nitrogen micrograms liter−1 (●) and phosphate micrograms liter−1 (⧫) and as dissolved oxygen milligrams liter−1 (▵) measured monthly in the Metauro (b) and Foglia (d) estuaries. All abiotic parameters were measured as previously reported by Fiorentini et al. (13).
Dendrograms (Fig. 2) group the strains by the different PhP types within each Vibrio species: the unweighted-linked clustering analysis identified nine clusters of common PhP types (C), which are defined by levels of similarity ranging from 75 to 97.5%. Single phenotypes (S) significantly different from the most common PhP types were also found. A range of diversity from 0.802 to 0.846 was determined among V. alginolyticus strains. Of interest, PhP type C7 was found to be common to both V. alginolyticus 457 and non-O1 V. cholerae 420 collected from Foglia estuary, while all V. parahaemolyticus strains belonged to a few different PhP types and elicited a low diversity. Finally, the non-O1 V. cholerae strains analyzed yielded a diversity index of 0.822. No evident relationship between the PhP types and the origin of the isolates was found. According to our findings of identical PhP types in both rivers and during different months it is possible to hypothesize that Vibrio strains belonging to various species may survive in largely different physicochemical conditions.
FIG. 2.
Dendrograms showing phenotypic similarity among isolated Vibrio strains of different species, their virulence properties, and sites and times of sampling. Abbreviations used: ID level, identity level; Hly, hemolysin; CT, cytotoxin; M and F, Metauro and Foglia estuary, respectively; S1 to S6, single PhP types; C1 to C9, common PhP types.
Pathogenic properties of Vibrio strains.
A total of 49 Vibrio strains characterized by the PhP system were assayed for their ability to adhere and to induce cytotoxicity in cultured mammalian cells. The 35.5% of Vibrio strains recovered from the Foglia estuary and the 66.7% of the strains recovered from the Metauro estuary were able to adhere to HEp-2 cells. A total of 23 strains had adhesive properties to HEp-2 cells (46.9%), 19 strains produced cytotoxic effects on cultured cells (38.8%), and 5 strains were able to release in the supernatant factors or toxins capable of causing cytopathic effects consisting of retraction and rounding up of cultured cells (10.2%). Adhesive and cytotoxic features were contemporaneously present in 45.5% of V. parahaemolyticus strains, 25% of non-O1 V. cholerae strains, and 18.8% of V. alginolyticus strains. Only one non-O1 V. cholerae strain showed all of the virulence properties we tested.
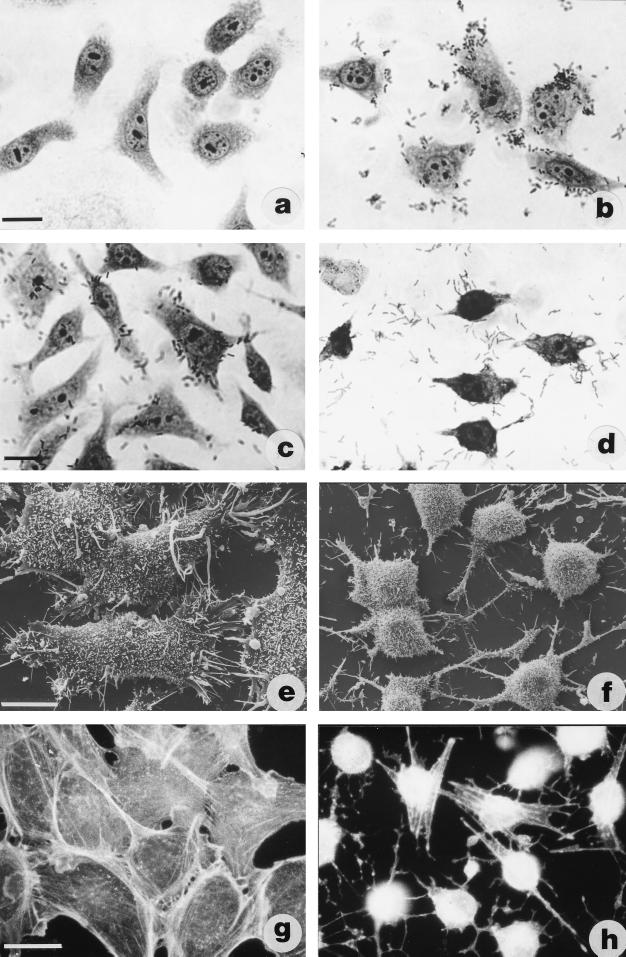
Two adhesiveness patterns were detected either by LM or SEM. Figure 3 shows the adhesion patterns of vibrios to HEp-2 cells, either “clustered and localized” (Fig. 3b, V. parahaemolyticus 86) or “diffuse” (Fig. 3c, V. alginolyticus 46). A large number of Vibrio strains isolated from both sampling sites appeared able to produce cytotoxic effects. In particular, 45% of the 49 PhP-typed Vibrio strains recovered from the Foglia were cytotoxic, while for strains isolated from the Metauro estuary this percentage fell to 28%. In Fig. 3d the cytotoxic effect of non-O1 V. cholerae FA7 can be seen, the HEp-2 cells becoming retracted and roundish within 24 h.
FIG. 3.
Phase-contrast micrographs of HEp-2 cells exposed to bacteria (a to d) showing different patterns of adhesion (b and c) or cytotoxicity (d). Note the clustered bacterial distribution on the cells in panel b and the diffuse one in panel c. SEM of control CHO cells (e) and CHO cells after treatment with cytotoxic supernatant (f). Cytotoxicity due either to bacteria or to soluble bacterial products causes cell rounding (d and f). Bar, 10 μm.
The cellular alterations induced by exposure to bacterial supernatants were investigated. As observed by SEM, CHO cells (Fig. 3e) after overnight exposure to V. alginolyticus 21 supernatant showed a significant rounding up, even if the cells remained attached to the substrate by thin protrusions (Fig. 3f). Such a change in cell shape was accompanied by a breakdown of the actin cytoskeleton (Fig. 3g and h). This effect was observed in 7 of 32 (21.8%) V. alginolyticus strains. This previously unreported in vitro effect could explain the in vivo pathogenicity elicited by some V. alginolyticus strains of clinical interest isolated from wounds and ear infections (4, 11, 16).
The high diversity of strains belonging to the same species, as shown by their phenotypical expression, may explain this strain-specific expression of virulence factors. It still remains unclear which are the most significant environmental factors able to induce a Vibrio strain to express a specific virulence for humans.
On the basis of our data, we conclude that toxigenic Vibrio strains isolated from the estuarine environment of the Northwest Adriatic coast, where seafood production and consumption and recreational and bathing areas are common, may significantly contribute to the onset of sporadic and epidemic outbreaks of diarrheal disease in humans. A long-term research program for the continuous monitoring and study of this specific coastal pollution is needed as an urgent preventive measure to protect human health.
Acknowledgments
We thank L. Ferrante and M. Rocchi for their support in performing the statistical analyses.
This work was partially supported by a grant from MURST-ISS, Prot. MURST, 4/15.1.-1, Research Project “Safeguard of Adriatic Sea” to C.F. and by grant 97.01187.PF49 from the Italian National Research Council CNR Target Project on Biotechnology.
REFERENCES
- 1.Alsina M, Blanch A R. A set of keys for biochemical identification of environmental Vibrio species. J Appl Bacteriol. 1994;76:79–85. doi: 10.1111/j.1365-2672.1994.tb04419.x. [DOI] [PubMed] [Google Scholar]
- 2.Alsina M, Blanch A R. Improvement and update of a set of keys for biochemical identification of environmental Vibrio species. J Appl Bacteriol. 1994;77:719–721. doi: 10.1111/j.1365-2672.1994.tb04419.x. [DOI] [PubMed] [Google Scholar]
- 3.Baumann P, Furniss L, Lee J V. Genus I. Vibrio. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 518–538. [Google Scholar]
- 4.Blake P A, Weaver R E, Hollis D G. Disease of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- 5.Bolinches J, Romalde J L, Toranzo A E. Evaluation of selective media for isolation and enumeration of vibrios from estuarine waters. J Microbiol Methods. 1988;8:151–160. [Google Scholar]
- 6.Caldini G, Neri A, Cresti S, Boddi V, Rossolini G M, Lanciotti E. High prevalence of Vibrio cholerae non-O1 carrying heat-stable enterotoxin encoding genes among Vibrio isolates from a temperate-climate river basin of central Italy. Appl Environ Microbiol. 1997;63:2934–2939. doi: 10.1128/aem.63.7.2934-2939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caruso G, Zaccone R, Genovese L, Crisafi E. Microbiological monitoring of Castellammare gulf (TP) water for their suitability in marine aquaculture. Microbiologica. 1998;21:169–182. [PubMed] [Google Scholar]
- 8.Colwell R R, Huq A. Vibrio in the environment: viable but not culturable Vibrio cholerae. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspective. Washington, D.C: American Society for Microbiology; 1994. pp. 117–133. [Google Scholar]
- 9.Epstein P R. Algal blooms in the spread and persistence of Vibrio cholerae. Biosystems. 1993;31:209–221. doi: 10.1016/0303-2647(93)90050-m. [DOI] [PubMed] [Google Scholar]
- 10.Epstein P R, Ford T E, Colwell R R. Marine ecosystem. Lancet. 1993;342:1216–1219. doi: 10.1016/0140-6736(93)92191-u. [DOI] [PubMed] [Google Scholar]
- 11.Farmer J J, III, Hickman-Brenner F W, Kelly M T. Vibrio. In: Lennette E H, Balows A, Hausler W J Jr, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C: American Society for Microbiology; 1985. pp. 282–301. [Google Scholar]
- 12.Filetici E, Bonadonna L, Ciccozzi M, Anastasio M P, Fantasia M, Shimada T. Phenotypic and genotypic biotyping of environmental strains of Vibrio cholerae non-O1 isolated in Italy. Appl Environ Microbiol. 1997;63:4102–4106. doi: 10.1128/aem.63.10.4102-4106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorentini C, Barbieri E, Falzano L, Matarrese P, Baffone W, Pianetti A, Katouli M, Kühn I, Möllby R, Bruscolini F, Casiere A, Donelli G. Occurrence, diversity and pathogenicity of mesophilic Aeromonas in estuarine waters of the Italian coast of Adriatic Sea. J Appl Microbiol. 1998;85:501–511. doi: 10.1046/j.1365-2672.1998.853517.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaper J B, Morris J G, Levine M M. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kühn I, Austin D A, Austin B, Blanch A R, Grimont P A D, Jofre J, Koblavi S, Larsen J L, Möllby R, Pedersen K, Tiainen T, Verdonck L, Swings J. Diversity of Vibrio anguillarum isolates from different geographical and biological habitats, determined by the use of combination of eight different typing methods. Syst Appl Microbiol. 1996;19:442–450. [Google Scholar]
- 16.Matsiota-Bernard P, Nauciel C. Vibrio alginolyticus wound infection after exposure to seawater in an air crash. Eur J Clin Microbiol Infect Dis. 1993;12:474–475. doi: 10.1007/BF01967448. [DOI] [PubMed] [Google Scholar]
- 17.Möllby R, Kuhn I, Katouli M. Computerized biochemical fingerprinting: a new tool for typing of bacteria. Rev Med Microbiol. 1993;4:231–241. [Google Scholar]
- 18.Morris J G, Takeda T, Tall B D, Losonsky G A, Bhattacharya S K, Forrest B D, Kay B A, Nishibuchi M. Experimental non-O group I Vibrio cholerae gastroenteritis in humans. J Clin Investig. 1990;85:697–705. doi: 10.1172/JCI114494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piersimoni C, Morbiducci V, Scalise C. Non-O1 Vibrio cholerae gastroenteritis and bacteremia. Lancet. 1991;337:791–792. doi: 10.1016/0140-6736(91)91409-n. [DOI] [PubMed] [Google Scholar]
- 20.Sakazaki R. Control of contamination with V. parahaemolyticus in sea food and isolation and identification of the vibrios. In: Hobbs B C, Christian J H B, editors. The microbial safety of food. New York, N.Y: American Press, Inc.; 1973. pp. 375–385. [Google Scholar]
- 21.Volterra L, Aulicino F A, Bonadonna L, De Mattia M, Di Girolamo I, Liberti R, Mancini L. Microbial analyses of Adriatic Sea mucilages. In: Vollenweider R A, Marchetti R, Viviani R, editors. Marine coastal eutrophication. New York, N.Y: Elsevier Science Publishing; 1992. pp. 551–556. [Google Scholar]
- 22.West P A, Lee J V. Ecology of Vibrio species, including Vibrio cholerae in natural water of Kent, England. J Appl Bacteriol. 1982;52:435–448. doi: 10.1111/j.1365-2672.1982.tb05074.x. [DOI] [PubMed] [Google Scholar]
- 23.West P A. The human pathogenic vibrios. A public health update with environmental perspectives. Epidemiol Infect. 1989;103:1–34. doi: 10.1017/s0950268800030326. [DOI] [PMC free article] [PubMed] [Google Scholar]