Abstract
Studies performed in the past in our laboratory have detailed the development of sulphur mustard lesions in the domestic, white pig using small glass chambers to achieve saturated vapour exposure under occluded conditions. We have now used this experimental model to produce cutaneous lesions for detailed histopathological studies following challenge with lewisite. Histological examination of resulting lesions have revealed that although the overall pattern of lesion development is similar to that seen following mustard challenge, the time-course of cellular events is very much compressed. The epidermis showed focal basal cell vacuolation with associated acute inflammation as early as one hour postexposure. Coagulative necrosis of the epidermis and papillary dermis was complete by 24 hours and followed the appearance of multiple coalescent blisters between six and 12 hours postexposure. At 48 hours, the lesions were full thickness burns with necrosis extending into the deep subcutaneous connective and adipose tissues. The study of lesions beyond 24 hours revealed early epithelial regeneration at the wound edge. The overall spontaneous healing rate of these biologically severe lesions was significantly faster than comparable sulphur mustard injuries and probably reflected a lack of alkylation of DNA and RNA.
Keywords: Lewisite, dichloro(2-chlorovinyl)arsine, domestic pig, histopathology, skin lesion(s), burn, chemical
Lewisite (dichloro(2-chlorovinyl)arsine) is an organic trivalent arsenical compound possessing significant systemic toxicity and marked vesicant (blistering) properties in man (Goldman & Dacre 1989). It was isolated in pure form by Lee-Lewis in 1918 in the USA (Maynard 1996) and although its use as a chemical weapon was proposed and the Former Soviet Union is known to have stockpiled significant quantities of Lewisite (particularly as a weaponized mixture with sulphur mustard) it was never actually used in war. According to Prentiss (1937), the first shipment was on its way to Europe when the 1918 armistice was agreed that ended the first World War; this shipment was apparently destroyed at sea. Despite this, interest in Lewisite has persisted as it is a lethal compound with properties which make it a candidate for use as a chemical weapon.
Compared to sulphur mustard, a well recognized vesicant chemical warfare agent, there has been little research performed to define the natural history of Lewisite-induced skin lesions in either experimental animals or man. Studies performed in the laboratories at CBD have previously shown that the pig can be used to model both the development and healing of sulphur mustard vapour-induced lesions in man (Brown & Rice 1997; Rice 1997). Furthermore, the pig has been widely used in wound healing research because its skin has a number of similarities to that of man (Montagna & Yun 1964) and has been previously recognized as a valid model for a range of cutaneous toxicity tests (Khan 1984).
The purpose of these studies was to define the development and early healing phases of skin lesions in the domestic, white pig induced by Lewisite vapour under occluded conditions by studying the histopathology of lesions at various time-points ranging from one hour to three weeks postexposure.
Materials and methods
Chemicals
Lewisite was synthesized at CBD and provided as a solution in hexane containing 12.0 mg/ml of pure Lewisite. Halothane and Euthatal (pentobarbitone sodium, 200 mg/ml) were supplied by Centaur Services (Castle Cary, Somerset), and all other chemicals were from Taab Laboratories Equipment Ltd, Reading, UK.
Animals
Fifteen adult, female domestic white pigs (body weight range 20–32 kg, mean 27 kg) were housed in straw bedded pens before the application of Lewisite and allowed access to food and water ab libitum. The care, husbandry and use of all experimental animals used in this study was subject to peer review within the Animal Ethics Committee and authorization under the Animals (Scientific Procedures) Act 1986.
Experimental procedure
Each animal received general anaesthesia consisting of inhaled halothane (1–3%) administered in a stream of nitrous oxide and oxygen. Whilst anaesthetized, an area measuring approximately 35 by 25 cm (875 cm2) of dorsal skin was prepared by gentle wet shaving and drying in readiness for dosing chamber application. Each animal was exposed to Lewisite vapour over four 10 cm2 areas at a dose of 0.3 mg/cm2 using an occluded glass fibre disc saturated with Lewisite in hexane solution (250 μl of a solution containing 12 mg/ml). The glass fibre discs were held in the roof of a circular glass chamber, inverted and glued using an acrylic adhesive to the shaved area of dorsal skin so that the glass fibre disc does not make contact with the skin (see Figure 1). Even exposure to saturated Lewisite vapour is achieved by allowing the liquid Lewisite to evaporate from the glass fibre disc using the animal's body heat. The occluded discs were left in situ until sacrifice or for 6 h before being carefully removed when the animals were recovered from general anaesthesia.
Figure 1.

Diagrammatic representation of the Lewisite dosing apparatus. The glass fibre disc was saturated with a solution of Lewisite in hexane.
The animals were humanely killed with an intravenous overdose of Euthatal (pentobarbitone sodium, 200 mg/ml) in groups of at least two animals at 1,2,4,6,9, and 12 h and 1,3, 7 and 21 days after the initial application of Lewisite dosing chambers.
Autopsy and tissue selection
Samples for routine histological examination were excised from each exposed site at autopsy according to the schedule shown diagrammatically in Figure 2. These blocks of skin tissue were fixed in 10% neutral buffered formalin and processed by standard techniques for observation by light microscopy. The staining technique employed was Harris' haematoxylin and eosin method.
Figure 2.

Diagrammatic representation of lesion sites and tissue excision protocol.
Calculation of areas of re-epithelialization
Since many of the sections of the exposed sites from the later time-points showed evidence of epithelial regeneration from the wound edge, calculations of the area and percentage re-epithelialization for each site were performed. The area of the zone of re-epithelialization (see Figure 3) was calculated by measuring values of R2 at four to six different points around the circumference of the lesion and then calculating the average area of re-epithelialization (A) using the following expression,
 |
and since the original exposed area = 10.00 cm2, then the percentage re-epithelialization (P) is given by,
 |
The figures presented in the Table 1 are based on the measured values of R2 and the calculations of area of re-epithelialization (A) and percentage healing (P) made from such values.
Figure 3.

Diagrammatic representation of the lesion healing zones.
Table 1.
Summary of measured values of R2 and the calculated areas and percentages of spontaneous healing

† Data derived frompreviously reported wound healing studies with sulphur mustard (Rice 1997).
Results
Skin sites excised post-exposure
At 1 and 2 h post-exposure
Skin sections examined at one hour unexpectedly showed small, discrete areas of basal cell vacuolation associated with early acute inflammatory cell infiltration. The majority of the epidermis, however, was histologically normal as was the dermis (including the adnexal structures and blood vessels) and the subcutaneous connective and adipose tissues.
By 2 h, the skin sections showed very similar features to those described above at one hour. The focal areas of epidermal damage were, however, now more conspicuous both in terms of the total number of epidermal cells involved and the degree of inflammatory cell response to the damage. The dermis and underlying tissues remained histologically normal.
At 4 h post-exposure
Again, the areas of epidermal degeneration noted at earlier time-points persisted but now involved large areas of the epidermis as well as the superficial epithelial portions of the adnexal structures. The papillary dermal collagen showed mild inflammatory cell infiltration and oedema accompanied by focal thrombosis of the subepidermal capillary network; individual endothelial cells were swollen with early pyknotic changes in their nuclei (see Figure 4).
Figure 4.
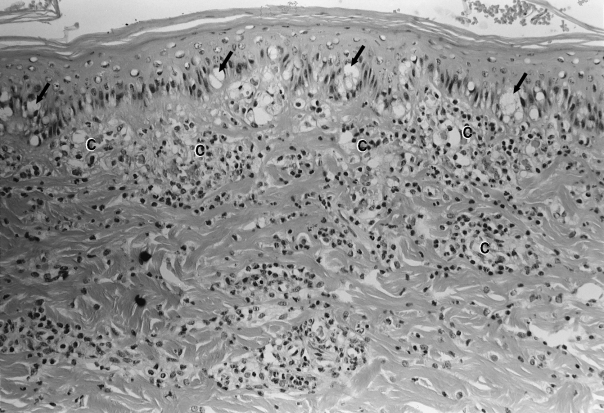
A section of dorsal skin showing advanced epidermal degeneration and clear vacuolation of basal keratinocytes (arrows). The underlying dermis is diffusely infiltrated by acute inflammatory cells and the papillary dermal capillaries (C) are necrotic and thrombosed. Haematoxylin and Eosin ×250.
The deep reticular dermis and subcutaneous fatty tissues remained histologically normal.
At 6 h post-exposure
The changes noted at four hours now involved the entire epidermis within the exposed zone. The epidermis also showed obvious areas of dermo-epidermal separation measuring between 0.003 and 0.048 cm (see Figure 5).
Figure 5.

A section of dorsal skin showing advanced epidermal degeneration and obvious areas of dermo-epidermal separation (arrows). The underlying dermis (D) is diffusely infiltrated by acute inflammatory cells and individual collagen bundles are beginning to become separated by the accumulation of oedema fluid. Haematoxylin and Eosin ×250.
The papillary dermis was now obviously oedematous and acutely inflamed with early destruction of individual collagen bundles. The superficial capillary network was completely thrombosed with no discernible cellular detail to the lining endothelial cells. The deeper aspects of the demis and subcutaneous fat were still histologically normal.
At 9 h post-exposure
The epidermis showed complete epidermal degeneration with extensive areas of dermo-epidermal separation leading to the formation of multiple small blisters measuring between 0.148 and 0.417 cm in diameter and 0.011 and 0.045 cm in depth (see Figure 6).
Figure 6.
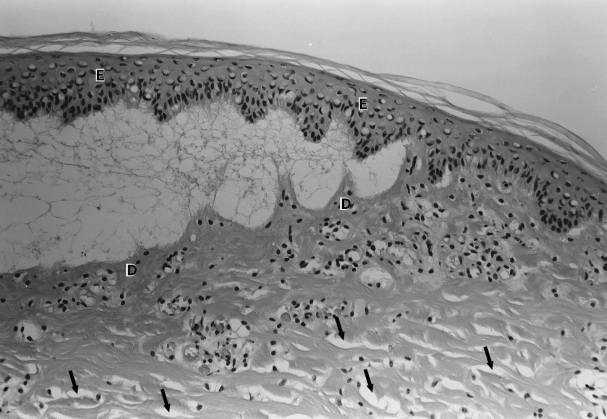
A section of dorsal skin showing the formation of a subepidermal blister, the roof of which is formed from degenerate epidermis (E) and the base by early necrotic dermis (D). As in Figure 4, separation of dermal collagen bundles by oedema fluid is also evident (arrows). Haematoxylin and Eosin ×250.
The histological changes to the papillary dermis, the reticular dermis and the subcutaneous connective tissues and fat were exactly similar to those described above at 6 h.
At 12 h post-exposure
The histological appearances at 12 h were not too different to those described at 9 h. The histological picture was still dominated by areas of dermo-epidermal separation and the formation of subepidermal blisters measuring between 0.456 and 0.677 cm in diameter and 0.012 and 0.081 cm in depth.
The lesions now also showed mild oedema and acute inflammation of the reticular dermis and subcutaneous fat with focal areas of coagulative necrosis.
At 1 day post-exposure
These sections revealed full thickness lesions involving the epidermis, dermis and subcutaneous connective and fatty tissues.
The epidermis was completely necrotic and had separated from the dermis in many areas forming coalescent blisters measuring between 0.273 and 0.761 cm in diameter and 0.018 and 0.085 cm in depth. Occasional blisters were noted that were > 1.000 cm in diameter; these clearly represented the coalescence of smaller blisters in close proximity.
Towards the centre of each lesion, the epidermis and papillary dermis showed established coagulative necrosis with the formation of an immature eschar. Oedema, acute inflammation and focal haemorrhage extended into the deep subcutaneous tissues.
In one or two sections of the four lesions examined there was evidence of early regeneration at the wound edge. Skin osites revealed full thickness lesions with an established eschar and coagulative necrosis extending focally into the deep reticular dermis and subcutaneous tissues.
All lesions revealed evidence of epithelial regeneration at the wound edge that was beginning to undermine the surface eschar. The calculated percentage healing rates derived from the histological measurements for these lesions are summarized in Table 1.
At 7 days post-exposure
These lesions showed exactly similar features to those described above at 3 days. The only significant difference was the degree of epithelial regeneration at the wound edge.
At 21 days post-exposure
In every case, the exposed region was covered by a well developed eschar composed of necrotic debris and coagulated fibrin. The eschar was undermined in all cases by tongues of regenerative epidermis that had migrated for varying distances beneath the surface scab (see Table 1). The tongues of regenerative epithelium all showed features of recent proliferation including acanthosis, loss of the rete ridge pattern, hyperkeratosis and parakeratosis (see Figure 7).
Figure 7.

A section of dorsal skin showing early migration of a tongue of regenerative epidermis (R) at the wound edge beneath the well established surface eschar (E). The inflammatory infiltrate which delineates the boundary between the nonviable surface eschar and viable dermis is clearly visible (arrows). Haematoxylin and Eosin ×125.
The dermis in the region of the exposed zone was mildly oedematous and composed of active granulation tissue supporting a complex network of new blood vessel formation and a large population of active fibroblasts. There was some evidence of mild oedema and active inflammation extending into the deep dermis directly beneath the exposed region but also spreading laterally beyond the confines of the exposed zone and into the subcutaneous fat.
Histological changes beyond the edges of exposure sites
Previous studies conducted in the pig with sulphur mustard vapour under similar conditions revealed areas of superficial epidermal necrosis which extended beyond the margins of exposure. In the current study, although there was initially macroscopic evidence of erythema around the margins of the dosing chambers, histological examination of skin samples excised from this boundary region revealed no evidence of any irreversible damage to the epidermis or dermis.
Rates of spontaneous healing
All lesions examined from one day postexposure onwards revealed evidence of epithelial regeneration from the wound edge; at this time, necrosis had extended to and involved the superficial adnexal structures, and therefore there was no significant epithelial healing from remnants of these structures as would be expected in a more superficial chemical burn.
Using the simple formulae outlined above, it was possible to calculate the area of epithelial regeneration in each case and from this figure determine the percentage of the original lesion area that had re-epithelialized at each time-point. These data were compared to similar data for sulphur mustard lesions derived from a previously reported study (Rice 1997) and show that although the lesions are full thickness, those induced by Lewisite vapour have a spontaneous healing rate which is approximately 20 times that of sulphur mustard injuries at three weeks postexposure (see Figure 8).
Figure 8.

Comparative spontaneous healing rates for sulphur mustard (▪) and Lewisite (•) vapour-induced lesions in the domestic white pig.
Discussion
In general terms this study has shown that the use of the current animal model, which had been used previously to study the development of sulphur mustard burns (Lindsay & Rice 1995; Brown & Rice 1997; Rice 1997), results in reproducible skin injuries after challenge with saturated Lewisite vapour under occluded conditions and that these injuries are comparable with those previously described in human skin (Davis 1944; McGown et al. 1987).
Furthermore, the overall pattern of pathological changes observed in this study is similar to that observed with sulphur mustard (Lindsay & Rice 1995; Brown & Rice 1997) and consist of epidermal and dermal necrosis of a coagulative type which may extend, albeit focally, into the subdermal connective and adipose tissues. Prior to the development of necrosis, the lesion is characterized in the pig by the development of small areas of dermo-epidermal separation which enlarge by coalescence to form macroscopically visible, subepidermal blisters.
Despite these similarities, this study has also highlighted two important differences between sulphur mustard and Lewisite-induced skin lesions. The first, is the time frame over which the lesion progresses. In this study, we have been able to identify significant changes at the light microscopic level as early as one hour postexposure. At this time there is significant vacuolation of basal epidermal cells associated by four hours postexposure with an early inflammatory response; these changes are indicative irreversible damage to the cell membrane and disruption of the cellular energy cycle. Lewisite is thought to bind to dihydrolipoic acid, a component of the pyruvate dehydrogenase complex (Peters 1953), preventing the formation of acetyl coenzyme A and a build up of pyruvate. Lewisite is also known to bind to and inhibit a wide range of important cellular macromolecules such as proteins and enzymes rich in sulphhydryl groups (Ehrlich 1909). The onset of dermo-epidermal separation, blister formation and necrotic changes in dermis, vasculature and adnexal structures also occur at earlier time-points compared to the developing sulphur mustard injury (Lindsay & Rice 1995; Brown & Rice 1997). This supports the theory that, although the overall pattern of lesion development for the two agents is similar, there are differences in the molecular mechanism of action.
The second difference between the skin burns induced by sulphur mustard and Lewisite highlighted by the current study is the difference between the spontaneous rates of epithelial healing (see Figure 8). It is well known that sulphur mustard is a powerful bifunctional alkylating agent capable of crosslinking DNA and RNA (Fox & Scott 1980). In a previously reported study of the healing of sulphur mustard injuries in the pig (Rice 1997), the delayed spontaneous epithelial healing of sulphur mustard was investigated and found to be dependant on two important factors:
• Alkylation of epidermal cells extended beyond the immediate region of exposure. Although epidermal cells in this area may not ultimately die, the level of chemical damage to the cell's DNA may be sufficient to delay or prevent effective replication. Surface healing with the growth of new skin (re-epithelialization) of ulcerated lesions following the rupture of blisters relies partly on regeneration from undamaged epidermis at the edge of the wound and partly on regeneration from intact hair shafts (Willems 1989).
• In addition to achieving effective epidermal regeneration, re-epithelialization itself is dependant upon the presence of an appropriate substrate on which epidermal cells can migrate (Woodley et al. 1985; Shakespeare & Shakespeare 1987). The papillary dermis and basement membrane are vital in this respect and not only provide a structural scaffold for the epidermis but also act as molecular signals for the subsequent maturation of the overlying new epidermis (Fleming 1991). The studies in pigs clearly demonstrate that the collagen of the papillary dermis is altered by exposure to sulphur mustard and in this altered state may no longer function normally (Lindsay & Rice 1995).
Unlike sulphur mustard, there is no evidence that Lewisite alkylates DNA and the increased spontaneous healing rate of these lesions may be explained purely in terms of the absence of alkylation of the DNA of keratinocytes at the wound edge. The difference in healing rate cannot be explained in terms of differences in either the size or biological severity of the two lesions.
Conclusions
Studies performed in the past in our laboratory have detailed the development of sulphur mustard lesions in the domestic, white pig using small glass chambers to achieve saturated vapour exposure under occluded conditions. We have now used this experimental model to produce cutaneous lesions for detailed histopathological study following challenge with lewisite.
Histological examination of resulting lesions has revealed that although the overall pattern of lesion development is similar to that seen following mustard challenge, the time-course of cellular events is very much compressed. The epidermis shows focal basal cell vacuolation with associated acute inflammation as early as one hour postexposure. Coagulative necrosis of the epidermis and papillary dermis is complete by 24 hours and follows the appearance of multiple coalescent blisters between six and 12 hours postexposure. At 48 hours, the lesions are full thickness burns with necrosis extending into the deep subcutaneous connective and adipose tissues.
The study of lesions beyond 24 hours reveals early epithelial regeneration at the wound edge. The overall spontaneous healing rate of these biologically severe lesions is significantly faster than comparable mustard injuries and probably reflects a lack of alkylation of DNA and RNA.
References
- 1.Brown RFR, Rice P. Histopathological changes in Yucatan minipig skin following challenge with sulphur mustard. A sequential study of the first twenty-four hours following challenge. Int. J. Exp. Pathol. 1997;78:9–20. doi: 10.1046/j.1365-2613.1997.d01-236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis J. Dermatologic aspects of vesicant war gases (dichloroethyl sulphide and dichlorovinyl arsine) J. Am. Med. Assoc. 1944;126:209. [Google Scholar]
- 3.Ehrlich P. Über den jetzigen Stand der Chemotherapie. Ber. Dt. Chem. Ges. 1909;42:17. [Google Scholar]
- 4.Fleming S. Cell adhesion and epithelial differentiation. J. Pathol. 1991;164(2):95. doi: 10.1002/path.1711640202. [DOI] [PubMed] [Google Scholar]
- 5.Fox M, Scott M. The genetic toxicology of nitrogen and sulphur mustard. Mutation Res. 1980;75:131–168. doi: 10.1016/0165-1110(80)90012-3. [DOI] [PubMed] [Google Scholar]
- 6.Goldman M, Dacre J. Lewisite: its chemistry, toxicology and biological effects. Rev. Environ. Contamin. Toxicol. 1989;110:75–115. doi: 10.1007/978-1-4684-7092-5_2. [DOI] [PubMed] [Google Scholar]
- 7.Khan MA. Mini-pig: advantages and disadvantages as a model in toxicity testing. J. Am. Coll. Toxicol. 1984;3:337–342. [Google Scholar]
- 8.Lindsay CD, Rice P. Changes in connective tissue macromolecular components of Yucatan mini-pig skin following application of sulphur mustard vapour. Hum. Exp. Toxicol. 1995;14:341–348. doi: 10.1177/096032719501400404. [DOI] [PubMed] [Google Scholar]
- 9.Maynard RL. Organic Arsenicals. In: Marrs TC, Maynard RL, Sidell FR, editors. Chemical Warfare Agents: Toxicology and Treatment. New York: John Willey & Sons; pp. 175–184. [Google Scholar]
- 10.McGown EL, van Ravensway T, Dumlao CR. Histologic changes in nude mouse skin and human skin xenografts following exposure to sulphhydryl reagents: arsenicals. Toxicol. Pathol. 1987;15:149–156. doi: 10.1177/019262338701500204. [DOI] [PubMed] [Google Scholar]
- 11.Montagna W, Yun YS. The skin of the domestic pig. J. Invest. Dermatol. 1964;43:11–21. [PubMed] [Google Scholar]
- 12.Peters R. Significance of biochemical lesions in the pyruvate oxidase system. Br. Med. Bull. 1953;9:116. doi: 10.1093/oxfordjournals.bmb.a074325. [DOI] [PubMed] [Google Scholar]
- 13.Prentiss AM. Chemicals in War. New York: McGraw-Hill Book Company; [Google Scholar]
- 14.Rice P. The surgical treatment of sulphur mustard burns. J. Defence Sci. 1997;2(3):343–347. [Google Scholar]
- 15.Shakespeare VA, Shakespeare PG. Growth of cultured human keratinocytes on fibrous dermal collagen: an electron scanning microscope study. Burns. 1987;13:343. doi: 10.1016/0305-4179(87)90122-7. [DOI] [PubMed] [Google Scholar]
- 16.Willems JL. Clinical management of mustard gas casualties. Annales Med. Militaris (Belgicae) 1989;3(Suppl.):1–61. [Google Scholar]
- 17.Woodley DT, O'Keefe EJ, Prunieras M. Cutaneous wound healing: a model for cell–matrix interactions. J. Am. Acad. Dermatol. 1985;12:420. doi: 10.1016/s0190-9622(85)80005-0. [DOI] [PubMed] [Google Scholar]


