Abstract
Parathymic lymph nodes (PTLNs) have been identified in several species, but in humans they have been noted only once before in a study 90 years ago using fetal material. We now report their occurrence in children. Human PTLNs are small but distinctive lymphatic organs located on the surface of the thymus (or sometimes between the upper and lower lobes of the thymus) and covered with the thymic capsule. Histologically, the medullary cords of these lymph nodes were found to be thin, with only small numbers of plasma cells. In addition, they had a well-developed paracortical area rich with high endothelial venules (HEV), but a thin cortex, including only a few undeveloped follicles. Flow cytometric analysis of PTLNs revealed that the ratios of T:B cells (14·6±9·3) and of CD4+:CD8+ T cells (4·9±1·4) in PTLNs were much higher than in other peripheral lymphoid tissues and in peripheral blood. Because of these characteristics of the human PTLNs, we propose that the human PTLNs might influence the functional differentiation of T cells.
INTRODUCTION
The only previous report that humans have a parathymic lymph node (PTLN) was in a study of human fetus thymic lymphatics by Severeanu in 1909.1 The presence of thymic lymphatics and a thymus-specific lymph node have been reported in other animals, namely, in sheep2 and in guinea-pig.3 According to these reports, there were various arrangements in the way in which the lymphatics enter the thymus-specific groups of the lymph nodes, and the way in which those lymphocytes produced in the thymus enter the PTLN. In the present study, attention was focused on the function of the PTLN and the possibility of its involvement with T-cell differentiation.
MATERIALS AND METHODS
Thymus and parathymic lymph node
Human thymus and parathymic lymph node materials were obtained, with the assistance of the cardiac surgery team of Mie University School of Medicine, from children up to 5 years of age undergoing cardiac surgery or from cadavers of children up to 2 years of age undergoing post-mortem examination in the Osaka Medical Examiner’s Office, the Medical Examiner’s Office of Hyogo Prefecture, and the Department of Forensic Medicine and Sciences, Mie University School of Medicine. For histological analysis, tissue was embedded in OCT compound (Miles Laboratories. Inc., IN) and frozen at −80° for frozen section or fixed in 10% buffered formalin and embedded in paraffin. Paraffin sections were stained with haematoxylin and eosin. For cell suspension, lymphocytes were separated by gently teasing cells out of the organ pieces. The cells were immediately used for analysis.
Antibodies
Non-labelled anti-CD3, anti-CD19, anti-CD45RA, anti-CD45RO and anti-Vα24 antibodies, control mouse immunoglobulin G (IgG), fluorescein isothiocyanate (FITC)-conjugated anti-CD4/phycoerythrin (PE)-conjugated anti-CD8 antibody, and FITC-conjugated anti-CD3/PE-conjugated anti-CD19 antibody were purchased from Immunotech SA (Marseille, France). Non-labelled anti-CD8 and anti-CD20 antibodies were purchased from Dako (Glostrup, Denmark). FITC-conjugated anti-CD4 antibody, FITC-conjugated anti-CD3 antibody, PE-conjugated anti-CD3 antibody, PE- conjugated rat anti-mouse IgG1 antibody, peridinin chlorophyll protein-conjugated (PerCP-conjugated) rat anti-mouse IgG1 antibody, and PerCP-conjugated rat anti-mouse IgG2a+b antibody were purchased from Becton Dickinson (San Jose, CA).
Immunohistochemistry
Acetone-fixed frozen sections were rehydrated in phosphate-buffered saline (PBS), and endogenous peroxidase activity was quenched in 0·3% H2O2 on absolute methanol at 25° for 20 min. The sections were immunostained with antibodies specifically reactive with surface antigens on T or B cells for 2 hr at room temperature. The immunoperoxidase procedure was performed according to the manufacturer’s instructions (Vector Laboratories). Peroxidase activity was detected with the diaminobenzidine peroxidase substrate. Immunoperoxidase slides were counterstained with haematoxylin.
Isolation of PTLN lymphocyte subsets
To obtain CD3+ CD4+ CD8− single-positive
and CD3+ CD4− CD8− double-negative T cells from PTLN, the following procedure of negative selection was applied. Dispersed PTLN cells were incubated for 30 min at 4° with mouse anti-human CD8 antibody and mouse anti-human CD19 antibody. Labelled cells were then removed with goat anti-mouse IgG-coupled magnetic beads (M-450 Dynabeads; Dynal AS, Oslo, Norway). The separated cells had a purity of over 97%.
Three-colour immunofluorescence
All fluorescence analyses were carried out at 4° in PBS. Dispersed thymocytes and lymph node cells (5×105) were incubated with an optimal amount of the non-labelled first antibody for 30 min, followed by two washes and subsequent incubation with PerCP-conjugated rat anti-mouse IgG for 30 min. After two additional washes, cells were incubated with directly labelled dual-colour antibodies for 30 min, and washed twice. All fluorescence analyses were carried out in a fluorescence-activated cell sorter (FACScan) flow cytometer (Becton Dickinson) using Cellquest software. Upon data acquisition a gate was set on intact cells by forward-/side-scatter analysis.
RESULTS
Parathymic lymph nodes in humans were located on the surface of the thymus, or between the upper and lower lobes of the thymus, covered with the common capsule of the thymus (Fig. 1a,b). Usually, several PTLNs were present in one individual; the ones we investigated ranged in size from 1–2 mm to 6–7 mm.
Figure 1.
Thymus and PTLNs obtained from a 3-month-old female autopsy case. (a) Macroscopic view of the thymus and the PTLNs (arrows). The PTLNs are observed on the surface of the apex of the cervical thymus or between the cervical and thoracic lobes. (b) Microscopic view of the PTLN of the cervical thymus (H&E stain). The PTLN is covered by the common capsule of the thymus. In this node, there is hardly any medullar cord to be found ×30. (c) High magnification view of a part of the PTLN as framed in (b). A small follicle in the subcapsular area and many HEVs in paracortical area are found; ×150.
We found these lymph nodes in all cases where the subject was 5 years of age or younger. In contrast, no PTLNs could be found in any adult cases in medico-legal autopsies.
The PTLN possessed an extremely thin cortex with a few follicles. The medullary cords were poorly developed with only small numbers of plasma cells, but the paracortical area was well developed and contained many high endothelial venules (HEVs) (Figs 1c and 2a). In contrast, the histological appearance of the cervical lymph nodes or mesenteric lymph nodes was quite different: the cortical parenchyma of these nodes consisted of an outer layer containing a number of large germinal centres while the medullary cords were well-developed and contained a number of plasma cells (Fig. 2b,c).
Figure 2.
Histological findings of the PTLN and mesenteric lymph nodes (H&E stain). Representative figures using both the PTLN and the mesenteric lymph nodes from the same donor (20-month-old female autopsy case) are shown. (a) A PTLN; this node possessed a thin cortex with a small number of follicles; a large part of this node is dominated by the paracortical area; ×30. (b) A small mesenteric lymph node (×30), and (c) a large mesenteric lymph node (×15). The cortical parenchyma of these nodes consisted of an outer layer containing a number of large germinal centres while the medullary cords were well-developed.
Immunohistochemical staining with anti-CD3 antibody and anti-CD20 antibody revealed that the superficial cortex possessed small-sized follicles containing B cells while the deeper paracortical area contained a large number of T cells (Fig. 3a,b). Moreover, anti-CD20+ B cells and CD3+ T cells were found to adhere to the walls of the HEV (Fig. 3c,d). Representative figures using frozen sections of the PTLN from Case 1 in Table 1 (16-day-old male undergoing cardiac surgery) are shown. The cortex of the PTLN in this case was much thinner than in older children and had no secondary follicles.
Figure 3.
Immunostaining of frozen sections of PTLN obtained from a 16-day-old male (undergoing open cardiac surgery). (a) Stained with anti-CD3 antibody; many of the PTLN cells are positive; ×150. (b) Stained with anti-CD20 antibody; the subcapsular area and a small primary follicle are stained; ×150. (c) High magnification view of HEV stained with anti-CD3 antibody. Adhesion between CD3-positive cells and high endothelial cells is found; ×450. (d) High magnification view of HEV stained with anti-CD20 antibody. Adhesion between CD20-positive cells and high endothelial cells is also found. CD20-positive cells are also present generally in the perivascular area; ×450. Arrowheads show the HEVs in the PTLN.
Table 1.
CD4+:CD8+ ratio and CD3+:CD19+ ratio of PTLN cells in the 11 individuals studied

*Abbreviations used in diagnosis are follows: TGA, transposition of the great arteries; DS, Down syndrome; TOF, tetralogy of Fallot; SIDS, sudden infant death syndrome; VSD, ventricular septal defect.
Two-colour immunofluorescence was used to characterize the cell distributions of each subpopulation of the PTLN lymphocytes. Typical data from the CD4/CD8 and CD3/CD19 two-colour immunofluorescence analyses are shown in Fig. 4(a,b). In this example (Case 8 in Table 1), 72% of the unseparated thymocytes were CD4+ CD8− T cells, 17% were CD4− CD8+ T cells, 9% were CD19+ B cells, and the remaining 1% were CD4− CD8− CD19− cells. In almost all cases we investigated, the ratios of T cells:B cells (14·6±9·3) and of CD4+ T cells:CD8+ T cells (4·9±1·4) in PTLNs were much higher than in other peripheral lymphoid tissues and in peripheral blood. This result is summarized in Table 1.
Figure 4.
Two-colour immunofluorescence analysis of PTLN cells. Analysis of surface CD4/CD8 expression (a) and CD3/CD19 expression (b) with the percentage in each quadrant.
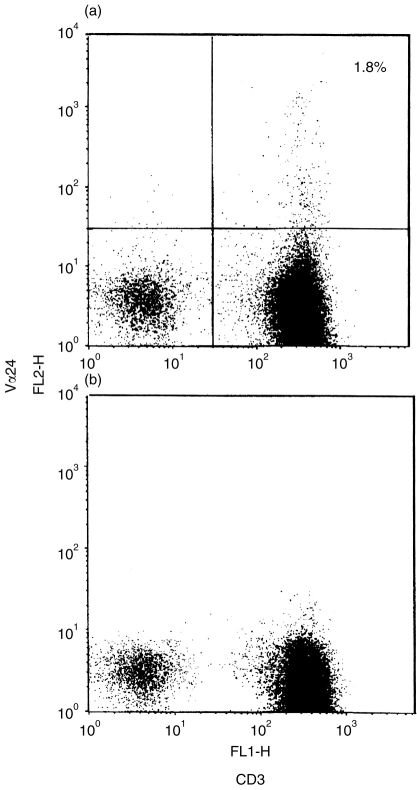
The expression of CD3, CD45RA, CD45RO, and interleukin-2 receptor β (IL-2Rβ) in each subpopulation of the PTLN lymphocytes was examined by three-colour immunofluorescence analysis (Fig. 5). CD45RA+ cells predominated among the CD4+ and CD8+ single-positive T cells (Fig. 5g,h). The percentage of CD45RO+ cells in the CD4+ CD8− subpopulation was higher than that in the CD4− CD8+ subpopulation (13% and 7%, respectively). B cells, which also expressed CD45RA antigen, predominated in the CD4− CD8− subpopulations, but a small number of double-negative CD3+ T cells was observed (Fig. 5f). High expressions of CD45RO and IL-2Rβ antigens were observed in the CD4− CD8− subpopulations (Fig. 5l,o). In order to determine the origin of these expressions of CD45RO and IL-2Rβ, we separated CD3+ CD4+ CD8− single-positive cells and CD3+ CD4− CD8− double-negative cells from whole PTLN lymphocytes using antibody coupled to paramagnetic beads. The purity of these two populations was 97%. The expressions of CD45RA, CD45RO and IL-2Rβ of separated CD3+ CD4− CD8− double-negative cells were shown in Fig. 6. The rate of IL-2Rβ-positive cells in CD3+ CD4− CD8− double-negative cells was higher than in other subpopulations. We also examined the frequency of Vα24-positive T cells which are considered to correspond to mouse natural killer (NK) T cells. In Case 8, the percentage of those cells was about 1·8% of all PTLN cells (Fig. 7a).
Figure 5.
Three-colour immunofluorescence analysis of the expressions of CD3, CD45RA, CD45RO and IL-2Rβ in PTLN cells subsets defined by CD4 and CD8 surface expression (see to Fig. 4a). Cells are gated into CD4− CD8−, CD4+ CD8− and CD4− CD8+ subsets, and the expressions of those surface antigens in each subset are shown as histograms.
Figure 6.
Three-colour immunofluorescence analysis of separated CD3+ CD4+ CD8− SP cells and CD3+CD4− CD8− double-negative cells from whole PTLN lymphocytes using a depletion technique with antibody coupled to paramagnetic beads. The purity of these two populations was 99%. The expressions of CD45RA, CD45RO and IL-2Rβ of separated single-positive and double-negative cells are shown as histograms.
Figure 7.
Two-colour immunofluorescence analysis of the expression of TCR Vα24 and CD3 in PTLN cells. (a) The gated cells are TCR Vα24-positive, and the percentage is about 1·8%. (b) Isotypic control IgG1 was used instead of anti-TCR Vα24 antibody.
DISCUSSION
This study is the first report on the histological structure and lymphocyte composition of human PTLN.
The human PTLN was found to have special morphological characteristics. These histological characteristics of the human PTLN, thin cortex with small numbers of follicles and undeveloped medullary cords with small numbers of plasma cells, were extremely similar to those of the sheep PTLN, which have been reported previously.2 The presence of a thymus-specific lymph node has also been reported in the human fetus,1 the guinea-pig,3,4 and ‘rats and mice’.5 According to these reports with human, sheep and guinea-pig material, these lymph nodes were located downstream from the thymus, and part of the cells produced in the thymus entered these nodes through the lymphatics. On the other hand, there were some reports that the PTLN in the mouse possessed afferent lymph from the peritoneal cavity and its efferent lymph went into the thymus.6,7 These reports concluded that the PTLNs in the mouse might influence cortical thymocytopoiesis of the neighbouring thymic cortex. It is, however, generally considered that the human thymus has no afferent lymphatics.8,9 Furthermore, the histological character of the human PTLN is extremely similar to that of the sheep PTLN that is located downstream from the thymus.2 Therefore, it seems likely that the human PTLN may be located between the efferent lymphatics of the thymus and the peripheral circulation, and that some of newborn T cells produced in the thymus may enter this node.
In the present study, we found that the CD4+:CD8+ T-cell ratio in human PTLNs was much higher than that in other peripheral lymph nodes10 and in the peripheral blood.11–13 Generally, the CD4+:CD8+ T-cell ratio in peripheral lymph nodes and in the afferent and efferent lymph of those nodes is higher than that of peripheral blood. It is known that 85–90% of the lymphocytes entering a lymph node are derived from the blood via HEV.14 Consequently, the difference between the lymphocyte subsets in the blood and those in the afferent and efferent lymph of peripheral lymph nodes may be caused by selective migration through the HEV.15,16 On the other hand, the CD4+:CD8+ T-cell ratio in each peripheral lymph node in goats differs according to the sites where those nodes belong. The helper activity of T cells in each lymph node may also be different.17 Moreover, the CD4+:CD8+ T-cell ratio of efferent lymph changes with in vivo antigen challenge.18 These observations suggest that the alteration of lymphocyte subsets may be related to the function of those lymph nodes in vivo. In the present study, we showed that the human PTLN has rich HEV in its subcortical area, and that a higher rate of CD4+ cells is present in this area of the PTLN than in other peripheral lymphoid organs. It is supposed that these characteristics might be particularly important for considering the role of the PTLN.
The PTLN contains only a small number of CD4− CD8− double-negative T cells. Because we could detect few if any T-cell receptor (TCR)-γ/δ-positive cells in the PTLN (data not shown), most of these double-negative cells were thought to express TCRα/β. In humans, TCRα/β double-negative T cells have been previously identified in the peripheral blood, thymus and skin.19–21 A majority of CD4− CD8− double-negative T-cell clones obtained from peripheral blood can produce T helper type 0 cytokines by stimulation with anti-CD3 antibody in vitro.22 In the present study, moreover, these double-negative T cells in the PTLN contained a small number of TCR Vα24-positive cells23,24 which are considered to correspond to mouse Vα14-positive NK T cells having an ability to secrete primary IL-4.25,26 These NK T cells in mouse and human are considered to influence the functional differentiation of conventional T cells and thymocytes by their ability to secrete cytokines and their cytotoxicity.27 TCR Vα24- positive cells were also contained in CD4 single-positive T cells in the PTLN. TCR Vα24-positive cells constituted about 1·8% of all PTLN cells in this study with material from a 1-year-old female.
Considering the above characteristics of the human PTLN, we propose the hypothesis that the PTLN might be related to the functional differentiation of newborn T cells produced in the thymus.
Acknowledgments
We thank to Mr Yasumitsu Kikui and Miss Naoko Mizuhara for their technical assistance. This study was supported in part by Grant-in-Aid for Encouragement of Young Scientists of the Japanese Ministry of Education, Science, Sports and Culture.
REFERENCES
- 1.Severeanu G. Die Lymphgefäße der Thymus. Arch Anat Entw Gesch. 1909:93. [Google Scholar]
- 2.Yamashita A, Miyasaka M, Trnka Z. Early post-thymic T cells: studies on lymphocytes in the lymph coming from thymus of the sheep. In: Morris B, Miyasaka M, editors. Immunology of the Sheep. Basel: Editions Roche; 1985. p. 162. [Google Scholar]
- 3.Harris PF, Templeton WR. Preliminary studies on the lymphatic drainage of the guinea-pig thymus with special reference to extrinsic vessels. J Anat Lond. 1966;100:694. [Google Scholar]
- 4.Harris PF, Templeton WR. Studies on the extrinsic lymphatic drainage of the guinea-pig thymus. Acta Anat. 1968;69:366. doi: 10.1159/000143088. [DOI] [PubMed] [Google Scholar]
- 5.Blau JN, Gaugas JM. Parathymic lymph nodes in rats and mice. Immunology. 1968;14:763. [PMC free article] [PubMed] [Google Scholar]
- 6.Eggli P, Schaffner T, Gerber HA, Hess MW, Cottier H. Accessibility of thymic cortical lymphocytes to particles translocated from the peritoneal cavity to parathymic lymph nodes. Thymus. 1986;8:129. [Google Scholar]
- 7.Mueller C, Tschumper A, Tschumper J-C, Hess MW, Cottier H. Parathymic lymph node: oriented proliferative response of the murine thymic cortex to intraperitoneal stimulation. Thymus. 1987;9:3. [PubMed] [Google Scholar]
- 8.Goldstein G, MacKay IR. The Human Thymus. London: Heinemann; 1969. p. 3. [Google Scholar]
- 9.Bloodworth JMB, Jr, Hiratsuka H, Hickey RC, Wu J. Ultrastructure of human thymus, thymic tumors, and myasthenia gravis. In: Sommers SC, editor. Pathology Annual. New York: Appleton-Century-Crofts; 1975. p. 329. [PubMed] [Google Scholar]
- 10.Bryan CF, Eastman PJ, Conner JB, Baier KA, Durham JB. Clinical utility of lymph node normal range obtained by flow cytometry. Annal NY Acad Sci. 1993;677:404. doi: 10.1111/j.1749-6632.1993.tb38799.x. [DOI] [PubMed] [Google Scholar]
- 11.Reichert T, Debruyère M, Deneys V, et al. Lymphocyte subset reference ranges in adult caucasians. Clin Immunol Immunopathol. 1991;60:90. doi: 10.1016/0090-1229(91)90063-g. [DOI] [PubMed] [Google Scholar]
- 12.Nagel JE, Chrest FJ, Adler WH. Enumeration of T lymphocyte subsets by monoclonal antibodies in young and aged humans. J Immunol. 1981;127:2086. [PubMed] [Google Scholar]
- 13.Cuadrado E, Barrena MJ. Immune dysfunction in Down’s syndrome: primary immune deficiency or early senscence of the immune system? Clin Immunol Immunopathol. 1996;78:209. doi: 10.1006/clin.1996.0031. [DOI] [PubMed] [Google Scholar]
- 14.Hall JG, Morris B. The origin of the cells in the efferent lymph from a single lymph node. J Exp Med. 1965;121:901. doi: 10.1084/jem.121.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacKay CR, Kimpton WG, Brandon MR, Cahill RNP. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J Exp Med. 1988;167:1755. doi: 10.1084/jem.167.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streeter PR, Berg EL, Rouse BTN, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 17.Dobrzanski MJ, Yang TJ. Size, CD4 and CD8 marker profiles and functions of lymphocyte subpopulations in mucosal-associated lymph nodes. Immunol Invest. 1991;20:487. doi: 10.3109/08820139109082629. [DOI] [PubMed] [Google Scholar]
- 18.Bujdoso R, Young P, Hopkins J, Allen D, McConnell I. Non-random migration of CD4 and CD8 T cells: changes in the CD4:CD8 ratio and interleukin 2 responsiveness of efferent lymph cells following in vivo antigen challenge. Eur J Immunol. 1989;19:1779. doi: 10.1002/eji.1830191003. [DOI] [PubMed] [Google Scholar]
- 19.De Toribio ML, De La Hera A, Regueiro JR, et al. α/β heterodimeric T-cell receptor expression early in thymocyte differentiation. J Mol Cell Immunol. 1988;3:347. [PubMed] [Google Scholar]
- 20.Groh V, Fabbi M, Hochstenbach F, Maziarz RT, Strominger JL. Double-negative (CD4−CD8−) lymphocytes bearing T-cell receptor α and β chains in normal human skin. Proc Natl Acad Sci USA. 1989;86:5059. doi: 10.1073/pnas.86.13.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Londei M, Verhoef A, De Berardinis P, Kissonerghis M, Grubeck-Loebenstein B, Feldmann M. Definition of a population of CD4−CD8− T cells that express the αβ T-cell receptor and respond to interleukins 2, 3, and 4. Proc Natl Acad Sci USA. 1989;86:8502. doi: 10.1073/pnas.86.21.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsikis PD, Cohen SBA, Murison JG, et al. Human αβ T-cell receptor CD4−CD8− T cell clones are predominantly TH0-like. Immunology. 1995;84:501. [PMC free article] [PubMed] [Google Scholar]
- 23.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4−8− T cells. J Exp Med. 1994;180:1171. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumida T, Sakamoto A, Murata H, et al. Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with systemic sclerosis. J Exp Med. 1995;182:1163. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 26.Arase H, Arase N, Nakagawa K, Good RA, Onoé K. NK1.1+ CD4+ CD8− thymocytes with specific lymphokine secretion. Eur J Immunol. 1993;23:307. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- 27.Arase H, Arase N, Kobayashi Y, Nishimura Y, Yonehara S, Onoé K. Cytotoxity of fresh NK1.1+ T cell receptor α/β thymocytes against a CD4+8+ thymocyte population association with intact Fas antigen expression on the target. J Exp Med. 1994;180:423. doi: 10.1084/jem.180.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]