Abstract
Isolated, living muscle fibres from either Xenopus or mouse were observed in a confocal microscope and t-tubules were visualized with sulforhodamine B. Observations were made before and after fatiguing stimulation. In addition, experiments were performed on fibres observed in an ordinary light microscope with dark-field illumination.
In Xenopus fibres, recovering after fatigue, t-tubules started to show dilatations 2-5 min post-fatigue. These swellings increased in size over the next 10-20 min to form vacuoles. After 2-3 h of recovery the appearance of the fibres was again normal and force production, which had been markedly depressed 10-40 min post-fatigue, was close to control. Vacuoles were not observed in mouse fibres, fatigued with the same protocol and allowed to recover.
In Xenopus fibres, fatigued in normal Ringer solution and allowed to recover in Ringer solution with 30-50 mM L-lactate substituting for chloride (lactate-Ringer), the number and size of vacuoles were markedly reduced. Also, force recovery was significantly faster. Replacement of chloride by methyl sulphate or glucuronate had no effect on vacuolation.
Resting Xenopus fibres exposed to 50 mm lactate-Ringer and transferred to normal Ringer solution displayed vacuoles within 5-10 min, but to a smaller extent than after fatigue. Vacuolation was not associated with marked force reduction.
Mouse fibres, fatigued in 50 mm lactate-Tyrode (l-lactate substituting for chloride in Tyrode solution) and recovering in normal Tyrode solution, displayed vacuoles for a limited period post-fatigue. Vacuolation had no effect on force production.
The results are consistent with the view that lactate, formed during fatigue, is transported into the t-tubules where it attracts water and causes t-tubule swelling and vacuolation. This vacuolation may be counteracted in vivo due to a gradual extracellular accumulation of lactate during fatigue.
Vacuole formation in frog fibres after fatiguing stimulation was first described by Gonzalez-Serratos et al. (1978) using electron microscopy; they also obtained evidence that the vacuole content was similar to the external fluid in ionic composition, suggesting that vacuoles have a t-tubular origin. We have studied vacuole formation in fatigued Xenopus muscle fibres using light and electron microscopy (Westerblad & Lännergren, 1990; Lännergren et al. 1990) and more recently with confocal microscopy (Lännergren et al. 1999). We demonstrated that vacuoles arise from local dilatations of t-tubules and have further shown that vacuole formation is a reversible process with a maximum about 20 min after the end of the stimulation period and with complete regress within about 2 h. In addition, membrane-bound vacuoles can develop in skeletal muscle fibres under other conditions: during the efflux of small molecules (e.g. glycerol) which have been loaded into fibres (Krolenko et al. 1995; Gallagher & Huang, 1997), after exposure to certain drugs (e.g. Horn et al. 1996), and in various disease states (e.g. Libelius et al. 1979; Malouf & Wilson, 1986). Thus, the development of vacuoles appears to be of physiological importance.
T-tubule vacuole formation would require an imbalance between fluid entry into the lumen of t-tubules and escape from their openings into the extracellular space. The most likely cause of water flow into the t-tubules is an accumulation of osmotically active particles in their lumen. During intense activity of muscle fibres there is a large increase in their metabolism with an accumulation of, for instance, creatine, inorganic phosphate and lactic acid. During the recovery period, creatine phosphate is quickly resynthesized from its breakdown products whereas lactic acid is generally exported. Lactic acid efflux can occur by two principal mechanisms: diffusion and specific transport (Juel, 1997). Undissociated lactic acid can diffuse readily across biological membranes, but because of the low pKa (negative log of the acid dissociation constant) of lactic acid (pKa= 3.86) most of the lactic acid occurs in the dissociated form, i.e. as lactate ions and hydrogen ions at the pH prevailing in fibres during fatigue. Most of the lactate transport occurs via a specific lactate-H+ cotransport system (Juel, 1997). Several lactate-H+ transporter isoforms (monocarboxylate transporters, MCTs) have been identified; of these MCT1 and MCT4 have been found in skeletal muscle. MCT1 occurs predominantly in high-oxidative fibres whereas MCT4 is more abundant in glycolytic fibres (Juel & Halestrap, 1999). Immunolocalization of MCTs has just begun and so far MCT1 has been found in high concentrations in t-tubule membranes in rat heart muscle cells (Jóhannsson et al. 1997). Thus, it seems probable that lactate transporters, and hence also lactate transport, occur to a large extent in the t-tubule wall of skeletal muscle fibres.
Force recovery after fatiguing activity is a relatively slow process in Xenopus muscle fibres with full tetanic force regained after 2-3 h, much longer than the time required to reverse the metabolic changes occurring during fatigue (Westerblad & Lännergren, 1988; Thompson & Fitts, 1992). Typically, force decreases after the end of fatiguing stimulation with a minimum at about 20 min post-fatigue and then gradually returns to the pre-fatigue level. Since the time course of force recovery is similar to the waxing and waning of vacuoles it was natural to assume that there is a causal relation between the two processes. However, in a recent study we found clear examples of increasing force production at a time when vacuole formation was progressing (Lännergren et al. 1999) and it appears that the relation between vacuolation and depressed force production is complex.
The working hypothesis for the present study was that vacuole formation is due to the osmotic effect of lactate accumulating in the t-tubules. Lactate accumulation would be due to an efficient transport across the t-tubule wall and a relatively slow escape from the t-tubule opening into the surrounding medium. We have tested this idea by allowing fatigued fibres to recover in a Ringer solution with a proportion of NaCl being replaced by sodium l(+)-lactate (30-50 mm lactate-Ringer). Compared to controls, this caused a large reduction in the number and size of vacuoles as observed with confocal microscopy. In order to try to resolve the question of a causal relation between depressed force and the presence of vacuoles, force production was measured in parallel with the morphological observations. Experiments were also performed on isolated mouse muscle fibres. Mouse fibres do not normally show vacuole formation after fatigue. However, after fatigue in lactate-Tyrode and recovery in normal Tyrode solution clear vacuoles were observed during the early part of recovery.
METHODS
Animals and preparations
Female clawed frogs (Xenopus laevis) and male mice (NMRI strain) were used in this study. Xenopus frogs were stunned, decapitated and pithed; mice were killed by rapid neck disarticulation. All procedures involving animals were approved by the local ethics committee. Single fibres were isolated from the lumbrical muscles of Xenopus or the flexor brevis muscles of mice. Platinum clips were attached to the tendons and the fibre was suspended horizontally between an adjustable hook and an Akers AE801 force transducer in the perfusion channel of a muscle bath. Fibres were stretched to the length where maximum tetanic force (P0) was obtained. The bottom of the perfusion channel consisted of a glass coverslip (thickness 0.17 mm). The fibre was flanked by platinum electrodes, used for stimulation. Tetani were evoked using 70-80 Hz, 350-500 ms stimulus trains. Force was measured as peak force of tetani. Fatigue was induced by repeated tetanic stimulation, initially at intervals of 4 s for 2 min, then 3 s for 2 min and 2.5 s for 2 min until force had fallen to approximately 40 % of the original (i.e. to 0.4P0). Force recovery was followed by giving a 350 ms tetanus at various times post-fatigue, usually at 2, 5, 10, 20, 30, 40 and 60 min and then at longer intervals. Force signals were recorded on a pen-recorder and also on a personal computer after digitization at a sampling frequency of 500 Hz.
Solutions
All experiments were performed at room temperature (24-26°C). Xenopus muscle fibres were superfused with a solution containing (mm): NaCl 115, KCl 2.5, CaCl2 1.8, EDTA 0.1, and sodium phosphate buffer 3 (pH 7.0-7.2). This solution will be referred to as normal Ringer. Most fatigue runs on Xenopus fibres were performed in this solution (with sulforhodamine B (Molecular Probes) added, see below). The composition of lactate-Ringer was similar to that of normal Ringer, but 30 or 50 mm NaCl was replaced by an equivalent amount of sodium l(+)-lactate. About the same number of experiments was performed with the two concentrations. Since we could not detect a clear difference between the effects of 30 and 50 mm lactate, both solutions will be referred to as lactate-Ringer. For some experiments 50 mm NaCl was replaced either by sodium glucuronate or sodium methyl sulphate (pH 7.2). A few experiments were made with cinnamate (α-cyano-4-hydroxycinnamic acid), 5 mm, dissolved in normal Ringer.
Some control experiments were performed on Xenopus fibres in bicarbonate-buffered Ringer solution (bicarbonate-Ringer) with the following composition (mm): NaCl 99, KCl 2.5, NaHCO3 20, CaCl2 1.8, sodium phosphate buffer 1, EDTA 0.1 (pH 7.3 when bubbled with 95 % O2-5 % CO2).
Mouse muscle fibres were superfused with a Tyrode solution containing (mm): NaCl 125, KCl 5, MgCl2 0.5, Na2HPO4 0.4, CaCl2 1.8, EDTA 0.1, NaHCO3 25, glucose 5.5 and fetal calf serum (about 0.2 %, Gibco). This solution was bubbled with 95 % O2-5 % CO2 (pH 7.4). In some experiments 50 mm NaCl was replaced by an equivalent amount of sodium l(+)-lactate.
Light microscopy
The muscle bath was placed on the stage of a modified upright microscope (Leitz Laborlux) and a coverslip was placed on top of the perfusion channel to give a flat surface. Illumination was from below with a dark-field arrangement (cf. Westerblad & Lännergren, 1990). White light was focused on the preparation with an aspheric condensor lens with the central light rays blocked by a disc, situated just below the condensor lens, so that the fibre was illuminated by a hollow cone of light. Either a ×4 (NA 0.10) or a ×10 (NA 0.25) objective was used for observation and recording. Images were obtained with a Hamamatsu CCD camera and displayed on a monitor; permanent records were made with a Sony Mavigraph video printer.
Confocal microscopy
The imaging system consisted of a BioRad MRC 1024 unit with a krypton-argon mixed gas laser run at 15 mW (BioRad Microscopy Division, UK) attached to a Nikon Diaphot 200 inverted microscope. The muscle bath was placed on the microscope stage with the fibre mounted as close to the glass bottom as possible. T-tubules were visualized using sulforhodamine B. This fluorescent dye enters the lumen of the t-tubules (Endo, 1966) and has no deleterious effects on the excitability of fibres (Lännergren et al. 1999). The dye (0.5 mm in Ringer solution) was applied for 10-20 min before the start of observations and was present throughout the experiments. The objective used was a Nikon Plan Apo ×60 (oil, NA 1.4). Excitation was at 568 nm with emission recorded at 585 nm. Usually only one region of the fibre was observed with repeated images of this site. For each image 3× Kalman averaging was used. In order to avoid damage to fibres the laser light intensity was kept low. Usually 3 % of the maximum laser power (obtained by passing the light through grey filters) was sufficient to get acceptable images.
Quantification of vacuoles
The number and sizes of vacuoles were analysed on digitized images using Scion Image (Scion Corporation, MD, USA). For this purpose we used confocal images obtained with the ×60 objective, which gives a pixel size of 0.2 μm × 0.2 μm. The confocal image was first smoothed with a filter which replaces each pixel with the weighted average of its 3 × 3 neighbourhood pixels. The image was then inverted and thresholded so that each pixel became either black (stained with fluorescent markers) or white (unstained). The background level was adjusted until normal t-tubular staining was no longer visible. Finally a wand tool was used to analyse the number and sizes of vacuoles. This tool finds an edge in the image and follows it in a counter-clockwise direction until it returns to the point where it first found the edge. The smallest particle counted by the wand tool was set to 10 pixels (i.e. 0.4 μm2).
Statistics
Values are presented as means ±s.e.m. Student's paired t tests were used to verify statistical significance; the significance level was set at 0.05 throughout.
Experimental procedure and validation of buffer usage
In fatigue-recovery experiments on Xenopus fibres, the fibre was always fatigued in normal Ringer. Recovery then took place in either the normal solution or with chloride ions replaced with lactate, methyl sulphate or glucuronate. In the latter experiments a change back to normal Ringer was performed 30 min after the end of fatiguing stimulation. Two fatigue runs were frequently produced in the same fibre and recovery occurred either in normal Ringer or with chloride ion substitution. In this way a single fibre could serve as its own control. The basis for using this protocol is that we have shown previously that Xenopus fibres can be fatigued, allowed to recover, and then be fatigued again with the second fatigue run being similar to the first regarding both the fatigue and recovery pattern (Lännergren & Westerblad, 1988). In the present study we managed to obtain comparable patterns of force reduction in two subsequent fatiguing stimulation periods in a total of 13 fibres; results from the second recovery period were not used in fibres that fatigued markedly more rapidly during the second fatiguing stimulation period. For two of the fibres, recovery after both fatigue runs took place in normal Ringer. In these two fibres forces as well as changes in the microscopic appearance were similar during the two recovery periods (data not shown). Furthermore, in experiments with double fatigue runs and recovery in different solutions, the order was randomized and we could not detect any systematic difference in forces or vacuolation depending on whether the modified or normal Ringer was used first. Thus it is possible to have the same fibre to serve as its own control with regard to both force and morphological changes.
The hypothesis of the present study is that lactate is transported across the t-tubule wall and causes vacuolation by an osmotic effect. Both lactate-H+ translocation by the lactate-proton transporter and lactate diffusion are markedly reduced at low pH (Juel, 1997). The pH buffering in the t-tubule lumen may then be critical for the export of lactate. It has previously been shown that intracellular pH changes during fatigue runs are smaller in mouse fibres (Westerblad & Allen, 1992a) than in Xenopus fibres (Westerblad & Lännergren, 1988) and it is notable that the extracellular medium was more efficiently buffered in the former case (bicarbonate-buffered Tyrode vs. phosphate-buffered Ringer solution). Also, vacuoles are formed post-fatigue in Xenopus fibres but not in mouse fibres (Lännergren et al. 1999). It is thus possible that intracellular lactic acid accumulation and vacuole formation in Xenopus fibres is aggravated by the relatively inefficient pH buffering of our normal Ringer solution. In order to investigate this possibility, a series of control experiments was performed where fibres were fatigued and allowed to recover in either the normal phosphate-buffered Ringer solution or in bicarbonate-buffered Ringer solution (bicarbonate-Ringer). A total of seven fibres were used in these control experiments and double fatigue runs were performed in two of these fibres with fatigue and recovery first in bicarbonate-Ringer and then in normal Ringer in one fibre and in the reverse order in the other. In these two latter experiments, substantial vacuolation was observed during all recovery periods and there was no noticeable difference between the two solutions. There was an extensive vacuolation post-fatigue also in the other fibres and mean data showed no significant difference between the bicarbonate-Ringer group (n = 4) and the normal Ringer group (n = 5): at 30 min of recovery the number of vacuoles per 100 μm2 was 2.43 ± 0.32 vs. 1.97 ± 0.45 and the size of vacuoles was 2.55 ± 0.32 vs. 2.25 ± 0.50 μm. Thus, it appears unlikely that vacuole formation in Xenopus fibres is due to inefficient pH buffering in the t-tubules and subsequent experiments were performed in the normal phosphate-buffered Ringer solution.
RESULTS
Light-microscopic appearance of Xenopus fibres recovering in different media
For the experiments with light microscopy, the recovery of three of the fibres was in normal Ringer after the first run and in lactate-Ringer after the second run. The order was reversed for the other two fibres. The extent of vacuolation, as determined from the change in transparency of the fibre, was somewhat variable but in three of the fibres there was a clear difference in the appearance during recovery in the two media. Figure 1A shows that during recovery in normal Ringer the fibre became whitish, or opaque with a fine granularity, and this appearance had a maximum at about 30 min recovery. Eventually, the fibre regained its original, transparent appearance. This change in transparency has been described previously and the increased opacity has been attributed to light-scattering by vacuoles (Westerblad & Lännergren, 1990). After complete recovery and an additional 1 h of rest, the fibre was fatigued again to the same force level, about 40 % of the rested force. Recovery after the second fatigue run (Fig. 1B) was in 30 mm lactate-Ringer and this time there was hardly any change in transparency. It thus appeared from these initial experiments that the presence of a relatively high concentration of lactate in the extracellular fluid can markedly reduce the formation of vacuoles during the recovery from fatiguing stimulation.
Figure 1. Light-microscopic appearance (dark-field) of a Xenopus muscle fibre recovering in different media.

A, Xenopus muscle fibre fatigued to 0.4P0 and allowed to recover in normal Ringer. After complete force recovery the fibre was rested for another 1 h and then fatigued again to the same force level and allowed to recover in Ringer solution with 30 mm sodium l-lactate replacing an equimolar amount of NaCl (B). Note that light-scattering (opacity) at 20-60 min recovery is less marked in the latter case. Scale bar represents 50 μm.
Confocal microscopy of recovering Xenopus fibres: effects of extracellular lactate
All fibres studied with confocal microscopy were exposed to sulforhodamine B and many images were obtained of one area. In this way dye-filled t-tubules were clearly visualized and the process of vacuole formation and regress could be followed in detail. Figure 2 shows an example of a fibre recovering in normal Ringer where a group of vacuoles could be followed from the first dilatation of t-tubules to near-complete regression. Vacuoles were never observed earlier than 2 min post-fatigue. In most fibres studied some swollen t-tubules could be seen at this time. Vacuoles reached their maximum size by 20-30 min and then regressed. Vacuolation is thus a reversible process and after recovery for 2-3 h both the appearance and the force production of fibres have returned to pre-fatigue conditions.
Figure 2. Confocal images of a Xenopus fibre recovering in normal Ringer.

Xenopus fibre fatigued and allowed to recover in normal Ringer. Localized swellings of t-tubules, which are the starting points of vacuoles, can be discerned at 5 min recovery, and clear vacuoles are formed at 10 min recovery. In the middle of this panel there are five vacuoles which can be followed through the rest of the recovery period. These vacuoles start to collapse between 60 and 100 min recovery and have disappeared 120 min post-fatigue. Scale bar represents 5 μm.
In six fibres duplicate runs were made with recovery both in normal Ringer and lactate-Ringer (lactate-Ringer for the first recovery period in four of the fibres). Figure 3 shows images of a fibre recovering in the two different solutions after fatigue runs in normal Ringer. In the left panel, recovery was in lactate-Ringer; for the right panel the fibre had recovered completely and was fatigued again and allowed to recover in normal Ringer. As can be seen, the extent of vacuolation was clearly less during recovery in the former case and regress of vacuoles occurred much sooner. Figure 4 summarizes data regarding the concentration of vacuoles from the six fibres subjected to dual fatigue runs, using the measuring approach described in Methods. Vacuoles were less abundant during recovery in lactate-Ringer and their average area was smaller: 1.51 ± 0.53 μm2 in lactate-Ringer vs. 2.84 ± 0.53 μm2 in normal Ringer at 30 min recovery. Thus, these experiments show that the presence of a high extracellular lactate concentration has a significant ‘protective’ effect against vacuole formation in fibres recovering from fatigue. Further, the extent of vacuolation did not seem to depend on the order in which the exposure to the different recovery media occurred.
Figure 3. Confocal images of a Xenopus fibre recovering in different media.

The fibre was fatigued in normal Ringer and a change was made immediately after the fatigue run to 30 mM lactate-Ringer (A). After complete force recovery and an extra 1 h of rest the fibre was fatigued again and allowed to recover in normal Ringer (B). Note fewer and smaller vacuoles at corresponding times in lactate-Ringer. Scale bar is 20 μm.
Figure 4. Measurements of vacuolation in lactate-Ringer vs. normal Ringer.
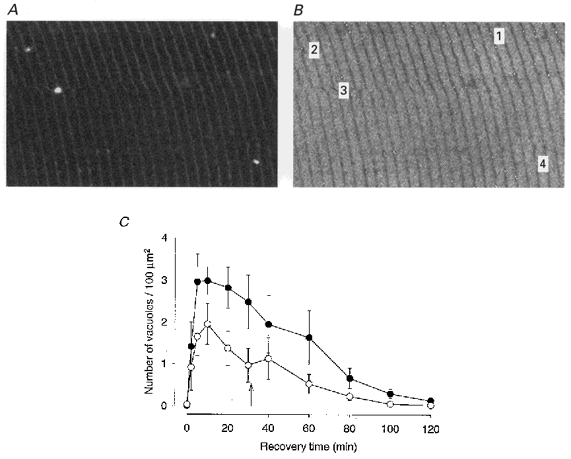
A and B illustrate a method for measuring the number of vacuoles in a given area. A, original confocal image in which four vacuoles can be seen. B, inverted image of the same area with labelling of profiles recognized by the analysis program. C, plot of number of vacuoles at various times of recovery. Data (means ±s.e.m.) for six Xenopus fibres in which recovery could be studied both in normal Ringer (•) and in lactate-Ringer (○). Arrow indicates time when a change was made from lactate-Ringer to normal Ringer.
Extracellular lactate accelerates force recovery after fatigue
Force recovery after fatigue in Xenopus fibres has a characteristic time course with a further decrease of tetanic tension after the end of stimulation until about 10-20 min post-fatigue, followed by a relatively slow recovery (Westerblad & Lännergren, 1986). In the present confocal experiments tetanic force was measured at various times after the end of fatigue runs in 14 fibres; in six of these two fatigue runs were performed with recovery in different media. Figure 5 shows a summary of the results where the effect of allowing fibres to recover in lactate-Ringer (n = 10) is compared to recovery in normal Ringer (n = 10). The effect was very clear-cut: maximum post-fatigue force depression was not as marked and the rate of recovery was faster when fibres recovered in lactate-Ringer compared to normal Ringer.
Figure 5. Force recovery is faster in lactate-Ringer than in normal Ringer.

Tetanic force production at various times of recovery. •, recovery in normal Ringer; ○, recovery in lactate-Ringer for 30 min. n = 10 for both groups. Arrow indicates time when a change was made from lactate-Ringer to normal Ringer.
Experiments on unfatigued fibres showed that tetanic force in lactate-Ringer was 93.8 ± 1.4 % (n = 4) of the control in normal Ringer. Thus, lactate in itself had a slight depressant effect and cannot explain the larger force production in lactate-Ringer at a given time during recovery.
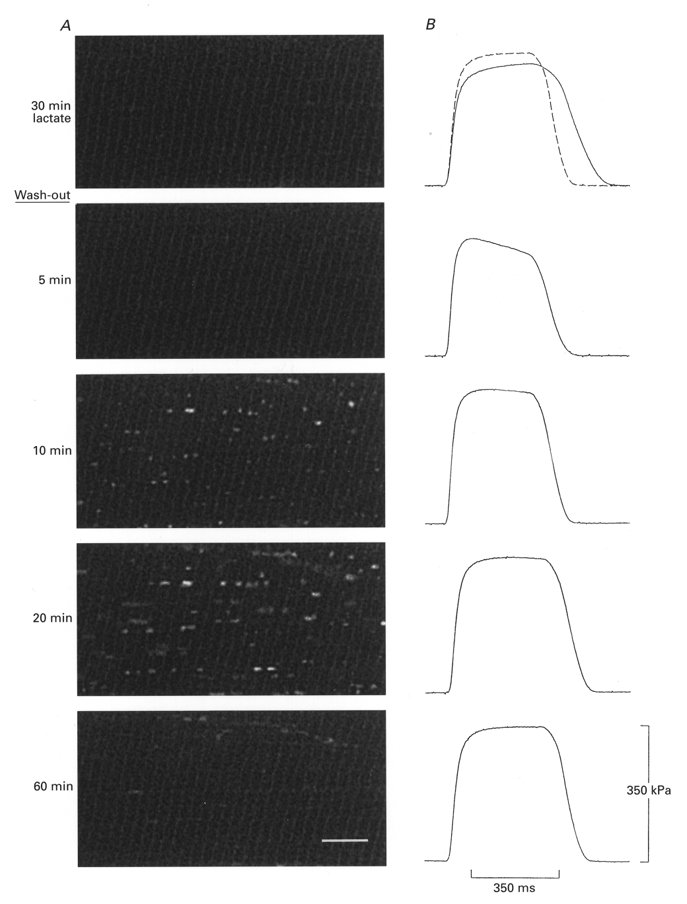
A change from lactate-Ringer to normal Ringer was made immediately after recording the 30 min recovery value. Force was measured again at 35 min in four fibres (not shown in Fig. 5) and at 40 min. The mean value for force measured about 5 min after the change (at 35 min, n = 4) was 23 ± 16 % lower and at 40 min (n = 10) 4 ± 7 % lower than the 30 min value. Thus, following the change from lactate-Ringer to normal Ringer there was a transient reversal of force recovery. Images were also obtained just before and after the change from lactate-Ringer to normal Ringer. One example is shown in Fig. 6. As can be seen, vacuolation increased after the change and vacuoles were clearly larger in the first image obtained after the change. Similar results were obtained in three other fibres. Thus, a change from lactate-Ringer to normal Ringer was associated with a transient increase in vacuolation and a transient force depression, suggesting a correlation between the two events.
Figure 6. Vacuoles become more prominent when a change is made from lactate-Ringer to normal Ringer.

Selected confocal images of a Xenopus fibre recovering after fatigue. A, image at 30 min recovery when fibre is still in 50 mm lactate-Ringer. B, same area at 35 min, 5 min after change to normal Ringer. Scale bar is 20 μm.
Anion substitutes other than lactate do not lessen vacuolation
In order to see if the effects on vacuolation and force recovery were specific for lactate or rather due to removal of a relatively large amount of chloride ions in the extracellular medium some experiments were done with other substitutes, namely methyl sulphate or glucuronate. Both ions have been used previously as impermeant chloride substitutes (Hutter & Noble, 1960; Vaughan-Jones, 1979). In two fibres recovery was studied in Ringer solution with 50 mm sodium methyl sulphate replacing 50 mm NaCl (methyl sulphate-Ringer) and in two other fibres 50 mm glucuronate-Ringer was applied during recovery. The results with the two substitutes were the same. Figure 7 shows that there was extensive vacuolation with the same time course as during recovery in normal Ringer. Thus, the ‘protective’ effect of lactate on vacuole formation is not due to substitution of a large amount of lactate ions (with low membrane permeability) for chloride ions. However, some minor effect of substitution is indicated by the transient increase in vacuolation immediately after the change back to normal Ringer.
Figure 7. Anion substitutes other than lactate do not protect against vacuolation.

A shows an area of a Xenopus fibre at 30 min recovery in normal Ringer after fatigue. B shows essentially the same area at 30 min recovery in 50 mm glucuronate-Ringer after a second fatigue run (in normal Ringer). Scale bar is 20 μm. C, mean values (±s.e.m.) for number of vacuoles in 50 mm glucuronate-Ringer (n = 2) and in 50 mm methyl sulphate-Ringer (n = 2) (○); • are mean values for recovery in normal Ringer. Arrow indicates when a change was made from experimental solution to normal Ringer.
Effects of washout after loading non-fatigued (resting) fibres with lactate
The monocarboxylate transporters can transfer lactate in both directions across the fibre membrane (Juel, 1997). Thus, an increase in intracellular lactate concentration can be achieved in a rested fibre without inducing most of the changes which occur during fatigue. Fibres were bathed in lactate-Ringer for 30 min (without stimulation) and a change was then made to normal Ringer. The result was similar in all experiments undertaken (n = 4): vacuoles started to develop after about 10 min, were clearly seen at 20 min, and then slowly receded over the next 20 min. One example is shown in Fig. 8. The extent of vacuolation, however, was much smaller than after a fatigue run with recovery in normal Ringer. Thus, vacuoles can develop during the presumed efflux of lactate from unfatigued fibres. Force was recorded just after the images were obtained and are shown in Fig. 8B. From these it can be seen that tetanic force was moderately depressed 5 min after the solution change. However, at the time when vacuoles were first seen (i.e. at 10 min) force had recovered to 99 % of control and was identical to the control at 20 min recovery when the vacuoles had grown further. Similar results were obtained in the other fibres. Thus, in these experiments vacuolation lagged behind a small, transient force depression.
Figure 8. Vacuolation can be induced in rested fibres exposed to lactate.
A, images of a rested (unstimulated) Xenopus fibre which was bathed in 50 mm lactate-Ringer for 30 min and then returned to normal Ringer. Vacuoles are clearly observed at 10 and 20 min after the change to normal Ringer. Scale bar is 10 μm. B, force records show that there was only a slight, transient force depression at 5 min after the change. Dashed line is control force in normal Ringer obtained before exposure to lactate-Ringer.
Cinnamate did not block vacuolation
Cinnamate (α-cyano-4-hydroxycinnamic acid) has been used to block transporter-dependent lactate flux in skeletal muscle (Mason & Thomas, 1988; Juel, 1997). Since an important part of our theory was that vacuole formation was due to efflux of lactate from the myoplasm into the t-tubule lumen, it was of interest to see if vacuolation occurred in the presence of this drug. Initial experiments, in which cinnamate (5 mm) was applied before the beginning of fatiguing stimulation, were unsuccessful in that fibres frequently failed during fatiguing stimulation and therefore the stimulation periods were abnormally short. In three experiments 5 mm cinnamate was applied immediately after the end of fatiguing stimulation. In all three fibres vacuolation was seen, which was similar to that observed during recovery in normal Ringer. Thus, cinnamate applied immediately after the end of fatigue did not protect against vacuole formation. Furthermore, the rate of force recovery after fatigue was not significantly different in the presence of cinnamate (data not shown).
Vacuole formation in mouse muscle fibres
In a previous report (Lännergren et al. 1999) we showed confocal images from a large number of muscle fibres after fatigue, in both Xenopus and mouse. In contrast to the situation in Xenopus fibres, we could never demonstrate any changes in t-tubule morphology in mouse fibres after fatigue, which may be attributed to a higher density of t-tubules in mammalian fibres and a shorter diffusion pathway from bottom to mouth of tubules, due to the smaller fibre radius. We now reasoned that if accumulation of lactate supplies the driving force for water movement into the t-tubule lumen, then the chance of observing vacuoles would be better if escape of lactate into the surrounding medium is prevented during the fatigue run. To test this idea we fatigued mouse fibres in Tyrode solution with a high lactate content (50 mm) and allowed them to recover in normal Tyrode solution. In three successful experiments, clear vacuoles could be seen early during recovery. These vacuoles originated from localized swellings of t-tubules. One example is shown in Fig. 9. Compared to Xenopus fibres the density of vacuoles was considerably lower. Also, the time course was different with the first sign of vacuole formation at about 2 min post-fatigue, a peak at about 5 min and a rapid regress thereafter.
Figure 9. Vacuole formation in a mouse muscle fibre.

A, selected images of an isolated mouse fibre which was fatigued in 50 mm lactate-Tyrode. Immediately after the fatigue run a change was made to normal Tyrode and images obtained every minute. Clear vacuoles were observed at 2 and 5 min recovery. Scale bar is 10 μm. B, force records show that tetanic force production recovered rapidly and had no obvious relation to vacuolation.
Force records were also obtained from the mouse fibres. The mean tetanic force was 84.8 ± 14.2 % and 93.4 ± 8.7 % of control at 5 and 10 min recovery, respectively, which is similar to the rate of force recovery after fatigue runs in normal Tyrode solution (Westerblad & Allen, 1992b).
DISCUSSION
The main novel findings of the present study are as follows. (1) Lactate in the extracellular medium during recovery reduces the number and size of vacuoles formed post-fatigue in Xenopus fibres. (2) Force recovery after fatigue in Xenopus fibres is markedly faster in lactate-Ringer than in normal Ringer. (3) Rested Xenopus fibres loaded with lactate display vacuolation after a change to normal Ringer but this vacuolation is not associated with a marked force decline. (4) In mouse fibres vacuoles form and regress after a fatigue run in lactate-Tyrode but this occurs much faster than in Xenopus fibres.
Our hypothesis was that lactate will accumulate during exhaustive activity. Lactate will leave the fibre via the t-tubular membrane, causing water inflow through osmosis into the t-tubules and resultant swelling. The findings above generally support this theory. Nagesser et al. (1992) have shown that the lactate concentration rises markedly to about 40 mm in Xenopus fibres during fatiguing stimulation with a stimulation pattern of the type we use here. Hence, a large lactate gradient develops across the membrane and this would be much reduced or abolished by the presence of 30-50 mm lactate on the outside, resulting in a very limited lactate efflux and therefore much less vacuole formation as observed. However, vacuole formation was not completely abolished and on average the reduction was about 60 %. Different reasons can be suggested for this. (1) Lactate-Ringer was applied too late and a stronger effect would have been seen if lactate was applied from the beginning or half-way into the fatigue run. An argument against this is that under normal conditions vacuoles never develop during fatigue runs and there is always a delay of at least 2 min before the first signs of vacuole formation are seen. (2) Due to efficient transport, the lactate concentration in the t-tubules is much higher than that measured in the fibre. This is possible, but then 50 mm would be more effective than 30 mm and we did not see any evidence for this. (3) Other osmotic forces than those associated with lactate production and transport might be of importance. During fatiguing stimulation of the type used here there is almost complete breakdown of phosphocreatine (PCr), which would increase the intracellular osmolarity by about 25 mosmol l−1 (Nagesser et al. 1992) and contribute to fibre swelling. After the end of stimulation PCr is resynthesized which would reduce the number of osmotically active particles in the myoplasm, and thus aid water movement into the t-tubules. (4) It is also possible that there are water fluxes associated with Na+-K+ transport. Nik-Zainal et al. (1999) have recently shown that vacuolation in fibres shrinking back from an osmotic glycerol load can be markedly reduced by inhibiting Na+-K+ transport with cardiac glycosides. Their interpretation was that Na+-K+ transport across the t-tubule wall might be involved in volume regulation and since fatigue is associated with marked swelling (Lännergren, 1990) such a mechanism might also operate here.
Our hypothesis is supported by the finding that exposing rested fibres to high lactate for 30 min and then returning them to normal Ringer resulted in vacuole formation, albeit to a much smaller degree. There may be several reasons why vacuolation is much less prominent in this case. It is quite likely that the intracellular lactate concentration is increased much less than during fatiguing stimulation. Another possibility is that extensive vacuolation not only requires a high lactate concentration in the t-tubules but also some change in the structures surrounding the tubules. We have previously shown that excitation-contraction coupling becomes susceptible to mechanical stress in Xenopus fibres after fatiguing stimulation and have localized the weak point to the triadic region (Bruton et al. 1995). We have further shown that the critical factor is increased [Ca2+]i and not some fatigue-related metabolic change since mechanical weakening also occurred in fibres which were activated with a series of tetani, given at long intervals to minimize metabolic changes (Bruton et al. 1996). Similar results have been obtained in mouse muscle fibres by Chin & Allen (1996). Lamb et al. (1995) have shown in mechanically skinned fibres that exposure to high [Ca2+] causes loss of excitation-contraction coupling and distortion of triad junctions. Thus, it is possible that during fatiguing stimulation the Ca2+ concentration in the triadic region rises to such a high level that structural changes are initiated which lead both to impaired excitation-contraction coupling and to weakening of the t-tubule scaffold so that the t-tubule wall bulges out more easily under the influence of water accumulation in the lumen.
The lactate accumulation hypothesis is also supported by the result in Xenopus fibres of changing back from lactate-Ringer to normal Ringer during recovery (Fig. 6). In those fibres, which still displayed vacuoles at 30 min, the change back caused increased vacuolation, which would be explained by a change from a situation where lactate efflux is impeded to one where it is facilitated.
It is pertinent to ask why vacuoles only become apparent some time after fatigue and are never seen during the period of fatiguing stimulation. One possible explanation is that during stimulation lactate is continuously produced and transported into the t-tubules at rates such that the concentration is roughly similar in the cytoplasm and the t-tubules; hence there is no major gradient between the two compartments. When stimulation is stopped, lactate production ceases but transport across the surface membrane and the t-tubule wall continues so that the concentration in the cytoplasm becomes lower than in the t-tubules and water is drawn into their lumen.
The lactate accumulation hypothesis is further supported by the findings in mouse fibres. In these, vacuoles are never observed after a fatigue run in normal Tyrode but were clearly seen after a fatigue run in 50 mm lactate-Tyrode (with recovery in normal Tyrode). The absence of vacuole formation under normal conditions may be due to a less extensive lactate accumulation in these fibres. This is supported by the finding that intracellular pH changes are small during fatigue runs in mouse fibres (Westerblad & Allen, 1992a; Bruton et al. 1998). If the t-tubules are an important export route, then lactate will exit more easily from mammalian than from frog fibres because there are twice as many tubules per sarcomere, also the diffusion distance from the bottom of tubules to the exterior is shorter because of the smaller radius of mouse fibres. With a large extracellular concentration of lactate during the fatigue run less lactate will be transported out during the run. Thus, at the beginning of recovery, when a change is made to normal Tyrode, there will be a large lactate gradient from inside to outside, lactate will be transported into the t-tubules, and these will swell.
The finding that cinnamate had no clear effect on vacuole formation might appear as evidence against the lactate accumulation hypothesis. We can think of some possible reasons for the lack of effect. One is that cinnamate is an extremely potent inhibitor of pyruvate transport into mitochondria (Halestrap, 1975); thus it may lead to a large increase in lactate production in the fibre (see Juel & Halestrap, 1999) and may by this action actually promote vacuole formation. Another possibility is that efflux of lactate into the t-tubules is mainly by diffusion, which would not be affected by cinnamate. This is possible, but then acid lactate-Ringer (pH = 6.2) should be more effective in preventing vacuolation and we saw no clear evidence for this (authors’ unpublished results). It is also possible that the cinnamate concentration that we used was too low. Mason & Thomas (1988) obtained results with 5 mm cinnamate in frog sartorius muscle which indicated that inhibition of active lactate transport was incomplete.
Relationship between vacuoles, lactate and force
A typical feature of Xenopus fibres is that after fatiguing stimulation, which causes the force output to be reduced to about 40 % of the original, recovery is not monotonical but force shows a further reduction, sometimes down to very low values, before it gradually returns to the pre-fatigue value (Westerblad & Lännergren, 1986). The time course of this post-contractile depression is very similar to the time course of vacuolation, which suggests a causative relation between the two events. We could show here that the extent of vacuolation was markedly reduced by the presence of lactate in the extracellular medium; at the same time force recovery was markedly faster, which strengthens this notion. Also, when a change was made during recovery from lactate-Ringer to normal Ringer vacuolation was increased for some time and at the same time there was a transient force depression. Thus, there are several pieces of evidence to suggest that delayed force recovery is related to vacuole formation. On the other hand, when the time course of the two events is scrutinized more closely we can find examples of force increasing at the same time as vacuoles are becoming more prominent (Lännergren et al. 1999). Results from the present study also argue against a direct causal relation: there was little force depression when vacuole formation was promoted in rested Xenopus fibres by exposing them to lactate and then changing back to normal Ringer (see Fig. 8). Further, in mouse fibres there was no clear temporal correlation between the presence of vacuoles and force reduction; also the force depression was slight (see Fig. 9). We thus find it likely that the primary event is a fatigue-induced defect in excitation-contraction coupling, possibly due to a weakening of structures surrounding the t-tubules. If the defect has developed, the formation of vacuoles will further depress excitation-contraction coupling and reduce force; in the absence of the defect, vacuoles will have little effect on excitation-contraction coupling and force.
Lactate causes a large inhibition of Ca2+- and caffeine-induced Ca2+ release in sarcoplasmic reticulum (SR) vesicles and skinned muscle fibres (Favero et al. 1997; Dutka & Lamb, 2000). However, this depressant effect of lactate appears to have little impact on the normal voltage-activated SR Ca2+ release either in skinned fibres (Dutka & Lamb, 2000) or in unfatigued, intact fibres (present results, Westerblad & Allen, 1992a). Furthermore, during recovery Xenopus fibres produced higher forces in the presence of lactate (see Fig. 5), thus the opposite to what would be expected if lactate inhibits SR Ca2+ release.
Physiological significance
It is unclear whether the vacuolation process that we observe in Xenopus fibres is a physiological phenomenon that occurs after fatiguing contractions in vivo. The fluid space around contracting fibres in a whole muscle is very restricted and during a long series of contractions, such as those used here, there will probably be a gradual build-up of lactate in this space. Since it was demonstrated in the present experiments that extracellular lactate has a protective effect against vacuole formation it is quite possible that the extent of vacuolation during the recovery period is much smaller. There is, however, an argument that after-effects of a bout of high-intensity contractions are of physiological significance and this is the presence of ‘low-frequency fatigue’, a state of reduced force production, especially at low activation frequencies, that may persist for a number of hours and which has been repeatedly documented both in animal and in human muscles. The cause of low-frequency fatigue is reduced Ca2+ release during activation, but the exact mechanism behind this reduction is unclear (Lännergren et al. 1996). We have shown previously that there is very pronounced ‘low-frequency fatigue’ after a fatigue run in Xenopus fibres (Lännergren et al. 1996). As discussed above there might be an indirect coupling between vacuole formation and post-fatigue force depression and it is then of importance to evaluate factors which influence the two events. Apart from this, the present results are of general importance in that they show that the t-tubular system of muscle fibres is not a rigid structure but is capable of marked, short-term plastic changes. The results also indicate that t-tubules are not only important for the inward spread of excitation but may also serve as an important export route from the fibre interior to the surrounding medium.
Acknowledgments
The study was supported by the Knut and Alice Wallenberg Foundation, the Swedish Medical Research Council (project no. 3642), the Swedish National Centre for Sports Research, and funds at the Karolinska Institute.
References
- Bruton JD, Lännergren J, Westerblad H. Mechano-sensitive linkage in excitation-contraction coupling in frog skeletal muscle. The Journal of Physiology. 1995;484:737–742. doi: 10.1113/jphysiol.1995.sp020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Lännergren J, Westerblad H. Effects of repetitive stimulation at long intervals on excitation-contraction coupling in frog skeletal muscle. The Journal of Physiology. 1996;495:15–22. doi: 10.1113/jphysiol.1996.sp021570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Lännergren J, Westerblad H. Effects of CO2-induced acidification on the fatigue resistance of single mouse muscle fibers at 28°C. Journal of Applied Physiology. 1998;85:478–483. doi: 10.1152/jappl.1998.85.2.478. [DOI] [PubMed] [Google Scholar]
- Chin ER, Allen DG. The role of elevations in intracellular [Ca2+] in the development of low frequency fatigue in mouse single muscle fibres. The Journal of Physiology. 1996;491:813–824. doi: 10.1113/jphysiol.1996.sp021259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. Effect of lactate on depolarization-induced Ca2+ release in mechanically skinned skeletal muscle fibers. American Journal of Physiology – Cell Physiology. 2000;278:C517–525. doi: 10.1152/ajpcell.2000.278.3.C517. [DOI] [PubMed] [Google Scholar]
- Endo M. Entry of fluorescent dyes into the sarcotubular system of the frog muscle. The Journal of Physiology. 1966;185:224–238. doi: 10.1113/jphysiol.1966.sp007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero TG, Zable AC, Colter D, Abramson JJ. Lactate inhibits Ca2+-activated Ca2+-channel activity from skeletal muscle sarcoplasmic reticulum. Journal of Applied Physiology. 1997;82:447–452. doi: 10.1152/jappl.1997.82.2.447. [DOI] [PubMed] [Google Scholar]
- Gallagher FA, Huang CL-H. Osmotic detubulation in frog muscle arises from a reversible vacuolation process. Journal of Muscle Research and Cell Motility. 1997;18:305–322. doi: 10.1023/a:1018670025321. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos HAV, Somlyo AV, McClellan G, Shuman H, Borrero LM, Somlyo AP. Composition of vacuoles and sarcoplasmic reticulum in fatigued muscle: electron probe analysis. Proceedings of the National Academy of Sciences of the USA. 1978;75:1329–1333. doi: 10.1073/pnas.75.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP. The mitochondrial pyruvate carrier: Kinetics and specificity for substrates and inhibitors. Biochemical Journal. 1975;148:85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JW, Jensen CB, White SL, Laska MN, Giera DD, Hoover DM. In vitro and in vivo ultrastructural changes induced by macrolide antibiotic LY281389. Fundamental and Applied Toxicology. 1996;32:205–216. [PubMed] [Google Scholar]
- Hutter OF, Noble D. The chloride conductance of frog skeletal muscle. The Journal of Physiology. 1960;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- Jóhannsson E, Nagelhus EA, McCullagh KJ, Sejersted OM, Blackstad TW, Bonen A, Ottersen OP. Cellular and subcellular expression of the monocarboxylate transporter MCT1 in rat heart. A high-resolution immunogold analysis. Circulation Research. 1997;80:400–407. [PubMed] [Google Scholar]
- Juel C. Lactate-proton cotransport in skeletal muscle. Physiological Reviews. 1997;77:321–358. doi: 10.1152/physrev.1997.77.2.321. [DOI] [PubMed] [Google Scholar]
- Juel C, Halestrap AP. Lactate transport in skeletal muscle – role and regulation of the monocarboxylate transporter. The Journal of Physiology. 1999;517:633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolenko SA, Amos WB, Lucy JA. Reversible vacuolation of the transverse tubules of frog muscle: a confocal fluorescence microscopy study. Journal of Muscle Research and Cell Motility. 1995;16:401–411. doi: 10.1007/BF00114505. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. The Journal of Physiology. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. Volume changes of isolated Xenopus muscle fibres associated with repeated tetanic contractions. The Journal of Physiology. 1990;420:116P. [Google Scholar]
- Lännergren J, Bruton JD, Westerblad H. Vacuole formation in fatigued single muscle fibres from frog and mouse. Journal of Muscle Research and Cell Motility. 1999;20:19–32. doi: 10.1023/a:1005412216794. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. The effect of temperature and stimulation scheme on fatigue and recovery in Xenopus muscle fibres. Acta Physiologica Scandinavica. 1988;133:83–89. doi: 10.1111/j.1748-1716.1988.tb08382.x. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H, Bruton JD. Slow recovery of force in single skeletal muscle fibres. Acta Physiologica Scandinavica. 1996;156:193–202. doi: 10.1046/j.1365-201X.1996.198000.x. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H, Flock B. Transient appearance of vacuoles in fatigued Xenopus muscle fibres. Acta Physiologica Scandinavica. 1990;140:437–445. doi: 10.1111/j.1748-1716.1990.tb09019.x. [DOI] [PubMed] [Google Scholar]
- Libelius R, Jirmanova I, Lundquist I, Thesleff S, Barnard EA. T-tubule endocytosis in dystrophic chicken muscle and its relation to muscle fiber degeneration. Acta Neuropathologica. 1979;48:31–38. doi: 10.1007/BF00691788. [DOI] [PubMed] [Google Scholar]
- Malouf NN, Wilson PF. Proliferation of the surface connected intracytoplasmatic membranous network in skeletal muscle disease. American Journal of Pathology. 1986;125:358–368. [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Thomas RC. A microelectrode study of the mechanism of L-lactate entry into and release from frog sartorius muscle. The Journal of Physiology. 1988;400:459–479. doi: 10.1113/jphysiol.1988.sp017132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesser AS, van der Laarse WJ, Elzinga G. Metabolic changes with fatigue in different types of single muscle fibres of Xenopus laevis. The Journal of Physiology. 1992;448:511–523. doi: 10.1113/jphysiol.1992.sp019054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nik-Zainal S, Skepper JN, Hockaday A, Huang CL-H. Cardiac glycosides inhibit detubulation in amphibian skeletal muscle fibres exposed to osmotic shock. Journal of Muscle Research and Cell Motility. 1999;20:45–53. doi: 10.1023/a:1005494114976. [DOI] [PubMed] [Google Scholar]
- Thompson LV, Fitts RH. Muscle fatigue in the frog semitendinosus: role for the high-energy phosphates and Pi. American Journal of Physiology. 1992;263:C803–809. doi: 10.1152/ajpcell.1992.263.4.C803. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones RD. Non-passive chloride distribution in mammalian heart muscle: Micro-electrode measurement of the intracellular chloride activity. The Journal of Physiology. 1979;295:83–109. doi: 10.1113/jphysiol.1979.sp012956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. The Journal of Physiology. 1992a;449:49–71. doi: 10.1113/jphysiol.1992.sp019074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. The Journal of Physiology. 1992b;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J. Force and membrane potential during and after fatiguing, intermittent tetanic stimulation of single Xenopus muscle fibres. Acta Physiologica Scandinavica. 1986;128:369–378. doi: 10.1111/j.1748-1716.1986.tb07990.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiologica Scandinavica. 1988;133:83–89. doi: 10.1111/j.1748-1716.1988.tb08383.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J. Reversible increase in light scattering during recovery from fatigue in Xenopus muscle fibres. Acta Physiologica Scandinavica. 1990;140:429–435. doi: 10.1111/j.1748-1716.1990.tb09018.x. [DOI] [PubMed] [Google Scholar]


