Abstract
BACKGROUND—In duodenal ulcer patients, intragastric acidity during omeprazole treatment is significantly lower before Helicobacter pylori eradication than after cure. AIMS—To determine if H pylori enhances the acid inhibitory potency of omeprazole in isolated parietal cells and on H+/K+-ATPase. METHODS—Rat parietal cells and pig gastric membrane vesicles enriched in H+/K+-ATPase activity were incubated with H pylori and the H pylori fatty acid cis 9,10-methyleneoctadecanoic acid (MOA), and the inhibitory effects of omeprazole on parietal cell acid production, H+/K+-ATPase enzyme activity, and ATPase mediated proton transport were assessed. RESULTS—In isolated parietal cells, H pylori and MOA increased the acid inhibitory potency of omeprazole 1.8 fold. H pylori did not affect the inhibitory potency of omeprazole on H+/K+-ATPase enzyme activity. In proton transport studies, H pylori (intact bacteria and sonicate) and MOA accelerated the onset of the inhibitory effect of omeprazole and enhanced the proton dissipation rate in response to omeprazole. H pylori itself increased proton permeability at the vesicle membrane. CONCLUSION—Our results show that H pylori augments the acid inhibitory potency of omeprazole in parietal cells and enhances omeprazole induced proton efflux rate from gastric membrane vesicles. We suggest that omeprazole unmasks the permanent effect of H pylori on proton permeability at the apical parietal cell membrane, which is counteracted in the absence of a proton pump inhibitor by a reserve H+/K+-ATPase capacity. Keywords: H pylori; parietal cells; omeprazole; H+/K+-ATPase
Full Text
The Full Text of this article is available as a PDF (161.5 KB).
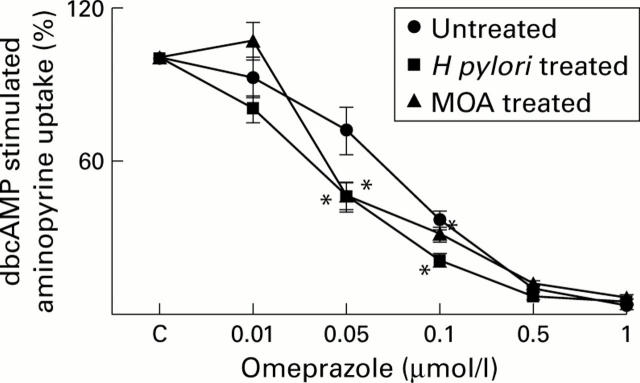
Figure 1 .
Effect of H pylori and cis 9,10-methyleneoctadecanoic acid (MOA) on the inhibitory action of omeprazole on dibuturyl cyclic adenosine monophosphate (dbcAMP) stimulated acid production in rat parietal cells. Gastric cells (2×106 cells, parietal cell content 28-35%) were incubated without (untreated), or with H pylori (50 cfu/gastric cell) or MOA (5 µmol/l). Thereafter, H pylori was removed from the cells. Omeprazole was added to the cells and acid production was initiated by dbcAMP (0.1 mmol/l). After 40 minutes of incubation at 37°C, intracellularly trapped aminopyrine was counted. dbcAMP stimulated aminopyrine uptake in untreated, H pylori, and MOA treated cells was 115 (37), 117 (40), and 124 (12) pmol aminopyrine/105 parietal cells, respectively. These values were set to 100%. Values are mean (SEM) of four cell preparations. C, control. *p<0.05 v omeprazole alone.
Figure 2 .
Effect of H pylori on the inhibitory effect of omeprazole on gastric H+/K+-ATPase activity. Gastric membrane vesicles were incubated without (untreated) or with H pylori 5×105 or 2.5×106 bacteria/µg membrane protein. Bacteria were then separated from the medium. Omeprazole was added to the vesicles and ATPase activity was initiated by adding ATP and the K+ ionophore valinomycin. Values are mean (SEM). C, control.
Figure 3 .
Effect of omeprazole on H+/K+-ATPase mediated H+ transport in gastric membrane vesicles exposed to H pylori cells. Gastric vesicles (60 µg protein/ml) were incubated with and without H pylori (8×105 bacteria/µg membrane protein) in 2 ml of 10 mmol/l piperazine-N,N'-bis [2-ethane-sulphonic acid]/Tris buffer containing 2 mmol/l MgCl2 and 150 mmol/l KCl. After 30 minutes of incubation, H pylori was separated by centrifugation. Acridine orange and omeprazole (10 µmol/l) were added and proton transport was initiated by adding ATP and valinomycin (arrow).
Figure 4 .

Effect of omeprazole on H+/K+-ATPase mediated H+ transport in gastric membrane vesicles exposed to H pylori cells. Gastric membrane vesicles were incubated with H pylori (1.6 and 8×105 bacteria/µg membrane protein) as described in fig 2. Proton transport was initiated by addition of ATP and valinomycin (arrow) and omeprazole was added (↑) when the pH gradient had reached its maximum value.
Figure 5 .
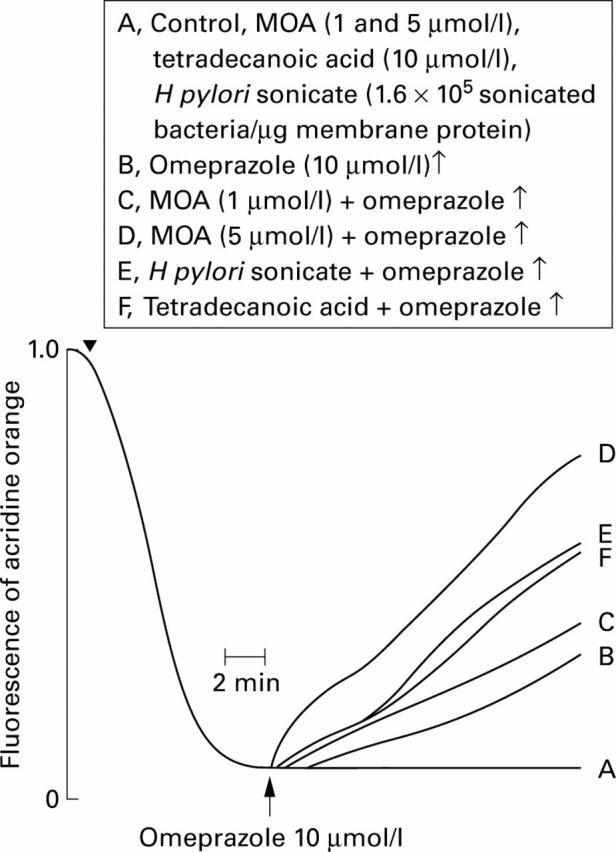
Effect of omeprazole on H+/K+-ATPase mediated H+ transport in gastric membrane vesicles exposed to a H pylori sonicate (1.6×105 sonicated bacteria/µg membrane protein), cis 9,10-methyleneoctadecanoic acid (MOA) (1 and 5 µmol/l), and tetradecanoic acid (10 µmol/l). Experimental conditions were as described in fig 2. Proton transport was initiated by addition of ATP and valinomycin (arrow) and omeprazole was added (↑) when the pH gradient had reached its maximum value.
Figure 6 .
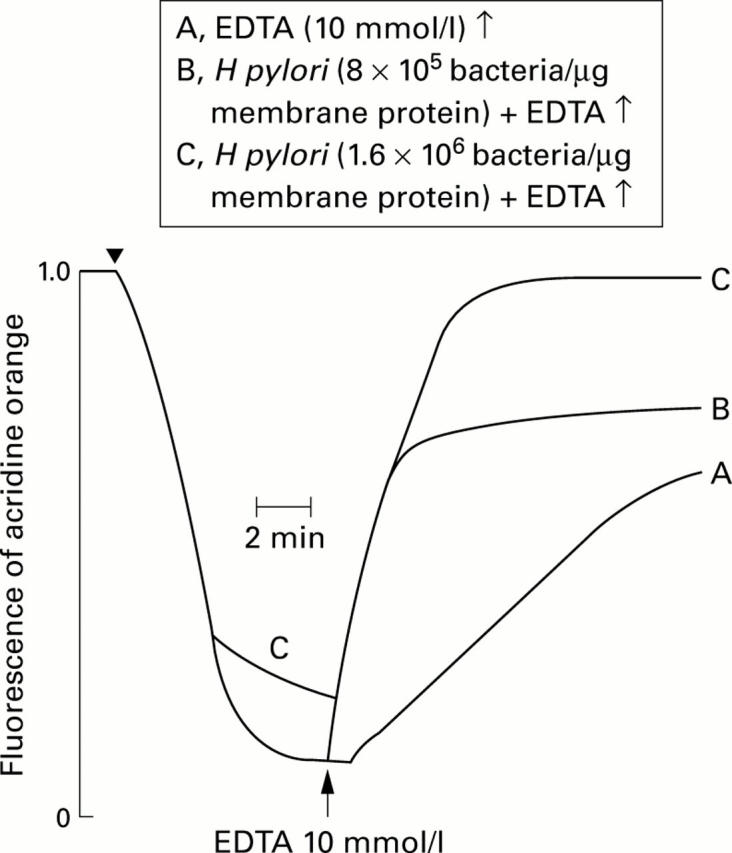
Effect of the magnesium chelating agent ethylenediaminetetraacetic acid (EDTA) on intravesicular acidity in gastric membrane vesicles treated with and without H pylori cells (8×105 and 1.6×106 bacteria/µg membrane protein). Experimental conditions were as described in fig 2. Proton transport was initiated by addition of ATP and valinomycin (arrow) and EDTA was added (↑) to stop the ATPase reaction when the pH gradient had reached its maximum value.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beil W., Birkholz C., Wagner S., Sewing K. F. Helicobacter pylori fatty acid cis 9,10-methyleneoctadecanoic acid increases [Ca2+]i, activates protein kinase C and stimulates acid secretion in parietal cells. Prostaglandins Leukot Essent Fatty Acids. 1998 Aug;59(2):119–125. doi: 10.1016/s0952-3278(98)90090-4. [DOI] [PubMed] [Google Scholar]
- Beil W., Birkholz C., Wagner S., Sewing K. F. Interaction of Helicobacter pylori and its fatty acids with parietal cells and gastric H+/K(+)-ATPase. Gut. 1994 Sep;35(9):1176–1180. doi: 10.1136/gut.35.9.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beil W., Hackbarth I., Sewing K. F. Mechanism of gastric antisecretory effect of SCH 28080. Br J Pharmacol. 1986 May;88(1):19–23. doi: 10.1111/j.1476-5381.1986.tb09466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beil W., Staar U., Schünemann P., Sewing K. F. Omeprazole, SCH 28080 and doxepin differ in their characteristics to inhibit H+/K+-ATPase driven proton accumulation by parietal cell membrane vesicles. Biochem Pharmacol. 1988 Dec 1;37(23):4487–4493. doi: 10.1016/0006-2952(88)90664-8. [DOI] [PubMed] [Google Scholar]
- Carter S. G., Karl D. W. Inorganic phosphate assay with malachite green: an improvement and evaluation. J Biochem Biophys Methods. 1982 Dec;7(1):7–13. doi: 10.1016/0165-022x(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Cave D. R., Vargas M. Effect of a Campylobacter pylori protein on acid secretion by parietal cells. Lancet. 1989 Jul 22;2(8656):187–189. doi: 10.1016/s0140-6736(89)90372-3. [DOI] [PubMed] [Google Scholar]
- Gillen D., el-Omar E. M., Wirz A. A., Ardill J. E., McColl K. E. The acid response to gastrin distinguishes duodenal ulcer patients from Helicobacter pylori-infected healthy subjects. Gastroenterology. 1998 Jan;114(1):50–57. doi: 10.1016/s0016-5085(98)70632-8. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., McCulloch R. K., Armstrong J. A., Wee S. H. Unusual cellular fatty acids and distinctive ultrastructure in a new spiral bacterium (Campylobacter pyloridis) from the human gastric mucosa. J Med Microbiol. 1985 Apr;19(2):257–267. doi: 10.1099/00222615-19-2-257. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Alpert L. C., Smith J. L., Yoshimura H. H. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am J Gastroenterol. 1988 Sep;83(9):974–980. [PubMed] [Google Scholar]
- Graham D. Y., Opekun A., Lew G. M., Evans D. J., Jr, Klein P. D., Evans D. G. Ablation of exaggerated meal-stimulated gastrin release in duodenal ulcer patients after clearance of Helicobacter (Campylobacter) pylori infection. Am J Gastroenterol. 1990 Apr;85(4):394–398. [PubMed] [Google Scholar]
- Labenz J., Tillenburg B., Peitz U., Idström J. P., Verdú E. F., Stolte M., Börsch G., Blum A. L. Helicobacter pylori augments the pH-increasing effect of omeprazole in patients with duodenal ulcer. Gastroenterology. 1996 Mar;110(3):725–732. doi: 10.1053/gast.1996.v110.pm8608881. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Forte J. G. A study of H+ transport in gastric microsomal vesicles using fluorescent probes. Biochim Biophys Acta. 1978 Apr 4;508(2):339–356. doi: 10.1016/0005-2736(78)90336-x. [DOI] [PubMed] [Google Scholar]
- Morris A., Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987 Mar;82(3):192–199. [PubMed] [Google Scholar]
- Moss S. F., Legon S., Bishop A. E., Polak J. M., Calam J. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet. 1992 Oct 17;340(8825):930–932. doi: 10.1016/0140-6736(92)92816-x. [DOI] [PubMed] [Google Scholar]
- Rademaker J. W., Hunt R. H. Helicobacter pylori and gastric acid secretion: the ulcer link? Scand J Gastroenterol Suppl. 1991;187:71–77. [PubMed] [Google Scholar]
- Robert A., Olafsson A. S., Lancaster C., Zhang W. R. Interleukin-1 is cytoprotective, antisecretory, stimulates PGE2 synthesis by the stomach, and retards gastric emptying. Life Sci. 1991;48(2):123–134. doi: 10.1016/0024-3205(91)90405-z. [DOI] [PubMed] [Google Scholar]
- Smith J. T., Pounder R. E., Nwokolo C. U., Lanzon-Miller S., Evans D. G., Graham D. Y., Evans D. J., Jr Inappropriate hypergastrinaemia in asymptomatic healthy subjects infected with Helicobacter pylori. Gut. 1990 May;31(5):522–525. doi: 10.1136/gut.31.5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdú E. F., Armstrong D., Fraser R., Viani F., Idström J. P., Cederberg C., Blum A. L. Effect of Helicobacter pylori status on intragastric pH during treatment with omeprazole. Gut. 1995 Apr;36(4):539–543. doi: 10.1136/gut.36.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S., Beil W., Westermann J., Logan R. P., Bock C. T., Trautwein C., Bleck J. S., Manns M. P. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997 Dec;113(6):1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- Wallmark B. Mechanism of action of omeprazole. Scand J Gastroenterol Suppl. 1986;118:11–17. doi: 10.3109/00365528609090881. [DOI] [PubMed] [Google Scholar]
- el-Omar E. M., Penman I. D., Ardill J. E., Chittajallu R. S., Howie C., McColl K. E. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995 Sep;109(3):681–691. doi: 10.1016/0016-5085(95)90374-7. [DOI] [PubMed] [Google Scholar]