Abstract
Correlated spiking activity and associated Ca2+waves in the developing retina are important in determining the connectivity of the visual system. Here, we show that GABA, via GABAB receptors, regulates the temporal characteristics of Ca2+ waves occurring before synapse formation in the embryonic chick retina. Blocking ionotropic GABA receptors did no affect these Ca2+ transients. However, when these receptors were blocked, GABA abolished the transients, as did the GABAB agonist baclofen. The action of baclofen was prevented by the GABAB antagonistp-3-aminopropyl-p-diethoxymethyl phosphoric acid (CGP35348). CGP35348 alone increased the duration of the transients, showing that GABAB receptors are tonically activated by endogenous GABA. Blocking the GABA transporter GAT-1 with 1-(4,4-diphenyl-3-butenyl)-3-piperidine carboxylic acid (SKF89976A) reduced the frequency of the transients. This reduction was prevented by CGP35348 and thus resulted from activation of GABAB receptors by an increase in external [GABA]. The effect of GABAB receptor activation persisted in the presence of activators and blockers of the cAMP–PKA pathway. Immunocytochemistry showed GABAB receptors and GAT-1 transporters on ganglion and amacrine cells from the earliest times when Ca2+ waves occur (embryonic day 8). Patch-clamp recordings showed that K+ channels on ganglion cell layer neurons are not modulated by GABAB receptors, whereas Ca2+ channels are; however, Ca2+channel blockade with ω-conotoxin-GVIA or nimodipine did not prevent Ca2+ waves. Thus, the regulation of Ca2+ waves by GABAB receptors occurs independently of N- and L-type Ca2+ channels and does not involve K+ channels of the ganglion cell layer. GABAB receptors are likely to be of key importance in regulating retinal development.
Keywords: retina, ganglion cells, Ca2+, spontaneous activity, GABAB receptors, patch-clamping
Spontaneous electrical activity plays a pivotal role in the development of the nervous system through the changes it produces in intracellular calcium concentration ([Ca2+]i). Electrical activity and associated changes in [Ca2+]i regulate the expression of ion channels and transmitter phenotype (Gu and Spitzer, 1995, 1997), dendritic geometry (Bodnarenko et al., 1995), neurite extension (Fields et al., 1990; Gomez and Spitzer, 1999), and synapse formation (Constantine-Paton et al., 1990; Kalil, 1990; Katz and Shatz, 1996). These experiments have shown that the frequency and kinetics of [Ca2+]i transients are key determinants of the development of the nervous system.
In the retina, spontaneous electrical activity takes the form of rhythmic bursts of action potentials that spread between adjacent ganglion and amacrine cells and produce transient elevations in [Ca2+]i. These waves have been described for both mammalian (Meister et al., 1991;Wong et al., 1993, 1995; Catsicas and Mobbs, 1995) and chick (Catsicas et al., 1998; Wong et al., 1998) retina and are necessary for the formation of the appropriate synaptic connections by ganglion cells within the lateral geniculate nucleus and optic tectum (Mooney et al., 1996; Wong, 1999). This activity is likely instructive in shaping cortical development (Weliky, 1999). However, the role of synchronized electrical activity and Ca2+waves within the retina is unknown.
At early developmental stages in the mammalian retina, initiation and propagation of correlated activity requires cholinergic transmission (Feller et al., 1996; Penn et al., 1998), whereas at later stages, GABAergic, glycinergic, and glutamatergic transmission can modulate wave generation (Fischer et al., 1998; Wong, 1999). In contrast, in the chick retina, Ca2+ wave production does not require cholinergic transmission, but endogenous ACh modulates the temporal pattern of the activity (Catsicas et al., 1998). Activation of other neurotransmitter receptors also changes the frequency with which Ca2+ waves are produced; however, activation of GABAA receptors does not contribute to wave production at early times (Catsicas et al., 1998; Wong et al., 1998). This is surprising on several counts. First, GABA is released during early chick retinal development (Bonness et al., 1996). Second, at these ages, GABA is depolarizing and activation of GABAA receptors leads to Ca2+ entry via voltage-gated channels (Yamashita and Fukuda, 1993). Third, in both mammalian and chick retina, the major source of ACh is a subpopulation of amacrines and displaced amacrines that also express and release GABA (O'Malley et al., 1992; Santos et al., 1998). Thus, GABA is a good candidate for modulating Ca2+ waves.
Here, we show that endogenous GABA modulates the frequency and duration of [Ca2+]i waves in the chick retina from the earliest times at which such activity can be detected [embryonic day 8 (E8)]. This effect is mediated by GABAB receptors in a cAMP-independent manner, influenced by the activity of the GABA transporter 1 (GAT-1), and is independent of the modulation of Ca2+ channels by GABAB receptors.
MATERIALS AND METHODS
Chicken White Leghorn eggs were incubated at 37°C and 60% humidity. Chicken embryos between E8 and E12 were decapitated, and their eyes removed.
Calcium imaging. The retinas were dissected from the eyecup at room temperature in Krebs' solution containing (in mm): 100 NaCl, 30 NaHCO3, 6 KCl, 1 MgCl2, 1 CaCl2, 3 NaH2PO4, and 20 glucose, pH 7.4 by gassing with 5% CO2–95% O2. After dissection, the retinas were loaded with the Ca2+-sensitive dye Calcium Green-1 AM (10 μm; Molecular Probes, Eugene, OR) in Krebs' solution in the presence of the dispersant Cremophor-EL (0.03%; Sigma, Poole, UK) for 1 hr at room temperature. After loading, the retinas were maintained in fresh Krebs' solution at room temperature for up to 2 hr before use. The central piece of the retina (∼5 × 5 mm) was transferred to a perfusion chamber on the stage of an inverted microscope and imaged at 35°C, held flat with a nylon mesh glued to a platinum harp, and continually superfused with Krebs' solution delivered through a peristaltic pump.
Imaging data acquisition and analysis. Retinas were imaged as flat mounts, with the ganglion cell surface facing the objective, using an inverted epifluorescence microscope (Axiovert 100; Zeiss, Oberkochen, Germany) equipped with a fluorescein filter set (XF22; Omega Optical, Stanmore, UK) and a Zeiss Fluar 40× lens (numerical aperture of 1.3). Fluorescence images were recorded using a slow-scan cooled CCD camera (model number 2000; Digital Pixell). Images were acquired at 1 Hz and analyzed off-line using Lucida 3.5 software (Kinetic Imaging Ltd., Liverpool, UK). The mean fluorescence (imaged field of view, ∼100 × 200 μm) was calculated and normalized to its initial value at time 0. Increases in the fluorescence of Calcium Green-1 reflect increases in [Ca2+]i.
Electrophysiology. Membrane currents from amacrine and ganglion cells in the ganglion cell layer (GCL) of E9–E12 chick retinas were measured in the whole-cell clamp configuration using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). Patch pipettes were pulled from thick-walled borosilicate glass with internal filament (GC150F; Clark Electromedical Instruments, Reading, UK) and had resistances of ∼10 MΩ. After dissection (see above), the extracellular matrix and major axon bundles overlying the GCL were removed mechanically in several small areas under a dissecting microscope using a pair of fine forceps. The central piece of the retina was transferred to a recording chamber (as above) on the stage of an upright microscope (BX50WI; Olympus Optical, London, UK) equipped with a 40× water immersion lens. The ganglion cell surface faced the objective, and the cells were visualized under differential interference contrast optics or epifluorescence (see below). K+ currents were elicited with 200 msec voltage steps from −140 to +50 mV in 10 mV increments from a holding potential of −90 mV. The steady-state current values were obtained by averaging the points between 30 and 130 msec into the voltage step. The effect of baclofen was determined by subtracting the currents evoked in baclofen from those in its absence. Ca2+currents were elicited with 40 msec voltage steps to 0 mV from the holding potential (−90 mV), alternated with −10 mV steps. This was repeated five times, and the data were averaged. Leak subtraction was effected by scaling the trace resulting from the small step and subtracting it from the trace obtained in response to the large depolarizing step. The steady-state current values were obtained by averaging the points between 20 and 30 msec into the voltage step. The mean access resistance was 30 ± 2 MΩ. In the case of Ca2+ currents, if the series resistance voltage errors exceeded 5 mV, data were discarded. Where outward K+ conductances were not blocked and the currents were large, voltages were corrected for the voltage error produced by current flow through the access resistance. The recordings were performed at room temperature. To investigate K+ currents, the external solution was Ringer's solution containing (in mm): 138 NaCl, 4 KCl, 0.41 MgCl2, 3 HEPES, 3 CaCl2, 1 NaH2PO4, and 5.6 glucose, pH 7.4 with NaOH; 1 μm TTX was added to block sodium currents. The internal solution consisted of (in mm): 108 KH2PO4, 4.5 MgCl2, 9 HEPES, 14 creatine phosphate, 4 MgATP, and 0.5 NaGTP, pH 7.3 (with 100 mm KOH). To investigate Ca2+ currents, the external solution was Ringer's solution containing (in mm): 135 NaCl, 2.5 KCl, 0.41 MgCl2, 3 CaCl2, 5 BaCl2, 3 HEPES, 6 glucose, and 1 μm TTX, pH 7.4 (with NaOH). The internal solution consisted of (in mm): 130 CsCl, 5 TEA-Cl, 0.1 CaCl2, 1 NMDG-EGTA, 10 HEPES, 4 MgATP, 0.5 NaGTP, and 14 creatine phosphate, pH 7.3 with CsOH. In half of the experiments, the CsCl in the internal solution was replaced by equal amounts of Cs-gluconate. This substitution did not affect the currents observed and helped to preserve the morphology of the cells. The internal solution was stored frozen, and Lucifer yellow (0.3% w/v; Sigma) was added just before use. At the end of the experiment, the morphology of the dye-filled cells was visualized using epifluorescence illumination, and images were acquired with a cooled CCD camera (C4880–80; Hamamatsu, Hamamatsu City, Japan). Two drugs, cAMP and adenosine 3′,5′-cyclic phosphorothioate-Rp (Rp-cAMPS), were introduced into cells via the patch-pipette. The diffusion time for these drugs into cells was rapid because they have molecular weights similar to Lucifer yellow (∼450 Da), which filled cells within a few seconds of moving into the whole-cell configuration.
Drugs and statistical analysis. During both imaging and electrophysiological experiments, drugs were applied by bath perfusion: GABA, bicuculline, forskolin, cAMP, 8-bromoadenosine 3′:5′-cAMP (8-bromo-cAMP), and nifedipine (all from Sigma); picrotoxin (PTX), baclofen, and nimodipine (all from Tocris Cookson, Bristol, UK);p-3-aminopropyl-p-diethoxymethyl phosphoric acid (CGP35348) (gift from Novartis, Basel, Switzerland); 1-(4,4-diphenyl-3-butenyl)-3-piperidine carboxylic acid (SKF89976A) (SmithKline Beecham, Harlow, UK); and Rp-cAMPS (Calbiochem-Novabiochem, Nottingham, UK). Each sample trace shows the sequential application of drugs, as indicated, to a single retina.
All the quantitative data presented were tested using Student'st test, and differences were considered statistically significant at p < 0.05. The results are means ± SEM.
Immunocytochemistry. Eyes from E6, E8, E10, and E18 chicks were isolated and fixed by immersion in 4% paraformaldehyde–PBS overnight at 4°C. After three rinses in PBS, the eyes were stored in PBS at 4°C. For sectioning, the eyes were embedded in liquid 4% agar–PBS in brass molds that ensured rapid heat dissipation from the agar block. Transverse sections 100-μm-thick were cut using a vibratome (Campden Instruments, Loughborough, UK) and kept in PBS at 4°C until processed for immunocytochemistry. The sections were permeabilized with 0.05% Triton X-100 in 0.05% BSA and PBS (blocking solution) and then incubated free-floating overnight at 4°C in blocking solution containing a polyclonal primary antibody raised against synthetic peptides of the rat sequences of either GABAB receptors (PC300; 1:500) or the neuronal GABA transporter GAT-1 (PC250L; 1:125) (both primary antibodies from Calbiochem-Novabiochem). After rinsing in PBS, the sections were incubated in either anti-guinea pig (GABAB) or anti-rabbit (GAT-1) biotinylated secondary antibodies for 2 hr at room temperature (both 1:150; Vector Laboratories, Peterborough, UK). The slices were then rinsed and incubated in Texas Red- or FITC-tagged streptavidin (1:25; Vector Laboratories) for 2 hr at room temperature. After rinsing off the excess streptavidin with PBS, the sections were mounted in Citifluor (Citifluor Ltd., London, UK) on glass slides and imaged on a confocal microscope (LSM510; Zeiss). Negative controls consisted of sections processed as described above but in the absence of primary antibody.
RESULTS
When spontaneous calcium activity first occurs, activating GABAA receptors depolarizes cells
As described previously, when experiments were performed at ∼35°C, regular spontaneous Ca2+transients propagated as waves that could be detected from E8 onward (Catsicas et al., 1998). These occurred in 50% of the retinas at E8 (n = 20 retinas) and in ∼80% of retinas from E9 onward (n = 86). The Ca2+transients had a mean duration (measured at half-maximal amplitude) of 5 ± 1 sec and occurred with a mean frequency of 1 ± 0.1 min−1 (Fig.1A). Yamashita and Fukuda (1993) have shown that activation of GABAAreceptors very early in development (E3) leads to depolarization of chick retinal cells and a transient increase in [Ca2+]i via voltage-gated L-type Ca2+ channels. This raises the possibility that ionotropic GABA receptors regulate Ca2+ waves early in development.
Fig. 1.

Ionotropic GABA receptors are expressed and depolarize retinal cells when Ca2+ waves can first be detected. A, Spontaneous Ca2+transients from an E9 retina in control situation. The levels of fluorescence averaged over the field of view were normalized to the basal level at the beginning of the recording session.B, Traces plotting the changes in fluorescence in an E6 (top) and an E12 (bottom) retina. Application of GABA (100 μm) elicited a transient increase in [Ca2+]i at both ages (left traces), which was largely prevented by coapplication of the GABAA receptor antagonist bicuculline (50 μm;middle traces). The response to GABA recovered after 15 min washout of bicuculline (right traces).C, Developmental profile of the response to GABA. The amplitude of the responses is plotted as a percentage of the maximum response, observed at E8. All responses observed were increases in [Ca2+]i. GABA responses were present at E4 and peaked at E8. By E14, GABA no longer evoked increases in [Ca2+]i(see Results). n = 3–6 retinas for each age. The experiments in B and C were done in HEPES-buffered Ringer's solution and performed at room temperature to prevent spontaneous Ca2+ transients.
We first investigated whether activation of GABAA receptors leads to an increase in [Ca2+]i at the time waves first occur. These experiments were performed at room temperature to prevent spontaneous Ca2+waves (such as in Fig. 1A). Between E4 (the earliest time investigated) and E12, application of GABA (100 μm) induces a transient increase in Ca2+ that is greatly and reversibly reduced by the GABAA receptor antagonist bicuculline (50 μm) (Fig.1B,C). The developmental profile of this response is shown in Figure 1C, in which the fluorescence changes evoked by 100 μm GABA were normalized to the mean of the data at E8, the stage when the responses were largest. At E4, five of six retinas responded to GABA. On subsequent days and up until E12, GABA produced increases in [Ca2+]i in all retinas tested (n = 23). From E14 (n = 3) onward, GABA-evoked changes in [Ca2+]i were no longer apparent. GABA-evoked currents can be recorded by whole-cell patch clamping at this age and beyond (our unpublished observation), and GABA is the major inhibitory neurotransmitter in the mature retina (Freed, 1992; Luckasiewicz and Shields, 1998), so the absence of a Ca2+ response at late times presumably reflects a switch in the action of GABA from depolarizing to hyperpolarizing or shunting. Thus, from E8 to E12, the period during which we have studied retinal Ca2+ waves, activation of GABAA receptors leads to transient increases in [Ca2+]i.
GABA affects spontaneous calcium transients independently of ionotropic GABA receptors
The data in Figure 1 raise the possibility that ionotropic GABA receptors are involved in the generation of Ca2+ waves. To address this, we investigated whether or not blocking ionotropic GABA receptors affects spontaneous Ca2+ transients. Application of bicuculline (50 μm) and PTX (100 μm), to block GABAA and GABACreceptors, in retinas maintained at 35°C was without effect on the frequency or duration of the transients (p = 0.18 for the frequency; p = 0.5 for the duration;n = 4) (Fig.2A,B). Thus, under control conditions, ionotropic GABA receptors do not regulate the temporal characteristics of spontaneous Ca2+ transients at this stage in development. In contrast, application of GABA (50 μm) in the presence of bicuculline and PTX reversibly abolished the Ca2+ transients (n = 4) (Fig.2A,B). Upon washout of the drugs, the transients recovered with a frequency initially higher than, and a duration similar to, that in control situation. These results show that GABA can modulate Ca2+ waves independently of ionotropic GABA receptors.
Fig. 2.

Blocking ionotropic GABA receptors does not affect spontaneous Ca2+ transients under control conditions. A, Ca2+ transients recorded from an E9 retina upon successive application of drugs, as indicated. The activity recovered in the presence of bicuculline and picrotoxin upon washout of GABA; some transients had an increased duration under these conditions. B, Histogram of the results pooled from four retinas at E9. The frequency (gray bars) and duration (black bars) of Ca2+transients in the presence of drugs is shown as a percentage change compared with the control condition. Application of the ionotropic GABA receptor antagonists bicuculline (bicu; 50 μm) and PTX (100 μm) did not affect the frequency of the transients. In contrast, application of GABA (50 μm) in the presence of bicuculline and PTX abolished the transients. After a 15 min wash of the drugs, the transients recovered with a frequency initially higher than in control (189 ± 29%;p = 0.09) and a duration comparable with that in control.
GABAB receptors modulate spontaneous calcium transients
In addition to ionotropic receptors, GABA can exert its actions through activation of GABAB receptors. Applying the GABAB receptor agonist baclofen (100 μm) reversibly blocked the Ca2+ transients (n = 4) (Fig. 3A). This effect was dose-dependent and prevented by coapplication of CGP35348 (100 μm), an antagonist at GABAB receptors (1 μmbaclofen, p = 0.01, n = 5; 10 μm baclofen, n = 3) (Fig.3B,D). To determine whether or not GABAB receptors are endogenously activated during spontaneous activity, we applied CGP35348 alone. Figure 3Cshows a sample trace from an E9 retina before (top), during (middle), and after (bottom) application of CGP35348. Although some transients with kinetics similar to those in control situation were seen in the presence of CGP35348 (middle of the trace), most had a greatly prolonged duration. The mean duration of the transients was measured at half-maximal amplitude over a 6 min period in seven retinas in the presence of CGP35348 and compared with that in control (Fig.3E). CGP35348 reversibly prolonged the duration of the transients approximately fivefold: control, 4.49 ± 0.57 sec; CGP, 22.79 ± 3.53 sec (p < 0.005); wash, 5.95 ± 1.73 sec. We cannot exclude the possibility that the Ca2+ transients observed in the presence of CGP35348 resulted from the merging of several unitary events and thus reflect an increase in transient frequency. However, it is more likely that they correspond to an increased duration of single events, because agents such as forskolin (see Fig. 5), which greatly increased the frequency of Ca2+ transients, never gave rise to longer lasting transients comparable with those seen in the presence of CGP35348. These results show that GABAB receptors are endogenously activated and regulate the duration (and/or frequency) of transients.
Fig. 3.

GABAB receptors modulate spontaneous Ca2+ transients. A, B,D, Activating GABAB receptors with the agonist baclofen (100 μm) reversibly abolished the transients. The effect of baclofen was dose-dependent (D) and prevented by application of the GABAB receptor antagonist CGP35348 (CGP, 100 μm; B, D,n = 3–4 retinas per condition). C,E, Blocking GABAB receptors prolongs spontaneous Ca2+ transients. Spontaneous Ca2+ transients in an E9 retina under control conditions (top trace), in the presence of CGP35348 (100 μm; middle trace), and after 10 min washout of CGP35348 (bottom trace). E, Pooled data taken from four retinas (comprising 4–10 transients for each condition) showing the duration of Ca2+transients as measured at half-peak.
Fig. 5.
GABAB receptors act in push–pull with a cAMP-dependent pathway. A, Spontaneous Ca2+ transients recorded from an E9 retina upon sequential application of forskolin and baclofen, as indicated.B, Pooled data taken from six retinas of the Ca2+ transient frequency normalized to control in each condition. Activating adenylate cyclase with forskolin (1 μm) increased the frequency of transients. This effect could be reversibly prevented by coapplication of baclofen (50 μm). The effect of both drugs reversed after 20 min wash.C, Bath application of 8-bromo-cAMP (500 μm, 30 min) mimicked the effect of forskolin (n = 2) and was antagonized by coapplication of baclofen (50 μm). Blocking protein kinase A with bath application of Rp-cAMPS (500 μm, 20–30 min) reduced the frequency of transients, which could be further decreased by baclofen (50 μm). The activity recovered only partially (D). D, Pooled data from three retinas showing the frequency of Ca2+ transients normalized to control.
Modulation of spontaneous calcium transients by GAT-1 transporters
To better understand the mechanisms regulating the concentration of ambient GABA, we next examined the effect of SKF89976A, a selective antagonist of the neuronal GABA transporter GAT-1 (Fig. 4). Application of SKF89976A (100 μm) reversibly reduced the frequency of Ca2+ transients by ∼80% (p < 0.001; n = 7) (Fig.4A). This is likely to occur via activation of GABAB receptors, because it mimics the effect of the GABAB receptor agonist baclofen (Fig.3A,B,D). In support of this interpretation, the effect of SKF89976A was prevented by coapplication of CGP35348 (100 μm); however, in the presence of SKF89976A and CGP35348, the frequency of transients exceeded that in control (p < 0.01;n = 5) (Fig. 4B). This “overshoot” in frequency could be prevented by application of the ionotropic GABA receptor antagonists bicuculline (50 μm) and PTX (100 μm) together with SKF89976A and CGP35348 (p = 0.19;n = 3) (Fig. 4B). These results suggest that, in basal conditions, GAT-1 transporters take up GABA maintaining it at a low concentration in the extracellular space thereby limiting the activation of GABABreceptors. However, when GAT-1 is inhibited, the rise in GABA concentration is sufficient to reduce the frequency of Ca2+ transients through increased activation of GABAB receptors. In the presence of CGP35348, this negative regulation is prevented, and the elevated levels of GABA increase the frequency of Ca2+ transients via activation of ionotropic GABA receptors.
Fig. 4.
Blocking neuronal GABA transport reduces the frequency of spontaneous Ca2+ transients.A, The frequency of transients was reversibly reduced with application of the GAT-1 transporter blocker SKF89976A (SKF; 100 μm; p < 0.001; n = 7). B, The effect of SKF was prevented by coapplication of CGP35348 (CGP; 100 μm). In the presence of SKF89976A and CGP35348, the frequency of Ca2+ transients increased approximately threefold compared with control levels (p < 0.01). This overshoot in the frequency was prevented if bicuculline (bicu; 50 μm) and PTX (100 μm) were applied together with SKF89976A and CGP35348 (n = 5 retinas). The duration of the transients in the presence of SKF89976A and CGP35348 was similar to that in control (data not shown).
GABAB receptors oppose the effects of cAMP-activating pathways to set the frequency of calcium waves
GABAB receptors commonly exert their actions through coupling to G-proteins of the Gi/o type. Their activation may lead to direct modulation of K+ and Ca2+channels by the Gβγsubunit or to modulation, generally a downregulation, of adenylate cyclase via the Gα subunit (Bettler et al., 1998, and references therein). Thus, GABABreceptors could modulate calcium waves by reducing cAMP levels. We have shown previously that forskolin, a constitutive activator of adenylate cyclase that raises cAMP levels in cells, increases the frequency of calcium waves in the embryonic chick retina (Catsicas et al., 1998); similar results were obtained by Stellwagen et al. (1999) in developing ferret retina. Here we show that 1 μm forskolin is sufficient to raise the frequency of calcium waves ∼2.5-fold (p < 0.05; n = 6) (Fig.5A,B) and that coapplication of 50 μm baclofen, a dose that in isolation would block calcium waves (Fig. 3), reversibly counteracted the effect of forskolin, resulting in a frequency similar to control (n = 6). The effect of forskolin could be mimicked by long perfusion (25 min) of 8-bromo-cAMP, a membrane-permeable analog of cAMP (500 μm; a fourfold increase in two retinas) (Fig. 5C), showing that the effect of forskolin on calcium waves is attributable to the activation of adenylate cyclase. As was the case for forskolin, coapplication of baclofen (50 μm) reduced the frequency of transients in the presence of 8-bromo-cAMP (by 80% compared with 8-bromo-cAMP alone in two retinas) (Fig.5C).
Many effects of cAMP are attributable to a signaling cascade involving the activation of protein kinase A (PKA). Thus, to investigate the participation of PKA in regulating spontaneous calcium waves, we used the membrane-permeable, specific inhibitor Rp-cAMPS to block its endogenous activation. Application of Rp-cAMPS (500 μm, 15–30 min) resulted in a decrease in transient frequency that could be further reduced by application of 50 μm baclofen (p = 0.001 comparing Rp-cAMPS with control;n = 3) (Fig. 5C,D). Upon washout of both drugs, the activity recovered only partially, except for one retina that could be imaged long enough (80 min wash) for the activity to recover to control level. The concentration of Rp-cAMPS used here is well above its ki for PKA (11 μm) and should result in a complete block of the kinase and, indeed, similar doses of Rp-cAMPS (100–500 μm) applied extracellularly block forskolin-induced, cAMP-dependent enhancement of synaptic transmission in neostriatal slices (Colwell and Levine, 1995) and vesicular recycling in dissociated granule cells (Chavis et al., 1998). Our results show that the cAMP–PKA signaling cascade is constitutively activated in this system and promotes calcium transients. Activating GABAB receptors with baclofen has an effect that counteracts that of cAMP pathway activators, such as forskolin and 8-bromo-cAMP, and is additive to that of blocking PKA with Rp-cAMPS. This suggests that GABAB receptors do not decrease the frequency of calcium waves by blocking adenylate cyclase and lowering cAMP levels.
Expression of GABAB receptors and GAT-1 transporters in developing chick retina
At times when we are first able to detect spontaneous Ca2+ waves (E8), the layers of processes [inner plexiform layer (IPL) and outer plexiform layer (OPL)] in the chick retina have yet to mature, and morphologically identifiable synapses are absent (Hughes and LaVelle, 1974; Hering and Kröger, 1996). Little is known of the distribution of GABAB receptors and GAT-1 transporters at these times. We thus examined their expression patterns between E6 and E18 using polyclonal antibodies raised against their mammalian counterparts (Figs. 6,7). At E6, GABABreceptor immunoreactivity was already apparent in the developing chick retina (Fig. 6). Presumptive ganglion cells in the emerging GCL were labeled. Plaques of immunoreactivity were also apparent throughout the thickness of the tissue. At E8, cells in the GCL, as well as in the inner part of the neuroblastic layer (NBL) in which amacrine and displaced ganglion cells are located, were immunopositive for GABAB receptors. Punctate staining was detected in the IPL, in the outer NBL, the location of presumptive horizontal cells, and in the nascent OPL. At E10, the staining was very similar to that at E8, but more immunoreactive plaques were present in the outer retina at the later time. By the time the retina has reached a mature morphology, at E18, the lamina-specific staining had become apparent in both IPL and OPL. Differential staining of cell bodies in the inner nuclear layer (INL) was also apparent, most cells in the amacrine sublayer of the INL, bordering the IPL, were heavily immunopositive, as well as putative horizontal cells bordering the inner part of the OPL. Cell bodies in the GCL and ganglion cell axons in the fiber layer (FL) were heavily labeled.
Fig. 6.

Expression of GABAB receptors in the developing chick retina. Confocal microscope sections of GABAB receptor immunostaining of transverse vibratome slices from E6, E8, E10, and E18 chick retina. Left panels, White-light micrographs from the slices in themiddle panels showing the structure of the retina for each age. Middle panels, Sections immunoreacted with the anti-GABAB receptor antibody. Right panels, Negative controls in which the primary antibody was omitted. At E6, GABABreceptor-immunoreactive cells, presumptive ganglion cells, were present at the inner margin of the NBL in which the GCL forms. Punctate immunostaining was also present through the thickness of the NBL. At E8, the GCL is separated from the NBL by a layer of processes, the IPL. GABAB receptor immunoreactivity was prominent in cells of the GCL. Cells at the inner margin of the NBL, presumptive amacrine cells, were also positive, as well as fibers of the IPL. Immunoreactivity was still present in the rest of the NBL, in particular surrounding distal cell bodies of the NBL outlining the prospective OPL (presumptive horizontal cells). At E10, the pattern of staining was essentially the same as at E8, except there were more immunoreactive plaques in the outer retina at later times. By E18, the retina has reached its mature morphology. GABAB receptor immunoreactivity was present in cells of the GCL (ganglion and displaced amacrine cells), of the inner part of the INL (amacrine and displaced ganglion cells), and bordering the OPL. The pattern of staining showed a clear lamina distribution in both OPL and IPL, and immunoreactivity was present in the FL. Some autofluorescence is apparent in the outer retina at the level of the outer nuclear layer (ONL) and OPL (see negative controls). Scale bar, 25 μm.
Fig. 7.
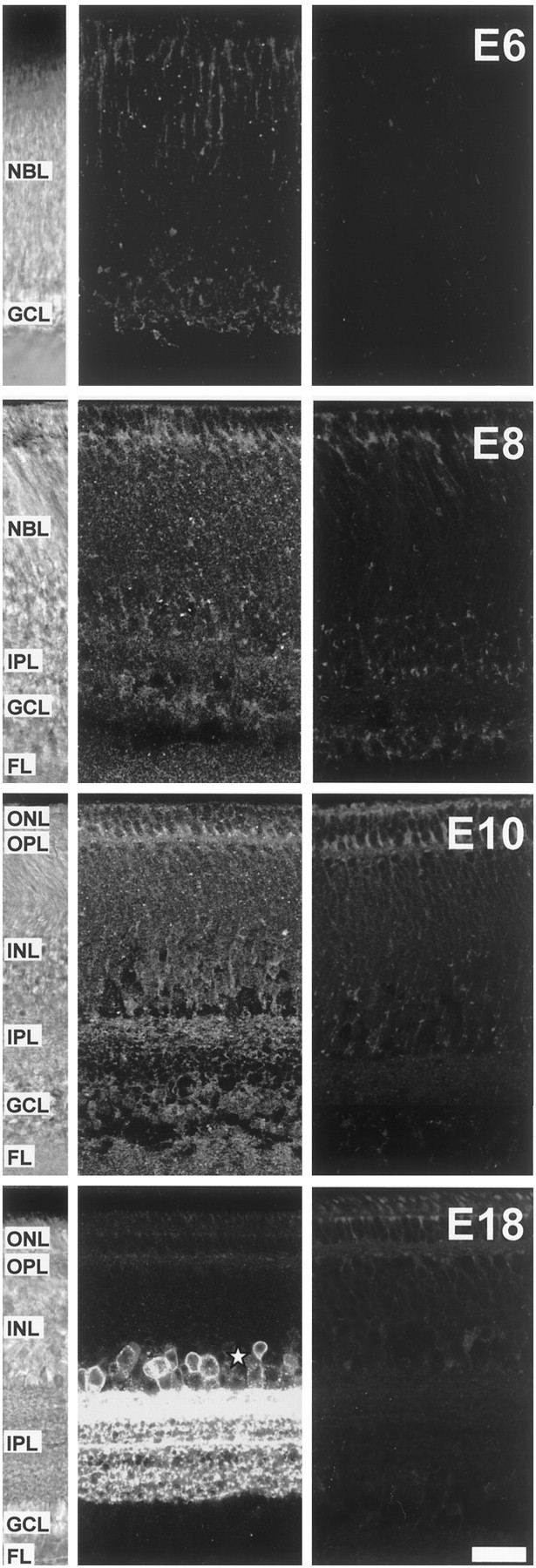
Expression of GAT-1 transporters in the developing chick retina. Confocal microscope sections of GAT-1 transporter immunostaining of transverse vibratome slices from E6, E8, E10, and E18 chick retina. Left panels, White-light micrographs from the slices in the middle panels showing the structure of the retina for each age. Middle panels, Sections immunoreacted with the anti-GAT-1 antibody. Right panels, Negative controls in which the primary antibody was omitted. At E6, GAT-1 immunostaining was punctate and restricted to the outer NBL and the GCL. At E8, immunoreactive puncta were distributed throughout the retina. By E10, GAT-1 lamina-specific staining was apparent in the IPL, with laminas closer to the INL more strongly labeled. At E18, GAT-1 staining is restricted to the IPL, in which it has a clear laminar distribution, and to somata in the proximal INL, presumptive amacrine cells (asterisk). Some autofluorescence is apparent in the outer retina at the level of the ONL and OPL (see negative controls). Scale bar, 20 μm.
At E6, GAT-1 transporter immunoreactivity (Fig. 7) was limited to a punctate staining in the outer NBL and in the GCL. At E8 and E10, this punctate staining was superimposed on a diffuse labeling present throughout the retina. By E10, staining in the IPL was apparent and lamina-specific, the laminas closer to the INL being more strongly labeled than the laminas closer to the GCL. This lamina-specific staining was conserved and striking at E18. By then, GAT-1 immunoreactivity had reached its mature pattern, similar to that described for the rat retina (Johnson et al., 1996), in which the staining is restricted to the plasma membrane of cells in the amacrine sublayer of the INL (asterisk) and the IPL. Immunoreactivity was absent from the GCL. These results show that both GABAB receptors and GAT-1 transporters are expressed in regions in which amacrine and ganglion cells and their processes are located, at a time and in a pattern that is compatible with their ability to regulate spontaneous calcium waves.
GABAB receptors modulate calcium currents in ganglion cells and displaced amacrine cells
GABAB receptors usually act to inhibit cells by increasing a K+ conductance or reduce neurotransmission by decreasing a Ca2+conductance (Bettler et al., 1998). We first investigated the presence of baclofen-induced K+ currents in the developing retina using the whole-cell patch-clamp technique to record from ganglion and amacrine cells in the GCL (Fig.8). K+currents were evoked by voltage steps between −140 and +50 mV (holding potential of −90 mV). Baclofen had no effect on the currents evoked at voltages between −140 and −50 mV (n = 6) (Fig.8A,B), showing that GABAB receptors do not activate K+ channels in GCL neurons. However, at more positive potentials, baclofen suppressed a small inward current described in more detail below.
Fig. 8.
GABAB receptors do not modulate K+ currents in ganglion cell layer neurons.A, Currents evoked in an E9 neuron by 200 msec voltage steps to the indicated potentials from a holding potential of −90 mV in control solution (left) and in the presence of 100 μm baclofen (right). B, Current–voltage relationship from six neurons evoked by voltage steps in 10 mV increments from −139 to +4 mV from a holding potential of −90 mV in the absence (control, filledcircles) and presence of 100 μm baclofen (open circles). These data have been corrected for any voltage error produced by current flow through the access resistance in the steady state.
Sun and Chiu (1999) have shown that N-type Ca2+ channels in neonatal rat optic nerve are regulated by GABAB receptors and control the magnitude of the Ca2+ influx associated with action potentials. We examined the possibility that GABAB receptors exert their action on spontaneous Ca2+ waves through a similar mechanism. Ca2+ currents were recorded from GCL neurons (Fig. 9) at room temperature in an external solution containing 3 mmCa2+ plus 5 mmBa2+ and 1 μm TTX. The presence of Ba2+ in the external solution together with 5 mm TEA in the patch pipette ensured K+ currents were minimal. Under these conditions, a depolarizing voltage step to 0 mV from a holding potential of −90 mV elicited an inward Ca2+ current. This current was evoked by voltage steps positive to −40 mV and reached maximum amplitude between 0 and +10 mV (Fig. 9A). We investigated the nature of this Ca2+ current using the dihydropyridines (DHP) nifedipine and nimodipine, blockers of L-type Ca2+ channels, and the peptide ω-conotoxin-GVIA (cgtxGVIA), a blocker of N-type Ca2+ channels (Fig. 9B). A saturating dose of either DHP (10 μm) blocked a relatively small portion, ∼15%, of the Ca2+ current (in DHP, 85 ± 6% of control; p = 0.03; n = 8). On the other hand, cgtxGVIA (5 μm) blocked ∼90% of the total current (in cgtxGVIA, 10 ± 1% of control;n = 3), showing that most of the Ca2+ current present in GCL neurons is of the N-type. As observed previously (Amico et al., 1995), the effect of cgtxGVIA was irreversible even after a prolonged wash (30 min). This high-voltage activated Ca2+ current was reduced by 100 μm baclofen in all cells tested (26 cells) (Fig. 9C). This dose of baclofen, which abolished spontaneous Ca2+ transients (Fig. 3), reversibly reduced the Ca2+ current by half (p < 0.001; n = 15 cells in which baclofen alone was tested; current, 51 ± 7% of control in six ganglion cells and 51 ± 6% in nine amacrine cells) (Fig.9C, inset). The magnitude of the block by baclofen in the presence of DHP was comparable with that in the absence of DHP: baclofen plus DHP, 50 ± 5% of DHP alone current (n = 6); baclofen alone, 51 ± 4% of control (n = 15). This could be taken to suggest that baclofen affects equally the N- and L-type current. However, given the small size of the L-type current, this requires further investigation. Blocking GABAB receptors with the specific antagonist CGP35348 (100 μm) reversibly increased the Ca2+ current by ∼10% (p < 0.01; n = 6) (Fig.9D, inset). Twenty of the cells recorded were filled with Lucifer yellow introduced via the patch pipette of which 10 were ganglion cells, as shown by the presence of an axon that ran in the fiber layer and the large diameter of their cell body (≥15 μm); an example of such a cell is shown in Figure 9E. It is likely, because of their smaller cell bodies (≤10 μm), their position on the outer margin of the GCL and the absence of an axon, that the remaining cells were displaced amacrines (Zhou, 1998). These results show that both ganglion cells and displaced amacrine cells possess N- and L-type Ca2+ channels that are negatively regulated by GABAB receptors.
Fig. 9.

Effect of GABAB receptors on the Ca2+ current in ganglion cell layer neurons.A, Current–voltage relationship from an E11 cell in response to depolarizing steps in 10 mV increments from a holding potential of −90 to +60 mV in control solution. B, Current traces in response to a depolarizing step to 0 mV from a holding potential of −90 mV in control solution and during the sequential application of the L-type Ca2+ channel blocker nimodipine (10 μm) and of the peptide N-type Ca2+ channel blocker cgtxGVIA (5 μm).wash indicates washout of nimodipine before application of cgtxGVIA. The effect of cgtxGVIA was irreversible. C, Current traces in response to a depolarizing step to 0 mV from a holding potential of −90 mV in control solution (5 mmBa2+ and 1 μm TTX;left) in the presence of baclofen (100 μm;middle) and after wash to control (right). The histogram shows pooled data from 15 cells between E9 and E12 in which the amplitude of the currents was normalized to the control current and plotted as percentage change from control. Baclofen reversibly decreased the current by ∼50% (p < 0.001). D, Current traces in response to a depolarizing step to 0 mV from a holding potential of −90 mV in control solution (left), in the presence of CGP35348 (100 μm; CGP;middle) and after wash (right). The histogram shows pooled data from six cells at E10–E11 in which the amplitude of the currents was normalized to the control current and plotted as percentage change from control. CGP35348 reversibly increased the current by ∼10% (p < 0.01). E, Example of a Lucifer yellow-filled ganglion cell in an E10 retina photographed after whole-cell patch-clamp recording in situ. The patch pipette, still attached to the cell, is visible to the left. The image is an overlay of four photographs taken at different focal planes to reveal the extent of the dendritic tree. An asterisk marks the axon, running more superficial than the dendrites in the fiber layer. Scale bar, 20 μm.
GABAB modulation of the calcium current in GCL neurons is independent of cAMP and the activation of PKA
The imaging experiments described above (Fig. 5) show that GABAB receptors can modulate spontaneous calcium waves irrespective of whether the cAMP–PKA signaling pathway is activated or blocked. To determine whether GABAB-mediated inhibition of Ca2+ currents is also independent of this signaling cascade, we next used whole-cell patch clamping of GCL neurons (Fig. 10), including in the patch pipette either cAMP (2 mm, to clamp intracellular cAMP levels) (Fig. 10A) or Rp-cAMPS (500 μm, to block PKA) (Fig. 10B). Under both conditions, baclofen produced a reduction in the Ca2+ current comparable with that in control conditions: change in control, 49 ± 4% (n = 15); in cAMP, 58 ± 4% (n = 10; p = 0.2); in Rp-cAMPS, 63 ± 6% (n = 6; p = 0.07). The density of Ca2+ current in cells exposed to Rp-cAMPS was smaller than that in control cells (control, 17.5 ± 1.5 pA/pF; Rp-cAMPS, 11 ± 1pA/pF; p = 0.01), suggesting the existence of a PKA-sensitive fraction in this current. How GABAB receptors modulate the Ca2+ current remains to be determined, but our results are consistent with the GABABreceptor exerting its effects on the Ca2+current via a direct G-protein modulation of the channels, a mechanism by which these receptors are known to operate in other circumstances (Herlitze et al., 1996; Ikeda, 1996).
Fig. 10.
GABAB receptors modulate Ca2+ currents in ganglion cell layer neurons in a cAMP–PKA-independent manner. A, B, Current traces in response to a depolarizing step to 0 mV from a holding potential of −90 mV in the presence of either cAMP (2 mm; A) or the PKA blocker Rp-cAMPS (500 μm; B, left traces) in the patch pipette, upon application of baclofen (100 μm;middle traces) and after wash of baclofen (right traces). The histograms show pooled data from 10 (A) or six (B) cells at E9–E11 in which the amplitude of the currents in baclofen was normalized to the currents in the presence of cAMP or Rp-cAMPS, respectively, and plotted as percentage change.
The effect of GABAB receptors on Ca2+ waves is independent of their action on Ca2+ channels
The effect the activation of GABAB receptors has on the Ca2+ channels could account for their effect on Ca2+ waves. However, blocking N- or L-type channels with cgtxGVIA (5 μm) or nimodipine (10 μm), respectively, had no effect on the frequency of Ca2+ waves (n= 4) (Fig.11A,B). Furthermore, baclofen (10 μm) still blocked Ca2+ waves in the presence of these antagonists (Fig. 11). This indicates that the action of GABAB receptors in modulating Ca2+ waves is independent of its action on N- and L-type channels. In addition, we have shown that there is no effect of GABAB receptor activation on the K+ currents of cells in the GCL (Fig. 8). This suggests that, if K+ channels mediate the effect of GABAB receptors on Ca2+ waves, they must do so at a site outside of the GCL.
Fig. 11.
Blocking N- or L-type Ca2+channels does not block spontaneous Ca2+ transients. Application of cgtxGVIA (5 μm; A) or nimodipine (10 μm; B) to block N- and L-type Ca2+ channels, respectively, did not affect the frequency of transients (n = 4 retinas). Coapplication of baclofen (10 μm) reversibly abolished the transients in both cases.
DISCUSSION
Developmental changes in the regulation of Ca2+waves by neurotransmitters
The pharmacology of Ca2+ waves in the chick retina is developmentally regulated. At late times (E16), glutamatergic inputs, perhaps from bipolar cells, may initiate or drive spontaneous activity in the GCL (Wong et al., 1998). The results of Wong et al. also suggest that glycinergic and GABAergic inputs from amacrine cells inhibit this activity at times when these neurotransmitters are hyperpolarizing or shunting and that cholinergic amacrine cells, while present appear, unlike in mammalian retina (Zhou, 1998), to exert little effect on the activity. We have shown, in contrast to Wong et al. (1998), that calcium waves are a robust phenomenon before synaptogenesis. At these times (E8–E12) (Fig.12), unlike later on, blockade of glutamate is without effect, cholinergic and glycinergic antagonists reduce activity (Catsicas et al., 1998), and, as we show here, GABA exerts its action via GABAB receptors rather than ionotropic receptors. These changing influences of neurotransmitters may result from differences in the level, pattern of expression, or type of receptor expressed, or from changes in the ionic permeability or the equilibrium potential of the permeant ions. At both E8 and E16, blocking gap junctions suppresses Ca2+waves (Catsicas et al., 1998; Wong et al., 1998).
Fig. 12.
Mechanisms regulating calcium waves during early development (E8–E12). Before synaptogenesis, the frequency of the calcium waves that propagate between ganglion and amacrine cells (GC and AC, respectively) is regulated by a number of the transmitter molecules released by amacrine cells, including ACh acting at nicotinic receptors, glycine (Gly), adenosine (data not shown), and GABA acting at GABAB receptors. The GABA transporter GAT-1 acts to keep GABA in the extracellular space at low levels. Glutamate receptors appear not to be involved in the regulation of calcium waves until after E12, at which time glutamate antagonists block wave activity probably through an action at the newly formed synapses between bipolar and ganglion cells (not shown). At both early and late times, gap junctions (GJ) are important for wave propagation because agents that block the junctions inhibit the waves, whereas agents known to influence coupling through modulation of intracellular levels of cAMP (forskolin, dopamine, and adenosine) increase wave frequency (see Discussion). Positive and negative signs indicate wave-promoting and wave-inhibiting signals, respectively. Shaded figures and pathways indicate that they are present but not instrumental in wave activity.
Recently, Stellwagen et al. (1999) have shown that, in ferret retina, adenosine strongly affects wave dynamics via A2 receptors and an elevation of cAMP and activation of PKA. We have shown here that, in chick, a similar cAMP–PKA-activated pathway exists in which elevation of cAMP with forskolin or 8-bromo-cAMP increases wave frequency, whereas blockade of PKA reduces it. We have shown that GABAB receptor activation opposes cAMP-activating pathways, which raises the possibility that the net frequency of Ca2+ waves, and possibly their overall dynamics, results from the balance of release of GABA, acting at GABAB receptors, and transmitters such as adenosine activating the cAMP pathway. Interestingly, a subpopulation of starburst amacrine cells displaced to the ganglion cell layer coexpresses ACh, GABA, and adenosine in the adult mammalian retina (Blazynski, 1989). If this were true also in the developing retina, it would make displaced starburst amacrines ideally suited for the fine-tuning of the properties of Ca2+waves. These same authors (Stellwagen et al., 1999) have shown that, although present, ionotropic GABA receptors do not modulate wave dynamics in neonatal ferret retina, a result similar to what we see in early chick retina (but see Fischer et al., 1998).
The source of GABA and its actions on Ca2+ waves
One of the major sources of GABA within the developing retina is the amacrine cell population (Massey and Redburn, 1987;Freed, 1992). The transporter GAT-1 is present in the chick retina throughout the period during which Ca2+waves occur and is strongly expressed by amacrine cells (Fig. 7). GAT-1 is also the predominant transporter of amacrine cells in the rat retina (Johnson et al., 1996), although GAT-3 is also found in some amacrines and is strongly expressed by Müller cells. The GAT-1 inhibitor SKF89976A reduces the frequency of the transients presumably because it induces an increase in the extracellular concentration of GABA. Because SKF89976A is not transported and thus cannot increase extracellular concentrations of the transmitter via heteroexchange, GABA release in its presence could occur through reversal of another GABA transporter or via vesicular release. The fact that the available blockers of GAT-3 transporters are translocated, and so lead to GABA release by heteroexchange, prevented us from investigating their role in regulating the extracellular GABA concentration.
When CGP35348 is coapplied with SKF89976A to prevent the activation of GABAB receptors, the frequency of the transients is elevated beyond that in control conditions but is restored to control levels when ionotropic GABA receptors are blocked with a mixture of bicuculline and PTX. This result may be explained by the depolarizing activity of GABA at ionotropic receptors, which one might expect to increase spontaneous activity, and a “damping” action at the GABAB receptor. The fact that blockers of ionotropic GABA receptors have no effect on transient frequency when the GAT-1 transporter operates probably reflects the low levels of GABA in the extracellular space under these conditions; although the affinity of GABAA receptors for GABA varies greatly with the subunits expressed, and we do not know which subunits are expressed at early times, our results suggest a higher affinity of GABAB receptors than GABAA receptors for GABA in the developing retina, as has been described previously (Sodickson and Bean, 1996). Although GABAC receptors are more sensitive to GABA than GABAA receptors (Bormann and Feigenspan, 1995), our observations show that endogenous activation of GABAC receptors does not modulate Ca2+ waves. Thus, under physiological conditions, GABA reaches the critical concentration required for the activation of GABAB receptors and may be a major factor determining the frequency and duration of Ca2+ waves at early times in the developing retina. A similar role for GABABreceptors has been proposed in the control of the GABAA-dependent network activity seen in rat hippocampus (McLean et al., 1996).
We have shown that GABAB receptors are expressed in chick retina in regions of ganglion and amacrine cells throughout the period when Ca2+ waves occur. Koulen et al. (1998) have described a similar distribution in developing rat retina. GABAB receptors, through coupling to G-proteins, can either directly modulate ion channels or negatively couple to adenylate cyclase (Bettler et al., 1998). Thus, the inhibitory effect of GABAB receptors on calcium waves could result from inhibition of Ca2+ channels, activation of K+ channels, or inhibition of cAMP production and its signaling cascade. Our results show that GABAB receptors do not act on K+ channels of GCL neurons but do affect their Ca2+ channels directly, downregulating Ca2+ influx in a cAMP-independent manner. This Ca2+ current and its modulation by GABAB receptors were observed in all the cells we recorded in the GCL and thus are present in both ganglion and amacrine cells. Surprisingly, blocking calcium channels with cgtxGVIA and nimodipine does not prevent the occurrence of calcium waves. Thus, GABAB receptors do not shut down wave activity via an action on GCL neuron K+ currents or via an action on N- or L-type Ca2+ channels in the GCL or elsewhere. The route by which Ca2+ enters cells involved in wave activity is unknown for any species so far examined. Further experiments are required to determine the mechanisms of the [Ca2+]iincrease. It is possible that these include both ligand- and voltage-gated channels.
Starburst amacrine cells are thought to drive wave activity in ganglion cells via ACh release and also possibly gap junctions. If GABAB receptors are present in starburst amacrine cells, it is possible they could reduce ACh release by clamping their membrane voltage to the resting potential through the activation of a K+ conductance. Thus, one might expect blocking the GABAB receptor to have a similar effect to that of raising extracellular ACh. Interestingly, we have shown previously that eserine, which blocks ACh breakdown by acetylcholinesterase, increases wave frequency (Catsicas et al., 1998). However, eserine raises the steady-state concentration of ACh rather than increasing the frequency of its release as might result from blocking the GABAB receptor. Zhou and Fain (1995)examined the actions of GABA on rabbit starburst cells and showed that the GABA-evoked currents found are produced by the activation of GABAA, and not GABAB or GABAC, receptors. However, these authors did not study the possible modulation of voltage-dependent channels by GABAB receptors.
The results we present here demonstrate the importance of GABA and GABAB receptors in the control of Ca2+ waves in the developing retina and point to substantial changes in the roles played by GABA in the regulation of this activity during development. Ca2+ waves appear before anatomically distinct synapses are established and during the period that ganglion and amacrine cell dendrites are forming. They are robust through the time when these cells undergo a period of massive cell death. Our results show that baclofen, CGP35348, and SKF89976A are useful tools with which to manipulate the frequency of Ca2+ waves at these crucial times in development. Experiments using these agents will help clarify the role of Ca2+ waves in the regulation of dendritic maturation, synapse formation, and cell death.
Footnotes
This work was supported by the Wellcome Trust and the Biotechnology and Biological Sciences Research Council. We thank D. Attwell, C. Auger, M. Hamann, M. Takahashi, and D. Rossi for helpful comments and suggestions.
Correspondence should be addressed to Marina Catsicas, Department of Physiology, University College London, Gower Street, London WC1E 6BT, UK. E-mail: m.catsicas@ucl.ac.uk.
REFERENCES
- 1.Amico C, Marchetti C, Nobile M, Usai C. Pharmacological types of calcium channels and their modulation by baclofen in cerebellar granules. J Neurosci. 1995;15:2839–2848. doi: 10.1523/JNEUROSCI.15-04-02839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettler B, Kaupmann K, Bowery N. GABAB receptors: drugs meet clones. Curr Opin Neurobiol. 1998;8:345–350. doi: 10.1016/s0959-4388(98)80059-7. [DOI] [PubMed] [Google Scholar]
- 3.Blazynski C. Displaced cholinergic, GABAergic amacrine cells in the rabbit retina also contain adenosine. Vis Neurosci. 1989;3:425–431. doi: 10.1017/s0952523800005927. [DOI] [PubMed] [Google Scholar]
- 4.Bodnarenko SR, Jeyarasasingam G, Chalupa LM. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate-mediated afferent activity. J Neurosci. 1995;15:7037–7045. doi: 10.1523/JNEUROSCI.15-11-07037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonness V, Catsicas M, Cunningham J, Hutson P, Murray F, Neal M, Mobbs P. Depolarization-evoked transmitter release and agonist-evoked increases in [Ca2+]i in the early development of the chick retina. J Physiol (Lond) 1996;494:79.P. [Google Scholar]
- 6.Bormann J, Feigenspan A. GABAC receptors. Trends Neurosci. 1995;18:515–519. doi: 10.1016/0166-2236(95)98370-e. [DOI] [PubMed] [Google Scholar]
- 7.Catsicas M, Mobbs P. Retinal development: waves are swell. Curr Biol. 1995;5:977–979. doi: 10.1016/s0960-9822(95)00192-8. [DOI] [PubMed] [Google Scholar]
- 8.Catsicas M, Bonness V, Becker D, Mobbs P. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr Biol. 1998;8:283–286. doi: 10.1016/s0960-9822(98)70110-1. [DOI] [PubMed] [Google Scholar]
- 9.Chavis P, Mollard P, Bockaert J, Manzoni O. Visualization of cyclic AMP-regulated presynaptic activity at cerebellar granule cells. Neuron. 1998;20:773–781. doi: 10.1016/s0896-6273(00)81015-6. [DOI] [PubMed] [Google Scholar]
- 10.Colwell CS, Levine MS. Excitatory synaptic transmission in neostriatal neurons: regulation by cyclic AMP-dependent mechanisms. J Neurosci. 1995;15:1704–1713. doi: 10.1523/JNEUROSCI.15-03-01704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- 12.Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- 13.Fields RD, Neale EA, Nelson PG. Effects of patterned electrical activity on neurite outgrowth from mouse sensory neurons. J Neurosci. 1990;10:2950–2964. doi: 10.1523/JNEUROSCI.10-09-02950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freed MA. GABAergic circuits in the mammalian retina. Prog Brain Res. 1992;90:107–131. doi: 10.1016/s0079-6123(08)63611-0. [DOI] [PubMed] [Google Scholar]
- 15.Fischer KF, Lukasiewicz PD, Wong RO. Age-dependent and cell-class specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci. 1998;18:3767–3778. doi: 10.1523/JNEUROSCI.18-10-03767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 17.Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- 18.Gu X, Spitzer NC. Breaking the code: regulation of neuronal differentiation by spontaneous calcium transients. Dev Neurosci. 1997;19:33–41. doi: 10.1159/000111183. [DOI] [PubMed] [Google Scholar]
- 19.Hering H, Kröger S. Formation of synaptic specialisations in the inner plexiform layer of the developing chick retina. J Comp Neurol. 1996;375:393–405. doi: 10.1002/(SICI)1096-9861(19961118)375:3<393::AID-CNE4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 21.Hughes WF, LaVelle A. On the synaptogenic sequence in the chick retina. Anat Rec. 1974;179:297–301. doi: 10.1002/ar.1091790302. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 23.Johnson J, Chen TK, Rickman DW, Evans C, Brecha NC. Multiple gamma-aminobutyric acid plasma membrane transporters (GAT-1, GAT-2, GAT-3) in the rat retina. J Comp Neurol. 1996;375:212–224. doi: 10.1002/(SICI)1096-9861(19961111)375:2<212::AID-CNE3>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalil RE. The influence of action potentials on the development of the central visual pathway in mammals. J Exp Biol. 1990;153:261–276. doi: 10.1242/jeb.153.1.261. [DOI] [PubMed] [Google Scholar]
- 25.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 26.Koulen P, Malitschek B, Kuhn R, Bettler B, Wässle H, Brandstätter JH. Presynaptic and postsynaptic localisation of GABAB receptors in neurons of the rat retina. Eur J Neurosci. 1998;10:1446–1456. doi: 10.1046/j.1460-9568.1998.00156.x. [DOI] [PubMed] [Google Scholar]
- 27.Luckasiewicz PD, Shields CR. A diversity of GABA receptors in the retina. Cell Dev Biol. 1998;9:293–299. doi: 10.1006/scdb.1998.0238. [DOI] [PubMed] [Google Scholar]
- 28.Massey SC, Redburn DA. Transmitter circuits in the vertebrate retina. Prog Neurobiol. 1987;28:55–96. doi: 10.1016/0301-0082(87)90005-0. [DOI] [PubMed] [Google Scholar]
- 29.McLean HA, Caillard O, Khazipov R, Ben-Ari Y, Gaiarsa JL. Spontaneous release of GABA activates GABAB receptors and controls network activity in the neonatal rat hippocampus. J Neurophysiol. 1996;76:1036–1046. doi: 10.1152/jn.1996.76.2.1036. [DOI] [PubMed] [Google Scholar]
- 30.Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- 31.Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 32.O'Malley DM, Sandell JH, Masland RH. Co-release of acetylcholine and GABA by the starburst amacrine cells. J Neurosci. 1992;12:1394–1408. doi: 10.1523/JNEUROSCI.12-04-01394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- 34.Santos PF, Carvalho AL, Carvalho AP, Duarte CB. Differential acetylcholine and GABA release from cultured chick retina cells. Eur J Neurosci. 1998;10:2723–2730. doi: 10.1046/j.1460-9568.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 35.Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stellwagen D, Shatz CJ, Feller MB. Dynamics of retinal waves are controlled by cyclic AMP. Neuron. 1999;24:673–685. doi: 10.1016/s0896-6273(00)81121-6. [DOI] [PubMed] [Google Scholar]
- 37.Sun BB, Chiu SY. N-type calcium channels and their regulation by GABAB receptors in axons of neonatal rat optic nerve. J Neurosci. 1999;19:5185–5194. doi: 10.1523/JNEUROSCI.19-13-05185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weliky M. Recording and manipulating the in vivo correlational structure of neuronal activity during visual cortical development. J Neurobiol. 1999;41:25–32. doi: 10.1002/(sici)1097-4695(199910)41:1<25::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- 40.Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- 41.Wong RO, Chernjavsky A, Smith SJ, Shatz CJ. Early functional neural networks in the developing retina. Nature. 1995;374:716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- 42.Wong WT, Sanes JR, Wong RO. Developmentally regulated spontaneous activity in the embryonic chick retina. J Neurosci. 1998;18:8839–8852. doi: 10.1523/JNEUROSCI.18-21-08839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita M, Fukuda Y. Calcium channels and GABA receptors in the early embryonic chick retina. J Neurobiol. 1993;24:1600–1614. doi: 10.1002/neu.480241205. [DOI] [PubMed] [Google Scholar]
- 44.Zhou ZJ. Direct participation of starburst amacrine cells in spontaneous rhythmic activities in the developing mammalian retina. J Neurosci. 1998;18:4155–4165. doi: 10.1523/JNEUROSCI.18-11-04155.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou ZJ, Fain G. Neurotransmitter receptors of starburst amacrine cells in rabbit retinal slices. J Neurosci. 1995;15:5334–5345. doi: 10.1523/JNEUROSCI.15-07-05334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]








