Abstract
The object of this study was to determine whether inhibition of capacitative calcium entry is essential for relaxation of the mouse anococcygeus via the NO/cyclic GMP signalling pathway.
In intact muscles, thapsigargin (Tg; 100 nM)-induced tone was relaxed by NO, sodium nitroprusside (SNP), 8-Br-cyclic GMP, and nitrergic field stimulation. The relaxations were similar in magnitude to those observed against carbachol (50 μM) tone and, with the exception of those to 8-Br-cyclic GMP, were reduced by the soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxodiazolo[4,3-a]quinoxalin-1-one (ODQ, 5 μM).
In single smooth muscle cells, loaded with Fura-2, both carbachol and Tg produced sustained elevations in cytoplasmic calcium levels ([Ca2+]i). SNP inhibited the rise in [Ca2+]i produced by carbachol, an effect attenuated by ODQ. In contrast, neither SNP nor 8-Br-cyclic GMP reduced the elevated [Ca2+]i associated with Tg.
In β-escin skinned preparations, NO had no effect on tone induced by calcium (1 μM in the presence of 100 μM GTP). Carbachol and Tg produced further increases in calcium/GTP-induced tone and, in both cases, this additional tone was relaxed by NO and 8-Br-cyclic GMP.
The results support the hypothesis that the NO/cyclic GMP pathway inhibits capacitative calcium entry by refilling the internal stores, since reduction in [Ca2+]i was not observed in the presence of Tg. However, as muscle relaxation was still observed, impairment of capacitative calcium entry cannot be considered obligatory for relaxation. Results from skinned tissues suggest that inhibition of calcium sensitization processes, perhaps associated with store-depletion, may be an important mechanism of NO/cyclic GMP-induced relaxation.
Keywords: Anococcygeus (mouse), β-escin, calcium sensitization, capacitative calcium entry, carbachol, cyclic GMP, nitric oxide, relaxation, smooth muscle, sodium nitroprusside
Introduction
Capacitative (store-operated) calcium entry is a ubiquitous calcium influx pathway activated following depletion of the calcium stores within the sarcoplasmic/endoplasmic reticulum (SR/ER; Putney, 1986, 1990; Berridge, 1995; Parekh & Penner, 1997). Physiologically, store depletion results from activation of G-protein-coupled receptors, with consequent generation of inositol 1,4,5-trisphosphate (IP3) and opening of IP3 receptor ion channels on the SR/ER membrane. Pharmacologically, the calcium stores can be depleted, and capacitative calcium entry activated, by a number of agents, most commonly by drugs which inhibit the SR/ER calcium ATPase, such as thapsigargin (Tg) and cyclopiazonic acid. Capacitative calcium entry is known to contribute to a diverse range of cellular functions including (depending on cell type) store re-filling, cell division, secretory processes, and contraction of smooth muscle. Despite this widespread and important role in cell signalling, several aspects of capacitative calcium entry remain unresolved (Putney & McKay, 1999), including the cellular mechanisms linking the SR/ER with the store-operated channels (SOCs) on the plasma membrane, and the molecular structure of the channels themselves.
A further area of controversy concerns the interaction between capacitative calcium entry and the NO/cyclic GMP signalling system. Initial observations that cyclic GMP might activate capacitative calcium entry in some cells (Bahnson et al., 1993; Xu et al., 1994) were challenged by other studies in which the cyclic nucleotide was found to be ineffective (Fasolato et al., 1993; Bian et al., 1996; Gilon et al., 1995; Parekh & Penner, 1997). More recently, an inhibitory effect of NO/cyclic GMP on capacitative calcium entry has been reported in blood platelets, vascular endothelium, and smooth muscle cells (Wayman et al., 1996b; Trepakova et al. 1999; Cohen et al., 1999; Kwan et al., 2000). Indeed, from the results of experiments carried out in rat aorta, Cohen et al. (1999) have proposed that inhibition of capacitative calcium entry may be essential for the vasorelaxant effects of NO and that the inhibition is secondary to cyclic GMP-induced refilling of the SR, thereby removing the primary drive for SOC opening. Central to this proposal is the observation that the SR calcium ATPase inhibitor Tg, which blocks calcium re-accumulation by the SR, prevented both NO-induced inhibition of capacitative calcium entry and vasorelaxation.
We have reported previously that capacitative calcium entry is important for sustained contractions of the mouse anococcygeus in response to both receptor agonists and inhibitors of the SR calcium ATPase (Wayman et al., 1996a; 1998; 1999) and, further, that capacitative calcium entry in this non-vascular smooth muscle is inhibited by the NO/cyclic GMP signalling pathway (Wayman et al., 1996b; Gibson et al., 1998). However, the cellular mechanisms of this inhibitory effect, and its relative contribution to NO-induced relaxation, have not yet been investigated. Given the recent observations of Cohen et al. (1999) in rat aorta, the object of the present study was to determine whether inhibition of capacitative calcium entry is also essential for relaxation of the mouse anococcygeus in response to activation of the NO/cyclic GMP signalling pathway.
Methods
Tension studies with intact tissues
Male mice (LACA: Tuck, Essex, U.K.; 25 – 35 g) were killed by stunning and exsanguination. The two anococcygeus muscles were dissected out separately and set up in 1 ml glass organ baths containing Krebs'-bicarbonate buffer (composition, mM): NaCl 118.1, KCl 4.7, MgSO4 1.0, KH2PO4 1.0; CaCl2 2.5, NaHCO3 25.0, glucose 11.1, which was maintained at 37°C and gassed with 95% O2: 5% CO2. A resting tension of 200 – 400 mg was placed on the tissue and changes in tension recorded with a Biegestab K30 force-displacement transducer attached to a pen-recorder (Graphtec WR3101). Muscles were allowed to equilibrate for 30 min before beginning experimental procedures. In order to prevent any effects due to release of noradrenaline from the sympathetic nerves within the tissue the Krebs' solution contained the α-adrenoceptor antagonist phentolamine (1 μM), and muscles were exposed to the adrenergic neurone blocking agent guanethidine (30 μM) for 10 min during the equilibration period. To inhibit calcium entry through voltage-operated calcium channels, 10 μM verapamil was included in the Krebs' solution in all experiments. Field stimulation of the nitrergic nerves within the tissue (Gibson et al., 1990; 10 s trains every 100 s; 1 – 10 Hz; 1 ms pulse width; 70 V) was applied via two parallel platinum electrodes running down either side of the tissue; these were attached to Grass S48 stimulators. When relaxations to drugs were to be investigated the Krebs' solution also contained the nitric oxide synthase inhibitor L-NG-nitroarginine (L-NOARG; 50 μM) in order to prevent any effects of NO released from the nitrergic nerves; when relaxations to nitrergic nerve stimulation were to be studied, L-NOARG was omitted.
To record relaxations to drugs or field stimulation, tone was first raised with either 50 μM carbachol or 100 nM Tg; due to the prolonged and persistent nature of the response to Tg the effects of only one concentration of the drug were observed in each muscle preparation. Relaxant drugs or field stimulation were then applied when a stable elevation of tone had been achieved. With the exception of NO, relaxant drugs were added cumulatively; each concentration was left in contact with the tissue until the response peaked, before addition of the next dose. With NO, the relaxations to each concentration were transient, most likely reflecting the unstable nature of NO in the presence of O2, and so tone was allowed to recover each time (without washout) before the next dose of NO was added to the bath. Responses were calculated as the per cent relaxation of tone compared with the level immediately before addition of the first dose of relaxant drug or before each train of field stimulation.
Tension studies with β-escin-skinned muscles
Mouse anococcygeus muscles were dissected and set up initially in Krebs' solution as detailed above, with the exception that the experiments were carried out at 25°C; experiments with skinned muscles are routinely carried out at temperatures of 20 – 25°C to help prolong tissue viability (Pfitzer et al., 1982; 1984; 1986). As before, the Krebs' solution contained phentolamine, L-NOARG and verapamil, and muscles were incubated with guanethidine (10 min) during the equilibration period. Following 30 min equilibration, the muscles were exposed to 50 μM carbachol to test their viability (a contraction of 300 mg tension or greater was regarded as satisfactory). The Krebs' solution was then replaced by a relaxing solution containing (mM): PIPES 20, MgCl2 7.1, KCl 108, EGTA 2, Na2ATP 5.9, creatine phosphate 2, creatine phosphokinase 4 units ml−1, E-64 1 μg ml−1, and FCCP 1 μM; pH 6.8, in which the muscle was allowed to equilibrate for a further 20 min before a 10 min exposure to 50 μM β-escin, which permeabilizes the cell membrane to calcium whilst leaving G-protein coupled receptors, and their effectors, intact (Iizuka et al., 1994). Changes in tension in these experiments were recorded using a Grass FT03 force-displacement transducer attached to a MacLab data acquisition system. Final concentrations of calcium in the bath, in the presence of 2 mM EGTA, were calculated using Chelator for Windows software.
Isolation of single smooth muscle cells and measurement of Fura-2 fluorescence
Anococcygeus muscles were dissected from male mice as outlined above. Single smooth muscle cells were dissociated enzymatically using a method based on that described for the rat anococcygeus (McFadzean & England, 1992). The muscles were incubated for 10 min at 37°C in a physiological salt solution (PSS) containing zero added calcium plus (in mM): NaCl 120, KCl 6, MgCl2 1.2, Na2HPO4 1.2, glucose 11, HEPES 10, pH 7.2. Following this, the tissues were incubated for 12.5 min at 37°C in PSS to which had been added (all from Sigma) bovine serum albumin (fatty acid free, 3.05 mg ml−1), papain (0.6 mg ml−1), collagenase (Type 1A; 0.8 mg ml−1) and dithioerythritol (12 mM). The tissues were then washed three times using enzyme-free PSS, and the single cells isolated by passing the muscle pieces through a wide-bore Pasteur pipette several times. The resultant cell suspension was centrifuged at approximately 180 g for 1.5 min, and the cell-free supernatant discarded. The pellet was suspended in PSS containing 0.75 mM calcium chloride. The cell suspension was then incubated, in the dark, with 2.5 μM Fura-2 acetoxymethylester (Fura-2/AM; Calbiochem) for 9 min at 37°C; the cell suspension was then diluted 10 fold with PSS containing 0.75 mM calcium chloride, centrifuged at approximately 180×g for 1 min, and the cell-free supernatant discarded. The pellet, containing the Fura-2 loaded cells, was resuspended in PSS containing 0.75 mM calcium chloride, and droplets of the cell-rich suspension were placed on alcohol-washed, poly-lysine-coated glass cover slips and stored at 4°C for at least 1.5 h. The glass cover slips were then placed in a small chamber to form the perfusion bath which was mounted on the stage of an inverted microscope (Nikon Diaphot 300). The cells were perfused with Krebs solution (see above) at 37°C using a gravity-fed system. Fura-2 fluorescence was recorded at 510 nm while alternating between the two excitation wavelengths (340 and 380 nm) using a Cairn Research Ltd spectrophotometer system. Fluorescence emission was collected only from the cell of interest. Because of the recognized uncertainties inherent in the measurement of absolute intracellular calcium concentrations, the results throughout this study are expressed as the ratio of fluorescence at 340 and 380 nm (R340/380). However, in some cells, the absolute values of cytosolic calcium concentration were obtained using the equation of Grynkiewicz et al. (1985); cells were permeabilized with ionomycin (50 μM) and exposed to PSS containing 10 mM calcium followed by PSS containing zero added calcium plus 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraaecetic acid (BAPTA; 1 mM). Using this procedure, the resting calcium concentration was estimated as 54±11 nM (n=6).
Statistics
Results are expressed as mean±s.e.mean and statistical analysis was by Student's t-test (paired or unpaired as appropriate), with a P value of 0.05 or less taken to indicate a significant difference.
Drugs used
Drugs/materials used during the study were: BAPTA (Sigma); carbachol (BDH); 8-Bromo-cyclic guanosine monophosphate (8-Br-cyclic GMP; Sigma); creatine phosphate (Sigma); creatine phosphokinase (Sigma); E-64 (trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane, Sigma); ethylene glycol-bis(β-aminoethyl ether)N,N,N′,N′,-tetraacetic acid (EGTA; Sigma); FCCP (carbonyl cyanide p-trifluoromethoxyphenyl-hydrazone, Sigma); guanethidine monosulphate (Sigma); HEPES, N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid] (Sigma); neomycin (Calbiochem); L-NG-nitroarginine (Sigma); nitric oxide (BDH); PIPES, piperazine-N,N′-bis[2-ethanesulphonic acid] (Sigma); 1H-[1,2,4]oxodiazolo[4,3-a]quinoxalin-1-one (ODQ; Tocris); phentolamine HCl (Sigma); SKF96365 (Affiniti Research Products); sodium nitroprusside (Sigma); thapsigargin (Tg; Sigma and Calbiochem); verapamil HCl (Sigma). The method for preparing saturated (3 mM) solutions of NO has been reported elsewhere (Gibson & Mirzazadeh, 1989). Drugs were dissolved in distilled water, except thapsigargin and ODQ which were dissolved in DMSO; the final bath concentration (less than 0.2% v v−1) of this solvent showed no significant biological effects.
Results
Tension studies with intact tissues
As reported previously (Wallace et al., 1999), both 50 μM carbachol and 100 nM Tg produced strong and sustained contractions of the mouse anococcygeus; there was no significant difference in the magnitude of the contractions produced by the two drugs (404±32 mg tension with carbachol; 332±33 mg tension with Tg, n=6 in each case). As expected from previous studies (Gibson et al., 1990), SNP (0.01 – 5 μM), NO (0.7 – 30 μM) and nitrergic field stimulation (1 – 10 Hz) produced concentration- or frequency-dependent relaxations when tone was raised with 50 μM carbachol (Figure 1). However, in contrast to the reported inability of NO to relax rat aorta contracted by Tg (Cohen et al., 1999), both SNP and NO produced potent relaxations of tone induced by Tg in the mouse anococcygeus; indeed, the concentrations – response curves were superimposable on those obtained against carbachol-induced tone (Figure 1). In the case of nitrergic field stimulation, relaxations at 1 and 2 Hz were actually greater against Tg-induced tone when compared with carbachol, although at 5 and 10 Hz they became similar (Figure 1).
Figure 1.
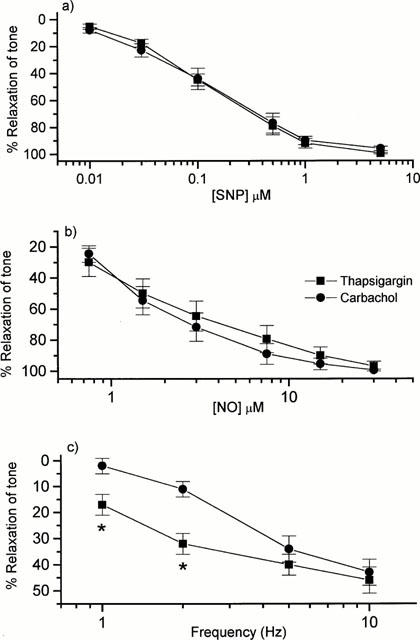
Concentration-response curves for relaxations of the mouse anococcygeus muscle to (a) sodium nitroprusside (SNP) or (b) nitric oxide (NO), and (c) frequency-response curve for relaxations to nitrergic field stimulation, when tone was raised with either 50 μM carbachol or 100 nM thapsigargin. Each point is the mean±s.e.mean from a minimum of five individual muscle preparations. *P<0.05 compared with corresponding value with carbachol tone.
To ensure that the relaxations of Tg-induced tone were due to activation of the guanylyl cyclase/cyclic GMP pathway, the effects of the soluble guanylyl cyclase inhibitor ODQ were investigated. Relaxations to SNP and NO were greatly reduced or abolished in the presence of 5 μM ODQ (Figure 2); although not shown, 5 μM ODQ also abolished relaxations to nitrergic field stimulation at all frequencies tested (1 – 10 Hz). The cell-permeable analogue of cyclic GMP, 8-Br-cyclic GMP (10 – 400 μM) also produced concentration-related relaxations of Tg-induced tone; as expected, these relaxations were unaffected by ODQ (Figure 2b), since the actions of the nucleotide analogue do not require increased guanylyl cyclase activity.
Figure 2.

Concentration-response curves for relaxations of the mouse anococcygeus muscle to (a) sodium nitroprusside (SNP) and nitric oxide (NO) or (b) 8-Br-cyclic GMP and the effect on these of the soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxodiazolo [4,3-a]quinoxalin-1-one (ODQ; 5 μM). Tone was raised with 100 nM thapsigargin (Tg). Each point is the mean±s.e.mean from a minimum of five individual muscle preparations.
The above results clearly demonstrated that, unlike the situation in rat aorta (Cohen et al., 1999), the SR calcium ATPase inhibitor Tg does not prevent, or reduce, the relaxant effects of the NO/cyclic GMP signalling system in the mouse anococcygeus.
Calcium fluorescence studies using single smooth muscle cells
We next investigated the interaction between Tg and the NO/cyclic GMP system on cytoplasmic calcium levels ([Ca2+]i) in single smooth muscle cells freshly dispersed from the anococcygeus. 50 μM carbachol produced a biphasic increase in [Ca2+]i (Figure 3a); as described previously (Wayman et al., 1999), the first transient increase represents calcium release from the SR, while the second sustained phase is due to calcium influx through SOCs. Our belief that this sustained phase of the carbachol response represents calcium entry through SOCs is based on observations that its time-course and pharmacology mirror that of the sustained responses initiated by Ca-ATPase inhibitors (Wayman et al., 1999); however, some caution is required since there is evidence that certain putative SOCs may be activated not only by store depletion per se but also by receptor – dependent, store-independent mechanisms (Birnbaumer et al., 1996; Putney & McKay, 1999). Application of 10 μM SNP during the sustained phase reduced the carbachol-induced increase in [Ca2+]i (by 72±16% after 2 min; n=12; Figure 3b); the inhibitory effect of SNP was attenuated (to 12±9%; n=7) in the presence of 5 μM ODQ (not shown). These results confirm that SNP, acting via guanylyl cyclase, can inhibit capacitative calcium entry in the mouse anococcygeus (Wayman et al., 1996b; Gibson et al., 1998). Tg, on the other hand, produced a slower increase in [Ca2+]i and a biphasic effect was less evident (Figure 3c); this could be related either to the rate of entry of Tg into the cell or to the much slower rate of depletion of the SR produced by the Ca ATPase inhibitor compared with receptor agonists such as carbachol. Application of 10 μM SNP, at a point when the Tg response had reached a stable plateau, produced no significant change in [Ca2+]i (n=9; P>0.05; Figure 3c).
Figure 3.

Recordings of the effects of carbachol (50 μM), thapsigargin (Tg; 100 nM) and sodium nitroprusside (SNP; 10 μM) on cytoplasmic calcium levels (measured as the Fura-2 ratio; R340/380) in single smooth muscle cells of the mouse anococcygeus. Each trace comprises the integrated data from (a) 6 cells, (b) 12 cells, and (c) 9 cells.
The above experiments were carried out with cells bathed in 2.5 mM calcium. In a second series of experiments, we used cells which were initially bathed in calcium-free solution before exposure to Tg and then calcium; such ‘calcium readmission' protocols are widely used in the study of capacitative calcium entry (Wallace et al., 1999). Data accumulated from a number of these experiments are shown in Figure 4. Under calcium-free conditions, application of Tg produced a small transient increase in [Ca2+]i, reflecting depletion of the stores (Figure 4). Subsequent addition of 2.5 mM calcium, 10 min after Tg, resulted in a larger and sustained increase in [Ca2+]i due to capacitative calcium entry (Wallace et al., 1999; Wayman et al., 1999). Application of either SNP (1 or 10 μM) or 8-Br-cyclic GMP (100 μM) during the sustained phase, in concentrations which produced powerful relaxations of the muscle, produced no reduction in [Ca2+]i (Figure 4b,c); in contrast, the general calcium entry blocking agent SKF96365 (20 μM) clearly reduced the [Ca2+]i towards basal levels (Figure 4a).
Figure 4.

Graphs showing the effects of thapsigargin (Tg; 100 nM), SKF96365 (20 μM), sodium nitroprusside (SNP; 1 μM) and 8 Br-cyclic GMP (100 μM) on cytoplasmic calcium levels (measured as the Fura-2 ratio; R340/380) in single smooth muscle cells from the mouse anococcygeus. Cells were bathed initially in calcium-free medium, before exposure to Tg; 2.5 mM calcium (Ca) was then added 10 min after Tg. Each point is the mean±s.e.mean from at least five individual cells.
Tension studies with β-escin-skinned muscles
The results so far indicated that the NO/cyclic GMP signalling system can relax the mouse anococcygeus without reducing [Ca2+]i. To investigate the nature of this calcium-independent relaxation we carried out some experiments with β-escin-skinned preparations, in which the plasma membrane is rendered permeable to calcium, whilst receptor-effector coupling is preserved.
In skinned muscles bathed in calcium-free relaxing solution, addition of calcium (0.1 – 50 μM) produced concentration-related contractions, resulting in a maximum increase in tension of 35±6 mg (n=10); 1 μM calcium produced a submaximal contraction (EC57) and was used in all subsequent experiments. Since the object of the study was to investigate the effect of the NO/cyclic GMP system in skinned muscles, the substrate for guanylyl cyclase, GTP (100 μM), was added at the peak of the calcium response (Figure 5a); this resulted in a further increase in tension (Figure 5a), but NO (96 μM) failed to cause any relaxation of this combined calcium/GTP-induced tone. Carbachol (50 μM) produced an additional increase in tension in the presence of calcium/GTP (Figure 5b), and in this case NO (96 μM) did produce significant relaxation of the carbachol-induced component. The relaxation produced by NO was concentration-related, the per cent relaxations of carbachol tone in the presence of 1.5, 6, 24 and 96 μM NO being 40±3%, 65±12%, 86±19% and 88±19% respectively. Similarly, 8-Br-cyclic GMP (20 μM) produced relaxations of this carbachol-induced tone (126±23% relaxation; n=5; P<0.05).
Figure 5.

Graphs showing the time-course of tension changes in mouse anococcygeus muscles skinned with β-escin. Muscles were bathed initially in a calcium-free relaxing solution and then exposed sequentially to calcium ions (Ca2+; 1 μM), GTP (100 μM) and then (a) nitric oxide (NO, 96 μM; n=6), b) carbachol (carb, 50 μM) followed by NO (n=6), and (c) thapsigargin (Tg, 100 nM) followed by NO (n=5). Results are expressed as a percentage of the peak contraction produced by calcium alone. Each point is the mean±s.e.mean *significant relaxation induced by NO (P<0.05 compared with value immediately before addition of NO).
Addition of 100 nM Tg, rather than carbachol, also enhanced the calcium/GTP contraction of permeabilized muscles (Figure 5c); in this case, Tg increased muscle tone by a maximum of 41±10% above the level induced by calcium/GTP (n=5; P<0.05). Similar results were obtained in experiments in which the EGTA concentration of the bathing medium was increased from 2 mM to 10 mM; here, in the presence of 1 μM calcium and 100 μM GTP, 100 nM Tg increased tone by 56±18% (n=5; P<0.05). As with carbachol, the Tg-induced component of the contraction in skinned muscles was relaxed significantly by 96 μM NO (Figure 5c), and by 20 μM 8-Br-cyclic GMP (100±18% relaxation; n=5; P<0.05). Although not shown, NO-induced relaxations of both carbachol and Tg tone were abolished in the presence of 5 μM ODQ.
Discussion
The most significant finding of the present study is that activation of the NO/cyclic GMP signalling pathway causes relaxation of Tg-induced tone in the mouse anococcygeus without reducing the elevated [Ca2+]i. This observation provides important information on the cellular mechanisms involved in the interactions between the NO/cyclic GMP pathway and capacitative calcium entry in the anococcygeus, and the relative contribution of these mechanisms to smooth muscle relaxation.
There is now good evidence that capacitative calcium entry underlies the raised [Ca2+]i associated with sustained contractions of the mouse anococcygeus in response to both receptor agonists and inhibitors of the SR Ca-ATPase (Wayman et al., 1996a; 1998; 1999; Wallace et al., 1999). We have previously proposed that the NO/cyclic GMP signalling pathway can inhibit capacitative calcium entry in this smooth muscle (Wayman et al., 1996b) and, consistent with this, in the present study SNP reduced the sustained, SOC-dependent increase in [Ca2+]i observed in the presence of carbachol. Inhibition of capacitative calcium entry by cyclic GMP has also been found in blood platelets (Trepakova et al., 1999), vascular endothelium (Kwan et al., 2000) and aortic smooth muscle (Cohen et al., 1999). In theory, such inhibition could result from a direct regulatory action on the SOC, or indirectly to enhanced refilling of the SR removing the primary drive for SOC opening. Some evidence obtained in previous studies on the mouse anococcygeus supports the latter mechanism, since SNP was found to enhance refilling of the SR following depletion of the calcium stores by exposure to carbachol in calcium-free medium (Gibson et al., 1994; Wayman et al., 1997); in contrast, the calcium channel blocking agent SKF96365 inhibited store refilling by preventing calcium moving into the cell through SOCs. Further support for the indirect mechanism comes from the present study, since neither SNP nor 8-Br-cyclic GMP could reduce [Ca2+]i in the presence of Tg, when store refilling via the SR Ca-ATPase would be prevented. Consequently, our results support the hypothesis (Cohen et al., 1999) that enhanced refilling of the SR, rather than direct SOC inhibition, underlies the reduction of capacitative calcium entry by the NO/cyclic GMP pathway.
However, while our results are consistent with the recent findings of Cohen et al. (1999) in terms of the mechanism by which NO/cyclic GMP inhibits capacitative calcium entry they do not support their suggestion that such inhibition is essential for smooth muscle relaxation. In the aorta, NO did not relax tone produced by Tg; in the anococcygeus, Tg-induced tone was relaxed by NO, SNP and nitrergic nerve stimulation, and in each case relaxation was markedly reduced by the soluble guanylyl cyclase inhibitor ODQ. The relaxations were similar against tone produced by either Tg or carbachol. Indeed, at the lower frequencies of field stimulation nitrergic relaxations were actually greater in the presence of Tg; one possible explanation for this is that carbachol might activate presynaptic muscarinic receptors on the nitrergic nerve terminals, which are known to inhibit nitrergic neurotransmission in the anococcygeus (Li & Rand, 1989). Thus, while inhibition of capacitative calcium entry may contribute to the relaxant effects of the NO/cyclic GMP signalling pathway in the anococcygeus it is certainly not obligatory; other cellular mechanisms must be involved, and the experiments with skinned tissues were carried out to provide information on what these mechanisms might be.
In addition to its effects on calcium metabolism, cyclic GMP is known to modulate excitation – contraction coupling in smooth muscle at several points beyond the processes leading to elevated [Ca2+]i; much of the evidence for this has come from experiments with skinned preparations (Pfitzer et al., 1982; 1984; 1986; Nishimura & van Breemen, 1989). Cyclic GMP-dependent phosphorylation of myosin light chain kinase has been reported, although this seems unlikely to explain relaxation since there was no change in enzyme activity associated with such phosphorylation (Nishikawa et al., 1984; Hathaway et al., 1985). In addition, there is some evidence of a direct action of cyclic GMP on the contractile proteins themselves, resulting in altered cross-bridge dynamics (Murphy & Walker, 1998; Chuang et al., 1998). However, the bulk of evidence favours the view that cyclic GMP, via protein kinase G, stimulates the activity of myosin light chain phosphatase thereby producing a form of ‘calcium desensitization' (Wu et al., 1996; Lee et al., 1997) by reducing the level of phosphorylated myosin at a given [Ca2+]i; as yet, however, it is not clear whether protein kinase G acts directly to phosphorylate myosin light chain phosphatase, or whether the action is indirect via phosphorylation of another protein, such as telokin, which regulates myosin light chain phosphatase activity (Lee et al., 1997; Wu et al., 1998; Surks et al., 1999). In the present study, NO had no effect on tone induced by calcium itself, but it did relax the further increase in calcium-induced tension produced by the muscarinic receptor agonist carbachol, the relaxation being abolished by ODQ and mimicked by 8-Br-cyclic GMP. This suggests that in the anococcygeus the NO/cyclic GMP pathway does not interact directly with the contractile proteins or myosin light chain phosphatase, but that it inhibits a ‘calcium sensitization' mechanism activated by carbachol. Two important questions for future study arise from our experiments with skinned preparations. The first concerns the nature of the calcium sensitization mechanism targetted by NO/cyclic GMP in the anococcygeus. Sensitization processes known to be activated by receptor agonists in smooth muscle include the RhoA/Rho kinase pathway and certain isoforms of protein kinase C (Somlyo & Somlyo, 2000). In relation to our results with the anococcygeus, one important recent observation is that protein kinase G phosphorylates RhoA and inhibits its calcium sensitization activity in vascular smooth muscle (Sauzeau et al., 2000); as in the anococcygeus, the cyclic GMP signal did not relax tone induced by calcium alone in β-escin-permeabilized muscle, but did relax the further increase in tone following calcium sensitization (Sauzeau et al., 2000). The second important question raised by the present study involves the observation that not only carbachol enhanced the contraction to calcium in skinned preparations, but so too did Tg. This was an unexpected result, but it raises the intriguing possibility that store depletion itself might be a stimulus not only for the opening of SOCs in the plasma membrane, but also for calcium sensitization. While identification of the precise cellular mechanisms underlying this effect of Tg awaits detailed investigation it may be noteworthy that, in addition to their role in calcium sensitization, small G proteins such as RhoA have been proposed as the intracellular signals mediating SOC activation (Parekh & Penner, 1997). Of relevance in terms of the present study was the observation that the Tg-induced contraction in skinned muscles was relaxed by both NO and 8-Br-cyclic GMP.
In conclusion, the results confirm that the NO/cyclic GMP signalling pathway can inhibit capacitative calcium entry in the mouse anococcygeus, and support the hypothesis that this inhibition results from enhanced refilling of the internal stores. However, unlike the rat aorta (Cohen et al., 1999), impairment of capacitative calcium entry is not obligatory for relaxation of the mouse anococcygeus via the NO/cyclic GMP pathway; experiments with skinned muscles suggest that a major component of the relaxation involves inhibition of calcium sensitization processes activated by receptor agonists and/or store depletion.
Acknowledgments
The authors thank the Wellcome Trust Pfizer (UK) and BBSRC for support.
Abbreviations
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraaecetic acid
- 8-Br-cyclic GMP
8-bromo-cyclic guanosine monophosphate
- [Ca2+]i
cytoplasmic calcium ion concentration
- E-64
trans-epoxysuccinyl-L-leucylamideo-(4-guanidino)butane
- EGTA
ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′,-tetraacetic acid
- ER
endoplasmic reticulum
- FCCP
carbonyl cyanide p-trifluoromethoxyphenyl-hydrazone
- HEPES
N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
- IP3
inositol 1,4,5-trisphosphate
- L-NOARG
L-NG-nitroarginine
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxodiazolo[4,3-a]quinoxalin-1-one
- PIPES
piperazine-N,N′-bis[2-ethanesulphonic acid]
- PSS
physiological salt solution
- SNP
sodium nitroprusside
- SOC
store-operated channel
- SR
sarcoplasmic reticulum
- Tg
thapsigargin
References
- BAHNSON T.D., PANDOL S.J., DIONNE V.E. Cyclic GMP modulates depletion-activated Ca2+ entry in pancreatic acinar cells. J. Biol. Chem. 1993;268:10808–10812. [PubMed] [Google Scholar]
- BERRIDGE M.J. Capacitative calcium entry. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIAN X.P., BIRD G. STJ., PUTNEY JNR. J.W. cGMP is not required for capacitative Ca2+ entry in Jurkat T-lymphocytes. Cell Calcium. 1996;19:351–354. doi: 10.1016/s0143-4160(96)90075-5. [DOI] [PubMed] [Google Scholar]
- BIRNBAUMER L., ZHU X., JIANG M., BOULAY G., PEYTON M., VANNIER B., BROWN D., PLATANO D., SADEGHI E., STEFANI E., BIRNBAUMER M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc. Natl. Acad. Sci. U.S.A. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUANG A.T., STRAUSS J.D., STEERS W.D., MURPHY R.A. cGMP mediates corpus cavernosum smooth muscle relaxation with altered cross-bridge function. Life Sciences. 1998;63:185–194. doi: 10.1016/s0024-3205(98)00259-8. [DOI] [PubMed] [Google Scholar]
- COHEN R.A., WEISBROD R.M., GERICKE M., YAGHOUBI M., BIERL C., BOLOTINA V.M. Mechanism of nitric oxide-induced vasodilatation. Circ. Res. 1999;84:210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- FASOLATO C., HOTH M., PENNER R. A GTP-dependent step in the activation mechanism of capacitative calcium influx. J. Biol. Chem. 1993;268:20737–20740. [PubMed] [Google Scholar]
- GIBSON A., MCFADZEAN I., TUCKER J.F., WAYMAN C. Variable potency of nitrergic-nitrovasodilator relaxations of the mouse anoccygeus against different forms of induced tone. Br. J. Pharmacol. 1994;113:1494–1500. doi: 10.1111/j.1476-5381.1994.tb17165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., MCFADZEAN I., WAYMAN C.P., WALLACE P. Capacitative calcium entry and the regulation of smooth muscle tone. Trends Pharmacol. Sci. 1998;19:266–269. doi: 10.1016/s0165-6147(98)01222-x. [DOI] [PubMed] [Google Scholar]
- GIBSON A., MIRZAZADEH S. N-methylhydroxylamine inhibits, and M& B22948 potentiates, relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs. Br. J. Pharmacol. 1989;96:637–644. doi: 10.1111/j.1476-5381.1989.tb11863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., MIRZAZADEH S., HOBBS A.J., MOORE P.K. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br. J. Pharmacol. 1990;99:602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILON P., OBIE J.F., BIAN X., BIRD G.S., DAGORN J.C., PUTNEY J.W., JR Role of cyclic GMP in the control of capacitative Ca2+ entry in rat pancreatic acinar cells. Biochem. J. 1995;311:649–656. doi: 10.1042/bj3110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HATHAWAY D.R., KONICKI M.V., COOLICAN S.A. Phosphorylation of myosin light chain kinase from vascular smooth muscle by cAMP- and cGMP-dependent protein kinases. J. Mol. Cell Cardiol. 1985;17:841–850. doi: 10.1016/s0022-2828(85)80098-5. [DOI] [PubMed] [Google Scholar]
- IIZUKA A., IKEBE M., SOMLYO A.V., SOMLYO A.P. Introduction of high molecular weight (IgG) proteins into receptor coupled, permeabilised smooth muscle. Cell Calcium. 1994;16:461–445. doi: 10.1016/0143-4160(94)90073-6. [DOI] [PubMed] [Google Scholar]
- KWAN H.-Y., HUANG Y., YAO X. Store-operated calcium entry in vascular endothelial cells is inhibited by cGMP via a protein kinase G-dependent mechanism. J. Biol. Chem. 2000;275:6758–6763. doi: 10.1074/jbc.275.10.6758. [DOI] [PubMed] [Google Scholar]
- LEE M.R., LI L., KITAZAWA T. Cyclic GMP causes Ca2+ desensitisation in vascular smooth muscle by activating the myosin light chain phosphatase. J. Biol. Chem. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Prejunctional inhibition of non-adrenergic non-cholinergic transmission in the rat anococcygeus muscle. Eur. J. Pharmacol. 1989;168:107–110. doi: 10.1016/0014-2999(89)90640-7. [DOI] [PubMed] [Google Scholar]
- MCFADZEAN I., ENGLAND S. Properties of the inactivating outward current in single smooth muscle cells from the rat anococcygeus. Pflugers Arch. 1992;421:117–124. doi: 10.1007/BF00374817. [DOI] [PubMed] [Google Scholar]
- MURPHY R.A., WALKER J.S. Inhibitory mechanisms for cross-bridge cycling: the nitric oxide cGMP signal transduction pathway in smooth muscle relaxation. Acta Physiol. Scand. 1998;164:373–380. doi: 10.1046/j.1365-201X.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- NISHIKAWA M., LANEROLLE DE P., LINCOLN T.M., ADELSTEIN R.S. Phosphorylation of mammalian myosin light chain kinases by the catalytic subunit of cyclic AMP-dependent protein kinase and by cyclic GMP-dependent protein kinase. J. Biol. Chem. 1984;259:8429–8436. [PubMed] [Google Scholar]
- NISHIMURA J., VAN BREEMEN C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem. Biophys. Res. Com. 1989;163:929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store depletion and calcium influx. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- PFITZER G., HOFMANN F., DISALVO J., RUEGG J.C. cGMP and cAMP inhibits tension development in skinned coronary arteries. Pflugers Arch. 1984;401:277–280. doi: 10.1007/BF00582596. [DOI] [PubMed] [Google Scholar]
- PFITZER G., MERKEL L., RUEGG J.C., HOFMANN F. Cyclic GMP-dependent protein kinase relaxes skinned fibers from guinea pig taenia coli but not from chicken gizzard. Pflugers. Arch. 1986;407:87–91. doi: 10.1007/BF00580726. [DOI] [PubMed] [Google Scholar]
- PFITZER G., RUEGG J.C., FLOCKERZI V., HOFMANN F. cGMP-dependent protein kinase decreases calcium sensitivity of skinned cardiac fibres. FEBS Lett. 1982;149:171–175. doi: 10.1016/0014-5793(82)81095-8. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W., JR A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W., JR Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W., JR, MCKAY R.R. Capacitative calcium entry channels. BioEssays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., LE JEUNE H., CARIO-TOUMANIANTZ C., SMOLENSKI A., LOHMANN S.M., BERTOGLIO J., CHARDIN P., PACAUD P., LOIRAND G. Cyclic GMP-dependent protein kinase signaling pathway inhibitsRhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. 2000;522:177–186. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SURKS H.K., MOCHIZUKI N., KASAI Y., GEORGESCU S.P., TANG K.M., ITO M., LINCOLN T.M., MENDELSOHN M.E. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase I alpha. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- TREPAKOVA E.S., COHEN R.A., BOLOTINA V.M. Nitric oxide inhibits capacitative calcium influx in human platelets by promoting sarcoplasmic/endoplasmic reticulum Ca2+-ATPase-dependent refilling of Ca2+ stores. Circ. Res. 1999;84:201–209. doi: 10.1161/01.res.84.2.201. [DOI] [PubMed] [Google Scholar]
- WALLACE P., AYMAN S., MCFADZEAN I., GIBSON A. Thapsigargin-induced tone and capacitative calcium influx in mouse anococcygeus smooth muscle cells. Naunyn Schmiedebergs Arch. Pharmacol. 1999;360:368–375. doi: 10.1007/s002109900100. [DOI] [PubMed] [Google Scholar]
- WAYMAN C.P., GIBSON A., MCFADZEAN I. Depletion of either ryanodine- or IP3-sensitive calcium stores activates capacitative calcium entry in mouse anococcygeus smooth muscle. Pflugers Arch. 1998;435:231–239. doi: 10.1007/s004240050506. [DOI] [PubMed] [Google Scholar]
- WAYMAN C.P., MCFADZEAN I., GIBSON A., TUCKER J.F. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br. J. Pharmacol. 1996a;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAYMAN C.P., MCFADZEAN I., GIBSON A., TUCKER J.F. Inhibition by sodium nitroprusside of a calcium store depletion-activated non-selective cation current in smooth muscle cells of the mouse anococcygeus. Br. J. Pharmacol. 1996b;118:2001–2008. doi: 10.1111/j.1476-5381.1996.tb15636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAYMAN C.P., MCFADZEAN I., GIBSON A., TUCKER J.F. Cellular mechanisms underlying carbachol-induced oscillations of calcium-dependent membrane current in smooth muscle cells from mouse anococcygeus. Br. J. Pharmacol. 1997;121:1301–1308. doi: 10.1038/sj.bjp.0701279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAYMAN C.P., WALLACE P., GIBSON A., MCFADZEAN I. Correlation between store-operated cation current and capacitative Ca2+ influx in smooth muscle cells from mouse anococcygeus. Eur. J. Pharmacol. 1999;376:325–329. doi: 10.1016/s0014-2999(99)00400-8. [DOI] [PubMed] [Google Scholar]
- WU X., HAYSTEAD T.A.J., NAKAMOTO R.K., SOMLYO A.V., SOMLYO A.P. Acceleration of myosin light chain dephosphorylation and relaxation of smooth muscle by telokin. J. Biol. Chem. 1998;18:11362–11369. doi: 10.1074/jbc.273.18.11362. [DOI] [PubMed] [Google Scholar]
- WU X., SOMLYO A.V., SOMLYO A.P. Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphatase. Biochem. Biophys. Res. Com. 1996;220:658–663. doi: 10.1006/bbrc.1996.0460. [DOI] [PubMed] [Google Scholar]
- XU X., STAR R.A., TORTORICI G., MUALLEM S. Depletion of intracellular Ca2+ stores activates nitric-oxide synthase to generate cGMP and regulate Ca2+ influx. J. Biol. Chem. 1994;269:12645–12653. [PubMed] [Google Scholar]


