Abstract
Transcription from many archaeal promoters can be reconstituted in vitro using recombinant TATA-box binding protein (TBP) and transcription factor B (TFB)—homologues of eukaryal TBP and TFIIB—together with purified RNA polymerase (RNAP). However, all archaeal genomes sequenced to date reveal the presence of TFE, a homologue of the α-subunit of the eukaryal general transcription factor, TFIIE. We show that, while TFE is not absolutely required for transcription in the reconstituted in vitro system, it nonetheless plays a stimulatory role on some promoters and under certain conditions. Mutagenesis of the TATA box or reduction of TBP concentration in transcription reactions sensitizes a promoter to TFE addition. Conversely, saturating reactions with TBP de-sensitizes promoters to TFE. These results suggest that TFE facilitates or stabilizes interactions between TBP and the TATA box.
INTRODUCTION
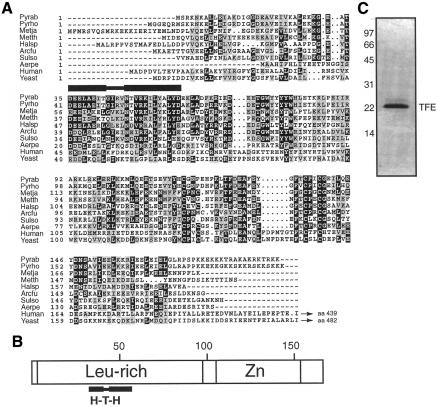
The archaeal basal transcription machinery is fundamentally related to the eukaryal RNA polymerase (RNAP) II machinery (Orphanides et al., 1996; Reeve et al., 1997; Bell and Jackson, 1998a). A range of in vitro transcription assays have demonstrated that a variety of archaeal promoters of varying strengths can be accurately transcribed in reactions containing archaeal TATA-box binding protein (TBP), transcription factor B (TFB) and RNAP (Hethke et al., 1996, 1999; Qureshi et al., 1997; Bell et al., 1998; Darcy et al., 1999; Bell and Jackson, 2000). However, it has become apparent that all archaeal genomes sequenced to date encode a homologue of a third eukaryal general transcription factor, TFIIE (Bell and Jackson, 1998b; Aravind and Koonin, 1999; Kyrpides and Ouzounis, 1999). The archaeal TFIIE homologue, TFE, is related to the N-terminal 20 kDa of the α-subunit of TFIIE (Figure 1A). Extensive analyses of eukaryal TFIIE function have been performed in vivo and in vitro (Ohkuma et al., 1995; Kuldell and Buratowski, 1997). In particular, deletion analyses have revealed that the C-terminal portion of TFIIEα is dispensable for viability in yeast but that the N-terminal portion is essential in vivo (Kuldell and Buratowski, 1997). Remarkably, the smallest truncated version of TFIIEα, which nonetheless permits yeast growth, corresponds closely to full-length archaeal TFE (Figure 1A). In addition, similar deletion analyses that examined TFIIE function in reconstituted in vitro transcription assays revealed an analogous requirement for the N-terminal portion of human TFIIEα for basal and activated transcription (Ohkuma et al., 1995). Thus, it appears that archaeal TFE corresponds to the minimal essential region of eukaryal TFIIEα (see Figure 1A). Although the nature of the essential function remains undetermined, it has been demonstrated that this region of TFIIEα can interact with TBP and RNAP II (Maxon et al., 1994; Yokomori et al., 1998). In archaea, the TFE open reading frame (ORF) contains an N-terminal, weakly conserved, helix–turn–helix motif within a leucine-rich region and a C-terminal zinc ribbon (Figure 1B). Intriguingly, while some archaeal TFEs possess putative zinc ribbons with four cysteine residues coordinating the metal ion, those of Sulfolobus solfataricus and Archaeoglobus fulgidus have the second cysteine substituted by aspartic acid or methionine, respectively. While these maintain the potential to coordinate a metal ion via polar side chains, the TFEs of Pyrococci and Methanobacterium thermoautotrophicum possess proline or glycine in the analogous position (Figure 1A). Thus, it is possible that not all archaeal TFEs possess a coordinated metal ion.
Fig. 1. Archaea possess a sequence homologue of the α-subunit of TFIIE. (A) An alignment of various archaeal TFE and eukaryal TFIIEa subunits is shown. Pyrab, Pyrococcus abyssi, DDBJ/EMBL/GenBank accession No. C75055; Pyrho, Pyrococcus horikoshii, accession No. B71106; Metja, Methanococcus jannaschii, accession No. Q58187; Metth, Methanobacterium thermoautrophicum, accession No. A69090; Halsp, Halobacterium sp. NRC-1, accession No. AAG19231; Arcfu, Archaeglobus fulgidus, accession No. E69344; Sulso, Sulfolobus solfatraicus, http://niji.imb.nrc.ca/sulfolobus; Aerpe, Aeropyrum pernix, accession No. F72503; human, accession No. P29083; yeast, accession No. P36100. Identical residues are boxed in black and homologous residues are shaded in grey. A helix–turn–helix structure is indicated by black horizontal bars. (B) Schematic representation of motifs found in archaeal TFE. (C) Coomassie blue-stained SDS–polyacrylamide gel containing 3 µg of purified recombinant TFE.
The reconstituted archaeal transcription assays performed to date demonstrate accurate transcription from a range of promoters in the presence of TBP, TFB and RNAP. Therefore, this indicates that TFE is not required for transcription from these promoters under the conditions employed. However, it is possible that TFE either plays a general stimulatory role in transcription or is required for a subset of promoters or under certain conditions. To determine whether TFE has a general role in archaeal transcription, we have purified recombinant S. solfatricus P2 TFE and assayed its activity in a reconstituted Sulfolobus in vitro transcription system.
RESULTS
Archaeal TFE interacts with RNAP and TBP
The ORF of S. solfataricus P2 TFE was identified in the S. solfataricus P2 genome sequence and amplified by PCR, followed by cloning and expression in Escherichia coli as a C-terminally His6-tagged fusion protein. This protein was purified (Figure 1C) and then used in protein–protein interaction assays to determine whether it could interact with components of the archaeal basal transcription machinery. First, 100 ng of TFE were mixed with 10 µg of S. solfataricus whole-cell extract, prior to incubation with Ni-NTA–agarose. Beads were washed extensively and bound protein eluted by boiling in SDS–PAGE loading buffer. Following electrophoresis, eluted proteins were detected by western blotting using antisera generated against TBP, TFB or the B-subunit of RNAP (Qureshi et al., 1997). The interaction assays were carried out in the presence of 50 µg/ml ethidium bromide to exclude the possibility that interactions were bridged by DNA rather than being direct (Lai and Herr, 1992). The results of these assays show that TFE can interact with TBP and RNAP, but not TFB in whole-cell extracts (Figure 2A). The assays were repeated using purified RNAP and purified recombinant TBP in place of extract (Figure 2B). These assays confirmed the results of the pull-downs from extract, indicating that TFE can interact directly with TBP and RNAP in the absence of DNA.
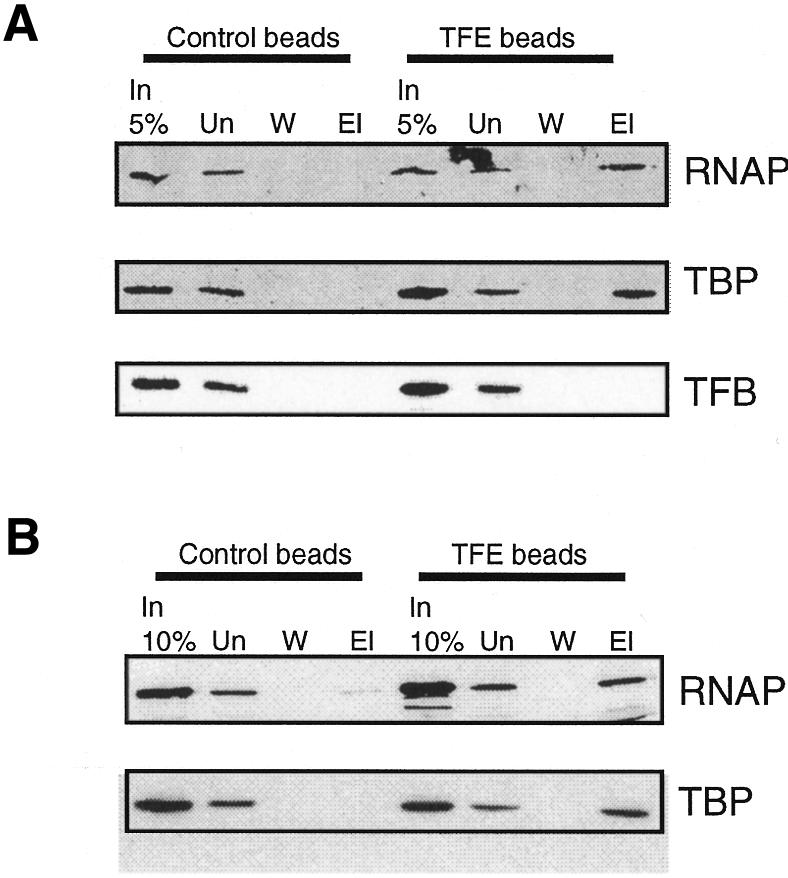
Fig. 2. TFE interacts with RNAP and TBP. (A) Ni-NTA pull-down assays were performed with 10 µg of S. solfataricus extract, as detailed in Methods, in the presence (TFE beads) or absence (control beads) of 100 ng recombinant TFE. The proteins pulled-down in the assay were detected by western blotting with antisera raised against TBP, TFB or RNAP B-subunit (Qureshi et al., 1997). Lanes contain 5% of input (In), unbound material (Un), wash (W) or material eluted from the beads (El). (B) Assays performed as above but with purified TBP and RNAP in place of extract.
TFE stimulates transcription on some promoters in vitro
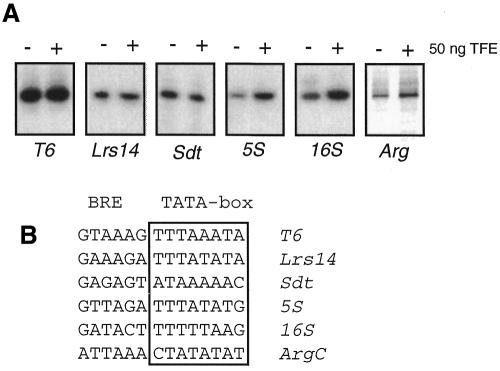
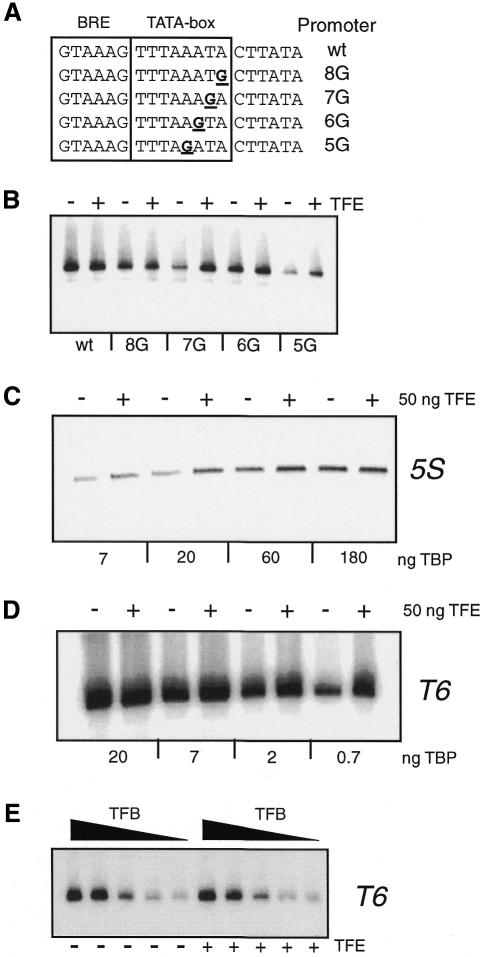
To determine whether TFE plays a role in transcription, reconstituted transcription assays, using highly purified Sulfolobus RNAP and purified recombinant TBP and TFB, were performed on six different promoters, either with no added TFE or supplemented with 50 ng of TFE. As these assays contained RNAP purified from cells, western blotting was performed using anti-TFE antisera to confirm that the RNAP preparation was essentially free of endogenous TFE (see Supplementary data available at EMBO reports Online). As seen in Figure 3A, under the reaction conditions employed, three promoters (T6, Lrs14 and Sdt) are largely unaffected by TFE. However, the yield of transcript from three other promoters (ArgC, 5S and 16S) is stimulated 2- to 3-fold by the addition of TFE. Examination of the sequence of the core elements of these three TFE-responsive promoters reveals a common deviation from the TATA-box consensus in having a G at position 8 of the template strand of the TATA box, or the dyad-related C at position 1 of the element (Figure 3B). Previous work with human TFIIEα has revealed that the α-subunit of TFIIE can stimulate the binding of TBP to the TATA element (Yokomori et al., 1998). We reasoned, therefore, that some non-consensus TATA-elements may confer sensitivity to TFE in our experiments. We note, however, that the Sdt promoter, which appears to be unaffected by TFE addition, also deviates from the TATA consensus. To test our hypothesis we made a series of guanine substitutions at positions 5, 6, 7 and 8 of the TATA box of the T6 promoter, and tested the effect of TFE on transcription of these promoter derivatives (Figure 4A and B). Somewhat surprisingly, substitution of guanine for adenine at positions 6 or 8 had only a small effect on transcription compared to wild-type T6 promoter. Furthermore, these promoters were largely insensitive to the addition of TFE. In contrast, guanine substitution at position 7 and 5 resulted in a significant reduction of transcription relative to wild type. Importantly, these promoters were now stimulated by the addition of TFE to transcription reactions. This suggests that the relative affinity of TBP for the TATA box may impart sensitivity to TFE addition to a promoter. In light of the above, we reasoned that by altering TBP concentration in transcription assays, promoters may become either TFE-sensitive (under limiting TBP concentrations) or TFE-insensitive (under saturating TBP concentrations). To test this idea, we varied TBP concentration in the transcription assays programmed with 5S or T6 promoters (Figure 4C and D). As demonstrated above, when the 5S promoter is transcribed in reactions containing 20 ng of TBP, the addition of TFE stimulates transcription ∼2-fold. However, as increasing TBP is added to a maximum of 180 ng the stimulatory effect is no longer detected (Figure 4C). Similarly, we varied TBP levels in transcription reactions programmed by the T6 promoter. The promoter is TFE-independent at 20 ng of TBP; however, at lower TBP levels the reactions are now stimulated by TFE (Figure 4D). In contrast, experiments in which TFB concentration was varied and TBP levels kept constant at a saturating level showed no significant differences in the presence or absence of TFE (Figure 4E). Together with the TATA-box substitution experiments in Figure 4, these data support the hypothesis that TFE can stimulate transcription under conditions where there are sub-optimal interactions between TBP and the TATA box.
Fig. 3. TFE stimulates transcription from some promoters. (A) In vitro transcriptions were carried out with the indicated promoters in reactions containing 20 ng TBP, 25 ng TFB and 200 ng RNAP for 10 min at 65°C. RNA products were detected by primer extension. (B) Sequence of TATA box and BRE of the promoters used in (A).
Fig. 4. TFE stimulates transcription under sub-optimal TBP–TATA-box interactions. (A) The sequence of wild-type and mutant T6 promoters is shown. The TATA box and BRE are boxed and the positions of sequence substitutions shown in bold and underlined. (B) The products of in vitro transcription of the promoters shown in (A) were detected by primer extension analysis and electrophoresed on a 8% denaturing polyacrylamide gel. (C) In vitro transcription reactions were performed on the 5S promoter, either with no TFE or supplemented with 50 ng of TFE as indicated. The reactions contained 25 ng TFB, 200 ng of RNAP and varying amounts of TBP as indicated. (D) In vitro transcription reactions were performed on the T6 promoter, either with no TFE or supplemented with 50 ng of TFE as indicated. Reaction conditions were as (C). (E) In vitro transcription assays performed on the T6 promoter. Reactions contained 20 ng TBP, 200 ng RNAP and either 40, 20, 10, 5 or 2.5 ng TFB.
TFE stimulates early stages in transcription initiation
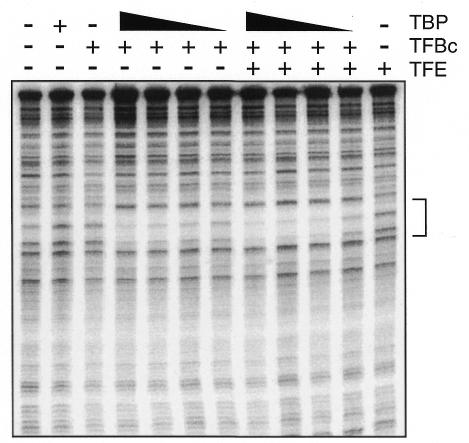
We next sought to determine whether TFE stimulates early stages in promoter recognition by performing DNase I footprinting of TBP–TFB–DNA complex formation on the T6 promoter at a range of TBP concentrations, in the presence or absence of TFE. As seen in Figure 5, TFE modestly enhances protection of the TATA-box region of the promoter; specifically, protection is observed at a 2-fold lower TBP concentration in the presence of TFE. This level of stimulation is in agreement with the level of stimulation of transcription seen on the T6 promoter under limiting TBP conditions (Figure 4C).
Fig. 5. TFE stimulates TBP–TFB–DNA complex formation. DNase I footprinting analysis was performed on the T6 promoter in the presence or absence of 25 ng TFE and 20 ng TFBc (TFB core domain) as indicated. TBP was present at 20, 15, 10 or 5 ng per reaction (lanes 4–7 and 8–11).
DISCUSSION
We find that TFE maximally exerts a 2- to 3-fold stimulatory effect on transcription, dependent on the promoter tested and reaction conditions. There appears to be a general correlation between detection of TFE-mediated stimulation and conditions where TBP–TATA-box interactions are sub-optimal. In agreement with this, we find that TFE interacts with TBP and that TFE appears to facilitate or stabilize recognition of the basal promoter elements by the basal machinery. How might this stimulation be mediated? One possibility is that TFE binds a DNA element in addition to TBP itself, and thereby facilitates cooperative binding of TBP and itself to DNA. However, extensive cross-linking analyses have been performed with the eukaryal basal transcription machinery, and there is no evidence for direct interactions between TFIIEα and promoter DNA in the vicinity of the TATA box (Kim et al., 2000). Consistent with this, we observe that there is no detectable change in the TBP/TFB footprint observed in the presence or absence of TFE (Figure 5). A second possibility is that TFE induces a conformational change in TBP which facilitates DNA binding. This too seems unlikely, as the crystal structures of TBP in solution and bound to DNA have been determined and there is very little structural difference between the two forms of the protein (DeDecker et al., 1996; Kosa et al., 1997). A related possibility is suggested by the observation that TBP from eukarya and archaea exists as a dimer in solution. It has been proposed that dimerization of TBP prevents TBP–DNA interactions. It is possible that the function of TFE may prevent dimerization of TBP and so favour TATA-box recognition, indeed such behaviour has recently been described for eukaryal TFIIA (Coleman et al., 1999).
The effect that we observe with TFE is relatively weak: while withholding TBP or TFB from transcription assays essentially abolishes transcription on most promoters (Qureshi et al., 1997), the omission of TFE exerts a small quantitative effect in vitro. It is conceivable that a range of archaeal promoters exist that are more TFE dependent; based on our findings, we would propose that such promoters would contain TATA boxes that correspond poorly to the consensus sequence. In addition it is possible that in vivo, under physiological TBP concentrations, TFE plays a more significant effect. Additionally, in archaeal cells, DNA is compacted by interaction with a range of small basic proteins, including direct homologues of eukaryal histones in some species (Reeve et al., 1997). Some of these proteins have been observed to repress transcription in vitro (Soares et al., 1998). It is possible that some of this repression results from competition for access to the TATA box between TBP and these non-specific DNA-binding proteins. If so, TFE could stimulate transcription in vivo by facilitating interaction between TBP and the TATA box. Experiments to reconstitute archaeal chromatin in vitro are underway to address this and other related issues.
METHODS
Cloning and purification of S. solfataricus P2 TFE
The ORF of TFE was identified by BLAST search of the publicly available S. solfataricus genome sequence (http://niji.imb.nrc.ca/sulfolobus) using the sequence of A. fulgidus TFE as query (Klenk et al., 1997). The ORF was amplified by PCR using primers TFE5 (5′-GGGGATCCCATATGGTTAACGCAGAGGATCGTTT-3′) and TFE3 (5′-GCCCTGACTCGAGATGATTTTTATTAGCTCCAAG-3′). The PCR product was digested with NdeI and XhoI, and ligated to NdeI–XhoI digested pET30a (Novagen). The resultant expression plasmid pET-ssTFE was transformed into BLR RIL and expression of His6-tagged TFE was induced during logarithmic growth of cells by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to 1 mM for 3 h. Cells were pelleted and resuspended in N300 (50 mM Tris pH 8.0, 300 mM NaCl, 10 mM β-mercaptoethanol), lysed by sonication and clarified by centrifugation. The supernatant was heated to 70°C for 30 min and re-centrifuged. The resultant supernatant had imidazole added to 20 mM and was then applied to a column containing Ni-NTA–agarose matrix (Qiagen). The matrix was washed with 10 column volumes of N300 + 45 mM imidazole, followed by elution by N300 + 500 mM imidazole. One millilitre fractions were collected and the presence of TFE determined by SDS–PAGE and Coomassie blue staining. Positive fractions were pooled and dialysed extensively against N300 + 10% glycerol + 10 µM ZnSO4. Polyclonal antisera were generated in a rabbit against TFE (three consecutive injections of 100 µg TFE at three-weekly intervals). Serum was collected and tested for the presence of anti-TFE antibodies using standard methodologies (Lane and Harlow, 1988)
Purification of TBP, TFB and RNAP
These proteins were purified as described previously (Qureshi et al., 1997; Bell and Jackson, 2000).
Transcription assays, EMSA and DNase I footprinting
These were performed as described previously (Bell et al., 1999) using TBP, TFB, TFBc and RNAP purified as described (Bell and Jackson, 2000)
Protein–protein interaction studies
These were performed in a volume of 500 µl of BB [90 mM KCl, 20 mM Tris pH 8.0, 10 % glycerol, 10 mM β-mercaptoethanol, 50 µg/ml ethidium bromide, 0.1% Triton X-100, 20 mM imidazole, 10 µg/ml bovine serum albumin (BSA)]. The reactions contained 100 ng purified TFE and either 10 µg S. solfataricus whole-cell extract, 1 µg of purified TBP or RNAP, as indicated in Figure 2. Reactions were shaken at room temperature together with 20 µl of a 50% slurry of Ni-NTA–agarose in BB. Beads were collected by centrifugation and washed 5 times with 1 ml of BB prior to resuspension in 40 µl 1× SDS–PAGE loading buffer and boiling. Eluted protein was subjected to SDS–PAGE and detected by western blotting as described previously.
Supplementary data
Supplementary data are available at EMBO reports Online.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We thank Jessica Downs for valuable comments on this manuscript. This work was supported by the Wellcome Trust.
REFERENCES
- Aravind L. and Koonin, E.V. (1999) DNA-binding proteins and evolution of transcription regulation in the archaea. Nucleic Acids Res., 27, 4658–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S.D. and Jackson, S.P. (1998a) Transcription and translation in Archaea: a mosaic of eukaryal and bacterial features. Trends. Microbiol., 6, 222–228. [DOI] [PubMed] [Google Scholar]
- Bell S.D. and Jackson, S.P. (1998b) Transcription in Archaea. Cold Spring Harb. Symp. Quant. Biol., 63, 41–51. [DOI] [PubMed] [Google Scholar]
- Bell S.D. and Jackson, S.P. (2000) The role of transcription factor B in transcription initiation and promoter clearance in the archaeon Sulfolobus acidocaldarius.J. Biol. Chem., 275, 12934–12940. [DOI] [PubMed] [Google Scholar]
- Bell S.D., Jaxel, C., Nadal, M., Kosa, P.F. and Jackson, S.P. (1998) Temperature, template topology, and factor requirements of archaeal transcription. Proc. Natl Acad. Sci. USA, 95, 15218–15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S.D., Cairns, S.S., Robson, R.L. and Jackson, S.P. (1999) Transcriptional regulation of an archaeal operon in vivo and in vitro. Mol. Cell, 4, 971–982. [DOI] [PubMed] [Google Scholar]
- Coleman R.A., Taggart, A.K.P., Burma, S., Chicca, J.J. and Pugh, B.F. (1999) TFIIA regulates TBP and TFIID dimers. Mol. Cell, 4, 451–457. [DOI] [PubMed] [Google Scholar]
- Darcy T.J., Hausner, W., Awery, D.E., Edwards, A.M., Thomm, M. and Reeve, J.N. (1999) Methanobacterium thermoautotrophicum RNA polymerase and transcription in vitro.J. Bacteriol., 181, 4424–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDecker B.S., O’Brien, R., Fleming, P.J., Geiger, J.H., Jackson, S.P. and Sigler, P.B. (1996) The crystal structure of a hyperthermophilic archaeal TATA-box binding protein. J. Mol. Biol., 264, 1072–1084. [DOI] [PubMed] [Google Scholar]
- Hethke C., Geerling, A.C.M., Hausner, W., deVos, W.M. and Thomm, M (1996) A cell-free transcription system for the hyperthermophilic Archaeon Pyrococcus furiosus. Nucleic Acids Res., 24, 2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hethke C. et al. (1999) Cell-free transcription at 95 degrees: thermostability of transcriptional components and DNA topology requirements of Pyrococcus transcription. Genetics, 152, 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T-Y., Ebright, R.H and Reinberg, D. (2000) Mechanism of ATP-dependent promoter melting by TFIIH. Science, 288, 1418–1421. [DOI] [PubMed] [Google Scholar]
- Klenk H.P. et al. (1997) The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature, 390, 364–370. [DOI] [PubMed] [Google Scholar]
- Kosa P.F., Ghosh, G., DeDecker, B.S. and Sigler, P.B. (1997) The 2.1-Å crystal structure of an archaeal preinitiation complex: TATA-box-binding protein/transcription factor (II)B core/TATA-box. Proc. Natl Acad. Sci. USA, 94, 6042–6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuldell N.H. and Buratowski, S. (1997) Genetic analysis of the large subunit of yeast transcription factor IIE reveals two regions with distinct functions. Mol. Cell. Biol., 17, 5288–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrpides N.C. and Ouzounis, C.A. (1999) Transcription in Archaea. Proc. Natl Acad. Sci. USA, 96, 8545–8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.S. and Herr, W. (1992) Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc. Natl Acad. Sci. USA, 89, 6958–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. and Harlow, E (1988) Antibodies, a Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Maxon M.E. and Tjian, R. (1994) Transcriptional activity of transcription factor IIE is dependent on zinc binding. Proc. Natl Acad. Sci. USA, 91, 9529–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxon M.E., Goodrich, J.A. and Tjian, R. (1994) Transcription factor IIE binds preferentially to RNA polymerase IIa and recruits TFIIH: a model for promoter clearance. Genes Dev., 8, 515–524. [DOI] [PubMed] [Google Scholar]
- Ohkuma Y., Hashimoto, S., Wang, C.K., Horikoshi, M. and Roeder, R.G. (1995) Analysis of the role of TFIIE in basal transcription and TFIIH-mediated carboxy-terminal domain phosphorylation through structure-function studies of TFIIE-α. Mol. Cell. Biol., 15, 4856–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanides G., Lagrange, T. and Reinberg, D. (1996) The general transcription factors of RNA polymerase II. Genes Dev., 10, 2657–2683 [DOI] [PubMed] [Google Scholar]
- Qureshi S.A., Bell, S.D. and Jackson, S.P. (1997) Factor requirements for transcription in the Archaeon Sulfolobus shibatae. EMBO J., 16, 2927–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J.N., Sandman, K. and Daniels, C.J. (1997) Archaeal histones, nucleosomes, and transcription initiation. Cell, 89, 999–1002 [DOI] [PubMed] [Google Scholar]
- Soares D., Dahlke, I., Li, W.T., Sandman, K., Hethke, C., Thomm, M. and Reeve, J.N. (1998) Archaeal histone stability, DNA binding, and transcription inhibition above 90°C. Extremophiles, 2, 75–81. [DOI] [PubMed] [Google Scholar]
- Yokomori K., Verrijzer, C.P. and Tjian, R. (1998) An interplay between TATA box-binding protein and transcription factors IIE and IIA modulates DNA-binding and transcription. Proc. Natl Acad. Sci. USA, 95, 6722–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.