Abstract
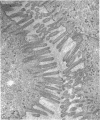
Jejunal biopsy specimens from patients with gluten-sensitive enteropathy (GSE) (obtained during gluten challenge) as well as from normal individuals and patients with other gastrointestinal abnormalities were cultured in vitro for 48 h in the presence or absence of a peptic-tryptic digest (P-T digest) of gliadin. In the absence of gliadin the alkaline phosphatase activity in the biopsy specimens obtained from normal control individuals increased from an initial value of 384 +/- 83 U to a 48 h value of 561 +/- 151 U (mean +/- SD) (difference significant at P < 0.01). The initial alkaline phosphatase activity of specimens obtained from patients with GSE was strikingly lower than that of normals, 117 +/- 79 U, and increased to a 48 h value of 399 +/- 203 U (difference significant at P < 0.01). The biochemical change in cultured biopsy specimens of GSE patients correlated with increases in the length and regularity of brush borders of epithelial cells as seen with the electron microscope. In the presence of a P-T digest of gliadin, the alkaline phosphatase activity of biopsy specimens of control individuals increased from an initial value of 384 +/- 83 U to a 48 h value of 578 +/- 156 U. In contrast, the alkaline phosphatase activity of biopsy specimens of patients with GSE in exacerbation showed a markedly diminished increase in activity during 48 h of culture; in this case the initial activity was 117 +/- 79 U and the final activity was 203 +/- 93 U. This inhibitory effect on increase of alkaline phosphatase activity during organ culture was specific in that a P-T digest of casein (a protein not toxic in vivo to patients with GSE) had no effect on alkaline phosphatase increases in culture. Finally, these results obtained with biopsy specimens taken from patients with GSE in exacerbation were compared with results obtained from patients with GSE in remission. Alkaline phosphatase activity of specimens obtained from the latter group of patients also increased during culture but in this instance P-T digest of gliadin in the culture medium had no significant inhibitory effect. In conclusion, the inhibitory effect of gliadin on intestinal epithelial cells in organ culture represents an in vitro model of gluten-sensitive enteropathy. Inasmuch as this effect of gliadin is not seen in cultures of specimens taken from patients in remission, it appears that gliadin is not directly toxic to GSE jejunal mucosa per se, but rather toxicity requires the participation of an endogenous effector mechanism which must first be stimulated in vivo.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRANDBORG L. L., RUBIN G. E., QUINTON W. E. A multipurpose instrument for suction biopsy of the esophagus, stomach, small bowel, and colon. Gastroenterology. 1959 Jul;37(1):1–16. [PubMed] [Google Scholar]
- BROWN A. L., Jr Microvilli of the human jejunal epithelial cell. J Cell Biol. 1962 Mar;12:623–627. doi: 10.1083/jcb.12.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg N. O., Dahlqvist A., Lindberg T., Nordén A. Intestinal dipeptidases and disaccharidases in celiac disease in adults. Gastroenterology. 1970 Oct;59(4):575–582. [PubMed] [Google Scholar]
- Bernardin J. E., Kasarda D. D., Mecham D. K. Preparation and characterization of alpha-gliadin. J Biol Chem. 1967 Feb 10;242(3):445–450. [PubMed] [Google Scholar]
- Booth C. C. The enterocyte in coeliac disease. Br Med J. 1970 Oct 3;4(5726):14–17. doi: 10.1136/bmj.4.5726.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning T. H., Trier J. S. Organ culture of mucosal biopsies of human small intestine. J Clin Invest. 1969 Aug;48(8):1423–1432. doi: 10.1172/JCI106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- FRAZER A. C., FLETCHER R. F., ROSS C. A., SHAW B., SAMMONS H. G., SCHNEIDER R. Gluten-induced enteropathy: the effect of partially digested gluten. Lancet. 1959 Sep 5;2(7097):252–255. doi: 10.1016/s0140-6736(59)92051-3. [DOI] [PubMed] [Google Scholar]
- FRAZER A. C. On the growth defect in coelic disease. Proc R Soc Med. 1956 Dec;49(12):1009–1013. doi: 10.1177/003591575604901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizer W. D., Laster L. Peptide hydrolase activities of the mucosa of human small intestine. J Clin Invest. 1969 Jan;48(1):210–228. doi: 10.1172/JCI105970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loeb P. M., Strober W., Falchuk Z. M., Laster L. Incorporation of L-leucine-14C into immunoglobulins by jejunal biopsies of patients with celiac sprue and other gastrointestinal diseases. J Clin Invest. 1971 Mar;50(3):559–569. doi: 10.1172/JCI106525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog F., Etzler M. E., Grey R. D. The differentiation of alkaline phosphatase in the small intestine. Ann N Y Acad Sci. 1969 Oct 14;166(2):447–465. doi: 10.1111/j.1749-6632.1969.tb46414.x. [DOI] [PubMed] [Google Scholar]
- Trier J. S., Browning T. H. Epithelial-cell renewal in cultured duodenal biopsies in celiac sprue. N Engl J Med. 1970 Dec 3;283(23):1245–1250. doi: 10.1056/NEJM197012032832302. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Haba G. L. INHIBITION BY PUROMYCIN OF AMINO ACID INCORPORATION INTO PROTEIN. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1721–1729. doi: 10.1073/pnas.45.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]