Abstract
The three-dimensional structure of a bacterial superantigen, Staphylococcus aureus enterotoxin H (SEH), bound to human major histocompatibility complex (MHC) class II (HLA-DR1) has been determined by X-ray crystallography to 2.6 Å resolution (1HXY). The superantigen binds on top of HLA-DR1 in a completely different way from earlier co-crystallized superantigens from S.aureus. SEH interacts with high affinity through a zinc ion with the β1 chain of HLA-DR1 and also with the peptide presented by HLA-DR1. The structure suggests that all superantigens interacting with MHC class II in a zinc-dependent manner present the superantigen in a common way. This suggests a new model for ternary complex formation with the T-cell receptor (TCR), in which a contact between the TCR and the MHC class II is unlikely.
Keywords: MHC class II/staphylococcal enterotoxin/superantigen/X-ray crystallography/zinc
Introduction
Superantigens are microbial or viral toxins that comprise a class of disease-associated, immunostimulatory molecules that bind major histocompatibility complex (MHC) class II molecules and stimulate a large number of T cells (up to 20% of all T cells). These properties are attributable to their unique ability to cross-link MHC class II and the T-cell receptor (TCR), forming a trimolecular complex (Papageorgiou and Acharya, 2000). Superantigen-induced T-cell activation leads to production of cytokines such as tumor necrosis factor (TNF) α and β and interleukin-2 (IL-2), which may result in acute toxic shock or scarlet fever (Marrack and Kappler, 1990). In contrast to con ventional antigens, superantigens bind as unprocessed molecules directly to the MHC molecule and the superantigen is then recognized by subsets of T cells bearing specific TCR Vβ profiles (Papageorgiou and Acharya, 2000).
Microbial superantigens are globular proteins of 22–29 kDa that are highly resistant to proteases and heat denaturation and are able to be absorbed by the epithelium as immunologically intact proteins (Hamad et al., 1997). Structural studies have revealed that the microbial superantigens have a remarkably conserved fold, although they display different binding modes to MHC class II molecules and can bind different TCRs. They have a common two-domain architecture (N- and C-terminal domains) with an α-helix part spanning the centre of the molecule (Papageorgiou and Achrya, 2000). The microbial superantigen family comprises the staphylococcal enterotoxins (SEs) A, B, C1-3, D, E, G, H, I, J, K, L, M and toxic shock syndrome toxin (TSST-1) produced by several strains of Staphylococcus aureus; and the streptococcal pyrogenic exotoxins (SPEs) A1–4, C, G, H, the streptococcal mitogenic exotoxin (SMEZ, SMEZ-2) and the streptococcal superantigen (SSA) all produced by several strains of Streptococcus pyogenes (Papageorgiou and Acharya, 2000).
Some of the superantigens have been suggested to interact in a zinc-dependent manner with MHC class II, including SEA, SED, SEE, SEH, SEI, SEJ, SPE-C, SPE-H and SMEZ-2 (Fraser et al., 1992; Abrahmsén et al., 1995; Roussel et al., 1997; Al-Daccak et al., 1998; Munson et al., 1998; Zhang et al., 1998; Nilsson et al., 1999; Proft et al., 1999; Arcus et al., 2000), and will be referred to as the zinc family. Several of these superantigens have been crystallized and their three-dimensional structures have been elucidated, but only the structure of SPE-CH35A has been determined in complex with its presenting molecule MHC class II at 3.2 Å resolution (Li et al., 2001). The structure of SEH has been solved and mutational studies have been performed (Nilsson et al., 1999; Håkansson et al., 2000). Although SEH has a single zinc-dependent binding site for MHC class II, in contrast to SEA, which has two MHC class II-binding sites (Abrahmsén et al., 1995), the affinity measured for the interaction is the highest measured to date for any superantigen, Bmax1/2 ∼0.5 nM (Nilsson et al., 1999).
In this study, we have crystallized SEH in complex with human MHC class II, HLA-DR1, in the presence of zinc and determined the three-dimensional structure to 2.6 Å resolution using X-ray crystallography. The structure shows a high-affinity zinc-dependent interaction between SEH and HLA-DR1 as well as an interaction between SEH and the main chain of the peptide presented by HLA-DR1.
Results
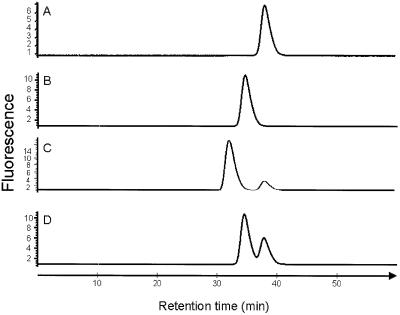
Both SEH and HLA-DR1 were expressed successfully in Escherichia coli and purified to homogeneity. The zinc ion dependency for binding of SEH to HLA-DR1 was shown by gel filtration analyses. SEH and HLA-DR1 form a stable complex in the presence of ZnCl2. In the presence of EDTA, no complex formation was detected, which clearly shows that the interaction between SEH and HLA-DR1 is stabilized by zinc ions (Figure 1). Crystals of the SEH– HLA-DR1 complex in the presence of zinc ions were grown by vapour diffusion at room temperature. Using X-ray diffraction from a single crystal, the three-dimensional structure of the complex was determined by molecular replacement, first looking at HLA-DR1 (Stern et al., 1994, 1DLH) then at SEH (Håkansson et al., 2000, 1ENF). The final model was refined at 2.6 Å resolution (Rwork = 0.202, Rfree = 0.258; 30–2.6 Å resolution, Table I) and included 88 water molecules (see Materials and methods).
Fig. 1. Gel filtration analyses of the HLA-DR1 complex, measuring the fluorescence. (A) SEH, (B) the HLA-DR1–peptide complex, (C) SEH and the HLA-DR1–peptide complex in the presence of zinc, and (D) SEH and the HLA-DR1–peptide complex in the presence of EDTA.
Table I. Structure determination statistics.
| Data collection | |
| Resolution range (Å) | 30–2.6 (31.4)a |
| Rsym (%)b | 7.1 (91.8)a |
| % Completeness | 96 (2185)a |
| Unique reflections total | 21 105 (3.55)a |
| Redundancy total | 3.54 (3.15)a |
|
I/σ |
14.4 |
| Refinement statistics | |
| R-factor (%) | 20.2 |
| Rfree (%)c | 25.8 |
| B-factor (Å2) | 42.3 |
| Water molecules | 88 |
| Model statistics | |
| Bond length r.m.s.d. (Å) | 0.007 |
| Bond angles r.m.s.d. (°) | 1.4 |
aLast shell, 2.6–2.69 Å.
bRsym = ΣhΣi|I(h,i) – <I(h)>|/ΣIhΣi(h,i), where I(h,i) is the intensity of the ith measurement of h and <I(h)> is the corresponding average value of all i measurements.
cRfree determined using 5% of all reflections.
Overall structure
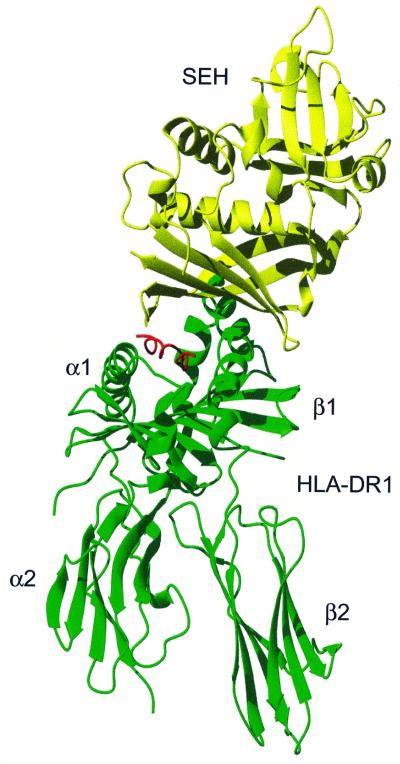
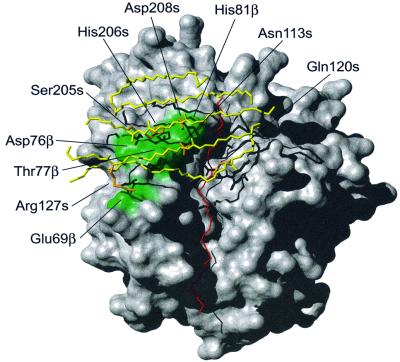
The complex consists of SEH (∼25 kDa) and the extracellular domains of HLA-DR1 (∼44 kDa) containing an α-chain (∼21 kDa), a β-chain (∼22 kDa) and the haemagglutinin (HA)-peptide (PKYVKQNTLKLAT). The complex has an extended overall structure, with the SEH molecule binding on top of the HLA-DR1 molecule (Figure 2). SEH binds with its concave β-sheet (β6, β7, β12) to the β1 helix in HLA-DR1 and to the presented antigen peptide. The SEH molecule covers almost half of the peptide-binding groove of HLA-DR1 (Figure 3). The total accessible area in the complex was 26391 Å2 calculated using GRASP (Nicholls et al., 1991), with a contacting region of 1461 Å2 between SEH and HLA-DR1.
Fig. 2. Ribbon representation of the HLA-DR1–SEH complex with HLA-DR1 (green), HA-peptide (red) and SEH (yellow).
Fig. 3. Surface representation of HLA-DR1 (grey) with the residues involved in hydrogen bonding to SEH marked in green. The β-sheets on SEH (β6, β7, β9, β10 and β12) that are interacting with HLA-DR1 are drawn in yellow and the antigen peptide is in red. Side chains on SEH forming hydrogen bonds are coloured orange.
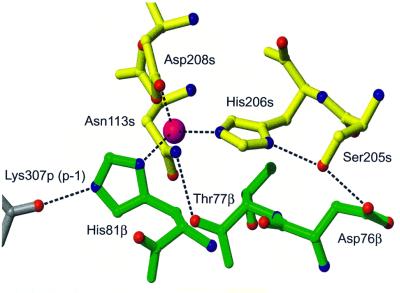
Zinc stabilizes the interaction by cross-linking HLA-DR1 and SEH through His81β on the β-chain of HLA-DR1 (d = 2.1 Å) and His206s (d = 2.1 Å) and Asp208s (d = 2.0 Å), both on the β12-strand on SEH (residues belonging to the β-chain of HLA-DR1 have ‘β’ as a suffix, residues belonging to the α chain of HLA-DR1 have ‘α’ as a suffix, residues belonging to SEH have ‘s’ as a suffix, and residues belonging to MHC-bound peptide have ‘p’ as a suffix). The zinc ion has three protein ligands, with a fourth possibly being a water molecule. However, no electron density for the water molecule is seen in the final 2.6 Å 2Fo – Fc map due to low occupancy. There are two hydrogen bonds in this area that connect the HLA-DR1 and SEH, Asp76β–Ser205s and Thr77β–Asn113s (Table II, Figure 4). Trp115s contributes to the stabilization of the interaction by making van der Waal’s contacts (defined as <4.0 Å) with His81β. Moreover, the orientation of the solvent-exposed His81β side chain is stabilized by a strong hydrogen bond to the main chain oxygen of Lys307p (p-1, 2.7 Å), shown already in the HLA-DR1–peptide structure (Stern et al., 1994). Superantigens have exploited this property of HLA-DR1 by using the His81β side chain as a perfect target for high-affinity zinc coordination. His206s is also stabilized by a hydrogen bond to the side chain of Ser205s (2.9 Å), which in addition forms the hydrogen bond to Asp76β as mentioned above. Another hydrogen bond is formed between Asp208s and Asn113s, which also interacts with Thr77β, described above. Thus this network of hydrogen bonds contributes significantly to stabilizing the zinc-interacting area of SEH (Figure 4).
Table II. Hydrogen bonds between HLA-DR1/peptide and SEH.
| SEH | Distance (Å) | |||
|---|---|---|---|---|
| HLA-DR1 | ||||
| E69 | OE2 | R127 | NH1 | 2.7 |
| D76 | OD2 | S205 | OG | 2.7 |
| T77 | O | N113 | ND2 | 2.7 |
| HA-peptide | ||||
| K307 | NZ | N210 | OD1 | 2.6 |
| K310 | N | Q120 | OE1 | 2.9 |
| K310 | O | Q120 | NE2 | 3.1 |
Fig. 4. The coordination and hydrogen bond pattern of selected residues in the region around the zinc ion (magenta) and the HA-peptide (grey)–HLA-DR1 (green)–SEH (yellow) complex. An additional hydrogen bond between Asp208s, OD1 and Asn113s, ND2 (2.7 Å) is observed but omitted in the picture for clarity.
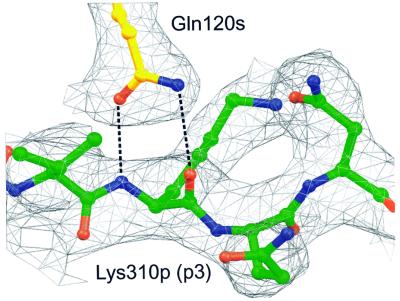
A large part of the SEH-interacting surface on HLA-DR1 is formed by the peptide. The C-terminal part of the β6 strand and the N-terminal part of the β7 strand, as well as the loop in between them on SEH (Trp115s–Gln120s) make van der Waal’s contacts to the N-terminal part (p-1→p3) of the peptide. Gln120s hydrogen-bonds via its side chain oxygen and nitrogen atoms to the backbone nitrogen and oxygen of Lys310p (p3), respectively (Figure 5). The side chain of Lys307p (p-1) extends out from the peptide-binding groove and its side chain nitrogen forms a hydrogen bond to the side chain of Asn210s. The parts of the β6 and β7 strands that do not interact with the peptide instead form several van der Waal’s contacts with the β1 helix of HLA-DR1. In this area, one hydrogen bond is observed between Arg127s and Glu69β.
Fig. 5. Electron density from the final 2.6 Å 2Fo – Fc map of the HA-peptide–SEH interaction, contoured at 1σ, with the HA-peptide (green) and SEH (yellow). Gln120s forms a bidentate hydrogen bond with Lys310p (p3).
Van der Waal’s contacts are also seen between the α-chain of HLA-DR1 and SEH. Gln57α and Glu55α both interact with Ile119s.
The structure of the HLA-DR1–HA-peptide complex is very similar to the structure published by Stern et al. (1994; 1DLH). Minor structural changes are seen close to the binding site of SEH as well as some changes in the residues exposed on the surface. Some important changes are seen for Glu55α and Gln57α of HLA-DR1 where the side chains have changed position to be able to make van der Waal’s contacts with SEH. A loop area in the C-terminal domain of the β2-chain of HLA-DR1 (Ser104β–His111β) is not observed in this complex structure, possibly due to disordering of the protein. The position of His81β that binds to the zinc ion together with Asp208s and His206s is hardly affected at all upon complex formation. In addition, the structure of the peptide presented by HLA-DR1 is not significantly affected upon binding to SEH. The SEH molecule also maintains a structure similar to the earlier published uncomplexed SEH structure (Håkansson et al., 2000, 1ENF). However, some minor structural changes are seen in the MHC-binding area of SEH and in the areas exposed to the solvent. In the uncomplexed crystal structure of SEH, Trp115s has two conformations, which is in contrast to the complex where the tryptophan residue adopts only one conformation stabilized by a van der Waal’s contact with His81β.
Discussion
The zinc family of superantigens
In this study, the crystal structure of SEH in complex with HLA-DR1 was determined to 2.6 Å resolution. SEH binds on top of HLA-DR1 through a zinc ion and also to the peptide presented by HLA-DR1. A common role for metal ions such as zinc is to increase the affinity of protein– protein interactions (Fraústo da Silva and Williams, 1994). The interaction between SEH and HLA-DR1 has been suggested previously to be zinc dependent (Nilsson et al., 1999). Alanine substitution of Asp208s, Asp167s and Asp203s decreased the binding to HLA-DR1. The structure of the complex shows that Asp208s is a zinc ligand. Asp167s and Asp203s possibly contribute to the interaction by increasing the negatively charged area and in that way increase the affinity for zinc (Ruessell and Fersht, 1987). Sequence alignment performed with CLUSTAL_W (Thompson et al., 1994) shows that all superantigens belonging to the zinc family share the zinc-binding motif with SEH, including His206s and Asp208s (Figure 6). In the zinc-binding area, Asn113s and Ser205s also contribute to the binding through hydrogen bonds to the β-chain of HLA-DR1. Asn113s is conserved in all superantigens belonging to the zinc family, and the interaction of this aspargine residue with HLA-DR is probably also conserved, as has been shown by this study and by the SPE-C–HLA-DR2a complex structure (Li et al., 2001, 1HQR), as well as in alanine substitution studies for SEA (Abrahmsén et al., 1995). In addition, both complex structures show that this aspargine residue contributes to the stability of the zinc site by making a hydrogen bond to the zinc-ligating aspartate residue (Figure 4; Li et al., 2001). Moreover, the zinc ligand, His81β, is widely conserved between different MHC class II molecules in the otherwise polymorphic β-chain.
Fig. 6. Sequence alignment of the zinc family of superantigens and SEB. Residues involved in the HLA-DR1–SEH interaction are marked in yellow. Numbering is according to SEH. The N-terminal parts of the sequences are not shown.
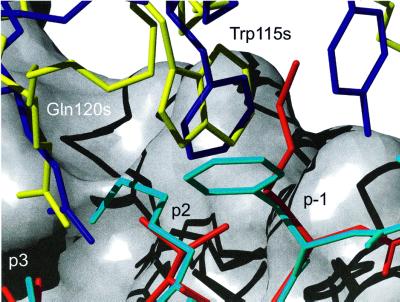
SEB does not belong to this zinc-binding family of superantigens according to the sequence alignment (Figure 6). The crystal structure of the SEB–HLA-DR1 complex has been determined previously (Jardetzky et al., 1994) and shows a completely different interaction between the superantigen and HLA-DR1 compared with the SEH–HLA-DR1 structure. SEB interacts with the α-chain of HLA-DR1 in a zinc-independent way and no interaction with the peptide is seen. The contact region for SEB–HLA-DR1 (924 Å2) is almost half of that observed for the SEH–HLA-DR1 complex. In accordance, the affinity between SEB and HLA-DR1 is low (Kd = 14 µM; Leder et al., 1998) compared with the high affinity between SEH and HLA-DR1 (Bmax1/2 ∼0.5 nM; Nilsson et al., 1999). The structure presented here demonstrates a site of interaction with the β-chain of HLA-DR1, which is different from the SEB site that is situated on its α-chain. Furthermore, the residues in SEB which are involved in the HLA-DR1 interaction are not present in SEH. Four out of nine of the superantigens belonging to the zinc family (SEA, SED, SEE and SEJ) have sequence similarities with SEB in its HLA-DR1-binding area. SEA was shown to bind HLA-DR1 through two binding sites, one zinc dependent and one SEB-like (Abrahamsén et al., 1995). The fact that SEA bears two binding sites for HLA-DR1 demonstrates the ability of SEA to cross-link different HLA-DR1 molecules (Tiedemann et al., 1996). In addition, the presence of two binding sites in SEA increases the affinity between the superantigen and HLA-DR1. However, in the case of SEH where only one binding site for HLA-DR1 is seen, the binding to the single site is even stronger than for the two sites together in SEA (Abrahmsén et al., 1995; Nilsson et al., 1999). The strong binding is due possibly to the interaction over the zinc ion and the hydrogen bonds around the zinc site, and to the peptide. In addition, the SEH-unique hydrogen bond from Arg127s to Glu69β possibly contributes to the higher affinity between SEH and HLA-DR1 compared with other superantigen–HLA-DR1 complexes. This strong interaction between SEH and HLA-DR1 was confirmed biochemically using gel filtration, where a stable complex was detected. Ser205s is another unique residue for SEH (except for SPE-C) and might therefore also contribute to the strong interaction with HLA-DR1 (Figure 4). The serine residue is positioned in a loop region between the β11 and β12 strands of SEH. According to structural alignment between SEA (Schad et al., 1995; 1ESF) and SEH, this region is also a loop area in SEA. The serine residue adopts very similar structures in SEH and SPE-C, as observed in the structure of the SPE-C–HLA-DR2a complex (Li et al., 2001). Sequence alignment indicates that all the streptococcal superantigens of the zinc family have only a single HLA-DR1-binding site, the zinc-dependent site (Proft et al., 1999). The similarity between SEH and SPE-C in their binding mode to HLA-DR molecules suggests that all streptococcal superantigens belonging to the zinc family bind like SEH and SPE-C to HLA-DR. Trp115s is conserved in eight out of nine superantigens in the zinc family according to sequence alignment (Figure 6). The probable function is to make van der Waal’s contacts with His81β, as is seen in SEH–HLA-DR1, and in that way to stabilize the zinc site. In SPE-C, a phenylalanine residue stabilizes the interaction in a similar way (Figure 7; Li et al., 2001).
Fig. 7. Comparison between the HLA-DR2a–MBP peptide–SPE-C complex (Li et al., 2001, 1HQR) and the HLA-DR1–HA-peptide–SEH complex. The superposition was made by fitting the α-chains of the HLA-DRs to each other. The MBP peptide is marked in cyan, the HA-peptide in red, SEH in yellow and SPE-C in blue. The β-chain of HLA-DR1 is represented as a surface (grey).
Superantigens interacting with the peptide
SEH makes several interactions with the HA-peptide presented by HLA-DR1. In particular, Gln120s hydrogen-bonds to the backbone of Lys310p (p3). Six out of nine superantigens belonging to the zinc family (SEA, SEE, SED, SEH, SPE-H and SMEZ-2) have a glutamine residue in this position, suggesting that most of the superantigens in this family interact with the peptide and possibly have a SEH-like binding interface with HLA-DR1. SPE-C has a glutamine residue in this position in the structural alignment (Figure 7), in contrast to the sequential alignment (Figure 6), and similar hydrogen bonds between the glutamine residue and the peptide observed in the SPE-C–HLA-DR2a complex (Li et al., 2001).
Three different human HLA-DR–peptide complexes have been structurally aligned with the SEH–HLA-DR1 structure (Dessen et al., 1997, 2SEB; Murthy and Stern, 1997, 1AQD; Li et al., 2000, 1FV1). The superposition shows that residues in position p3 of the peptides vary but the direction of the side chain is similar. The SEH molecule does not overlap with any of the side chains of the different peptides, although it is in very close contact with the lysine in the p2 position of the myelin basic protein (MBP) peptide. However, the structure of SPE-C in complex with HLA-DR2a–MBP peptide shows that the side chain of this lysine radically changes its position (6.5 Å; Li et al., 2001) when the superantigen is present and therefore it does not prevent the superantigen from binding to the peptide. Thus, the zinc family of superantigens seems to be able to bind many different peptides in a similar way. This is supported by the fact that the SPE-C–HLA-DR2a structure and the SEH–HLA-DR1 structure have similar interactions with the peptide although completely different peptides are present (Figure 7). This leads to the conclusion that although superantigen binding to MHC class II involves the interaction with the antigen peptide, the superantigen binding is not peptide specific since the major interaction is with the backbone of the peptide. However, the different peptides may modulate the affinity between the superantigen and MHC class II. Furthermore, the presence of a peptide in the peptide-binding groove is of importance for binding, which has been shown for some superantigens belonging to the zinc family (Dowd et al., 1995; Wen et al., 1996). In contrast to the zinc family of superantigens, a peptide-specific T-cell response has been suggested for TSST-1 in the complex structure between TSST-1 and HLA-DR1 (Kim et al., 1994).
No TCR–HLA-DR1 interaction in the predicted ternary structure
SEH binds on top of HLA-DR1 and forms an extended structure. A superposition of the crystal structure of the HLA-DR1–TCR complex (Hennecke et al., 2000; 1FYT) on the HLA-DR1–SEH crystal structure strongly suggests that an interaction between HLA-DR1 and TCR is not possible since SEH takes the position of TCR. SEH interacts with residues in the peptide presented by HLA-DR1 that TCR interacts with in the TCR–HLA-DR1 crystal structure (Hennecke et al., 2000). A model where SEB binds outside the peptide-binding groove interacting both with HLA-DR1 and with the TCR has been proposed (Li et al., 1998). This model shows that there is an interaction between the TCR and HLA-DR1, which was supported by substitution studies on HLA-DR1 (Labrecque et al., 1994). However, the proposed TCR-interacting area on HLA-DR1, in the presence of SEB, is the area where SEH interacts with HLA-DR1 in the SEH–HLA-DR1 complex structure. This clearly shows that TCR cannot interact with HLA-DR1 in the HLA-DR1–SEH–TCR complex in the same way as it does in the predicted HLA-DR1–SEB–TCR complex. The lack of interaction between TCR and HLA-DR1 in the HLA-DR1–SEH–TCR complex possibly is compensated by the strong interaction between SEH and HLA-DR1 compared with other superantigens. This superantigen–MHC class II structure suggests a new mode of ternary complex formation.
Materials and methods
Cloning, protein overexpression and purification
SEH was cloned and expressed in E.coli as described by Nilsson et al. (1999). The cultivation was carried out in a bioreactor (Forsberg et al., 1997; Håkansson et al., 2000) and the protein was purified using anion-exchange chromatography (Håkansson et al., 2000). The SEH protein was >95% pure according to Coomassie-stained SDS–PAGE and isoelectric focusing. The yield was 860 mg/l growth medium.
The HLA-DR1 α-chain (DR*0101) and β-chain (DRB1*0101) genes were reconstructed for T7 polymerase-directed expression of their extracellular domains in E.coli (Frayser et al., 1999). For protein overexpression, plasmids encoding the DRα and DRβ polypeptides were transformed separately into E.coli BL21(DE3)pLys and BL21(DE3) cells, respectively. Individual colonies were grown in 1 l of growth medium (Frayser et al., 1999), with the exception of not adding 35 µg/ml chloramphenicol to the DRβ cultivation. Expression was induced by addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at an OD600 nm of 0.6–0.8. The cells were harvested after 3 h by centrifugation (6000 g, 30 min) and lysed. The inclusion bodies were isolated by a differential centrifugation–detergent wash procedure (Frayser et al., 1999). The subunits were purified separately under denaturing and reducing conditions using anion-exchange chromatography on an XK26 column (350 ml), Q Sepharose-HP (Pharmacia Biotech), according to Frayser et al. (1999). The yield was 60 mg/l growth medium for the α-subunit and 12 mg/l growth medium for the β-subunit. Both subunits were 90% pure according to Gelcode-stained SDS–PAGE. Purified subunits were diluted dropwise with constant stirring to a final concentration of 0.08 µM into a standard folding solution: 20 mM Tris–HCl, 30% (w/v) glycerol, 0.5 mM EDTA, 3 mM reduced glutathione, 0.3 mM oxidized glutathione pH 8.5. Folding mixtures were kept for 3 days at 4°C in the presence of 1 µM HA-peptide (PKYVKQNTLKLAT), from influenza haemagglutinin residues 306 to 318 (the same peptide as in the crystal structure of the HLA-DR1–peptide complex, 1DLH; Stern et al., 1994). DEAE-Sephadex A-50 was added to the folding mixtures at 2 g of dry resin/l, swollen overnight at 4°C, collected in a glass funnel and transferred to an XK 50 column (Pharmacia Biotech). The column was washed with 10 column volumes of 20 mM Tris–HCl pH 8.0, and the protein was eluted with 0.5 M NaCl, 20 mM Tris–HCl pH 8.0. The eluate was exchanged into 20 mM Tris–HCl pH 8.0, using a Variperm L (ECV,10 ml; ICV, 2 ml) (YMC Europe GMBH). The folded heterodimer was then purified using anion-exchange chromatography on a 6 ml Resource Q column (Amersham Pharmacia Biotech) in 20 mM Tris–HCl, with a linear gradient over 10 column volumes of 0–400 mM NaCl, and the buffer of the eluate was changed to TBS (25 mM Tris, 150 mM NaCl pH 7.4). After refolding, buffer exchange and concentration of the heterodimer using evaporation, the final yield was 15%. Functional interaction of HLA-DR1 and SEH was detected by gel filtration using a Superdex 200 column (7.5 × 300 mm, Amersham Pharmacia Biotech). HLA-DR1 (2.5 µM) was mixed with 2.5 µM SEH in the presence of 100 µM ZnCl2 and left overnight at 4°C. The protein solution was applied to the gel filtration column using a running buffer of TBS, 100 µM ZnCl2 at 0.025 ml/min, 18°C. The excitation was at 280 nm and the fluorescence was detected by emission at 350 nm. The same procedure was repeated using EDTA instead of ZnCl2 to show the dependence of the complex formation on zinc.
Crystallization and data collection
Concentrated HLA-DR1–peptide trimer (4.3 mg/ml) was mixed with highly concentrated SEH to a final concentration of 5 mg/ml protein (ratio 1:1) and 1 mM ZnCl2. Crystals were grown by vapour diffusion by mixing 1.5 µl of protein solution with 1.5 µl of well solution containing 0.05 mM mono-potassium dihydrogen phosphate, 20% (w/v) polyethylene glycol (PEG) 8000, pH 5.1 at 18°C. The crystals were formed in space group C2, with a = 123.62 Å; b = 122.84 Å; c = 48.51 Å; β = 100.13, and contained one complex in the asymmetric unit. The crystals were flash-frozen in 0.05 mM mono-potassium dihydrogen phosphate, 30% (w/v) PEG 8000, 10% (w/v) PEG 400 and 10% (w/v) sucrose.
The native data set was collected at beamline I711, at the MAX-laboratory in Lund, Sweden (Cerenius et al., 2000), using a Mar345 imaging plate detector system. The data sets were evaluated in the XDS program packages (Kabsch, 1993) and scaled using the CCP4 suite of programs (CCP4, 1994).
Structure determination and refinement
The structure of the SEH–HLA-DR1 complex was solved by a molecular replacement technique using AMoRe (Navaza, 1994) with the SEH (Håkansson et al., 2000, 1ENF) and the HLA-DR1–HA-peptide (Stern et al., 1994, 1DLH) structures. The model was built in O (Jones et al., 1991) and the refinements were made using CNS (Brünger et al., 1998). The final model includes residues A3–A182 in the α-chain, B3–B103 and B112–B190 in the β-chain, 13 residues in the peptide and residues D2–D213 in SEH. The final R-factor (Rfree) is 0.202 (0.258) and good geometry of the model indicates a well-determined structure. Details of the structural data are given in Table I. All molecule illustrations were produced using MOLMOL (Koradi et al., 1996). The coordinates of the SEH–HLA-DR1 complex have been deposited in the Protein Data Bank with the accession No. 1HXY.
Acknowledgments
Acknowledgements
We would like to express our gratitude for the gift of the HLA-DR1 plasmid from the laboratory of Professor Lawrence Stern and thank Mia Frayser for helping us with expression issues of HLA-DR1. We would also like to thank Dr Peter Seijer-Andersen for helpful discussions, and Dr Annelie Sjöberg, Dr Helén Carlsson-Nyhlén, Ulla Larsson-Lorek and Ulf Niss at Active Biotech Research AB for assistance with the expression and purification of SEH and HLA-DR1. This project was partly funded by the Swedish Natural Sciences Research Council (NFR).
References
- Abrahmsén L., Dohlsten,M., Segren,S., Björk,P., Jonsson,E. and Kalland,T. (1995) Characterisation of two distinct MHC class II binding sites in the superantigen staphylococcal enterotoxin A. EMBO J., 14, 2978–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Daccak R. et al. (1998) Staphylococcal enterotoxin D is a promiscuous superantigen offering multiple modes of interactions with the MHC class II receptors. J. Immunol., 160, 225–232. [PubMed] [Google Scholar]
- Arcus V.L., Proft,T., Sigrill,J.A., Baker,H.M., Fraser,J.D. and Baker,E.N. (2000) Conservation and variation in superantigen structure and activity highlighted by the three-dimensional structure of two new superantigens from Streptococcus pyogenes. J. Mol. Biol., 299, 157–168. [DOI] [PubMed] [Google Scholar]
- Brünger A.T. et al. (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta. Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- Cerenius Y., Ståhl,K., Svensson,L.A., Ursby,T., Oskarsson,A., Albertsson,J. and Liljas,A. (2000) The crystallography beamline I711 at MAX II. J. Synchotron Radiat., 7, 203–208. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project No. 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–763. [DOI] [PubMed] [Google Scholar]
- Dessen A., Lawrence,C.M., Cupo,S., Zaller,D.M. and Wiley,D.C. (1997) X-ray structure of HLA-DR4 (DRA*0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity, 7, 473–481. [DOI] [PubMed] [Google Scholar]
- Dowd J.E., Jenkins,R.N. and Karp,D.R. (1995) Inhibition of antigen-specific T cell activation by staphylococcal enterotoxins. J. Immunol., 154, 1024–1031. [PubMed] [Google Scholar]
- Forsberg G., Forsgren,M., Jaki,M., Norin,M., Sterky,C., Enhörning,Å., Larsson,K., Ericsson,M. and Björk,P. (1997) Identification of framework residues in a secreted recombinant antibody fragment that control production level and localisation in Escherichia coli. J. Biol. Chem., 272, 12430–12436. [DOI] [PubMed] [Google Scholar]
- Fraser J.D., Urban,R.G., Strominger,J.L. and Robinson,H. (1992) Zinc regulates the function of two superantigens. Proc. Natl Acad. Sci. USA, 89, 5507–5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraústo da Silva J.J.R. and Williams,R.J.P. (1994) The Biological Chemistry of the Elements. Clarendon Press, Oxford, UK.
- Frayser M., Sato,A.K., Xu,L. and Stern,L.L. (1999) Empty and peptide-loaded class II major histocompatibility complex proteins produced by expression in Escherichia coli and folding in vitro.Protein Expr. Purif., 15, 105–114. [DOI] [PubMed] [Google Scholar]
- Håkansson M., Petersson,K., Nilsson,H., Forsberg,G., Björk,P., Antonsson,P. and Svensson,L.A. (2000) The crystal structure of staphylococcal enterotoxin H: implication for binding properties to MHC class II and TcR molecules. J. Mol. Biol., 302, 527–537. [DOI] [PubMed] [Google Scholar]
- Hamad A.R.A., Marrack,P. and Kappler,J.W. (1997) Transcytosis of staphylococcal superantigen toxin. J. Exp. Med., 185, 1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke J., Carfi,A. and Wiley,D.C. (2000) Structure of covalentely stabilized complex of a human αβ T-cell receptor, influenza HA peptide and MHC class II molecule, HLA-DR1. EMBO J., 19, 5611–5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T.S., Brown,J.H., Gorga,J.C., Stern,L.J., Urban,R.G., Chi,Y., Stauffacher,C., Strominger,J.L. and Wiley,D.C. (1994) Three-dimensional structure of human class II histocompatibility molecule complexed with superantigen. Nature, 368, 711–718. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Zou, J-Y., Cowtan,S.W. and Kjeldegaard,M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- Kabsch W. (1993) Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr., 26, 795–800. [Google Scholar]
- Kim J., Urban,R.G., Strominger,J.L. and Wiley,D.C. (1994) Toxic shock syndrome toxin-1 complexed with class II major histocompatibility molecule HLA-DR1. Science, 266, 1870–1874. [DOI] [PubMed] [Google Scholar]
- Koradi R., Billeter,M. and Wüthrich,K. (1996) MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graphics, 14, 51–55. [DOI] [PubMed] [Google Scholar]
- Labrecque N., Thibodeau,J., Mourad,W. and Sékaly,R.P. (1994) T cell receptor–major histocompatibility complex class II interaction is required for the T cell response to bacterial superantigens. J. Exp. Med., 180, 1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder L. et al. (1998) A muatational analysis of the binding of staphylococcal enterotoxin B and C3 to the T cell receptor β chain and major histocompatibility complex class II. J. Exp. Med., 187, 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Llera,A., Tsuchiya,D., Leder,L., Ysern,X., Schlievert,P.M., Karjalainen,K. and Mariuzza,R.A. (1998) Three-dimensional structure of the complex between a T cell receptor β chain and the superantigen staphylococcal enterotoxin B. Immunity, 9, 807–816. [DOI] [PubMed] [Google Scholar]
- Li Y., Martin,H. and Mariuzza,A.R. (2000). Structural basis for the binding of an immunodominant peptide from myelin basic protein in different registers by two HLa-DR2 alleles. J. Mol. Biol., 304, 177–188. [DOI] [PubMed] [Google Scholar]
- Li Y., Li,H., Dimasi,N., McCormick,J.K., Martin,R., Schuck,P., Schlievert,P.M. and Mariuzza,R.A. (2001) Crystal structure of a superantigen bound to the high-affinity, zinc-dependent site on MHV class II. Immunity, 14, 93–104. [DOI] [PubMed] [Google Scholar]
- Marrack P. and Kappler,J. (1990) The staphylococcal enterotoxins and their relatives. Science, 248, 705–711. [DOI] [PubMed] [Google Scholar]
- Munson S.H., Tremaine,M.T., Betley,M.J. and Welch,R.A. (1998) Identification and characterisation of staphylococcal enterotoxin types G and I from Staphylococcus aureus.Infect. Immun., 66, 3337–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V.L. and Stern,L.J. (1997) The class II MHC protein HLA-DR1 in complex with an endogenous peptide: implications for the structural basis of the specificity of peptide binding. Structure, 5, 1385–1396. [DOI] [PubMed] [Google Scholar]
- Navaza J.(1994) AMoRe: an automated package for molecular replacement. Acta Crystallogr. A, 50, 157–163. [Google Scholar]
- Nicholls A., Sharp,K. and Honi,B. (1991) Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins, 11, 281–296. [DOI] [PubMed] [Google Scholar]
- Nilsson H., Björk,P., Dohlsten,M. and Antonsson,P. (1999) Staphylo coccal enterotoxin H displays unique MHC class II-binding properties. J. Immunol., 163, 6686–6693. [PubMed] [Google Scholar]
- Papageorgiou C.A. and Acharya K.R. (2000) Microbial superantigens: from structure to function. Trends Microbiol., 8, 369–375. [DOI] [PubMed] [Google Scholar]
- Proft T., Moffatt,S.L., Berkhan,C.J. and Fraser,J.D. (1999) Identification and charcterisation of novel superantigens from Streptococcus pyogenes. J. Exp. Med., 189, 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel A. anderson,B.F., Baker,H.M., Fraser,J.D. and Baker,E.N. (1997) Crystal structure of streptococcal superantigen SPE-C: dimerization and zinc binding suggest a novel mode of interaction with MHC class II molecules. Nature Struct. Biol., 4, 635–642. [DOI] [PubMed] [Google Scholar]
- Ruessell J.L. and Fersht,A.R. (1987) Rational modification of enzyme catalysis by engineering surface charge. Nature 328, 496–500. [DOI] [PubMed] [Google Scholar]
- Schad E.M., Zaitseva,I., Zaitsev,V.N., Dohlsten,M., Kalland,T., Schlievert,P.M., Ohlendorf,D.H. and Svensson,L.A. (1995) Crystal structure of the superantigen staphylococcal enterotoxin type A. EMBO J., 14, 3292–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern L.J., Brown,J.H., Jardetzky,T.S., Gorga,J.C., Urban,R.G., Strominger,J.L. and Wiley,D.C. (1994) Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature, 368, 215–221. [DOI] [PubMed] [Google Scholar]
- Tiedemann R.E. and Fraser,J.D. (1996) Cross-linking of MHC class II molecules by staphylococcal enterotoxin A is essential for antigen-presenting cell and T cell activation. J. Immunol., 157, 3958–3966. [PubMed] [Google Scholar]
- Thompson J.D., Higgins,D.G. and Gibson,T.J. (1994) CLUSTAL_W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen R., Cole,G.A., Surman,S., Blackman,M.A. and Woodland,D.L. (1996) Major histocompatibility complex class II-associated peptides control the presentation of bacterial superantigens to T cells. J. Exp. Med., 183, 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Iandolo,J.J. and Stewart,G.C. (1998) The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej). FEMS Microbiol. Lett., 168, 227–233. [DOI] [PubMed] [Google Scholar]