Abstract
The cornea of human subjects and of anaesthetised cats was stimulated with a jet of air of controlled flow, temperature and CO2 concentration delivered by a gas aesthesiometer.
In humans, the intensity and magnitude of various components of the sensory experience (intensity of the sensation, degree of irritation, magnitude of burning and stinging pain, magnitude of the cold and warm components of the sensation) were measured using separate visual analog scales. In anaesthetised cats, the impulse response to the same stimuli was recorded from single mechanosensory, polymodal and cold-sensitive corneal fibres in the ciliary nerves.
Intensity-response curves for mechanical stimulation showed that all parameters of the sensation experienced by humans increased with the intensity of the stimulus. Mechanical stimuli recruited mainly phasic mechanosensory and polymodal afferents in the cat.
Acidic stimulation with gas mixtures of increasing CO2 concentration evoked irritation, burning and to a lesser extent stinging pain of a magnitude roughly proportional to the intensity of the stimulus in humans. CO2 primarily recruited polymodal afferents and weakly excited cold-sensitive fibres in the cat's cornea.
Heat stimuli evoked in humans a sensation profile similar to CO2 but accompanied by a warmth component. In the cat's cornea, heat excited only polymodal fibres and silenced cold-sensitive corneal units.
Cold stimuli applied to the human cornea elicited a sensation of cooling that became irritant at the lowest temperatures. Corneal cold-sensitive fibres of the cat were activated in a manner proportional to the temperature drop, while polymodal nociceptor fibres were recruited only by the lowest temperatures. Topical menthol (0.2 mm) applied to humans evoked and later eliminated cold sensations produced by cold stimuli while the irritation sensation caused by low temperature stimuli still persisted.
Human subjects were able to identify masked mechanical, thermal and chemical stimuli applied to the cornea.
Irritation and cold sensations can therefore be evoked separately from the cornea by selective activation of mechanosensory, polymodal and cold corneal sensory afferents. Stimulation with different forms of energy usually leads to combined activation and/or inhibition of the different populations of sensory afferent fibres, evoking blended sensations that include irritation and thermal components in a variable degree.
Peripheral nociceptive neurones are primarily characterised by their response to one or several forms of energy at intensities near or within the range of tissue injury (Sherrington, 1906; Bessou & Perl, 1969). In the skin and deep tissues of mammals including man, various functional subclasses of nociceptive neurone have been categorised, based upon the form of noxious energy (mechanical, chemical, thermal) by which they are preferentially activated (see Campbell & Meyer, 1996). Microneurography and microstimulation in humans have provided convincing evidence that excitation by noxious stimuli of the peripheral axons of some types of primary nociceptive neurones innervating the skin is accompanied by pain sensations (Torebjörk et al. 1984; Ochoa & Torebjörk, 1989; Handwerker & Kobal, 1993). However, it is unclear whether a qualitatively different experience is evoked by selective excitation of each one of the various subpopulations of primary nociceptive neurones.
The contribution to pain of primary sensory neurones activated by innocuous mechanical and thermal stimulation is also still a matter of controversy. It is generally accepted that in the skin mechanical stimulation of neurones preferentially responding to low intensity mechanical forces does not elicit pain except when there is abnormal processing of peripheral information by the central nervous system (CNS) (Torebjörk et al. 1992). Likewise, warming of the skin becomes painful only when the population of polymodal nociceptive neurones is recruited (Van Hees & Gybels, 1981; Campbell & LaMotte, 1983; Adriaensen et al. 1984).
The origin of pain produced by cold is less certain. Selective activation of cutaneous cold receptors by moderate temperature reductions is the basis of thermal sensations of cooling (Hensel & Zotterman, 1951; Hensel & Boman, 1960). More intense temperature drops apparently excite a subpopulation of polymodal nociceptors located in vascular and perivascular tissues that may be responsible for the sensation of burning pain produced by intense cutaneous cooling (Kenshalo & Duclaux, 1977; LaMotte & Thalhammer, 1982; Yarnitski & Ochoa, 1990; Arndt & Klement, 1991; Campero et al. 1996). In contrast, it has been reported that cooling the cornea of the eye, which is innervated by cold-sensitive and polymodal sensory neurones (Belmonte et al. 1991; Gallar et al. 1993), always elicits a sensation of irritation devoid of a thermal component (Kenshalo, 1960; Beuerman & Tanelian, 1979).
The cornea is an avascular tissue of very simple structure, innervated almost exclusively by thin myelinated and unmyelinated trigeminal ganglion neurones that belong to the classes of high-threshold mechanosensory, polymodal and cold primary sensory neurones. A particular advantage is that corneal nerve terminals are easily accessible to non-noxious and noxious stimuli of different types (Belmonte & Gallar, 1996; Belmonte et al. 1997). Therefore the cornea is an amenable model for the analysis of the quality of sensations evoked by activation of specific subclasses of primary nociceptive neurones by their appropriate stimuli.
In the present work, we applied to the cornea of human subjects selective mechanical, thermal and chemical (acidic) stimuli and analysed the characteristics of the evoked sensation. The functional types of corneal nerve fibre recruited by these stimuli were also studied in anaesthetised cats. Preliminary results have been reported in abstract form (Acosta et al. 1997).
METHODS
Aesthesiometer
A gas aesthesiometer described in a previous publication (Belmonte et al. 1999) was used to apply selective mechanical, chemical and thermal stimulation of the cornea. Air jets of 3 s duration were applied to the corneal surface, separated by 2 min pauses. Mechanical stimulation consisted of a series of nine pulses of air at flows varying from 0 to 264 ml min−1. For chemical stimulation, the change in corneal pH produced by CO2 applied to the corneal surface was used (Chen et al. 1995). Nine pulses of a mixture of air and CO2 at different concentrations (0–80 % CO2) were employed. Thermal stimulation was performed by applying to the cornea 10 pulses of air at different temperatures in the nozzle of the probe (−10 to +90 °C), which caused the corneal surface temperature to vary from −5 to +3 °C around the basal value (Fig. 1A), measured by infrared thermography as previously described (Belmonte et al. 1999). Corneal thermography confirmed that stimulation was restricted to the cornea, with the area of detectable temperature change (> 0.5 °C) ranging between 1.7 and 92.2 mm2(n = 71; 11–60 % of the total surface of the cornea in humans; Pepose & Ubels, 1992). During selective chemical and thermal stimulation, flows 6.25 ml min−1 below the mechanical threshold of the subject were used. During mechanical and chemical stimulation, the air was heated at the tip of the probe to the temperature required by each value of flow to prevent changes in corneal basal temperature (Belmonte et al. 1999). Room temperature and humidity ranged between 23 and 25 °C and 50 and 58 %, respectively.
Figure 1. Temperature change at the corneal surface during thermal stimulation.
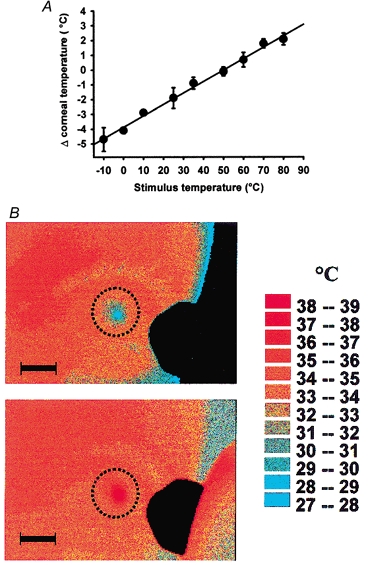
A, corneal surface temperature changes during stimulation with 3 s air jets of different temperatures (−10 to +80 °C), applied at flows below 150 ml min−1, n = 6–7. The 50 °C point is the average of 32 determinations performed at variable flows (0–267 ml min−1). The regression curve (y = 0.08x − 3.9; r2=0.994) was used to estimate the corneal temperature change evoked by the stimulus in the experiments in which no simultaneous thermography was performed. B, example of thermography temperature profiles in the ocular surface at the end of a stimulation with an air jet of 3 s duration, at a subthreshold flow (39 ml min−1). Stimulus temperature at the tip of the probe was −10 °C in the upper and +80 °C in the lower picture. Scale bars, 10 mm. Dotted lines show the limits of the cornea.
Psychophysical experiments
Sixteen human subjects (8 male and 8 female) aged 19–27 years (mean, 22.6 ± 0.1 years) participated in the study. The research followed the tenets of the Declaration of Helsinki. The subjects gave written informed consent to a protocol approved by our Institute's ethics committee and were free to terminate the session at any time. They received financial compensation for their participation in the study. None of them had a history of corneal or ocular pathology; three wore eyeglasses (less than −2 dioptres) to correct myopia.
Stimulation procedure
The subject was seated comfortably in front of a slit-lamp table, with the head supported by a head holder. The probe was advanced towards the eye with the slit-lamp table commands, and the tip placed perpendicular to the centre of the cornea, at a distance of 5 mm from the corneal surface. The subject was asked to blink immediately before application the stimulus. He/she identified the onset of the stimulus by the click produced by the opening of the valve in the probe.
At the beginning of each experimental series, the mechanical threshold was determined using the method of levels (Yarnitsky & Ochoa, 1990). Then, the series of stimuli of each modality at random intensities was applied with a 2 min interval between stimuli. Subjects ignored the modality of stimulation that was applied. In each subject, both eyes were studied in a 2 day protocol. On day 1, mechanical and heat stimuli were sequentially applied first in one eye then in the other. On day 2, CO2 and cold stimulation were performed. Four subjects, on two different days, were additionally treated with a drop of a viscoelastic eye solution (Hylashield, 0.1 % Hylan-A) or Hylashield with 0.2 mm menthol (kindly provided by Biomatrix Inc., Ridgefield, NJ, USA) applied in the conjunctival sac prior to cold stimulation. This viscoelastic solution was used to ensure a longer presence of menthol on the corneal surface.
Immediately after each stimulating pulse, the subject marked the magnitude of the various components of the sensation in separate 10 cm, horizontal visual analog scales (VAS) without marks. In preliminary experiments five different subjects were asked to define in their own words the qualities of the sensation felt after stimulation of the cornea with the gas aesthesiometer. ‘Diffuse sensation of burning’, ‘localised, punctate stinging sensation’, ‘refreshing or cooling’ and ‘non-painful warming’ were the four descriptors most often chosen by the subjects to describe the sensations experienced. Based on this information, six parameters were selected and tested on separate VAS scales: (1) intensity of the sensation; (2) degree of irritation produced by the stimulus; (3) magnitude of the burning component of the sensation; (4) magnitude of the stinging component of the sensation; (5) magnitude of innocuous warming; (6) magnitude of innocuous cooling. In the VAS, 0 was assigned to ‘no sensation’ and 10 to ‘maximal sensation’. Prior to the experiment, the parameters to be rated were explained to the subjects.
The intensity threshold for the chemical and thermal stimuli was taken as the lowest value that evoked a response ≥ 0.5 in the VAS.
Stimulus identification experiments
For each subject, mechanical, chemical, heat and cold stimuli of a magnitude rated in the previous experiments with the same subjective intensity value were applied on a separate day and their modality revealed to the subject. Subsequently, each stimulus was randomly applied twice to each eye and the subject was invited immediately after each application to try to identify its modality. Results were presented as a percentage of positive identifications.
Electrophysiological experiments
Experiments were performed in 16 adult cats of either sex anaesthetised with sodium pentobarbitone (Nembutal, 40 mg kg−1, i.p.). Animals were maintained under anaesthesia by i.v. infusion of dilute Nembutal (2.5 mg kg−1 h−1) throughout the experiment. Rectal temperature, end-tidal CO2 concentration and blood pressure were continuously monitored and maintained within physiological limits. At the end of the experiment, animals were killed with an overdose of anaesthetic. The experiments were carried out in accordance with the guidelines laid down by the ARVO statement for the use of animals in ophthalmic research and the Universidad Miguel Hernández Ethical Committee.
Single-unit corneal afferent activity was recorded in thin nerve filaments, dissected from the ciliary nerves at the back of the eye, using Ag–AgCl electrodes and conventional electrophysiological equipment, as described elsewhere (Belmonte et al. 1991).
Mechanical threshold was measured using a calibrated von Frey-type aesthesiometer (Cochet-Bonnet aesthesiometer) provided with a 0.12 mm diameter nylon filament of adjustable length (force, 0.11–1.96 mN. The receptive field was subsequently mapped using a suprathreshold force value (0.16–0.25 mN above threshold). Responsiveness to cold was explored applying a drop of saline at 4 °C onto the corneal surface. The receptive fields of cold-sensitive units were then located and mapped with the tip of an ice-cooled metal bar (0.5 mm diameter). The chemical sensitivity of the mechanically identified units was assayed by applying a 30 s pulse of gas containing 98.5 % CO2 (at 80 ml min−1 flow) to the receptive field. The conduction velocity of each unit was determined from the latency of the nerve impulse evoked by a 0.1–0.5 ms, 0.5–3 mA, suprathreshold electric shock, applied to the receptive area with a pair of silver electrodes, and the conduction distance, measured with an 8.0 gauge thread placed along the trajectory of the nerve. When the characterisation of the unit was complete, the probe of the gas aesthesiometer was placed 5 mm away from the receptive field on the cat's cornea. The same stimulation protocol used in the experiments with humans was applied. The impulse response of the unit was recorded continuously until completion of the experimental protocol. Fibre threshold to the stimulus was calculated as the minimum stimulus intensity required to evoke consistently at least one nerve impulse.
A total of 105 corneal units (85 with latencies corresponding to the Aδ range and 20 C-units) were studied. Thirty-five responded to touching of the corneal surface with the Cochet-Bonnet aesthesiometer and were insensitive to a 30 s CO2 pulse and to cold saline, and so were classified as mechanosensory units. Sixty-two fibres were classified as polymodal units because in addition to displaying mechanical sensitivity they also produced a train of impulses during the application of CO2. These fibres were also activated by heat. Eight fibres were initially identified as cold units based on their prominent ongoing activity in the absence of intended stimulation that increased markedly after application of a drop of cold saline to the cornea. In a separate group of four cold-sensitive and seven polymodal units, the responses to cold and chemical stimulation were tested before and after treatment with a 50 μl drop of 0.2–0.6 mm menthol applied to the unit's receptive field.
Neural discharges and traces of the stimulating pulses were recorded on FM tape for later off-line computer analysis (CED 1401plus and Spike 2 program, Cambridge Electronic Design Ltd, Cambridge, UK). The frequency of spontaneous discharges and changes in mean firing frequency (impulses s−1 (imp s−1)) evoked by application of the various stimuli were measured.
Analysis of data
Data are expressed as means ±s.e.m. Pearson's correlation was used to determine association between variables. Linear regression and the r2 coefficient were applied to see how well the regression model described the data. Differences between groups were compared with parametric or non-parametric tests for paired or unpaired data, two-way repeated measures (RM) ANOVA and Tukey's test. Significance was set at P < 0.05.
RESULTS
General
Stimulation with pulses of air at a nozzle temperature of 50 °C did not modify the basal corneal temperature (34.4 ± 0.1 °C, n = 94 measurements performed in both eyes of 3 subjects), except when air flows over 264 ml min−1 were attained. Air at temperatures below or above this neutral value (from −10 to +80 °C at the nozzle of the probe), applied to the centre of the cornea, produced a central point of highest temperature change (range, −7.5 to +3.4 °C) surrounded by almost concentric areas of progressively smaller temperature variation that were restricted to the cornea (Fig. 1B).
Mechanical stimulation
The minimum flow of air at neutral temperature required to evoke a sensation was 79 ± 5 ml min−1 (median, 83 ml min−1; range, 33–208 ml min−1; n = 32, both eyes of 16 subjects). Threshold flow varied greatly between individuals, but for the same individual similar values were measured in the two eyes and on different days (data not shown). The sensation evoked by threshold flow was always defined as slightly unpleasant.
As shown in Fig. 2A, subjects reported an increasing intensity of the sensation with increases in the strength of the stimulus, the correlation between the two parameters being significant (Pearson's correlation coefficient, 0.987; P < 0.001). The same was also true for the parameters ‘irritation’, ‘stinging’ and ‘burning’, which increased in proportion to the magnitude of the stimulus and reached similar maximal values (Fig. 2B–D).
Figure 2. Relationship between the amplitude of mechanical stimulation and VAS values in human subjects.
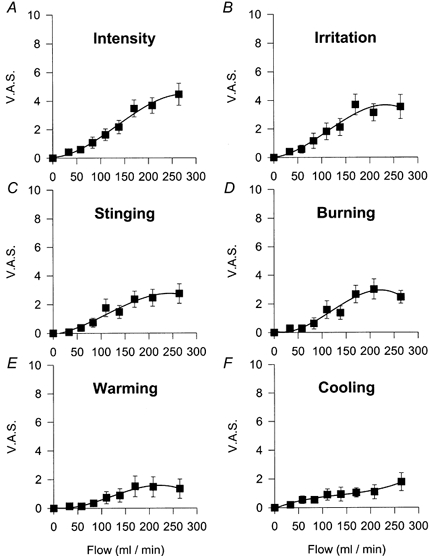
A, intensity. B, irritation. C, stinging. D, burning. E, warming. F, cooling. Data are means ±s.e.m., n = 16 subjects.
Subjects assigned low VAS values to the thermal components of the sensation evoked by mechanical stimuli (Fig. 2E–F). Moreover, after a given stimulus, some individuals assigned the same VAS value to warmth and cold, suggesting that temperature sensations associated with mechanical stimulation were not clearly defined.
When mechanical stimuli were applied to the cornea of the cat, they evoked in 35 out of 58 polymodal units a sustained, irregular discharge of impulses that usually outlasted the duration of the stimulating pulse and occurred at a mean threshold of 79 ± 9 ml min−1, n = 35. Eight out of 35 mechanosensory units responded with one to three impulses with no relation to the intensity of the stimulus. In seven cold-sensitive fibres a modest increase in the ongoing frequency of discharge was elicited with air pulses over 62 ± 8 ml min−1 that was not related to flow values.
Stimulation with CO2
The mean CO2 concentration necessary to evoke a sensation in 16 subjects was 21 ± 3 % (median, 10 %; range, 10–50 %; n = 32). The threshold sensation evoked by CO2 was defined by subjects as irritating. The intensity of the sensation experienced increased with the magnitude of the stimulus (Pearson's correlation coefficient, 0.940; P < 0.001; Fig. 3A). The same was true for the curve of irritation (Fig. 3B). The sensation of burning pain was more prominent than that of stinging pain (Fig. 3C and D), while the warming component attributed to the sensation was slightly greater than the cooling component, although both were of low magnitude (Fig. 3E and F).
Figure 3. Relationship between the magnitude of chemical stimulation and VAS values in human subjects.
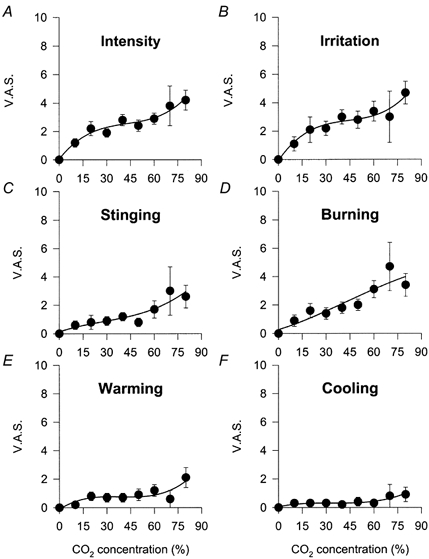
A, intensity. B, irritation. C, stinging. D, burning. E, warming. F, cooling. Data are means ±s.e.m., n = 16 subjects.
In the cat cornea, about half of the polymodal units (28 out of 54) produced within the first second an impulse discharge in response to CO2 pulses of increasing CO2 concentration. The discharge usually outlasted the duration of the pulse. Mean CO2 threshold concentration was 34 ± 3 % CO2 with a maximal mean firing frequency obtained at 52 ± 3 % CO2. Mechanosensory fibres fired only occasionally during the application of CO2 pulses, without any correspondence with CO2 concentration. Moderate increases of ongoing firing frequency were obtained in four out of five cold units during the application of CO2 pulses, with a threshold CO2 concentration of 21 ± 4 %.
Stimulation with hot air
Heat threshold had a mean value of +1.2 ± 0.2 °C above the basal corneal temperature (range, +1 to +2.5 °C; n = 18). The threshold sensation evoked by heat was defined as irritating. The magnitude of irritation was parallelled by the subjective assessment of intensity, reaching similar maximal values (Fig. 4A and B). Stimulation with heat also evoked a sensation of burning and, to a lesser degree, stinging pain as was the case for chemical stimulation (Fig. 4C and D). Likewise, the thermal attribute of the sensation was predominantly warmth (Fig. 4E and F).
Figure 4. Relationship between the magnitude of heat stimulation and VAS values in human subjects.

A, intensity. B, irritation. C, stinging. D, burning. E, warming. F, cooling. VAS data (means ±s.e.m., n = 16 subjects) are plotted against the change in corneal temperature.
Heat pulses applied to the cornea of the cat evoked a tonic impulse discharge in one-third of polymodal units (12 out of 35). No consistent impulse response to heat was observed in mechanosensory fibres. In three cold-sensitive fibres the ongoing activity decreased or stopped with heat stimulation.
Stimulation with cold air
Cooling threshold was established at −2.4 ± 0.4 °C below the corneal surface temperature (range, −4.75 to −1 °C; n = 12). The threshold sensation evoked by cold was defined as cooling and in some cases as slightly unpleasant. With pulses of air of decreasing temperature, a dissociation between the curves for subjective intensity and irritation was observed (Fig. 5A and B). Only the lowest tested temperature evoked a sensation of irritation in all the studied subjects. At the same levels, a moderate stinging pain sensation was also reported with almost no burning component (Fig. 5C and D). With cold stimulation, a warming sensation was never signalled (Fig. 5E), whereas the cooling sensation was prominent, being the dominant quality evoked by cold stimulation, with a magnitude proportional to the intensity of the stimulus (Fig. 5F).
Figure 5. Relationship between the magnitude of cold stimulation and VAS values in human subjects.
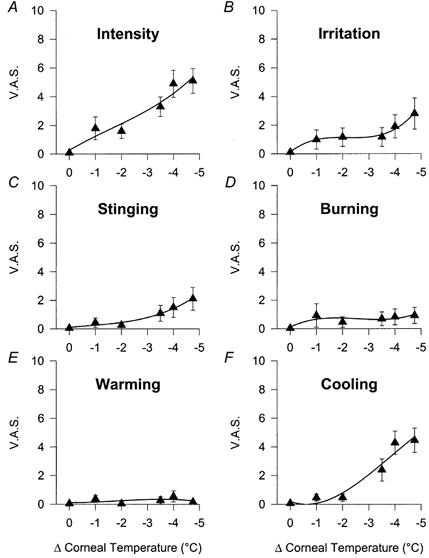
A, intensity. B, irritation. C, stinging. D, burning. E, warming. F, cooling. Data are means ±s.e.m., n = 16 subjects.
A quarter of corneal polymodal units in the cat (6 out of 26) produced a low frequency discharge during the application of the lowest temperature cold stimuli (corresponding to 29 °C in the human cornea). In contrast, cold did not evoke a consistent response in mechanosensory units.
All cold-sensitive units responded to a decrease of the corneal surface temperature of −1 °C, with the maximal response at a mean temperature change of −4.75 °C. The response to cold pulses was composed of a brief, high frequency impulse discharge followed by sustained firing at lower frequency for the duration of the pulse and a post-stimulus silence lasting several seconds.
Effect of menthol
Menthol applied topically to the eye produced an immediate sensation of cooling and mild irritation followed in some cases by a feeling of light discomfort sometimes with a burning component. Cold stimulation performed 15–30 min after menthol application showed that in comparison with the curves obtained in the same eyes treated with the control viscoelastic solution, the intensity curve flattened and the extreme cold stimuli also produced a mild but significant sensation of irritation (Fig. 6A and B). Warm sensations were not reported, but the most striking observation was that no cold sensations were elicited by cooling of the cornea in the subjects pretreated with topical menthol (Fig. 6E and F). The control viscoelastic solution itself, which reduces corneal evaporation (Acosta et al. 1999), also had a mild attenuating effect on the response to cold stimuli (compare Fig. 5 and Fig. 6).
Figure 6. Effect of 0.2 mm menthol on the VAS response to cold stimulation.
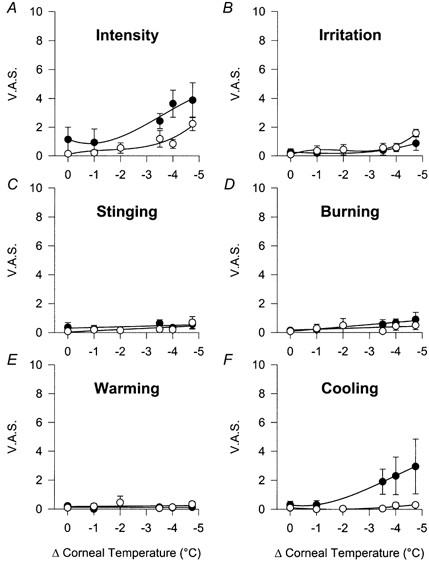
A, intensity. B, irritation. C, stinging. D, burning. E, warming. F, cooling. The subject's cornea was treated with Hylashield alone (•) and Hylashield plus 0.2 mm menthol (^) prior to stimulation. Data are means ±s.e.m., n = 4 subjects. In menthol-treated subjects, the value of irritation at -4.75 °C below the basal corneal temperature was significantly different from the value at this basal corneal temperature (34.4 °C; P < 0.05, paired t test).
In single-fibre recordings in the cat, four cold units increased their firing frequency immediately after application of menthol, with a discharge that lasted for 20–75 s. Five minutes after application of menthol, the response to cooling was reduced to 29 ± 18 % of the control value (P = 0.033, paired t test, data not shown). No changes in the thermal or chemical responsiveness of polymodal units (n = 7) were observed after menthol.
Profiles of the sensations evoked by the different stimuli
Figure 7 represents the VAS values for irritation, stinging and burning pain, warming and cooling components evoked by the different stimulus modalities. The data selected for each stimulus modality are those corresponding to a mechanical stimulus of air flow at 208 ml min−1, a chemical stimulus of 80 % CO2, a heat stimulus of +2.5 °C and a cold stimulus of −4 °C. These stimuli were selected because they evoked a mean VAS value of subjective intensity of 4 VAS units in the previously described experiments in which stimulus-response curves were obtained.
Figure 7. Comparison between components of the sensation evoked by different modalities of stimulation.
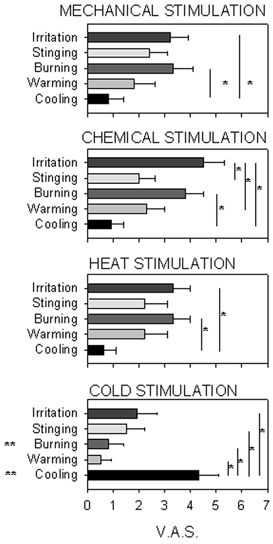
Data are means ± s.e.m., n = 9 subjects. Values of the different components of sensation evoked by selective mechanical, chemical, heat and cold stimuli with the same mean subjective intensity (4 VAS units, see text) were compared. *P < 0.05, significant difference between the magnitudes of the sensation components signalled by the end of the vertical lines inside each modality of stimulation. **P < 0.05 significant difference between the magnitudes of the sensation component comparing all the modalities of stimulation (P < 0.05, Tukey's test after two-way RM ANOVA).
Mechanical, chemical and heat stimuli had a low cooling component and high irritation and burning pain components. In contrast, intense cold stimuli had a prominent cooling component significantly greater than the sensation components associated with irritation and pain. Moreover, cross-comparison between data obtained with the different stimulus modalities showed that with cold stimulation, values for cooling were significantly higher and for the burning pain component significantly lower than in the case of the other modalities (P < 0.05, Tukey's test).
Identification of stimulus modality
Table 1 gives the percentage of correct identifications made by nine subjects to whom mechanical, chemical, heat and cold stimuli were randomly presented twice for each eye. Chemical and cold stimuli were accurately identified in more than 70 % of the cases. The incidence of correct identification was slightly lower for heat stimuli. Mechanical stimuli were confounded with other types of stimuli in about half of the trials.
Table 1.
Percentage of correct identification of the stimulus modality applied to the human cornea
| Stimulus reported | |||||
|---|---|---|---|---|---|
| Stimulus applied | Mechanial | Chemical | Heat | Cold | No stimulus or undefined |
| Mechanial | 47±7% | 17±7% | 17±8% | 19±9% | 0% |
| Chemical | 8±4% | 75±7% | 17±7% | 0% | 0% |
| Heat | 3±3% | 31±9% | 64±9% | 0% | 3±3% |
| Cold | 11±7% | 17±7% | 0% | 72±10% | 0% |
| Sham | 0% | 6±6% | 6±6% | 0% | 88±7% |
Data are means ± s.e.m. of the percentage of correct identifications determined in 9 subjects.
Correlation between sensation parameters and single-fibre activity
Table 2 compares the stimulus intensities necessary to excite primary corneal sensory units in the cat (firing threshold) with those required for stimulus detection in humans and the proportion of each type of corneal sensory afferent that was recruited by the different stimulus modalities applied with the gas aesthesiometer. The sensation threshold for mechanical stimulation in humans was similar to that required to activate polymodal units in the cat, whereas the threshold value of corneal mechanosensory fibres was higher. In the case of chemical stimulation, the sensation threshold in humans was below the CO2 concentration necessary to recruit polymodal nociceptive units and near that required to activate cold units with CO2 in the animal experiments. For heat stimulation, the sensation threshold in humans was close to the heat threshold of polymodal afferents in the cat. The sensation threshold for cold stimulation in humans had an intermediate value between that of cold-sensitive and polymodal fibres. Irritation evoked by cold stimulation appeared at temperature values required to excite polymodal fibres with cold.
Table 2.
Sensation threshold in human and firing threshold of corneal units in the cat
| Human | Cat | |||
|---|---|---|---|---|
| Stimulus applied | Sensation threshold | Mechanosensory | Polymodal units | Cold-sensitive units |
| Mechanical (ml min−1) | 79±5 | 109±24(23%) | 79±9(60%) | 62±8(25%) |
| Chemical (%CO2) | 21±3 | (3%)† | 34±3(54%) | 21±4(80%) |
| Heat (°C) | +1.2±0.2 | (4%)† | +2±0(36%) | (−60%)* |
| Cold (°C) | −2.4±0.4 | (4%)† | −2.7±0.8(9%) | −1±0(100%) |
Data are means ± s.e.m. Percentage of corneal sensory units recruited by each type of stimulation is also shown in parentheses. Data correspond to units that changed their basal firing frequency by more than 30% in response to at least one-third of the pulses of each modality.
For values below 5% thresholds were not calculated.
Percentage of units that reduced their firing discharge during application of heat stimuli.
VAS values for the intensity and irritation components of the sensation evoked by increasing mechanical stimulation correlated well with the discharge of polymodal nociceptors (P = 0.00715 and 0.0178, respectively; Pearson's correlation; Fig. 8A) but were not correlated with the impulse activity of mechanosensory or cold-sensory units (data not shown). Although the firing frequency of polymodal nociceptive fibres in the cat was augmented with pulses of CO2 of increasing concentration, no significant correlation was found between the impulse response in polymodal fibres and VAS scores evoked in humans by similar stimuli. The same was also true for the firing frequency evoked by CO2 and heat in mechanosensory and cold-sensory units and the human psychophysical responses evoked by these stimuli. In contrast, for cold stimuli the correlation between intensity and cooling VAS values with cold-sensory fibre impulse activity was significant (P = 0.049 and 0.045, respectively; Fig. 8B). A positive correlation was also found between the VAS values of the irritation component in humans and the gross discharge of all types of corneal afferent fibre evoked by cold stimulation (P = 0.002).
Figure 8. Correlation between VAS ratings in humans and impulse response of corneal units in cats.
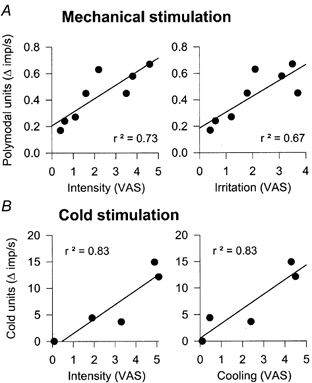
A, mechanical stimulation. B, cold stimulation. Data were obtained using an identical stimulation protocol for the psychophysical and the electrophysiological experiments. Pearson's correlation and linear regression (continuous lines) were statistically significant.
DISCUSSION
This study shows that two main sensations can be evoked by mechanical, thermal or chemical stimulation of the cornea: one of ‘irritation’ that was obtained by application of mechanical force, acid, heat and extreme cold, and one of ‘cooling’ that was elicited by application of moderate cold. Thus, the data suggest that in the human cornea, as occurs in the skin (Wolf & Hardy, 1941; Chery-Croze, 1983; Chen et al. 1996), separate peripheral and central afferent channels exist for the perception of pain and innocuous cold.
Characteristics of the stimulus and sensation ratings
The gas aesthesiometer used in this study (Belmonte et al. 1999) was suitable for restricting stimulation to only one modality and to the limits of the corneal tissue. Only mechanical stimulation with high flows of air produced additional cooling of the corneal surface. Thermography also showed that stimuli were restricted to the cornea. Therefore, sensations evoked by the various stimulus modalities in humans and neural discharges in corneal fibres of cats can be safely ascribed to the independent effects of mechanical, chemical and thermal stimuli on corneal nerve endings.
The subjective parameters selected to determine the magnitude, affective quality and thermal attributes of the evoked sensation in humans were based on the descriptors most often chosen by the subjects in previous corneal stimulation experiments (Lele & Weddell, 1956; Kenshalo, 1960; Beuerman et al. 1977; Belmonte et al. 1999). Information about intensity and irritation was collected separately in an effort to discriminate between the capacity to detect the magnitude of the stimulus and the degree of unpleasantness elicited by that stimulation (Price, 1988). It is difficult to determine to what extent the subjects confused intensity with irritation, or the sensation of warmth with that of burning pain, but the fact that often these parameters were seen changing independently of each other suggests that they reflect separate sensory experiences.
Mechanical stimulation
The magnitude of mechanical stimulation with increasing gas flows was accurately reflected in the subjective stimulus-intensity curve. There was a complete overlap between intensity and irritation curves for low and moderate flow values. This suggests that no innocuous sensations can be evoked by mechanical stimulation of the cornea even with near-threshold forces, excluding the possibility that touch is a separate modality from pain in the human cornea as previously suggested (Lele & Weddell, 1956; Draeger, 1984). Pure mechanosensory units responded to increasing gas flows with only one or two spikes irrespective of the magnitude of the stimulus, suggesting that they mainly signal the onset of high intensity stimulation. Therefore, encoding of mechanical stimulus intensity was presumably produced by activation of polymodal units, which exhibited a somewhat higher capability of translating the stimulus force into firing frequency changes. Nevertheless, the limited encoding by polymodal fibres of the magnitude of the stimulus observed in our electrophysiological experiments suggests that progressive recruitment of units is an important mechanism for intensity perception in the cornea as occurs in other tissues (Torebjörk & Hallin, 1974, 1976; Van Hees & Gybels, 1981; see Price, 1988). This is not surprising considering the high density of nerve endings and the partial overlapping of receptive fields of polymodal neurones in the cornea (Belmonte & Giraldez, 1981; Beuerman & Kupke, 1982; Brock et al. 1998; De Felipe et al. 1999). The ongoing discharge of cold-sensory fibres was slightly altered by all levels of mechanical stimuli with the gas jet, but particularly when high flows were applied. This may explain the separation of intensity and irritation curves for the highest flow values, which was coincidental with the occurrence of a distinct sensation of cooling, additionally suggesting that the firing of cold-sensory fibres modified corneal pain sensation. Changes in the quality of sensations evoked by interactions between mechanical and thermal sensory inputs have been reported in other somatosensory territories (Bini et al. 1984; Craig et al. 1996). Co-activation of cold fibres by air pulses could also be the reason for the confusion between mechanical and cold stimuli sometimes observed in the stimulus identification experiments.
Chemical stimulation
Our data in the cat confirm that CO2 application is an appropriate chemical stimulus for polymodal nociceptors, possibly through the production of a local pH drop (Belmonte et al. 1991; Steen et al. 1992; Gallar et al. 1993; Chen et al. 1995). Increasing concentrations of CO2 augmented the subjective magnitude of the sensation and the degree of irritation experienced by the subject, with a complete overlap between the curves of intensity and irritation, suggesting that irritation is the sole sensation evoked by activation of corneal polymodal nociceptors. Correlation between the firing frequency of polymodal fibres in the cat and subjective values of the various parameters of the sensation in humans was poor. This may indicate again that progressive recruitment of polymodal fibres by higher CO2 concentrations was more important for the encoding of the intensity of the stimulus than increasing the firing of individual units. Nevertheless, it is also possible that short CO2 pulses that stimulate corneal fibres in the human cornea were insufficient to evoke a similar response in the cat, which has a mechanical threshold, measured with the Cochet-Bonnet aesthesiometer, higher than that in humans (Chan-Ling, 1989). Also, the possibility cannot be excluded that mechano-insensitive nociceptors (Schaible & Schmidt, 1985; Handwerker et al. 1991; Meyer et al. 1991; Brock et al. 1998; Weidner et al. 1999), which were not detected with our fibre sampling procedure, contributed to the encoding of CO2 stimuli.
Subjects described the sensation evoked by CO2 in terms of both stinging and burning pain. Activation of Aδ and C polymodal nociceptors by electrical or natural stimuli applied to the skin of the hand during microneurographic studies in humans (Ochoa & Torebjörk, 1989) produced pricking and burning pain, respectively. The cornea possesses both Aδ and C polymodal nociceptors and it could be speculated that like in the skin, Aδ mechanosensory and polymodal fibres mediate sensations of stinging pain while C fibres evoke predominantly burning pain sensations. Chemical stimulation weakly excited cold-sensory fibres (Gallar et al. 1993). Accordingly, a weak conscious cold sensation was elicited at the highest CO2 concentration value.
Thermal stimulation
Stimulation with hot air at flows below mechanical threshold produced a distinct sensation of irritation that overlapped fully with the intensity curve and had stinging and burning pain components. These noxious sensations are attributable to the excitation of polymodal nociceptors (Bessou & Perl, 1969; Croze et al. 1976), which in the cornea belong to the Aδ and C fibre type and are activated by temperatures over 37–39 °C (Belmonte & Giraldez, 1981; Gallar et al. 1993) as well as by stimulation with CO2. The main difference in terms of fibre recruitment between CO2 and heat stimuli is that heating completely silenced cold-sensory fibres. This may explain why a feeling of warmth accompanied this stimulus modality, and was interpreted as being different from chemical stimulation in the experiments of stimulus identification.
Sensations evoked by cold stimuli had a well-defined profile and were clearly distinguishable from those elicited by other stimulus modalities. While changes in stimulus intensity were well detected and correlated closely with the magnitude of the cooling component attributed to the stimulus, irritation was reported only with the lowest temperatures and was of moderate value. Lele & Weddell (1956) described in human subjects defined thermal sensations evoked by stimulation of the cornea with cold air. These results were challenged by Kenshalo (1960), who compared the sensation evoked by the cooled bulb of a thermometer applied to the human cornea, conjunctiva, forehead skin and lip and concluded that in the cornea, in contrast to the other tissues, cold evoked a specific quality of irritation, without a defined thermal component. Likewise, Beuerman and colleagues (Beuerman et al. 1977; Beuerman & Tanelian, 1979) presented data showing that only irritation was evoked by a jet of saline directed at the human cornea at temperatures between 28 and 30 °C. This led to a general acceptance that irritation is the sole sensation that can be evoked from the cornea with any form of stimulating energy.
In our study, in which the sensation evoked by cooling was divided into various subcomponents, it became evident that small corneal temperature decreases were detected and interpreted mainly as a thermal experience. Cold stimuli selectively activated cold-sensory fibres of the cat cornea, in a manner proportional to the temperature reduction. Sensory fibres responding to cold in the cornea present some differences to those of the episclera, such as larger receptive fields, more prominent dynamic responses to cooling and sensitivity to capsaicin (Gallar et al. 1993). Based on the psychophysical data of Kenshalo (1960) indicating the absence of temperature sensations arising from the cornea, it was tentatively concluded (Gallar et al. 1993; Belmonte & Gallar, 1996; Belmonte et al. 1997) that corneal cold-sensitive fibres are ‘cold nociceptors’ similar to those described in the tooth (Jiväsjärvi & Kniffki, 1987). The present results indicate that this is not the case and that corneal fibres responding to cold appear to behave as innocuous thermal receptors sensitive to temperature reductions, giving rise to thermal sensations of cooling in the human cornea.
At low temperature values (around 29 °C at the corneal surface), cold stimuli also recruited a reduced proportion (about 25 %) of corneal polymodal nociceptors. The possibility that noxious low temperatures of the skin are detected by a subpopulation of nociceptors has been proposed repeatedly (Torebjörk, 1974; Georgopoulos, 1976, 1977; LaMotte & Thalhammer, 1982; Saumet et al. 1985; Arndt & Klement, 1991; Klement & Arndt, 1992; Campero et al. 1996). The present data in the cornea favour this interpretation. The action of menthol on corneal sensitivity further supports the idea that low temperatures evoke a blended sensation resulting from the simultaneous activation of specific cold thermoreceptors and a fraction of polymodal nociceptors. Menthol induces a cold sensation in the skin and mucosae due to a rather selective initial activation of cold receptor fibres (Hensel & Zotterman, 1951) followed by an inhibition of the impulse discharge (Schäfer et al. 1986). Likewise, in the cat cornea, menthol produced a transient excitation of cold-sensory fibres followed by a prolonged reduction in their responsiveness to cooling. In agreement with these observations, topical application of menthol to human eyes evoked a transient sensation of ‘freshness’ often followed by a feeling of warmth and the disappearance of any thermal sensation upon stimulation of the cornea with cold air. These effects would explain the absence after menthol of thermal sensations evoked by cold air in the human cornea but the persistence of a mild irritation with the lowest temperature stimuli, which are expected to maximally recruit the fraction of polymodal fibres sensitive to cold. Furthermore, the frequent reporting of warm sensations in the eye following ocular instillation of menthol supports the hypothesis that an inhibition of cold-sensory fibres rather than an activation of ‘warm receptors’ (never identified in the cornea), is responsible for warm sensations arising from this tissue, as was suggested by the experiments involving stimulation with hot air.
Sensations evoked by combined activation of corneal fibres
The present experiments showed that stimuli that produced a selective activation of polymodal nociceptive fibres of the cornea, such as CO2 or heat, evoked a distinct sensation of irritation and pain. When mechanical force was applied pure mechanosensory fibres were also recruited and the quality of the unpleasant sensation changed, suggesting that the sensory input provided by this class of mechanosensory Aδ fibres does not simply add to the inflow supplied by polymodal Aδ fibres but elicits a pain quality that is distinct from that produced by polymodal fibres of either conduction velocity. Pure mechanosensory fibres may contribute to the evocation in the cornea of a sharp pain sensation as occurs in the skin following application of acute noxious stimuli (Head, 1920; Lewis & Pochin, 1937; Bishop & Landau, 1958; Price et al. 1977; Meyer & Campbell, 1981). Activation or inhibition of thermosensitive (cold) fibres concomitant with the discharge of nociceptive fibres changes the quality of the irritating sensation by the addition of a thermal component that can be distinguished as independent by the subject if appropriately explored. Finally, the demonstration that selective stimulation of corneal cold-sensitive fibres evokes a pure thermal sensation, resolves in favour of Weddell and co-workers (Weddell, 1955; Lele & Weddell, 1956) the controversy about the presence or not of sensory modalities other other than pain in the human cornea (Kenshalo, 1970).
This study provides direct evidence that the cornea is equipped with thermal and nociceptive sensory nerve endings functionally similar to those of the skin and external mucosae. Their separate activation evoked qualities of sensation interpreted either as innocuous thermal sensations or irritant sensations. The advantageous characteristics of this tissue for the application of separate stimulus modalities further allowed us to establish that selective stimulation of Aδ and C polymodal nociceptors produces a pain sensation that changes in quality when mechanosensory and/or thermal fibres are activated concomitantly. However, the ability to distinguish the thermal component of the stimulus was maintained, thus supporting the hypothesis that separate sensory channels exist for the transmission to the CNS of the modality and the spatial-temporal characteristics of peripheral non-noxious and noxious stimuli.
Acknowledgements
This work was supported by grants SAF99-0066-C02-01 and SAF99-0066-C02-02 from the CICYT, Spain. The authors wish to thank Dr Martin Schmelz for his assistance in the thermography measurements, Mr Alfonso Perez-Vegara for his technical assistance and Dr Adolfo Aracil for his collaboration in the initial experiments.
References
- Acosta MC, Gallar J, Belmonte C. Relationship between neural activity and sensations evoked by noxious stimulation of the cornea. Society for Neuroscience Abstracts. 1997;23:1257. [Google Scholar]
- Acosta MC, Gallar J, Belmonte C. The influence of eye solutions on blinking and ocular comfort at rest and during work at video display terminals. Experimental Eye Research. 1999;68:663–669. doi: 10.1006/exer.1998.0656. [DOI] [PubMed] [Google Scholar]
- Adriaensen H, Gybels J, Handwerker HO, Van Hees J. Suppression of C-fibre discharges upon repeated heat stimulation may explain characteristics of concomitant pain sensations. Brain Research. 1984;302:203–211. doi: 10.1016/0006-8993(84)90232-4. [DOI] [PubMed] [Google Scholar]
- Arndt JO, Klement W. Pain evoked by polymodal stimulation of hand veins in humans. Journal of Physiology. 1991;440:467–478. doi: 10.1113/jphysiol.1991.sp018719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Investigative Ophthalmology and Visual Science. 1999;40:513–519. [PubMed] [Google Scholar]
- Belmonte C, Gallar J. Corneal nociceptors. In: Belmonte C, Cervero F, editors. Neurobiology of Nociceptors. New York: Oxford University Press; 1996. pp. 146–183. [Google Scholar]
- Belmonte C, Gallar J, Pozo MA, Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat's cornea. Journal of Physiology. 1991;437:709–725. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Garcia-Hirschfeld J, Gallar J. Neurobiology of ocular pain. Progress in Retina and Eye Research. 1997;16:117–156. [Google Scholar]
- Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. Journal of Physiology. 1981;321:355–368. doi: 10.1113/jphysiol.1981.sp013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibres to noxious stimuli. Journal of Neurophysiology. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Beuerman RW, Kupke K. Neural regeneration following experimental wounds of the cornea in the rabbit. In: Hollyfield JG, editor. The Structure of the Eye. Amsterdam: Elsevier NH; 1982. pp. 319–330. [Google Scholar]
- Beuerman RW, Maurice DM, Tanelian DL. Thermal stimulation of the cornea. In: Anderson M, editor. Pain in the Trigeminal Region. Amsterdam: Elsevier NH; 1977. pp. 413–422. [Google Scholar]
- Beuerman RW, Tanelian DL. Corneal pain evoked by thermal stimulation. Pain. 1979;7:1–14. doi: 10.1016/0304-3959(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Bini G, Cruccu G, Hagbarth KE, Schady W, Torebjörk E. Analgesic effect of vibration and cooling on pain induced by intraneural electrical stimulation. Pain. 1984;18:239–248. doi: 10.1016/0304-3959(84)90819-4. [DOI] [PubMed] [Google Scholar]
- Bishop GH, Landau WM. Evidence for a double peripheral pathway for pain. Science. 1958;128:712–713. doi: 10.1126/science.128.3326.712. [DOI] [PubMed] [Google Scholar]
- Brock JA, McLachlan EM, Belmonte C. Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea. Journal of Physiology. 1998;521:211–217. doi: 10.1111/j.1469-7793.1998.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JN, LaMotte RH. Latency to detection of first pain. Brain Research. 1983;266:203–208. doi: 10.1016/0006-8993(83)90650-9. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Cutaneous nociceptors. In: Belmonte C, Cervero F, editors. Neurobiology of Nociceptors. New York: Oxford University Press; 1996. pp. 117–145. [Google Scholar]
- Campero M, Serra J, Ochoa JL. C-polymodal nociceptors activated by noxious low temperature in human skin. Journal of Physiology. 1996;497:565–572. doi: 10.1113/jphysiol.1996.sp021789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Ling T. Sensitivity and neural organization of the cat cornea. Investigative Ophthalmology and Visual Science. 1989;30:1075–1082. [PubMed] [Google Scholar]
- Chen CC, Rainville P, Bushnell MC. Noxious and innocuous cold discrimination in humans: evidence for separate afferent channels. Pain. 1996;68:33–43. doi: 10.1016/S0304-3959(96)03180-6. [DOI] [PubMed] [Google Scholar]
- Chen X, Gallar J, Pozo MA, Baeza M, Belmonte C. CO2 stimulation of the cornea: a comparison between human sensation and nerve activity in polymodal afferents of the cat. European Journal of Neuroscience. 1995;7:1154–1163. doi: 10.1111/j.1460-9568.1995.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Chery-Croze S. Relationship between noxious and cold stimuli and the magnitude of pain sensation in man. Pain. 1983;15:265–269. doi: 10.1016/0304-3959(83)90061-1. [DOI] [PubMed] [Google Scholar]
- Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- Croze SR, Duclaux R, Kenshalo DR. The thermal sensitivity of the polymodal nociceptors in the monkey. Journal of Physiology. 1976;263:539–569. doi: 10.1113/jphysiol.1976.sp011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe C, Gonzalez GG, Gallar J, Belmonte C. Quantification and immunocytochemical characteristics of trigeminal ganglion neurones projecting to the cornea: effect of corneal wounding. European Journal of Pain. 1999;3:31–39. doi: 10.1053/eujp.1998.0100. [DOI] [PubMed] [Google Scholar]
- Draeger J. Measurement and Clinical Importance. Vienna: Springer-Verlag; 1984. Corneal Sensitivity. [Google Scholar]
- Gallar J, Pozo MA, Tuckett RP, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat's cornea. Journal of Physiology. 1993;468:609–622. doi: 10.1113/jphysiol.1993.sp019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. Journal of Physiology. 1976;39:71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP. Stimulus-response relations in high-threshold mechanothermal fibres innervating primate glabrous skin. Brain Research. 1977;128:547–552. doi: 10.1016/0006-8993(77)90181-0. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Forster C, Kirchhoff C. Discharge patterns of human C-fibres induced by itching and burning stimuli. Journal of Neurophysiology. 1991;66:307–315. doi: 10.1152/jn.1991.66.1.307. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Kobal G. Psychophysiology of experimentally induced pain. Physiological Reviews. 1993;73:639–671. doi: 10.1152/physrev.1993.73.3.639. [DOI] [PubMed] [Google Scholar]
- Head H. Studies in Neurology. London: Oxford University Press; 1920. [Google Scholar]
- Hensel H, Boman KKA. Afferent impulses in cutaneous sensory nerves in human subjects. Journal of Neurophysiology. 1960;23:564–578. doi: 10.1152/jn.1960.23.5.564. [DOI] [PubMed] [Google Scholar]
- Hensel H, Zotterman Y. The response of the cold receptors to constant cooling. Acta Physiologica Scandinavica. 1951;22:96–113. doi: 10.1111/j.1748-1716.1951.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Jyväsjärvi E, Kniffki KD. Cold stimulation of teeth: a comparison between the responses of cat intradental A delta and C fibres and human sensation. Journal of Physiology. 1987;391:193–207. doi: 10.1113/jphysiol.1987.sp016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenshalo DR. Comparison of thermal sensitivity of the forehead, lip, conjunctiva and cornea. Journal of Applied Physiology. 1960;15:987–991. doi: 10.1152/jappl.1960.15.6.987. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR. Psychophysical studies of temperature sensitivity. Contributions to Sensory Physiology. 1970;4:19–74. doi: 10.1016/b978-0-12-151804-2.50008-5. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Duclaux R. Response characteristics of cutaneous cold receptors in the monkey. Journal of Neurophysiology. 1977;40:319–332. doi: 10.1152/jn.1977.40.2.319. [DOI] [PubMed] [Google Scholar]
- Klement W, Arndt JO. The role of nociceptors of cutaneous veins in the mediation of cold pain in man. Journal of Physiology. 1992;454:378–382. doi: 10.1113/jphysiol.1992.sp019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JN. Response properties of high-threshold cutaneous cold receptors in the primate. Brain Research. 1982;244:279–287. doi: 10.1016/0006-8993(82)90086-5. [DOI] [PubMed] [Google Scholar]
- Lele PP, Weddell G. The relationship between neurohistology and corneal sensibility. Brain. 1956;79:119–154. doi: 10.1093/brain/79.1.119. [DOI] [PubMed] [Google Scholar]
- Lewis T, Pochin EE. The double pain response of the human skin to a single stimulus. Clinical Science. 1937;3:67–79. [Google Scholar]
- Meyer RA, Campbell JN. Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science. 1981;213:1527–1529. doi: 10.1126/science.7280675. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Research. 1991;561:252–261. doi: 10.1016/0006-8993(91)91601-v. [DOI] [PubMed] [Google Scholar]
- Ochoa J, Torebjörk E. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. Journal of Physiology. 1989;415:583–599. doi: 10.1113/jphysiol.1989.sp017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepose JS, Ubels JL. The cornea. In: Hart ML Jr, editor. Adler's Physiology of the Eye. London: Mosby-Year Book, Inc.; 1992. pp. 29–71. [Google Scholar]
- Price DD. Psychological and Neural Mechanisms of Pain. New York: Raven Press; 1988. [DOI] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely R. Peripheral supression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- Saumet JL, Chery-Croze S, Duclaux R. Response of cat skin mechanothermal nociceptors to cold stimulation. Brain Research Bulletin. 1985;15:529–532. doi: 10.1016/0361-9230(85)90046-2. [DOI] [PubMed] [Google Scholar]
- Schäfer K, Braun HA, Isenberg C. Effect of menthol on cold receptor activity. Journal of General Physiology. 1986;88:757–776. doi: 10.1085/jgp.88.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. Journal of Neurophysiology. 1985;54:1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New York: Scribner; 1906. [Google Scholar]
- Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in the rat skin. Journal of Neuroscience. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjörk E. Afferent C units responding to mechanical thermal and chemical stimuli in human non-glabrous skin. Acta Physiologica Scandinavica. 1974;92:374–390. doi: 10.1111/j.1748-1716.1974.tb05755.x. [DOI] [PubMed] [Google Scholar]
- Torebjörk E, Hallin RG. Identification of afferent C units in intact human skin nerves. Brain Research. 1974;67:387–403. doi: 10.1016/0006-8993(74)90489-2. [DOI] [PubMed] [Google Scholar]
- Torebjörk E, Hallin RG. A new method for classification of C-unit activity in intact human skin nerves. In: Bonica JJ, Fessard A, editors. Advances in Pain Research and Therapy. vol. 1. New York: Raven Press; 1976. pp. 29–34. [Google Scholar]
- Torebjörk HE, LaMotte RH, Robinson CJ. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: simultaneous recordings in humans of sensory judgements of pain and evoked responses in nociceptors with C-fibres. Journal of Neurophysiology. 1984;51:325–339. doi: 10.1152/jn.1984.51.2.325. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in human. Journal of Physiology. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hees J, Gybels JM. C nociceptor activity in human nerve during painful and non painful skin stimulation. Journal of Neurology, Neurosurgery and Psychiatry. 1981;44:600–607. doi: 10.1136/jnnp.44.7.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weddell G. Somesthesis and the chemical senses. Annual Review of Psychology. 1955;6:119–136. doi: 10.1146/annurev.ps.06.020155.001003. [DOI] [PubMed] [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjörk HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. Journal of Neuroscience. 1999;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Hardy JD. Studies on pain: observations on pain due to local cooling and on factors involved in ‘cold pressor’ effect. Journal of Clinical Investigation. 1941;20:521–533. doi: 10.1172/JCI101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113:893–902. doi: 10.1093/brain/113.4.893. [DOI] [PubMed] [Google Scholar]


