Adventitious infections are among the most pervasive problems encountered in the use of laboratory mice and rats. Small laboratory rodents are susceptible to more than 50 viral, bacterial, parasitic, and fungal agents, which include well-known organisms such as mouse hepatitis virus and Mycoplasma pulmonis (Bhatt and others 1986; Compton and others 1993; Lindsey and others 1991; Percy and Barthold 1993) as well as emerging agents such as the recently discovered mouse and rat parvoviruses and Helicobacter species (Fox and Lee 1997; Jacoby and others 1996). Some of these agents incite overt disease, but most cause subclinical infections that can, nonetheless, significantly alter research results. They usually gain entry through infected animals or animal products such as serum, cells, or transplantable tumors. Expanding exchanges of animals and animal products among laboratories throughout the world have amplified the risks for introducing infection, especially among colonies that are not protected by stringent preventive measures. Risks from infection also are increasing due to the availability of genetically engineered mice, whose responses to infection and disease can be unpredictable. Hundreds of novel mouse strains have been developed, and their use will continue to grow as advances in biotechnology increase their value to research. This trend is adding significantly to the size of institutional rodent colonies and to the complexity of rodent health care. The prevention of deleterious infections among valuable, densely housed rodents, such as transgenic mice, requires timely testing, appropriate housing, rapid diagnosis, and control of worrisome outbreaks. These measures, and the use of animals with known microbial profiles, are sound insurance against tainted research.
Assessment Tools
There is wide agreement in the scientific community that advances in laboratory animal health have enhanced the reliability of animal-based research. However, attempts to assess the effectiveness of specific pathogen-free (SPF1) housing, husbandry, and health care have been sparse. The last broad survey on the microbial status of mouse and rat colonies in the United States, which focused on the prevalence of infections in commercial breeding colonies (Casebolt and others 1988), was published about 10 years ago. A 1993 survey of laboratory animal use from the National Center for Research Resources (NCRR 1997) described responses about health care staffing for animal resources but did not inquire about the health status of animal colonies. Because of the increasing reliance of biomedical science on genetically engineered rodents and the deleterious influence of infection on rodent-based research, we recently surveyed major research centers throughout the United States for the presence of detrimental infectious agents in mouse and rat colonies. A summary of the results was published in July 1997 (Jacoby and Lindsey 1997). This article provides a more complete synopsis of survey results, which indicate that risks from infection remain high despite improvements in rodent health care.
The current survey queried animal resource directors at institutions comprising the top 102 recipients of National Institutes of Health (NIH1) funds in 1996. Seventy-two institutions (71%) from 35 different states responded. Sixty-five of the respondents were colleges or universities, but 6 research institutes and 1 teaching hospital were included (Table 1). Collectively, they received more than $5.2 billion in annual NIH support during fiscal year 1995-1996 (slightly more than half of the NIH extramural research budget)—about $2.1 billion of which involved animal-related research—and used more than 3 million mice and 1 million rats.
Table 1.
Categories of respondents to survey of studies funded in 1996 by the National Institutes of Health
| No. surveyed | No. responded | % | |
| Colleges and universities | 93 | 65 | 70 |
| Research institutes | 7 | 6 | 86 |
| Teaching hospitals | 2 | 1 | 50 |
| Totals | 102 | 72 | 71 |
Survey Results
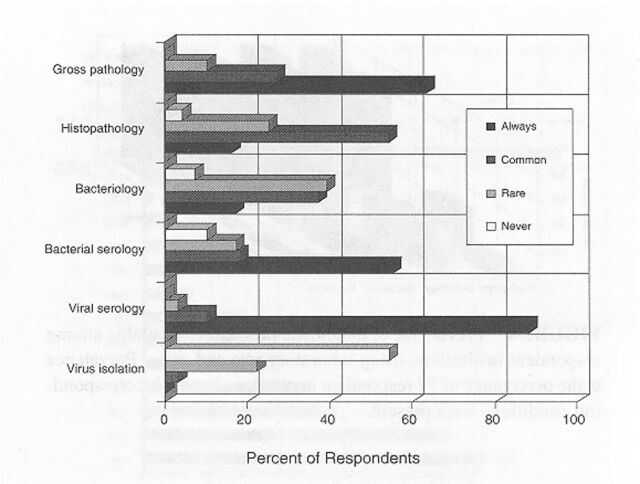
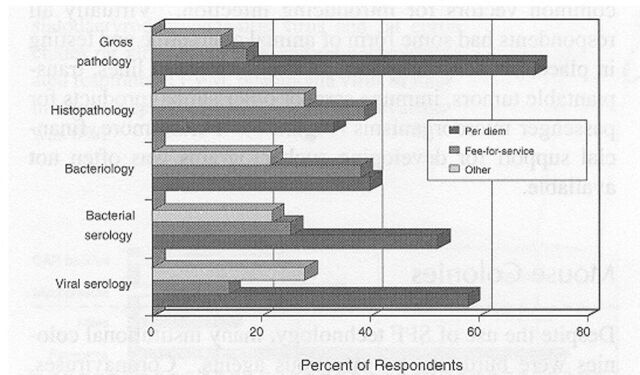
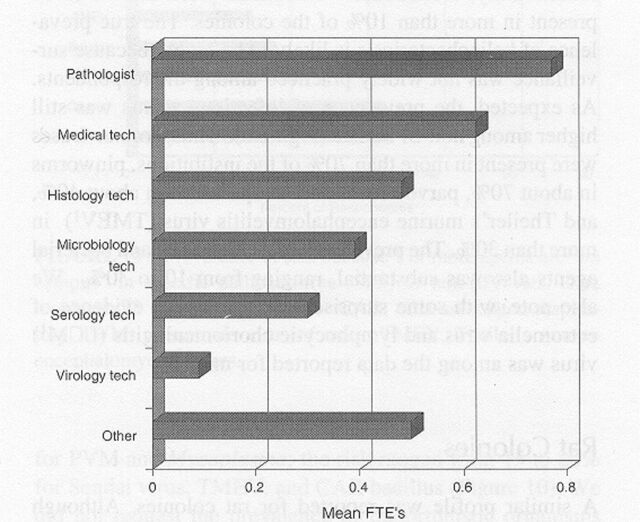
Among the respondents, 70% of the mouse colonies and 60% of the rat colonies were maintained under SPF conditions, designed to prevent the entry of common infectious agents (Table 2). Surveillance for infection was most intense for resident colonies and rodents obtained from noncommercial sources such as other research institutions. The typical frequency for surveillance testing among SPF mouse and rat colonies was 3 to 4 times per year. Serology was the primary means for detection of viral infections, whereas culture, serology, and microscopy were used collectively to detect bacterial or parasitic infections (Figure 1). Few institutions used molecular tests for diagnosis, so data about such tests were excluded. However, the development and application of molecular diagnostics is likely to undergo rapid growth in the near future as addressed in a companion article (Weisbroth and others 1998). Approximately half of the respondents recovered costs for health monitoring through per diem charges, and half charged investigators directly or supported surveillance with institutional funds (Figure 2). Many institutions had some capacity for on-site diagnostic laboratory support, most frequently directed by a pathologist (Figure 3).
Table 2.
Source of animals and frequency of testing based on survey of studies funded in 1996 by the National Institutes of Health
| Mouse | Rat | |||
| SPF1 | Non-SPF | SPF | Non-SPF | |
| Animals housed as (%) | 70 | 30 | 60 | 40 |
| Respondents (%) who test | ||||
| Vendor animals | 38 | 25 | 32 | 26 |
| Nonvendor animals | 78 | 56 | 74 | 51 |
| Resident animals | 92 | 60 | 88 | 60 |
| Rooms tested (%) | ||||
| Annually | 14 | 12 | 12 | 10 |
| Semiannually | 22 | 31 | 24 | 15 |
| Quarterly | 45 | 36 | 37 | 19 |
| Animals tested/room/time point | 3.6 | 2.1 | 2.6 | 1.7 |
| Animals tested/room/year | 23 | 7.5 | 12 | 6 |
| Source of sentinel animals (%) | ||||
| Procured by resource | 90 | 57 | 83 | 56 |
| Donated by investigator | 35 | 31 | 29 | 28 |
SPF, specific pathogen-free.
Figure 1.
Diagnostic testing of laboratory rats and mice: frequency of use and prevalence of users. Data represent 72 survey respondents.
Figure 2.
Sources of cost recovery for diagnostic testing of laboratory rats and mice. Data represent 72 survey respondents.
Figure 3.
Staffing for diagnostic laboratories that test rats and mice, expressed as full-time equivalents (FTEs). Data represent 72 survey respondents.
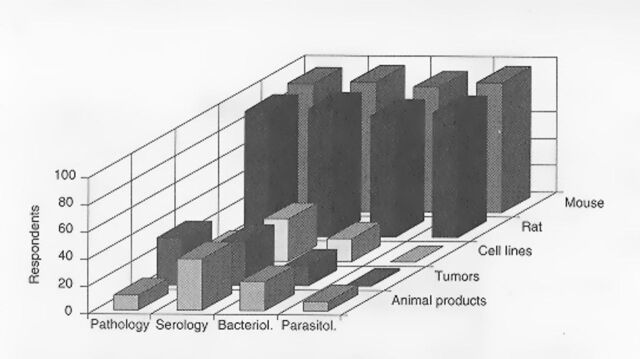
The survey also asked about measures to monitor animals and animal products obtained from external sources, common vectors for introducing infection. Virtually all respondents had some form of animal quarantine and testing in place, but few had programs for testing cell lines, transplantable tumors, immune sera, or other animal products for passenger microorganisms (Figure 4). Furthermore, financial support for developing such programs was often not available.
Figure 4.
Prevalence of diagnostic procedures available among respondent institutions using laboratory rats and mice. Prevalence is the percentage of 72 respondent institutions in which corresponding conditions were present.
Mouse Colonies
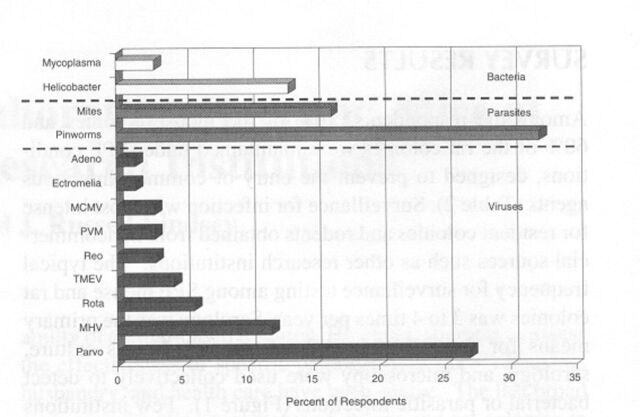
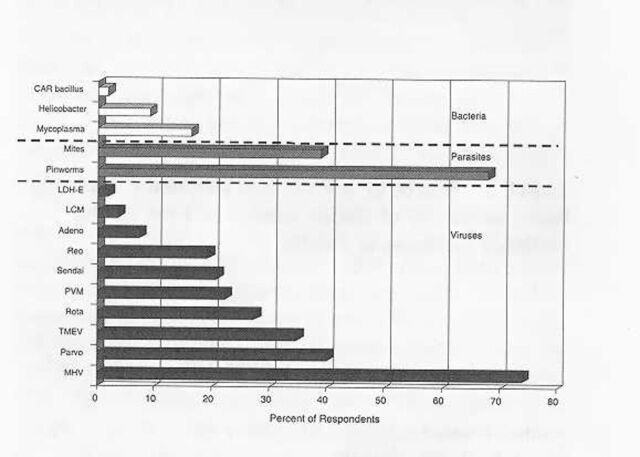
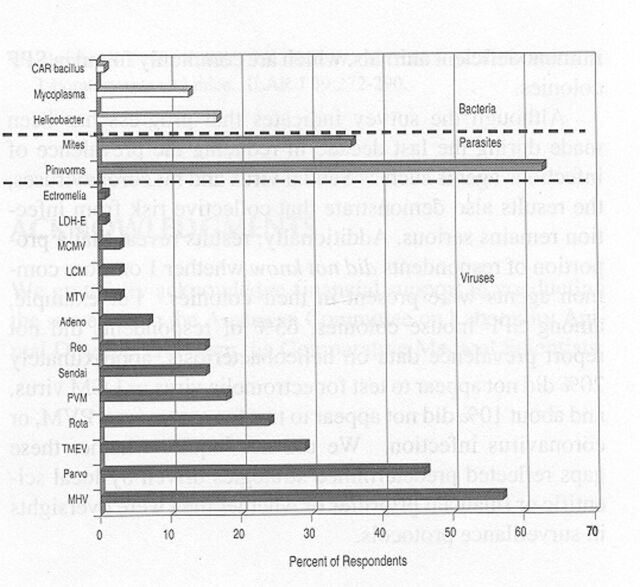
Despite the use of SPF technology, many institutional colonies were burdened by infectious agents. Coronaviruses, parvoviruses, and ecto- and endoparasites were reported in 10 to 35% of the SPF mouse colonies (Figure 5). Additionally, Helicobacter infections were known to be present in more than 10% of the colonies. The true prevalence of helicobacteriosis is likely to be higher because surveillance was not widely practiced among the respondents. As expected, the prevalence of infectious agents was still higher among non-SPF mice (Figure 6). Thus, coronaviruses were present in more than 70% of the institutions, pinworms in about 70%, parvoviruses and ectoparasites in about 40%, and Theiler's murine encephalomyelitis virus (TMEV1) in more than 30%. The prevalence of 5 other viral and bacterial agents also was substantial, ranging from 10 to 30%. We also note, with some surprise, that serological evidence of ectromelia virus and lymphocytic choriomeningitis (LCM1) virus was among the data reported for mice.
Figure 5.
Prevalence of infectious agents in specific pathogen-free mice. Prevalence is the percentage of 72 respondent institutions in which corresponding conditions were present. MCMV, mouse cytomegalovirus; MHV, mouse hepatitis virus; Parvo, minute virus of mice+mouse parvovirus; PVM, pneumonia virus of mice; TMEV, Theiler's murine encephalomyelitis virus. The prevalence of cilia-associated respiratory bacillus, lactic dehydrogenase-elevating virus, lymphocytic choriomeningitis virus, and Sendai virus infections among institutional SPF colonies was 0%.
Figure 6.
Prevalence of infectious agents in non-specific pathogen-free mice. Data represent 72 survey respondents. CAR, cilia-associated respiratory; LCM, lymphocytic choriomeningitis virus; LDH-E, lactic dehydrogenase-elevating virus; MHV, mouse hepatitis virus; PVM, pneumonia virus of mice; TMEV, Theiler's murine encephalomyelitis virus. The prevalence of mouse cytomegalovirus and ectromelia virus infections was 0%.
Rat Colonies
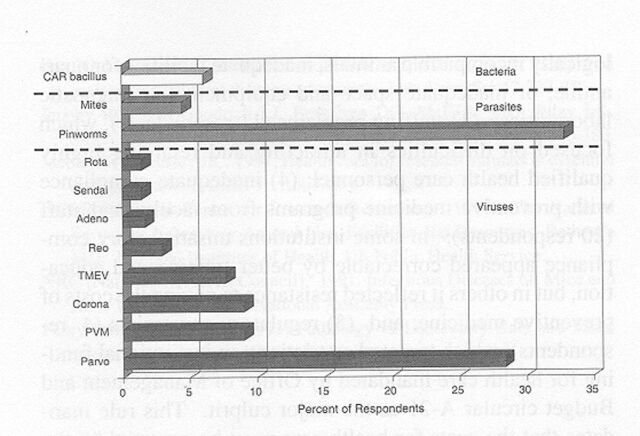
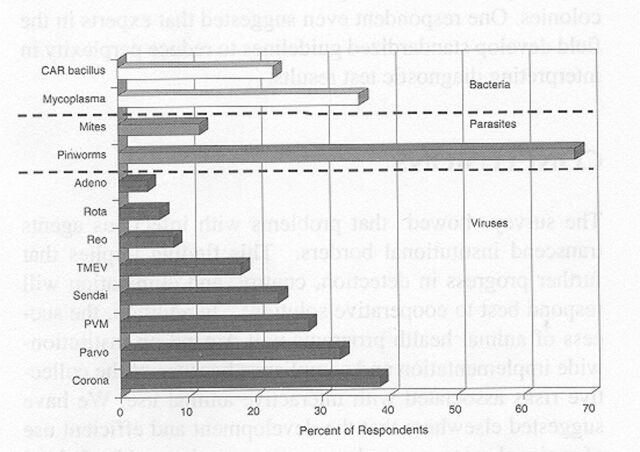
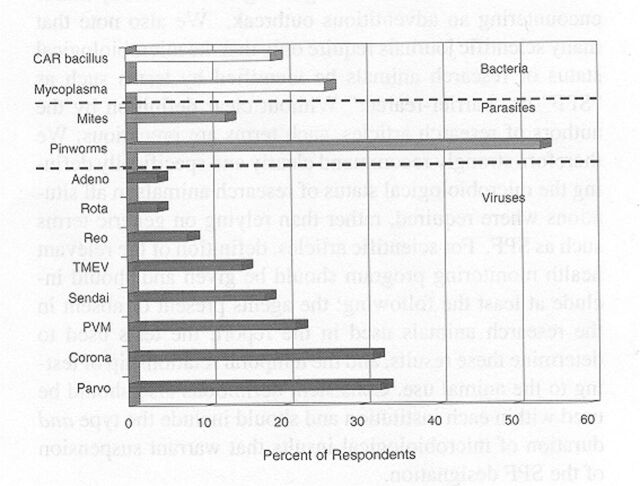
A similar profile was reported for rat colonies. Although mycoplasmosis appeared to have been eliminated among SPF rats, almost 30% of the colonies had parvovirus infection, and 5 to 10% were infected with coronaviruses, pneumonia virus of mice (PVM1), or TMEV (Figure 7). About one third of the colonies also were at risk for pinworms. Infection among non-SPF rats was higher, as expected. The prevalence of coronaviruses, parvoviruses, PVM, Sendai virus, cilia-associated respiratory (CAR1) bacillus, and Mycoplasma pulmonis ranged from 20 to about 40% and approached 70% percent for pinworms (Figure 8).
Figure 7.
Prevalence of infectious agents in non-specific pathogen-free rats. Data represent 72 survey respondents. CAR, cilia-associated respiratory; PVM, pneumonia virus of mice; TMEV, Theiler's murine encephalomyelitis virus.
Figure 8.
Prevalence of infectious agents in specific pathogen-free rats. Data represent 72 survey respondents. Corona includes sialodacryrodacryoadenitis virus and rat coronavirus; Parvo includes rat virus, H-1 virus, and rat parvovirus. CAR, cilia-associated respiratory; PVM, pneumonia virus of mice; TMEV, Theiler's murine encephalomyelitis virus. The prevalence of mycoplasmosis was 0%.
Evaluation of Infection Risk
Because many respondents operated both SPF and non-SPF colonies, we assumed a risk from cross-infection because 1or more infectious agents were “on campus.” By this criterion, mouse colonies at more than half of the respondents' centers were at risk for coronavirus infection or endoparasitism, more than one third were at risk for parvovirus infection and ectoparasitism, and more than 10% were at risk for at least 7 other viral and bacterial infections (Figure 9). The corresponding risk among rat colonies exceeded 50% for pinworms, 30% for coronaviruses and parvoviruses, and 20% for PVM and Mycoplasma; the risk ranged from 15 to 20% for Sendai virus, TMEV, and CAR bacillus (Figure 10). We did not request the prevalence of opportunistic organisms such as Pneumocystis carinii, which have been difficult to detect by routine testing. Such agents, however, are believed to be widespread in mice and rats and are pathogenic in immunodeficient animals, which are commonly found in SPF colonies.
Figure 9.
Prevalence of risk because 1 or more agents are “on campus” in specific pathogen-free (SPF) or non-SPF mice. Data represent 72 survey respondents. CAR, cilia-associated respiratory; LCM, lymphocytic choriomeningitis virus; LDH-E, lactic dehydrogenase- elevating virus; MCMV, mouse cytomegalovirus; MTV, mouse thymic virus; PVM, pneumonia virus of mice; TMEV, Theiler's murine encephalomyelitis virus.
Figure 10.
Prevalence of risk because 1 or more agents are “on campus” in specific pathogen-free (SPF) or non-SPF rats. Data represent 72 survey respondents. CAR, cilia-associated respiratory; PVM, pneumonia virus of mice; TMEV, Theiler's murine encephalomyelitis virus.
Although the survey indicates that progress has been made during the last decade in reducing the prevalence of infectious agents such as Sendai virus and rat coronaviruses, the results also demonstrate that collective risk from infection remains serious. Additionally, results reveal that a proportion of respondents did not know whether 1 or more common agents were present in their colonies. For example, among SPF mouse colonies, 65% of respondents did not report prevalence data on helicobacteriosis, approximately 20% did not appear to test for ectromelia virus or LCM virus, and about 10% did not appear to test for parvovirus, PVM, or coronavirus infection. We did not inquire whether these gaps reflected predetermined strategies driven by local scientific or financial priorities or whether they were oversights in surveillance protocols.
Definitions
The survey also implied that there was considerable disparity in the definition of an SPF colony. Because variation existed in agents, testing frequency and methods, and numbers of animals tested, a uniform standard for “SPFness' was not easily discernible. Additionally, we do not know of any standardized criteria for downgrading an SPF colony that is encountering an adventitious outbreak. We also note that many scientific journals require only that the microbiological status of research animals be identified by terms such as “SPF” or “barrier-reared.” Without clear definition by the authors of research articles, such terms are precarious. We therefore strongly recommend clearly and specifically defining the microbiological status of research animals in all situations where required, rather than relying on generic terms such as SPF. For scientific articles, definition of the relevant health monitoring program should be given and should include at least the following: the agents present or absent in the research animals used in the report, the tests used to determine these results, and the temporal relationship of testing to the animal use. Consistent definitions also should be used within each institution and should include the type and duration of microbiological insults that warrant suspension of the SPF designation.
Concerns
Survey respondents also were asked to summarize major concerns about preventive health care for rodents at their institutions. Fifty-one of the 72 institutions reported 1 or more factors, apart from the presence of infection per se. The factors fell into 6 categories: (1) inadequate financing for health care (34 respondents), the most commonly cited problem that typically included shortcomings in facilities and staffing; (2) inadequate facilities (19 respondents), which included inadequate means for separating microbiologically incompatible animals, inadequate facilities for quarantine, or inadequate space and equipment for diagnostic laboratories; (3) staffing problems (11 respondents), which focused on difficulties in attracting and retaining highly qualified health care personnel; (4) inadequate compliance with preventive medicine programs from faculty and staff (20 respondents): in some institutions unsatisfactory compliance appeared correctable by better training and education, but in others it reflected resistance to paying the costs of preventive medicine; and (5) regulatory constraints (4 respondents), which targeted restrictions on institutional funding for health care mandated by Office of Management and Budget circular A-21 as the major culprit. This rule mandates that the costs for health care must be assumed by the users or the institution exclusive of federal funds obtained from indirect cost recovery. Six respondents also raised the cogent point that the quality and interpretive power of diagnostic tests need further improvement because test results are frequently the basis for major health care decisions in rodent colonies. One respondent even suggested that experts in the field develop standardized guidelines to reduce perplexity in interpreting diagnostic test results.
Conclusions
The survey showed that problems with infectious agents transcend institutional borders. This finding implies that further progress in detection, control, and elimination will respond best to cooperative solutions. In addition, the success of animal health programs will depend on institution-wide implementation and compliance because of the collective risks associated with interactive animal use. We have suggested elsewhere that the development and efficient use of regional resources and consortiums, enhanced by federal funding, can improve quality and reduce cost, especially when exchanges of animals and animal products occur among neighboring institutions (Jacoby and Lindsey 1997). Complementary responsibilities for testing and diagnosis, including on-line sharing of results, will enhance disease prevention and control programs while reducing logistical and financial redundancy among participating institutions. Encouragement of regional cooperation and innovation can place the required resources within reach of virtually every biomedical research center in the country at an affordable cost.
Acknowledgments
We gratefully acknowledge financial support in conducting the survey from the American Committee on Laboratory Animal Diseases and from the Comparative Medical Scientists.
Footnotes
Abbreviations used in this paper: CAR, cilia-associated respiratory; LCM, lymphocytic choriomeningitis; NIH, National Institutes of Health; PVM, pneumonia virus of mice; SPF, specific pathogen-free; TMEV, Theiler's murine encephalomyelitis virus.
References
- Bhatt PN, Jacoby RO, Morse HC, New AE.editors. Viral and mycoplasmal infections of laboratory rodents: Effects on biomedical research. 1986. Orlando FL: Academic Press. [Google Scholar]
- Casebolt DB, Lindsey JR, Cassell GH. Prevalence rates of infectious agents among commercial breeding populations of rats and mice. Lab Anim Sci 1988. 38:327-329. [PubMed] [Google Scholar]
- Compton SR, Barthold SW, Smith AL. The cellular and molecular pathogenesis of coronaviruses.Lab Anim Sci 199343:15-28 [PubMed] [Google Scholar]
- Fox JG, Lee A. The role of Helicobacter species in newly recognized gastrointestinal tract diseases of animals.Lab Anim Sci 199747:222-255 [PubMed] [Google Scholar]
- Jacoby RO, Ball-Goodrich LJ, Besselsen DG, McKisic MD, Riley LK, Smith AL. 1996. Rodent parvovirus infections. Lab Anim Sci 46:370-380. [PubMed] [Google Scholar]
- Jacoby RO, Lindsey JR. Health care for research animals is essential and affordable. FASEB J 199711:609-614 [DOI] [PubMed] [Google Scholar]
- NCRR [National Center for Research Resources]. The National Survey of Laboratory Animal Use, Facilities and Resources. 1997. Bethesda MD: National Institutes of Health, US Public Health Service. [Google Scholar]
- NRC [National Research Council]. Infectious Diseases of Mice and Rats. 1991. Washington DC: National Academy Press. [Google Scholar]
- Percy DH, Barthold SW. 1993. Pathology of Laboratory Rats and Mice. Ames IA: Iowa State University Press. [Google Scholar]
- Weisbroth SH, Peters R, Riley LK, Shek W. Microbiological assessment of laboratory rats and mice. ILAR J 39:272-290. [DOI] [PubMed] [Google Scholar]