Abstract
The congenital muscular dystrophies (CMD) are a heterogeneous group of autosomal recessive disorders presenting in infancy with muscle weakness, contractures, and dystrophic changes on skeletal-muscle biopsy. Structural brain defects, with or without mental retardation, are additional features of several CMD syndromes. Approximately 40% of patients with CMD have a primary deficiency (MDC1A) of the laminin α2 chain of merosin (laminin-2) due to mutations in the LAMA2 gene. In addition, a secondary deficiency of laminin α2 is apparent in some CMD syndromes, including MDC1B, which is mapped to chromosome 1q42, and both muscle-eye-brain disease (MEB) and Fukuyama CMD (FCMD), two forms with severe brain involvement. The FCMD gene encodes a protein of unknown function, fukutin, though sequence analysis predicts it to be a phosphoryl-ligand transferase. Here we identify the gene for a new member of the fukutin protein family (fukutin related protein [FKRP]), mapping to human chromosome 19q13.3. We report the genomic organization of the FKRP gene and its pattern of tissue expression. Mutations in the FKRP gene have been identified in seven families with CMD characterized by disease onset in the first weeks of life and a severe phenotype with inability to walk, muscle hypertrophy, marked elevation of serum creatine kinase, and normal brain structure and function. Affected individuals had a secondary deficiency of laminin α2 expression. In addition, they had both a marked decrease in immunostaining of muscle α-dystroglycan and a reduction in its molecular weight on western blot analysis. We suggest these abnormalities of α-dystroglycan are caused by its defective glycosylation and are integral to the pathology seen in MDC1C.
Introduction
The congenital muscular dystrophies (CMD) are a heterogeneous group of autosomal recessively inherited diseases, presenting at birth or within the first 6 mo of life with hypotonia, muscle weakness, and the variable appearance of contractures and characterized by dystrophic changes on skeletal-muscle biopsy (Dubowitz 1995). The differing degrees of motor developmental delay, physical disability, muscle pathology, and elevation of serum creatine kinase (CK), along with the variable presence of mental retardation and structural brain defects, underscores the heterogeneous nature of this condition. Recent epidemiological data suggest that the incidence of CMD is 4.65×10-5 and that its prevalence is 8×10-6 (Mostacciuolo et al. 1996), indicating that it is among the most frequent autosomal recessively inherited neuromuscular diseases.
One of the main objectives of the International Consortium on CMD has been to delineate and subdivide the various forms of CMD (Dubowitz 1994, 1995, 1997, 1999; Muntoni et al., in press). Seven genetically distinct entities have been identified so far. The most common form of CMD (∼40% of cases) is due to a primary deficiency in the laminin α2 chain of merosin (laminin-2), resulting from mutations in the LAMA2 gene (MDC1A [MIM 156225]; Tome et al. 1994; Helbing-Leclerc et al. 1995). Other CMD disease genes identified so far are ITGA7, leading to integrin α7 deficiency ([MIM 600536]; Hayashi et al. 1998); COL6A2, coding for the α2 chain of collagen VI, in the Ullrich variant (UCMD [MIM 254090]; Camacho Vanegas et al. 2001); and fukutin, responsible for Fukuyama congenital muscular dystrophy (FCMD [MIM 253800], Fukuyama et al. 1981; Kobayashi et al. 1998). Other forms of CMD that have been mapped but for which the disease genes remain elusive are muscle-eye-brain disease (MEB [MIM 236670]), mapped to chromosome 1p32-34 (Cormand et al. 1999); a form with rigidity of the spine (RSMD1 [MIM 602771]), mapped to 1p35-36 (Moghadaszadeh et al. 1998); and a variant characterized by muscle hypertrophy (MDC1B [MIM 604801]), mapped to 1q42 (Muntoni et al. 1998; Brockington et al. 2000). The primary role of abnormalities of the extracellular matrix in these diseases is exemplified by the defects in genes for laminin α2, integrin α7, and collagen VI. In addition, basal lamina abnormalities have also been demonstrated in FCMD by means of electron microscopy (Ishii et al. 1997).
Merosin consists of three laminin chains, α2β1γ1, with α2 forming a link between α-dystroglycan and the basal lamina (Henry et al. 1999). α-dystroglycan is a heavily glycosylated peripheral-membrane component of the dystrophin-associated glycoprotein complex (DAPC), which, in addition to laminin α2, binds perlecan and agrin in the extracellular matrix, whereas β-dystroglycan, derived from the same gene (Holt et al. 2000), is a transmembrane protein that links to dystrophin intracellularly (Ibraghimov-Beskrovnaya et al. 1992). Dystroglycan therefore plays a pivotal role in linking the actin-associated cytoskeleton to components of the extracellular matrix (Ervasti et al. 1991; Ibraghimov-Beskrovnaya et al. 1992), and disruption of this axis is associated with several forms of muscular dystrophy (Campbell 1993).
FCMD is one of a number of CMD forms that have been shown to have a secondary reduction in the expression of laminin α2 (Hayashi et al. 1993). Recently, a profound depletion of skeletal and cardiac muscle α-dystroglycan has also been reported in FCMD (Hayashi et al. 2001). FCMD is common in Japan, because of a founder haplotype containing a 3.0-kb retrotransposal insertion into the 3′ UTR of the FCMD gene (Kobayashi et al. 1998). Fukutin, a protein of unknown function, is predicted to be a secreted protein that has sequence similarities to a family of proteins involved in modifying cell-surface molecules such as glycoproteins and glycolipids (Aravind et al. 1999). Fukutin also contains a conserved DxD motif that is found in many glycosyltransferases (Aravind et al. 1999; Breton and Imberty 1999).
Although FCMD is rare outside Japan, other CMD syndromes with secondary laminin α2 deficiency with or without brain involvement are not, including MDC1B (Brockington et al, 2000), MEB (Haltia et al. 1997), and other, as-yet-unmapped variants (Talim et al. 2000; Villanova et al. 2000). We have used sequence analysis to identify fukutin homologues in the human genome as candidate genes for these variants of CMD.
In the present study, we report the identification of a gene encoding a new member of the fukutin-related-protein family (FKRP [EMBL accession number AJ314847]). This gene is mutated in a severe form of CMD, which we have called “MDC1C.” Patients harboring mutations in this gene had a secondary deficiency of laminin α2 and a profound reduction in α-dystroglycan immunostaining. In addition, the molecular weight of α-dystroglycan was reduced in muscle. Together, these findings suggest that this protein is abnormally glycosylated in MDC1C and is central to the pathology seen in this disorder.
Families, Material, and Methods
Family 1
We recently reported the clinical features of the two children in this Scottish family and suggested that they are affected by a novel form of CMD (Mercuri et al. 2000). Both children presented soon after birth with hypotonia and feeding difficulties. They never acquired the ability to walk, because of severe weakness, which also affected their facial muscles. Weakness affected the arms more than the legs, with prominent wasting of the deltoids and pectoralis muscles, whereas both calf and quadriceps muscles were hypertrophied. Cognitive development, intelligence, and vision were normal, as was brain magnetic resonance imaging. Serum CK was markedly elevated (9,000–14,550 IU/L, normal values <190 IU/liter). The older sibling died suddenly at age 7 years, following an upper respiratory tract infection. The younger sibling is now 4 years old and recently had a gastrostomy because of feeding difficulties.
Family 2
This is a consanguineous family from Libya with one affected child. In addition, two children on the paternal side of the family had died in the first decade of life with a similar illness. She presented in the first few weeks of life with hypotonia and feeding difficulties, followed by motor delay. On examination at age 16 mo, she could not walk and was weaker in her arms than in her legs. She had calf hypertrophy and facial weakness. Her serum CK was 2,478 IU/liter and she had a myopathic EMG. Her intelligence was normal.
Family 3
The affected child in this family is a 6.5-year-old girl from southern England who is currently unable to stand or walk. She presented with hypotonia in the first few weeks of life, followed by delayed motor milestones. She could stand with support between the ages of 4 and 6 years. Her intelligence was normal, her serum CK was markedly elevated, and she had a muscle biopsy at age 9 mo that was dystrophic. At age 6 years, she had marked hypertrophy of the leg muscles with wasting and weakness of shoulder-girdle muscles, particularly her deltoid, pectoralis, and sternocleidomastoid muscles. Serum CK was elevated (2,695 IU/liter). A brain MRI showed normal results; an echocardiogram showed mildly impaired left-ventricular function.
Family 4
The third child of this Sudanese consanguineous family is affected. A detailed clinical description of this patient and those from families 5, 6, and 7 has recently been submitted elsewhere (S. Quijano-Roy, L. Galan, A Ferreiro, F. Cheliout-Heraut, F. Gray, M. Fardeau, A. Barois, P. Guicheney, N. B. Romero, and B. Estournet, unpublished data). He presented at birth with hypotonia, and his motor development was severely delayed; he never acquired the ability to walk. Calf and tongue hypertrophy were noticed at the ages of 4 and 10 years, respectively. At age 14 years, he went into respiratory failure and has been ventilated via a tracheostomy ever since. His cognitive development and brain MRI were normal; he developed a transient cardiac insufficiency after a respiratory infection at age 11 years, but current cardiac function is normal. Serum CK was elevated (2,277 IU/liter) at age 10 years. He underwent three muscle biopsies (at the ages of 2, 3, and 9 years) that showed a dystrophic picture with normal expression of dystrophin and sarcoglycans.
Laminin α2 expression was reduced with an antibody against the 80-kDa fragment and was absent with an antibody against the 300-kDa fragment. No muscle was available to study the expression of α-dystroglycan.
Family 5
The only child from this nonconsanguineous family of Caribbean origin is affected. She was symptomatic at birth with weakness and hypotonia, and her motor development was severely delayed. She could walk only with assistance between the ages of 3 and 6 years. Hypertrophy of the calves had been noticed in infancy, and macroglossia appeared around puberty. She developed progressive contractures in the elbows, knees, and fingers. Her cardiac function was normal; however, she developed restrictive respiratory insufficiency leading to nasal ventilation at age 14 years and tracheostomy at age 17 years. Spinal surgery was performed at age 16 years because of progressive scoliosis. Her serum CK was 7,760 IU/liter at age 5 years. She underwent three muscle biopsies (at the ages of 14 mo, 8 years, and 19 years) that showed a dystrophic picture with normal expression of dystrophin and sarcoglycans. Laminin α2 expression was reduced with an antibody against the 80-kDa fragment and was almost absent with an antibody against the 300-kDa fragment. α-dystroglycan expression was virtually absent in her muscle. Her brain MRI and mental function were normal.
Family 6
This is a 25-year-old patient, who was the first child of a nonconsanguineous French couple. She presented at birth with hypotonia, followed by delayed motor milestones. She could stand with support between the ages of 17 mo and 7 years; hypertrophy of her calves was noticed at age 6 mo, and hypertrophy of her tongue was noticed in her second decade of life. This eventually required a partial glossectomy at age 23 years because of progressive difficulties in chewing and swallowing. Intelligence was normal. A serum CK at age 2 years was markedly elevated. A brain MRI was normal, but a mild impairment of her cardiac left-ventricular function was documented on echocardiography at age 14 years. She has been ventilated with a tracheostomy since age 13 years. A muscle biopsy showed a reduction of laminin α2 with an antibody that recognizes the 80-kDa fragment. No muscle was available to study the expression of α-dystroglycan.
Family 7
The patient in this French family died at age 10 years because of respiratory complications. She presented at birth with hypotonia and delayed motor milestones; she never acquired the ability to walk. Marked hypertrophy of the calves and tongue was noticed in the first years of life. Progressive feeding difficulties led to a gastrostomy at age 9 years; her intelligence and brain MRI were normal, but echocardiography showed that her left-ventricular function was at the lower limit of normal at age 9 years. She died shortly afterward because of respiratory complications. Laminin α2 was reduced with an antibody that recognizes the 80-kDa fragment. There was no muscle to study the expression of α-dystroglycan.
Database Screening
We performed BLAST analysis for new fukutin homologues, using the BLAST server at NCBI. The identification of transcribed sequences (ESTs) and their genomic location was also performed using BLAST analysis. Sequence alignments were obtained using the ClustalW Service at the European Bioinformatics Institute.
Northern Blot Analysis
We determined the expression of FKRP and an estimate of transcript size by northern blot analysis using a blot containing poly(A)+ RNA isolated from eight tissues (Human Multiple Tissue northern blot I, Clontech). An 836-bp DNA probe containing bp 253–1088 of the FKRP coding sequence was amplified with primers FKRP-2F (GAGCTGGTAGACTCCTTCCT) and FKRP-4R (CCTTCTCCCATACGAAGC). The blot was hybridized with the radiolabeled (32P) probe in ExpressHyb hybridization solution (Clontech), according to the manufacturer's instructions.
Radiation Hybrid Mapping
The Genebridge 4 Radiation Hybrid DNA panel (UK-MRC HGMP Resource Centre) and primers FKRP-2F and FKRP-4R, amplifying a 836-bp fragment of FKRP, were used to define the chromosomal localization of FKRP. LOD scores were calculated using the RHyME program on the UK-MRC HGMP Resource Centre Web site.
Genotyping
Genomic DNA was extracted from whole blood by standard methods. Linkage to the FKRP locus was assessed by genotyping subjects for the dinucleotide repeat markers D19S219 and D19S606. Genotyping was performed using an ABI 377 Automated sequencer and was analyzed using Genescan 3.1 and Genotyper 2.0 (Applied Biosystems).
Mutational Analysis
A 1.7-kb fragment containing the FKRP coding sequence was amplified using Advantage-GC Genomic Polymerase Mix (Clontech) and primers FKRP-1F (AAAGGGAATTGAGAAAGAGC) and FKRP-5 (GCTCACACAGAGCTTCTCC). PCR products were separated by agarose gel electrophoresis, were purified (Qiagen), and were used for direct sequencing. Sequencing reactions were performed using a ABI Prism BigDye Terminator Cycle Sequencing kit (Applied Biosystems) and primers FKRP-1R (GCAGGAAGGAGTCTACCAG), FKRP-2R (CCGAGAGGTTGAAGAGGT), FKRP-3R (CTCCTCGTAGAGGTAGGC), FKRP-4R, and FKRP-5R. Sequencing products were separated on an ABI377 automated sequencer (Applied Biosystems) and analyzed using SeqEd (Applied Biosystems).
Immunohistochemistry
Unfixed frozen 8-mm sections were incubated with monoclonal antibodies to β-spectrin; laminins α2, β1, and γ1 (Chemicon); α- and δ-sarcoglycan (Novocastra); perlecan (Chemicon); and α-dystroglycan (V1A4-1 Upstate Biotechnology). All primary antibodies were applied for 1 h and were revealed with an appropriate biotinylated secondary antibody (Amersham 1:200) for 30 min, followed by streptavidin conjugated to Alexa (Molecular Probes) for 20 min. All dilutions and washings were made in phosphate buffered saline. Sections were mounted in aqueous mountant and were viewed with epifluorescence using a Leica Aristoplan microscope.
Immunoblotting
Muscle proteins were extracted in sample buffer consisting of 1 M Tris HCl, 1% SDS, glycerol, 2-mercaptoethanol, plus a cocktail of protease inhibitors (antipain, aprotinin, and leupeptin). Soluble protein was resolved using a NuPage Pre-cast gel (4%–12% Bis-Tris; Invitrogen) and then was transferred electrophoretically to nitrocellulose membrane (Sartorius). Nitrocellulose strips were blocked in 5% milk powder in Tris-buffered saline buffer; were probed with antibodies to α-dystroglycan (V1A4 1:1,000), β-dystroglycan (Novocastra 1:25), and α- and γ-sarcoglycan (Novocastra 1:50, 1:100); and were washed and incubated with HRP-anti-mouse (1:50,000; Jackson). Membranes were visualized using chemiluminescence (ECL+Plus, Amersham).
Results
Database Screening
Comparison of the mouse fukutin sequence with sequence databases using TBLASTN (Altschul et al. 1997) revealed a murine expressed sequence tag (EST; AA016785) that showed significant similarity (E=8×10-3) across the proposed fukutin active-site region. Reciprocal BLAST searches using a corrected version of this sequence as a query demonstrated its significance to fukutin homologues (Aravind and Koonin 1999), in particular Rickettsia prowazekii RP689 (E=2×10-5) and putative orthologous sequences in Homo sapiens and Drosophila melanogaster (fig. 1b). Sequence analysis of FKRP predicts the presence of a hydrophobic transmembrane-spanning region (amino acids 4–28) followed by a “stem region” and the putative catalytic domain. A similar molecular organization is found in several Golgi-resident glycosyltransferases (Munro 1998).
Figure 1.
Sequence of the human FKRP cDNA and analysis of the primary sequence. A, Partial nucleotide sequence and open reading frame are shown. The 4-kb cDNA encodes a protein of 495 amino acids, with a predicted molecular weight of 54.6 kDa. B,Multiple sequence alignment of fukutin homologues. Missense and nonsense mutations that were found to occur within the region aligned are indicated by red asterisks (*) and slashes (/), respectively. Amino acid residues are colored according to an 80% consensus: plus signs (+) indicate positively charged residues (H, K, and R; blue), minus signs (−) indicate negatively charged residues (D and E; red), black asterisks indicate serine or threonine residues (light blue on grey background), “a” indicates aromatic residues (F, H, W, and Y; blue on yellow background), “b” indicates big residues (E, F, H, I, K, L, M, Q, R, W, and Y; blue on yellow background), “c” indicates charged residues (DEHKR; pink), “h” indicates hydrophobic residues (A, C, F, G, H, I, L, M, T, V, W, and Y; black on yellow background), “l” indicates aliphatic residues (I, L, and V; grey on yellow), “p” indicates polar residues (C, D, E, H, K, N, Q, R, S, and T; blue), and s indicates small residues (A, C, D, G, N, P, S, T, and V; green). Residues that are conserved in >80% of sequences are shown as yellow on black. Residues excised from the alignment are indicated by numbers in parentheses. Predicted secondary structures are indicated below the alignment (e/E, extended or β-strand structure; h/H, helix). Residue numbers and GeneInfo identifiers (or else cosmid names) are shown following the alignment. Ca = Candida albicans; Ce = Caenorhabditis elegans; Dm = Drosophila melanogaster; HELP1 = haemolysin erythrocyte lysis protein 1; Hi = Haemophilus influenzae; Hs = Homo sapiens; Ll = Lactobacillus lactis lactis; Pi = Prevotella intermedia; Rp = Rickettsia prowazekii; Sc = Saccharomyces cerevisiae; Sp = Streptococcus pyogenes; Tb = Trypanosoma brucei. Fukutin homologues partially encoded by ESTs in T. brucei (EST AQ655742), T. cruzi(AI077222), and Leishmania major (AQ852136), as well as a Bacillus subtilis sequence similar to L. lactis TrpF C-terminal domain (GeneInfo number 1750106; bases 12108–12419), are not shown. Two C. elegans sequences, marked with a dagger (†), are alternative gene predictions that differ from published versions by the prediction of additional exons.
Radiation-Hybrid Mapping
Radiation-hybrid mapping using the Genebridge 4 Hybridization DNA panel gave a maximum two-point LOD score of 17.25 with marker D19S219. The most likely order of markers is centromere-D19S219-FKRP-D19S606-telomere on chromosome 19q13.3. D19S219 and D19S606 are placed 3 cM apart on the Généthon map.
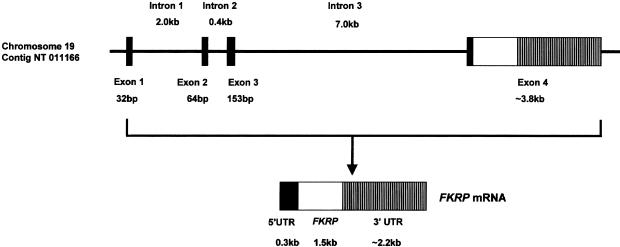
Genomic Organization and Northern Blot Analysis
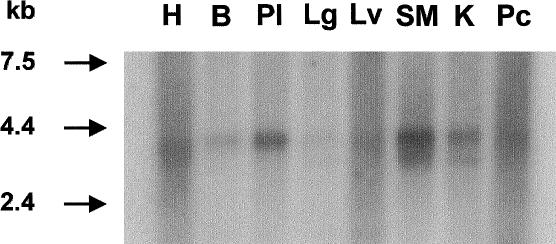
The genomic organization of the FKRP gene was determined by comparing the cDNA sequence with a contiguous segment of chromosome 19 in contig NT-011166.2. The 12-kb gene is composed of three noncoding exons and a single large exon of 3.8-kb that contains part of the 5′ untranslated region and the entire open frame and 3′ untranslated region (fig. 2). Northern blot analysis demonstrated a transcript of ∼4.0-kb in all tissues studied. The FKRP transcript is expressed predominantly in skeletal muscle, placenta, and heart and relatively weakly in the remaining tissues (fig. 3). An additional transcript of ∼3.6 kb was visible in skeletal muscle, kidney, and lung.
Figure 2.
Schematic representation of the FKRP gene. The gene consists of 4 exons and spans no more than 12 kb. The entire coding sequence is located within exon 4. The 5′ UTR is blackened, the FKRP open reading frame is unblackened, and the 3′ UTR is hatched.
Figure 3.
Northern blot analysis demonstrating the expression of FKRP mRNA. A 4.0-kb transcript is seen in all tissues studied. H = heart; B = brain; Pl = placenta; Lg = lung; Lv = liver; SM = skeletal muscle; K = kidney; Pc = pancreas. It is most highly expressed in skeletal muscle, placenta, and heart and is expressed relatively weakly in the remaining tissues. An additional transcript of ∼3.6 kb is visible in skeletal muscle, kidney, heart, and lung.
Mutation Analysis of the FKRP Gene
We initially studied the FKRP gene in 25 families with CMD with brain involvement. No family's results were consistent with linkage to the FKRP locus. In addition, direct sequencing of the FKRP coding region in noninformative families showed that it was normal. We concluded that FKRP was not a major locus for these disorders, at least in our patient population.
Next, we looked at families with CMD with a secondary deficiency in laminin α2 and no brain involvement. In seven families that shared a common, severe phenotype, we identified mutations in the coding region of FKRP that are expected to affect protein function (fig. 4 and table 1). In brief, we identified 10 different mutations—8 missense and 2 nonsense. Both affected children from consanguineous families 2 and 4 had homozygous missense changes, as did the affected child from nonconsanguineous family 5. The affected children in the remaining four families were compound heterozygotes. Families 1 and 3 had one missense and one frameshift mutation, whereas families 6 and 7 had two missense mutations (table 1). In family 1, the C1154A change was present in the mother, but the A926G change was unexpectedly not found in the father’s lymphocyte DNA, suggesting a germline mutation (fig. 4). The changes were considered to be pathogenic, because they segregated with the disease in a recessive fashion and were not present in 100 control chromosomes. A small number of silent changes were identified and are listed in table 1.
Figure 4.
An example of mutation analysis in FKRP. a, The pedigree of family 1, showing the segregation of two heterozygous mutations, A926G and C1154A. Both affected children are compound heterozygotes, whereas the unaffected sib has inherited only one of the at-risk alleles. The father unexpectedly did not have the C1154A mutation in his lymphocyte DNA and most likely is a germline mosaic. Haplotype analysis at this and other loci confirmed paternity (data not shown). b, DNA sequence analysis of FKRP, identifying the mutations in family 1.
Table 1.
Mutations and Polymorphisms Found in the FKRP Gene
| Change | Inheritance | ProteinEffect |
| Mutations: | ||
| Family 1 (British): | ||
| A926G | Heterozygous | Tyr309Cys |
| C1154A | Heterozygous | Ser385Stop |
| Family 2 (Libyan): | ||
| C1378T | Homozygous | Pro448Leu |
| Family 3 (British): | ||
| G1016A | Heterozygous | Arg339His |
| 165InsGGAG | Heterozygous | Asp60Stop |
| Family 4 (Sudanese): | ||
| C649A | Homozygous | Pro217Thr |
| Family 5 (Caribbean): | ||
| A1394C | Homozygous | Tyr465Ser |
| Family 6 (French): | ||
| C341G | Heterozygous | Ala114Gly |
| G1201A | Heterozygous | Asp401Asn |
| Family 7 (French): | ||
| C947G | Heterozygous | Pro316Arg |
| T983C | Heterozygous | Tyr328Ser |
| Polymorphisms: | ||
| C-34T | Heterozygous | None |
| C63A | Heterozygous | None |
| C135T | Heterozygous | None |
| C249T | Heterozygous | None |
| G648A | Heterozygous | None |
| G654A | Heterozygous | None |
| G1341A | Heterozygous | None |
Muscle Biopsy: Immunohistochemistry and Immunoblot Analysis
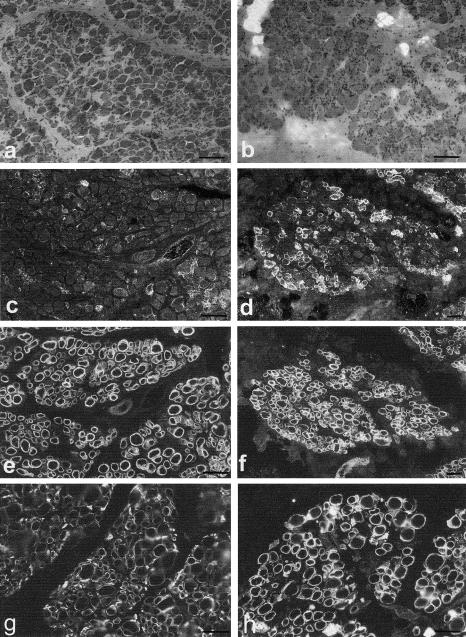
The skeletal muscle biopsies showed a severe dystrophic picture in all cases (a haematoxylin- and eosin-stained section from families 1 and 2 is shown in fig. 5a–b). Antibodies to α- and β-dystroglycan in families 1 and 2 showed preserved β-dystroglycan (fig. 5e–f) but a severe depletion of α-dystroglycan on most fibers in family 1 (fig. 5c), whereas >50% of fibers showed a reduction in family 2 (fig. 5d). Only the muscle from family 1 showed reduced labeling for laminin α2 (fig. 5g), β1, and γ1, and this tended to be on the larger rather than smaller fibers. In this family, only a minority of fibers with a severe depletion of α-dystroglycan were also associated with a reduction in laminin α2, β1, and γ1 labeling. However, the muscle from family 2 did not show any reduction in laminin α2 (fig. 5h), β1, and γ1 labeling, suggesting that the absence of α-dystroglycan in a large proportion of fibers is not directly associated with a reduction in these laminin chains. Immunolabeling of the membrane-associated proteins β spectrin and dystrophin, along with the glycosylated proteins α- and δ-sarcoglycan, were within normal limits. Perlecan immunolabeling in families 1 and 2 was indistinguishable from controls. A marked reduction in α-dystroglycan expression was reported in two of our patients from France, the only other families from which muscle was available.
Figure 5.
Muscle sections, from families 1 (a) and 2 (b), stained with haematoxylin and eosin. Immunolabeling for α- and β-dystroglycan is shown in c and e, repectively (family 1), and in d and f, respectively (family 2). α-dystroglycan was markedly reduced on most fibers in family 1, whereas >50% of fibers showed a reduction in family 2. β-dystroglycan was normally expressed in all fibers in both families. Immunolabeling for laminin α2 is shown in g (family 1) and h (family 2). A reduction in laminin α2 expression on the larger fibers was evident only in family 1. Bar = 100 μm.
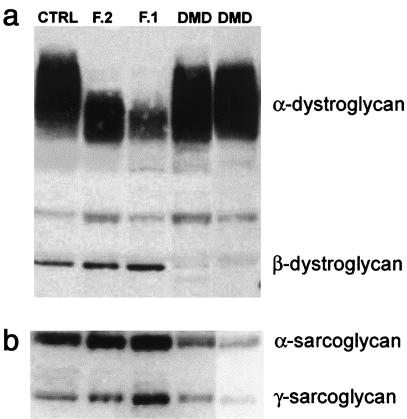
Immunoblot analysis of α-dystroglycan in families 1 and 2, adult control muscle, and Duchenne muscular dystrophy (DMD) muscle was performed. A broad band of ∼156 kDa was seen in normal muscle that was reactive with antibody V1A4-1, which recognizes a glycosylated epitope (fig. 6a). Families 1 and 2 showed a narrowing of the broad reactive band with a marked shift towards a lower molecular weight. Only minor alterations in the molecular weight of α-dystroglycan were apparent in the DMD muscle, despite the presence of a similar number of immature fibers to MDC1C, as judged by the expression of neonatal myosin (not shown). Both MDC1C muscles showed the same abnormality in α-dystroglycan on western blot. This suggests that the reduction in the mean α-dystroglycan molecular weight observed in MDC1C was not simply a reflection of an immature muscle but likely reflects differences in the pattern of glycosylation in these MDC1C patients. The reduction in β-dystroglycan in DMD was expected, given the absence of dystrophin in these patients. The molecular weight of the heavily glycosylated membrane proteins α- and γ-sarcoglycan, along with β-dystroglycan, were normal (figs. 6a–b).
Figure 6.
a, Immunoblot analysis of muscle proteins extracted from families 1 and 2 (F1 and F2) showed a marked shift in the average molecular weight of α- but not β-dystroglycan. Control adult muscle (CTRL), together with muscle from patients with Duchenne muscular dystrophy (DMD), are included for comparison. The significant reduction in α-dystroglycan molecular weight was only observed in patients with MDC1C. b, The molecular weights of α- and γ-sarcoglycan are comparable with those of control and DMD muscle.
Discussion
We have identified a new member of the fukutin family of proteins, FKRP, whose gene maps to chromosome 19q13.3. FKRP, like fukutin, contains sequence motifs identifying it as a putative sugar transferase (Aravind and Koonin 1999; Breton and Imberty 1999). Mutations in this gene give rise to a very distinct form of congenital muscular dystrophy, which previously has been recognized as a separate clinical entity (Mercuri et al. 2000; S. Quijano-Roy, L. Galan, A Ferreiro, F. Cheliout-Heraut, F. Gray, M. Fardeau, A. Barois, P. Guicheney, N. B. Romero, and B. Estournet, unpublished data). The characteristic clinical features of this condition are onset in the first few weeks of life; severe weakness and wasting of the shoulder-girdle muscles; hypertrophy and weakness of the leg muscles, with inability to walk; calf and thigh hypertrophy; and severe restrictive respiratory involvement, leading to respiratory failure in the second decade of life. Several patients had signs of heart involvement, and this suggests that cardiac muscle is also affected in this disorder. Other invariable features were preserved intelligence, normal brain structure on imaging, and marked elevation of serum CK. A secondary reduction in laminin α2 was also observed, although this was less clear in some patients because of the severe generalized muscle damage secondary to the dystrophic muscle process. In keeping with established nomenclature, we have called this disease “MDC1C” (MDC1A represents CMD with a primary laminin α2 deficiency; MDC1B has a secondary laminin α2 deficiency and calf hypertrophy linked to 1q42). We did not observe patients with two null mutations in FKRP, a finding paralleled in FCMD, where it has been suggested that the presence of two such mutations would be embryonic lethal (Kondo-Iida et al. 1999).
Patients with MDC1C had a severe reduction in α-dystroglycan immunolabeling that was much more dramatic than the reduction in laminin α2, the only additional protein in which we could detect an altered pattern of expression. The muscle biopsy from family 1 showed that fibers deficient in laminin α2 were also deficient in α-dystroglycan immunolabeling, but the converse was not true. This suggests that whatever relationship exists between the reduction in α-dystroglycan and laminin α2, the deficiency in α-dystroglycan occurs first. Perlecan expression was within normal limits in families 1 and 2, suggesting that other components of the basement membrane are not grossly perturbed. The normal immunolabeling of β spectrin and dystrophin confirmed that the alterations in α-dystroglycan were not due to generalized membrane damage, which is often a feature of dystrophic muscle. These observations, together with the reduction in the average weight of α-dystroglycan, raise the possibility that FKRP is involved in the glycosylation of α-dystroglycan. The normal expression of the glycosylated proteins α- and γ-sarcoglycan suggests that the pathogenesis of this disorder is closely linked to the altered expression of α-dystroglycan, rather than a more generalized effect on glycosylated membrane proteins.
Dystroglycan is widely expressed, but its glycosylation pattern varies in a tissue-specific and developmental pattern (Henry and Campbell 1999; Leschziner et al. 2000), and this may provide a possible mechanism by which defects in individual glycosyltransferases can result in a tissue-specific phenotype. Many of α-dystroglycan’s binding partners, including laminin α2, recognize carbohydrate moieties, and their disruption would be expected to have a profound effect on its function (Durbeej et al. 1998; Montanaro et al. 1999). This is supported by the recent finding that the gene mutated in the recessive myodystrophy (myd) mouse encodes a glycosyltransferase, LARGE, and results in the altered glycosylation of α-dystroglycan (Grewal et al. 2001). The myd mouse carries an out-of-frame deletion in the LARGE gene that is predicted to give rise to a truncated protein lacking either of its two catalytic domains, in effect creating a functional “knockout.” The myd phenotype is characterized by proximal muscle weakness, reduced life span, elevated serum creatine kinase, and a skeletal-muscle pathology with foci of degeneration and regeneration. These features are shared by MDC1C; however, the myd mouse also shows signs of CNS involvement (sensorineural deafness) that is not apparent in MDC1C (Mathews et al. 1995). In addition, patients with MDC1C have a variable cardiac involvement not seen in the myd mouse. Skeletal-muscle α-dystroglycan expression in the myd mouse was found to be virtually absent, using the same antibody as was used in the present study. Because this antibody recognizes a glycosylated epitope, these observations suggest that an abnormality in the glycosylation of dystroglycan may underlie the skeletal-muscle pathology seen in this animal. However, no further comparisons can be made, because data were not presented on the immunohistochemical expression of either α-dystroglycan or laminin α2 (Grewal et al. 2001), and the depletion of α-dystroglycan on immunoblotting was more marked than that observed in MDC1C. The skeletal muscle from patients with FCMD also shows a severe immunohistochemical reduction of α-dystroglycan and almost undetectable levels of α- but not β-dystroglycan, when tested by immunoblotting. The biosynthesis of glycoproteins is a complex pathway involving the controlled activity of many different enzymes in the synthesis of a variety of carbohydrate structures. It is probable that FKRP, like fukutin and LARGE, participates somewhere along one or more of these pathways in the processing of α-dystroglycan. The more severe reduction of α-dystroglycan seen in both FCMD and the myd mouse, compared with that seen in MDC1C, probably represents the differing involvement of the respective glycosyltransferase in the synthesis of the glycosylated epitope recognized by the V1A4-1 antibody.
The altered processing of α-dystroglycan in MDC1C, like FCMD and the myd mouse, is likely to be integral to the pathology in this disease. However, although we could find no evidence of other abnormalities in the processing of other proteins, such as β-dystroglycan and α- and γ-sarcoglycan, the activities of glycosyltransferases are known to be permissive, and the existence of additional substrates and the contribution to the pathology that their altered processing would produce cannot be ignored.
The altered processing of α-dystroglycan due to its abnormal glycosylation in MDC1C, together with the recent corresponding findings in FCMD and the myd mouse, identifies a novel pathogenic mechanism in muscular dystrophy and opens a new avenue of research into these diseases.
Acknowledgments
We wish to thank the following organizations for financial support: the Muscular Dystrophy Campaign of Great Britain and Northern Ireland (support to F.M.), the European Community (Myo-Cluster: GENRE grant QLG1 CT 1999 00870 [to F.M.]), the Wellcome Trust (support to D.J.B.), the Association Française contre les Myopathies, and INSERM (French INSERM/AFM Research network on rare disorders) and the German Research Society (DFG) to T.V. (STR498/3-1). D.J.B. is a Wellcome Trust Senior Fellow. P.P. was supported by a University of Padua Fellowship. The expert technical assistance of Dr. Lucy Feng is also gratefully acknowledged.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- BLAST, http://www.ncbi.nlm.nih.gov/BLAST/
- ClustalW Service at the European Bioinformatics Institute, http://www2.ebi.ac.uk/clustalw/ (for sequence alignments)
- EMBL, http://www.ebi.ac.uk/embl/ (for human FKRP [accession number AJ314847])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MDC1A [MIM 156225], MEB [MIM 236670], FCMD [MIM 253800], ITGA7 [MIM 600536], UCMD [MIM 254090], and MDC1B [MIM 604801])
- UK-MRC HGMP Resource Centre, http://www.hgmp.mrc.ac.uk/Registered/Webapp/rhyme/index.html (for the RHyME program)
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Koonin E V (1999) The fukutin protein family-predicted enzymes modifying cell-surface molecules. Curr Biol 9:R836–R883 [DOI] [PubMed] [Google Scholar]
- Breton C, Imberty A (1999) Structure/function studies of glycosyltransferases. Curr Opin Struct Biol 9:563–571 [DOI] [PubMed] [Google Scholar]
- Brockington M, Sewry CA, Herrmann R, Naom I, Dearlove A, Rhodes M, Topaloglu H, Dubowitz V, Voit T, Muntoni F (2000) Assignment of a form of congenital muscular dystrophy with secondary merosin deficiency to chromosome 1q42. Am J Hum Genet 66:428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho Vanegas O, Bertini E, Zhang RZ, Petrini S, Minosse C, Sabatelli P, Giusti B, Chu ML, Pepe G (2001) Ullrich scleroatonic muscular dystrophy is caused by recessive mutations in collagen type VI. Proc Natl Acad Sci USA 98:7516–7521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KP (1995) Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell 80:675–679 [DOI] [PubMed] [Google Scholar]
- Cormand B, Avela K, Pihko H, Santavuori P, Talim B, Topaloglu H, de la Chapelle A, Lehesjoki AE (1999) Assignment of the muscle-eye-brain disease gene to 1p32-p34 by linkage analysis and homozygosity mapping. Am J Hum Genet 64:126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz V (1994) 22nd ENMC sponsored workshop on congenital muscular dystrophy held in Baarn, The Netherlands, 14–16 May 1993. Neuromuscul Disord 4:75–81 [DOI] [PubMed] [Google Scholar]
- ——— (1995) Muscle disorders in childhood (2nd edition). W. B. Saunders, London [Google Scholar]
- ——— (1997) 50th ENMC international workshop: congenital muscular dystrophy. 28 February 1997 to 2 March 1997, Naarden, The Netherlands. Neuromuscul Disord 7:539–547 [PubMed] [Google Scholar]
- ——— (1999) 68th ENMC international workshop (5th international workshop): On congenital muscular dystrophy, 9–11 April 1999, Naarden, The Netherlands. Neuromuscul Disord 9:446–454 [DOI] [PubMed] [Google Scholar]
- Dubowitz V, Fardeau M (1995) Proceedings of the 27th ENMC sponsored workshop on congenital muscular dystrophy. Neuromuscul Disord 5:253–258 [DOI] [PubMed] [Google Scholar]
- Durbeej M, Henry MD, Campbell K (1998) Dystroglycan in development and disease. Curr Opin Cell Biol 10:594–601 [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP (1991) Membrane organization of the dystrophin-glycoprotein complex. Cell 66:1121–1131 [DOI] [PubMed] [Google Scholar]
- Fukuyama Y, Osawa M, Suzuki H (1981) Congenital progressive muscular dystrophy of the Fukuyama type—clinical, genetic and pathological considerations. Brain Dev 3:1–29 [DOI] [PubMed] [Google Scholar]
- Grewal PK, Holzfeind PJ, Bittner RE, Hewitt JE (2001) Mutant glycosyltransferase and altered glycosylation of α-dystroglycan in the myodystrophy mouse. Nat Genet 28:151–154 [DOI] [PubMed] [Google Scholar]
- Haltia M, Leivo I, Somer H, Pihko H, Paetau A, Kivela T, Tarkkanen A, Tome F, Engvall E, Santavuori P (1997) Muscle-eye-brain disease: a neuropathological study. Ann Neurol 41:173–180 [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ, Ozawa E, Goto Y, Nonaka I, Tsukahara T, Wang JZ, Hoffman EP, Arahata K (1998) Mutations in the integrin α7 gene cause congenital myopathy. Nat Genet 19:94–97 [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Engvall E, Arikawa-Hirasawa E, Goto K, Koga R, Nonaka I, Sugita H, Arahata K (1993) Abnormal localization of laminin subunits in muscular dystrophies. J Neurol Sci 119:53–64 [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Ogawa M, Tagawa K, Noguchi S, Ishihara T, Nonaka I, Arahata K. (2001) Selective deficiency of α-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology 57:115–121 [DOI] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J,Tome FM, Schwartz K, Fardeau M, Tryggvason K (1995) Mutations in the laminin α 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet 11:216–218 [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP (1999) Dystroglycan inside and out. Curr Opin Cell Biol 11:602–607 [DOI] [PubMed] [Google Scholar]
- Holt KH, Crosbie RH, Venzke D P, Campbell KP (2000) Biosynthesis of dystroglycan: processing of a precursor propeptide. FEBS Lett 468:79–83 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, Segawa M, Yoshioka M, Saito K, Osawa M, Hamano K, Sakakihara Y, Nonaka I, Nakagome Y, Kanazawa I, Nakamura Y, Tokunaga K, Toda T (1998) An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 394:388–392 [DOI] [PubMed] [Google Scholar]
- Kondo-Iida E, Kobayashi K, Watanabe M, Sasaki J, Kumagai T, Koide H, Saito K, Osawa M, Nakamura Y, Toda T (1999) Novel mutations and genotype-phenotype relationships in 107 families with Fukuyama-type congenital muscular dystrophy (FCMD). Hum Mol Genet 8:2303–2309 [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP (1992) Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355:696–702 [DOI] [PubMed] [Google Scholar]
- Ishii H, Hayashi YK, Nonaka I, Arahata K (1997) Electron microscopic examination of basal lamina in Fukuyama congenital muscular dystrophy. Neuromuscul Disord 7:191–197 [DOI] [PubMed] [Google Scholar]
- Leschziner A, Moukhles H, Lindenbaum M, Gee SH, Butterworth J, Campbell KP, Carbonetto S (2000) Neural regulation of α-dystroglycan biosynthesis and glycosylation in skeletal muscle. J Neurochem 74:70–80 [DOI] [PubMed] [Google Scholar]
- Mathews KD, Rapisarda D, Bailey HL, Murray JC, Schelper RL, Smith R (1995) Phenotypic and pathologic evaluation of the myd mouse: a candidate model for facioscapulohumeral dystrophy. J Neuropathol Exp Neurol 54:601–606 [DOI] [PubMed] [Google Scholar]
- Mercuri E, Sewry CA, Brown SC, Brockington M, Jungbluth H, DeVile C, Counsell S, Manzur A, Muntoni F (2000) Congenital muscular dystrophy with secondary merosin deficiency and normal brain MRI: a novel entity? Neuropediatrics 31:186–189 [DOI] [PubMed] [Google Scholar]
- Moghadaszadeh B, Desguerre I, Topaloglu H, Muntoni F, Pavek S, Sewry C, Mayer M, Fardeau M, Tome FM, Guicheney P (1998) Identification of a new locus for a peculiar form of congenital muscular dustrophy with early rigidity of the spine, on chromosome 1p35-36. Am J Hum Genet 62:1439–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanaro F, Lindenbaum M, Carbonetto S (1999) α-Dystroglycan is a laminin receptor involved in extracellular matrix assembly on myotubes and muscle cell viability. J Cell Biol 145:1325–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostacciuolo ML, Miorin M, Martinello F, Angelini C, Perini P, Trevisan CP (1996) Genetic epidemiology of congenital muscular dystrophy in a sample from north-east Italy. Human Genetics 97:277–279 [DOI] [PubMed] [Google Scholar]
- Munro S (1998) Localization of proteins to the Golgi apparatus. Trends Cell Biol 8:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni F, Blake D, Brockington M, Brown S, Hayashi HY, Merlini L, Topaloglu H, Sabatelli P, Voit T, Guicheney P. 6th ENMC sponsored workshop on congenital muscular dystrophy held in Nardeen, The Netherlands, 27–28 October 2000. Neuromuscul Disord (in press) [Google Scholar]
- Muntoni F, Taylor J, Sewry CA, Naom I, Dubowitz V (1998) An early onset muscular dystrophy with diaphragmatic involvement, early respiratory failure and secondary α2 laminin deficiency unlinked to the LAMA2 locus on 6q22. Europ J Paed Neurol 2:19–26 [DOI] [PubMed] [Google Scholar]
- Talim B, Ferreiro A, Cormand B, Vignier N, Oto A, Gogus S, Cila A, Lehesjoki AE, Pihko H, Guicheney P, Topaloglu H (2000) Merosin-deficient congenital muscular dystrophy with mental retardation and cerebellar cysts unlinked to the LAMA2, FCMD and MEB loci. Neuromuscul Disord 10:548–552 [DOI] [PubMed] [Google Scholar]
- Tome FM, Evangelista T, Leclerc A, Sunada Y, Manole E, Estournet B, Barois A, Campbell KP, Fardeau M (1994) Congenital muscular dystrophy with merosin deficiency. C R Acad Sci III 317:351–357 [PubMed] [Google Scholar]
- Villanova M, Mercuri E, Bertini E, Sabatelli P, Morandi L, Mora M, Sewry C, Brockington M, Brown SC, Ferreiro A, Maraldi NM, Toda T, Guicheney P, Merlini L, Muntoni F (2000) Congenital muscular dystrophy associated with calf hypertrophy, microcephaly and severe mental retardation in three Italian families: evidence for a novel CMD syndrome. Neuromuscul Disord 10:541–547 [DOI] [PubMed] [Google Scholar]