Abstract
The development of molecular markers and genomic resources has facilitated the isolation of genes responsible for rare monogenic epilepsies in human and mouse. Many of the identified genes encode ion channels or other components of neuronal signaling. The electrophysiological properties of mutant alleles indicate that neuronal hyperexcitability is one cellular mechanism underlying seizures. Genetic heterogeneity and allelic variability are hallmarks of human epilepsy. For example, mutations in three different sodium channel genes can produce the same syndrome, GEFS+, while individuals with the same allele can experience different types of seizures. Haploinsufficiency for the sodium channel SCN1A has been demonstrated by the severe infantile epilepsy and cognitive deficits in heterozygotes for de novo null mutations. Large-scale patient screening is in progress to determine whether less severe alleles of the genes responsible for monogenic epilepsy may contribute to the common types of epilepsy in the human population. The development of pharmaceuticals directed towards specific epilepsy genotypes can be anticipated, and the introduction of patient mutations into the mouse genome will provide models for testing these targeted therapies.
Keywords: ion channel, seizure, sodium channel, mutation detection
INTRODUCTION
Epilepsy is one of the most common neurological disorders, affecting approximately 3% of individuals at some time in their lives, and is a significant medical burden to patients and to society (36). A strong genetic influence, long suspected, has been confirmed during the past few years by the mapping and isolation of more than 40 genes responsible for monogenic epilepsy in human families and mouse models.
Human epilepsy is a heterogeneous disorder defined by recurrent unprovoked seizures, the clinical manifestation of abnormal synchronized neuronal discharges in the brain. The primary seizure types are generalized seizures, which involve the entire brain from the outset, and partial (focal) seizures, which begin in a localized brain region (19). Classification of epilepsy syndromes combines information on seizure type, age at onset, etiology, clinical course, and electroencephalographic (EEG) findings (20). Idiopathic epilepsy lacks antecedent disease or injury to the central nervous system and is of presumed genetic origin. The current classifications are not well correlated with genetic causes, since the same mutations can produce different syndromes in different individuals, and a single syndrome can be generated by mutations in more than one gene.
All of the genes thus far identified as causing idiopathic epilepsy are molecular components of neuronal signaling. The functional effects of the mutant alleles provide direct evidence for neuronal hyperexcitability as one cellular mechanism underlying seizures. A major challenge for the future is to determine whether these monogenic epilepsy genes also contribute to the common epilepsies that do not have clear patterns of inheritance, and if so, to determine the identity and frequency of the responsible alleles. Identification of the genetic basis for inherited epilepsies provides new therapeutic targets for this frequently debilitating disorder.
In this chapter, we describe the recent progress in identification of idiopathic epilepsy genes in human and mouse, the functional effects of mutated alleles, and the preliminary efforts to evaluate the role of these genes in common epilepsies. Additional information can be found in several excellent reviews (3, 10, 13, 30, 33, 41, 45, 60, 64, 67, 70).
METHODS FOR ISOLATION OF MONOGENIC EPILEPSY GENES
Progress in molecular neurobiology during the past two decades identified many functional candidate genes for epilepsy based on their role in generation and transmission of electrical signals in neurons. Chromosomal map positions provided the key connection between candidate genes and human disorders. During the 1980s and 1990s, cDNA clones were isolated and mapped to specific human chromosome positions using somatic cell genetics, fluorescent in situ hybridization (FISH), and PCR analysis of radiation hybrid panels. During the same period, the development of polymorphic molecular markers for human linkage analysis made it possible to map the loci for clinical epilepsy syndromes found in large family pedigrees. The coincidence of chromosomal positions of candidate genes and disease loci led to the identification of several monogenic human epilepsy genes. The identified human epilepsy genes are listed in Table 1.
TABLE 1.
Identified genes responsible for human monogenic idiopathic epilepsy
| Year | Gene | Chromosome | MIM | Mode | Types of mutant alleles | Clinical syndrome |
|---|---|---|---|---|---|---|
| 2001 | GABRG2 GABAA receptor | 5q31 | 604233 | AD | Missense | GEFS+3 |
| 2001 | SCN2A sodium channel alpha subunit | 2q24 | 604233 | AD | Missense | GEFS+ |
| 2000 | SCN1A sodium channel alpha subunit | 2q24 | 604233 | AD | Missense null, missense | GEFS+2 |
| AD | SMEI | |||||
| 2000 | CHRNB2 acetylcholine recepter beta subunit | 1p21 | 605375 | AD | Missense | ADNFLE3 |
| 1998 | SCN1B sodium channel beta 1 subunit | 19q13 | 604233 | AD | Missense | GEFS+1 |
| 1998 | KCNQ2 potassium channel | 20q13 | 602235 | AD | Missense, null | BFNC1 (EBN1) |
| 1998 | KCNQ3 potassium channel | 8q24 | 121201 | AD | Missense | BFNC2 (EBN2) |
| 1995 | CHRNA4 acetylcholine receptor alpha | 20q13 | 600513 | AD | Missense | ADNFLE1 |
AD, autosomal dominant; GEFS+, Generalized epilepsy with febrile seizures plus; SMEI, severe myoclonic epilepsy of infancy; ADNFLE, autosomal dominant nocturnal frontal lobe epilepsy; BFNC, benign familial neonatal convulsions.
Mouse epilepsy genes have been isolated using experimental crosses with thousands of informative meioses that define small nonrecombinant regions of 0.5 to 1 Mb. Isolation of large-insert clones spanning the nonrecombinant region and identification of genes in the nonrecombinant region have been streamlined by new genomic resources. The availability of ordered BAC clone contigs spanning the mouse genome has eliminated the need to screen clone libraries, and the gene content of most regions can now be obtained electronically from the assembled sequence of the corresponding human chromosome region. With these methods, the time required to map and clone an epilepsy mutation has been greatly reduced, and several spontaneous mouse mutations associated with well-characterized seizures have been cloned (Table 2). A publicly funded initiative to generate additional seizure models in the mouse by chemical mutagenesis will provide increased opportunities for applying these methods to identification of epilepsy genes.
TABLE 2.
Epilepsy genes identified in spontaneous mouse mutants. For details see the Mouse Genome Database (MGD) website at www.informatics.jax.org. The chromosomal locations of the human orthologs are indicated
| Category | Gene | Mouse chr | Protein | Mutant | Mutation | Modea | Seizure type | Human chromosome |
|---|---|---|---|---|---|---|---|---|
| Channels receptors | Cacna1a | 8 | Voltage-gated calcium channel α subunit | tottering leaner rolling-Nagoya | Missense truncation | AR | Spike wave, focal motor | 19p13 |
| Cacnb4 | 2 | Voltage-gated calcium channel β4 subunit | lethargic | Null | AR | Spike wave | 2q22 | |
| Cacna2d2 | 9 | Voltage-gated calcium channel α2δ2 subunit | ducky torpid | Null | AR | Spike wave | 3p21 | |
| Cacng2 | 15 | Voltage-dependent calcium channel | stargazer | Null | AR | Spike wave | 22 | |
| γ2 subunit OR receptor transporter | waggler | |||||||
| Kcnj6 | 16 | G-protein gated inwardly-rectifying K+ channel (GIRK2) | weaver | Missense | AR | Tonic-clonic | 21q22 | |
| Itpr1 | 6 | Inositol 1,4,5-triphosphate receptor | opisthotonos | In-frame deletion | AR | Tonic-clonic | 3p26 | |
| pH Homeostasis | Slc9a1 | 4 | Na+/H+ exchanger | slow wave epilepsy | Null | AR | Spike wave tonic-clonic | 1p36 |
| Intracellular transport | Myo5a | 9 | Myosin Va | dilute-neurological | Null | AR | 15q21 | |
| Ap3d | 10 | Adaptor-related protein complex AP-3, delta | mocha | Null | AR | 19p13 | ||
| Myelination | Pmp22 | 11 | Peripheral myelin protein | trembler | Several | AD | Tonic-clonic | 17p12 |
| Plp | X | Myelin proteolipid protein | jimpy | Several | XR | Xq21 | ||
| Membrane protein | Mass1 | 13 | Monogenic audiogenic seizure susceptibility 1 | Frings | Truncation | AR | Audiogenic | 7 |
AR, autosomal recessive; AD, autosomal dominant; XR, X-linked recessive.
IDENTIFICATION OF MUTATIONS IN ION CHANNEL GENES
The propagation of the electrical impulse in neurons is initiated by the transient opening of voltage-gated sodium channels and influx of sodium ions along a concentration gradient. The impulse is terminated by the transient opening of voltage-gated potassium channels that permit the efflux of potassium and restoration of the resting potential of the cell. Voltage-gated calcium channels in the axon terminal convert the electrical signal to a chemical signal via influx of calcium ions, leading to release of synaptic vesicles containing neurotransmitters. This release activates ligand-gated receptors in the postsynaptic membrane and initiates an electrical impulse in the downstream neuron. The shared domain structure of the voltage-gated potassium, sodium, and calcium channels demonstrates their evolutionary origin from a common ancestral protein (38). Predictions that mutations in these channels and receptors could produce disregulated neuronal firing have been confirmed by the identification of disease-causing mutations in human and mouse.
Voltage-Gated Sodium Channels
The voltage-gated sodium channels contain a large pore-forming transmembrane α subunit of 260 kDa that is capable of generating a sodium current in response to membrane depolarization (15). The α subunit can associate with three auxiliary β subunits of 35 kDa that influence the rate of channel inactivation and intracellular localization (15). Four of the ten α subunit genes in the mammalian genome are expressed at high levels in the central nervous system: SCN1A, SCN2A, SCN3A, and SCN8A (47).
GENERALIZED EPILEPSY WITH FEBRILE SEIZURES PLUS
The first evidence of sodium channel mutations in epilepsy was obtained in 1998 by analysis of a large Australian family with 378 members, including 42 with a history of epilepsy (76). The phenotype in affected family members was highly variable and included febrile (fever-induced) seizures persisting beyond the usual termination age of six years, generalized epilepsy involving absence seizures, myoclonic, atonic or tonic-clonic seizures, and partial epilepsy. This syndrome was designated Generalized Epilepsy with Febrile Seizures Plus (GEFSP; MIM 604233) (63). The locus GEFS+1 was mapped to chromosome 19q13, where the sodium channel β1 subunit gene SCN1B had been previously mapped. Exon sequencing identified a missense mutation, C121W, that cosegregated with the disease and was not observed in 96 controls (76). In functional assays, the mutant protein failed to accelerate the recovery from inactivation of the associated α subunit (76) (Table 3). Coexpression of mutant and wild-type β subunits with the α subunit produced an intermediate rate of inactivation (48), indicating that the inactive mutant subunit can compete for binding to the α subunit in heterozygotes and accounting for the dominant inheritance of the disorder. In heterozygotes, the association of inactive β subunits with α subunits is predicted to produce a population of channels that would inactivate slowly and generate “persistent current.” A neuron with persistent sodium current will require a smaller depolarization to initiate firing, and thus may be considered to be in a hyperexcitable state.
TABLE 3.
Electrophysiological effects of dominantly inherited epilepsy mutations in voltage-gated sodium channels
| Gene | Syndrome | Mutant allele (domain) |
Properties of isolated mutant channel |
Predicted cellular effects |
Types of seizures in family members |
Reference for electrophysiology |
|---|---|---|---|---|---|---|
| SCN2A | GEFS+ | R187W (D2S6) trans-membrane | Reduced rate of inactivation | Increased persistent current leading to lower threshold for firing of action potentials | Febrile, brief afebrile generalized tonic and tonic/clonic | (72) |
| SCN2A (mouse) | Temporal lobe epilepsy | GAL/QQQ(D2S4/5) cytoplasmic linker | Reduced rate of inactivation | Increased persistent current leading to lower threshold for firing of action potentials | Focal seizures originating in hippocampus | (42) |
| SCN1B | GEFS+ | C121W extra-cellular (beta) | Reduced rate of inactivation of associated alpha subunits | Increased persistent current leading to lower threshold for firing of action potentials | Febrile, absense, myoclonic-astatic | (48, 74) |
| SCN1A | GEFS+ | R1648H (D4S4) trans-membrane | Rapid recovery from inactivation | Propensity for repetitive firing | Febrile, absence, myoclonic, generalized tonic/clonic | (66) |
| SCN1A | GEFS+ | T875M (D2S4) trans-membrane | Enhanced slow inactivation | Reduced channel activity | Febrile, absense, generalized tonic/clonic | (66) |
| SCN1A | SMEI | Null | Complete loss of activity | Reduced channel activity to 50% in affected heterozygotes | Febrile, generalized tonic/clonic, absence, myoclonic, partial | (18) |
A second locus, GEFS+2, was mapped in 1999 to a 20-cM interval of chromosome 2q24 that contained the α subunit genes SCN1A, SCN2A, and SCN3A by analysis of two large families (6, 50). Screening affected individuals from both families using conformation sensitive gel electrophoresis of amplified exons identified two missense mutations in the SCN1A gene, R1648H and T875M (27). Both mutations changed evolutionarily invariant residues located in the voltage-sensing S4 segments of the protein. Introduction of these mutations into the SCN1A channel and examination of the kinetic properties in Xenopus oocytes demonstrated that the R1648H mutation accelerated the recovery from inactivation and decreased the use dependence of channel activity (66). This accelerated recovery could lead to rapid firing patterns and neuronal hyperexcitability. Similar effects were observed when the corresponding mutation was introduced into SCN4A (2). The second mutation, T875M, increased the likelihood of inactivation by the slow inactivation mode, which would reduce the proportion of channel protein available for opening. The effect of this “functional hypomorph” may be similar to the null mutations described below. Four additional missense mutations in SCN1A have been identified in families with GEFS+, but their functional effects have not been described (26, 75).
SCN1A is physically located in a 1 Mb cluster with SCN2A and SCN3A (26). The three genes share 85% amino acid sequence identity and are coexpressed in neurons, but they differ in subcellular distribution and levels of expression in different types of neurons. A mutation in SCN2A was recently identified in a Japanese family with GEFS+ (72). The mutation, R187W, resulted in delayed channel inactivation, which could increase sodium influx and neuronal excitability. The mutation was not observed in 224 alleles from unaffected individuals. Overlapping clinical syndromes thus result from certain mutations in three sodium channel genes, SCN1A, SCN2A, and SCN1B.
SEVERE MYOCLONIC EPILEPSY OF INFANCY
Children with Severe Myoclonic Epilepsy of Infancy (SMEI) experience febrile seizures that progress to frequent severe afebrile seizures, delayed psychomotor development, ataxia, and myoclonic episodes. The elevated incidence of epilepsy in relatives of children with SMEI suggested a genetic predisposition in some cases (9, 64a). Because of the association of SCN1A with febrile seizures in GEFS+, Claes and colleagues screened Belgian children with SMEI for mutations in SCN1A (18). Seven de novo mutations were identified in affected children that were not present in either parent. Six of these mutations are frameshift or nonsense mutations resulting in null alleles with complete loss of function. These observations demonstrate for the first time that quantitative deficiency in a sodium channel can cause disease. The haploinsufficiency of human SCN1A contrasts with the recessive inheritance of null alleles of other sodium channels in the mouse (47). This work demonstrates that de novo mutations may be responsible for sporadic cases of epilepsy. In the future, it will be worthwhile including transcriptional regulatory regions of the sodium channel genes in mutational screening; this will require that the transcription start sites of these genes be identified.
TEMPORAL LOBE EPILEPSY IN THE Q54 MOUSE
Specific disease mechanisms can be tested in mouse models by introducing a gain-of-function mutation by microinjection of a transgene construct. The mutation GAL879-881QQQ in sodium channel SCN2A is located in the S4–S5 linker of transmembrane domain 2 and results in delayed inactivation and increased persistent current in Xenopus oocytes. Transgenic mice carrying this mutation exhibit a progressive seizure disorder that begins between 1 and 2 months of age and has several features of human temporal lobe epilepsy (42). Continuous EEG and video monitoring detected focal seizure activity originating in the hippocampus. During seizures the mice exhibit behavioral arrest and stereotyped repetitive behaviors. There is progressive cell loss and gliosis in the CA1–CA3 and hilus, reminiscent of the hippocampal sclerosis seen in patients with temporal lobe epilepsy (40). Recordings of hippocampal CA1 neurons from presymptomatic mice detected a 50% increase in the amount of persistent sodium current between action potentials, which may increase the resting membrane potential of the cells and lead to hyperexcitability. The lifespan of these mice is greatly reduced. The Q54 mice can be genotyped presymptomatically, making them a valuable model for early interventions, and for determining whether cell loss and gliosis precede seizure activity. The Q54 mouse provides another example of seizures resulting from delayed inactivation of a voltage-gated sodium channel.
The sodium channel mutations demonstrate three types of genetic heterogeneity in epilepsy: (a) mutation in a single gene can generate different syndromes (SCN1A in GEFS+and SMEI); (b) a single mutation can generate different types of seizures within a family (affected individuals in GEFS+ families); (c) mutations in different genes can produce the same syndrome (SCN1A, SCN2A, and SCN1B in GEFS+).
The electrophysiological effects of six sodium channel mutations have been measured in in vitro assays (Table 3). The abnormal properties of the isolated channels predict at least three different mechanisms of abnormal firing at the cellular level. The most common observation was delayed channel inactivation resulting in persistent sodium current. At the cellular level, persistent current may lead to seizures by reducing the threshold for firing of successive action potentials. This is also the most common defect associated with disease mutations of the skeletal and cardiac muscle sodium channels (3). An unusual mechanism was observed for the R1648H form of SCN1A, which recovers from inactivation more rapidly than wild-type channels and may increase the frequency of firing of action potentials. Surprisingly, alleles of SCN1A associated with decreased activity, T875M and the null mutations in SMEI, also predispose to seizures (Table 3). This effect of low activity may be unique to SCN1A due to aspects of intracellular localization, regional expression pattern, or a unique role in inhibitory neurons. It would be interesting to determine whether mice with reduced levels of the channels SCN2A and SCN8A are susceptible to seizures. SCN3A and SCN8A, which are expressed at high levels throughout the human brain, have not yet been screened for mutations in epilepsy families.
Voltage-Gated Potassium Channels
Two classes of voltage-gated potassium channels have been associated with seizures, the Kv channels and the KCNQ channels. The Kv channel KCN1A is involved in the recovery phase of the action potential. Like the delayed inactivation mutants of SCN1A and SCN2A, loss of function of this channel would result in prolonged sodium currents. Targeted inactivation of KCN1A in the mouse resulted in development of spontaneous tonic-clonic seizures that occur with high frequency beginning at 3 weeks of age and continue throughout adult life (65). Mutations of human KCN1A, which have as their primary effect an episodic ataxia (EA1), also predispose to seizures (78). Targeted inactivation of the Kv3.2 channel in the mouse resulted in increased seizure susceptibility in homozygotes (43).
The KCNQ2 and KCNQ3 proteins interact to generate the M-type current, a slowly activating and deactivating potassium conductance that contributes to sub-threshold electroexcitability of neurons and their responsiveness to synaptic inputs. The effect of the M current is to reduce neuronal excitability. Loss-of-function mutations for the potassium channels KCNQ2 and KCNQ3 have been identified in families with inherited benign neonatal convulsions, EBN1 (MIM#121200) and EBN2 (MIM#121201) (Table 1).
Voltage-Gated Calcium Channels
The voltage-gated calcium channel is composed of a large α subunit and three accessory subunits, β, γ, and α2δ. Combinations of the products of multiple genes for each subunit generate a large number of molecular isoforms of the channel. Complete deficiency of the alpha subunit results in severe ataxia and late onset neurodegeneration (29a). Seven spontaneous mouse mutations in calcium channel subunits produce spike-wave epilepsy and ataxia, suggesting that human orthologs may be involved in absence epilepsy. Three mutations in the α subunit gene Cacna1a were identified by positional cloning. The tottering allele substitutes leucine for a highly conserved proline in the S5–S6 linker of domain II (29). The Nagoya mutation is an arginine-to-glycine substitution in the voltage-sensing S4 segment of domain III (49). Leaner mice have a splice site mutation in the coding region for the C-terminal domain of Cacna1a, which results in truncation of the open reading frame and expression of aberrant C-terminal sequences (29). A significant reduction in calcium current density was recorded from Purkinje cells of tottering mice (28). A splice site mutation in the β4 subunit gene Cacnb4 that produces a truncated, nonfunctional protein was identified in the mouse mutant lethargic (12). lethargic mice exhibit absence seizures, lethargy, and ataxia, and go through a crisis during the third week of life. Both lethargic and tottering mice exhibit decreased glutamatergic synaptic transmission in thalamic neurons, suggesting that the α and β4 subunits are required for neurotransmitter release specific to glutaminergic synapses (14). A survey of human epilepsies identified two potential disease mutations in the ortholog CACNB4 (25). Spontaneous mutations of the α2δ2 subunit arose in the mouse mutants ducky (4a) and torpid, which exhibit abnormal gait and seizures as well as dysgenesis of hindbrain and spinal cord (4, 5). Positional cloning of the mouse mutant stargazer identified a new protein with 25% amino acid sequence identity to the muscle-specific γ subunit of the voltage-gated calcium channel (44). Coexpression of the mutant subunit with the wild-type α subunit resulted in minor alterations in channel properties (16, 41a, 44). Recent work indicates that another function of the stargazin protein is synaptic localization of AMPA receptors, which are missing from cerebellar granule cells of stargazer mice (16).
Ligand-Gated GABAA Receptors
GABAA receptors are ligand-gated chloride channels composed of a pentameric assembly of homologous subunits (46). Since GABAA receptors mediate synaptic inhibition, mutations with reduced activity could produce neuronal hyperexcitability. The most abundant receptor isoform in brain is the α1β2γ2 receptor. No mutations were detected in these subunit genes by direct mutation screening (53) or association analysis (61) in families with idiopathic generalized epilepsy. However, in 2001 the third locus for Generalized Epilepsy with Febrile Seizures+, GEFS+3, was mapped to chromosome 5q34 in two large pedigrees from France and Australia (7, 74). The α1 and γ2 genes are both located in this chromosome region, and exon sequencing identified γ2 mutations in both families. In the French family, the mutation K289M in the extracellular domain was present in all 13 affected members, 2 obligate carriers, and 1 asymptomatic individual (7). Affected individuals in the Australian family carry the mutation R43Q, located in a high-affinity benzodiazepine-binding domain (74).
The functional effects of the γ2 mutations were tested by electrophysiological analysis in Xenopus oocytes. Channels formed by coinjection of α1, β2, γ2, and γ2 subunit mRNAs generate large inward currents over a range of GABA concentrations. The effect of the K289M mutation was to reduce maximum current amplitude by 90% at all GABA concentrations (7). This is consistent with the pre-diction that loss of GABAA receptor activity would lead to hyperexcitability and seizure susceptibility. The R43Q subunit exhibited normal activity but failed to be activated by diazepam (74). Oocytes injected with equal amounts of wild-type and mutant γ2 together with α1 and β2 subunits displayed intermediate diazepam potentiation. The pathological effect of this mutation suggested to the authors that the GABAA receptor may be regulated in vivo by an unidentified endogenous diazepam-like molecule. The discovery of the γ2 mutations is likely to stimulate renewed attention to the other GABAA receptor subunits as candidates for epileptic disorders.
Ligand-Gated Acetylcholine Receptors
Like the GABAA receptor, the neuronal nicotinic acetylcholine receptor is a pentameric assembly of homologous subunits that mediates rapid synaptic transmission. This receptor has a nonselective cation pore. The major isoform in the brain is composed of α4 and β2 subunits encoded by the CHRNA4 and CHRNB2 genes. Mutations in both of these subunits have been associated with the disorder autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE, MIM 600513), characterized by brief seizures during light sleep that originate in the frontal lobe. The locus ENFL1 was mapped to chromosome 20q13 in an Australian pedigree (55). The α4 subunit mutation S248F was identified in this family and was the first known human epilepsy mutation (69). A second α4 mutation was later found in a Norwegian family (68).
The ADNFLE locus ENFL3 was mapped to chromosome 1p21 in a three-generation pedigree from southern Italy (32). The acetylcholine β2 subunit gene had previously been mapped to this location, and molecular analysis identified the amino acid substitution V287L (23). The mutation was present in eight affected individuals and four unaffected family members, demonstrating incomplete penetrance. The mutated residue is located in the second transmembrane domain that forms the channel pore. The electrophysiological effects of V287L were examined by patch clamp analysis of transfected HEK cells (23). Application of nicotine to wild-type α4β2 channels results in rapid activation of an inward current that is followed by desensitization. The rate of desensitization was reduced in the V287L channel, and an intermediate rate of desensitization was observed when equal amounts of wild-type and mutant β2 were injected. Slowed desensitization could lead to prolonged currents and increased neuronal excitability in response to cholinergic stimulation. Another mutation at the same amino acid residue, V287M, was identified in a Scottish family (54). V287M increased channel sensitivity to acetylcholine by approximately tenfold, with no change in the desensitization properties of the channel measured in Xenopus oocytes (54). This property is also consistent with increased neuronal excitability.
ION CHANNEL MUTATIONS AND NEURONAL HYPEREXCITABILITY
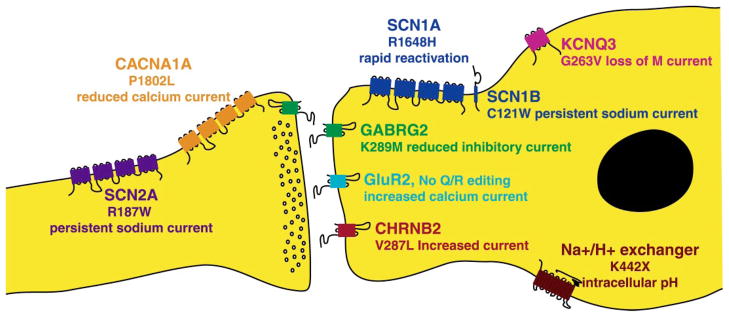
Many of the functional defects in the mutated voltage-gated and ligand-gated channels described above are predicted to increase the intrinsic excitability of neurons. Increased excitability could lead to increased neuronal firing and to episodes of synchronized firing by large numbers of neurons that constitute a seizure. The characteristics of these mutant channels strongly support this hypothesis regarding the origin of seizures. Examples of mutations predicted to predispose to neuronal hyperexcitability are shown in Figure 1.
Figure 1.
Mechanisms of ion channel mutations in idiopathic epilepsy. The functional effects of representative mutations in neuronal channels and their contributions to neuronal hyperexcitability are indicated.
SEIZURES IN MICE WITH TARGETED INACTIVATION OF ENDOGENOUS GENES
Homologous recombination in embryonic stem cells has been used for targeted inactivation (knock-out) of several thousand mouse genes during the past decade. Approximately two dozen of these lines, or 1% of the total, have been described as exhibiting spontaneous seizures (57) (for update, see Table 4 in the Supplemental Material link in the online version of this chapter or at http://www.annualreviews.org/). These genes may be considered candidate genes for human disorders mapped to the corresponding chromosome regions. If the inactivated genes are representative of the genome, several hundred genes might be targets for epilepsy mutations.
RNA Editing of Glutamate Receptor Subunits
The contribution of RNA editing of ligand-gated glutamate receptors to seizure susceptibility has been revealed by targeted mutations in the GluR2, GluR6, and Adar2 genes of the mouse (58). GluR2 and GluR6 are related subunits of the neuronal AMPA and kainate glutamate receptors, respectively. The GluR2 and GluR6 transcripts are edited at the Q/R site, resulting in substitution of an arginine residue for glutamine that reduces calcium permeability and changes current/voltage relationships of the channel. Mice with a noneditable allele of the GluR2 gene exhibit severe epilepsy and early lethality (11), and mice with a noneditable site in the GluR6 gene demonstrated increased sensitivity to kainate-induced seizures (73). Severe epilepsy was also observed in mice lacking the enzyme that edits the Q/R site, ADAR2 (37). The seizures and lethality of the Adar2 null mice could be rescued by a pre-edited allele of the GluR2 gene, indicating that this transcript is the physiologically most important substrate for ADAR2 (37). Although glutamate receptor mutations have not been identified in human or mouse epilepsy, these experiments demonstrate that their coding sequences and intronic elements, as well as the editing enzymes, are potential targets for epileptogenic mutations. The voltage-gated sodium and calcium channels of Drosophila undergo RNA editing at multiple sites (58), but efforts to detect editing of the corresponding sites of the mammalian channels have been unsuccessful.
The Sodium/Hydrogen Transporter SLC9A
Targeted inactivation of the sodium/hydrogen transporter, a ubiquitously expressed transmembrane protein that functions in regulation of intracellular pH by export of H+ ions in exchange for extracellular sodium ions, confirmed the earlier studies of a spontaneous mouse mutant, slow wave epilepsy (swe) (22) (Table 3). Homozygous swe mice exhibit spontaneous generalized tonic-clonic seizures as well as 3/sec spike-wave discharges accompanied by behavioral arrest that resemble human absence seizures. Survival and seizure intensity in swe homozygotes are influenced by genetic background. The targeted allele of Slc9A results in a similarly severe phenotype (8), demonstrating the sensitivity of neurons to intracellular pH.
IDENTIFYING GENES RESPONSIBLE FOR HUMAN EPILEPSIES LACKING CLEAR MODES OF INHERITANCE
The genes identified so far in human epilepsies have been found in families with clear Mendelian forms of inheritance and sufficient numbers of meioses to support positional cloning. However, Mendelian syndromes in large families comprise only a small proportion of all epilepsy. In most forms of epilepsy the genetic influences are complex and may involve the combined effects of multiple genes and environmental factors, each with a small effect on susceptibility. Identification of the genes involved in epilepsies with complex inheritance presents major challenges.
Linkage Analysis Using Collections of Small Families
Phenotype definition for linkage analysis is one of the most difficult problems in study design when multiple families are combined for analysis. Observed cosegregation of a spectrum of clinical features in a Mendelian inheritance pattern can provide the basis for defining a syndrome, as was done for GEFS+ (64), autosomal dominant partial epilepsy with auditory features (52), and familial partial epilepsy with variable foci (77). Alternatively, families can be selected for analysis based on the correspondence of the symptoms of affected family members with the defined clinical epilepsy syndromes (20). This approach raises difficult questions about how to define the phenotype. For example, in studies of the families of probands with juvenile myoclonic epilepsy, affected family members also have a range of idiopathic generalized epilepsy syndromes such as pyknolepsy, juvenile absence, and awakening grand mal. In the absence of clear information about which syndromes should be assumed to result from the susceptibility gene, many studies have used several alternative phenotype definitions (e.g., juvenile myoclonic epilepsy only, all idiopathic generalized epilepsies, or idiopathic generalized epilepsies plus EEG abnormalities without clinical seizures). Similarly, in the absence of a clear genetic model, LOD scores are sometimes estimated under multiple different models of penetrance and mode of inheritance. The use of multiple phenotype definitions and mode-of-inheritance assumptions inflates the type 1 error rates, and hence adjustment must be made to correct for this (39). Despite these problems, consistent evidence has been obtained for linkage of a susceptibility gene to the HLA region of chromosome 6p in families ascertained through subjects with juvenile myoclonic epilepsy.
In a linkage study of 130 families with idiopathic generalized epilepsy, Sander et al. provided evidence for a novel susceptibility locus on 3q26 (62). Suggestive LOD scores were also obtained for regions of chromosomes 2 and 14. Evidence for a susceptibility gene on chromosome 18 was obtained from a genome scan of 91 families with idiopathic generalized epilepsy (24). This study concluded that genetic classification cuts across syndrome classifications and that several interacting genes influence risk for idiopathic epilepsies (24). These complexities may explain why the gene on chromosome 6p has yet to be identified.
Association Studies Using Candidate Genes
Mild mutations in genes already identified as causing autosomal dominant forms of epilepsy, when inherited together with other predisposing mutations, may produce a state of neuronal excitability sufficient to generate seizures. To test the role of identified monogenic epilepsy genes, several studies have screened patients and controls for coding variants. These studies can potentially detect common variants that are present at higher frequencies in patients than in controls, as well as rare variants found only in patients, either of which could underlie the common epilepsies (56).
Based on the identification of SCN1A mutations in GEFS+2, we screened 226 additional patients for mutations in SCN1A (26). The sample included probands from 165 families containing multiple affected individuals (83 childhood or juvenile absence epilepsy, 72 juvenile myoclonic epilepsy, 4 generalized tonic clonic seizures, and 6 febrile seizures) and 61 sporadic patients with generalized epilepsy. One substitution affecting an evolutionary conserved residue (W1204R) was identified in a GEFS+family with febrile seizures, JME, and other generalized seizures. Seven other coding variants were detected, but three were discordant with disease, three were observed in sporadic cases and could not be followed up, and one was a common polymorphism, T1067A, with the same allele frequencies of 0.66 and 0.33 in patients and in controls. In a similar study, Wallace and colleagues tested 53 probands with phenotypes consistent with GEFS+, including 36 familial and 17 isolated cases (75). Six mutations (three in SCN1A and three in SCN1B) were identified in the familial samples. No mutations were found in the sporadic samples. They estimate that SCN1A and SCN1B account for 17% of familial GEFS+.
Based on the calcium channel β4 subunit gene mutation in the lethargic mouse, we screened 90 human families with dominantly inherited idiopathic generalized epilepsy for mutations in CACNB4, including 19 with childhood absence epilepsy, 22 with juvenile absence epilepsy, and 49 with juvenile myoclonic epilepsy. The premature terminating mutation R482X that eliminates 38 amino acids at the C-terminal end was identified in one patient with juvenile myoclonic epilepsy. The amino acid substitution C104F was identified in one family with generalized epilepsy and praxis-induced seizures, and also in another family with episodic ataxia (25). Neither mutation was observed in 510 control chromosomes. Electrophysiological analysis in Xenopus oocytes revealed that the R482X mutation increased the rate of inactivation of the coexpressed α1A subunit. This is predicted to reduce the net inward flow of calcium into neurons during the rapid alteration in membrane potential associated with an action potential. The C104F mutation did not significantly alter channel kinetics. It is difficult to prove disease causality for rare variants identified in small families that do not alter evolutionarily conserved residues or have dramatic effects on protein function. We consider R482X and C104F to be “potential disease mutations,” and have proceeded to test them in a mouse model by generating transgenic mice expressing the R482X and C104F cDNAs in neurons. If these mutations are responsible for the dominant disorders in the original families, we would predict the development of seizures or seizure-susceptibility in the transgenic mice. These studies are still in progress.
KCNQ2 and KCNQ3 are mutated in BNFC types 1 and 2. However, screening of a large collection of patients with common forms of idiopathic generalized epilepsy failed to identify additional mutations (35, 71). Analysis of noncoding polymorphisms in the voltage-gated calcium channel CACNA1A provided evidence for possible association with generalized idiopathic epilepsy (17).
Another approach was taken to test the acetylcholine receptor subunits as candidate genes in a group of small families with benign epilepsy of childhood with centrotemporal spikes (51). A partial genome scan was carried out using markers in the chromosome regions containing the receptor subunit genes. Analysis using an “affecteds only” model gave evidence for linkage to chromosome 15q14 in an estimated 70% of families. It will be interesting to see whether this finding is confirmed in other studies.
FUTURE PROSPECTS
Additional Monogenic Epilepsy Genes in Human
At least 15 additional loci have been mapped in human (see Table 5 in the Supplemental Material link in the online version of this chapter or at http://www.annualreviews.org), and responsible genes will likely be identified in the near future. The assembled, annotated human genome sequence provides a nearly complete list of candidate genes for the nonrecombinant intervals identified by linkage analysis in large families, and testing these genes for mutations can be carried out on a large scale by manual methods such as CSGE or SSCP gels or with automated methods such as dHPLC. In view of the severe clinical phenotypes of SMEI patients with reduced expression of SCN1A, it would be worthwhile to screen for variation in transcriptional regulatory regions. This will require experimental identification of the transcription start sites for the candidate genes, information not yet available for most of the epilepsy genes in Tables 1 and 2. Indirect evidence for hypomorphic alleles can be obtained by determining the ratio of allelic transcripts in RNA from individuals who are heterozygotes for SNPs in transcribed sequences. Underrepresentation of one allele could be caused by a mutation in regulatory sequences or reduced mRNA stability due to a transcribed variant. The mouse calcium channel mutations lethargic (12) and spike wave epilepsy (22) both reduce mRNA stability in addition to changing the protein sequence.
New Monogenic Epilepsies from Large-Scale Chemical Mutagenesis in the Mouse
A large-scale effort is in progress to generate novel mouse mutants with neurological disorders by in vivo chemical mutagenesis with ethylnitroso urea (ENU). The NIH-sponsored mutagenesis center at the Jackson Laboratory is screening for mutations that decrease the threshold for seizures after electroconvulsive shock (31). Already several seizure-prone models have emerged (e.g., http://www.jax.org/resources/documents/nmf/), and all mutants will be available to interested investigators. Genetic mapping of large numbers of mutations and isolation of the affected genes has promise for identification of additional molecular pathways involved in epileptogenesis. Improvements in imaging and automated behavioral monitoring in rodents will increase the utility of these models for understanding the pathogenesis of seizures.
Large-Scale Mutation Detection in Patients with Common Forms of Epilepsy
Application of efficient large-scale genomic screening methods to large populations of patients with common types of epilepsy will permit identification of underlying genetic variation correlated with the disease. “Resequencing chips” can detect any variant in a target sequence such as a panel of candidate genes, using genomic DNA as substrate (34). Evaluating the functional significance of rare variants is a major challenge (59), as gene frequencies may not differ between patients and controls for common susceptibility alleles and functional assays are not available for many proteins. Testing candidates by generating mouse models carrying the human mutations is feasible, but the high cost and effort involved will limit the application of this approach.
Development of Individualized Pharmacogenetic Therapies
A major motivation for research on epilepsy genetics is development of better pharmacological treatment for this debilitating disorder. An example of the future possibilities of allele-specific therapy for ion channel mutations is provided by recent work on an allele of the cardiac sodium channel gene SCN5A in the Brugada syndrome (1). In vitro analysis of the D1790G mutation in SCN5A predicted that the mutant channel would be resistant to the commonly used drug lidocaine, but would be responsive to another inhibitor, flecainide. Administration of flecainide to patients carrying the mutation confirmed the prediction, enabling these patients to receive an effective therapeutic agent that might not have been tried without knowledge of their specific mutation. This is an encouraging example of the practical applications that may be expected as the mutations responsible for epilepsy in individual patients are identified.
The past five years have seen many breakthroughs, with the identification of epilepsy genes providing new insight into molecular mechanisms of the disease. Emerging technologies for high-throughput mutation screening will greatly expand the ability to detect patient mutations. Continuing progress into the genetic basis of epilepsy will provide the basis for development of urgently needed new therapies.
Acknowledgments
Jane Santoro provided expert assistance with preparation of the manuscript. We gratefully acknowledge research support from the National Institutes of Health (GM24872, NS34509, and NS20656), the March of Dimes, the Epilepsy Foundation, and the Muscular Dystrophy Association of America.
Footnotes
This chapter is dedicated to Roslyn Klaif in appreciation of her courage and inspiration.
Visit the Annual Reviews home page at www.AnnualReviews.org
LITERATURE CITED
- 1.Abriel H, Wehrens XH, Benhorin J, Kerem B, Kass RS. Molecular pharmacology of the sodium channel mutation D1790G linked to the long-QT syndrome. Circulation. 2000;102:921–25. doi: 10.1161/01.cir.102.8.921. [DOI] [PubMed] [Google Scholar]
- 2.Alekov AL, Rahman MM, Mitrovic N, Lehmann-Horn F, Lerche H. A sodium channel mutation causing epilepsy in man exhibits subtle defects in fast inactivation and activation in vitro. J Physiol. 2000;529:533–39. doi: 10.1111/j.1469-7793.2000.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashcroft F. Ion Channels and Disease. San Diego: Academic; 2000. [Google Scholar]
- 4.Balaguero N, Barclay J, Mione M, Canti C, Brodbeck J, et al. Reduction in voltage-dependent calcium channel function in cerebellar purkinje cells of the mouse mutant ducky, which has a null mutation for the calcium channel accessory subunit α2δ2. Soc Neurosci Abstr. 2000;26:365. [Google Scholar]
- 4a.Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar purkinje cells. J Neurosci. 2001;21:6095–104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barclay J, Rees M. Genomic organization of the mouse and human α2δ2 voltage-dependent calcium channel subunit genes. Mamm Genome. 2000;11:1142–44. doi: 10.1007/s003350010211. [DOI] [PubMed] [Google Scholar]
- 6.Baulac S, Gourfinkel-An I, Picard F, Rosenberg-Bourgin M, Prud’homme JF, et al. A second locus for familial generalized epilepsy with febrile seizures plus maps to chromosome 2q21–q33. Am J Hum Genet. 1999;65:1078–85. doi: 10.1086/302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, et al. irst genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ 2-subunit gene. Nat Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- 8.Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, et al. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol. 1999;276:C788–95. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- 9.Benlounis A, Nabbout R, Feingold J, Parmeggiani A, Guerrini R, et al. Genetic predisposition to severe myoclonic epilepsy in infancy. Epilepsia. 2001;42:204–9. [PubMed] [Google Scholar]
- 10.Berkovic S, Ottman R. Molecular genetics of the idiopathic epilepsies: the next steps. Epileptic Disord. 2001;2:179–81. [PubMed] [Google Scholar]
- 11.Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, et al. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–80. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- 12.Burgess DL, Jones JM, Meisler MH, Noebels JL. Mutation of the Ca2+ channel β subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–92. doi: 10.1016/s0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- 13.Burgess DL, Noebels JL. Calcium channel defects in models of inherited generalized epilepsy. Epilepsia. 2000;41:1074–75. doi: 10.1111/j.1528-1157.2000.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 14.Caddick SJ, Wang C, Fletcher CF, Jenkins NA, Copeland NG, Hosford DA. Excitatory but not inhibitory synaptic transmission is reduced in lethargic (Cacnb4Ih) and tottering (Cacna1atg) mouse thalami. J Neurophysiol. 1999;81:2066–74. doi: 10.1152/jn.1999.81.5.2066. [DOI] [PubMed] [Google Scholar]
- 15.Catterall WA. Molecular properties of brain sodium channels: an important target for anticonvulsant drugs. Adv Neurol. 1999;79:441–56. [PubMed] [Google Scholar]
- 16.Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–43. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 17.Chioza B, Wilkie H, Nashef L, Blower J, McCormick D, et al. Association between the α1a calcium channel gene CACNA1A and idiopathic generalized epilepsy. Neurology. 2001;56:1245–46. doi: 10.1212/wnl.56.9.1245. [DOI] [PubMed] [Google Scholar]
- 18.Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–32. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comm. Classif. Terminol. Int. League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 20.Comm. Classif. Terminol. Int. League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 21.Deleted in proof
- 22.Cox GA, Lutz CM, Yang CL, Biemesderfer D, Bronson RT, et al. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell. 1997;91:139–48. doi: 10.1016/s0092-8674(01)80016-7. [DOI] [PubMed] [Google Scholar]
- 23.De Fusco M, Becchetti A, Patrignani A, Annesi G, Gambardella A, et al. The nicotinic receptor β2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet. 2000;26:275–76. doi: 10.1038/81566. [DOI] [PubMed] [Google Scholar]
- 24.Durner M, Keddache MA, Tomasini L, Shinnar S, Resor SR, et al. Genome scan of idiopathic generalized epilepsy: evidence for major susceptibility gene and modifying genes influencing the seizure type. Ann Neurol. 2001;49:328–35. [PubMed] [Google Scholar]
- 25.Escayg A, De Waard M, Lee DD, Bichet D, Wolf P, et al. Coding and non-coding variation of the human calcium- channel β4-subunit gene CACNB4 in patients with idiopathic generalized epilepsy and episodic ataxia. Am J Hum Genet. 2000;66:1531–39. doi: 10.1086/302909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escayg A, Heils A, MacDonald BT, Haug K, Sander T, Meisler MH. A novel SCN1A mutation associated with generalized epilepsy with febrile seizures plus-and prevalence of variants in patients with epilepsy. Am J Hum Genet. 2001;68:866–73. doi: 10.1086/319524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–45. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher CF, Frankel WN. Ataxic mouse mutants and molecular mechanisms of absence epilepsy. Hum Mol Genet. 1999;8:1907–12. doi: 10.1093/hmg/8.10.1907. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher CF, Lutz CM, O’Sullivan TN, Shaughnessy JD, Jr, Hawkes R, et al. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–17. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- 29a.Fletcher CF, Tottene A, Lennon VA, Wilson SM, Dubel SJ, et al. Dystonia and cerebellar atrophy in Cacna1a null mice lacking P/Q calcium channel activity. FASEB J. 2001;15:1288–90. doi: 10.1096/fj.00-0562fje. [DOI] [PubMed] [Google Scholar]
- 30.Frankel WN. Detecting genes in new and old mouse models for epilepsy: a prospectus through the magnifying glass. Epilepsy Res. 1999;36:97–110. doi: 10.1016/s0920-1211(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 31.Frankel WN, Taylor L, Beyer B, Tempel BL, White HS. Electroconvulsive thresholds of inbred mouse strains. Genomics. 2001;74:306–12. doi: 10.1006/geno.2001.6564. [DOI] [PubMed] [Google Scholar]
- 32.Gambardella A, Annesi G, De Fusco M, Patrignani A, Aguglia U, et al. A new locus for autosomal dominant nocturnal frontal lobe epilepsy maps to chromosome 1. Neurology. 2000;55:1467–71. doi: 10.1212/wnl.55.10.1467. [DOI] [PubMed] [Google Scholar]
- 33.Gardiner RM. Impact of our understanding of the genetic aetiology of epilepsy. J Neurol. 2000;247:327–34. doi: 10.1007/s004150050598. [DOI] [PubMed] [Google Scholar]
- 34.Hacia JG. Resequencing and mutational analysis using oligonucleotide microarrays. Nat Genet. 1999;21:42–47. doi: 10.1038/4469. [DOI] [PubMed] [Google Scholar]
- 35.Haug K, Hallmann K, Horvath S, Sander T, Kubisch C, et al. No evidence for association between the KCNQ3 gene and susceptibility to idiopathic generalized epilepsy. Epilepsy Res. 2000;42:57–62. doi: 10.1016/s0920-1211(00)00164-9. [DOI] [PubMed] [Google Scholar]
- 36.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–68. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 37.Higuchi M, Maas S, Single FN, Hartner J, Rozov A, et al. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 38.Hille B. Ionic Channels of Excitable Membranes. 3 Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 39.Hodge SE, Abreu PC, Greenberg DA. Magnitude of type I error when single-locus linkage analysis is maximized over models: a simulation study. Am J Hum Genet. 1997;60:217–27. [PMC free article] [PubMed] [Google Scholar]
- 40.Houser CR. Neuronal loss and synaptic reorganization in temporal lobe epilepsy. Adv Neurol. 1999;79:743–61. [PubMed] [Google Scholar]
- 41.Junaid MA, Pullarkat RK. Biochemistry of neuronal ceroid lipofuscinoses. Adv Genet. 2001;45:93–106. doi: 10.1016/s0065-2660(01)45005-x. [DOI] [PubMed] [Google Scholar]
- 41.Kang M-G, Chen C-C, Felix R, Letts VA, Frankel WN, et al. Biochemical and biophysical evidence for 2 subunit association with neuronal voltage-activated Ca2+ channels. J Biol Chem. 2001 doi: 10.1074/jbc.M100787200. In press. [DOI] [PubMed] [Google Scholar]
- 42.Kearney JA, Plummer NW, Smith MR, Kapur J, Cummins TR, et al. A gain-of-function mutation in the sodium channel gene Scn2a results in seizures and behavioral abnormalities. Neuroscience. 2001;102:307–17. doi: 10.1016/s0306-4522(00)00479-6. [DOI] [PubMed] [Google Scholar]
- 43.Lau D, Vega-Saenz de Miera EC, Contreras D, Ozaita A, Harvey M, et al. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci. 2000;20:9071–85. doi: 10.1523/JNEUROSCI.20-24-09071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, et al. The mouse stargazer gene encodes a neuronal Ca2+ channel gamma subunit. Nat Genet. 1998;19:340–47. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 45.McNamara JO. Emerging insights into the genesis of epilepsy (Review) Nature. 1999;399:A15–22. doi: 10.1038/399a015. [DOI] [PubMed] [Google Scholar]
- 46.Mehta AK, Ticku MK. An up- date on GABAA receptors. Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 47.Meisler MH, Kearney J, Escayg A, Mac-Donald BT, Sprunger LK. Sodium channels and neurological disease: insights from Scn8a mutations in the mouse. Neuroscientist. 2001;7:136–45. doi: 10.1177/107385840100700208. [DOI] [PubMed] [Google Scholar]
- 48.Moran O, Conti F. Skeletal muscle sodium channel is affected by an epileptogenic β1 subunit mutation. Biochem Biophys Res Commun. 2001;282:55–59. doi: 10.1006/bbrc.2001.4502. [DOI] [PubMed] [Google Scholar]
- 49.Mori M, Konno T, Ozawa T, Murata M, Imoto K, Nagayama K. Novel interaction of the voltage-dependent sodium channel (VDSC) with calmodulin: Does VDSC acquire calmodulin-mediated Ca2+-sensitivity? Biochemistry. 2000;39:1316–23. doi: 10.1021/bi9912600. [DOI] [PubMed] [Google Scholar]
- 50.Moulard B, Guipponi M, Chaigne D, Mouthon D, Buresi C, Malafosse A. Identification of a new locus for generalized epilepsy with febrile seizures plus (GEFS+) on chromosome 2q24–q33. Am J Hum Genet. 1999;65:1396–400. doi: 10.1086/302621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neubauer BA, Fiedler B, Himmelein B, Kampfer F, Lassker U, et al. Centrotemporal spikes in families with rolandic epilepsy: linkage to chromosome 15q14. Neurology. 1998;51:1608–12. doi: 10.1212/wnl.51.6.1608. [DOI] [PubMed] [Google Scholar]
- 52.Ottman R, Risch N, Hauser WA, Pedley TA, Lee JH, et al. Localization of a gene for partial epilepsy to chromosome 10q. Nat Genet. 1995;10:56–60. doi: 10.1038/ng0595-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters HC, Kämmer G, Volz A, Kaupmann K, Ziegler A, et al. Mapping, genomic structure, and polymorphisms of the human GABABR1 receptor gene: evaluation of its involvement in idiopathic generalized epilepsy. Neurogenetics. 1998;2:47–54. doi: 10.1007/s100480050051. [DOI] [PubMed] [Google Scholar]
- 54.Phillips HA, Favre I, Kirkpatrick M, Zuberi SM, Goudie D, et al. CHRNB2 is the second acetylcholine receptor subunit associated with autosomal dominant nocturnal frontal lobe epilepsy. Am J Hum Genet. 2001;68:225–31. doi: 10.1086/316946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips HA, Scheffer IE, Berkovic SF, Hollway GE, Sutherland GR, Mulley JC. Localization of a gene for autosomal dominant nocturnal frontal lobe epilepsy to chromosome 20q13.2. Nat Genet. 1995;10:117–18. doi: 10.1038/ng0595-117. [DOI] [PubMed] [Google Scholar]
- 56.Pritchard JK. Are rare variants responsible for susceptibility to complex diseases? Am J Hum Genet. 2001;69:124–37. doi: 10.1086/321272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puranam RS, McNamara JO. Seizure disorders in mutant mice: relevance to human epilepsies. Curr Opin Neurobiol. 1999;9:281–87. doi: 10.1016/s0959-4388(99)80041-5. [DOI] [PubMed] [Google Scholar]
- 58.Reenan RA. RNA world meets behavior: AI pre-mRNA editing in animals. Trends Genet. 2001;17:53–56. doi: 10.1016/s0168-9525(00)02169-7. [DOI] [PubMed] [Google Scholar]
- 59.Risch N, Spiker D, Lotspeich L, Nouri N, Hinds D, et al. A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet. 1999;65:493–507. doi: 10.1086/302497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan SG. Ion channels and the genetic contribution to epilepsy. J Child Neurol. 1999;14:58–66. doi: 10.1177/088307389901400104. [DOI] [PubMed] [Google Scholar]
- 61.Sander T, Peters C, Kammer G, Samochowiec J, Zirra M, et al. Association analysis of exonic variants of the gene encoding the GABAB receptor and idiopathic generalized epilepsy. Am J Med Genet. 1999;88:305–10. doi: 10.1002/(sici)1096-8628(19990820)88:4<305::aid-ajmg5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 62.Sander T, Schulz H, Saar K, Gennaro E, Concetta Riggio M, et al. Genome search for susceptibility loci of common idiopathic generalised epilepsies. Hum Mol Genet. 2000;9:1465–72. doi: 10.1093/hmg/9.10.1465. [DOI] [PubMed] [Google Scholar]
- 63.Scheffer IE, Berkovic SF. Generalized epilepsy with febrile seizures plus a genetic disorder with heterogeneous clinical phenotypes. Brain. 1997;120:479–90. doi: 10.1093/brain/120.3.479. [DOI] [PubMed] [Google Scholar]
- 64.Scheffer IE, Berkovic SF. Genetics of the epilepsies. Curr Opin Pediatr. 2000;12:536–42. doi: 10.1097/00008480-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 64a.Singh R, Andermann E, Whitehouse WP, Harvey AS, Keene DL, et al. Severe myoclonic epilepsy of infancy: extended spectrum of GEFS+? Epilepsia. 2001;42:837–44. doi: 10.1046/j.1528-1157.2001.042007837.x. [DOI] [PubMed] [Google Scholar]
- 65.Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, et al. Deletion of the Kv1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–19. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 66.Spampanato J, Escayg A, Meisler MH, Goldin AL. Functional effects of two voltage-gated sodium channel mutations that cause generalized epilepsy with febrile seizures plus type 2. J Neurosci. 2001 doi: 10.1523/JNEUROSCI.21-19-07481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stafstrom CE, Tempel BL. Epilepsy genes: the link between molecular dysfunction and pathophysiology. Ment Retard Dev Disabil Res Rev. 2000;6:281–92. doi: 10.1002/1098-2779(2000)6:4<281::AID-MRDD7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 68.Steinlein OK, Magnusson A, Stoodt J, Bertrand S, Weiland S, et al. An insertion mutation of the CHRNA4 gene in a family with autosomal dominant nocturnal frontal lobe epilepsy. Hum Mol Genet. 1997;6:943–47. doi: 10.1093/hmg/6.6.943. [DOI] [PubMed] [Google Scholar]
- 69.Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11:201–3. doi: 10.1038/ng1095-201. [DOI] [PubMed] [Google Scholar]
- 70.Steinlein OK, Noebels JL. Ion channels and epilepsy in man and mouse. Curr Opin Genet Dev. 2000;10:286–91. doi: 10.1016/s0959-437x(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 71.Steinlein OK, Stoodt J, Biervert C, Janz D, Sander T. The voltage gated potassium channel KCNQ2 and idiopathic generalized epilepsy. NeuroReport. 1999;10:1163–66. doi: 10.1097/00001756-199904260-00001. [DOI] [PubMed] [Google Scholar]
- 72.Sugawara T, Tsurubuchi Y, Agarwala KL, Ito M, Fukuma G, et al. A missense mutation of the Na+ channel αII subunit gene Nav1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc Natl Acad Sci USA. 2001;98:6384–89. doi: 10.1073/pnas.111065098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vissel B, Royle GA, Christie BR, Schiffer HH, Ghetti A, et al. The role of RNA editing of kainate receptors in synaptic plasticity and seizures. Neuron. 2001;29:217–27. doi: 10.1016/s0896-6273(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 74.Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, et al. Mutant GABAA receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 75.Wallace RH, Scheffer IE, Barnett S, Richards M, Dibbens L, et al. Neuronal sodium-channel α1-subunit mutations in generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2001;68:859–65. doi: 10.1086/319516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta 1 subunit gene SCN1B. Nat Genet. 1998;19:366–70. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- 77.Xiong L, Labuda M, Li DS, Hudson TJ, Desbiens R, et al. Mapping of a gene determining familial partial epilepsy with variable foci to chromosome 22q11–q12. Am J Hum Genet. 1999;65:1698–710. doi: 10.1086/302649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zuberi SM, Eunson LH, Spauschus A, De Silva R, Tolmie J, et al. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain. 1999;122:817–25. doi: 10.1093/brain/122.5.817. [DOI] [PubMed] [Google Scholar]