Abstract
Safe and potent new adjuvants are needed for vaccines that are administered to mucosal surfaces. This study was performed to determine if interleukin-1α (IL-1α) combined with other proinflammatory cytokines provided mucosal adjuvant activity for induction of systemic and mucosal anti-human immunodeficiency virus (HIV) peptide antibody when intranasally administered with an HIV peptide immunogen. Nasal immunization of BALB/c mice with 10 μg of an HIV env peptide immunogen with IL-1α, IL-12, and IL-18 on days 0, 7, 14, and 28 induced peak serum anti-HIV peptide immunoglobulin G1 (IgG1) and IgA titers of 1:131,072 and 1:7,131, respectively (P = 0.05 versus no adjuvant). The use of cholera toxin (CT) as a mucosal adjuvant induced serum IgG1 and IgA titers of 1:32,768 and 1:776, respectively. The adjuvant combination of IL-1α, IL-12, and IL-18 induced anti-HIV peptide IgA titers of 1:1,176, 1:7,131, and 1:4,705 in saliva, fecal extracts and vaginal lavage, respectively. Titers induced by the use of CT as an adjuvant were 1:223, 1:1,176, and 1:675, respectively. These results indicate that the proinflammatory cytokines IL-1α, IL-12, and IL-18 can replace CT as a mucosal adjuvant for antibody induction and are important candidates for use as mucosal adjuvants with HIV and other vaccines.
Human immunodeficiency virus (HIV) infection is predominantly associated with mucosal transmission of HIV after sexual contact with an HIV-infected partner (2, 3). The appropriate anti-HIV antibody response may play a crucial role in protection against mucosal transmission of HIV. For example, passive transfer of neutralizing anti-HIV immunoglobulin G (IgG) monoclonal antibodies protected against a vaginal challenge of rhesus macaques with pathogenic simian/HIV strain SHIV 89.6PD (27) or oral exposure of neonatal macaques to SHIV-vpu (6). Reports of HIV-exposed but seronegative individuals suggest that HIV-specific serum and mucosal IgA may also play a role in resistance to mucosal transmission of HIV (29, 30, 35; M. Clerici, S. Mazzoli, L. Lopalco, et al., Int. Conf. AIDS 12:263, abstr. 21124).
Anti-HIV IgA purified from exposed seronegative individuals has the ability to neutralize primary HIV isolates (29, 35) and inhibit HIV-1 transcytosis across human epithelial cell monolayers (15). Taken together, these reports suggest that mucosal and serum anti-HIV IgG and IgA responses against the appropriate HIV antigen may play an important role in vaccine-induced protection against mucosal HIV transmission.
Intranasal immunization may provide a practical means of vaccination for the induction of anti-HIV IgG and IgA responses. Indeed, others have reported nasal immunization of nonhuman primates with SIV antigens was an effective route of immunization for the induction of SIV-specific serum and mucosal IgG and IgA responses (19). The use of a mucosal adjuvant is needed for the induction of antigen-specific IgG and IgA responses after nasal immunization because mucosal immunization in the absence of adjuvant will induce either low or undetectable antigen-specific immune responses or antigen-specific tolerance (14, 54).
The most widely studied mucosal adjuvant is cholera toxin (CT) (16). CT is not safe for use in humans (24), and CT used as a nasal adjuvant in mice is associated with accumulation of CT and the coadministered vaccine antigen in the olfactory neurons (50). Additionally, nasal administration of higher doses of CT and tetanus toxoid in mice was associated with antigen-specific IgE-associated pathological changes in the lung and occasionally death (40). Similar reports of IgE-associated adverse effects have been reported for gastrically administered CT (42). These numerous reports of adverse effects associated with the use of CT as a mucosal adjuvant mandate that safe and effective mucosal adjuvants are needed for the successful development of vaccines that can be safely administered to humans via mucosal routes.
We recently reported that interleukin-1α (IL-1α) and IL-1β exhibit mucosal adjuvant activity for the induction of serum IgG and mucosal IgA antibody responses when nasally administered with protein antigens (45). We also determined that combinations of the proinflammatory cytokines IL-1α, IL-12, IL-18, and granulocyte-macrophage colony-stimulating factor (GM-CSF) provided mucosal adjuvant activity for the induction of HIV-specific cell-mediated immune responses when nasally administered to mice with an HIV peptide immunogen (44). It was not clear if these proinflammatory cytokines could provide mucosal adjuvant activity for the induction of HIV-specific antibody when nasally administered with HIV synthetic peptide immunogens. We therefore evaluated IL-1α, IL-12, IL-18, and GM-CSF for their ability to induce antigen-specific serum and mucosal IgG and IgA after nasal immunization with a model HIV env peptide immunogen.
MATERIALS AND METHODS
Animals.
Female BALB/c mice, 16 to 18 g, were purchased from Frederick Cancer Research and Developmental Center, National Cancer Institute, Frederick, Md. Mice were housed in filter-topped cages and provided food and water ad libitum. Procedures were approved by Duke University’ s Institutional Animal Care and Use Committee.
Immunization.
Mice were intranasally immunized as previously described (45–47). Briefly, mice (three to five mice per group) were immunized intranasally with the indicated amount of HIV immunogen and the indicated adjuvant in a total volume of 15 μl of sterile distilled water or physiologic saline (7.5 μl/nostril) while mice were under isoflurane anesthesia (IsoFlo USP; Solvay Animal Health, Inc., Mendota Heights, Minn.). The mucosal adjuvant CT was obtained from List Biological Laboratories, Inc. (Campbell, Calif.). Recombinant murine cytokines were purchased from PeproTech (Rocky Hill, N.J.), BioSource International (Camarillo, Calif.), or Chemicon (Temecula, Calif.) and used at doses previously evaluated for their ability to support the induction of HIV-specific cytotoxic T lymphocytes (CTL) after nasal immunization with HIV peptides (44).
HIV synthetic peptides.
Synthetic HIV peptides C4-V3MN and C4E9V-V389.6P were used as HIV immunogens (7, 17, 18, 47; R. De Lorimier, L. Spicer, and B. Haynes, unpublished data). The peptides are linear peptides containing a T-helper epitope from the fourth constant domain of HIV-1 gp120 and a B-cell epitope from the V3 loop of HIV-1 gp120. The amino acid sequence for C4-V3MN is KQIINMWQEVGKAMYATRPNYNKRKRIHIGPGRAFYTTK. The amino acid sequence for C4E9V-V389.6P is KQIINMWQVVGKAMYATRPNNNTRERLSIGPGRAFYARR. Peptides used for immunization of mice were purchased from Synpep (Dublin, Calif.) and purified to a single species by high-pressure liquid chromatography and verified by mass spectroscopy. Peptides of this design induce antibodies that are specific for the V3 loop of HIV, neutralize SHIV in a V3-specific fashion, and provide measurable protection against high virus loads and CD4+ lymphocyte depletion after challenge with SHIV 89.6P (23, 25).
Sample collection.
The collection of serum and vaginal samples was performed as previously described (46, 47). Fecal extracts were prepared be collecting fresh fecal pellets and preparing an extract by mixing the fecal pellet with fecal extract buffer (phosphate-buffered saline [PBS], 10% normal goat serum, 0.1% Kathon) and vortexing for 30 min. The vortexed mixture was centrifuged at 10,000 rpm in a microcentrifuge, and the supernatant was collected and stored at −30°C until assayed via enzyme-linked immunosorbent assay (ELISA). Salivary secretions were collected by injecting mice intraperitoneally with 250 μl of carbochol (12 μg/ml in PBS; carbamylcholine chloride; Sigma, St. Louis, Mo.) to induce salivation. Saliva samples were collected with a 100-μl pipette. Saliva secretions were centrifuged at 10,000 rpm in a microcentrifuge, and the supernatant was collected and stored at −30°C until assayed via ELISA.
ELISA.
Antigen-specific end-point antibody titers were determined using ELISA as previously described (46, 47) except that black ELISA plates were used (black microflour 2 ELISA plates; Dynex, Thermo Labsystems, Franklin, Mass.) and the fluorescent alkaline phosphatase substrate Attophos (Roche Molecular Biochemicals, Indianapolis, Ind.) was used. ELISA plates were read on a FluoroCount plate reader (Packard Instrument Company, Meriden, Conn.). Samples were considered positive for antigen-specific antibody when the relative light unit (RLU) value for the sample dilution was threefold higher than the RLU for a naive sample at the same dilution.
Statistical analysis.
Statistical analysis was determined using t test analysis for the data in Fig. 1. Because the comparison of activity between control groups (nasal immunization with peptide without adjuvant to nasal immunization with peptide plus adjuvant) was planned a priori, t tests were performed to compare each experimental group to the control group (39, 43). Statistical analysis for Fig. 2 to 5 was determined using SAS software. The comparison of multiple groups was performed using the more stringent general linear model procedure. Statistical significance was achieved at P = 0.05. Error bars represent standard deviation.
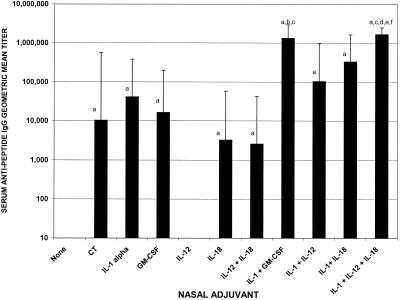
FIG. 1.
Serum antipeptide IgG anti-C4-V3 MN geometric mean titers after nasal immunization with 10 μg of C4-V3 MN immunogen with and without adjuvants. Female BALB/c mice were nasally immunized with 10 μg of C4-V3 MN peptide immunogen alone or combined with 1 μg of CT, 4 μg of IL-1α, 4 μg of GM-CSF, 100 ng of IL-12, 400 ng of IL-18, 20 ng of IL-12 and 400 ng of IL-18, 4 μg of IL-1α and 4 μg of GM-CSF, 4 μg of IL-1α and 100 ng of IL-12, 4 μg of IL-1α and 400 ng of IL-18, or 4 μg of IL-1α and 20 ng of IL-12 and 400 ng of IL-18. On day 35, serum samples were collected and tested via ELISA for the presence of anti-HIV peptide IgG endpoint titers. There were three mice per group. Letters above the bars indicate significance: a, greater than nasal immunization with no adjuvant, P < 0.05; b, greater than nasal immunization with GM-CSF adjuvant, P < 0.05; c, greater than nasal immunization with IL-1α adjuvant, P < 0.05; d, greater than nasal immunization with IL-18 adjuvant, P < 0.05; e, greater than nasal immunization with IL-12 plus IL-18 adjuvant, P < 0.05; f, greater than nasal immunization with CT adjuvant, P < 0.05.
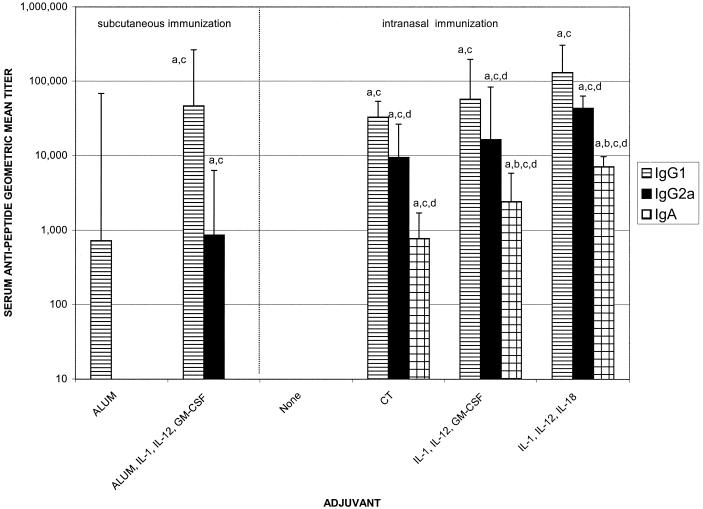
FIG. 2.
Serum antipeptide IgG1, IgG2a, and IgA geometric mean titers after immunization with 10 μg of C4E9V-V3 89.6P peptide with and without adjuvant. Female BALB/c mice were nasally immunized with 10 μg of C4E9V-V3 89.6P peptide immunogen alone or combined with1 μg of CT; 4 μg of IL-1α and 100 ng of IL-12 and 4 μg of GM-CSF; 4 μg of IL-1α and 20 ng of IL-12 and 400 ng of IL-18; 4 μg of IL-1α and 20 ng of IL-12 and 400 ng of IL-18 and 4 μg of GM-CSF on days 0, 7, 14, and 28. Control animals were immunized on the same schedule via the subcutaneous route with 10 μg of peptide formulated with alum or alum and 4 μg of IL-1α and 100 ng of IL-12 and 4 μg of GM-CSF. Serum samples were collected on day 35 and assayed via ELISA for the presence of anti-HIV IgG and IgA. There were five mice per group. Letters above the bars indicate significance:a, greater than nasal immunization with no adjuvant, P < 0.05; b, greater than nasal immunization with CT adjuvant, P < 0.05; c, greater than subcutaneous immunization with alum adjuvant, P < 0.05; d, greater than subcutaneous immunization with alum plus IL-1α, IL-12, and GM-CSF adjuvants, P < 0.05.
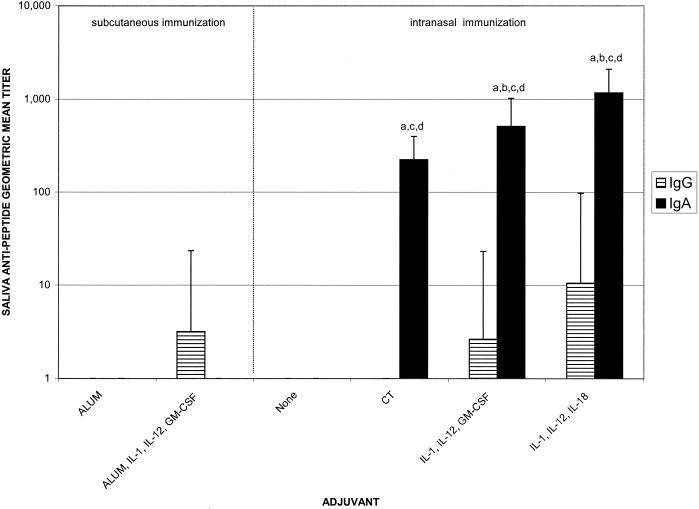
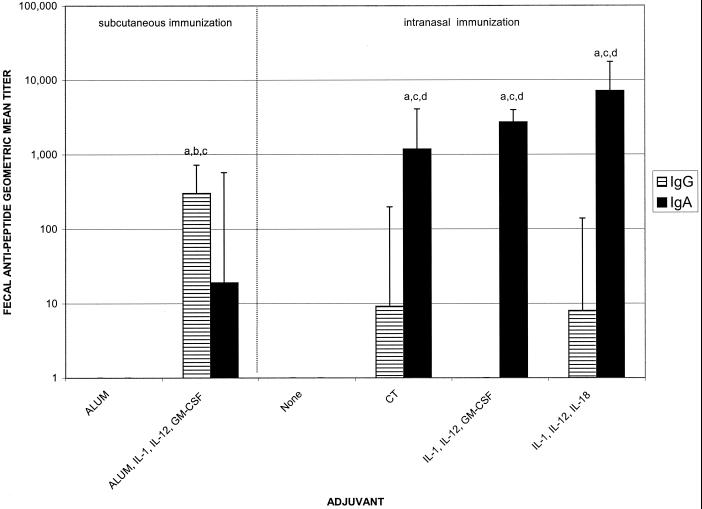
FIG. 5.
Salivary antipeptide IgG and IgA geometric mean titers after immunization with 10 μg of C4E9V-V3 89.6P peptide with and without adjuvant. Immunization and sample collections were performed as described in the legend for Fig. 2. There were five mice per group. Letters above the bars indicate significance: a, greater than nasal immunization with no adjuvant, P < 0.05; b, greater than nasal immunization with CT adjuvant, P < 0.05; c, greater than subcutaneous immunization with alum adjuvant, P < 0.05; d, greater than subcutaneous immunization with alum plus IL-1α, IL-12, and GM-CSF adjuvants, P < 0.05.
RESULTS
Adjuvant activity of cytokines and cytokine combinations intranasally administered with HIV synthetic peptide immunogens.
To determine if proinflammatory cytokines provided mucosal adjuvant activity for the induction of peptide-specific antibody when intranasally administered with an HIV synthetic peptide immunogen and if combinations of cytokines provided mucosal adjuvant activity superior to that provided by individual cytokines, female BALB/c mice were nasally immunized with 10 μg of HIV C4-V3MN immunogen alone or in the presence of CT or cytokines on days 0, 7, 14, and 28. The doses of cytokines used as adjuvants are based on previous studies in our laboratory and are listed in the figure legends (44).
Although IL-12 used alone did not exhibit mucosal adjuvant activity at the dose tested, all other cytokines, used either individually or in combination, provided significant adjuvant activity for the induction of HIV-specific serum IgG compared to immunization in the absence of adjuvant (P < 0.05) (Fig. 1). IL-1α, GM-CSF, and IL-18 exhibited mucosal adjuvant activity when used as mucosal adjuvants, as determined by the induction of serum antipeptide IgG titers of 1:41,285, 1:16,384, and 1:3,250, respectively (Fig. 1). The peak serum antipeptide IgG responses were detected in mice that received the combination of IL-1α plus IL-12 plus IL-18 or IL-1α plus GM-CSF, with geometric mean endpoint IgG titers of 1:1,664,510 and 1:1,321,122, respectively (Fig. 1).
The serum antipeptide IgG titer induced with IL-1α plus IL-12 plus IL-18 was significantly greater than the serum IgG titer induced by the use of CT, IL-1α, IL-12, IL-18, or the combination of IL-12 plus IL-18 (P < 0.05). The serum IgG titer induced by the combination of IL-1α plus GM-CSF was significantly greater that the serum IgG titer induced by the use of IL-1α or GM-CSF alone (P < 0.05). Cytokines administered intranasally with HIV peptide immunogens supported the induction of antipeptide IgA responses in the vaginal secretions, with peak IgA titers being detected when IL-1α plus GM-CSF (1:203) or IL-1α or IL-12 plus IL-18 (1:128) were used as adjuvants (data not shown).
Comparison of antipeptide IgG and IgA responses after subcutaneous or intranasal immunization with HIV env immunogen and cytokine adjuvants.
The next experiment was performed for a number of reasons. First, this experiment was performed to determine if nasal immunization with cytokine adjuvants was capable of inducing systemic antipeptide IgG responses comparable to those induced with systemic immunization using an adjuvant approved for human use, alum. Second, this experiment was performed using a different HIV immunogen, C4(E9V)-V3 89.6P, to confirm that cytokines exhibit mucosal adjuvant activity for the induction of both systemic and mucosal antibody responses when intranasally administered with different HIV peptide immunogens. Third, this experiment was performed to evaluate antipeptide IgG and IgA in diverse mucosal compartments. Towards this end, antipeptide IgG and IgA responses were monitored in the female reproductive tract, saliva, and fecal extracts. Finally, we wanted to determine if the antipeptide IgG subclass profile induced by immunization with the C4(E9V)-V3 89.6P immunogen was influenced by different routes of immunization and/or different adjuvants.
Female BALB/c mice (five mice/group) were immunized with 10 μg of C4E9V-V3 89.6P immunogen by the subcutaneous route with alum or alum plus the cytokines IL-1α plus IL-12 plus GM-CSF or by the nasal route with no adjuvant, CT, or the cytokine combinations of IL-1α plus IL-12 plus GM-CSF or IL-1α plus IL-12 plus IL-18. Cytokine combinations were used because they produced the best adjuvant activity, as determined by the experiment in Fig. 1. IL-12 was added to the combination of IL-1α plus GM-CSF because the addition of IL-12 to the combination of IL-1α plus IL-18 produced the highest serum antipeptide IgG titer that was statistically greater than serum IgG titers induced by the use of CT or IL-1α alone as nasal vaccine adjuvants (Fig. 1).
Serum IgG1 and IgG2a responses were monitored to determine if the cytokines had any effect on the antipeptide IgG subclass distribution profile. Nasal immunization with HIV immunogen alone did not induce any detectable antipeptide IgG1 or IgG2a response (Fig. 2). Nasal immunization with HIV immunogen plus IL-1α plus IL-12 plus IL-18 or IL-1α plus IL-12 plus GM-CSF induced serum IgG1 and IgG2a antipeptide titers that were greater than those induced by nasal immunization alone or subcutaneous immunization with peptide and alum (Fig. 2, P < 0.5).
The combination of IL-1α plus IL-12 plus IL-18 induced the highest serum IgG1 response of all groups tested, with a geometric mean titer of 1:131,072 (P < 0.05 versus no adjuvant for nasal immunization, P < 0.05 versus alum). This cytokine combination also induced the peak antipeptide serum IgG2a, with a geometric mean titer of 1:43,237 (P < 0.05 versus no nasal adjuvant; Fig. 2). In fact, the serum antipeptide IgG2a response induced by any of the nasal adjuvant groups was significantly increased compared to the IgG2a response induced when mice were immunized subcutaneously with alum formulated with IL-1α or IL-12 plus GM-CSF (P < 0.05, Fig. 2), suggesting that the route of immunization plays a role in the IgG subclass of antibody induced. IL-1α, IL-12, and GM-CSF formulated with the HIV immunogen on alum and administered subcutaneously provided significant adjuvant activity and induced a serum antipeptide IgG1 geometric mean titer of 1:46,340 (P < 0.05 versus alum, Fig. 2), a 64-fold increase of the antipeptide titer induced by the use of alum alone, suggesting that adsorbed cytokines can augment the adjuvanticity of alum.
Serum antipeptide IgA responses were also monitored in all groups. Antipeptide serum IgA was only detected in mice nasally immunized with HIV immunogen and adjuvants. The peak serum antipeptide IgA response, a geometric mean titer of 1:7,131, was induced with the use of IL-1α plus IL-12 plus IL-18 as nasal adjuvants. In fact, the cytokine adjuvant combinations induced serum IgA responses that were significantly increased compared to serum IgA responses induced by the use of CT as a mucosal adjuvant (P < 0.05) (Fig. 2).
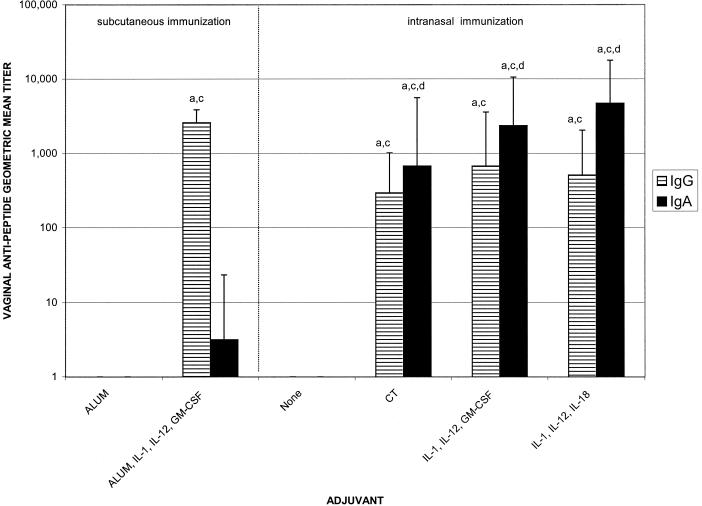
To evaluate the induction of antipeptide mucosal antibody responses after nasal immunization with HIV peptide immunogen and cytokine adjuvants, vaginal, fecal, and salivary secretions were tested for the presence of antipeptide IgG and IgA. Peak antipeptide IgA responses in all mucosal compartments tested were induced via nasal immunization with HIV immunogen and IL-1α plus IL-12 plus IL-18 as mucosal adjuvants (Fig. 3 to 5). In vaginal secretions, the cytokine combinations of IL-1α plus IL-12 plus GM-CSF and IL-1α plus IL-12 plus IL-18 were as effective as CT for the induction of antipeptide IgG (1:675, 1:512, and 1:294, respectively) and IgA (1:2,352, 1:4,705, and 1:675, respectively) (Fig. 3). The IgA responses induced by all of the nasal adjuvant groups (CT or cytokine combinations) were significantly greater than the IgA responses induced by either of the subcutaneous groups or nasal immunization with peptide alone (P < 0.05) (Fig. 3). Subcutaneous immunization using alum plus IL-1α plus IL-12 plus GM-CSF induced vaginal IgG titers of 1:2,580 but only minimal IgA (1:3). In fecal samples, nasal immunization with HIV peptide immunogen and CT, IL-1α plus IL-12 plusGM-CSF or IL-1α plus IL-12 plus IL-18 induced comparable antipeptide IgA titers of 1:1,176, 1:2,702, and 1:7,131, respectively (Fig. 4). The fecal antipeptide IgA responses induced by nasal immunization plus adjuvant (CT or cytokine combinations) were significantly greater than the fecal IgA responses induced by subcutaneous immunization with peptide plus alum alone or with IL-1α plus IL-12 plus GM-CSF (1:19), or nasal immunization with peptide alone (P < 0.05). Although subcutaneous immunization with peptide formulated with alum plus cytokines did not induce significant antipeptide IgA responses, it did induce antipeptide IgG responses (1:304) that were greater than those induced by any other group (P < 0.05) (Fig. 4).
FIG. 3.
Vaginal antipeptide IgG and IgA geometric mean titers after immunization with 10 μg of C4E9V-V3 89.6P peptide with and without adjuvant. Immunization and sample collections were performed as described in the legend to Fig. 2. There were five mice per group. Letters above the bars indicate significance: a, greater than nasal immunization with no adjuvant, P < 0.05; b, greater than nasal immunization with CT adjuvant, P < 0.05; c, greater than subcutaneous immunization with alum adjuvant, P < 0.05; d, greater than subcutaneous immunization with alum plus IL-1α, IL-12, and GM-CSF adjuvants, P < 0.05.
FIG. 4.
Fecal antipeptide IgG and IgA geometric mean titers after immunization with 10 μg of C4E9V-V3 89.6P peptide with and without adjuvant. Immunization and sample collections were performed as described in the legend for Fig. 2. There were five mice per group. Letters above the bars indicate significance: a, greater than nasal immunization with no adjuvant, P < 0.05; b, greater than nasal immunization with CT adjuvant, P < 0.05; c, greater than subcutaneous immunization with alum adjuvant, P < 0.05; d, greater than subcutaneous immunization with alum plus IL-1α, IL-12, and GM-CSF adjuvants, P < 0.05.
Salivary secretions were also monitored for the presence of antipeptide IgG and IgA responses. Nasal immunization with HIV peptide and IL-1α plus IL-12 plus GM-CSF or IL-1α plus IL-12 plus IL-18 induced antipeptide IgA responses (1:512 and 1:1,176, respectively) that were significantly greater than the antipeptide IgA response induce by the use of CT as an adjuvant (1:223) or any other immunization group (P < 0.05) (Fig. 5).
DISCUSSION
In this study we report that cytokines may be useful as mucosal adjuvants for the induction of antipeptide serum IgG and mucosal IgA antibody responses when combined with HIV immunogens and administered via the nasal route. The combination of IL-1α or IL-12 plus IL-18 provided the single best nasal adjuvant activity, as determined by the induction of serum antipeptide IgG, IgA, and mucosal antipeptide IgA responses. IL-1α plus GM-CSF and IL-1α plus IL-12 plus GM-CSF also provided significant adjuvant activity when intranasally administered with HIV immunogens. Our results suggest that cytokines may be used to replace CT as mucosal HIV vaccine adjuvants.
In a previous study, we reported that nasal immunization with HIV immunogen and CT induced serum IgG and mucosal IgA responses, while subcutaneous immunization using complete Freund’s adjuvant (CFA) as an adjuvant induced antipeptide serum IgG responses but not mucosal IgA responses (47). However, in the present study, subcutaneous immunization with HIV immunogen and alum plus cytokine adjuvants induced low but detectable antipeptide IgA responses in fecal and vaginal secretions. This difference may be attributed to a number of factors, including the use of a more sensitive fluorescent ELISA protocol, the use of cytokines as adjuvants, and the use of alum. Our results are in agreement with others who have reported that cytokine (IL-12) adsorbed to alum with HIV gp120 has adjuvant activity in vivo (21).
Because HIV infection occurs at mucosal surfaces, the induction of anti-HIV IgA in mucosal secretions may play a crucial role in vaccine-induced protection against mucosally transmitted HIV. In this study, vaginal secretions collected from mice that contained antipeptide IgA did not exhibit HIV-neutralizing activity (data not shown). This may be a technical issue associated with the collection of the mouse vaginal lavage samples, since our collection process dilutes that vaginal sample at least 10-fold (47). It is possible that in their undiluted form, the vaginal antipeptide IgA antibodies could neutralize HIV, since this peptide has been reported to induce anti-HIV neutralizing antibodies in guinea pigs and monkeys (23, 25). It is also difficult to evaluate neutralizing anti-HIV antibody responses in mouse serum due to a high level of nonspecific neutralization of HIV detected in naive mouse serum.
Although neutralization of HIV via secretory IgA would likely protect at the mucosal surface, it is important to mention that anti-HIV IgA responses may contribute to protection against HIV infection in the absence of classical HIV-neutralizing activity via immune exclusion (4, 32), intracellular virus neutralization (12, 29), and transepithelial transport of immune complexes (22, 38). Indeed, anti-HIV IgA has been reported to block HIV transcytosis across human epithelial monolayers (10). It is also important to mention that a nonneutralizing antirotavirus IgA protected mice against gastric infection with rotavirus via intracellular virus neutralization (12).
Mucosal vaccine-induced protection against HIV would likely require persistent mucosal anti-HIV IgA antibody responses. In our previous study (47), nasal immunization with HIV peptide and the mucosal adjuvant CT induced high-titered serum IgG and vaginal IgG and IgA responses that remained at near peak levels through day 90 of the immunization regimen (55 days after the last boost). Both serum and vaginal antipeptide IgG responses remained detectable through day 196 (161 days after the last boost), while vaginal IgA responses were no longer detectable at day 196. In the present study, we did not address the persistence of mucosal anti-HIV peptide antibody responses induced with the use of cytokines adjuvants. Preliminary studies currently being performed in our laboratory suggest that nasal immunization of nonhuman primates with HIV immunogens and cytokines induces antigen-specific antibody responses (48). We will use the nonhuman primate as a model to develop nasal immunization strategies that induce both systemic and mucosal antibody responses that persist over time.
Some cytokines evaluated in our study were active when delivered intranasally while others were not. For example, IL-1, IL-18, and GM-CSF exhibited mucosal adjuvant activity for the induction of HIV-specific antibody responses when delivered intranasally with 10 μg of C4-V3MN HIV immunogen, while IL-12 was not effective when used alone (Fig. 1). Mucosal epithelial cells express receptors for IL-1 and GM-CSF (13, 34), and therefore, IL-1 and GM-CSF are able to bind to their specific receptors on the mucosal epithelial cells and exert their biological/adjuvant activity. It is not clear if mucosal epithelial cells express IL-12 receptors. However, we found that the adjuvant combination of IL-1α plus IL-12 plus IL-18 was the only group to induce serum antipeptide IgG responses greater than those induced by the individual components or CT, suggesting that IL-12 provided adjuvant activity when combined with IL-1α and IL-18 (Fig. 1). One possible explanation for this observation is that IL-1 increased the permeability of nasal mucosa and allowed IL-12 to cross the mucosal epithelium and exert its biological activity in the nasally associated lymphoid tissue. It is important to mention that our conclusions are based on the doses of cytokines tested in our studies; other doses of the same cytokines may exhibit different adjuvant activities. Indeed, others have reported that IL-12 exhibits mucosal adjuvant activity after nasal administration (11, 26). However, the use of liposomes in the IL-12 formulation, a higher dose of IL-12 (1 μg), and more frequent administration may have permitted the IL-12 to be active when administered by the nasal route.
Others have reported that cytokines administered with HIV peptide immunogens were able to augment the induction of anti-HIV antibody responses (1). In this other study, cytokines were formulated with peptide in incomplete Seppic adjuvant (ISA) and administered subcutaneously. Subcutaneous administration of immunogen, ISA, and cytokines determined that IL-1β was the most effective single cytokine (cytokines tested included IL-2, IL-4, IL-1β, IL-7, IL-12, and GM-CSF) for enhancement of anti-HIV IgG1 responses in BALB/c mice (1). More recently, this group has determined that IL-12 (8) or IL-12 plus GM-CSF (9) enhanced the induction of HIV-specific CTL after intrarectal immunization with HIV peptide immunogen and the mucosal adjuvant CT. However, their vaccination protocol did not induce anti-HIV peptide antibody responses, and they used cytokines to augment the adjuvant activity of CT, while we evaluated cytokines to replace CT.
The safety of nasal vaccine adjuvants is a major concern when developing new adjuvants for use in humans. Of the cytokines evaluated in this current study, IL-1α, IL-12, and GM-CSF have been evaluated in human clinical trials after administration by parenteral routes (5, 20, 31, 33, 36, 37, 41, 49, 51–53). Adverse effects associated with the use of IL-1α, IL-12, and GM-CSF by parenteral routes include fever, chills, nausea, and fatigue (5, 31, 36, 49, 52). Despite reports of adverse effects with human clinical trials of IL-1, IL-12, and GM-CSF, we believe recombinant cytokines may be useful as nasal vaccine adjuvants in humans for several reasons. First, for use as nasal vaccine adjuvants, cytokines would be administered locally and not systemically, and the adverse effects associated with parenteral administration may be attenuated or absent after local administration. Second, many of the adverse effects reported for IL-1, IL-12, and GM-CSF involved repeated or continuous administration of recombinant cytokine. With nasal immunization, the cytokines would be coadministered with the vaccine only at the time of immunization for a total of two or three immunizations. Preliminary studies in our laboratory have determined that the cytokines IL-1α and GM-CSF provided nasal adjuvant activity in nonhuman primates in the absence of detectable adverse events (fever and weight loss) (47).
Thus, our study has demonstrated that cytokine combinations containing IL-1α, IL-12, IL-18, and/or GM-CSF could substitute completely for CT as a nasal adjuvant for the induction of systemic and mucosal anti-HIV antibody. Combinations of these cytokines are prime candidates for use as adjuvants with HIV and other vaccines. Additional studies are under way in nonhuman primates to evaluate the safety and efficacy of recombinant cytokines as nasal vaccine adjuvants.
Acknowledgments
We acknowledge Tom Tlusty for expert technical assistance and Shila K. Nordone for assistance with the statistical evaluations.
This work was supported by NIH 2 PO1 AI35351 and the Research Center on HIV and AIDS, Durham Veterans Administration Hospital. C.P.B. was supported by the Interdisciplinary Training Program on AIDS, Division of Infectious Diseases, Duke University Medical Center.
REFERENCES
- 1.Ahlers, J. D., N. Dunlop, D. W. Alling, P. L. Nara, and J. A. Berzofsky. 1997. Cytokine-in-adjuvant steering of the immune response phenotype to Hiv-1 vaccine constructs — granulocyte-macrophage colony-stimulating factor and Tnf-alpha synergize with Il-12 to enhance induction of cytotoxic T lymphocytes. J. Immunol. 158:3947–3958. [PubMed] [Google Scholar]
- 2.Anonymous. 2000. AIDS epidemic update: December 2000. Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS, Geneva, Switzerland.
- 3.Anonymous. 2000. Report on the global HIV/AIDS epidemic. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland.
- 4.Apter, F. M., W. I. Lencer, R. A. Finkelstein, J. J. Mekalanos, and M. R. Neutra. 1993. Monoclonal immunoglobulin A antibodies directed against cholera toxin prevent the toxin-induced chloride secretory response and block toxin binding to intestinal epithelial cells in vitro. Infect. Immun. 61:5271–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins, M. B., M. J. Robertson, M. Gordon, M. T. Lotze, M. DeCoste, J. S. DuBois, J. Ritz, A. B. Sandler, H. D. Edington, P. D. Garzone, J. W. Mier, C. M. Canning, L. Battiato, H. Tahara, and M. L. Sherman. 1997. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 3:409–417. [PubMed] [Google Scholar]
- 6.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett, J. A., S. S. Wasserman, C. B. Hicks, R. T. Dodge, K. J. Weinhold, C. O. Tacket, N. Ketter, A. E. Wittek, T. J. Palker, and B. F. Haynes. 1998. Safety and immunogenicity of an HLA-based HIV envelope polyvalent synthetic peptide immunogen. AIDS 12:1291–1300. [DOI] [PubMed] [Google Scholar]
- 8.Belyakov, I. M., J. D. Ahlers, B. Y. Brandwein, P. Earl, B. L. Kelsall, B. Moss, W. Strober, and J. A. Berzofsky. 1998. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Investig. 102:2072–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belyakov, I. M., J. D. Ahlers, J. D. Clements, W. Strober, and J. A. Berzofsky. 2000. Interplay of cytokines and adjuvants in the regulation of mucosal and systemic HIV-specific CTL. J. Immunol. 165:6454–6462. [DOI] [PubMed] [Google Scholar]
- 10.Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of Hiv transcytosis across tight epithelial barriers by anti-Hiv envelope protein dIgA or IgM. Immunity 9:277–287. [DOI] [PubMed] [Google Scholar]
- 11.Boyaka, P. N., M. Marinaro, R. J. Jackson, S. Menon, H. Kiyono, E. Jirillo, and J. R. McGhee. 1999. IL-12 is an effective adjuvant for induction of mucosal immunity. J. Immunol. 162:122–128. [PubMed] [Google Scholar]
- 12.Burns, J. W., M. Siadat-Pajouh, A. A. Krishnaney, and H. B. Greenberg. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272:104–107. [DOI] [PubMed] [Google Scholar]
- 13.Christ, A. D., and R. S. Blumberg. 1997. The intestinal epithelial cell: immunological aspects. Springer Semin. Immunopathol. 18:449–461. [DOI] [PubMed] [Google Scholar]
- 14.Czerkinsky, C., F. Anjuere, J. R. McGhee, A. George-Chandy, J. Holmgren, M. P. Kieny, K. Fujiyashi, J. F. Mestecky, V. Pierrefite-Carle, C. Rask, and J. B. Sun. 1999. Mucosal immunity and tolerance: relevance to vaccine development. Immunol. Rev. 170:197–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devito, C., K. Broliden, R. Kaul, L. Svensson, K. Johansen, P. Kiama, J. Kimani, L. Lopalco, S. Piconi, J. J. Bwayo, F. Plummer, M. Cleric, and J. Hinkula. 2000. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 165:5170–5176. [DOI] [PubMed] [Google Scholar]
- 16.Elson, C. O. 1996. Cholera toxin as a mucosal adjuvant., p.59–72. In H. Kiyono, P. L. Ogra, and J. R. McGhee (ed.), Mucosal vaccines. Academic Press, New York, N.Y.
- 17.Hart, M. K., T. J. Palker, T. J. Matthews, A. J. Langlois, N. W. Lerche, M. E. Martin, R. M. Scearce, C. McDanal, D. P. Bolognesi, and B. F. Haynes. 1990. Synthetic peptides containing T and B cell epitopes from human immunodeficiency virus envelope gp120 induce anti-HIV proliferative responses and high titers of neutralizing antibodies in rhesus monkeys. J. Immunol. 145:2677–2685. [PubMed] [Google Scholar]
- 18.Hart, M. K., K. J. Weinhold, R. M. Scearce, E. M. Washburn, C. A. Clark, T. J. Palker, and B. F. Haynes. 1991. Nov 1. Priming of anti-human immunodeficiency virus (HIV) CD8+ cytotoxic T cells in vivo by carrier-free HIV synthetic peptides. Proc. Natl. Acad. Sci. USA 88:9448–9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imaoka, K., C. J. Miller, M. Kubota, M. B. McChesney, B. Lohman, M. Yamamoto, K. Fujihashi, K. Someya, M. Honda, J. R. McGhee, and H. Kiyono. 1998. Nasal immunization of nonhuman primates with simian immunodeficiency virus p55gag and cholera toxin adjuvant induces Th1/Th2 help for virus-specific immune responses in reproductive tissues. J. Immunol. 161:5952–5958. [PubMed] [Google Scholar]
- 20.Janik, J. E., L. L. Miller, D. L. Longo, G. C. Powers, W. J. Urba, W. C. Kopp, B. L. Gause, B. D. Curti, R. G. Fenton, J. J. Oppenheim, K. C. Conlon, J. T. Holmlund, M. Sznol, W. H. Sharfman, R. G. Steis, S. P. Creekmore, W. G. Alvord, A. E. Beauchamp, and, J. W. Smith, 2nd. 1996. Phase II trial of interleukin 1 alpha and indomethacin in treatment of metastatic melanoma. J. Natl. Cancer Inst. 88:44–49. [DOI] [PubMed] [Google Scholar]
- 21.Jankovic, D., P. Caspar, M. Zweig, M. Garciamoll, S. D. Showalter, F. R. Vogel, and A. Sher. 1997. Adsorption to aluminum hydroxide promotes the activity of Il-12 as an adjuvant for antibody as well as type 1 cytokine responses to Hiv-1 Gp120. J. Immunol. 159:2409–2417. [PubMed] [Google Scholar]
- 22.Kaetzel, C. S., J. K. Robinson, K. R. Chintalacharuvu, J.-P. Vaerman, and M. E. Lamm. 1991. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: A local defense function for IgA. Proc. Natl. Acad. Sci. USA 88:8796–8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letvin, N. L., S. Robinson, D. Rohne, M. K. Axthelm, J. W. Fanton, M. Bilska, T. J. Palker, H. X. Liao, B. F. Haynes, and D. C. Montefiori. 2001. Vaccine-elicited V3 loop-specific antibodies in rhesus monkeys and control of a simian-human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate envelope. J. Virol. 75:4165–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine, M. M., J. B. Kaper, R. E. Black, and M. L. Clements. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol. Rev. 47:510–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao, H. X., B. Etemad-Moghadam, D. C. Montefiori, Y. Sun, J. Sodroski, R. M. Scearce, R. W. Doms, J. R. Thomasch, S. Robinson, N. L. Letvin, and B. F. Haynes. 2000. Induction of antibodies in guinea pigs and rhesus monkeys against the human immunodeficiency virus type 1 envelope: neutralization of nonpathogenic and pathogenic primary isolate simian/human immunodeficiency virus strains. J. Virol. 74:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marinaro, M., P. N. Boyaka, R. J. Jackson, F. D. Finkelman, H. Kiyono, E. Jirillo, and J. R. McGhee. 1999. Use of intranasal IL-12 to target predominantly Th1 responses to nasal and Th2 responses to oral vaccines given with cholera toxin. J. Immunol. 162:114–121. [PubMed] [Google Scholar]
- 27.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nature Med. 6:207–210. [DOI] [PubMed] [Google Scholar]
- 28.Mazanec, M. B., C. S. Kaetzel, M. E. Lamm, D. Fletcher, and J. G. Nedrud. 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 89:6901–6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzoli, S., L. Lopalco, A. Salvi, D. Trabattoni, S. Lo Caputo, F. Semplici, M. Biasin, C. Ble, A. Cosma, C. Pastori, F. Meacci, F. Mazzotta, M. L. Villa, A. G. Siccardi, and M. Clerici. 1999. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J. Infect. Dis. 180:871–875. [DOI] [PubMed] [Google Scholar]
- 30.Mazzoli, S., D. Trabattoni, S. L. Caputo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3:1250–1257. [DOI] [PubMed] [Google Scholar]
- 31.Meropol, N. J., N. J. Petrelli, B. J. Lipman, M. Rodriguez-Bigas, W. Hicks, H. O. Douglass, J. L. Smith, M. Rasey, L. E. Blumenson, L. Vaickus, F. A. Hayes, and J. M. Agosti. 1996. Granulocyte-macrophage colony-stimulating factor as infection prophylaxis in high-risk oncologic surgery. Am. J. Surg. 172:299–302. [DOI] [PubMed] [Google Scholar]
- 32.Michetti, P., M. J. Mahan, J. M. Slauch, J. J. Mekalanos, and M. R. Neutra. 1992. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 60:1786–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemunaitis, J., F. R. Appelbaum, K. Lilleby, W. C. Buhles, C. Rosenfeld, Z. R. Zeigler, R. K. Shadduck, J. W. Singer, W. Meyer, and C. D. Buckner. 1994. Phase I study of recombinant interleukin-1 beta in patients undergoing autologous bone marrow transplant for acute myelogenous leukemia. Blood 83:3473–3479. [PubMed] [Google Scholar]
- 34.Panja, A., S. Goldberg, L. Eckmann, P. Krishen, and L. Mayer. 1998. The regulation and functional consequence of proinflammatory cytokine binding on human intestinal epithelial cells. J. Immunol. 161:3675–3684. [PubMed] [Google Scholar]
- 35.Pastori, C., C. Barassi, S. Piconi, R. Longhi, M. L. Villa, A. G. Siccardi, M. Clerici, and L. Lopalco. 2000. HIV neutralizing IgA in exposed seronegative subjects recognise an epitope within the gp41 coiled-coil pocket. J. Biol. Regul. Homeostatic Agents 14:15–21. [PubMed] [Google Scholar]
- 36.Rinehart, J., E. Hersh, B. Issell, P. Triozzi, W. Buhles, and J. Neidhart. 1997. Phase 1 trial of recombinant human interleukin-1beta (rhIL-1beta), carboplatin, and etoposide In patients with solid cancers: Southwest Oncology Group Study 8940. Cancer Investig. 15:403–410. [DOI] [PubMed] [Google Scholar]
- 37.Robertson, M. J., C. Cameron, M. B. Atkins, M. S. Gordon, M. T. Lotze, M. L. Sherman, and J. Ritz. 1999. Immunological effects of interleukin 12 administered by bolus intravenous injection to patients with cancer. Clin. Cancer Res. 5:9–16. [PubMed] [Google Scholar]
- 38.Robinson, J. K., T. G. Blanchard, A. D. Levine, S. N. Emancipator, and M. E. Lamm. 2001. A mucosal IgA-mediated excretory immune system in vivo. J. Immunol. 166:3688–3692. [DOI] [PubMed] [Google Scholar]
- 39.Rosner, B. 1994. Fundamentals of biostatistics, p.319. Wadsworth Publishing, New York, N.Y.
- 40.Simecka, J. W., R. J. Jackson, H. Kiyono, and J. R. McGhee. 2000. Mucosally induced immunoglobulin E-associated inflammation in the respiratory tract. Infect. Immun. 68:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmons, S. J., B. A. Tjoa, M. Rogers, A. Elgamal, G. M. Kenny, H. Ragde, M. J. Troychak, A. L. Boynton, and G. P. Murphy. 1999. GM-CSF as a systemic adjuvant in a phase II prostate cancer vaccine trial. Prostate 39:291–297. [DOI] [PubMed] [Google Scholar]
- 42.Snider, D. P., J. S. Marshall, M. H. Perdue, and H. Liang. 1994. Jul 15. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J. Immunol. 153:647–657. [PubMed] [Google Scholar]
- 43.Sokal, R. R., and F. J. Rohlf. 1995. Comparison among means: planned comparsions, p.229–230. W. H. Freeman and Company, New York, N.Y.
- 44.Staats, H. F., C. P. Bradney, W. M. Gwinn, S. S. Jackson, G. D. Sempowski, H.-X. Liao, N. L. Letvin, and B. F. Haynes. 2001. Cytokine requirements for the induction of systemic and mucosal cytotoxic T lymphocytes after nasal immunization. J. Immunol. 167:5386–5394. [DOI] [PubMed] [Google Scholar]
- 45.Staats, H. F., and F. A. Ennis. 1999. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J. Immunol. 162:6141–6147. [PubMed] [Google Scholar]
- 46.Staats, H. F., S. P. Montgomery, and T. J. Palker. 1997. Intranasal immunization is superior to vaginal, gastric, or rectal immunization for the induction of systemic and mucosal anti-Hiv antibody responses. AIDS Res. Hum. Retroviruses 13:945–952. [DOI] [PubMed] [Google Scholar]
- 47.Staats, H. F., W. G. Nichols, and T. J. Palker. 1996. Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A). J. Immunol. 157:462–472. [PubMed] [Google Scholar]
- 48.Staats, H. F., T. G. Tlusty, M. A. Egan, S. Y. Chong, G. D. Sempowski, H. X. Liao, Z. R. Israel, J. H. Eldridge, and B. F. Haynes. 2001. Cytokine adjuvants for the induction of anti-HIV mucosal IgA and cell-mediated immune responses. Foundation for AIDS Vaccine Research and Development, Philadelphia, Pa.
- 49.Stasi, R., A. Pagano, E. Terzoli, and S. Amadori. 1999. Recombinant human granulocyte-macrophage colony-stimulating factor plus erythropoietin for the treatment of cytopenias in patients with myelodysplastic syndromes. Br. J. Haematol. 105:141–148. [PubMed] [Google Scholar]
- 50.van Ginkel, F. W., R. J. Jackson, Y. Yuki, and J. R. McGhee. 2000. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165:4778–4782. [DOI] [PubMed] [Google Scholar]
- 51.Veltri, S., and J. W. Smith, 2nd. 1996. Interleukin 1 trials in cancer patients: a review of the toxicity, antitumor and hematopoietic effects. Stem Cells 14:164–176. [DOI] [PubMed] [Google Scholar]
- 52.Verschraegen, C. F., A. P. Kudelka, W. Termrungruanglert, C. G. de Leon, C. L. Edwards, R. S. Freedman, J. J. Kavanagh, and S. Vadhan-Raj. 1996. Effects of interleukin-1 alpha on ovarian carcinoma in patients with recurrent disease. Eur. J. Cancer 32A:1609–1611. [DOI] [PubMed] [Google Scholar]
- 53.Weisdorf, D., E. Katsanis, C. Verfaillie, N. K. C. Ramsay, R. Haake, L. Garrison, and B. R. Blazar. 1994. Interleukin-1 alpha administered after autologous transplantation: a phase I/II clinical trial. Blood 84:2044–2049. [PubMed] [Google Scholar]
- 54.Xiao, B. G., and H. Link. 1997. Mucosal tolerance: a two-edged sword to prevent and treat autoimmune diseases. Clin. Immunol. Immunopathol. 85:119–128. [DOI] [PubMed] [Google Scholar]