Abstract
l-Tryptophan degradation by indoleamine 2,3-dioxygenase (IDO) might have an important role in gamma interferon (IFN-γ)-induced antimicrobial effects. In the present study, the effects of Toxoplasma gondii infection on IDO were investigated by using wild-type and IFN-γ-gene-deficient (knockout) (IFN-γ KO) mice. In wild-type C57BL/6J mice, enzyme activities and mRNA levels for IDO in both lungs and brain were markedly increased and lung l-tryptophan concentrations were dramatically decreased following T. gondii infection. In contrast, these metabolic changes did not occur in T. gondii-infected IFN-γ KO mice or in uninfected IFN-γ KO mice. The levels of inducible nitric oxide synthase (iNOS) induction in infected IFN-γ KO mice were high in lungs and low in brain compared to those in infected wild-type mice. The extent of increased mRNA expression of T. gondii surface antigen gene 2 (SAG2) induced in lungs and brain by T. gondii infection was significantly enhanced in IFN-γ KO mice compared to wild-type mice on day 7 postinfection. Treatment with N-nitro-l-arginine methyl ester, an iNOS inhibitor, increased the levels of SAG2 mRNA in brain but not in lungs and of plasma l-kynurenine after T. gondii infection. This in vivo study provides evidence that l-tryptophan depletion caused by T. gondii is directly mediated by IFN-γ in the lungs, where iNOS is not induced by IFN-γ. This study suggests that there is an antitoxoplasma mechanism of cross-regulation between iNOS and IDO and that the expression of the main antiparasite effector mechanisms for iNOS and/or IDO may vary among tissues.
Toxoplasma gondii, an intracellular protozoan parasite, is a major pathogen of opportunistic infectious disease and causes severe encephalitis, pneumonia, and retinochoroiditis in infants, during pregnancy, and in immunocompromised hosts, such as patients with AIDS or patients treated with immunosuppressive drugs (25, 32, 43). Previous studies indicated that various cytokines have important roles in the regulation of T. gondii replication. In immunocompetent hosts, interleukin-12 (IL-12) produced by activated macrophages triggers cell-mediated immunity in early T. gondii infection. IL-12 is the critical initiator of natural killer cell gamma interferon (IFN-γ) production, which is greatly augmented by tumor necrosis factor alpha (TNF-α), IL-1β, and IL-18 (5, 20, 21). Tissue growth factor β, IL-4, and IL-10 have crucial roles in limiting potentially harmful T-cell-induced inflammatory responses in early T. gondii infection (13, 19, 34). Many studies have demonstrated that IFN-γ production by natural killer cells is a major mechanism of innate defense against T. gondii infection (39). IFN-γ inhibits T. gondii replication in various human and mouse cells. Furthermore, a previous study indicated that IFN-γ-gene-deficient (knockout) (IFN-γ KO) mice lost the ability to inhibit T. gondii growth (22).
Some in vitro studies demonstrated that IFN-γ-induced antitoxoplasma activity in human cells, such as macrophages, fibroblasts, glioblastoma cells, and epithelial cells, depends on the induction of indoleamine 2,3-dioxygenase (IDO), which is the rate-limiting enzyme of the l-tryptophan (l-TRP)-l-kynurenine (l-KYN) pathway. IDO is induced by IFN-γ-dependent and/or -independent mechanisms, depending on the variety of immune stimuli in mammalian extrahepatic tissues (8, 10, 29, 30, 31). IFN-γ-induced IDO-dependent antitoxoplasma effects were postulated to be secondary to the degradation of the essential amino acid l-TRP (7, 29). Furthermore, the antimicrobial effects induced by IDO-dependent mechanisms were also demonstrated for both Chlamydia psittaci and group B streptococcal infections (4, 26). On the other hand, recent studies also indicated that IFN-γ-induced antitoxoplasma activity is mediated by nitric oxide (NO). Indeed, there is induction of inducible NO synthase (iNOS) in T. gondii replication in murine cells, such as macrophages and microglial cells (1, 23), but not in human macrophages (24). In an in vivo study with iNOS-deficient mice, the protective role of NO against T. gondii infection was tissue specific rather than systemic (38). Thus, IDO and iNOS are involved in the immunomodulatory roles of IFN-γ, and evidence suggests that these pathways are functionally cross-regulated. The mechanisms of IFN-γ-induced antitoxoplasma activity are complex and might vary by cell type, tissue, and species. In the present study, changes in l-TRP-l-KYN pathway metabolism via IDO induction following toxoplasma infection were characterized in vivo. In addition, the role of IFN-γ in the response to IDO induced by toxoplasma infection was also evaluated by using IFN-γ KO mice.
MATERIALS AND METHODS
Materials.
l-TRP, l-KYN, methylene blue, and ascorbic acid were obtained from Sigma Chemical Co. (St. Louis, Mo.). All other chemicals, of analytical grade, were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Animals.
Wild-type female C57BL/6J mice (18 to 20 g) were obtained from Japan SLC Inc. (Hamamatsu, Japan). IFN-γ KO mice were obtained from Jackson Laboratory (Bar Harbor, Maine). Animals were kept in a temperature-controlled facility at 25°C with a 12-h on-12-h off light cycle and allowed free access to food and water. All experiments were performed according to the guidelines for animal experiments set by Gifu University School of Medicine. Mice were killed at 0, 4, 7, 14, 21, and 28 days after infection by intraperitoneal injection of sodium pentobarbital (50 mg/kg of body weight). Blood was taken from the abdominal vena cava, and plasma was separated by low-speed centrifugation (1,000 × g, 10 min). Tissues were placed in polypropylene tubes and immediately frozen by immersion in liquid nitrogen. All samples were frozen at −80°C until analysis.
T. gondii infection.
Parasites were harvested from the brains of mice chronically infected with T. gondii cysts (Fukaya strain). Brain tissue was dispersed in saline. The final concentration of the infectious agent was adjusted to a dose of 20 cysts per 0.2 ml, which was injected intraperitoneally into mice.
Inhibitor studies.
N-Nitro-l-arginine methyl ester (l-NAME) and 6-chloro-dl-tryptophan were dissolved in 0.5% carboxymethyl cellulose sodium salt. l-NAME (100 mg/kg) and/or 6-chloro-dl-tryptophan (125 mg/kg) was administered orally twice a day. Treatment was started 4 days after T. gondii infection.
Determination of l-TRP and l-KYN concentrations.
Plasma was mixed with 3 volumes of 3% perchloric acid, and tissue samples were sonicated in 4 volumes of 3% perchloric acid. After centrifugation, the concentrations of l-TRP and l-KYN in the supernatants were measured by using high-performance liquid chromatography (HPLC) with a 5-mm octyldecyl silane column (150 mm by 2.1 mm; Eicom, Kyoto, Japan) and a spectrophotometric detector or a fluorescence spectrometric detector as described previously (11). UV signals were monitored at 280 nm for l-TRP and 355 nm for l-KYN. The fluorescence excitation and emission wavelengths were set at 270 and 360 nm, respectively, for quantification of l-TRP in tissues. The mobile phase consisted of 2.5% acetonitrile in 0.1 M sodium acetate (pH 3.9) and was filtered through a 0.45-μm-pore-size HA-type filter obtained from Millipore Corp. (Bedford, Mass.). The flow rate was maintained at 0.75 ml/min throughout the chromatographic run.
Enzyme assay.
IDO activity was measured as described previously (11). Briefly, tissues were homogenized with a Polytron homogenizer (Kinematica AG, Lucerne, Switzerland) in 1.5 volumes of ice-cold 0.14 M KCl-20 mM potassium phosphate buffer (pH 7.0). The homogenate samples were centrifuged at 7,000 × g and 4°C for 10 min. An aliquot of supernatant was taken for the measurement of IDO activity. The reaction mixture contained 50 μl of enzyme preparation and 50 μl of substrate solution. The composition of the substrate solution was 100 mM potassium phosphate buffer (pH 6.5), 50 μM methylene blue, 20 μg of catalase, 50 mM ascorbate, and 0.4 mM l-TRP. After incubation of the reaction mixture at 37°C, samples were acidified with 3% perchloric acid and centrifuged at 7,000 × g and 4°C for 10 min. The concentrations of the enzymatic products were measured by using HPLC. Enzyme activity was expressed as the product content per hour per milligram of tissue protein.
Determination of nitrite and nitrate.
NO release was determined spectrophotometrically by measuring the accumulation of nitrite and nitrate. Nitrate was measured as nitrite after enzymatic conversion by nitrate reductase as described previously (28). Nitrite was measured by using the Griess reaction (14).
Quantification of IDO mRNA by RT-PCR.
Total RNA from tissues was rapidly isolated by using an ISOGEN kit for RNA isolation (Nippon Gene, Tokyo, Japan). Total RNA (1 μg) was used for the synthesis of the first strand of cDNA. Reverse transcription (RT)-PCR was performed by using an mRNA selective PCR kit (Takara Biomedicals, Tokyo, Japan). The sample was reverse transcribed in a final volume of 50 μl at 50°C for 30 min. The subsequent PCR was performed at 85°C for 1 min, 55°C for 1 min, and 72°C for 1 min. The final cycle was performed at 72°C for 4 min. The nucleotide sequences for the forward and reverse primers for mouse IDO, iNOS, T. gondii surface antigen gene 2 (SAG2), β-actin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown in Table 1. The final PCR products were electrophoresed on 2.5% agarose gels and visualized by using UV light illumination after ethidium bromide staining. The signal intensities of the bands with the expected sizes were analyzed by using an image analyzer (Bio-Profile system; M&S Instrument Trading Inc., Tokyo, Japan). The optimal conditions for PCR of IDO, iNOS, SAG2, β-actin, and GAPDH cDNA fragments were examined by using mice infected with T. gondii. The signal intensities of IDO, iNOS, and SAG2 were normalized to that of β-actin or GAPDH amplified under identical conditions and were expressed as a percentage of the control value.
TABLE 1.
Primer sequences for RT-PCR
| Mouse molecule | Primera | Sequence | PCR product (bp) | Source or reference |
|---|---|---|---|---|
| IDO | F | 5"-CACTGAGCACGGACGGACTGAGA | 400 | This study |
| R | 5"-TCCAATGCTTTCAGGTCTTGACGC | |||
| iNOS | F | 5"-AAGCTGCATGTGACATCGACCCGT | 598 | 6 |
| R | 5"-GCATCTGGTAGCCAGCGTACCGG | |||
| SAG2 | F | 5"-ATGAGTTTCTCAAAGACCACGAGCCTA | 560 | 12 |
| R | 5"-TTACACAAACGTGATCAACAAACCTGC | |||
| β-Actin | F | 5-ATGGATGACGATATCGCT | 569 | 27 |
| R | 5-ATGAGGTAGTCTGTCAGGT | |||
| GAPDH | F | 5"-ACGACCCCTTCATTGACCTCAACT | 320 | 2 |
| R | 5"-ATATTTCTCGTGGTTCACACCCAT |
F, forward; R, reverse.
Statistical analyses.
Results were expressed as the mean and standard error of the mean (SEM) or as a percentage of the control value. Intergroup comparisons were made by using one-way analysis of variance, followed by the Mann-Whitney test or Scheffe's F post hoc test. A P value of less than 0.05 was considered statistically significant.
RESULTS
Temporal profiles of IDO induction in wild-type mice infected with T. gondii.
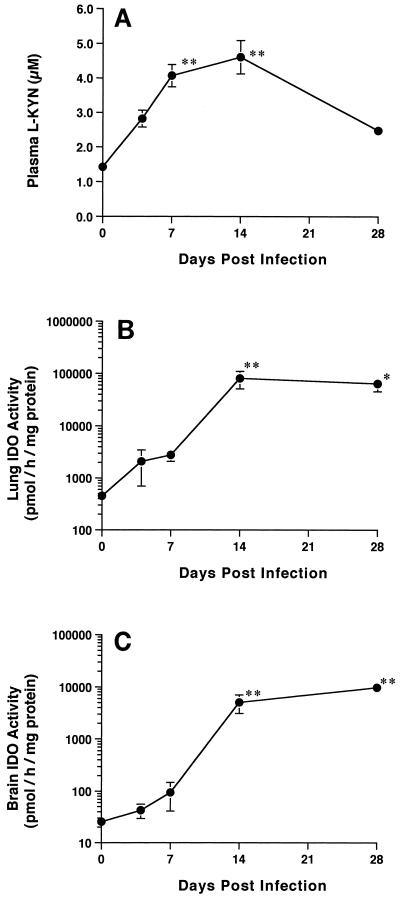
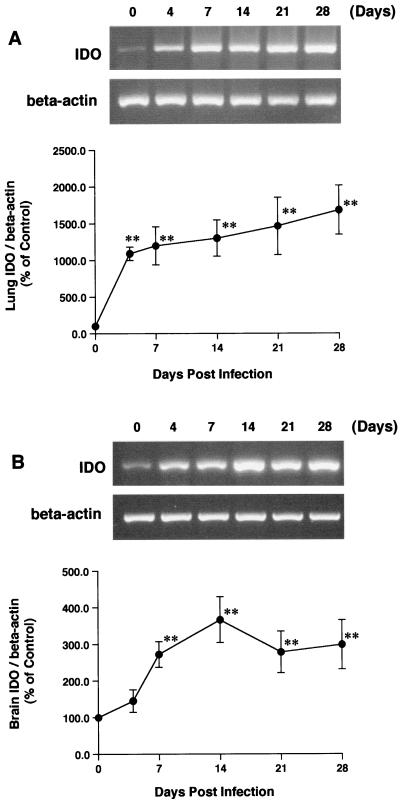
We investigated the time course of changes in plasma l-KYN concentrations and the levels of IDO activity and mRNA expression in the lungs and brain in wild-type mice infected with T. gondii (Fig. 1 and 2). Plasma l-KYN levels were significantly increased at least as early as day 7 following T. gondii infection (1.42 ± 0.09 versus 4.07 ± 0.33 μM, day 0 versus day 7, respectively; P < 0.05), with a maximal elevation on day 14 (3.24-fold), and were decreased on day 28 following T. gondii infection. Lung IDO activity was markedly increased (179-fold) on day 14 (from 450 ± 79 to 80,900 ± 29,500 pmol/h/mg of protein), and this level was maintained until day 28. Brain IDO activity was also increased (198-fold) on day 14 (from 25.5 ± 5.1 to 5,049 ± 1,936 pmol/h/mg of protein) and was maintained at that level until day 28. Furthermore, the lung IDO mRNA expression level was rapidly increased (2.13-fold) on day 4 and gradually increased until day 28. The brain IDO mRNA expression level was markedly increased on day 14, and this level was maintained until day 28.
FIG. 1.
Accumulation of plasma l-KYN (A) and marked induction of IDO activity in lungs (B) and brain (C) by T. gondii infection in wild-type mice. Note the logarithmic scale of the ordinate. Samples were analyzed on days 0, 4, 7, 14, and 28 following intraperitoneal injection of 20 cysts of T. gondii. Each point represents the mean and SEM for results from three to six samples. In a preliminary study (not shown), there were no significant changes in parameters measured in wild-type mice treated with brain homogenate without T. gondii infection at any time examined. ∗, P < 0.05. ∗∗, P < 0.005.
FIG. 2.
Induction of IDO mRNA expression in lungs (A) and brain (B) following T. gondii infection in wild-type mice. Total RNA was isolated from the lungs and brain of wild-type mice on the indicated days following intraperitoneal injection of T. gondii and used for RT-PCR. Each point represents the mean and SEM for results from three to six samples. ∗∗, P < 0.005 (for comparisons with controls).
Effects of IFN-γ KO on degradation of l-TRP and accumulation of l-KYN in the lungs and brain following T. gondii infection.
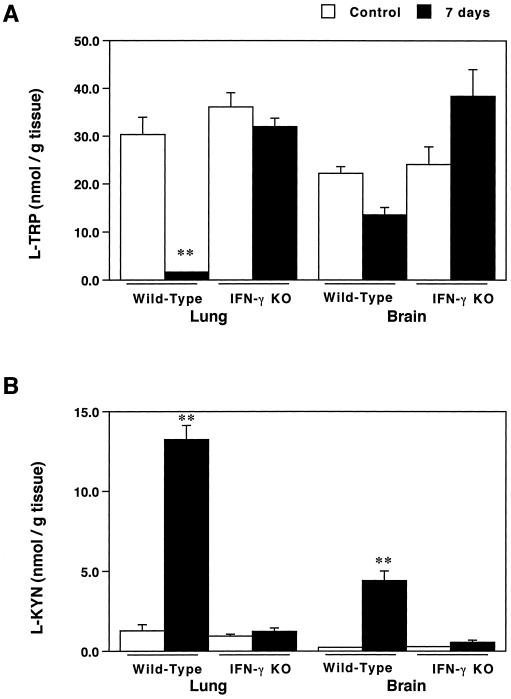
In the lungs, there were marked increases in l-KYN concentrations and almost complete l-TRP depletion in wild-type mice 7 days after T. gondii infection. In the brain, l-TRP concentrations tended to decrease, but the decrease was not statistically significant, and l-KYN concentrations were markedly increased on day 7 after T. gondii infection. In contrast, infected IFN-γ KO mice did not have these metabolic changes in the lungs or brain (Fig. 3).
FIG. 3.
Effects of IFN-γ gene deficiency on the degradation of l-TRP and the accumulation of l-KYN in lungs and brain after T. gondii infection. The concentrations of l-TRP (A) and l-KYN (B) in supernatants of homogenates of lungs and brain from wild-type and IFN-γ KO mice on days 0 and 7 following intraperitoneal injection of T. gondii were measured by using HPLC. Each bar represents the mean and SEM for results from four to eight samples. ∗∗, P < 0.005 (for comparisons with day 0 postinfection). It is especially noteworthy that IDO activity in the lungs was dramatically increased (more than 100-fold) by toxoplasma infection. Surprisingly, the l-TRP concentration in the lungs was completely depleted.
Differential effects of IFN-γ deficiency on IDO and iNOS induction in T. gondii-infected mice.
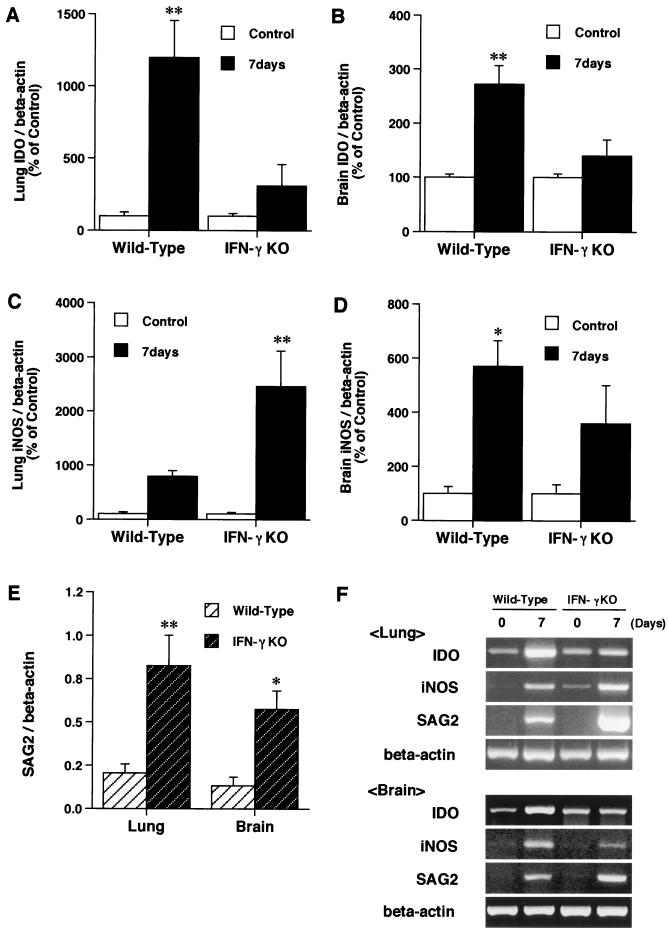
All IFN-γ KO mice died 9 to 11 days after T. gondii infection, whereas wild-type mice all survived until day 28 (data not shown). On day 7 after T. gondii infection, the level of SAG2 mRNA expression in IFN-γ KO mice was significantly higher than that in wild-type mice for both the lungs and brain (Fig. 4E).
FIG. 4.
Effects of IFN-γ gene deficiency on the induction of IDO and iNOS and tachyzoite SAG2 mRNA expression by T. gondii infection. The expression of IDO mRNA (A and B), iNOS mRNA (C and D), and SAG2 mRNA (E) was analyzed by using RT-PCR with total RNA from the lungs and brain of wild-type and IFN-γ KO mice on days 0 and 7 following intraperitoneal injection of T. gondii. Each bar represents the mean and SEM for results from three to six samples. ∗, P < 0.05 (for comparisons with day 0 postinfection). ∗∗, P < 0.005 (for comparisons with day 0 postinfection). (F) Representative gel photograph.
To investigate whether IDO and iNOS could be induced in IFN-γ KO mice infected with T. gondii as well as in wild-type mice, IDO and iNOS mRNA expression levels in the lungs and brain in IFN-γ KO mice were compared with those in wild-type mice. The markedly enhanced IDO mRNA expression seen in wild-type mice on day 7 after infection was not seen in IFN-γ KO mice for both the lungs and brain (Fig. 4A and B). In contrast, iNOS mRNA expression in the lungs after infection in IFN-γ KO mice was significantly higher than that in infected wild-type mice, although a significant increase in iNOS mRNA expression in the brain after infection was not observed in IFN-γ KO mice relative to wild-type mice (Fig. 4C and D).
Effect of an inhibitor of iNOS on SAG2 expression in wild-type mice after infection.
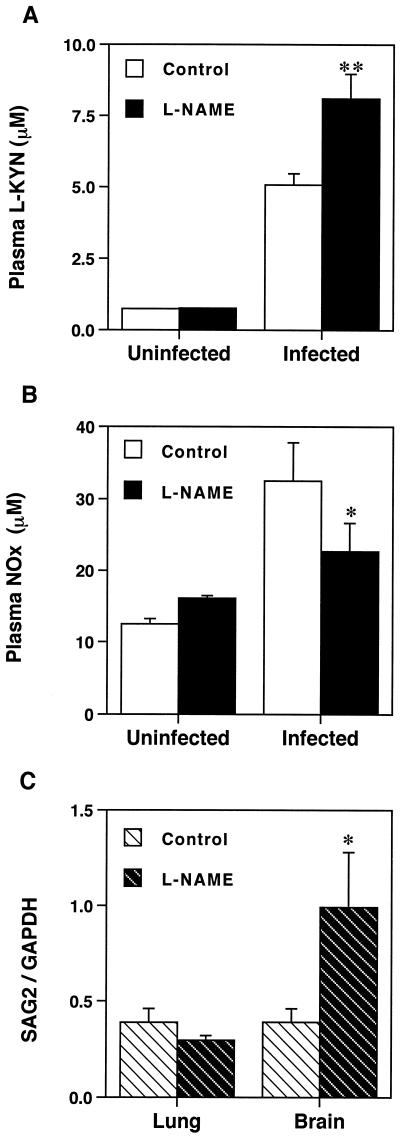
l-NAME inhibited the increase in the plasma nitrate concentration and enhanced the increase in the plasma l-KYN concentration after T. gondii infection. On the other hand, l-NAME markedly enhanced the level of SAG2 mRNA expression on day 7 in the brain but not in the lungs (Fig. 5).
FIG. 5.
Effects of an iNOS inhibitor on the plasma l-KYN and nitrate concentrations and tachyzoite SAG2 mRNA expression in T. gondii infection. Plasma l-KYN (A) and nitrate (NOx) (B) concentrations and tachyzoite SAG2 mRNA expression (C) in uninfected and infected mice treated with saline (Control) or l-NAME on day 7 following intraperitoneal injection of T. gondii were examined. Each bar represents the mean and SEM for results from four to six samples. ∗, P < 0.05 (for comparisons with controls). ∗∗, P < 0.005 (for comparisons with controls).
DISCUSSION
The present in vivo study demonstrates for the first time that IDO activity depends on IFN-γ synthesis following T. gondii infection and that antitoxoplasma activities induced by iNOS and/or IDO are regulated in a tissue-specific manner.
IFN-γ acts as an antimicrobial agent by activating macrophages, lymphocytes, and other cells following T. gondii infection. Some in vitro studies (8, 29, 30, 31) indicate that IFN-γ-induced antitoxoplasma activities are involved in IDO-dependent mechanisms by IDO induction, which is mainly induced by IFN-γ. IDO converts l-TRP to N-formylkynurenine, and strong IDO induction results in l-TRP depletion. Indeed, especially in human fibroblasts, epithelial cells, and glioblastoma cells, IDO induction by IFN-γ results in l-TRP depletion and inhibition of parasite growth. On the other hand, a recent study indicates that reactive nitrogen intermediates, including NO produced by IFN-γ, are important factors for antitoxoplasma activities (16). In murine macrophages and microglia cells, NO production is the main antiparasite effector mechanism, whereas iNOS induction is not involved in defense against toxoplasma in human macrophages (1, 23, 24). A previous study demonstrated that acute T. gondii replication was enhanced in the brain but not in the peritoneal cavity in iNOS-deficient mice (38). Thus, the mechanisms of IFN-γ-induced antitoxoplasma activities are complex. The expression of the main antiparasite effector mechanisms for iNOS and/or IDO seems to depend on the cell type, tissue, and species.
In the present study, IFN-γ KO mice had significantly shorter survival than wild-type mice in the early T. gondii infection period (data not shown), consistent with the results of a previous study (22). Furthermore, the levels of SAG2 mRNA expression in the lungs and brain were markedly increased in IFN-γ KO mice compared to wild-type mice (Fig. 4E). In wild-type mice, IDO activities in the lungs and brain were markedly increased by T. gondii infection (Fig. 1B and C). Similar results were obtained with IDO mRNA (Fig. 2).
The increased levels of lung IDO activity were maintained for at least 28 days, whereas plasma l-KYN levels increased to a maximum on day 14 and declined thereafter (Fig. 1). Previous studies indicated that l-KYN accumulates in the lungs, where IDO is induced the most, and might enter the circulation to reach the liver or to undergo further metabolism (35, 40). We speculate that the lungs and other systemic tissues produce l-KYN in response to toxoplasma infection and that elevated plasma l-KYN levels result from increased tissue production. Therefore, decreased plasma l-KYN levels might reflect decreased l-KYN accumulation in the lungs and other systemic tissues due to tissue substrate depletion by significant IDO induction on day 14 following toxoplasma infection.
l-TRP in the lungs was almost completely depleted by toxoplasma infection, and brain l-TRP levels tended to decrease. These results were due to the relatively low levels of IDO activity in the brain. Indeed, absolute values of IDO activity in the lungs are at least 10 times higher than those in the brain following toxoplasma infection. It is possible, however, that l-TRP degradation in the brain was caused at the local level. Although the localization of IDO was not demonstrated in the present study, increases in IDO activity in the lungs and brain following toxoplasma infection might arise from both resident cells and secondary infiltrated cells. Indeed, previous observations indicated that IDO immunoreactivity is markedly increased in the spinal cord of macaques with poliovirus infection, in areas of intense inflammatory response in which macrophage or microglia cell proliferation has occurred (18, 37).
In IFN-γ KO mice, in which enhanced T. gondii replication was found, the IDO induction, l-TRP depletion, and l-KYN accumulation caused by toxoplasma infection in the lungs of wild-type mice were completely absent (Fig. 3 and 4A). In contrast, lung iNOS induction was more prominent in infected IFN-γ KO mice than in infected wild-type mice (Fig. 4C). These results demonstrate that iNOS induction is not associated with an IFN-γ-mediated mechanism for resistance against T. gondii in the lungs. Therefore, we speculated that l-TRP depletion caused by IDO induction and/or other activities is more important than iNOS induction for antitoxoplasma mechanisms in the lungs. On the other hand, in the brain, l-TRP depletion did not occur in spite of IDO induction, and l-KYN accumulation occurred in wild-type mice following toxoplasma infection (Fig. 3). Furthermore, iNOS mRNA expression in the brain was enhanced in infected wild-type mice but not in infected IFN-γ KO mice (Fig. 4D). A previous study demonstrated that acute T. gondii replication was not suppressed in the brain in iNOS-deficient mice (38). Therefore, it is likely that the antitoxoplasma activity caused by iNOS induction in the brain is more dominant than that caused by l-TRP depletion and other factors, such as phagocytic NADPH oxidase. These results obtained in the lungs and brain suggest that the IFN-γ-induced mechanisms of resistance against T. gondii are different among tissues.
The present study also assessed the effects of 6-chloro-dl-tryptophan, an IDO inhibitor, and l-NAME, an iNOS inhibitor, on T. gondii replication. 6-Chloro-dl-tryptophan was not adequate as an IDO inhibitor when daily oral administration (5 mg/mouse) was performed in this study. In fact, 6-chloro-dl-tryptophan slightly attenuated the increases in lung and brain IDO activities (data not shown) (36). On the other hand, inhibition of iNOS induction increased plasma l-KYN concentrations. Furthermore, l-NAME did not have any effect on T. gondii replication in the lungs, although the level of T. gondii replication was increased by l-NAME in the brain. The present result of increased brain T. gondii replication in l-NAME-treated T. gondii-infected mice was in agreement with the result of the above-mentioned study with iNOS-deficient mice (38).
A previous in vitro study demonstrated that the levels of IFN-γ-induced IDO activity and IFN-γ-induced antitoxoplasma activities were significantly increased by treatment with an iNOS inhibitor (9). It is also known that NO can inhibit the activity of IDO in vitro (41). In acute toxoplasma infection, NO may prevent total depletion of tryptophan and/or other indoleamines, which are important in the functions of the host cell, and may also prevent the accumulation of potentially toxic metabolites in the kynurenine pathway. These data suggest that there is cross-regulation between iNOS and IDO.
It has not yet been established whether the physiologic role of IDO regulation by IFN-γ is beneficial or detrimental. Several studies suggest that IDO induction by IFN-γ exerts antimicrobial and antiproliferative effects, possibly by depletion of l-TRP. Indeed, transfected murine cells expressing the IDO gene inhibit toxoplasma replication following IDO induction (15). However, local accumulation of kynurenine metabolites, in particular, quinolinic acid, following IDO induction may also represent a potentially detrimental event. In fact, quinolinic acid is a potent excitotoxin, and its overproduction has been linked to neuronal damage occurring in brain inflammation (17), initiation of lipid peroxidation (33), and other conditions (3, 42). Indeed, quinolinic acid concentration was significantly increased by T. gondii infection (S. Fujigaki and K. Saito, unpublished observation). Therefore, it is possible that increased levels of quinolinic acid and other kynurenine pathway metabolites play a detrimental role following T. gondii infection. Although it is beyond the scope of the present study to determine whether the effects of these responses are toxic or beneficial, l-TRP depletion caused by strong IDO induction does occur in certain tissues following toxoplasma infection in an in vivo animal model.
In summary, the present study demonstrates that both lung and brain IDO activities increase as a result of toxoplasma infection and that l-TRP can be depleted in the lungs under such pathological conditions. In addition, the results obtained from IFN-γ KO mice suggest that IFN-γ is one of the most important cytokines for the induction of IDO and for antitoxoplasma activities in vivo. This study suggests that there is an antitoxoplasma mechanism of cross-regulation between iNOS and IDO and that the expression of the main antiparasite effector mechanisms for iNOS and/or IDO may vary among tissues. Further in vivo studies on the role of IDO and iNOS induction and on the consequences of increases in kynurenine pathway metabolites following toxoplasma infection are warranted.
Acknowledgments
We thank A. Makioka for providing T. gondii. We also thank John Cole for proofreading the English of the manuscript.
This study was supported in part by grant-in-aid 13680840 from the Ministry of Education, Science and Culture and by grants (Research on HIV/AIDS, Health Sciences Research) from the Ministry of Health and Welfare to K. Saito.
Editor: J. M. Mansfield
REFERENCES
- 1.Adams, L. B., J. B. Hibbs, Jr., R. R. Taintor, and J. L. Krahenbuhl. 1990. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J. Immunol. 144:2725-2729. [PubMed] [Google Scholar]
- 2.Alcolea, S., M. Theveniau-Ruissy, T. Jarry-Guichard, I. Marics, E. Tzouanacou, J. P. Chauvin, J. P. Briand, A. F. Moorman, W. H. Lamers, and D. B. Gros. 1999. Downregulation of connexin 45 gene products during mouse heart development. Circ. Res. 84:1365-1379. [DOI] [PubMed] [Google Scholar]
- 3.Beskid, M., L. Finkiewicz-Murawiejska, Z. Obminski, and B. Wolska. 1991. Quinolinic acid: a modulator of the heart calcium channel in the rat and a binder of calcium ions. Exp. Pathol. 41:110-114. [DOI] [PubMed] [Google Scholar]
- 4.Byrne, G. I., L. K. Lehmann, and G. J. Landry. 1986. Induction of tryptophan catabolism is the mechanism for gamma interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect. Immun. 53:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, G., R. Kastelein, and C. A. Hunter. 2000. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect. Immun. 68:6932-6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colle, J. H., P. B. Falanga, M. Singer, B. Hevin, and G. Milon. 1997. Quantitation of messenger RNA by competitive RT-PCR: a simplified read out assay. J. Immunol. Methods 210:175-184. [DOI] [PubMed] [Google Scholar]
- 7.Dai, W., H. Pan, O. Kwok, and J. P. Dubey. 1994. Human indoleamine 2,3-dioxygenase inhibits Toxoplasma gondii growth in fibroblast cells. J. Interferon Res. 14:313-317. [DOI] [PubMed] [Google Scholar]
- 8.Daubener, W., K. Pilz, S. Seghrouchni Zennati, T. Bilzer, H. G. Fischer, and U. Hadding. 1993. Induction of toxoplasmostasis in a human glioblastoma by interferon gamma. J. Neuroimmunol. 43:31-38. [DOI] [PubMed] [Google Scholar]
- 9.Daubener, W., V. Posdziech, U. Hadding, and C. R. MacKenzie. 1999. Inducible anti-parasitic effector mechanisms in human uroepithelial cells: tryptophan degradation vs. NO production. Med. Microbiol. Immunol. (Berlin) 187:143-147. [DOI] [PubMed] [Google Scholar]
- 10.Fujigaki, S., K. Saito, K. Sekikawa, S. Tone, O. Takikawa, H. Fujii, H. Wada, A. Noma, and M. Seishima. 2001. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism. Eur. J. Immunol. 31:2313-2318. [DOI] [PubMed] [Google Scholar]
- 11.Fujigaki, S., K. Saito, M. Takemura, H. Fujii, H. Wada, A. Noma, and M. Seishima. 1998. Species differences in l-tryptophan-kynurenine pathway metabolism: quantification of anthranilic acid and its related enzymes. Arch. Biochem. Biophys. 358:329-335. [DOI] [PubMed] [Google Scholar]
- 12.Gazzinelli, R. T., I. Eltoum, T. A. Wynn, and A. Sher. 1993. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J. Immunol. 151:3672-3681. [PubMed] [Google Scholar]
- 13.Gazzinelli, R. T., I. P. Oswald, S. L. James, and A. Sher. 1992. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J. Immunol. 148:1792-1796. [PubMed] [Google Scholar]
- 14.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 15.Habara-Ohkubo, A., T. Shirahata, O. Takikawa, and R. Yoshida. 1993. Establishment of an antitoxoplasma state by stable expression of mouse indoleamine 2,3-dioxygenase. Infect. Immun. 61:1810-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi, S., C. C. Chan, R. Gazzinelli, and F. G. Roberge. 1996. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J. Immunol. 156:1476-1481. [PubMed] [Google Scholar]
- 17.Heyes, M. P., K. Saito, J. S. Crowley, L. E. Davis, M. A. Demitrack, M. Der, L. A. Dilling, J. Elia, M. J. Kruesi, A. Lackner, et al. 1992. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 115:1249-1273. [DOI] [PubMed] [Google Scholar]
- 18.Heyes, M. P., K. Saito, D. Jacobowitz, S. P. Markey, O. Takikawa, and J. H. Vickers. 1992. Poliovirus induces indoleamine-2,3-dioxygenase and quinolinic acid synthesis in macaque brain. FASEB J. 6:2977-2989. [DOI] [PubMed] [Google Scholar]
- 19.Hunter, C. A., L. Bermudez, H. Beernink, W. Waegell, and J. S. Remington. 1995. Transforming growth factor-beta inhibits interleukin-12-induced production of interferon-gamma by natural killer cells: a role for transforming growth factor-beta in the regulation of T cell-independent resistance to Toxoplasma gondii. Eur. J. Immunol. 25:994-1000. [DOI] [PubMed] [Google Scholar]
- 20.Hunter, C. A., R. Chizzonite, and J. S. Remington. 1995. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J. Immunol. 155:4347-4354. [PubMed] [Google Scholar]
- 21.Hunter, C. A., C. S. Subauste, V. H. Van Cleave, and J. S. Remington. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 62:2818-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, L. L., and P. C. Sayles. 1995. Strong cytolytic activity of natural killer cells is neither necessary nor sufficient for preimmune resistance to Toxoplasma gondii infection. Nat. Immun. 14:209-215. [PubMed] [Google Scholar]
- 23.Jun, C. D., S. H. Kim, C. T. Soh, S. S. Kang, and H. T. Chung. 1993. Nitric oxide mediates the toxoplasmastatic activity of murine microglial cells in vitro. Immunol. Investig. 22:487-501. [DOI] [PubMed] [Google Scholar]
- 24.Langermans, J. A., M. E. Van der Hulst, P. H. Nibbering, P. S. Hiemstra, L. Fransen, and R. Van Furth. 1992. IFN-gamma-induced l-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J. Immunol. 148:568-574. [PubMed] [Google Scholar]
- 25.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211-222. [DOI] [PubMed] [Google Scholar]
- 26.MacKenzie, C. R., U. Hadding, and W. Daubener. 1998. Interferon-gamma-induced activation of indoleamine 2,3-dioxygenase in cord blood monocyte-derived macrophages inhibits the growth of group B streptococci. J. Infect. Dis. 178:875-878. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery, R. A., and M. J. Dallman. 1991. Analysis of cytokine gene expression during fetal thymic ontogeny using the polymerase chain reaction. J. Immunol. 147:554-560. [PubMed] [Google Scholar]
- 28.Moshage, H., B. Kok, J. R. Huizenga, and P. L. Jansen. 1995. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin. Chem. 41:892-896. [PubMed] [Google Scholar]
- 29.Murray, H. W., A. Szuro-Sudol, D. Wellner, M. J. Oca, A. M. Granger, D. M. Libby, C. D. Rothermel, and B. Y. Rubin. 1989. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect. Immun. 57:845-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagineni, C. N., K. Pardhasaradhi, M. C. Martins, B. Detrick, and J. J. Hooks. 1996. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect. Immun. 64:4188-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfefferkorn, E. R. 1984. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. USA 81:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pomeroy, C., and G. A. Filice. 1992. Pulmonary toxoplasmosis: a review. Clin. Infect. Dis. 14:863-870. [DOI] [PubMed] [Google Scholar]
- 33.Rios, C., and A. Santamaria. 1991. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem. Res. 16:1139-1143. [DOI] [PubMed] [Google Scholar]
- 34.Roberts, C. W., D. J. Ferguson, H. Jebbari, A. Satoskar, H. Bluethmann, and J. Alexander. 1996. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect. Immun. 64:897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito, K., J. S. Crowley, S. P. Markey, and M. P. Heyes. 1993. A mechanism for increased quinolinic acid formation following acute systemic immune stimulation. J. Biol. Chem. 268:15496-15503. [PubMed] [Google Scholar]
- 36.Saito, K., S. P. Markey, and M. P. Heyes. 1994. 6-Chloro-d,l-tryptophan, 4-chloro-3-hydroxyanthranilate and dexamethasone attenuate quinolinic acid accumulation in brain and blood following systemic immune activation. Neurosci. Lett. 178:211-215. [DOI] [PubMed] [Google Scholar]
- 37.Saito, K., T. S. Nowak, Jr., S. P. Markey, and M. P. Heyes. 1993. Mechanism of delayed increases in kynurenine pathway metabolism in damaged brain regions following transient cerebral ischemia. J. Neurochem. 60:180-192. [DOI] [PubMed] [Google Scholar]
- 38.Scharton-Kersten, T. M., G. Yap, J. Magram, and A. Sher. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1261-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 40.Takikawa, O., R. Yoshida, R. Kido, and O. Hayaishi. 1986. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J. Biol. Chem. 261:3648-3653. [PubMed] [Google Scholar]
- 41.Thomas, S. R., D. Mohr, and R. Stocker. 1994. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J. Biol. Chem. 269:14457-14464. [PubMed] [Google Scholar]
- 42.Veneziale, C. M., P. Walter, N. Kneer, and H. A. Lardy. 1967. Influence of l-tryptophan and its metabolites on gluconeogenesis in the isolated, perfused liver. Biochemistry 6:2129-2138. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, C. B., J. S. Remington, S. Stagno, and D. W. Reynolds. 1980. Development of adverse sequelae in children born with subclinical congenital Toxoplasma infection. Pediatrics 66:767-774. [PubMed] [Google Scholar]