Abstract
Stool specimens from 156 Maryland nursing home residents, who became ill during 20 outbreaks of gastroenteritis from November 1987 through February 1988, were analyzed. All tested negative for astroviruses, enteroviruses, Group A rotaviruses, Sapporo-like caliciviruses, and enteric bacteria (i.e., Salmonella, Clostridium, and Shigella species). Eighty-two (52%) were positive for Norwalk-like viruses (NLVs), members of the family Caliciviridae. Six distinct genetic clusters within genogroups I and II of the NLVs were detected; a genogroup II (GII) virus closely related to the Camberwell virus in the NLV GII/4 genetic cluster was the predominant strain. Serologic evidence of infection with ≥1 NLV was detected in 61 (56%) of 109 patients tested against 3 NLV antigens (i.e., Norwalk, Hawaii, and Toronto viruses). Sixteen (80%) outbreaks met the definition for an NLV outbreak. Taken together with a retrospective analysis of bacterial gastroenteritis in this same setting, these data support a major role for NLVs as etiologic agents of gastroenteritis in elderly persons.
Gastroenteritis outbreaks in nursing homes have been associated with both bacteria (including Salmonella, Campylobacter, Escherichia, Staphylococcus, and Clostridium species) and enteric viruses (including caliciviruses, rotaviruses, and astroviruses) [1–21], but the relative contribution of each has not been evaluated over an extended period. Acute epidemic viral gastroenteritis is characterized by a self-limited illness of 24–48 h duration, with a combination of ≥1 signs and symptoms, including nausea, vomiting, diarrhea, abdominal cramps, fever, chills, and headache [22]. Although this illness is generally mild, it can be debilitating in elderly persons, who are at increased risk for hospitalization [23–25]. In an analysis of 25,538 diarrhea-related deaths in the United States during 1979–1987, 51% of the deaths occurred in individuals .74 years old [26]. A recent study of 90 nonbacterial outbreaks from the Centers for Disease Control and Prevention (CDC) showed that 43% of the outbreaks occurred in hospitals or nursing homes and that Norwalk-like viruses (NLVs), members of the family Caliciviridae, were detected in 86 (96%) of the outbreaks [7, 27]. Similarly, in The Netherlands, 41 (59%) of 69 reported outbreaks of gastroenteritis occurred in nursing homes, and NLVs were detected in 60 (87%) of them [20]. Large-scale epidemiologic studies in England and Wales and in Sweden also documented the importance of NLV illness among elderly persons [28, 29].
This study was initiated in 1987 to elucidate the viral agents associated with gastroenteritis outbreaks in Maryland nursing homes during the winter season of 1987–1988. The winter season was selected because such outbreaks typically peaked at that time. Previous routine screening for enteric bacteria (i.e., Salmonella, Clostridium, and Shigella species) failed to identify an etiologic agent responsible for the majority of gastroenteritis outbreaks in Maryland nursing homes. Our initial attempts also were unsuccessful from an etiologic viewpoint, because they yielded evidence for only a small number of viral infections. We decided to reexamine the stool specimens because of the recent development of efficient diagnostic techniques, such as reverse-transcription (RT) polymerase chain reaction (PCR) [27, 30, 31], and were able to identify NLVs as the predominant pathogen in these outbreaks. The nucleotide sequences of the 1987–1988 NLVs analyzed in the present study were compared with those of NLVs that have been circulating globally in the past decade, to gain insight into the evolution of these ubiquitous diarrheal pathogens.
Materials and Methods
Study design
Nursing homes in Maryland are required to report gastroenteritis outbreaks to the Maryland Department of Health and Mental Hygiene (DHMH). An outbreak is defined as the onset of diarrhea (≥2 loose stools in 24 h) within a 7-day period in ≥3 residents from a single ward or unit or in ≥3% of the residents in a facility. Stool specimens taken from patients involved in gastroenteritis outbreaks in Maryland nursing homes are tested routinely for Salmonella, Clostridium, and Shigella species. From November 1987 to March 1988, 31 gastroenteritis outbreaks were reported to the DHMH. In the present study, stool specimens were collected for virologic testing from residents who experienced acute gastroenteritis during 20 outbreaks that occurred in 20 different nursing homes during this period. In 13 of 20 nursing home outbreaks, paired acute and convalescent serum samples also were collected. An outbreak was considered to be causally associated with an agent if ≥50% of the individuals in the outbreak developed either a serologic response to that agent or shed the agent in stool material or both [32]. During the period of this study, 252 nursing homes were licensed in 23 Maryland counties and in the city of Baltimore.
Analysis of serum antibody responses
Serum antibody titers to Norwalk virus (NV; Hu/NLV/GI/Norwalk/1968/US), Hawaii virus (HV; Hu/NLV/GII/Hawaii/1971/US), or Toronto virus (TV; Hu/NLV/GII/Toronto 24/1991/US) were measured by an ELISA, using recombinant (r) virus–like particles (VLPs) as antigen [33–35]. The rNV VLPs were provided by Dr. M. K. Estes (Baylor College of Medicine, Houston). In addition, antibody titers to NV were determined by a biotin-avidin immunoassay (BAI), similar to that described elsewhere [36], that used an NV-positive stool specimen as antigen. Antibody titers to Group Arotavirus and adenovirus were measured by complement fixation, by use of protocols similar to those described elsewhere, with minor modifications [37–39].
Immune electron microscopy and rotavirus ELISA detection
Immune electron microscopy was used to examine representative stool specimens for the presence of viruses with the use of human immune serum gamma globulin as the source of antibodies, as described elsewhere [40]. For the detection of rotavirus antigen in stool, a pre/post ELISA was performed [41].
Primers and RT-PCR
All stool specimens were screened by RT-PCR for the presence of enteroviruses (EV), human astroviruses, and caliciviruses. Primer pair EV/PCR-1 and EV/PCR-2, deduced from a highly conserved site in the 5′ noncoding region of known enteroviruses, was used to detect enteroviruses (including poliovirus) [42]. Primer pair Mon340/348, which corresponds to a region just upstream of the protease-encoding region of astroviruses located in open-reading frame (ORF) 1a, was used to detect astroviruses [43]. Two calicivirus primer pairs were used to detect caliciviruses. The primer pair P289/P290 recently has been described as a “broadly reactive” primer pair that detects both Sapporo-like viruses (SLVs) and NLVs in the family Caliciviridae and corresponds to a highly conserved region of the ORF1 that includes the YGDD motif of the RNA-dependent RNA polymerase encoded within the p289 primer [44]. A second calicivirus primer set, Mon431/Mon432 and Mon433/Mon434 (designated “region B” primer set), also corresponds to the RNA-dependent RNA polymerase region but is located near the 3′ end of ORF1 (primer sequences and RT-PCR protocol provided by Dr. Stephan Monroe, personal communication). In addition, ≥1 NLV-positive specimen from each outbreak was analyzed further in ORF2 with primers Jsn5/Jsn6, Mon381/Mon383 [45], and Mon381/Mon382 [46].
The NLV genogroups and genetic clusters were designated according to the system of Ando et al. [47]. In brief, genetic clusters in genogroup I or II (designated as GI or GII, followed by the cluster number and shown here in parentheses after the cryptogram of the strain) are represented by Hu/NLV/GI/Norwalk/1968/US (GI/1), Hu/NLV/GI/ Southampton/1991/UK (GI/2), Hu/NLV/GI/Desert Shield 395/1990/SA (GI/3), Hu/NLV/GI/Chiba 407/1987/JP (GI/4), Hu/NLV/GI/Musgrove/1989/UK (GI/5), Hu/NLV/GI/Hesse 3/1997/GE (GI/6), Hu/NLV/GI/Winchester/1994/UK (GI/7), Hu/NLV/GII/Hawaii/1971/US (GII/1), Hu/NLV/GII/Melksham/1994/UK (GII/2), Hu/NLV/GII/Toronto 24/1991/CA (GII/3), Hu/NLV/GII/Bristol/1993/UK (GII/4), Hu/NLV/GII/Hillingdon/1990/UK (GII/5), Hu/NLV/GII/Seacroft/1990/UK (GII/6), Hu/NLV/GII/Leeds/1990/UK (GII/7), and Hu/NLV/GII/Amsterdam/1998/NL (GII/8). The GenBank accession numbers for these viruses are M87661, L07418, U04469, AB022679, AJ277614, AF093797, AJ277609, U07611, X81879, U02030, X76716, AJ277607, AJ277620, AJ277608, and AF195848, respectively. The NLVs identified in this study are designated as MD (for Maryland), followed by an outbreak and specimen number.
RNA was purified from 200 µL of a Freon-extracted 10% stool suspension with the Ultraspec 3 reagent (Biotecx Laboratories). The RNA was resuspended with 30 µL of diethylpyrocarbonate-treated water. The same RNA extraction was used to perform EV-, astrovirus-, and calicivirus-specific RT-PCRs. Each RT-PCR used a premade PCR buffer (MasterAmp PCR Premixes kit; Epicentre); PCR Buffer D was used for primer sets p289/p290 and Mon340/Mon348, whereas buffer G was used for the other RT-PCRs. For EV and calicivirus, the parameters of the PCR were set as described elsewhere [7, 45]. For astrovirus-specific PCR, the parameters were similar to those described elsewhere [43], except that the number of amplifications was extended to 40. In all RT-PCR experiments, several controls were included in which water was added to an aliquot of the “master mix” and was subjected to the same amplification parameters.
Sequence analysis
Amplicons obtained from NLV-positive stools were gel-purified by use of a Qiaex Gel Extraction Kit (Qiagen). The nucleotide sequencing was determined using the Big Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin-Elmer), and the sequencing products were resolved on an automated sequencer (ABI 310; Applied Biosystems). Complete ORF2 sequences of NLVs in the GII/4 cluster were determined by use of consensus primers based on the ORF2 sequences of Bristol virus (BV), HV, Lordsdale virus (LV; GenBank accession number, X86557), and Camberwell virus (CV; GenBank accession number, AF145896) (table 1). Complete ORF2 sequences of NLV viruses in the GII/3 cluster were determined by use of consensus primers deduced from TV, Mexico virus (GenBank accession number U22498), and OTH-25 virus (GenBank accession number L23830) (table 1). The genome of nursing home virus MD 145-12 (with the exception of the 5′ and 3′ termini) was determined from direct sequence analysis of RT-PCR amplicons obtained by use of consensus primers deduced from the genomes of LV [48] and HV [49] (table 1). The 5′ end sequence of MD 145-12 was determined with the 5′ end RACE system (Life Technologies). The 3′ end sequence was determined by direct sequence analysis of a DNA fragment obtained by RT-PCR amplification across the 5′ and 3′ ends of the genome joined with RNA ligase [50].
Table 1.
Primers used for reverse-transcription polymerase chain reaction and sequence analysis of Norwalk-like viruses (NLVs).
| NLV strain, ORF, primer | Sequence | Sense | Positiona |
|---|---|---|---|
| 145-12 | |||
| 1 | |||
| FW1 | GTGAATGAAGATGGCGTCTA | + | 1–20 |
| RT1 | TGGGGGAACGCTGAAGGCCGTGT | − | 318–340 |
| FW2 | AACTAGTGGTTTCTTATAGTGTCA | + | 237–260 |
| RT2 | AAAAGCTGTATAGGGGAACGAT | − | 586–607 |
| FW3 | TACACTCCCCAGTATCTCATCT | + | 533–554 |
| RT3 | AAACTCCAAAGAGCTCTGCCA | − | 912–932 |
| FW4 | GGACTTTTGCAGGCATAGTGGAAT | + | 876–899 |
| RT4 | TTGTTTTCAATTGCCTCGAGGT | − | 1239–1260 |
| FW5 | TGGCTATGGTGAGATCCATCGA | + | 1203–1224 |
| RT5 | CCCTGTGAGGGAGGCTGCGAT | − | 1538–1558 |
| FW6 | AGGGAAGACCCACCTTGCCAG | + | 1501–1521 |
| RT6 | TATGTGCGAGAAATCAGGGCT | − | 1925–1945 |
| FW7 | CAGGCCAACCTGACATGTGGA | + | 1893–1913 |
| RT7 | CATCAGACTCAAGTGTGTAAGT | − | 2291–2312 |
| FW8 | AAGAGGTGCCAGATAGTGTA | + | 2261–2280 |
| RT8 | CTTTGCTTGAAAAGGCTGTGTGT | − | 2668–2690 |
| FW9 | GCAAGAAGGGGAAGAACAAGACT | + | 2631–2653 |
| RT9 | CAACCTGAACCAAAGTTGACTA | − | 3054–3075 |
| FW16-1 | GCGACCGAAGAGGACTTCTGTGA | + | 2813–2835 |
| RT16-2 | GACAGAATTCGCCCGATTTGTGTA | − | 3174–3197 |
| FW10 | TTCGAGGCCCCACCAAGCATCT | + | 3023–3044 |
| RT10 | GATGTAGGGACAGCCACAGTCA | − | 3437–3458 |
| FW11 | TCCAACGCCAAAAGTATGGA | + | 3401–3420 |
| RT11 | TGGGCTCTGTAAATGGTTTCA | − | 3771–3791 |
| FW12 | GGTGGCCCTTCATTGCAACAAGT | + | 3734–3756 |
| RT12 | GTCGCTAAGTCCGAGCCCCAGA | − | 4122–4143 |
| FW13 | GATGAGTTGGTCAAAACTGACA | + | 4070–4091 |
| RT13 | ATGGAGTTCCACTGGGAGGTACA | − | 4479–4501 |
| FW14 | TGATGGATGTGGGTGACTTCA | + | 4422–4442 |
| RT14 | ATTTGCCTAAGTATTGAGCTCT | − | 4794–4815 |
| FW15 | CCTGACCTTCCTGCGGAGAACTGT | + | 4732–4755 |
| RT15 | GCTGCGGACCGATCAGAT | − | 5114–5131 |
| 2 | |||
| NH1-FW | GGAGGGCGATCGCAATCTGGCT | + | 5050–5071 |
| NH3-RT | GCCCGCTACAGGTGCCGCAATAG | − | 5191–5213 |
| NH2-FW | GTTATGGCTCTGGAGCCCGTTGT | + | 5160–5182 |
| NH5-RT | GTAGGGGGATCAACACAGGTTCCA | − | 5536–5559 |
| NH4-FW | CATATAATTGTAGATGTTAG | + | 5511–5530 |
| NH7-RT | ACGCCATCAGTCGTGCATCTGCCA | − | 5873–5896 |
| NH6-FW | AGTGCTTTTGTTGTCCAACCA | + | 5847–5867 |
| NH9-RT | AGCTTTGGAGTGAAGTGGACACT | − | 6147–6169 |
| NH8-FW | CCACAAAGCTACAGTGAGCACT | + | 6122–6143 |
| NH11-RT | CTTCCTGGTAGAAGTGCAGCACCCAT | − | 6449–6474 |
| NH10-FW | GTATCCCAACATGAATTTGGA | + | 6410–6430 |
| NH12-RT | ATGCCAATCCAGCAAAGAAAGCTCCA | − | 6709–6734 |
| 3 | |||
| FW17 | TGATTCTTGGGTCAACCAGTTCTA | + | 6632–6655 |
| RT17 | AGCCTCAATTTGTGCTTGGAGCAT | − | 6890–6913 |
| FW18 | AATCAATGCTGGGGCTGGGGCCAT | + | 6766–6789 |
| RT18 | GAAAAGCGGCCTGCATTGTATGT | − | 7076–7098 |
| FW19 | CTCTGGACTGGAGCGGAACAAGGTA | + | 7026–7050 |
| RT19 | GATGTATTGAGAGCCCCCCTCAT | − | 7337–7359 |
| FW20 | CTGTAAGGACTAGGAACTGGGT | + | 7284–7305 |
| RT20 | ATAGTTTAGCGGCCGCTTTTTTTTTAAAGAC | − | 7550–polyA (+NotI) |
| GII/3 | |||
| 2 | |||
| NH1FW-TV | TGGGAGGGCGATCGCAATCTGGC | + | 814–836 |
| NH3RT-TV | CCACTGGCTCTAGCGCCATTGCCTC | − | 923–947 |
| NH2FW-TV | TAATGATGGTGCCGCCTGCCTCGT | + | 883–906 |
| NH5RT-TV | TCCAGCTAGGACCACTTGCACTTCA | − | 1159–1183 |
| NH4FW-TV | TTTGGCTCATCTTGCTAGAATGTA | + | 1114–1137 |
| NH7RT-TV | GCACCGGAAACCTGGAATTAGACATT | − | 1558–1583 |
| NH6FW-TV | AAAACCTTTTACCCTCCCCATT | + | 1522–1543 |
| NH9RT-TV | GCTGGTATGTCCTCTGCAGGGTC | − | 1826–1848 |
| NH8FW-TV | ATTCAACTATCATTGGCACATACA | + | 1774–1797 |
| NH11RT-TV | TGGGTCAAAGTCATCAGATTCAGT | − | 1994–2017 |
| NH10FW-TV | AGTGGACACAACATCTGGCCGCTT | + | 1939–1962 |
| NH13RT-TV | CCCAATTTGTGTAGCTTGGCCTCA | − | 2335–2358 |
| NH12FW-TV | AATCAGCCCCCGCCCAAACACAGGTG | + | 2265–2290 |
| NH14RT-TV | CCAGCCAATCCTGCTATAAAAGCT | − | 2505–2528 |
NOTE. From positions 1–7550 (MD145-12) and positions 814–2528 (GII/3), primers are grouped as consecutive pairs (+ or −) for each open-reading frame (ORF).
The alignment and genetic analysis of the partial sequences in ORFs 1 and 2 or the complete ORF2 sequences were performed by use of PILEUP, DISTANCES, and GROWTREE programs from the Genetic Computer Group (GCG) suite (Oxford Molecular Group) [51]. The complete ORF2 sequences of strains MD 101-2, MD 134-7, and MD 134-10 have been deposited into GenBank and assigned accession numbers AY03012, AY030098, and AY030313, respectively. In addition, the complete genomic sequence of MD 145-12 has been deposited into GenBank and assigned the accession number AY032605. Sequence information for other viruses is available on request.
Results
Seasonal Occurrence of Epidemic Gastroenteritis in Maryland Nursing Homes
A review of the number of gastroenteritis outbreaks reported to DHMH from January 1986 (1 year and 10 months before the collection of specimens began for this study) to December 2000 demonstrated that a total of 868 gastroenteritis outbreaks was reported among ∼250 licensed nursing homes. Pathogenic bacteria (Salmonella species, Clostridium difficile, and Shigella species) were associated with 24 (2.7%) of the 868 outbreaks. Of the remaining 844 outbreaks, 840 were designated as “presumed viral,” and 4 were associated with a common food source and thus were designated as foodborne of unknown etiology. The monthly distribution of the 840 presumed viral and 24 bacterial gastroenteritis outbreaks over this 15-year period is illustrated in figure 1. During this period, 16, 7, and 1 outbreaks of Salmonella species, Clostridium difficile, and Shigella species, respectively, were identified. The distribution of the bacterial gastroenteritis outbreaks did not show a seasonal occurrence, whereas the presumed viral outbreaks did (figure 1). The number of presumed viral outbreaks per year is summarized in table 2. More than 80% of the presumed viral outbreaks occurred during the cooler months of the year. The clinical features of this “winter” illness included an acute onset of diarrhea or vomiting or both, with illness generally lasting 24–48 h. Both residents and employees were affected, and the outbreaks typically lasted 1 −2 weeks.
Figure 1.
Comparison of the monthly distribution of 840 outbreaks of acute presumed viral gastroenteritis (GE) outbreaks (blue) and 24 outbreaks of acute bacterial GE outbreaks of Salmonella (red), Clostridium difficile (pink), and Shigella (green) species reported to the Maryland State Department of Health and Mental Hygiene over a 15-year period (1986–2000).
Table 2.
Incidence and seasonal occurrence of presumed viral gastroenteritis outbreaks from July 1986 to June 2000 in Maryland nursing homes.
| Yeara | Total no. of outbreaks reported |
No. of outbreaks occurring November–March (%) |
|---|---|---|
| 1986–1987 | 23 | 17 (73.9) |
| 1987–1988 | 36 | 31 (86.0) |
| 1988–1989 | 37 | 32 (86.4) |
| 1989–1990 | 74 | 68 (91.8) |
| 1990–1991 | 33 | 23 (69.6) |
| 1991–1992 | 85 | 67 (78.8) |
| 1992–1993 | 43 | 36 (83.7) |
| 1993–1994 | 60 | 44 (73.3) |
| 1994–1995 | 50 | 40 (80.0) |
| 1995–1996 | 92 | 78 (84.7) |
| 1996–1997 | 70 | 56 (80.0) |
| 1997–1998 | 53 | 43 (81.1) |
| 1998–1999 | 65 | 50 (76.9) |
| 1999–2000 | 86 | 73 (84.8) |
| Total | 807 | 658 (81.5) |
Shown from July to June, to analyze “winter” seasons that began in November and continued through March of the following year. Thus, excluded in this analysis are 14 outbreaks from January to June 1986 and 19 outbreaks from July to December 2000.
This study was limited to the 4-month “winter” peak period from November 1987 to February 1988 (table 3). During this winter season, a total of 31 outbreaks of gastroenteritis was reported to DHMH, with the following monthly distribution: 10 in November, 11 in December, 8 in January, 1 in February, and 1 in March. Information concerning the number of ill residents during each outbreak was available for residents of 23 nursing homes; these residents experienced an overall attack rate of 26% (963/3697). The attack rate range was 5%-59% (median, 27%). Among employees in 17 outbreaks for which information was available, the attack rate range was 0.6%-26% (median, 9%), and the overall attack rate was 10.1% (274/2705). In outbreak 134, the age range among ill residents was 47–99 years (mean, 82 years); in outbreak 138, it was 69–88 years (mean, 81 years); and in outbreak 145, it was 73–98 years (mean, 86 years). Of the 34 individuals involved in these 3 outbreaks, 25 (73%) were female.
Table 3.
Summary of the serologic responses of nursing home residents with gastroenteritis to the Norwalk, Hawaii, and Toronto viruses and identification of Norwalk-like viruses (NLVs) in stool specimens.
| Outbreak designation (date of onset) |
County in Maryland |
No. of individuals from whom paired serum samples were collected during outbreak |
No. of individuals with serologic response to indicated antigen (% positive)a |
No. of individuals from whom stool was collected in outbreak |
No. of individuals with stools positive for NLVs (% positive)a |
|||
|---|---|---|---|---|---|---|---|---|
| Norwalk virus, GI/1 |
Recombinant Hawaii virus, GII/1 |
Recombinant Toronto virus, GII/3 |
No. of stools positive for NLV |
Genogroup or genetic clusterb |
||||
| 101 (5 Nov 1987) | Baltimore | 0 | ND | ND | ND | 4 | 3 (75) | GII/3 |
| 104 (9 Nov 1987) | Hartford | 0 | ND | ND | ND | 10 | 9 (90) | GII/4 |
| 110 (16 Nov 1987) | Anne Arundel | 11 | 1 (9) | 8 (73) | 8 (73) | 9 | 6 (66) | GII/1 |
| 115 (23 Nov 1987) | Allegany | 0 | ND | ND | ND | 4 | 2 (50) | GII/4 |
| 117 (24 Nov 1987) | Montgomery | 4 | 0 | 1 (33) | 2 (67) | 8 | 4 (50) | GII/4 |
| 118 (24 Nov 1987) | Montgomery | 4 | 0 | 0 | 1 (25) | 3 | 2 (66) | GII/4 |
| 120 (30 Nov 1987) | Baltimore | 11 | 2 (18) | 4 (36) | 5 (45) | 12 | 8 (66) | GII/4 |
| 128 (8 Dec 1987) | Queen Ann’s | 0 | ND | ND | ND | 8 | 1 (13) | GII/4 |
| 134 (16 Dec 1987) | Baltimore | 7 | 0 | 4 (67) | 4 (67) | 10 | 7 (70) | GII/3or GII/4c |
| 136 (16 Dec 1987) | St. Mary’s | 0 | ND | ND | ND | 5 | 2 (40) | GII/4 |
| 137 (17 Dec 1987) | Baltimore | 15 | 3 (20) | 2 (13) | 5 (33) | 5 | 0 | Negative |
| 138 (18 Dec 1987) | Allegany | 8 | 1 (13) | 0 | 0 | 11 | 6 (54) | GII/4 |
| 140 (18 Dec 1987) | Montgomery | 0 | ND | ND | ND | 7 | 6 (86) | GII/4 |
| 143 (21 Dec 1987) | Baltimore | 11 | 2 (18) | 3 (27) | 5 (45) | 13 | 7 (53) | GII/4 |
| 145 (22 Dec 1987) | Baltimore | 11 | 2 (18) | 7 (64) | 6 (55) | 13 | 6 (46) | GII/4 or GII/8d |
| 147 (28 Dec 1987) | Baltimore | 13 | 0 | 8 (62) | 9 (69) | 13 | 5 (38) | GII/4 |
| 1 (7 Jan 1988) | Carroll | 5 | 0 | 1 (20) | 3 (60) | 5 | 0 | Negative |
| 4 (12 Jan 1988) | Baltimore | 0 | ND | ND | ND | 5 | 4 (80) | GII/4 |
| 9 (18 Jan 1988) | Carroll | 8 | 4 (50) | 0 | 0 | 6 | 2 (33) | GI/3 |
| 14 (20 Jan 1988) | Montgomery | 4 | 1 (25) | 0 | 0 | 5 | 2 (40) | GI/2 |
| Total | 112 | 16/112 (14)e | 38/109 (35)e | 48/109 (44)e | 156 | 82/156 (52) | ||
NOTE. The total number of individuals in outbreaks 117, 134, and 138 who were tested for serologic responses to the recombinant Hawaii and Toronto viruses were 3, 6, and 7, respectively. GI, genogroup I; GII, genogroup II; ND, not determined.
Outbreaks in which ≥50% of the individuals showed aserologic response to the indicated antigen or who were positive for NLVs in stool are underlined.
Designated according to the system of Ando et al. [47].
Of the 7 NLVs detected, 6 were GII/3 and 1 was GII/4.
Of the 6 NLVs detected, 5 were GII/4 and 1 was GII/8.
Data are no. positive/no. tested (%).
Association of Outbreaks with Enteric Viruses, as Determined by Serologic Analysis
Role of NLVs
NLVs have been established as a major cause of epidemic nonbacterial gastroenteritis [22, 27, 32, 52, 53]. The NLVs have a positive-sense RNA genome that is organized into 3 ORFs: ORF1 encodes a nonstructural polyprotein, ORF2 encodes the major capsid protein, and ORF3 encodes a minor capsid protein [22]. NLVs are genetically diverse and consist of ≥2 distinct genetic groups, designated GI and GII, with the prototype NV strain belonging to GI [54, 55]. Each genogroup has been further subdivided into genetic clusters [47].
We initially examined paired acute and convalescent serum samples from 112 patients in the 13 outbreaks (from which paired serum samples were available) that occurred between November 1987 and March 1988, using a BAI in which a known NV-positive human stool specimen was used as the antigen. Sixteen (14%) of the patients developed a ≥4-fold serologic response to the GI NV (table 3). Serologic responses to NV were detected in ≥1 individuals involved in 8 of the 13 outbreaks (table 3). However, only 1 outbreak (designated number 9) met the criterion for a NV outbreak. These paired serum samples were examined subsequently by use of an immunoassay that employed rVLPs obtained by expression of the NV capsid protein in the baculovirus system [34], and comparable results were obtained (data not shown).
An ELISA for the detection of serologic responses to HV (a GII NLV) using rHV VLPs as the antigen was employed to examine paired serum samples available from 109 of 112 nursing home residents with gastroenteritis. Thirty-eight (35%) of 109 individuals examined developed a serologic response to HV (table 3). A serologic response to HV was detected in ≥1 individual in 9 of 13 outbreaks (table 3), and 4 of 9 met the criterion for an HV outbreak. None of the individuals involved in outbreak 9 (associated with NV in the BAI immunoassay) developed a serologic response to HV. Thus, serologic responses to NV and HV occurred independently for the most part (P = :41, Fisher’s exact test, 2-tailed).
Serologic responses to TV (a GII NLV) were examined in the nursing home patients with an ELISA, by use of rTV VLPs as the antigen. Forty-eight (44%) of 109 individuals tested developed a serologic response to TV (table 3). Six (46%) of 13 outbreaks met the definition for a TV outbreak, 4 of which also met the definition of an HV outbreak (table 3). Ten of 13 outbreaks included ≥1 individual who developed a serologic response to TV. None of the individuals involved in outbreak 9 developed a serologic response to TV; in addition, serologic responses to NV and TV did not correlate when all serum samples were compared (P = :60, Fisher’s exact test, 2-tailed). In contrast, of 109 individuals tested for both HV and TV infection, 32 developed a response to both viruses, 6 developed a response to HV only, 16 developed a response to TV only, and 55 did not develop a significant response to either virus (data not shown). The high degree of correlation between the rHV and rTV ELISA assays in detecting a serologic response (P < :0001, Fisher’s exact test, 2-tailed) is consistent with the presence of shared antigens that are displayed when the VLPs are adsorbed to the solid phase of a microtiter plate [45]. In summary, serologic evidence of infection with ≥1 NLV was detected in 61 (56%) of 109 patients tested against each of the 3 NLV antigens (NV, HV, and TV).
Role of rotaviruses and adenoviruses
Acute and convalescent serum samples from 112 patients were screened for antibody responses to rotavirus (Group A) and adenovirus (type 2). No serologic evidence for infection with these viruses was found.
Association of Outbreaks with Enteric Pathogens and Their Characterization, as Determined by Analysis of Stool Specimens
Detection of NLVs
Stool specimens from 156 ill patients in 20 outbreaks were analyzed by RT-PCR. NLVs were detected from patients in 18 of 20 outbreaks, and the percentages of individuals who tested positive for NLVs within an outbreak ranged from 13% to 90%. Within the 18 NLV-positive outbreaks, when the region B primer set and the p289/p290 primers were used, the overall detection rates were 46.2% and 38.3%, respectively. The detection rate increased to 52.0% when the results of both primer sets were combined (table 3).
Association of outbreaks with the NLVs by serologic and virologic methods combined
All 20 outbreaks involved ≥1 individual with evidence of an NLV infection by either detection of viral RNA in stool material or by analysis of paired serum samples (table 3). With a stricter criterion, which states that 50% of the individuals tested must show evidence for infection with an agent in order to establish an etiologic association, we deduced that 16 (80%) of 20 outbreaks were caused by an NLV.
Genetic characterization of the polymerase region of the NLVs
When in sufficient quantity, the polymerase region PCR products obtained with both primer sets were sequenced and compared with nucleotide sequences available in GenBank. GI NLVs were detected in 2 outbreaks; GII NLV strains were detected in 16 outbreaks (table 3). The simultaneous occurrence of GI and GII viruses with in the same outbreak was not observed. The sequence of part of the polymerase region of a GI virus in outbreak 9 was described elsewhere and was deposited in to Gen-Bank under accession number U07612 as strain MD1 [56]. This virus shared highest identity with Desert Shield–like viruses in the genetic cluster GI/3. The GI virus in outbreak 14 was most related in its polymerase region to Southampton-like viruses in genetic cluster GI/2. The GII viruses detected in 14 separate outbreaks were most closely related to CV, a BV-like (GII/4) NLV strain associated with gastroenteritis in Australia in 1994 [57]. A partial polymerase region sequence of a virus in outbreak 140 (designated MD6) was described elsewhere and was deposited in GenBank under accession number U07613 [56]. Other GII viruses involved in outbreaks 101 (virus MD 101-2), 110 (virus MD 110-3), and 145 (virus MD 145-9) shared the highest sequence relatedness in their polymerase region with OTH-25 (GenBank accession number L23830), Arg320 (GenBank accession number AF190818), and SN88JN (GenBank accession number AF218947), respectively.
Two different GII viruses were cocirculating within outbreaks 134 and 145. During outbreak 134, 6 individuals shed a TV-like (GII/3) NLV (represented by virus MD 134-10), whereas 1 individual shed a BV-like (GII/4) NLV (virus MD 134-7). In outbreak 145, 5 individuals shed a BV-like (GII/4) NLV (represented by virus MD 145-12), whereas 1 individual shed an Amsterdam-like (GII/8) NLV (virus MD 145-9). The individuals involved in outbreaks 134 and 145 who shed the single “distinct” virus in these outbreaks each developed a serologic response to the rHV and rTV antigens but not to NV (data not shown). Specific primers were deduced from the ORF2 sequences of distinct representative viruses in outbreaks 134 and 145 to examine whether 2 genetically different NLVs could be detected in the same stool specimen. No evidence for a dual infection was observed (data not shown).
Genetic characterization of the ORF2 5′ end region from selected NLVs
Sequence analysis of the ORF2 region was performed to confirm the genetic typing results predicted from the analysis of the ORF1 polymerase region. This was not possible for the GI/2 (Southampton-like) strain from outbreak 14, which did not yield an ORF2 amplicon. Recently, a genetic classification system for the NLVs was proposed on the basis of the complete capsid protein sequence [47]. Comparison of the 5′ end region of the ORF2 of selected viruses from nursing home patients with those from reference strains representing the NLV genetic clusters of Ando et al. [47] showed that members from ≥5 distinct genetic clusters were detected. These NLV clusters were GI/3, GII/1, GII/3, GII/4, and GII/8, represented by Desert Shield virus, HV, TV, BV, and Amsterdam virus, respectively. Although the ORF2 could not be sequenced from the NLV in outbreak 14, it is likely that this GI virus belonged to a sixth genetic cluster, GI/2, because of its relatedness to Southampton virus in the polymerase region. The genetic relatedness of the capsid protein from representative Maryland nursing home NLVs with those of reference viruses from the NLV genetic clusters of Ando et al. [47] was examined by use of evolutionary distance programs in the GCG suite. The predominance of the GII/4 viruses and the marked diversity of the circulating viruses in the nursing home setting is illustrated in a dendrogram that includes reference viruses for each genetic cluster (figure 2). The analysis also includes the Alphatron virus (Hu/NLV/Alphatron/1998/NL), which has not yet been assigned to a genogroup or genetic cluster [58].
Figure 2.
Phylogram created with the DISTANCES and GROW-TREE programs (both part of the Genetic Computer Group suite [Oxford Molecular Group] [51]), using the uncorrected distances algorithms, on the basis of the alignment of 176 bases near the 5′ end of open-reading frame 2. The GenBank numbers of the reference strains are Bristol (X76716), Lordsdale (X86557), Camberwell (U46500), Hawaii (U07611), Melksham (X81879), Hillingdon (AJ277607), Amsterdam (AF195848), Leeds (AJ277608), Toronto (U02030), Sea-croft (AJ277620), Alphatron (AF195847), Musgrove (AJ277614), Chiba (AB022679), Southampton (L07418), Hesse (AF093797), Norwalk (M87661), Desert Shield (U04469), and Winchester (AJ277614). The scale bar represents 10 substitutions per 100 residues.
The geographical and temporal distributions of the 20 nursing home outbreaks in Maryland were analyzed (data not shown). No evidence was found for a pattern in the spread of outbreaks through the state (e.g., from west to east).
Genetic stability of the complete capsid protein of NLVs from 2 distinct genetic clusters
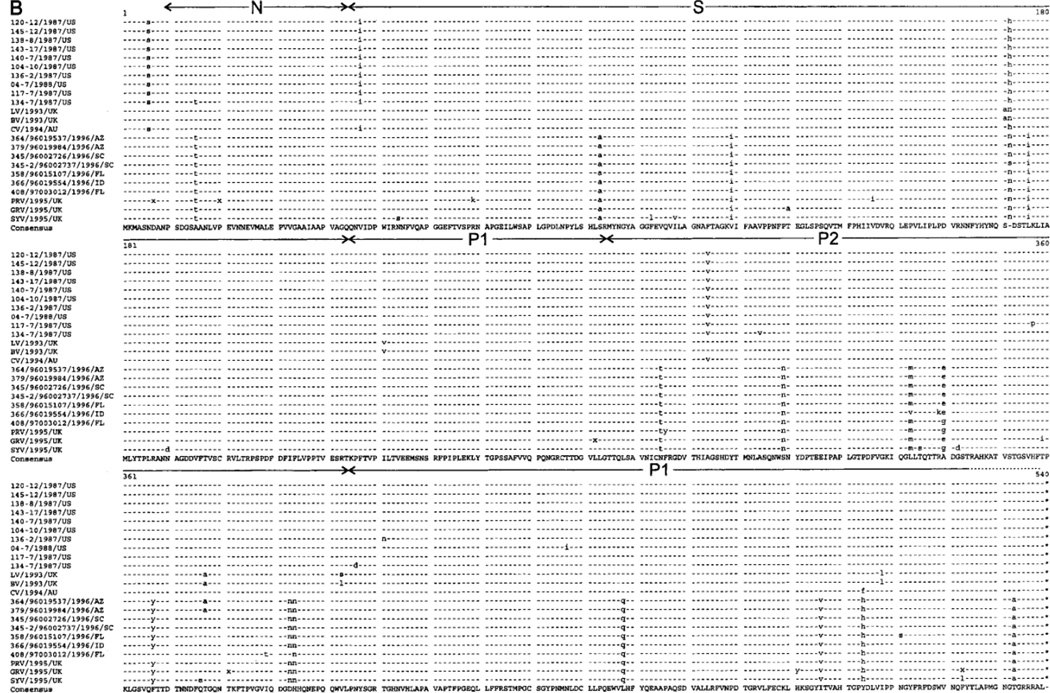
The genetic analysis of the partial sequence of ORF2 showed 96%–99.6% nucleic acid identity among the 14 BV-like strains and 100% nucleotide identity between the 2 TV-like strains (figure 2A). To examine genetic variation in the entire capsid protein, the complete ORF2 sequence of representative GII/4 (BV-like) NLVs from outbreaks 104, 117, 120, 136, 138, 140, 143, 145, and 04 and representative GII/3 (TV-like) NLVs in outbreaks 101 and 134 was determined. Outbreak 134 included 1 individual who shed a GII/4 virus and 6 individuals who shed a TV-like GII/3 virus. The deduced amino acid sequences of the capsid proteins from the GII/3 viruses in outbreaks 101 and 134 differed by 2 aa (figure 3A), which suggests that a highly related strain caused these outbreaks at a 42-day interval. A search of GenBank found that the capsid protein of the MD GII/3 viruses in outbreaks 101 and 134 showed the highest amino acid sequence relatedness (99% identity) with OTH-25, an NLV that was associated with gastroenteritis in Japan in 1989. The MD 101-2 and MD 134-10 capsid proteins showed 4 and 6 aa differences with OTH-25, respectively. Comparison with more recent GII/3 viruses, such as the Birmingham132 virus (GenBank accession number AJ277611), which was associated with illness in the United Kingdom in 1995, showed evidence for further variation, having a difference with MD 101-2 of ≥20 aa.
Figure 3.
Alignments base don the entire open-reading frame 2-aa sequences of the nursing home and reference viruses, using the PILEUP and PRETTY programs from the Genetic Computer Group suite (Oxford Molecular Group) [51]. The GenBank numbers of the reference viruses in panel A are OTH-25 (L23830), MXV (U22498), TV (U07611), Rbh (A89098), AUV (U46039), Arg320 (AF190817), and Bham132 (CAB89092). GenBank numbers of the reference viruses in panel B are LV (X86557), BV (X76716), CV (AF145896), 364/1996/AZ (AF080553), 379/1996/AZ (AF080556), 345/1996/SC (AF080549), 358/1996/FL (AF080552), 366/1996/ID (AF080554), 408/1996/FL (AF080558), PRV (AJ27761), GRV (AJ004864), and SYV (AJ277619). AUV, Auckland virus; GRV, Grimsby virus; PRV, Park Royal virus; SYV, Symgreen virus. Regions that correspond to the N (N-terminal), S (shell), P1 (protruding), and P2 structural domains of the Norwalk virus capsid protein that were defined by Prasad et al. [60] are indicated.
The capsid proteins of the GII/4 strains from outbreaks 104, 120, 138, 140, 143, and 145, which occurred within 71 days of each other, also have identical deduced amino acid sequences (figure 3B). These viruses showed 1 and 9 aa differences from CV (Australia, 1994) and LV (United Kingdom, 1993), respectively. The GII/4 viruses from outbreaks 136, 117, and 04 differed by 1 aa from each other and from the “identical” GII/4 viruses in the 6 outbreaks above (104, 120, 138, 140, 143, and 145). The GII/4 virus MD 134-7, which was derived from a predominantly GII/3 outbreak, differed by 3 aa from the other BV-like strains and was the most divergent among the GII/4 viruses in this study. These data demonstrate that a predominant GII/4 BV-like virus circulated among the nursing homes in Maryland and caused 6 outbreaks (104, 120, 138, 140, 143, and 145) and possibly outbreaks 115, 118, 128, and 147.
Noel et al. [59] showed the worldwide circulation of a “common” strain related to BV and LV in the late 1990s. We compared the deduced capsid protein sequence from the predominant GII/4 strain (represented by MD 145-12) from the Maryland nursing homes with the 1995–1996 US common strains (figure 3B). The capsid sequence of the predominant Maryland nursing home strains differed by 19 aa from the 1995–1996 US common strains, which resulted in an overall amino acid identity of 96%. Twelve of 19 variations were located in the carboxy-terminal half of the capsid protein, of which 8 were located in the region that corresponds to the highly variable P2 subdomain of NV [60]. In contrast, the single conservative amino acid substitution between MD 145-12 and CV was amino acid residue 504, located within the highly conserved P1 subdomain.
Comparison of the genome of the predominant nursing home NLV strain MD145-12 with that of CV
The genome of the MD 145-12 virus was sequenced to examine whether the marked conservation with CV in the capsid protein extended throughout the genome. The genome of MD 145-12 is 7556 nt in length and is similar in organization to other NLVs, with 3 major ORFs. The overall nucleotide identity between the MD 145-12 and CV genomes was 96.7%, with ∼96% nucleotide identity within each ORF, when each was analyzed independently. Amino acid sequence comparisons showed 98.6%, 99.8%, and 97.6% identity in ORF1, ORF2, and ORF3, respectively. The distribution of amino acid differences between the 2 viruses in the 3 ORFs is illustrated in figure 4. Of note, the N-terminal protein encoded in ORF1 appeared to be the most highly variable protein, containing 12 (54%) of 22 aa differences between the 2 ORF1 coding sequences. The second most variable protein was that encoded in ORF3, a minor structural protein [63]. The predicted NTPase, proteinase, and capsid proteins were the most conserved, containing only 1 aa difference each between the 2 viruses.
Figure 4.
Variations in amino acids between MD145-12 and Camberwell virus (CV). Blue and red bars indicate the conservative and nonconservative amino acid substitutions, respectively. Arrows and numbers indicate the location of the putative proteolytic cleavage sites within the open-reading frame 1 nonstructural polyprotein and are based on the previously identified cleavage sites of the Southampton and Camberwell viruses [61, 62].
Role of other agents
Immune electron microscopy was performed on a subset of 38 stool specimens (selected from 12 outbreaks), to examine whether other potential agents of gastroenteritis were present. Small, 27-nm viruses were observed in only 1 stool specimen that was later identified by RT-PCR and sequence analysis as being positive for a Toronto-like virus in outbreak 134. Enteroviruses, rotaviruses, astroviruses, Salmonella species, Clostridium species, and Shigella species were not detected by the methods used in the present study.
Discussion
Gastroenteritis is an important illness among elderly persons in residential settings because these individuals are at greater risk for severe illness that can lead to complications in already-debilitated patients. The identification of NLVs as the major etiologic agent of epidemic gastroenteritis in Maryland nursing homes during a winter season is consistent with recent large-scale epidemiologic studies that have identified such facilities as frequent settings for NLV gastroenteritis outbreaks [7, 20, 27–29]. NLVs were the only viral pathogen detected in this study, which indicates that, at least during this time frame, rotaviruses and astroviruses were not important agents. Coupled with these recent reports, it appears that the NLVs may emerge as the major etiologic agents of gastroenteritis in nursing homes. Furthermore, analysis of stool specimens collected over this single season, as well as from gastroenteritis outbreaks that occurred over a 15-year period in this setting, indicated that bacteria (Salmonella, Clostridium, and Shigella species) were not major agents of epidemic gastroenteritis. Presumed viral gastroenteritis outbreaks affected, on average, ∼20% of Maryland nursing homes each year over a 15-year period. Extrapolating from our data that 80% of nursing home outbreaks over a single winter season were caused by NLVs, it is likely that there may be a heavier disease burden from this illness in US nursing homes than previously recognized.
Presumed viral gastroenteritis outbreaks in the nursing homes typically had a marked seasonal occurrence in the cooler, “winter” months of the year, which is consistent with the description of “winter vomiting disease” first reported by Zahorsky in 1929 [64]. The reason for this seasonal occurrence is not known, but it has been described in other surveys of epidemic gastroenteritis in countries with temperate climates [65]. Transmission of NLVs occurs primarily through the fecal-oral route and is often associated with contaminated food or water [22]. However, airborne transmission of NLVs through respiratory droplets also has been proposed [66], and viruses with a cool weather seasonal occurrence are often spread by this route [65]. Although the sources for the NLVs in the Maryland nursing home outbreaks were not identified, the predominance and genetic diversity of these viruses suggests that NLVs from the community were readily introduced into the nursing homes.
The predominant virus associated with illness in this study was a BV-like virus in the NLV GII/4 genetic cluster, detected in 14 of 20 outbreaks. The predominance of a GII NLV was consistent with several studies that have shown the worldwide predominance of GII NLVs in recent years [21, 47, 54, 67–81]. None of the viruses in the Maryland nursing homes showed a close genetic relatedness with the “animal” NLVs that have been characterized thus far [82–84] or with the SLVs. The predominant nursing home virus (represented by strain MD 145-12) was similar to a distinct, common NLV strain that was detected in the United States and globally during 1995–1996 [59]. Apparently, viruses in genetic cluster GII/4 were already of major importance nearly a decade earlier in epidemic gastroenteritis in Maryland nursing homes. Analysis of the capsid proteins from viruses within the 1995–1996 global outbreak season [59] and those within the 1987–1988 nursing home winter season suggests that the predominant NLVs within an outbreak season are highly conserved. This observation is consistent with other molecular epidemiologic studies that have examined the genetic stability of NLVs within outbreaks [22]. However, the 19 aa differences between the predominant viruses from these 2 outbreak settings that occurred at different times suggests that evolution and selection does occur within the capsid proteins of each genetic cluster. In this regard, it is striking that the 1987–1988 predominant nursing home strain showed only 1 aa difference with the capsid protein of CV (GII/4), an NLV that was associated with disease in Australia in 1994, just before the emergence of the 1995–1996 common strain. If it is assumed that the NLVs associated with gastroenteritis in humans are maintained by passage in the human population, this particular capsid protein remained essentially unchanged in its amino acid sequence for ≥7 years. As expected, the overall nucleotide identity of the MD 145-12 and CV genomes was more variable (∼96%), which indicates that these viruses were not identical and that each had undergone evolution. Furthermore, the rate of nucleotide variation was similar in all 3 ORFs, which indicates that polymerase “errors” were distributed evenly throughout the RNA genome during replication. The marked conservation of capsid sequences between CV and MD 145-12 virus is noteworthy because of the apparently higher rate of evolution in certain other proteins encoded by the genome. The capsid protein, which is presumably under selective pressure from the host immune system, sustained fewer relative changes, compared with both the ORF1 and ORF3-encoded proteins. Most notably, the presently uncharacterized “N-terminal” protein encoded by ORF1 was a major site for amino acid variation between the viruses. Because NLVs are positive-strand RNA viruses and, thus, are subject to evolution as a quasi species, selective pressures for adaptation presumably exist. At present, it is not known why certain NLVs emerge to cause outbreaks that can be extensive, sometimes involving hundreds or thousands of individuals. Although NLVs apparently are able to remain viable under harsh environmental conditions (e.g., in sewage and in the tissues of oysters) for possibly long periods, replication and, thus, evolution in human hosts is constant, as evidenced by seroprevalence studies and by the frequency of disease [22]. The identification of viral determinants involved in adaptation and virulence of the NLVs, as well as other caliciviruses, may lead to a better understanding of how certain NLVs, such as the GII/4 viruses, have emerged as major epidemic gastroenteritis strains.
The present study allowed for an analysis of the genetic stability of viruses associated with gastroenteritis outbreaks occurring within the same season for 2 different genetic clusters. The entire capsid protein gene was sequenced from the 2 most frequently detected viruses in the nursing home setting—GII/3 (TV-like) NLVs (implicated in outbreaks 101 and 134) and GII/4 (BV-like) NLVs, with the latter being the predominant virus in the season. As described above, the sequence of the capsid protein of the majority of viruses representing the predominant GII/4 virus was identical among outbreaks. However, amino acid differences were detected sometimes when viruses from different outbreaks were compared, which indicates either that evolution was occurring as the virus was transmitted in this setting or that certain GII/4 viruses originated from a source that was different from the predominant GII/4 strain. In this study, 2 of 20 outbreaks (outbreaks 134 and 145, which occurred within the same town, but in separate nursing homes) showed the presence of NLVs from 2 distinct genetic clusters within the outbreak. The occurrence of .1 NLV genetic cluster within the same specimen or outbreak has been observed elsewhere and often has been associated with contaminated water or sewage [85–89]. Thus, simultaneous exposure to multiple NLVs can occur. Clearly, the molecular epidemiology of the NLVs is complex, and the identification of virus and host factors involved in transmission will remain challenging. Furthermore, the biological significance of NLV genetic clusters and the effect of genetic variations within these clusters remain unclear, because it has been difficult to assess the role of neutralizing antibodies and cell-mediated immune responses in the absence of a cell culture system and animal model. The development of such systems to study immunity and pathogenesis remains an important goal for elucidating the natural history of these viruses.
The identification of NLVs as epidemiologically important pathogens in nursing home gastroenteritis outbreaks and the study of their genetic diversity and evolution are useful for the development of potential vaccine strategies or therapeutic agents. Furthermore, knowledge of the molecular characteristics of circulating viruses can yield important insight into the source and spread of viruses in a community and may lead to the development of better control measures for the prevention of NLV outbreaks.
Acknowledgments
We thank Robert M. Chanock and Ebenezer Israel, for their support; Edward Kassira, Carmela Groves, Ernest Williams, and Ali Salimi, for their contributions to this study; Tamie Ando, for helpful discussions regarding the Norwalk-like virus genetic typing system; and Stephan Monroe, for sharing the region B reverse-transcription polymerase chain reaction protocol before publication.
References
- 1.Smith JL. Foodborne illness in the elderly. J Food Prot. 1998;61:1229–1239. doi: 10.4315/0362-028x-61.9.1229. [DOI] [PubMed] [Google Scholar]
- 2.Standaert SM, Hutcheson RH, Schaffner W. Nosocomial transmission of Salmonella gastroenteritis to laundry workers in a nursing home. Infect Control Hosp Epidemiol. 1994;15:22–26. doi: 10.1086/646813. [DOI] [PubMed] [Google Scholar]
- 3.Tallis G, Ng S, Ferreira C, Tan A, Griffith J. A nursing home outbreak of Clostridium perfringens associated with pureed food. Aust N Z J Public Health. 1999;23:421–423. doi: 10.1111/j.1467-842x.1999.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 4.Augustin AK, Simor AE, Shorrock C, McCausland J. Outbreaks of gastroenteritis due to Norwalk-like virus in two long-term care facilities for the elderly. Can J Infect Control. 1995;10:111–113. [PubMed] [Google Scholar]
- 5.Cubitt WD, Holzel H. An outbreak of rotavirus infection in a long-stay ward of a geriatric hospital. J Clin Pathol. 1980;33:306–308. doi: 10.1136/jcp.33.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cubitt WD, Pead PJ, Saeed AA. A new serotype of calicivirus associate with an outbreak of gastroenteritis in a residential home for the elderly. J Clin Pathol. 1981;34:924–926. doi: 10.1136/jcp.34.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fankhauser RL, Noel JS, Monroe SS, Ando T, Glass RI. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J Infect Dis. 1998;178:1571–1578. doi: 10.1086/314525. [DOI] [PubMed] [Google Scholar]
- 8.Gellert GA, Waterman SH, Ewert D, et al. An outbreak of acute gastroenteritis caused by a small round structured virus in a geriatric convalescent facility. Infect Control Hosp Epidemiol. 1990;11:459–464. doi: 10.1086/646212. [DOI] [PubMed] [Google Scholar]
- 9.Gray JJ, Wreghitt TG, Cubitt WD, Elliot PR. An outbreak of gastroenteritis in a home for the elderly associated with astrovirus type 1 and human calicivirus. J Med Virol. 1987;23:377–381. doi: 10.1002/jmv.1890230410. [DOI] [PubMed] [Google Scholar]
- 10.Halvorsrud J, Orstavik I. An epidemic of rotavirus-associated gastroenteritis in a nursing home for the elderly. Scand J Infect Dis. 1980;12:161–164. doi: 10.3109/inf.1980.12.issue-3.01. [DOI] [PubMed] [Google Scholar]
- 11.Humphrey TJ, Cruickshank JG, Cubitt WD. An outbreak of calicivirus associated gastroenteritis in an elderly persons home: a possible zoonosis? J Hyg (Lond) 1984;93:293–299. doi: 10.1017/s0022172400064822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X, Turf E, Hu J, et al. Outbreaks of gastroenteritis in elderly nursing homes and retirement facilities associated with human caliciviruses. J Med Virol. 1996;50:335–341. doi: 10.1002/(SICI)1096-9071(199612)50:4<335::AID-JMV9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan JE, Schonberger LB, Varano G, Jackman N, Bied J, Gary GW. An outbreak of acute nonbacterial gastroenteritis in a nursing home: demonstration of person-to-person transmission by temporal clustering of cases. Am J Epidemiol. 1982;116:940–948. doi: 10.1093/oxfordjournals.aje.a113496. [DOI] [PubMed] [Google Scholar]
- 14.Lewis DC, Lightfoot NF, Cubitt WD, Wilson SA. Outbreaks of astrovirus type 1 and rotavirus gastroenteritis in a geriatric in-patient population. J Hosp Infect. 1989;14:9–14. doi: 10.1016/0195-6701(89)90128-x. [DOI] [PubMed] [Google Scholar]
- 15.Marrie TJ, Lee SH, Faulkner RS, Ethier J, Young CH. Rotavirus infection in a geriatric population. Arch Intern Med. 1982;142:313–316. [PubMed] [Google Scholar]
- 16.Martiquet P, Njoo H. Two institutional outbreaks of Norwalk-like gastroenteritis—Ontario. Can Dis Wkly Rep. 1989;15:113–116. [PubMed] [Google Scholar]
- 17.Marx A, Shay DK, Noel JS, et al. An outbreak of acute gastroenteritis in a geriatric long-term-care facility: combined application of epidemiological and molecular diagnostic methods. Infect Control Hosp Epidemiol. 1999;20:306–311. doi: 10.1086/501622. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez EM, Parrott C, Rolka H, Monroe SS, Dwyer DM. An outbreak of viral gastroenteritis in a nursing home: importance of excluding ill employees. Infect Control Hosp Epidemiol. 1996;17:587–592. doi: 10.1086/647390. [DOI] [PubMed] [Google Scholar]
- 19.Simor AE, McArthur M, McGeer A, Shurtleff S, Jabar M. Calicivirus gastroenteritis in a geriatric long-term care facility—Ontario. Can Dis Wkly Rep. 1990;16:239–240. 243. [PubMed] [Google Scholar]
- 20.Vinje J, Altena SA, Koopmans MP. The incidence and genetic variability of small round-structured viruses in outbreaks of gastroenteritis in The Netherlands. J Infect Dis. 1997;176:1374–1378. doi: 10.1086/517325. [DOI] [PubMed] [Google Scholar]
- 21.Wright PJ, Gunesekere IC, Doultree JC, Marshall JA. Small round-structured (Norwalk-like) viruses and classical human caliciviruses in southeastern Australia, 1980–1996. J Med Virol. 1998;55:312–320. [PubMed] [Google Scholar]
- 22.Green KY, Chanock RM, Kapikian AZ. Human caliciviruses. In: Knipe DM, Howley P, editors. Fields virology. Philadelphia: Lippincott, Williams, and Wilkins; 2001. pp. 847–874. [Google Scholar]
- 23.Crossley KB, Peterson PK. Infections in the elderly. Clin Infect Dis. 1996;22:209–215. doi: 10.1093/clinids/22.2.209. [DOI] [PubMed] [Google Scholar]
- 24.Gangarosa RE, Glass RI, Lew JF, Boring JR. Hospitalizations involving gastroenteritis in the United States, 1985: the special burden of the disease among the elderly. Am J Epidemiol. 1992;135:281–290. doi: 10.1093/oxfordjournals.aje.a116282. [DOI] [PubMed] [Google Scholar]
- 25.Garibaldi RA. Residential care and the elderly: the burden of infection. J Hosp Infect. 1999;43(Suppl):S9–S18. doi: 10.1016/s0195-6701(99)90061-0. [DOI] [PubMed] [Google Scholar]
- 26.Lew JF, Glass RI, Gangarosa RE, Cohen IP, Bern C, Moe CL. Diarrheal deaths in the United States, 1979 through 1987: a special problem for the elderly. JAMA. 1991;265:3280–3284. [PubMed] [Google Scholar]
- 27.Glass RI, Noel J, Ando T, et al. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J Infect Dis. 2000;181(Suppl 2):S254–S261. doi: 10.1086/315588. [DOI] [PubMed] [Google Scholar]
- 28.Dedman D, Laurichesse H, Caul EO, Wall PG. Surveillance of small round structured virus (SRSV) infection in England and Wales, 1990-5. Epidemiol Infect. 1998;121:139–149. doi: 10.1017/s0950268898001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedlund KO, Rubilar-Abreu E, Svensson L. Epidemiology of calicivirus infections in Sweden, 1994–1998. J Infect Dis. 2000;181(Suppl 2):S275–S280. doi: 10.1086/315585. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Graham DY, Wang K, Estes MK. Norwalk virus genome cloning and characterization. Science. 1990;250:1580–1583. doi: 10.1126/science.2177224. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Wang J, Graham DY, Estes MK. Detection of Norwalk virus in stool by polymerase chain reaction. J Clin Microbiol. 1992;30:2529–2534. doi: 10.1128/jcm.30.10.2529-2534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan JE, Gary GW, Baron RC, et al. Epidemiology of Norwalk gastroenteritis and the role of Norwalk virus in outbreaks of acute nonbacterial gastroenteritis. Ann Intern Med. 1982;96:756–761. doi: 10.7326/0003-4819-96-6-756. [DOI] [PubMed] [Google Scholar]
- 33.Green KY, Kapikian AZ, Valdesuso J, Sosnovtsev S, Treanor JJ, Lew JF. Expression and self-assembly of recombinant capsid protein from the antigenically distinct Hawaii human calicivirus. J Clin Microbiol. 1997;35:1909–1914. doi: 10.1128/jcm.35.7.1909-1914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang X, Wang M, Graham DY, Estes MK. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66:6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leite JP, Ando T, Noel JS, et al. Characterization of Toronto virus capsid protein expressed in baculovirus. Arch Virol. 1996;141:865–875. doi: 10.1007/BF01718161. [DOI] [PubMed] [Google Scholar]
- 36.Gary GW, Jr, Kaplan JE, Stine SE, Anderson LJ. Detection of Norwalk virus antibodies and antigen with a biotin-avidin immunoassay. J Clin Microbiol. 1985;22:274–278. doi: 10.1128/jcm.22.2.274-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandt CD, Kim HW, Jeffries BC, et al. Infections in 18,000 infants children in a controlled study of respiratory tract disease II. Variation in adenovirus infections by year and season. Am J Epidemiol. 1972;95:218–227. doi: 10.1093/oxfordjournals.aje.a121389. [DOI] [PubMed] [Google Scholar]
- 38.Kapikian AZ, Cline WL, Mebus CA, et al. New complement-fixation test for the human reovirus-like agent of infantile gastroenteritis: Nebraska calf diarrhoea virus used as antigen. Lancet. 1975;1(7915):1056–1061. doi: 10.1016/s0140-6736(75)91827-9. [DOI] [PubMed] [Google Scholar]
- 39.Sever JL. Application of a microtechnique to viral serological investigations. J Immunol. 1962;88:320–329. [PubMed] [Google Scholar]
- 40.Kapikian AZ, Dienstag JL, Purcell RH. Immune electron microscopy as a method for the detection, identification and characterization of agents not cultivatable in an in vitro system. In: Rose NR, Friedman H, editors. Manual of clinical immunology. 2d. Washington, DC: American Society for Microbiology; 1980. pp. 70–83. [Google Scholar]
- 41.Yolken R, Wilde J. Assays for detecting human rotavirus. In: Kapikian AZ, editor. Viral infections of the gastrointestinal tract. New York: Marcel Dekker; 1994. pp. 251–278. [Google Scholar]
- 42.Yang CF, De L, Yang SJ, et al. Genotype-specific in vitro amplification of sequences of the wild type 3 polioviruses from Mexico and Guatemala. Virus Res. 1992;24:277–296. doi: 10.1016/0168-1702(92)90124-r. [DOI] [PubMed] [Google Scholar]
- 43.Belliot G, Laveran H, Monroe SS. Detection and genetic differentiation of human astroviruses: phylogenetic grouping varies by coding region. Arch Virol. 1997;142:1323–1334. doi: 10.1007/s007050050163. [DOI] [PubMed] [Google Scholar]
- 44.Jiang X, Huang PW, Zhong WM, Farkas T, Cubitt DW, Matson DO. Design and evaluation of a primer pair that detects both Norwalk- and Sapporolike caliciviruses by RT-PCR. J Virol Methods. 1999;83:145–154. doi: 10.1016/s0166-0934(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 45.Noel JS, Ando T, Leite JP, et al. Correlation of patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J Med Virol. 1997;53:372–383. doi: 10.1002/(sici)1096-9071(199712)53:4<372::aid-jmv10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 46.Belliot G, Noel JS, Li J-F, et al. Characterization of capsid genes, expressed in the baculovirus system, of three new genetically distinct strains of “Norwalk-like viruses”. J Clin Microbiol. 2001;39:4288–4295. doi: 10.1128/JCM.39.12.4288-4295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ando T, Noel JS, Fankhauser RL. Genetic classification of “Norwalk-like viruses. J Infect Dis. 2000;181(Suppl 2):S336–S348. doi: 10.1086/315589. [DOI] [PubMed] [Google Scholar]
- 48.Dingle KE, Lambden PR, Caul EO, Clarke IN. Human enteric Caliciviridae: The complete genome sequence and expression of virus-like particles from a genetic group II small round structured virus. J Gen Virol. 1995;76:2349–2355. doi: 10.1099/0022-1317-76-9-2349. [DOI] [PubMed] [Google Scholar]
- 49.Pletneva MA, Sosnovtsev SS, Green KY. The genome of Hawaii virus and its relationship with other members of the caliciviridae. Virus Genes. 2001;23:5–16. doi: 10.1023/a:1011138125317. [DOI] [PubMed] [Google Scholar]
- 50.Sosnovtsev S, Green KY. RNA transcripts derived from a cloned full-length copy of the feline calicivirus genome do not require VPg for infectivity. Virology. 1995;210:383–390. doi: 10.1006/viro.1995.1354. [DOI] [PubMed] [Google Scholar]
- 51.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greenberg HB, Valdesuso J, Yolken RH, et al. Role of Norwalk virus in outbreaks of nonbacterial gastroenteritis. J Infect Dis. 1979;139:564–568. doi: 10.1093/infdis/139.5.564. [DOI] [PubMed] [Google Scholar]
- 53.Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, Chanock RM. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972;10:1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green SM, Lambden PR, Caul EO, Clarke IN. Capsid sequence diversity in small round structured viruses from recent UK outbreaks of gastroenteritis. J Med Virol. 1997;52:14–19. [PubMed] [Google Scholar]
- 55.Wang J, Jiang X, Madore HP, et al. Sequence diversity of small, round-structured viruses in the Norwalk virus group. J Virol. 1994;68:5982–5990. doi: 10.1128/jvi.68.9.5982-5990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lew JF, Kapikian AZ, Valdesuso J, Green KY. Molecular characterization of Hawaii virus and other Norwalk-like viruses: evidence for genetic polymorphism among human caliciviruses. J Infect Dis. 1994;170:535–542. doi: 10.1093/infdis/170.3.535. [DOI] [PubMed] [Google Scholar]
- 57.Cauchi MR, Doultree JC, Marshall JA, Wright PJ. Molecular characterization of Camberwell virus and sequence variation in ORF3 of small round-structured (Norwalk-like) viruses. J Med Virol. 1996;49:70–76. doi: 10.1002/(SICI)1096-9071(199605)49:1<70::AID-JMV12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 58.Vinje J, Koopmans MP. Simultaneous detection and genotyping of “Norwalk-like viruses” by oligonucleotide array in a reverse line blot hybridization format. J Clin Microbiol. 2000;38:2595–2601. doi: 10.1128/jcm.38.7.2595-2601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noel JS, Fankhauser RL, Ando T, Monroe SS, Glass RI. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J Infect Dis. 1999;179:1334–1344. doi: 10.1086/314783. [DOI] [PubMed] [Google Scholar]
- 60.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 61.Seah EL, Marshall JA, Wright PJ. Open reading frame 1 of the Norwalk-like virus Camberwell: completion of sequence and expression inmammalian cells. J Virol. 1999;73:10531–10535. doi: 10.1128/jvi.73.12.10531-10535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu BL, Viljoen GJ, Clarke IN, Lambden PR. Identification of further proteolytic cleavage sites in the Southampton calicivirus polyprotein by expression of the viral protease in E. coli. J Gen Virol. 1999;80:291–296. doi: 10.1099/0022-1317-80-2-291. [DOI] [PubMed] [Google Scholar]
- 63.Glass PJ, White LJ, Ball JM, Leparc-Goffart I, Hardy ME, Estes MK. Norwalk virus open reading frame 3 encodes a minor structural protein. J Virol. 2000;74:6581–6591. doi: 10.1128/jvi.74.14.6581-6591.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zahorsky J. Hyperemesis hiemis or the winter vomiting disease. Arch Pediatr. 1929;46:391–395. [Google Scholar]
- 65.Mounts AW, Ando T, Koopmans M, Bresee JS, Noel J, Glass RI. Cold weather seasonality of gastroenteritis associated with Norwalk-like viruses. J Infect Dis. 2000;181(Suppl 2):S284–S287. doi: 10.1086/315586. [DOI] [PubMed] [Google Scholar]
- 66.Sawyer LA, Murphy JJ, Kaplan JE, et al. 25- to 30-nm virus particle associated with a hospital outbreak of acute gastroenteritis with evidence for airborne transmission. Am J Epidemiol. 1988;127:1261–1271. doi: 10.1093/oxfordjournals.aje.a114918. [DOI] [PubMed] [Google Scholar]
- 67.Beller M, Ellis A, Lee SH, et al. Outbreak of viral gastroenteritis due to a contaminated well: international consequences. JAMA. 1997;278:563–568. [PubMed] [Google Scholar]
- 68.Bon F, Fascia P, Dauvergne M, et al. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J Clin Microbiol. 1999;37:3055–3058. doi: 10.1128/jcm.37.9.3055-3058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Green J, Gallimore CI, Norcott JP, Lewis D, Brown DW. Broadly reactive reverse transcriptase polymerase chain reaction for the diagnosis of SRSV-associated gastroenteritis. J Med Virol. 1995;47:392–398. doi: 10.1002/jmv.1890470416. [DOI] [PubMed] [Google Scholar]
- 70.Kilgore PE, Belay ED, Hamlin DM, et al. A university outbreak of gastroenteritis due to a small round-structured virus: application of molecular diagnostics to identify the etiologic agent and patterns of transmission. J Infect Dis. 1996;173:787–793. doi: 10.1093/infdis/173.4.787. [DOI] [PubMed] [Google Scholar]
- 71.Levett PN, Gu M, Luan B, et al. Longitudinal study of molecular epidemiology of small round-structured viruses in a pediatric population. J Clin Microbiol. 1996;34:1497–1501. doi: 10.1128/jcm.34.6.1497-1501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maguire AJ, Green J, Brown DW, Desselberger U, Gray JJ. Molecular epidemiology of outbreaks of gastroenteritis associated with small round-structured viruses in East Anglia, United Kingdom, during the 1996–1997 season. J Clin Microbiol. 1999;37:81–89. doi: 10.1128/jcm.37.1.81-89.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moe CL, Gentsch J, Ando T, et al. Application of PCR to detect Norwalk virus in fecal specimens from outbreaks of gastroenteritis. J Clin Microbiol. 1994;32:642–648. doi: 10.1128/jcm.32.3.642-648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pang XL, Joensuu J, Vesikari T. Human calicivirus-associated sporadic gastroenteritis in Finnish children less than two years of age followed prospectively during a rotavirus vaccine trial. Pediatr Infect Dis J. 1999;18:420–426. doi: 10.1097/00006454-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Patterson W, Haswell P, Fryers PT, Green J. Outbreak of small round structured virus gastroenteritis arose after kitchen assistant vomited. Commun Dis Rep CDR Rev. 1997;7:R101–R103. [PubMed] [Google Scholar]
- 76.Saito H, Saito S, Kamada K, et al. Application of RT-PCR designed from the sequence of the local SRSV strain to the screening in viral gastroenteritis outbreaks. Microbiol Immunol. 1998;42:439–446. doi: 10.1111/j.1348-0421.1998.tb02307.x. [DOI] [PubMed] [Google Scholar]
- 77.Schvoerer E, Bonnet F, Dubois V, et al. A hospital outbreak of gastroenteritis possibly related to the contamination of tap water by a small round structured virus. J Hosp Infect. 1999;43:149–154. doi: 10.1053/jhin.1999.0632. [DOI] [PubMed] [Google Scholar]
- 78.Steele AD, Phillips J, Smit TK, Peenze I, Jiang X. Snow Mountain-like virus identified in young children with winter vomiting disease in South Africa. J Diarrhoeal Dis Res. 1997;15:177–182. [PubMed] [Google Scholar]
- 79.Vinje J, Koopmans MP. Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in The Netherlands. J Infect Dis. 1996;174:610–615. doi: 10.1093/infdis/174.3.610. [DOI] [PubMed] [Google Scholar]
- 80.Wolfaardt M, Taylor MB, Booysen HF, Engelbrecht L, Grabow WO, Jiang X. Incidence of human calicivirus and rotavirus infection in patients with gastroenteritis in South Africa. J Med Virol. 1997;51:290–296. doi: 10.1002/(sici)1096-9071(199704)51:4<290::aid-jmv6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 81.Yamazaki K, Oseto M, Seto Y, et al. Reverse transcription-polymerase chain reaction detection and sequence analysis of small round-structured viruses in Japan. Arch Virol Suppl. 1996;12:271–276. doi: 10.1007/978-3-7091-6553-9_29. [DOI] [PubMed] [Google Scholar]
- 82.Liu BL, Lambden PR, Gunther H, Otto P, Elschner M, Clarke IN. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J Virol. 1999;73:819–825. doi: 10.1128/jvi.73.1.819-825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sugieda M, Nagaoka H, Kakishima Y, Ohshita T, Nakamura S, Nakajima S. Detection of Norwalk-like virus genes in the caecum contents of pigs. Arch Virol. 1998;143:1215–1221. doi: 10.1007/s007050050369. [DOI] [PubMed] [Google Scholar]
- 84.van Der Poel WH, Vinje J, van Der Heide R, Herrera MI, Vivo A, Koopmans MP. Norwalk-like calicivirus genes in farm animals. Emerg Infect Dis. 2000;6:36–41. doi: 10.3201/eid0601.000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brugha R, Vipond IB, Evans MR, et al. A community outbreak of food-borne small round-structured virus gastroenteritis caused by a contaminated water supply. Epidemiol Infect. 1999;122:145–154. doi: 10.1017/s0950268898001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gray JJ, Green J, Cunliffe C, et al. Mixed genogroup SRSV infections among a party of canoeists exposed to contaminated recreational water. J Med Virol. 1997;52:425–429. [PubMed] [Google Scholar]
- 87.Hafliger D, Hubner P, Luthy J. Outbreak of viral gastroenteritis due to sew-age-contaminated drinking water. Int J Food Microbiol. 2000;54:123–126. doi: 10.1016/s0168-1605(99)00176-2. [DOI] [PubMed] [Google Scholar]
- 88.Lodder WJ, Vinje J, van De Heide R, de Roda Husman AM, Leenen EJ, Koopmans MP. Molecular detection of Norwalk-like caliciviruses in sewage. Appl Environ Microbiol. 1999;65:5624–5627. doi: 10.1128/aem.65.12.5624-5627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sugieda M, Nakajima K, Nakajima S. Outbreaks of Norwalk-like virus-associated gastroenteritis traced to shellfish: coexistence of two genotypes in one specimen. Epidemiol Infect. 1996;116:339–346. doi: 10.1017/s0950268800052663. [DOI] [PMC free article] [PubMed] [Google Scholar]