Abstract
Iatrogenic hyperadrenocorticism (or iatrogenic Cushing's syndrome) is an adrenal disorder that may result from long-term administration of glucocorticoids for therapeutic purposes, most often given to treat allergic or immune-mediated disorders. Prolonged treatment with synthetic glucocorticoids can suppress hypothalamic corticotrophin releasing hormone and plasma adrenocorticotrophic hormone (ACTH), thus causing a functional inactivity of the adrenal cortex. The result is a clinical syndrome of hyperadrenocorticism but with basal and ACTH-stimulated plasma cortisol concentrations that are consistent with spontaneous hypoadrenocorticism (Addison's disease).
Whilst iatrogenic hyperadrenocorticism is relatively frequent in dogs, the diagnosis of iatrogenic hyperadrenocorticism in cats is very uncommon because this species has been found to be remarkably resistant to prolonged administration of glucocorticoids. To the author's knowledge, there are only two published clinical cases of feline iatrogenic Cushing's syndrome. This report describes a case of iatrogenic hyperadrenocorticism in a cat, and shows how normalisation of the adrenal function was achieved with supportive treatment and withdrawal of glucocorticoid administration.
A10-year-old, female, neutered cross-bred cat was presented with a 1-week history of anorexia and lethargy. Over the previous 2 days, the signs had become more severe and haematochezia had also developed. One month previously, the cat had been treated with methylprednisolone acetate (Depo-medrone, 20 mg sc weekly for 4 weeks) for a stomatitis which was presumed to be immune mediated. It had begun to eat ravenously and gain weight as soon as the treatment was initiated. Polyuria and polydipsia were not reported.
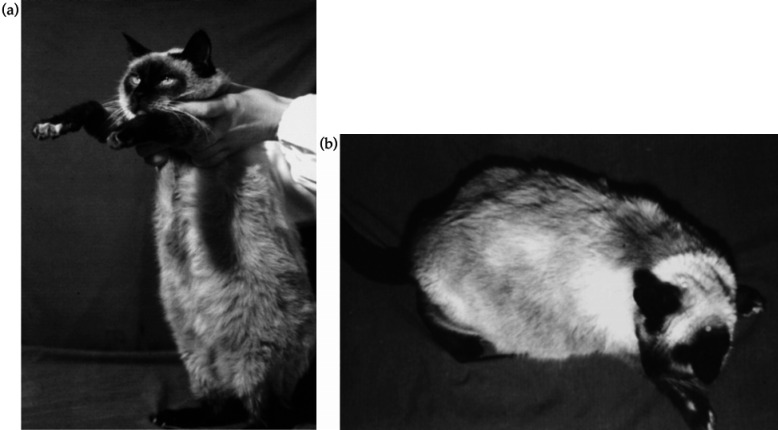
On physical examination, the patient was found to be grossly overweight (5.5 kg) with a pot-bellied appearance [Fig 1(a, b)], mental depression and reluctance to move. Generalised muscle wasting and poor condition of the coat were also observed. Measurement of the rectal temperature (38.9°C) revealed the presence of fresh blood in the rectum. The skin showed a slight reduction in elasticity, and excessive bruising followed venipuncture for blood testing. Examination of the oral cavity did not show any significant abnormalities that could be related to chronic gingivitis/stomatitis.
Fig 1.
The general appearance of the patient at presentation: the cat was grossly overweight (5.5 kg) with a pendulous abdomen (a) and poor condition of the coat (b).
Results of complete blood count (CBC), serum biochemistry and urine analysis are shown in Table 1(a). The only haematological abnormalities were leukocytosis, characterised by a neutrophilia with left shift, and a mild thrombocytopenia. The serum chemistry changes were elevated liver enzymes with mild hyperproteinaemia and polyclonal gammopathy, hyperbilirubinaemia, hyperglycaemia and hyperlipidaemia (cholesterol and triglycerides). Measurement of serum electrolytes revealed significant hypokalaemia and hypochloridaemia. Hypocalcaemia was also found. Serum fructosamine concentration (229 μmol/l) was within the reference range, indicating that the hyperglycaemia was unlikely to result from diabetes mellitus and was probably stress related. Urinalysis was characterised by the presence of glucose, proteins, blood and leukocytes. Urine culture did not give rise to any significant aerobic or anaerobic bacterial growth. Faeces appeared normal in consistency and faecal examination did not reveal parasitic infection. The cat was found negative for the presence of antibodies to feline immunodeficiency virus (FIV) and for feline leukaemia virus antigen (FeLV) (Snap-Combi FeLV-FIV, IDEXX Labs. Inc., Westbrook, MA, USA). The patient was then hospitalised and given lactated Ringer's solution at maintenance level (200 ml/24 h) and enrofloxacin (Baytril; Bayer) (30 mg iv, followed by 15 mg po s.i.d. for 4 days) for suspected urinary tract infection (UTI) based on urine sediment analysis.
Table 1.
Results of laboratory tests
| SI units | (a) Day 0 | (b) Day 3 | (c) Day 5 | (d) Follow-up | Reference range | |
|---|---|---|---|---|---|---|
| Complete blood count | ||||||
| Erythrocytes | ×1012/l | 6.79 | – | 6.88 | 9.24 | 5.0–10.0 |
| Haemoglobin | g/l | 113 | – | 123 | 134 | 80–150 |
| Haematocrit | l/l | 0.35 | – | 0.35 | 0.46 | 0.24–0.45 |
| MCV | fl | 52.3 | – | 51.7 | 49.7 | 39–55 |
| MCH | pg | 16.7 | – | 17.8 | 14.5 | 13–17 |
| MCHC | g/l | 319 | – | 345 | 291 | 320–360 |
| Reticulocytes | % | 0 | – | 0 | 0 | 0–1.5 |
| Platelets | 109/l | 180 | – | 195 | 332 | 190–400 |
| White blood cells | 109/l | 23.9 | – | 12.6 | 8.4 | 5.5–15.4 |
| Neutrophils | 109/l | 19.9 | – | 10.5 | 5.7 | 2.5–12.5 |
| Band neutrophils | 109/l | 0.4 | – | 0.1 | 0 | 0–0.3 |
| Lymphocytes | 109/l | 3.0 | – | 1.61 | 2.0 | 1.5–7.0 |
| Monocytes | 109/l | 0.3 | – | 0.25 | 0.3 | 0–0.85 |
| Eosinophils | 109/l | 0.3 | – | 0.13 | 0.4 | 0–0.75 |
| Basophils | 109/l | 0 | – | 0 | 0 | 0–0.2 |
| Serum biochemistry | ||||||
| Total protein | g/l | 83 | – | – | 85 | 60–82 |
| Albumin | g/l | 36 | – | – | 49 | 25–39 |
| Globulins | g/l | 47 | – | – | 36 | 26–50 |
| Total bilirubin | μmol/l | 18.48 | – | – | 5.71 | 2.0–15.0 |
| Amylase | U/l | 1646 | – | – | 834 | 700–2000 |
| Urea | mmol/l | 6.4 | – | – | 8.2 | 5–10 |
| Glucose | mmol/l | 10.4 | – | – | 6.7 | 3.5–9.0 |
| Cholesterol | mmol/l | 6.12 | – | – | 3.83 | 1.5–6.0 |
| Triglycerides | mmol/l | 1.44 | – | – | 0.41 | 0.6–1.2 |
| Creatinine | μl | 75 | – | – | 105 | 75–180 |
| AST | U/l | 127 | – | – | 25 | 10–59 |
| ALT | U/l | 159 | – | – | 44 | 10–75 |
| ALP | U/l | 110 | – | – | 11 | 0–90 |
| GGT | U/l | 3.94 | – | – | 0.2 | 0–6 |
| LDH | U/l | 2125 | – | – | 59 | 63–193 |
| CK | U/l | 56 | – | – | 249 | 0–580 |
| Fructosamine | μl | 229 | – | – | 159 | 228–341 |
| Sodium | mmol/l | 155 | – | – | 146 | 145–157 |
| Potassium | mmol/l | 2.8 | – | – | 4.6 | 3.7–5.8 |
| Chloride | mmol/l | 109 | – | – | 114 | 112–129 |
| Calcium | mmol/l | 1.7 | – | – | 2.6 | 1.8–3.0 |
| Phosphorus | mmol/l | 1.4 | – | – | 1.44 | 1.03–2.82 |
| Urinalysis | ||||||
| Specific gravity | 1.025 | – | 1.030 | 1.025 | 1.001–1.080 | |
| pH | 6.5 | – | 6.0 | 5.5 | 4.5–8.5 | |
| Glucose | + | – | Neg | Neg | Neg | |
| Bilirubin | + | – | Neg | Neg | Neg | |
| Ketones | Neg | – | Neg | Neg | Neg | |
| Protein | ++ | – | + | Neg | Neg to trace | |
| Blood | ++ | – | Neg | Neg | Neg | |
| Leukocytes | ++ | – | + | Neg | Neg | |
| Bacteria | Neg | – | Neg | Neg | Neg | |
| Crystals | Neg | – | Neg | Neg | Variable | |
| Serology | ||||||
| FIV | Neg | – | – | – | – | |
| FeLV | Neg | – | – | – | – | |
| ACTH stimulation test | ||||||
| Pre-stimulation | nmol/l | – | 0.8 | – | 102 | 5.5–165 |
| Post-stimulation (30′) | nmol/l | – | 2.2 | – | 107 | 55–220 |
| Post-stimulation (60′) | nmol/l | – | 2.5 | – | 121 | 55–220 |
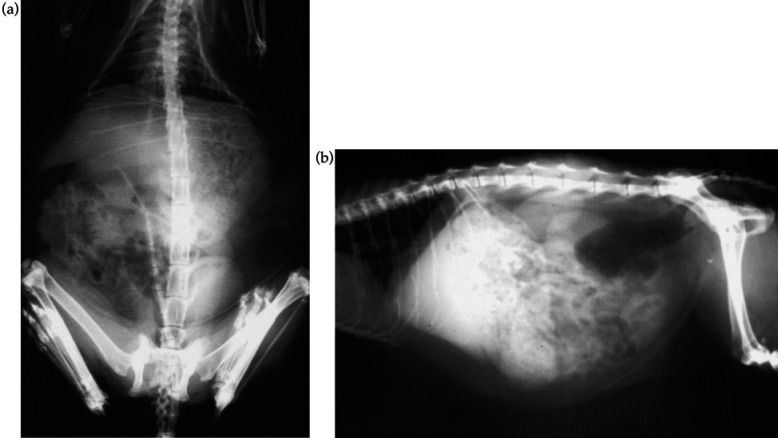
Dorso-ventral and lateral abdominal radiographs revealed marked abdominal contrast, due to the presence of excessive peri-visceral fat, an overfilled stomach, full bladder and moderate hepatomegaly [Fig 2(a, b)]. No other radiographic abnormalities were found. After a 24-h fast, radiographic studies were undertaken after a barium meal, but no abnormalities were detected. Haematochezia and dyschezia resolved after the second day of hospitalisation.
Fig 2.
Dorso-ventral (a) and lateral (b) abdominal radiographs showing the presence of excessive peri-visceral fat, overfilled stomach, full bladder and a moderate hepatomegaly.
On the third day, an adrenocorticotrophic hormone (ACTH) stimulation test was performed by measuring plasma cortisol prior to, 30 and 60 min after im administration of 125 μg of the synthetic polypeptide tetracosactrin (Synacthen; CIBA), according to the method described by Willard et al (1999). Results of the ACTH stimulation test are shown in Table 1(b). The very low resting cortisol concentration and the failure of the adrenal glands to respond to the synthetic ACTH administration were compatible with a corticosteroid-induced adrenal suppression.
On Day 5, the patient was eating and behaving normally. The CBC and urinalysis carried out the same day did not reveal any apparent abnormality [Table 1(c)] and the cat was discharged, with instructions to feed a restriction diet (Feline r/d, Hill's) in order to decrease the caloric intake and reduce the obesity.
Follow-up
The cat was re-examined 4 months later. The owner reported that the animal had progressed well. The patient had lost 1.3 kg and was no longer considered to be overweight (Fig 3). The condition of the coat and the skin had improved and muscle tone was normal. All parameters of CBC, serum biochemistry, urinalysis and serum fructosamine were within the normal ranges [Table 1(d)]. A second ACTH stimulation test was performed and results are shown in Fig 4. Compared with the results obtained 4 months previously, basal plasma cortisol was higher and a clear response of the adrenal glands to the ACTH administration was found.
Fig 3.
The general appearance of the patient at re-examination (4 months later). The patient had lost 1.3 kg and was no longer considered to be overweight. The condition of the coat and the skin had ameliorated and muscle tone was normal.
Fig 4.
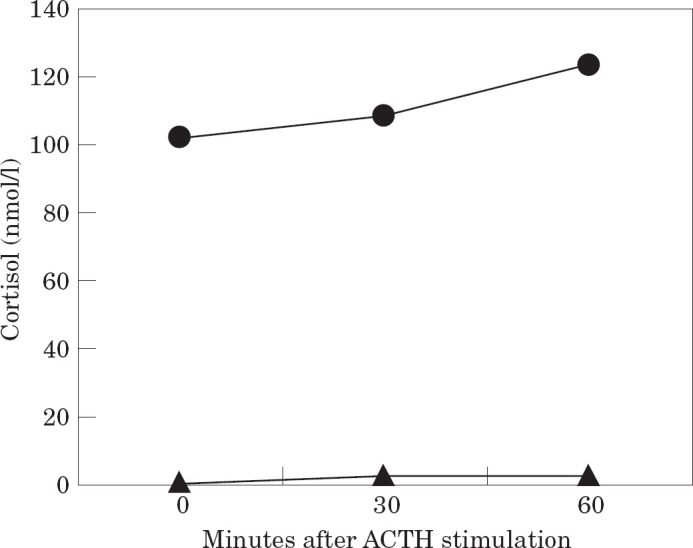
Results of the ACTH stimulation test carried out on the third day of hospitalisation (▴) and the day of re-examination of the patient (•, 4 months later). The test was performed by measuring plasma cortisol prior to, 30 and 60 min after im administration of 125 μg of tetracosactrin (Synacthen; CIBA).
Discussion
It is suggested that, compared with dogs, cats rarely develop hyperadrenocorticism (Scott et al 1982) and, as far as the author is aware, only two clinical cases of iatrogenic Cushing's syndrome have been reported previously, after a chronic sc administration of dexamethasone (Greene et al 1995) or triamcinolone acetate (Shaer & Ginn 1999). In this patient, some of the most frequent reported clinical signs in naturally occurring hyperadrenocorticism (Duesberg & Peterson 1997) had been noticed: increased appetite (followed by obesity, lethargy and anorexia), hair loss with symmetrical alopecia, muscle wasting, fragile skin and hepatomegaly. Polyuria and polydipsia are often reported in cats with hyperadrenocorticism (Zerbe et al 1987, Nelson et al 1988, Usher 1991, Childers & Raunch 1993, Valentine & Silber 1996, Watson & Herrtage 1998), but were not reported in this patient. As the polyuria and polydipsia are secondary to the osmotic diuresis induced by hyperglycaemia and associated glucosuria, rather than directly associated with steroid effects as in dogs, it might be possible that the diabetogenic effect of glucocorticoid excess was not present for long enough in this cat for these clinical signs to develop. In fact, the fructosamine level suggested that hyperglycaemia had been present for no more than 3–4 days over a 2–3-week period, based on the half-life of fructosamine (Crenshaw et al 1996). For this reason, the hyperglycaemia may have been related to glucose intolerance mediated by stress or exogenous glucocorticoids, rather than diabetes mellitus, by increasing gluconeogenesis and decreasing glucose utilisation in the peripheral tissues (Middleton & Watson 1985, Behrend & Kemppainen 1997). Another explanation for the hyperglycaemia could be the concomitant hepatopathy, accompanied by decreased glucocorticoid and glucocorticoid clearance (Yoshiya et al 1987, Shaw & Ihle 1997).
The possibility of liver involvement was supported by the presence of elevated liver enzymes and hepatomegaly at the day of presentation. Potential adverse effects of glucocorticoids on liver function have been well described in dogs after experimental administration of prednisone (Badylak & Van Vleet 1981, Solter et al 1994), but in cats a marked elevation of liver enzymes has been often reported to be uncommon both in patients with Cushing's disease (Nelson et al 1988, Immik et al 1992) and in those receiving glucocorticoids (Scott et al 1982). However, Shaer & Ginn (1999) have documented a case of hepatopathy associated with iatrogenic hyperadrenocorticism in a cat, characterised by a significant elevation of liver enzymes and mild hepatomegaly has also been described by Nelson et al (1988) in cats affected by Cushing's disease. These findings suggest that, whilst cats are usually resistant to the development of side-effects associated with glucocorticoids, hepatopathy can occur in this species following steroid therapy. The radiographic findings of perivisceral fat, full bladder and muscle hypotrophia have been previously observed in feline hyperadrenocorticism (Watson & Herrtage 1998).
The poor condition of the coat and fragile skin can be related to the side-effects of glucocorticoids even though it is less commonly seen in cats than dogs, occurring in approximately 50–60% of the patients with hyperadrenocorticism (Duesberg & Peterson 1997, Watson & Herrtage 1998). The tendency to bruising after venipuncture is common both in canine and feline Cushing's syndrome and this may be related to the steroid-induced suppression of tissue granulation that eventually leads to a poor wound healing (Feldman & Nelson 1996). Fragile skin and bruising has also been reported in a cat that had been on long-term corticosteroid therapy (triamcinolone acetate) for an indolent ulcer (Canfield et al 1992).
The presence of fresh blood in the faeces (haematochezia) suggested colonic or rectal ulceration. Gastrointestinal complications characterised by colonic ulceration or even perforation have been associated with the use of corticosteroids in dogs (Toombs et al 1980). In this case, although potentially related to the hyperadrenocorticism, the intestinal bleeding was only transient and did not give rise to any significant haematological alteration.
Urinary tract infections are common both in dogs and cats with hyperadrenocorticism. Proteinuria, blood and leukocytes indicated a possible UTI, and urine culture is indicated in all patients with suspected UTI. In this case, there was no growth on bacterial culture but with the sediment analysis, antibiotic therapy was commenced while awaiting for results of urine bacteriology. The concurrent leukocytosis and neutrophilia could be related either to a UTI or to the methylprednisolone usage, but the latter is not usually associated with a left shift (Willard et al 1999). The mild glucosuria was probably a consequence of the transient hyperglycaemia.
Although of minor diagnostic relevance, hypokalemia may occur in canine Cushing's due to the mineralcorticoid effect of steroids (Willard et al 1999), and has also been described in a case of iatrogenic feline hyperadrenocorticism, together with a general decreased concentration of serum electrolytes (Greene et al 1995).
Synthetic glucocorticoids have the capacity to suppress the hypothalamic-pituitary-adrenal axis (HPA). A similar effect has been observed after prolonged treatment with megestrol acetate or proligestone (Peterson 1987, Church et al 1994). If serum ACTH remains diminished for a sufficient time, the adrenal cells that synthesise cortisol (zona fasciculata and reticularis) became atrophic, with subsequent decline of cortisol concentration and poor responsiveness to exogenous ACTH. Thus, the ACTH stimulation test is the ideal procedure for identifying animals with iatrogenic hyperadrenocorticism. Synthetic ACTH (tetracosactrin) appears to stimulate the adrenal cortex more consistently than natural pituitary extract (ACTH gel) at the recommended dose of 125 μg per cat (Sparkes et al 1990, Peterson & Kemppainen 1993). Although intravenous administration of synthetic ACTH gives rise to a greater degree of stimulation (Peterson & Kemppainen 1992), some authors have observed a faster cortisol response after im administration of cosyntropin compared with iv injection (Smith & Feldman 1987). This finding has been interpreted as a possible consequence of the pain associated with the im injection that may eventually lead to an endogenous corticotrophin-cortisol priming effect (Robson et al 1995). However, the present author opted for the im route to avoid further venous bruising in this patient. According to Duesberg & Peterson (1997), plasma cortisol concentration tends to peak earlier (30 min) and return to baseline values in cats more quickly than in dogs. Thus, the test should require at least two blood samples at 30 and 60 min to ensure the detection of maximal adrenal response (Willard et al 1999). Nevertheless, results in Fig 4 show that cortisol level at 60 min after stimulation is higher than at 30 min, suggesting that the peak cortisol response may occur later, as observed by Sparkes et al (1990). Results of the ACTH stimulation test, performed on Day 3 and the day of re-examination, clearly demonstrate that the adrenal cortex was severely depressed when the cat was referred to the hospital, but could recover spontaneously after suspension of any anti-inflammatory drug for a period of 4 months.
The treatment of iatrogenic Cushing's disease usually involves the discontinuation of corticosteroid administration until the HPA recovers (Kaufman 1984, Kimmerle & Rolla 1985). It has also been reported that the adrenocortical hypofunction resulting from iatrogenic Cushing's in dogs may be successfully restored using electroacupuncture (Lin et al 1991). Although animals on chronic oral treatment should be tapered off their steroids when discontinuing the drug, the present author thought that a sudden suspension could be carried out given the long-acting formulation of the corticosteroid used.
Polyphagia is assumed to be a direct effect of glucocorticoids (Feldman & Nelson 1996) and weight gain is a consequence. Suspension of corticosteroid treatment should then be followed by a normalisation of the appetite and a reduction of bodyweight. However, given the severe obesity and lethargy observed in this patient, a restriction diet was prescribed in addition to withdrawal of the drug.
To the best of the author's knowledge, this is the first clinical report of steroid-induced hyperadrenocorticism in a cat after sc administration of methylprednisolone acetate. This cat received the recommended dose of methylprednisolone acetate (20 mg) on a weekly basis rather than fortnightly (Ettinger & Feldman 2000), but clinical signs developed after a relatively short period of time (4 weeks). In an experimental study, normal cats receiving a weekly dose of 20 mg of methylprednisolone acetate sc for 4 weeks did not develop the typical clinical signs of hyperadrenocorticism (Scott et al 1979). However, there may be a variability in the likelihood of developing clinical signs following the use of methylprednisolone acetate, and therefore this product should be used cautiously in cats.
The first two clinical reports of feline iatrogenic Cushing's disease have been published over the last 5 years, and this suggests that this complication may have been underestimated in the past, possibly due to the belief that this species is highly resistant to side-effects of glucocorticoids.
Acknowledgements
The author gratefully acknowledges Prof Tim J Gruffydd-Jones and Dr Andy Sparkes for reviewing this manuscript, and for their valuable advice and encouragement. The author also wishes to thank Dr Lieta Marinelli who referred the clinical case, and Prof Gianfranco Gabai for the cortisol radioimmunoassay. The cat was visited and assisted at the Animal Hospital of the University of Padova, Italy.
References
- Badylak SF, van Vleet JF. (1981) Sequential morphologic and clinicopathologic alterations in dogs with experimentally induced glucocorticoid hepatopathy. American Journal of Veterinary Research 42, 1310–1318. [PubMed] [Google Scholar]
- Behrend EN, Kemppainen RJ. (1997) Glucocorticoid therapy: Pharmacology, indications and complications. Veterinary Clinics of North America: Small Animal Practice 27, 187–213. [DOI] [PubMed] [Google Scholar]
- Canfield PJ, Hinchliffe JM, Yager JA. (1992) Probable steroid-induced skin fragility in a cat. Australian Veterinary Practitioner 22, 164–170. [Google Scholar]
- Childers HE, Raunch A. (1993) What is your diagnosis? [abdominal mass in a cat]. Journal of the American veterinary Medical Association 203, 507–508. [PubMed] [Google Scholar]
- Church DB, Watson DJ, Emslie DR, Middleton DJ. (1994) Effects of proligestone and megestrole acetate on plasma adrenocorticotrophic hormone, insulin and insulin-like growth factor-1 concentrations in cats. Research in Veterinary Science 56, 175–178. [DOI] [PubMed] [Google Scholar]
- Crenshaw KL, Peterson ME, Hebb LA, Moroff SD, Nichols R. (1996) Serum fructosamine concentration as an index of glycemia in cats with diabetes mellitus and stress hyperglycemia. Journal of Veterinary Internal Medicine 10, 360–364. [DOI] [PubMed] [Google Scholar]
- Duesberg C, Peterson ME. (1997) Adrenal disorders in cats. Veterinary Clinics of North America: Small Animal Practice 27, 321–347. [DOI] [PubMed] [Google Scholar]
- Ettinger SJ, Feldman EC. (2000) Oral and salivary gland disorders. In: Textbook of Veterinary Internal Medicine (5th edn). WB Saunders, Philadelphia, pp. 1114–1121. [Google Scholar]
- Feldman EC, Nelson RW. (1996) Hyperadrenocorticism (Cushing's syndrome). In: Canine and Feline Endocrinology and Reproduction (2nd edn). WB Saunders, Philadelphia, pp. 187–265. [Google Scholar]
- Greene CE, Carmichael KP, Gratzek A. (1995) Iatrogenic hyperadrenocorticism in a cat. Feline Practice 23, 7–12. [Google Scholar]
- Immink WFGA, van Toor AJ, Vos JH, van der Linde-Sipman JS, Lubberink AA. (1992) Hyperadrenocorticism in four cats. Veterinary Quarterly 15, 81–85. [DOI] [PubMed] [Google Scholar]
- Kaufman J. (1984) Diseases of the adrenal cortex of dogs and cats. Modern Veterinary Practice 65, 513–516. [PubMed] [Google Scholar]
- Kimmerle R, Rolla AR. (1985) Iatrogenic Cushing's syndrome due to dexamethasone nasal drops. American Journal of Medicine 79, 535–537. [DOI] [PubMed] [Google Scholar]
- Lin JH, Su HL, Chang SH, Shien YS, Wu LS. (1991) Treatment of iatrogenic Cushing's syndrome in dogs with electro-acupuncture stimulation of stomach 36. American Journal of Chinese Medicine 19, 9–15. [DOI] [PubMed] [Google Scholar]
- Middleton DJ, Watson ADJ. (1985) Glucose intolerance in cats given short-term therapies of prednisolone and megestrol acetate. American Journal of Veterinary Research 46, 2623–2625. [PubMed] [Google Scholar]
- Nelson RW, Feldman EC, Smith MC. (1988) Hyperadrencorticism in cats: Seven cases (1978–1987). Journal of American Veterinary Medical Association 193, 245–250. [PubMed] [Google Scholar]
- Peterson ME. (1987) Effects of megestrol acetate on glucose tolerance and growth hormone secretion in the cat. Research in Veterinary Science 42, 354–357. [PubMed] [Google Scholar]
- Peterson ME, Kemppainen RJ. (1992) Comparison of intravenous and intramuscular routes of administering cosyntropin for corticotropin preparations (tetracosactrin and cosyntropin) in healthy cats. American Journal of Veterinary Research 53, 1752–1755. [PubMed] [Google Scholar]
- Peterson ME, Kemppainen RJ. (1993) Dose-response relationship between plasma concentrations of corticotropin and cortisol after administration of incremental doses of cosyntropin for corticotropin stimulation testing in cats. American Journal of Veterinary Research 54, 300–304. [PubMed] [Google Scholar]
- Robson M, Taboada J, Wolfsheimer K. (1995) Adrenal gland function in cats. The Compendium. Small Animal 17, 1205–1214. [Google Scholar]
- Schaer M, Ginn PE. (1999) Iatrogenic Cushing's syndrome and steroid hepatopathy in a cat. Journal of the American Animal Hospital Association 35, 48–51. [DOI] [PubMed] [Google Scholar]
- Scott DW, Kirk RW, Bentinck-Smith J. (1979) Some effects of short-term methylprednisolone therapy in normal cats. Cornell Veterinarian 69, 104–115. [PubMed] [Google Scholar]
- Scott DW, Manning TO, Reimers TJ. (1982) Iatrogenic Cushing's syndrome in the cat. Feline Practice 12, 30–36. [Google Scholar]
- Shaw DH, Ihle SL. (1997) Hepatobiliary and exocrine pancreatic disorders. In: Small Animal Internal Medicine. William & Wilkins, Baltimore, p. 299. [Google Scholar]
- Smith MC, Feldman EC. (1987) Plasma endogenous ACTH concentrations and plasma cortisol responses to synthetic ACTH and dexamethasone sodium phosphate in healthy cats. American Journal of Veterinary Research 48, 1719–1724. [PubMed] [Google Scholar]
- Solter PF, Hoffman WE, Chambers MD, Schaeffer DJ, Kuhlenschmidt MS. (1994) Hepatic total 3a-hydroxy bile acids concentration and enzyme activities in prednisone-treated dogs. American Journal of Veterinary Research 55, 1086–1092. [PubMed] [Google Scholar]
- Sparkes AH, Adams DT, Douthwaite JA, Gruffydd-Jones TJ. (1990) Assessment of adrenal function in cats: Response to intravenous synthetic ACTH. Journal of Small Animal Practice 31, 2–5. [Google Scholar]
- Toombs JP, Caywood DD, Lipowitz AJ, Stevens JB. (1980) Colonic perforation following neurosurgical procedures and corticosteroid therapy in four dogs. Journal of American Veterinary Medical Association 177, 68–72. [PubMed] [Google Scholar]
- Usher DG. (1991) Hyperadrenocorticism in a cat. Canadian Veterinary Journal 32, 326. [PMC free article] [PubMed] [Google Scholar]
- Valentine RW, Silber AS. (1996) Feline hyperadrenocorticism: A rare case. Feline Practice 24, 6–11. [Google Scholar]
- Watson PJ, Herrtage ME. (1998) Hyperadrenocorticism in six cats. Journal of Small Animal Practice 39, 175–184. [DOI] [PubMed] [Google Scholar]
- Willard MD, Tvedten H, Turnwald GH. (1999) Endocrine, metabolic and lipidic disorders. In: Small Animal Clinical Diagnosis by Laboratory Methods (3rd edn). WB Saunders, Philadelphia, pp. 166–167. [Google Scholar]
- Yoshiya K, Kishimoto T, Ishikawa Y, Utsunomiya J. (1987) Insulin response following intravenous glucose administration in dogs with obstructive jaundice. Journal of Surgical Research 43, 271–277. [DOI] [PubMed] [Google Scholar]
- Zerbe CA, Nachreiner RF, Dunstan RW, Dalley JB. (1987) Hyperadrenocorticism in a cat. Journal of American Veterinary Medical Association 193, 245–250. [PubMed] [Google Scholar]