Abstract
Background: A previous study showed greater adhesion by platelets of healthy full term infants to subendothelial extracellular matrix (ECM) under flow conditions compared with healthy adult platelets.
Aim: To investigate the adhesion and aggregation of platelets from preterm infants on ECM under defined shear conditions.
Methods: In vitro platelet function was investigated in 106 preterm infants, 74 full term infants, and 26 healthy adults. Blood samples were obtained from all infants within 24 hours of birth, and weekly until discharge from preterm infants only. Citrated whole blood was placed in ECM precoated tissue culture plates and subjected to shear stress (1300 s-1) for two minutes using a rotating Teflon cone. Platelet adhesion (surface coverage) and aggregation (average size) to ECM were assayed using an image analyser. Assays for von Willebrand factor (vWF) antigen, ristocetin cofactor, and vWF collagen-binding activity were performed on samples from an additional 70 preterm infants, 23 healthy full term infants, and 24 healthy adults. Preterm infants with hyaline membrane disease (HMD) were analysed separately in both cohorts.
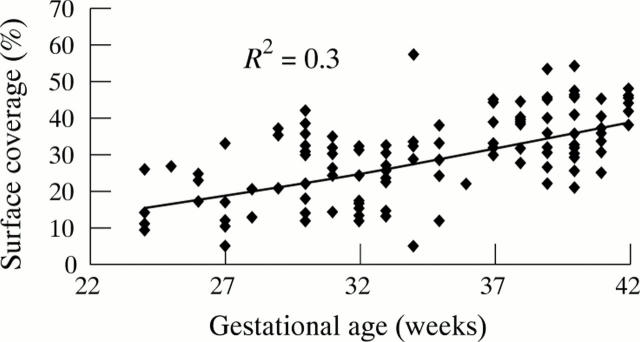
Results: Platelets from preterm infants displayed significantly less platelet adhesion than those from full term infants but similar aggregation and levels of vWF antigen, ristocetin cofactor, and collagen binding activity. Mean surface coverage was 22.0 (8.4)% for preterm infants with HMD, 28.7 (8.0)% for healthy preterm infants, and 35.7 (7.9)% for full term infants. Surface coverage in the preterm infants correlated with gestational age during the first 24 hours only, and did not reach full term levels during 10 weeks of follow up.
Conclusion: Platelet adhesion to ECM is significantly poorer in preterm than in full term infants, and poorer in preterm infants with HMD than in healthy preterm infants. Intrinsic platelet properties rather than the concentration or activity of vWF may be responsible for this difference.
Full Text
The Full Text of this article is available as a PDF (103.9 KB).
Figure 1 .
Surface coverage during the first 48 hours of life correlated with gestational age.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarts P. A., Bolhuis P. A., Sakariassen K. S., Heethaar R. M., Sixma J. J. Red blood cell size is important for adherence of blood platelets to artery subendothelium. Blood. 1983 Jul;62(1):214–217. [PubMed] [Google Scholar]
- Alkhamis T. M., Beissinger R. L., Chediak J. R. Artificial surface effect on red blood cells and platelets in laminar shear flow. Blood. 1990 Apr 1;75(7):1568–1575. [PubMed] [Google Scholar]
- Andrew M. Developmental hemostasis: relevance to hemostatic problems during childhood. Semin Thromb Hemost. 1995;21(4):341–356. doi: 10.1055/s-2007-1000655. [DOI] [PubMed] [Google Scholar]
- Andrew M., Paes B., Milner R., Johnston M., Mitchell L., Tollefsen D. M., Castle V., Powers P. Development of the human coagulation system in the healthy premature infant. Blood. 1988 Nov;72(5):1651–1657. [PubMed] [Google Scholar]
- Beumer S., IJsseldijk M. J., de Groot P. G., Sixma J. J. Platelet adhesion to fibronectin in flow: dependence on surface concentration and shear rate, role of platelet membrane glycoproteins GP IIb/IIIa and VLA-5, and inhibition by heparin. Blood. 1994 Dec 1;84(11):3724–3733. [PubMed] [Google Scholar]
- Blanchette V. S., Rand M. L. Platelet disorders in newborn infants: diagnosis and management. Semin Perinatol. 1997 Feb;21(1):53–62. doi: 10.1016/s0146-0005(97)80020-1. [DOI] [PubMed] [Google Scholar]
- Díaz-Ricart M., Carretero M., Castillo R., Ordinas A., Escolar G. Digital image analysis of platelet-extracellular matrix interactions: studies in von Willebrand disease patients and aspirin-treated donors. Haemostasis. 1994 Jul-Aug;24(4):219–229. doi: 10.1159/000217105. [DOI] [PubMed] [Google Scholar]
- Frojmovic M. M. Platelet aggregation in flow: differential roles for adhesive receptors and ligands. Am Heart J. 1998 May;135(5 Pt 2 SU):S119–S131. doi: 10.1016/s0002-8703(98)70240-6. [DOI] [PubMed] [Google Scholar]
- Goto S., Ikeda Y., Saldívar E., Ruggeri Z. M. Distinct mechanisms of platelet aggregation as a consequence of different shearing flow conditions. J Clin Invest. 1998 Jan 15;101(2):479–486. doi: 10.1172/JCI973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshaupt B., Muntean W., Sedlmayr P. Hyporeactivity of neonatal platelets is not caused by preactivation during birth. Eur J Pediatr. 1997 Dec;156(12):944–948. doi: 10.1007/s004310050748. [DOI] [PubMed] [Google Scholar]
- Israels S. J., Gowen B., Gerrard J. M. Contractile activity of neonatal platelets. Pediatr Res. 1987 Mar;21(3):293–295. doi: 10.1203/00006450-198703000-00019. [DOI] [PubMed] [Google Scholar]
- Katz J. A., Moake J. L., McPherson P. D., Weinstein M. J., Moise K. J., Carpenter R. J., Sala D. J. Relationship between human development and disappearance of unusually large von Willebrand factor multimers from plasma. Blood. 1989 May 15;73(7):1851–1858. [PubMed] [Google Scholar]
- Kenet G., Lubetsky A., Shenkman B., Tamarin I., Dardik R., Rechavi G., Barzilai A., Martinowitz U., Savion N., Varon D. Cone and platelet analyser (CPA): a new test for the prediction of bleeding among thrombocytopenic patients. Br J Haematol. 1998 May;101(2):255–259. doi: 10.1046/j.1365-2141.1998.00690.x. [DOI] [PubMed] [Google Scholar]
- Kundu S. K., Heilmann E. J., Sio R., Garcia C., Davidson R. M., Ostgaard R. A. Description of an in vitro platelet function analyzer--PFA-100. Semin Thromb Hemost. 1995;21 (Suppl 2):106–112. doi: 10.1055/s-0032-1313612. [DOI] [PubMed] [Google Scholar]
- Mull M. M., Hathaway W. E. Altered platelet function in newborns. Pediatr Res. 1970 May;4(3):229–237. doi: 10.1203/00006450-197005000-00001. [DOI] [PubMed] [Google Scholar]
- Rajasekhar D., Barnard M. R., Bednarek F. J., Michelson A. D. Platelet hyporeactivity in very low birth weight neonates. Thromb Haemost. 1997 May;77(5):1002–1007. [PubMed] [Google Scholar]
- Reimers R. C., Sutera S. P., Joist J. H. Potentiation by red blood cells of shear-induced platelet aggregation: relative importance of chemical and physical mechanisms. Blood. 1984 Dec;64(6):1200–1206. [PubMed] [Google Scholar]
- Ruggeri Z. M. Mechanisms of shear-induced platelet adhesion and aggregation. Thromb Haemost. 1993 Jul 1;70(1):119–123. [PubMed] [Google Scholar]
- Ruggeri Z. M. von Willebrand factor. J Clin Invest. 1997 Feb 15;99(4):559–564. doi: 10.1172/JCI119195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkman B., Linder N., Savion N., Tamarin I., Dardik R., Kennet G., German B., Varon D. Increased neonatal platelet deposition on subendothelium under flow conditions: the role of plasma von Willebrand factor. Pediatr Res. 1999 Feb;45(2):270–275. doi: 10.1203/00006450-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Shenkman B., Savion N., Dardik R., Tamarin I., Varon D. Testing of platelet deposition on polystyrene surface under flow conditions by the cone and plate(let) analyzer: role of platelet activation, fibrinogen and von Willebrand factor. Thromb Res. 2000 Aug 15;99(4):353–361. doi: 10.1016/s0049-3848(00)00255-3. [DOI] [PubMed] [Google Scholar]
- Shenkman B., Schneiderman J., Tamarin I., Kotev-Emeth S., Savion N., Varon D. Testing the effect of GPIIb-IIIa antagonist in patients undergoing carotid stenting: correlation between standard aggregometry, flow cytometry and the cone and plate(let) analyzer (CPA) methods. Thromb Res. 2001 May 15;102(4):311–317. doi: 10.1016/s0049-3848(01)00259-6. [DOI] [PubMed] [Google Scholar]
- Siekmann J., Turecek P. L., Schwarz H. P. The determination of von Willebrand factor activity by collagen binding assay. Haemophilia. 1998;4 (Suppl 3):15–24. doi: 10.1046/j.1365-2516.1998.0040s3015.x. [DOI] [PubMed] [Google Scholar]
- Varon D., Dardik R., Shenkman B., Kotev-Emeth S., Farzame N., Tamarin I., Savion N. A new method for quantitative analysis of whole blood platelet interaction with extracellular matrix under flow conditions. Thromb Res. 1997 Feb 15;85(4):283–294. doi: 10.1016/s0049-3848(97)00014-5. [DOI] [PubMed] [Google Scholar]
- Weinstein M. J., Blanchard R., Moake J. L., Vosburgh E., Moise K. Fetal and neonatal von Willebrand factor (vWF) is unusually large and similar to the vWF in patients with thrombotic thrombocytopenic purpura. Br J Haematol. 1989 May;72(1):68–72. doi: 10.1111/j.1365-2141.1989.tb07654.x. [DOI] [PubMed] [Google Scholar]