Haemophilus ducreyi causes chancroid, a genital ulcer disease (GUD) that is common in many developing countries (13, 14, 26, 68, 80, 103). UNAIDS and the World Health Organization estimate that the annual global incidence of chancroid is approximately 6 million cases (108). Although rare in the United States (24), chancroid persists in some urban areas and is frequently not recognized (65). The male-to-female ratio among patients with proven chancroid ranges from 3:1 in areas where it is endemic to as high as 25:1 in outbreak situations (67). Female sex workers serve as a reservoir for H. ducreyi and are thought to play an important part in transmission by infecting many male partners (67).
In addition to the morbidity associated with GUD, chancroid is a public health problem because H. ducreyi and the human immunodeficiency virus (HIV) facilitate each other’s transmission (39, 82, 111). In areas of chancroid endemicity, the relative risk of acquiring HIV infection for patients with GUD ranged from an odds ratio of 3 to an odds ratio of 18.2 (39, 82, 111). Conversely, HIV infection increases the risk of acquisition of GUD (20, 52). Per individual sexual act, GUD is estimated to enhance HIV transmission 10- to 100-fold (48, 81). H. ducreyi infection enhances HIV transmission by several possible mechanisms, including establishment of an accessible portal of viral entry, promotion of viral shedding from the ulcer, an increase in the viral load in blood and semen, and recruitment of CD4 cells and macrophages into the skin (34, 51, 55, 58, 89, 111). Mathematical models suggest that the mutual enhancement of transmission of HIV and GUD played a major role in accelerating the HIV epidemic in sub-Saharan Africa (81).
The association between chancroid and HIV transmission stimulated several laboratories to investigate H. ducreyi pathogenesis during the past 15 years. These studies have resulted in the identification of several potential virulence determinants, including lipooligosaccharides (LOS), which resemble human glycosphingolipids, pili, heat shock proteins, iron-regulated proteins or receptors, outer membrane proteins (OMPs), toxins, and other secreted products. Many of these studies are described in several comprehensive reviews (8, 67, 106); others are presented elsewhere (27, 35, 36, 61, 71, 83, 99, 110). Rather than review potential virulence determinants in detail, we will provide an overall picture of how the organism interacts initially with the human host, a comparison of human and animal models to study H. ducreyi infection, and the role of putative virulence determinants in these models.
THE ORGANISM
H. ducreyi was originally classified as a Haemophilus species because of its growth requirements, biochemical properties, and antigenic relatedness to other species in the group (67). However, by rRNA analysis, H. ducreyi is only remotely related to true haemophili such as Haemophilus influenzae and is now classified in the Actinobacillus cluster (4B) of the Pasteurellaceae (29, 31). Members of the species form a homogeneous DNA hybridization group and share many of the same surface antigens, suggesting that there is limited diversity within the species (23, 67, 106).
Recently, the genome of an H. ducreyi strain that is virulent in humans, 35000HP (HP refers to human passaged) (7, 89), was sequenced (unpublished observations; www.microbial-pathogenesis.org). The genome is composed of a single 1.7-Mb chromosome. A total of 1,693 putative open reading frames (ORFs) have been identified. The closest homologues of 66% of the genes were identified in H. influenzae or Pasteurella multocida as determined by BLAST analyses. Although there is substantial homology observed for many genes, there is little long-range conservation of the order of genes or operons in the chromosome when H. ducreyi is compared to these related species. Additionally, given the different spectrum of diseases caused by the different members of the Pasteurellaceae family, it is perhaps not surprising to note that the genes encoding the H. ducreyi hemolysin (71, 105) and the cytolethal distending toxin (CDT) (27) are absent from the H. influenzae (38) and P. multocida (64) genomes and that there are no homologues of the genes encoding the P. multocida toxin (74) or the Mannheimia (Pasteurella) hemolytica RTX-like leukotoxin genes (21) in the H. ducreyi genome.
NATURAL INFECTION
H. ducreyi is a strict human pathogen and naturally infects genital and nongenital skin, mucosal surfaces, and regional lymph nodes (67). H. ducreyi is thought to enter the skin through breaks in the epithelium that occur during intercourse (67, 96). Lack of circumcision is associated with infection in men (46, 67). H. ducreyi preferentially infects mucosal epithelium but also infects keratinized stratified squamous epithelium (46, 67). The transmission rate per sexual act is unknown. However, 70% of women who are secondary contacts of men with chancroid are infected (77), suggesting that the transmission rate is high. Erythematous papules form at each entry site within several hours to days and evolve into pustules in 2 to 3 days. After several weeks, the pustules ulcerate, and patients develop 1 to 4 painful ulcers and may frequently have suppurative lymphadenopathy. Patients typically do not seek medical attention until they have had ulcers for 1 to 3 weeks (25, 46, 67), probably about 3 to 6 weeks after inoculation. Because chancroid is most prevalent in countries with scarce resources and since the ulcers are quite painful, few biopsies have been done on confirmed chancroidal ulcers (1, 54, 55, 63). Since patients present at the ulcerative stage, little is known about the initial stages of natural infection other than historical information provided by patients.
HUMAN INFECTION MODEL
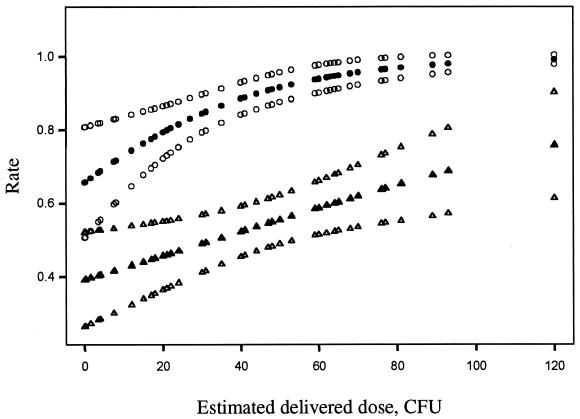
Given that the initial stages of chancroid are not usually painful or associated with regional lymphadenitis and that the organism does not disseminate, as well as historical precedence from the 1940s (32, 45), human inoculation experiments seemed reasonable and ethical. In the current human infection model (7, 89, 90), bacteria are delivered to the epidermis and dermis of the upper arm by puncture wounds made by the tines of an allergy testing device, simulating the presumed natural route of infection, abrasions that occur during intercourse. The estimated delivered dose (EDD) required to initiate papule formation may be as few as 1 to 2 CFU and the effect of EDDs on the probability of papule formation is dose dependent (5) (Fig. 1). Papules form within 24 h of inoculation and evolve into pustules in 2 to 5 days or resolve spontaneously. For subject safety and practicality, subjects are infected until the pustules become painful or ulcerate or until they have been infected for 14 days. Most subjects with pustules experience pain about 7 to 9 days after inoculation, at which time the experiment is terminated.
FIG. 1.
EDDs and probabilities of papule formation (filled circles) and 95% CI (open circles) and probabilities of pustule formation (filled triangles) and 95% CI (open triangles) as predicted by logistic regression. The papule formation rate was based on a total of 243 sites from 116 volunteers who participated in human challenge trials prior to 1 January 2001. The pustule formation rate was based on a total of 220 sites that achieved a definite outcome (pustule or resolved) from 108 of the 116 volunteers.
Although the papule formation rate in males and females is nearly identical, the pustule formation rate is dependent on both EDD (Fig. 1) and gender (16). The rate of pustule formation is statistically significantly higher in males than females (estimated odds ratio for male/female effect, 2.16; 95% confidence interval [CI], 1.08 to 4.29) (16). Thus, gender differences in susceptibility to disease progression may contribute to the high male-to-female ratios seen in naturally occurring chancroid. The usual EDDs employed in the human model are on the order of 101 to 102 CFU.
The major strength of the human model is the use of a relevant target of infection, human skin. The kinetics of papule and pustule formation resemble natural infection (7, 67), and the histopathology of experimental lesions is nearly identical to that of natural ulcers (54, 55, 63, 72, 89). However, the model can only be used to study the first 2 weeks of an infection that in nature is probably present for 3 to 6 weeks before patients seek treatment (25, 46, 67). Serum antibody responses and blastogenic responses of peripheral blood mononuclear cells to H. ducreyi, which occur late in the ulcerative stage of natural infection (25, 109), do not occur in this time frame (6, 7, 89, 90). Other limitations of the model are the artificial route of inoculation and the inability to study ulcers, lymphadenitis, mucosal infection, or infection of genital epithelium. Because infection of genital and nongenital keratinized stratified squamous epithelium occurs naturally, infection of the arm is likely a minor limitation.
BACTERIA-HOST INTERACTIONS IN EXPERIMENTAL INFECTION
The depth of the abrasion required to initiate natural infection is unknown. Most chancroid occurs on mucosal surfaces, where the epidermis is only a few cells thick and not keratinized, and very superficial abrasions would probably give the organism access to the dermis. A puncture wound is required to initiate infection in the human model, as up to 106 CFU placed on intact keratinized arm skin does not cause disease (90). How H. ducreyi interacts with the host in the experimental infection model is probably somewhat dependent on where the bacteria are delivered in the skin. The tines of the allergy testing device are 1.9 mm long, and the epidermis of fixed uninfected upper arm skin is up to 0.15 mm thick. Confocal microscopy of biopsies obtained immediately after inoculation with an EDD of 103 CFU shows that the bacteria are deposited along the length of the puncture wounds made by the allergy testing device to both the epidermis and the dermis (11). Thus, the bacteria are allowed to interact with the epidermis and dermis in the model, with the caveat that most of the inoculum is likely delivered to the dermis.
Due to the low EDD normally used in the model, bacteria cannot be seen within the first 24 h of infection (11). At 24 h, micropustules are already present in the epidermis of what clinically is a papule. By 48 h, the bacteria are seen in the epidermal micropustules and in the dermis, where they are predominantly extracellular and colocalize with polymorphonuclear leukocytes (PMNs), macrophages, collagen, and fibrin (11) (Fig. 2). At the pustular stage of disease, PMNs coalesce to form an abscess, and the macrophages form a collar at the base of the pustule (11). The relationships between H. ducreyi and PMNs, macrophages, collagen, and fibrin are maintained throughout the papular and pustular stages. The bacteria do not appear to interact with keratinocytes, fibroblasts, or dendritic cells throughout experimental infection (11, 12). Thus, evasion of phagocytosis and phagocytic killing appears to be a major mechanism of bacterial survival, while invasion of host cells is not a major feature of pathogenesis in experimental infection.
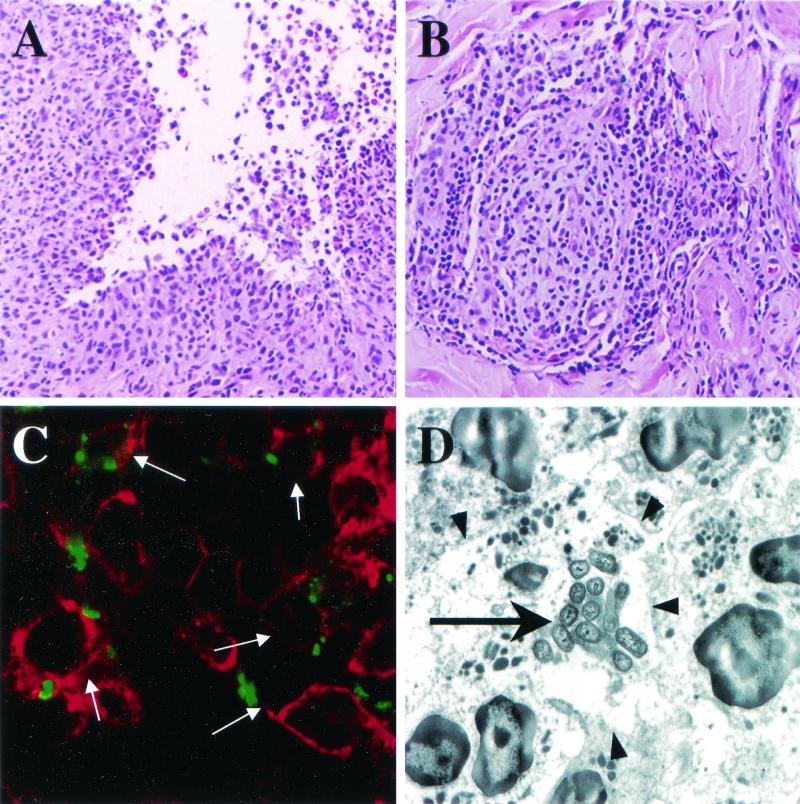
FIG. 2.
Histopathology and localization of H. ducreyi in pustules at the clinical end point. (A and B) Hematoxylin and eosin stain demonstrating the two major components of the inflammatory response, PMNs eroding through the epidermis (A) and a perivascular infiltrate of mononuclear cells (B). (C) Confocal microscopic image of tissue stained with polyclonal anti-H. ducreyi antiserum (green) and anti-CD45 monoclonal antibody (red). Arrows point to leukocytes with associated bacteria. Note that the bacteria are found on the borders of leukocytes but not within them. (D) Transmission electron micrograph of a cluster of bacteria (large arrow) surrounded by PMNs. Arrowheads point to the membranes of the PMNs. Note that the bacteria are between PMNs and near necrotic cell debris but are not engulfed by the PMNs.
In addition to the infiltrate of PMNs and macrophages that coalesce to form intraepidermal pustules, a dermal infiltrate of mononuclear cells is recruited within 24 h of infection (Fig. 2) (72). The mononuclear cells consist primarily of macrophages and T cells that are predominantly (60 to 80%) CD4+ cells of the αβ lineage and that express the memory marker CD45RO. Twenty to 40% of the T cells are CD8+, and a minor population of B cells, few NK cells, and no plasma cells are present throughout infection. Pustular lesions have increased numbers of dendritic cells in the epidermis and in hair follicles and eccrine ducts (72). This infiltrate is accompanied by HLA-DR expression on mononuclear and dendritic cells and expression of cytokine mRNAs for gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-8 (IL-8), features that resemble a delayed-type hypersensitivity (DTH) response. IL-2 mRNA is present in all lesions while IL-4 and IL-5 mRNAs are found in 66% of the lesions (72). This histopathology is nearly identical to that of natural infection except that CD4 and CD8 cells are present in equal numbers in chancroidal ulcers (1, 54, 55, 63, 72).
The fact that the mononuclear cell infiltrate in experimental infection occurs within 24 h of inoculation and resembles a DTH response was intriguing in that the volunteers have no prior history of chancroid. Before we had determined the location of the bacteria in the lesions, we had thought that the DTH response suggested the existence of an intracellular life stage, as has been noted for most other organisms that provoke a DTH response (72). We also speculated that epitopes shared by H. ducreyi and related members of the Pasteurellaceae that colonize humans elicited an infiltrate of cross-reactive memory cells to the skin (72). To determine the antigen specificity of the T cells, 21 T-cell lines were derived from biopsies of pustules obtained at the end point (42). Approximately half of the lines respond to H. ducreyi lysates, and these lines are predominantly CD4+ and produce IFN-γ or IFN-γ and IL-10 but no IL-4 or IL-5 in response to antigen. The lines show little response to antigens prepared from other members of the Pasteurellaceae and respond to different fractions of H. ducreyi whole cells separated by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The lack of cross-reactivity and the response of the lines to different antigen fractions suggest that subjects are sensitized to H. ducreyi during the course of experimental infection.
WORKING MODEL OF EXPERIMENTAL INFECTION
Based on data from the experimental infection model (11, 42, 72, 89), from observations made about H. ducreyi in vitro (49), and by analogy to other systems (9, 53, 56, 59, 60, 76, 86, 91), we now propose the following working model of experimental infection. H. ducreyi enters the skin through wounds and stimulates keratinocytes, fibroblasts, endothelial cells, melanocytes, or the immunologically reactive cells of the “dermal perivascular unit” to secrete IL-6 and IL-8 (49, 59, 72, 86). IL-8 leads to the accumulation of PMNs and macrophages within the epidermis and dermis (72), while IL-6 induces IL-2 and IL-2 receptor expression in T cells (49) and leads to recruitment of CD4 cells to the lesions (72, 89). Fibrin and collagen deposition occur as part of the normal process of wound repair (11) and provide a matrix for the infiltrating PMNs and macrophages (56). H. ducreyi lipoproteins and LOS activate macrophages to secrete IL-12 and TNF-α (72), a potent inducer of E-selectin on the endothelium, which in concert with chemokines produced by the endothelial cells and macrophages select for homing of memory (or effector) cells to the skin within 24 h of inoculation (53, 76, 91). IFN-γ (72) produced by T cells also induces E-selectin on the endothelium (53). IFN-γ and TNF-α (72) stimulate keratinocytes to produce IL-8 and other chemokines, amplifying the process (9). Immature dendritic cells are induced by inflammatory cytokines and bacterial products such as LOS to migrate to the regional lymph nodes (60), where they sensitize naive T cells to H. ducreyi antigens. H. ducreyi-specific memory T cells eventually home to the lesion (42). However, the development of an antigen-specific response does not seem to influence bacterial clearance.
The immune response to H. ducreyi has many features of a type 1 response (87), which usually facilitates phagocytosis, antibody responses, and bacterial clearance for extracellular bacterial pathogens. The antigen-specific CD4+ cells recruited to the skin (42) may eventually provide help for the development of antibody responses that usually occur late in the ulcerative stage of disease (25). The possible function of recruited CD8+ cells against this extracellular pathogen is less clear. When the PMNs and macrophages fail to clear the organism, the type 1 response is sustained, and the products released from the phagocytes probably damage the skin. Thus, experimental chancroid is an example of immunopathogenesis.
ANIMAL MODELS
Early attempts at developing animal models included intradermal injection of large doses (107 to 109 CFU) of H. ducreyi in mice and rabbits housed at room temperature (69, 107). Subsequent studies showed that the bacteria failed to replicate in these models, and disease was likely due to the LOS content of the inoculum (22). H. ducreyi does not grow at temperatures above 35°C in vitro. Housing rabbits at reduced temperatures (15 to 17°C) allows H. ducreyi to replicate (78). In the temperature-dependent rabbit model (TDRM), intradermal injections of 104 to 105 CFU in the back yield lesions within 48 h that progress to necrotic eschars within 1 week. Lesions persist up to 2 weeks before resolving. In the macaque model, 107 CFU of H. ducreyi are injected intradermally into the foreskin of male primates, who develop lesions within 1 to 2 days of infection (104). Exudative ulcers reminiscent of human chancroid develop in 1 to 2 weeks, and inguinal lymphadenopathy develops in most primates. However, female macaques challenged by injection adjacent to the vaginal opening fail to develop ulcers. Administration of exogenous iron decreases the infectious dose required for ulceration by a factor of 10 in primates (95). In the swine model, an EDD of 104 CFU of H. ducreyi is inoculated on the ears of pigs, using the allergy testing device employed in the human model (50). Papules develop within 2 days of inoculation, pustules form by 7 days, and sites ulcerate by 14 days of infection. Immunosuppression of swine with cyclophosphamide decreases ulceration, suggesting that the immune response contributes to lesion development (84). Table 1 shows a comparison of the TDRM, swine, and macaque models with the human model of infection and naturally occurring chancroid. The larger doses or EDDs required for infection in the animal models suggest that H. ducreyi is a less efficient pathogen for animals than for humans.
TABLE 1.
Comparison of experimental infection models and naturally occurring chancroid
| Model | Gender susceptibility | Site of infection | Route of infection | Dose (CFU) | Reduced temp required | Duration (wks) | Final outcome | Lymphadenopathy | Antibody response | Cutaneous response | Protection from second challenge |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Macaque | Males only | Foreskin | Intradermal | 107 | No | 3 | Wet ulcer | Yes | Yes | PMNs; lymphocytes, plasma cells, macrophages | NR |
| TDRM | NRa | Back | Intradermal | 104-105 | Yes | 2 | Eschar | No | Yes | PMNs, lymphocytes, plasma cells | Yes |
| Swine | NR | Ear | Puncture | 104 (EDD) | No | 4 | Dry ulcer | No | Yes | PMNs, T cells, macrophages | No |
| Human | Bothb | Arm | Puncture | 1-100 (EDD) | No | 2 | Pustule | No | No | PMNs, T cells, macrophages | No |
| Natural disease | Bothc | Genital skin and mucosa; nongenital skin | Sexually transmitted | Unknown | No | Weeks to months | Wet ulcer | 10-50% | Yes (late in ulcerative stage) | PMNs, T cells, macrophages | No |
NR, not reported.
Higher pustule formation rate was seen in males than in females.
Higher prevalence is seen in males than in females.
PMNs and lymphocytes are recruited to infected sites in the TDRM, macaque, and swine models (50, 78, 104). In contrast to the human model and to most recent studies of natural infection (1, 54, 55, 63, 72), numerous plasma cells are present in ulcers of the macaque model. Few plasma cells are present in ulcers from the TDRM. The presence or absence of B and plasma cells has not been determined in the swine model (50). Numerous T cells are seen in lesions from the swine model and in the TDRM, provided that rabbits are immunized with adjuvants or candidate vaccines prior to challenge (30). Macrophages are present in the swine and macaque models but their presence is not reported in the TDRM. The overall histopathology of lesions in the swine model is most similar to human experimental infection (50, 89), perhaps in part because the bacteria are inoculated in the same manner in these two models.
All three species of animals develop serum antibodies to H. ducreyi antigens within 1 to 2 weeks of infection (50, 78, 104). No serum antibody response develops after 2 weeks of human experimental infection, even in subjects who are challenged twice (6, 72, 89). Antibody responses in naturally infected patients seem to develop after 3 weeks of ulceration (25). Thus, serum antibody responses appear to be delayed in humans relative to the experimental animal models.
There is little evidence that natural infection confers protective immunity to H. ducreyi in that patients may be infected repeatedly (15, 46, 67). In small studies, neither humans nor swine are protected from rechallenge with the homologous strain after experimental infection (6, 50). Rabbits are protected from subsequent homologous strain challenge after one round of infection (47); this issue has not been addressed in macaques.
By immunoelectron microscopy, H. ducreyi are present in ulcers in the swine model (84). The bacteria are rare and found primarily in and near necrotic macrophages, PMNs, and keratinocytes. No localization studies have been reported with the macaque model or TDRM. Thus, few comparisons can be made between the animal models and the human experimental model in terms of host-bacterial interactions.
Several vaccine trials have been performed in the TDRM. Partial protection against subsequent challenge is seen with the recombinant D15 antigen, purified pilus (FtpA), recombinant hemolysin, and outer membrane vesicles (30, 33, 47, 100). LOS affords no protection (30). The mechanism(s) of protection for these vaccines in the TDRM is not yet established, and the significance of these findings for human disease is unclear.
A point of confusion in the literature regarding the human and animal models is that different criteria are used to define disease. In the human model, papules, pustules, and ulcers are determined by the clinical appearance of the lesions, a necessity in a clinical trial (72, 89, 90). Thus, the papule and pustule formation rates reported for the human model are clinical outcomes. The TDRM generally uses an integer scoring system ranging from 0 (no disease) to 4 (necrosis or ulcer) and also measures clinical outcomes (30, 78). Outcomes in the swine model are measured by a histologic scoring system ranging from 0 (no disease) to 5 (ulceration or epidermal necrosis and dermal erosion accompanied by confluence of immune cells) (85). Lesions in the swine model may achieve a score of 5 between days 2 and 14 (50, 84). A criticism of the human model is that it studies only the early stages of infection. However, pustules in the human model, which clinically appear as early as 2 days after inoculation, histologically resemble ulcers in the swine scoring system.
ROLE OF BACTERIAL COMPONENTS IN DISEASE
To test the role of putative virulence determinants in the human model, we performed several mutant-parent comparison trials in the 35000 or 35000HP background. In these trials, subjects are inoculated with multiple doses of the parent on one arm and an isogenic mutant on the other arm and serve as their own controls. A group of subjects is usually challenged with an EDD of the parent that causes a pustule formation rate of 70% (approximately 70 CFU) and with twofold serial dilutions of the mutant that span the parent dose (140, 70, and 35 CFU). If we observe similar pustule formation rates at sites infected with both the mutant and the parent, we repeat the experiment. If the results are confirmed, we conclude that there is no major difference in the virulence of the mutant and the parent and terminate the trial. If pustules do not develop at sites inoculated with the mutant, we increase the dose of the mutant in the next group(s) of subjects until the EDD of the mutant is at least 10-fold higher than that of the parent. These trials are usually accomplished with six to nine subjects and are not powered to detect a partial role of a virulence determinant in pustule formation.
We have completed 12 isogenic mutant-parent comparisons in the human model. Mutants that lack the hemoglobin receptor (HgbA), peptidoglycan-associated lipoprotein (PAL), or an OMP that is the major known determinant of serum resistance (DsrA) form papules at rates similar to those of the parent but are attenuated in pustule formation (4, 17, 40) (Table 2). Surprisingly, mutants that do not make hemolysin, CDT, both hemolysin and CDT, sialylated or paragloboside-like LOS, the major outer membrane protein (MOMP), fine tangled pili (FtpA), and superoxide dismutase C form papules and pustules at rates similar to those of the parent (3, 16a, 73, 101, 113-115). Thus, the human model can discriminate between virulent and attenuated isolates, but many putative virulence determinants are not required for pustule formation in experimental infection.
TABLE 2.
Mutants that are attenuated in the human model
| Virulence determinant | Dose range (CFU) | No. of sites | Formation rate (% [P value])a
|
Source | Reference | |
|---|---|---|---|---|---|---|
| Papule | Pustule | |||||
| Hemoglobin receptor | Elkins | 5 | ||||
| Parent | 48-60 | 18 | 89 | 55 | ||
| Mutant | 20-500 | 27 | 89 (1.0) | 0 (<0.0001) | ||
| PAL | Spinola | 40 | ||||
| Parent | 41-89 | 18 | 100 | 72 | ||
| Mutant | 28-800 | 27 | 93 (0.36) | 11 (<0.0001) | ||
| DsrA | Elkins | 17 | ||||
| Parent | 70-80 | 12 | 92 | 58 | ||
| Mutant | 35-800 | 18 | 67 (0.12) | 0 (0.0004) | ||
For simplicity, the 95% CI for papule and pustule formation rates are not shown.
Are the results of these trials consistent with the working model of pathogenesis? The DsrA mutant is highly sensitive to the killing of normal human serum (36) and may have been killed by serum that transudates into the wound. The data suggest that the hemoglobin receptor is required for efficient heme transport in vivo and that in the absence of HgbA, the organism was likely starved for heme and/or iron and then died. These mutants are cleared, and the recruitment of inflammatory cells to the skin is not sustained. PAL is a major lipoprotein in the H. ducreyi outer membrane, and the PAL mutant has an unstable outer membrane (40). Lipoproteins usually have major proinflammatory effects, including the ability to initiate innate and adaptive immunity through activation of Toll-like receptors on macrophages (19, 62, 66). The PAL mutant may have been attenuated because it was structurally less fit to evade the host response and/or because it may not elicit as vigorous an inflammatory response as the parent.
Why did so many putative virulence determinants have little role in experimental human infection? An obvious reason is that the organism has redundant virulence mechanisms. For example, H. ducreyi expresses two OmpA homologues, called MOMP and OmpA2 (57), and lack of expression of MOMP may not have been sufficient to affect virulence. A second possibility is that a particular virulence determinant may not be required for pustule formation but is required at a later stage of infection, which cannot be studied in the human model. Alternatively, the bacteria are forcibly introduced by the tines of the allergy testing device into the skin. The function of a candidate adhesin, such as a pilus, may be masked by the route of inoculation (3).
There are several discrepancies between the human challenge model and in vitro models that are used to identify candidate virulence determinants. In most in vitro assays, 105 to 107 CFU are usually allowed to interact with 105 eukaryotic cells. In the human model, subjects are usually inoculated with less than 100 CFU. The in vitro models may be showing the effects of pharmacological doses of the organism relative to the physiological doses that cause human infection. Experimental human infection also seems to utilize host cell targets in a way that is different from in vitro models. For example, the paragloboside residues of H. ducreyi LOS mediate attachment to and invasion of keratinocytes (43), and CDT and hemolysin have cytopathic effects for epithelial cells or fibroblasts in vitro (27, 28, 70, 112). In the human challenge model, neither keratinocytes nor fibroblasts seem to be a major target of bacterial attachment or invasion. The bacteria are quickly surrounded by PMNs and macrophages within 24 to 48 h of inoculation, and rapid formation of micropustules may effectively preclude the bacteria from major interactions with keratinocytes and fibroblasts during pustule formation. CDT and hemolysin cause lymphocyte death in vitro (41, 98, 112). However, CDT does not affect the metabolic activity of PMNs or the phagocytic capacity of PMNs (98), and hemolysin does not lyse PMNs (112). If the primary strategy for bacterial survival in the model is evasion of phagocytosis and the lymphocytic response does not greatly influence bacterial clearance, an isolate that cannot make CDT and/or hemolysin would not be impaired in its ability to cause papules and pustules.
Due to subject safety considerations, the experimental model cannot address the potential role of any putative virulence determinants at the ulcerative stage of disease. For example, it is possible that CDT and hemolysin contribute to the chronic nature of the chancroidal ulcer by killing fibroblasts and epithelial cells, which are intimately involved in wound healing.
Isogenic mutants have also been tested for virulence in the TDRM and swine models of infection. To our knowledge, eight isogenic mutants tested in the human model have also been tested in the TDRM (ftpA, lbgB/losB), the swine model (cdtC hhdB double mutant, dsrA, sodC), or both animal models (cdtC, hhdB, hgbA/hupA). The results in the animal models are in general consistent with those seen in the human model (3, 4, 16a, 33, 73, 85, 92-94, 114, 115; Thomas C. Kawula, personal communication; I. Leduc, D. W. Cameron, and S. M. Spinola, Program Abstr. 12th Meet. Int. Soc. Sex. Transm. Dis. Res., abstr. P386, p. 126, 1997). However, the sodC mutant, which is attenuated for survival in swine (85), is not attenuated in the human model for either pustule development or bacterial survival (16a). As stated earlier, H. ducreyi does not seem to be as efficient a pathogen for animals as for humans, and the mutant-parent comparison trials in the human model are not designed to detect partial contributions to pustule formation. A relatively small decrease in virulence may lead to a more noticeable pathogenic change in the animal models.
FUTURE DIRECTIONS
Although H. ducreyi is extracellular in the human model, we do not know if the relationships established in experimental infection are true for natural infection. Bacterial localization studies have not been done in the available animal models, and we do not know the extent to which these models resemble experimental or natural human infection. Thus, it is critical to examine biopsies from patients with culture-proven chancroid to determine the relationship between H. ducreyi and the host at the ulcerative stage and to do similar studies in the animal models. These data will help put the experimental models in perspective and aid in the development of relevant in vitro models.
Several of the steps outlined in the working model of experimental infection are hypothetical. We are presently determining which chemokine-chemokine receptor pathways are responsible for homing of both naive and memory cells to sites of experimental infection. We are especially interested in determining if the coreceptors for HIV entry into CD4-positive cells, CCR5 and CXCR4, are upregulated on T cells and macrophages that infiltrate experimental lesions. We are also pursuing detailed cytokine analysis on the single-cell level to define the nature of the activation state of the CD4 and CD8 cells within lesions. The study of the host response to experimental H. ducreyi infection should give insights into basic mechanisms of pathogen-induced inflammation in the skin.
A potential use of the human challenge model will be to determine the effects of in vivo growth on bacterial gene transcription during human infection. In vivo, H. ducreyi has a minimal doubling time of 16.5 + 3.8 h (102). After 10 to 12 bacterial generations, approximately 105 CFU are present in pustules. Bacterial transcripts consistently can be amplified by reverse transcription-PCR from samples containing 102 CFU (102). Amplification techniques, such as selective capture of transcribed sequences (44), together with the genome sequence of 35000HP will allow us to examine whether specific H. ducreyi genes are differentially transcribed in vivo and may provide additional insights into H. ducreyi virulence as well as the function of the numerous unannotated genes in the genome.
Importantly, H. ducreyi is surrounded by PMNs and macrophages but is able to resist phagocytosis in experimental infection (11, 12). Resistance does not appear to be mediated by the major LOS glycoforms, since a mutant whose LOS consists only of the heptose trisaccharide core and 2-keto-3-deoxyoctulosonic acid (KDO) is fully virulent in the human challenge model (113). However, while the H. ducreyi glycosyltransferases responsible for synthesis of all of the LOS glycoforms visible on silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels have been identified (10, 18, 37, 43, 93, 97; S. Sun, N. K. Scheffler, B. W. Gibson, J. Wang, and R. S. Munson, Jr., submitted for publication), there are several homologues of H. influenzae lsg glycosyltransferase genes (2, 75, 88) present in the genome. It has been proposed that H. ducreyi produces a loose capsular structure (8), but there is no capsule-like gene cluster in the genome sequence and it is unlikely that H. ducreyi elaborates a classical capsule. Many of the genes responsible for the synthesis of the enterobacterial common antigen-like polysaccharide (79) are present in the H. ducreyi genome. It will be interesting to determine whether the carbohydrate products produced by these newly identified glycosyltransferases play a role in resistance to phagocytosis by PMNs.
Given the data from the human challenge trials, there should be some optimism about the prospects for vaccine development. Antibodies that are bactericidal or promote opsonophagocytosis may afford protection against infection. Although the organism seems to have prevented the host from developing an effective immune response, the challenge will be to select immunogens that evoke responses that lead to organism clearance and protection from experimental challenge. If such immunogens are identified, they can be subsequently tested in the field.
Acknowledgments
This work was supported by grants AI31494 and AI27863 (to S.M.S.) and AI38444 and AI45091 (to R.S.M.) from the National Institutes of Allergy and Infectious Diseases (NIAID). M.E.B. was supported by Public Health Service grant AI09971 from the NIAID. The human challenge trials were also supported by grant MO1RR00750 to the GCRC at Indiana University and the Sexually Transmitted Diseases Clinical Trials Unit through contract NO1-AI75329 from the NIAID.
We thank Barry Katz and Jaroslaw Harezlack for the analyses of papule and pustule formation rates; Brad Allen, Tie Chen, Tricia Humphreys, Ray Johnson, Kevin Mason, and Will Ray for their thoughtful criticism of the manuscript; our collaborators, trainees, and technicians who contributed to this work; and the volunteers who participated in the human challenge trials.
Editor: D. A. Portnoy
Footnotes
Dedicated to the memory of Floyd W. Denny, Jr.
REFERENCES
- 1.Abeck, D., A. L. Freinkel, H. C. Korting, R. M. Szeimis, and R. C. Ballard. 1997. Immunohistochemical investigations of genital ulcers caused by Haemophilus ducreyi. Int. J. STD AIDS 8:585-588. [DOI] [PubMed] [Google Scholar]
- 2.Abu Kwaik, Y., R. E. McLaughlin, M. A. Apicella, and S. M. Spinola. 1991. Analysis of Haemophilus influenzae type b lipooligosaccharide-synthesis genes that assemble or expose a 2-keto-3-deoxyoctulosonic acid epitope. Mol. Microbiol. 5:2475-2480. [DOI] [PubMed] [Google Scholar]
- 3.Albritton, W. L. 1989. Biology of Haemophilus ducreyi. Microbiol. Rev. 53:377-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Tawfiq, J. A., M. E. Bauer, K. R. Fortney, B. P. Katz, A. F. Hood, M. Ketterer, M. A. Apicella, and S. M. Spinola. 2000. A pilus-deficient mutant of Haemophilus ducreyi is virulent in the human model of experimental infection. J. Infect. Dis. 181:1176-1179. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq, J. A., K. R. Fortney, B. P. Katz, C. Elkins, and S. M. Spinola. 2000. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 181:1049-1054. [DOI] [PubMed] [Google Scholar]
- 6.Al-Tawfiq, J. A., J. Harezlak, B. P. Katz, and S. M. Spinola. 2000. Cumulative experience with Haemophilus ducreyi in the human model of experimental infection. Sex. Transm. Dis. 27:111-114. [DOI] [PubMed] [Google Scholar]
- 7.Al-Tawfiq, J. A., K. L. Palmer, C.-Y. Chen, J. C. Haley, B. P. Katz, A. F. Hood, and S. M. Spinola. 1999. Experimental infection of human volunteers with Haemophilus ducreyi does not confer protection against subsequent challenge. J. Infect. Dis. 179:1283-1287. [DOI] [PubMed] [Google Scholar]
- 8.Al-Tawfiq, J. A., A. C. Thornton, B. P. Katz, K. R. Fortney, K. D. Todd, A. F. Hood, and S. M. Spinola. 1998. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 178:1684-1687. [DOI] [PubMed] [Google Scholar]
- 9.Barker, J. N., V. Sarma, R. S. Mitra, R. S. Dixit, and B. J. Nickoloff. 1990. Marked synergism between tumor necrosis factor-alpha and interferon-gamma in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J. Clin. Investig. 85:605-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer, B. A., S. R. Lumbley, and E. J. Hansen. 1999. Characterization of a waaF (RfaF) homolog expressed by Haemophilus ducreyi. Infect. Immun. 67:899-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer, M. E., and S. M. Spinola. 2000. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect. Immun. 68:2309-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behets, F. M.-T., J. Andriamiadana, D. Randrianasolo, R. Randriamanga, D. Rasamilalao, C.-Y. Chen, J. B. Weiss, S. A. Morse, G. Dallabetta, and M. S. Cohen. 1999. Chancroid, primary syphilis, genital herpes, and lymphogranuloma venereum in Antananarivo, Madagascar. J. Infect. Dis. 180:1382-1385. [DOI] [PubMed] [Google Scholar]
- 14.Behets, F. M.-T., A. R. Brathwaite, T. Hylton-Kong, C.-Y. Chen, I. Hoffman, J. B. Weiss, S. A. Morse, G. Dallabetta, M. S. Cohen, and J. P. Figueroa. 1999. Genital ulcers: etiology, clinical diagnosis, and associated human immunodeficiency virus infection in Kingston, Jamaica. Clin. Infect. Dis. 28:1086-1090. [DOI] [PubMed] [Google Scholar]
- 15.Blackmore, C. A., K. Limpakarnjanarat, J. G. Rigau-Perez, W. L. Albritton, and J. R. Greenwood. 1985. An outbreak of chancroid in Orange County, California: descriptive epidemiology and disease-control measures. J. Infect. Dis. 151:840-844. [DOI] [PubMed] [Google Scholar]
- 16.Bong, C. T. H., J. Harezlak, B. P. Katz, and S. M. Spinola. 2002. Men are more susceptible to pustule formation than women in the experimental model of Haemophilus ducreyi infection. Sex. Transm. Dis. 29:114-118. [DOI] [PubMed] [Google Scholar]
- 16a.Bong, C. T. H., K. R. Fortney, B. P. Katz, A. F. Hood, L. R. San Mateo, T. H. Kawula, and S. M. Spinola. 2002. The superoxide dismutase C mutant of Haemophilus ducreyi is virulent in human volunteers. Infect. Immun. 70:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bong, C. T. H., R. E. Throm, K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2001. A DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 69:1488-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozue, J. A., M. V. Tullius, J. Wang, B. W. Gibson, and R. S. Munson, Jr. 1999. Haemophilus ducreyi produces a novel sialyltransferase: identification of the sialyltransferase gene and construction of mutants deficient in the production of the sialic acid-containing glycoform of the lipooligosaccharide. J. Biol. Chem. 274:4106-4114. [DOI] [PubMed] [Google Scholar]
- 19.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R.-B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 20.Brunham, R. C., and A. R. Ronald. 1991. Epidemiology of sexually transmitted diseases in developing countries, p. 61-80. In J. N. Wasserheit, S. O. Aral, K. K. Holmes, and P. J. Hitchcock (ed.), Research issues in human behavior and sexually transmitted diseases in the AIDS era. American Society for Microbiology, Washington, D.C.
- 21.Burrows, L. L., E. Olah-Winfield, and R. Y. Lo. 1993. Molecular analysis of the leukotoxin determinants from Pasteurella hemolytica serotypes 1 to 16. Infect. Immun. 61:5001-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campagnari, A. A., L. M. Wild, G. E. Griffiths, R. J. Karalus, M. A. Wirth, and S. M. Spinola. 1991. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect. Immun. 59:2601-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casin, I., F. Grimont, P. A. D. Grimont, and M.-J. Sanson-Le Pors. 1985. Lack of deoxyribonucleic acid relatedness between Haemophilus ducreyi and other Haemophilus species. Int. J. Syst. Bacteriol. 35:23-25. [Google Scholar]
- 24.Centers for Disease Control and Prevention. 2000. Summary of notifiable diseases, United States, 1999. Morb. Mortal. Wkly. Rep. 48(53):84. [Google Scholar]
- 25.Chen, C.-Y., K. J. Mertz, S. M. Spinola, and S. A. Morse. 1997. Comparison of enzyme immunoassays for antibodies to Haemophilus ducreyi in a community outbreak of chancroid in the United States. J. Infect. Dis. 175:1390-1395. [DOI] [PubMed] [Google Scholar]
- 26.Chen, C. Y., R. C. Ballard, C. M. Beck-Sague, Y. Dangor, F. Radebe, S. Schmid, J. B. Weiss, V. Tshabalala, G. Fehler, Y. Htun, and S. A. Morse. 2000. Human immunodeficiency virus infection and genital ulcer disease in South Africa. The herpetic connection. Sex. Transm. Dis. 27:21-29. [DOI] [PubMed] [Google Scholar]
- 27.Cope, L. D., S. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purven, R. S. Munson, Jr., T. Lagergard, J. D. Radolf, and E. J. Hansen. 1997. A diffusible cytotoxin of Haemophilus ducreyi. Proc. Natl. Acad. Sci. USA 94:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes-Bratti, X., E. Chaves-Olarte, T. Lagergard, and M. Thelestam. 1999. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J. Clin. Investig. 103:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Ley, J., W. Mannheim, R. Mutters, K. Piechulla, R. Tytgat, P. Segars, M. Bisgaard, W. Frederikson, K.-H. Hinz, and M. Vanhoucke. 1990. Inter- and intrafamilial similarities of the rRNA cistrons of the Pasteurellaceae. Int. J. Syst. Bacteriol. 40:126-137. [DOI] [PubMed] [Google Scholar]
- 30.Desjardins, M., L. Filion, S. Robertson, L. Kobylinski, and D. Cameron. 1996. Evaluation of humoral and cell-mediated inducible immunity to Haemophilus ducreyi in an animal model of chancroid. Infect. Immun. 64:1778-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewhirst, F. E., B. I. Paster, I. Olsen, and G. J. Fraser. 1992. Phylogeny of 54 representative strains of species in the family Pasteurellaceae as determined by comparison of 16S rRNA sequences. J. Bacteriol. 174:2002-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dienst, R. B. 1948. Virulence and antigenicity of Haemophilus ducreyi. Am. J. Syph. Gon. Ven. Dis. 32:289-291. [PubMed] [Google Scholar]
- 33.Dutro, S. M., G. E. Wood, and P. A. Totten. 1999. Prevalence of, antibody response to, and immunity induced by Haemophilus ducreyi hemolysin. Infect. Immun. 67:3317-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dyer, J. R., J. J. Eron, I. F. Hoffman, P. Kazembe, P. L. Vernazza, E. Nkata, C. C. Daly, S. A. Fiscus, and M. S. Cohen. 1998. Association of CD4 cell depletion and elevated blood and seminal plasma human immunodeficiency virus type 1 (HIV-1) RNA concentrations with genital ulcer disease in HIV-1-infected men in Malawi. J. Infect. Dis. 177:224-227. [DOI] [PubMed] [Google Scholar]
- 35.Elkins, C., C.-J. Chen, and C. E. Thomas. 1995. Characterization of the hgbA locus encoding a hemoglobin receptor from Haemophilus ducreyi. Infect. Immun. 63:2194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkins, C., K. J. Morrow, and B. Olsen. 2000. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect. Immun. 68:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filiatrault, M. J., B. W. Gibson, B. Schilling, S. Sun, R. S. Munson, Jr., and A. A. Campagnari. 2000. Construction and characterization of Haemophilus ducreyi lipooligosaccharide (LOS) mutants defective in expression of heptosyltransferase III and beta-1,4-glucosyltransferase: identification of LOS glycoforms containing lactosamine repeats. Infect. Immun. 68:3352-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. Tomb, B. A. Dougherty, J. M. Merrick, K. McKenny, G. Sutton, W. FitzHugh, C. Fields, J. D. Gocayne, J. Scott, R. Shirley, L. Liu, A. Glodek, J. M. Kelly, J. F. Weidman, C. A. Phillips, T. Spriggs, E. Hedblom, M. D. Cotton, T. R. Utterback, M. C. Hanna, D. T. Nguyen, D. M. Saudek, R. C. Brandon, L. D. Fine, J. L. Fritchman, J. L. Fuhrmann, N. S. M. Geoghagen, C. L. Gnehm, L. A. McDonald, K. V. Small, C. M. Fraser, H. O. Smith, and C. J. Venter. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 39.Fleming, D. T., and J. N. Wasserheit. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75:3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fortney, K. R., R. S. Young, M. E. Bauer, B. P. Katz, A. F. Hood, R. S. Munson, Jr., and S. M. Spinola. 2000. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelfanova, V., E. J. Hansen, and S. M. Spinola. 1999. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect. Immun. 67:6394-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelfanova, V., T. L. Humphreys, and S. M. Spinola. 2001. Characterization of Haemophilus ducreyi-specific T-cell lines from lesions of experimentally infected human subjects. Infect. Immun. 69:4224-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibson, B. W., A. A. Campagnari, W. Melaugh, N. J. Phillips, M. A. Apicella, S. Grass, J. Wang, K. L. Palmer, and R. S. Munson, Jr. 1997. Characterization of a transposon Tn916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J. Bacteriol. 179:5062-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 96:11554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenblatt, R. B., E. S. Sanderson, H. S. Kupperman, Q. Hair, and P. Fried. 1944. The experimental prophylaxis of chancroid disease-II. Am. J. Syph. Gon. Ven. Dis. 28:165-178. [Google Scholar]
- 46.Hammond, G. W., M. Slutchuk, J. Scatliff, E. Sherman, J. C. Wilt, and A. R. Ronald. 1980. Epidemiologic, clinical, laboratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev. Infect. Dis. 2:867-879. [DOI] [PubMed] [Google Scholar]
- 47.Hansen, E. J., S. R. Lumbley, J. A. Richardson, B. K. Purcell, M. K. Stevens, L. D. Cope, J. Datte, and J. D. Radolf. 1994. Induction of protective immunity to Haemophilus ducreyi in the temperature-dependent rabbit model of experimental chancroid. J. Immunol. 152:184-192. [PubMed] [Google Scholar]
- 48.Hayes, R. J., K. F. Schulz, and F. A. Plummer. 1995. The cofactor effect of genital ulcers on the per-exposure risk of HIV transmission in sub-Saharan Africa. J. Trop. Med. Hyg. 98:1-8. [PubMed] [Google Scholar]
- 49.Hobbs, M. M., T. R. Paul, P. B. Wyrick, and T. H. Kawula. 1998. Haemophilus ducreyi infection causes basal keratinocyte cytotoxicity and elicits a unique cytokine induction pattern in an in vitro human skin model. Infect. Immun. 66:2914-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hobbs, M. M., L. R. San Mateo, P. E. Orndorff, G. Almond, and T. H. Kawula. 1995. Swine model of Haemophilus ducreyi infection. Infect. Immun. 63:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jessamine, P. G., and A. R. Ronald. 1990. Chancroid and the role of genital ulcer disease in the spread of human retrovirus. Med. Clin. N. Am. 74:1417-1431. [DOI] [PubMed] [Google Scholar]
- 52.Kaul, R., J. Kimani, N. J. D. Nagelkerke, F. A. Plummer, J. J. Bwayo, R. C. Brunham, E. N. Ngugi, and A. Ronald. 1997. Risk factors for genital ulcerations in Kenyan sex workers. Sex. Transm. Dis. 24:387-392. [DOI] [PubMed] [Google Scholar]
- 53.Kelly, K. A. 1997. Modulation of leukocyte-endothelial cell interactions by infectious agents. Bull. Inst. Pasteur 95:147-159. [Google Scholar]
- 54.King, R., S. H. Choudhri, J. Nasio, J. Gough, N. J. D. Nagelkerke, F. A. Plummer, J. O. Ndinya-Achola, and A. R. Ronald. 1998. Clinical and in situ cellular responses to Haemophilus ducreyi in the presence or absence of HIV infection. Int. J. STD AIDS 9:531-536. [DOI] [PubMed] [Google Scholar]
- 55.King, R., A. Gough, J. Nasio, F. Ndinya-Achola, F. Plummer, and J. Wilkins. 1996. An immunohistochemical analysis of naturally occurring chancroid. J. Infect. Dis. 174:427-430. [DOI] [PubMed] [Google Scholar]
- 56.Kirsner, R. S., and W. H. Eaglstein. 1993. The wound healing process. Dermatol. Clinics 11:629-640. [PubMed] [Google Scholar]
- 57.Klesney-Tait, J., T. J. Hiltke, I. Maciver, S. M. Spinola, J. D. Radolf, and E. J. Hansen. 1997. The major outer membrane protein of Haemophilus ducreyi consists of two OmpA homologs. J. Bacteriol. 179:1764-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kreiss, J. K., R. Coombs, F. Plummer, K. K. Holmes, B. Nikora, W. Cameron, E. Ngugi, J. O. Ndinya-Achola, and L. Corey. 1989. Isolation of human immunodeficiency virus from genital ulcers in Nairobi prostitutes. J. Infect. Dis. 160:380-384. [DOI] [PubMed] [Google Scholar]
- 59.Kupper, T. S. 1990. Immune and inflammatory processes in cutaneous tissues. J. Clin. Investig. 86:1783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanzavecchia, A., and F. Sallusto. 2000. Dynamics of T lymphocyte responses: intermediates, effectors and memory cells. Science 290:92-97. [DOI] [PubMed] [Google Scholar]
- 61.Lewis, D. A., J. Klesney-Tait, S. R. Lumbley, C. K. Ward, J. L. Latimer, C. A. Ison, and E. J. Hansen. 1999. Identification of the znuA-encoded periplasmic zinc transport protein of Haemophilus ducreyi. Infect. Immun. 67:5060-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 63.Magro, C. M., A. N. Crowson, M. Alfa, A. Nath, A. Ronald, J. O. Ndinya-Achola, and J. Nasio. 1996. A morphological study of penile chancroid lesions in human immunodeficiency virus (HIV)-positive and -negative African men with a hypothesis concerning the role of chancroid in HIV transmission. Hum. Pathol. 27:1066-1070. [DOI] [PubMed] [Google Scholar]
- 64.May, B. J., Q. Zhang, L. L. Li, M. L. Paustian, T. S. Whittam, and V. Kapur. 2001. Complete genome sequence of Pasteurella multocida, Pm70. Proc. Natl. Acad. Sci. USA 98:3460-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mertz, K. J., D. Trees, W. C. Levine, J. S. Lewis, B. Litchfield, K. S. Pettus, S. A. Morse, M. E. St. Louis, J. B. Weiss, J. Schwebke, J. Dickes, R. Kee, J. Reynolds, D. Hutcheson, D. Green, I. Dyer, G. A. Richwald, J. Novotny, I. Weisfuse, M. Goldberg, J. A. O'Donnell, and R. Knaup. 1998. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. J. Infect. Dis. 178:1795-1798. [DOI] [PubMed] [Google Scholar]
- 66.Modlin, R. L., H. D. Brightbill, and P. J. Godowski. 1999. The toll of innate immunity on microbial pathogens. N. Engl. J. Med. 340:1834-1835. [DOI] [PubMed] [Google Scholar]
- 67.Morse, S. A. 1989. Chancroid and Haemophilus ducreyi. Clin. Microbiol. Rev. 2:137-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morse, S. A., D. L. Trees, Y. Htun, F. Radebe, K. A. Orle, Y. Dangor, C. M. Beck-Sague, S. Schmid, G. Fehler, J. B. Weiss, and R. C. Ballard. 1997. Comparison of clinical diagnosis and standard laboratory and molecular methods for the diagnosis of genital ulcer disease in Lesotho: association with human immunodeficiency virus infection. J. Infect. Dis. 175:583-589. [DOI] [PubMed] [Google Scholar]
- 69.Odumeru, J. A., G. M. Wiseman, and A. R. Ronald. 1984. Virulence factors of Haemophilus ducreyi. Infect. Immun. 43:607-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palmer, K. L., W. E. Goldman, and R. S. Munson, Jr. 1996. An isogenic hemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol. Microbiol. 21:13-19. [DOI] [PubMed] [Google Scholar]
- 71.Palmer, K. L., and R. S. Munson, Jr. 1995. Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol. Microbiol. 18:821-830. [DOI] [PubMed] [Google Scholar]
- 72.Palmer, K. L., C. T. Schnizlein-Bick, A. Orazi, K. John, C.-Y. Chen, A. F. Hood, and S. M. Spinola. 1998. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J. Infect. Dis. 178:1688-1697. [DOI] [PubMed] [Google Scholar]
- 73.Palmer, K. L., A. C. Thornton, K. R. Fortney, A. F. Hood, R. S. Munson, Jr., and S. M. Spinola. 1998. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyi infection. J. Infect. Dis. 178:191-199. [DOI] [PubMed] [Google Scholar]
- 74.Petersen, S. K. 1990. The complete nucleotide sequence of Pasteurella multocida toxin gene and evidence for transcriptional repressor, TxaR. Mol. Microbiol. 4:821-830. [DOI] [PubMed] [Google Scholar]
- 75.Phillips, N. J., T. J. Miller, J. J. Engstrom, W. Melaugh, R. McLaughlin, M. A. Apicella, and B. W. Gibson. 2000. Characterization of chimeric lipopolysaccharides from Escherichia coli JM109 transformed with lipooligosaccharide synthesis genes (lsg) from Haemophilus influenzae. J. Biol. Chem. 275:4747-4758. [DOI] [PubMed] [Google Scholar]
- 76.Picker, L. J., J. R. Treer, B. Ferguson-Darnell, P. A. Collins, P. R. Bergstresser, and L. W. M. M. Terstappen. 1993. Control of lymphocyte recirculation in man. J. Immunol. 150:1122-1136. [PubMed] [Google Scholar]
- 77.Plummer, F. A., H. Nsanze, P. Karasira, L. J. D'Costa, J. Dylewski, and A. R. Ronald. 1983. Epidemiology of chancroid and Haemophilus ducreyi in Nairobi, Kenya. Lancet ii:1293-1295. [DOI] [PubMed] [Google Scholar]
- 78.Purcell, B. K., J. A. Richardson, J. D. Radolf, and E. J. Hansen. 1991. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J. Infect. Dis. 164:359-367. [DOI] [PubMed] [Google Scholar]
- 79.Rick, P. D., and R. P. Silver. 1996. Enterobacterial common antigen and capsular polysaccharides, p. 104-122. In F. C. Niedhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 80.Risbud, A., K. Chan-Tack, D. Gadkari, R. R. Gangakhedkar, M. E. Shepherd, R. Bollinger, S. Mehendale, C. Gaydos, A. Divekar, A. Rompalo, and T. C. Quinn. 1999. The etiology of genital ulcer disease by multiplex polymerase chain reaction and relationship to HIV infection among patients attending sexually transmitted disease clinics in Pune, India. Sex. Transm. Dis. 26:55-62. [DOI] [PubMed] [Google Scholar]
- 81.Robinson, N. J., D. W. Mulder, B. Auvert, and R. J. Hayes. 1997. Proportion of HIV infections attributable to other sexually transmitted diseases in a rural Ugandan population: simulation model estimates. Int. J. Epidemiol. 26:180-189. [DOI] [PubMed] [Google Scholar]
- 82.Royce, R. A., A. Sena, W. Cates, and M. S. Cohen. 1997. Current concepts: sexual transmission of HIV. N. Engl. J. Med. 336:1072-1078. [DOI] [PubMed] [Google Scholar]
- 83.San Mateo, L. R., M. M. Hobbs, and T. H. Kawula. 1998. Periplasmic copper-zinc superoxide dismutase protects Haemophilus ducreyi exogenous superoxide. Mol. Microbiol. 27:391-404. [DOI] [PubMed] [Google Scholar]
- 84.San Mateo, L. R., K. L. Toffer, P. E. Orndorff, and T. H. Kawula. 1999. Neutropenia restores virulence to an attenuated Cu,Zn-superoxide dismutase-deficient Haemophilus ducreyi strain in the swine model of chancroid. Infect. Immun. 67:5345-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.San Mateo, L. R., K. L. Toffer, P. E. Orndorff, and T. H. Kawula. 1999. Immune cells are required for cutaneous ulceration in a swine model of chancroid. Infect. Immun. 67:4963-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schroder, J.-M. 1995. Cytokine networks in the skin. J. Investig. Dermatol. 105(Suppl.):20S-24S. [PubMed] [Google Scholar]
- 87.Spellburg, B., and J. E. Edwards, Jr. 2001. Type1/type2 immunity in infectious diseases. Clin. Infect. Dis. 32:76-102. [DOI] [PubMed] [Google Scholar]
- 88.Spinola, S. M., Y. Abu Kwaik, A. J. Lesse, A. A. Campagnari, and M. A. Apicella. 1990. Cloning and expression in Escherichia coli of a Haemophilus influenzae type b lipooligosaccharide synthesis gene(s) that encodes a 2-keto-3-deoxyoctulosonic acid epitope. Infect. Immun. 58:1558-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spinola, S. M., A. Orazi, J. N. Arno, K. Fortney, P. Kotylo, C.-Y. Chen, A. A. Campagnari, and A. F. Hood. 1996. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J. Infect. Dis. 173:394-402. [DOI] [PubMed] [Google Scholar]
- 90.Spinola, S. M., L. M. Wild, M. A. Apicella, A. A. Gaspari, and A. A. Campagnari. 1994. Experimental human infection with Haemophilus ducreyi. J. Infect. Dis. 169:1146-1150. [DOI] [PubMed] [Google Scholar]
- 91.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301-314. [DOI] [PubMed] [Google Scholar]
- 92.Stevens, M., S. Porcella, J. Klesney-Tait, S. Lumbley, S. Thomas, M. Norgard, J. Radolf, and E. Hansen. 1996. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect. Immun. 64:1724-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stevens, M. K., J. Klesney-Tait, S. Lumbley, K. A. Walters, A. M. Joffe, J. D. Radolf, and E. J. Hansen. 1997. Identification of tandem genes involved in lipooligosaccharide expression by Haemophilus ducreyi. Infect. Immun. 65:651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stevens, M. K., J. L. Latimer, S. R. Lumbley, C. K. Ward, L. D. Cope, T. Lagergard, and E. J. Hansen. 1999. Characterization of a Haemophilus ducreyi mutant deficient in expression of cytolethal distending toxin. Infect. Immun. 67:3900-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sturm, A. W. 1997. Iron and virulence of Haemophilus ducreyi in a primate model. Sex. Transm. Dis. 24:64-68. [DOI] [PubMed] [Google Scholar]
- 96.Sullivan, M. 1940. Chancroid. Am. J. Syph. Gon. Ven. Dis. 24:482-521. [Google Scholar]
- 97.Sun, S., B. Schilling, L. Tarantino, M. V. Tullius, B. W. Gibson, and R. S. Munson. 2000. Cloning and characterization of the lipooligosaccharide galactosyltransferase II gene of Haemophilus ducreyi. J. Bacteriol. 182:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Svensson, L. A., A. Tarkowski, M. Thelestam, and T. Lagergard. 2001. The impact of Haemophilus ducreyi cytolethal distending toxin on cells involved in immune response. Microb. Pathog. 30:157-166. [DOI] [PubMed] [Google Scholar]
- 99.Thomas, C. E., B. Olsen, and C. Elkins. 1998. Cloning and characterization of tdhA, a locus encoding a TonB-dependent heme receptor from Haemophilus ducreyi. Infect. Immun. 66:4254-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas, K. L., I. Leduc, B. Olsen, C. E. Thomas, D. W. Cameron, and C. Elkins. 2001. Cloning, overexpression, purification, and immunobiology of an 85-kilodalton outer membrane protein from Haemophilus ducreyi. Infect. Immun. 69:4438-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Throm, R. E., J. A. Al-Tawfiq, K. R. Fortney, B. P. Katz, A. F. Hood, E. J. Hansen, and S. M. Spinola. 2000. Evaluation of an isogenic MOMP-deficient mutant in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:2602-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Throm, R. E., and S. M. Spinola. 2001. Transcription of candidate virulence genes of Haemophilus ducreyi during infection of human volunteers. Infect. Immun. 69:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Totten, P. A., J. M. Kuypers, C.-Y. Chen, M. J. Alfa, L. M. Parsons, S. M. Dutro, S. A. Morse, and N. B. Kiviat. 2000. Etiology of genital ulcer disease in Dakar, Senegal, and comparison of PCR and serologic assays for detection of Haemophilus ducreyi. J. Clin. Microbiol. 38:268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Totten, P. A., W. R. Morton, G. H. Knitter, A. M. Clark, N. B. Kiviat, and W. E. Stamm. 1994. A primate model for chancroid. J. Infect. Dis. 169:1284-1290. [DOI] [PubMed] [Google Scholar]
- 105.Totten, P. A., D. V. Norn, and W. E. Stamm. 1995. Characterization of the hemolytic activity of Haemophilus ducreyi. Infect. Immun. 63:4409-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trees, D. L., and S. A. Morse. 1995. Chancroid and Haemophilus ducreyi: an update. Clin. Microbiol. Rev. 8:357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tuffrey, M., D. Abeck, F. Alexander, A. P. Johnson, R. C. Ballard, and D. Taylor-Robinson. 1988. A mouse model of Haemophilus ducreyi infection (chancroid). FEMS Lett. 50:207-209. [Google Scholar]
- 108.UNAIDS. 1997. Sexually transmitted diseases: policies and principles for prevention and care. World Health Organization, New York, N.Y.
- 109.Van Laer, L., J. Vingerhoets, G. Vanham, L. Kestens, J. Bwayo, J. Otido, P. Piot, and E. Roggen. 1995. In vitro stimulation of peripheral blood mononuclear cells (PBMC) from HIV− and HIV+ chancroid patients by Haemophilus ducreyi antigens. Clin. Exp. Immunol. 102:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ward, C., S. Lumbley, J. Latimer, L. Cope, and E. Hansen. 1998. Haemophilus ducreyi secretes a filamentous hemagglutinin-like protein. J. Bacteriol. 180:6013-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wasserheit, J. N. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 112.Wood, G. E., S. M. Dutro, and P. A. Totten. 1999. Target cell range of Haemophilus ducreyi hemolysin and its involvement in invasion of human epithelial cells. Infect. Immun. 67:3740-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Young, R. S., M. J. Filiatrault, K. R. Fortney, A. F. Hood, B. P. Katz, R. S. Munson, Jr., A. A. Campagnari, and S. M. Spinola. 2001. Haemophilus ducreyi lipooligosaccharide mutant defective in expression of β-1,4-glucosyltransferase is virulent in humans. Infect. Immun. 69:4180-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Young, R. S., K. Fortney, J. C. Haley, A. F. Hood, A. A. Campagnari, J. Wang, J. A. Bozue, R. S. Munson, Jr., and S. M. Spinola. 1999. Expression of sialylated or paragloboside-like lipooligosaccharides is not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 67:6335-6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Young, R. S., K. R. Fortney, V. Gelfanova, C. L. Phillips, B. P. Katz, A. F. Hood, J. L. Latimer, R. S. Munson, Jr., E. J. Hansen, and S. M. Spinola. 2001. Expression of cytolethal distending toxin and hemolysin is not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 69:1938-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]