Abstract
To determine the role of interleukin-12 (IL-12) in primary and secondary immunity to a model intracellular bacterium, we have comprehensively evaluated infection with Francisella tularensis LVS in three murine models of IL-12 deficiency. Mice lacking the p40 protein of IL-12 (p40 knockout [KO] mice) and mice treated in vivo with neutralizing anti-IL-12 antibodies survived large doses of primary and secondary LVS infection but never cleared bacteria and exhibited a chronic infection. In dramatic contrast, mice lacking the p35 protein (p35 KO mice) of heterodimeric IL-12 readily survived large doses of primary sublethal LVS infection as well as maximal secondary lethal challenge, with only a slight delay in clearance of bacteria. LVS-immune wild-type (WT) lymphocytes produced large amounts of gamma interferon (IFN-γ), but p35 KO and p40 KO lymphocytes produced much less; nonetheless, similar amounts of NO were found in all cultures containing immune lymphocytes, and all immune lymphocytes were equally capable of controlling intracellular growth of LVS in vitro. Purified CD4+ and CD8+ T cells from both WT and p40 KO mice controlled intracellular growth, even though T cells from WT mice produced much more IFN-γ than those from p40 KO mice, and p40 KO T cells did not adopt a Th2 phenotype. Thus, while IL-12 p70 stimulation of IFN-γ production may be important for bacteriostasis, IL-12 p70 is not necessary for appropriate development of LVS-immune T cells that are capable of controlling intracellular bacterial growth and for clearance of primary or secondary LVS infection. Instead, an additional mechanism dependent on the IL-12 p40 protein, either alone or in another complex such as the newly discovered heterodimer IL-23, appears to be responsible for actual clearance of this intracellular bacterium.
People with defects in interleukin-12 (IL-12) production or IL-12 receptor expression, as well as in expression of gamma interferon (IFN-γ) receptors, appear to be unusually susceptible to Mycobacteria and Salmonella infections (9, 24, 33). However, the specific contributions of each cytokine to susceptibility remain incompletely understood. IFN-γ is clearly a key cytokine in responses to intracellular pathogens such as Mycobacteria and Salmonella, in part through activation of macrophages that results in nitric oxide (NO) production (at least in mice) and killing of intracellular parasites (reviewed in reference 39). IL-12 is a 70-kDa cytokine comprised of two disulfide-linked proteins (p35, which is constitutively expressed, and p40, which is inducible). IL-12 has a variety of biological functions, including stimulation of natural killer cell activity and induction of Th1 T-cell development (reviewed in reference 16). IL-12 production by antigen-presenting cells (including macrophages, dendritic cells, and B cells) greatly increases IFN-γ production by natural killer cells and T cells. IL-12 also upregulates expression of its own receptor on activated T cells, NK cells, and activated B cells, resulting in further IFN-γ production (41). Thus, this positive feedback loop alone might readily explain the importance of IL-12 in intracellular infections, through regulation of IFN-γ production.
Experimental studies on the role of IL-12 in intracellular infections have been performed using several different models of IL-12 deficiency, including in vivo depletion of IL-12 from normal mice by treatment with neutralizing anti-IL-12 antibodies (49); targeted mutation of the p35 chain of the IL-12 molecule in the mouse genome, resulting in p35 knockout (KO) mice (30); and targeted mutation of the p40 chain (p40 KO mice) (26). In some cases, infection of any IL-12-deficient mouse with a pathogen that stimulates cell-mediated immunity resulted in death. These include Leishmania major infection in p35 KO, p40 KO, and anti-IL-12-treated mice (28, 30, 40) and Toxoplasma gondii infection in p40 and anti-IL-12-treated mice (17, 50). In other cases, there are discrepancies in the phenotype of infection between p35 KO and p40 KO mice. For example, p40 KO mice infected intravenously with the fungal pathogen C. neoformans exhibited higher infection burdens, poorer granuloma formation in lungs, and earlier death than p35 KO mice, although both died more rapidly than wild-type (WT) mice (8). Of note, IFN-γ production was deficient but comparable in both types of KO mice (8). These results suggested a more complex picture of IL-12 dependence than might be expected based only on IL-12's role in Th1 T-cell development and IFN-γ production.
To further study the contributions of IL-12 and its subunits to protective immunity to intracellular bacteria, we have characterized murine infection with Francisella tularensis LVS in all three models of IL-12 deficiency. To our knowledge, this is the first such comprehensive evaluation of primary and secondary intracellular bacterial infection using the same infections in all three circumstances. LVS is a well-characterized intracellular bacterium that replicates in macrophages and disseminates to organs of the reticuloendothelial system (primarily the spleen, liver, lung, and lymph nodes [for a review, see reference 45]). Murine LVS infection initiated intradermally (i.d.) or subcutaneously is sublethal, while infection initiated intraperitoneally (i.p.) is lethal (13, 15). Similar to virtually all other intracellular pathogens, innate resistance to primary sublethal (i.d.) infection with LVS is clearly dependent on early production of IFN-γ and tumor necrosis factor alpha (TNF-α) (1, 11, 12, 25), and IL-12 is produced within a day after primary or secondary LVS infection (43); specific long-term protective immunity is dependent on Th1 T cells (51). Here we show that anti-IL-12-treated or p40 KO mice infected with LVS exhibit a chronic infection despite development of normal T-cell function, while resolution of LVS infection in p35 KO mice is nearly normal. Thus, clearance of this intracellular bacterial infection is not dependent on IL-12 p70 but on an unrelated function of p40.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free male BALB/cByJ and C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Male IL-12a− (p35) and IL-12b− (p40) KO mice in a C57BL/6J background were purchased from the Induced Mutant Resource of Jackson Laboratories; male IL-12b− (p40) KO mice in a BALB/c background were a generous gift from Dorothy Scott, Center for Biologics Research and Evaluation (CBER), Food and Drug Administration (FDA). All mice were housed in sterile microisolator cages in a barrier environment at the CBER, fed autoclaved food and water ad libitum, and routinely tested for common murine pathogens by a diagnostic service provided by the Division of Veterinary Services, CBER. Within an experiment, all WT and KO mice were carefully age matched (± 1 week), as some age-related differences in responses to F. tularensis LVS have been previously noted. In conducting the research described in this report, the investigators adhered to a protocol approved by the Animal Care and Use Committee of CBER.
Bacteria and growth conditions.
F. tularensis LVS (ATCC 29684; American Type Culture Collection, Rockville, Md.) was cultured on modified Mueller-Hinton (MH) agar plates or in modified MH broth (Difco Laboratories, Detroit, Mich.) supplemented with ferric pyrophosphate and IsoVitalex as previously described (Becton Dickinson, Cockeysville, Md.) (3, 15). One-milliliter aliquots of bacteria were frozen in broth alone at −70°C and periodically thawed for use, and viable bacteria were quantified by plating serial dilutions on MH agar plates. The number of CFU after thawing varied less than 10% over a 12-month period.
In vivo bacterial infections.
Mice were given 0.5 ml i.p. or 0.1 ml i.d. of the indicated dilution of LVS; actual doses of inoculated bacteria were simultaneously determined by plate count. All materials, including bacteria, were diluted in phosphate-buffered saline (PBS) (BioWhittaker, Walkersville, Md.) containing <0.01 ng of endotoxin per ml. In vivo organ burden and serum studies used groups of three to five mice, as indicated. Experiments enumerating numbers of CFU in organs of various mice were performed as previously described (15); briefly, spleens, livers, and lungs were removed aseptically and emulsified in a Stomacher (Tekmar, Cincinnati, Ohio) in 5 to 10 ml of sterile PBS, and appropriate dilutions were plated on MH plates.
Antibodies and in vivo antibody treatments.
The following neutralizing antibodies, purified and in azide-free and low-endotoxin format, used for blocking studies were purchased from BD PharMingen (San Diego, Calif.): anti-IFN-γ (clone R4-6A2), anti-IL-12 (clone C17.8, rat immunoglobulin G2a [IgG2a]), and anti-IL-4 (clone 11B11, rat IgG1). Antibodies for in vivo depletion of IL-12 (clones C15.6 and C15.1, both rat IgG1 [49]) were produced as ascites in BALB/c nu/nu mice, precipitated from clarified ascites with 50% ammonium sulfate, quantified using a capture enzyme-linked immunosorbent assay (ELISA) specific for rat IgG1 using monoclonal rat IgG1 as a standard (PharMingen), and tested for endotoxin levels using a kit from BioWhittaker; all ascites preparations contained ⩽10 EU of endotoxin per ml. Hybridoma cells producing both anti-IL-12 antibodies were a generous gift of Maria Wysocka and Giorgio Trincheri (Wistar Institute, Philadelphia, Pa.). Anti-IL-4 antibody (clone 11B11) used for in vivo treatments was a generous gift from Jerko Barbic and Mary Leef and was also produced as ascites and tested as described above. To deplete mice of IL-12, mice were treated i.p. with 500 μg each of C15.6 and C15.1 monoclonal antibodies or control anti-IL-4 antibodies on days −1 and 0 relative to the day of infection with LVS and then weekly thereafter. The treatment regimen used, employing a combination of two anti-IL-12 monoclonal antibodies on days −1 and 0 relative to infection and weekly thereafter, has previously been shown to effectively neutralize in vivo IL-12 bioactivity from peripheral sites in mice (40, 49).
Characterization of antibody response.
Sera were obtained via the lateral tail vein and pooled from the indicated groups of mice both before (prebleed) and after LVS infection at the indicated time points. Titers of specific anti-LVS serum antibodies were determined by ELISA using Immulon 1 plates coated with whole LVS bacteria and isotype-specific detection antibodies as previously described (37). End-point titer was defined as the lowest dilution of immune serum that had a mean optical density at 405 nm that was greater than the mean value of the matched dilution of normal prebleed mouse serum plus 3 standard deviations of the mean and was also greater than 0.050 (37).
In vitro assessment of control of intracellular bacterial growth in bone marrow-derived macrophages (BMMφ) and T-cell enrichment.
The in vitro culture system used to analyze the ability of immune lymphocytes to control intracellular replication of LVS bacteria and validation of the culture system's reflection of known parameters of in vivo control of bacterial growth have been described in detail elsewhere (4). Briefly, BMMφ derived from femurs of the indicated mice were cultured in 24-well plates (Costar, Corning, N.Y.) in complete medium (Dulbecco's modified Eagle's medium, 10% fetal bovine serum, glutamine, and nonessential amino acids) containing 10% L929 supernatant for 7 to 8 days, until a confluent monolayer was obtained. Macrophage monolayers were infected with F. tularensis LVS at a multiplicity of infection of 1:20 (bacteria to BMMφ) for 2 h and then treated for 45 min with medium containing 50 μg of gentamicin per ml to eliminate extracellular bacteria. Single-cell suspensions of lymphocytes (5 × 106/well; approximately one lymphocyte to two BMMφ) obtained by standard techniques from either naive or LVS-immune mice (mice previously infected with a sublethal dose of LVS i.d., as indicated) were then added to duplicate or triplicate infected macrophage cultures. CD4+ and CD8+ T cells were enriched from LVS-immune splenic lymphocyte preparations using a MACS Midi system and the appropriate magnetic beads according to the manufacturer's instructions (Miltenyi Biotec, Auburn, Calif.); the purity of the resulting T-cell preparations was assessed by flow cytometry as previously described (51) using the appropriate fluorochrome-labeled antibodies (BD PharMingen) in one- and two-color staining protocols. Following incubation of the infected macrophage-lymphocyte cultures, supernatants were harvested after 72 h and stored at −70°C until assay (see below). Growth of LVS in BMMφ was monitored as previously described (4) by lysing cultures with water at the indicated time points and plating serial dilutions of the lysate on MH agar plates.
Quantification of cytokines and NO in BMMφ culture supernatants.
Culture supernatants were assayed for IFN-γ, IL-12, IL-18, TNF-α, IL-4, and IL-10 by standard sandwich ELISAs. All antibody pairs and recombinant standards were purchased from BD PharMingen, and ELISAs were performed according to the manufacturer's instructions. Cytokines were quantified by comparison to recombinant standards using four-parameter fit regression in the SoftMax Pro ELISA analysis software (Molecular Devices, Sunnyvale, Calif.).
NO was detected in culture supernatants by the Griess reaction as previously described (20). Briefly, equal volumes of culture supernatants, diluted as necessary, were incubated with commercial Griess reagent (Sigma, St. Louis, Mo.) for 5 min at room temperature, and absorbance at 490 nm was measured. NO2 was quantified by comparison to serially diluted NaNO2 as a standard using four-parameter fit regression as described above.
RESULTS
Characterization of primary i.d. F. tularensis LVS infection in anti-IL-12-treated normal mice.
Following in vivo depletion of IL-12 from normal mice by anti-IL-12 treatment, mice were infected with sublethal (104 or 105) i.d. doses of F. tularensis LVS or a dose (106) that approaches the i.d. 50% lethal dose (LD50) (4, 10, 13, 15). Serum samples were obtained both before and after LVS infection, and survival was monitored; other mice were sacrificed at various time intervals to determine organ burdens. Overall survival results are shown in Table 1. Mice treated with either PBS or anti-IL-4 antibodies and infected with LVS exhibited a typical dose-dependent survival pattern, and their survival rates at the highest dose were not significantly different from each other (by Fisher's exact test; two-sided P value of >0.05). Anti-IL-12-treated mice exhibited decreased overall survival of the highest dose studied, 106 LVS i.d., compared to either PBS-treated mice, anti-IL-4-treated mice, or both control groups combined (P < 0.05 in all three cases). However, there were no differences in survival between control or anti-IL-12-treated mice at the lower two doses of LVS i.d. studied (P > 0.05 in all cases).
TABLE 1.
Survival of primary LVS infection by BALB/cByJ mice treated with anti-IL-12 antibodies
| Treatmenta | i.d. dose of LVS (CFU) | No. of deaths/total |
|---|---|---|
| PBS | 104 | 0/5 |
| 105 | 0/5 | |
| 106 | 6/29 | |
| Anti-IL-4 antibody | 105 | 0/5 |
| 106 | 7/16 | |
| Anti-IL-12 antibodies | 104 | 2/10 |
| 105 | 1/5 | |
| 106 | 20/29 |
BALB/cByJ mice were treated i.p. with either 0.5 ml of PBS, 1 mg of anti-IL-4 antibody or a combination of 500μg of C15.G anti-IL-12 antibody plus 500 μg of C15.1 anti-IL-12 antibody on days −1, 0, 7, 14, and 21. On day 0, mice were infected i.d. with the indicated dose of LVS; actual doses were confirmed by plate count at the time of infection. Since deaths of normal mice from i.d. LVS infection occur between days 4 and 7, survival was monitored through at least day 11, at which time selected mice were sacrificed to assess bacterial burdens; most mice were monitored through day 24 before they were sacrificed. The combined results of five independent experiments, all with five or more mice per group, are shown.
Impacts on both organ burdens and circulating cytokine levels in anti-IL-12-treated mice were also detected. Bacterial burdens in the spleens of mice treated with anti-IL-12 and infected with 106 LVS i.d. were not different from those of control mice at day 3 (Fig. 1). However, on day 18, numbers of bacteria in spleens of anti-IL-12-treated mice had declined but were significantly higher (by Student's t test; P < 0.05) than numbers in either PBS-treated or anti-IL-4-treated mice (Fig. 1A). Similar trends were evident in the other major sites of LVS infection, livers and lungs, of anti-IL-12-treated mice (data not shown). Remaining mice sacrificed on days 24 to 28, after the last available treatment with anti-IL-12, still contained substantial numbers of bacteria in all organs (data not shown).
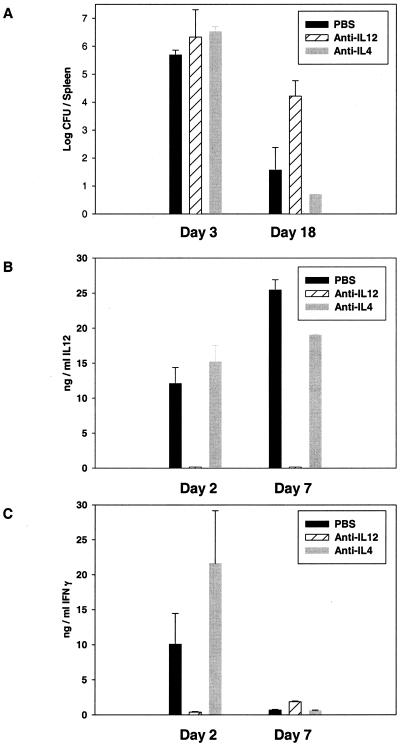
FIG. 1.
Bacterial burdens and serum cytokine levels in anti-IL-12-treated mice following primary sublethal F. tularensis LVS infection. BALB/cByJ mice were treated with PBS or anti-cytokine antibodies as described in Table 1 and infected with 106 LVS i.d. At the indicated times after infection, groups of four mice were sacrificed for determination of bacterial burdens in spleens (A) and to obtain serum for assessment of IL-12 p40 protein (B) or IFN-γ (C) by ELISA. No IL-12 p40 or IFN-γ was detected in prebleed serum samples from any mice; the limit of detection was approximately 150 pg/ml. Results from one representative experiment of four independent experiments of similar design are shown.
Circulating levels of IL-12 and IFN-γ in treated and infected mice were also studied. Large amounts of IL-12 were readily detected in serum from PBS- and anti-IL-4-treated mice both 2 and 7 days after LVS infection; in contrast, no IL-12 was detected in anti-IL-12-treated mice at any time point, in this or other similar experiments (data not shown), confirming the effectiveness and maintenance of depletion (Fig. 1B). On day 2, large amounts of IFN-γ were present in the sera of PBS- or anti-IL-4-treated mice, but barely detectable amounts that were significantly different from those of either control group (by Student's t test; P < 0.05) were found in the sera of anti-IL-12-treated mice. By day 7, serum levels of IFN-γ had greatly declined in PBS- or anti-IL-4-treated mice and were less than the very low levels still detected in anti-IL-12-treated mice (Fig. 1C). In more limited studies, similar differences in bacterial burdens and serum cytokine levels were seen in mice treated with anti-IL-12 and infected with a 104 dose of LVS i.d. (data not shown), despite the lack of impact of anti-IL-12 treatment on overall survival of this lower dose of LVS (Table 1).
Characterization of primary i.d. and secondary i.p. F. tularensis LVS infection and specific anti-LVS antibody responses in p35 and p40 IL-12 KO mice.
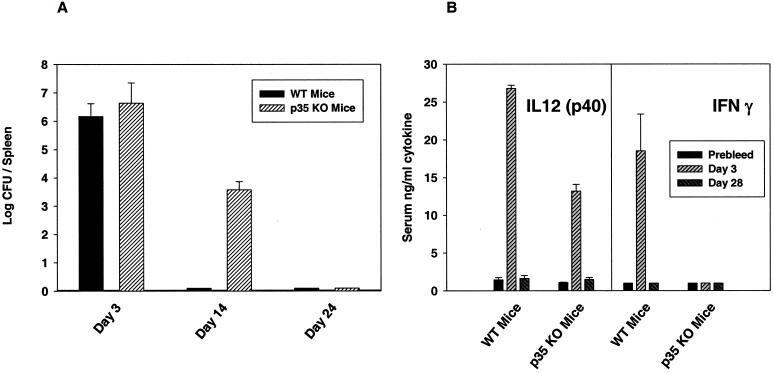
The course of LVS infection in anti-IL-12-treated mice was compared to that in IL-12 KO mice, using mice in which IL-12 production and normal bioactivity were eliminated by targeted mutation of either the p35 chain of IL-12 (p35 KO mice) or the p40 chain of IL-12 (p40 KO mice). Use of KO mice also permitted the study of secondary infection, which was greatly limited in anti-IL-12-treated mice by the difficulty and expense of maintaining anti-IL-12 treatment in vivo. Since C57BL/6J mice are approximately 1 log more resistant to both primary and secondary LVS infection than the BALB/cByJ mice described above (10, 13, 15, 25), correspondingly higher doses of LVS were studied in the C57-p35 KO mice. As shown in Table 2, C57-p35 KO mice readily survived primary i.d. LVS infection at rates comparable to those of WT C57BL6/J mice, with the exception of the highest dose studied, 107 LVS i.d. (significantly different by Fisher's exact test; P < 0.05). Selected mice were sacrificed between days 24 and 28, and no bacteria were detected in spleens, livers, and lungs of any C57BL/6J WT or C57-p35 KO mice (<50 CFU/organ). Further, virtually all C57BL/6J WT as well as C57-p35 KO mice survived maximal secondary lethal challenge of 5 × 106 LVS i.p. (equivalent to 500,000 LD50s) given on day 30 (Table 2), indicating the development of protective immunity in both cases. Importantly, bacteria were also cleared from all organs of both C57BL/6J WT and C57-p35 KO mice within 2 months after secondary lethal challenge (see footnotes to Table 2). To examine bacterial burdens in more detail, other mice were infected with 106 LVS i.d. and sacrificed at earlier time points. As illustrated in Fig. 2A, C57BL/6J WT and C57-p35 KO mice had comparable levels of bacteria in spleens at day 3. By day 14 C57BL/6J WT mice had no detectable bacteria in spleens, while C57-p35 KO mice exhibited declining numbers of bacteria that were eliminated by day 24; similar trends were evident in livers and lungs (data not shown). Sera from both C57BL/6J WT and C57-p35 LVS-infected KO mice had large amounts of IL-12 p40 protein at day 3 after i.d. LVS infection, but very low levels by day 28 (Fig. 2B); as expected, C57-p35 KO mice had no detectable complete IL-12 p70 (<50 pg/ml in all cases; data not shown). While sera from LVS-infected C57BL/6J WT mice had large amounts of serum IFN-γ on day 3 that declined below detectable levels by day 28, sera from LVS-infected C57-p35 KO mice had no detectable IFN-γ at either time point (Fig. 2B).
TABLE 2.
Survival of primary and secondary LVS infection by IL-12 p35 KO micea
| Mouse strain | i.d. dose of LVS on day 0 (primary infection) (CFU) | No. of deaths/total | i.p. dose of LVS on day 30 (secondary challenge) (CFU) | No. of deaths/total | Bacterial clearance by day 90 |
|---|---|---|---|---|---|
| C57BL/6J | 0 (PBS only) | 0/5 | 5 × 106 | 5/5 | NA |
| 105 | 2/5 | 5 × 106 | 0/3 | Yes | |
| 106 | 0/5 | 5 × 106 | 1/5 | Yes | |
| 3 × 106 | 2/8 | 5 × 106 | 0/4b | Yes | |
| 107 | 1/5 | 5 × 106 | 0/4 | Yes | |
| C57-p35 KO | 105 | 0/5 | 5 × 106 | 1/5 | Yes |
| 106 | 1/5 | 5 × 106 | 0/4 | Yes | |
| 3 × 106 | 2/8 | 5 × 106 | 0/4b | Yes | |
| 107 | 5/5 | 5 × 106 | NA | NA |
The indicated mice were infected on day 0 i.d. with the indicated dose of LVS; actual doses were confirmed by plate count at the time of infection. On day 30 after primary infection, surviving mice were lethally challenged with 5 × 106 (500,000 LD50s) CFU of LVS i.p. On day 90 (60 days after secondary challenge), surviving mice were sacrificed and assessed for bacterial burdens. The limit of detection was 50 CFU/organ (1.7 logs). NA, not applicable. The results of two independent experiments, with either five or eight mice per group, are shown.
Because some mice were sacrificed for use in other experiments, only the total shown were challenged.
FIG. 2.
Bacterial burdens and serum cytokine levels in p35 KO mice following primary sublethal F. tularensis LVS infection. C57BL/6J WT or C57-p35 KO mice were infected with 106 LVS i.d. At the indicated times after infection, groups of four mice were sacrificed for determination of bacterial burdens in spleens (A) and to obtain serum for assessment of IL-12 p40 protein or IFN-γ (B) by ELISA; the limit of detection was approximately 150 pg/ml. Results from one representative experiment of four independent experiments of similar design are shown.
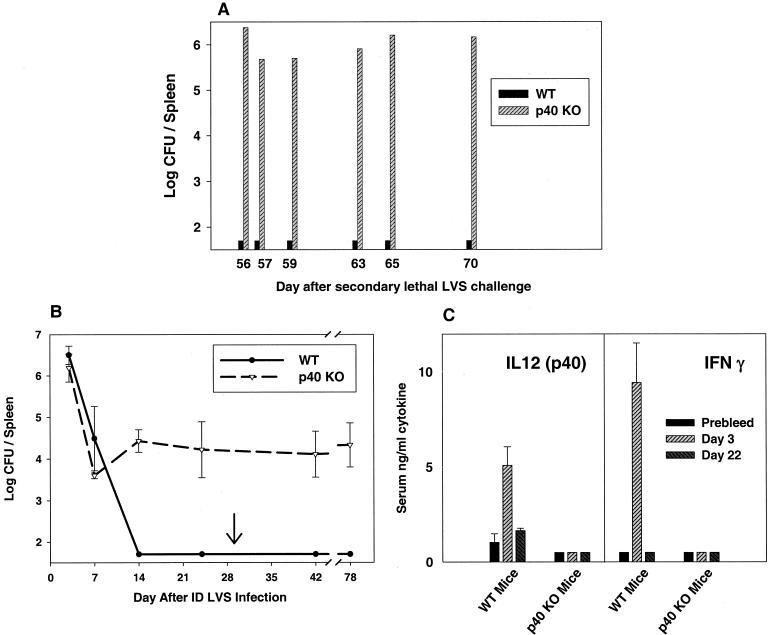
Similar studies were conducted in p40 KO mice, which were available in both the BALB/c and C57BL/6J background. As shown in Table 3, p40 KO mice survived primary sublethal i.d. LVS infection at rates comparable to those of either strain of WT mice. However, when selected mice were sacrificed between days 24 and 28, bacteria were detected in spleens, livers, and lungs of p40 KO but not WT mice (see below). Virtually all WT as well as p40 KO mice survived maximal lethal secondary LVS i.p. challenge given on day 28 for about 2 months after lethal challenge (Table 3). However, 50 days after secondary lethal challenge some p40 KO mice in the BALB/c background exhibited obvious morbidity, and according to protocol, were humanely sacrificed (when their ability to obtain food and water was judged to be compromised). Consistent levels of bacteria were found in spleens (Fig. 3A) as well as livers and lungs (data not shown) of i.d. LVS-infected BALB/c-p40 KO mice, while no bacteria were detected in comparable BALB/cByJ WT mice (randomly chosen from the matched infection group). Visible signs of illness were not observed in LVS-infected p40 KO mice on the C57BL/6J background, likely reflecting the slightly greater resistance of mice of that inbred strain.
TABLE 3.
Survival of primary and secondary LVS infection by IL-12 p40 KO micea
| Mouse strain | i.d. dose of LVS on day 0 (primary infection) (CFU) | No. of deaths/total | i.p. dose of LVS on day 28 (secondary challenge) (CFU) | No. of deaths/total (through day 50) | Bacterial clearance after day 50 |
|---|---|---|---|---|---|
| BALB/cByJ | 0 (PBS only) | 0/5 | 106 | 5/5 | NA |
| 104 | 0/8 | 106 | 1/8 | Yes | |
| 105 | 1/8 | 106 | 1/7 | Yes | |
| C57BL/6J | 106 | 0/8 | 106 | 0/4b | Yes |
| BALB/c-p40 KO | 104 | 0/6 | 106 | 2/6 | No |
| 105 | 2/5 | 106 | 0/3 | No | |
| 106 | 0/5 | 106 | 3/5 | No | |
| C57-p40 KO | 106 | 2/8 | 106 | 1/3b | No |
The indicated mice were infected i.d. with various doses of LVS on day 0. On day 28 after primary infection, surviving mice were lethally challenged i.p. with 106 LVS, and survival through day 50 was recorded. Visibly ill mice were sacrificed at various time points thereafter (ranging from days 56 to 70), and spleens, livers, and lungs were assessed for bacterial burdens (see Fig. 5). The limit of detection was 50 CFU/organ (1.7 logs). NA, not applicable. The results of two independent experiments, with either five or eight mice per group, are shown.
Because some mice were sacrificed for use in other experiments, only the total shown were challenged.
FIG. 3.
Bacterial burdens and serum cytokine levels in p40 KO mice following primary and secondary F. tularensis LVS infection. BALB/cByJ WT or BALB/c-p40 KO mice were infected with 106 LVS i.d. and challenged on day 30 (arrow) with 106 LVS i.p. (1,000,000 LD50s for BALB/cByJ mice). At the indicated times after secondary lethal challenge, individual mice were sacrificed for determination of bacterial burdens in spleens (A). Other C57BL/6J WT or C57-p40 KO mice, in groups of three, were sacrificed at the indicated time points after primary or secondary (arrow) LVS infection for determination of bacterial burdens in spleens (B), or serum was obtained after primary LVS infection for assessment of IL-12 p40 protein (C, left panel) or IFN-γ (C, right panel) by ELISA; the limit of detection was approximately 150 pg/ml. Results from one representative experiment of two (B) or three (C) independent experiments of similar design are shown.
To examine the bacterial burdens in p40 KO mice in more detail, other mice were infected with a sublethal dose of 106 LVS i.d. and sacrificed at various time points after both primary infection and secondary lethal i.p. challenge. As illustrated in Fig. 3B, i.d. LVS-infected C57BL/6J WT and p40 KO mice had comparable levels of bacteria in spleens on days 3 and 7; by day 14, however, C57BL/6J WT mice had no detectable bacteria in spleens, while C57-p40 mice exhibited detectable burdens that were maintained through day 24. All remaining infected mice survived and were given a maximal secondary lethal challenge on day 30 of 106 CFU i.p. (equivalent to 100,000 LD50s in nonimmune mice). C57BL/6J WT mice cleared secondary LVS challenge by day 42 (12 days after challenge), but C57-p40 mice contained large numbers of bacteria in spleens through day 78 (Fig. 3B). Similar results were obtained in livers and lungs (data not shown). Sera from LVS-infected C57BL/6J WT mice had large amounts of IL-12 p40 protein on day 3 after i.d. LVS infection, but very low levels by day 22. As expected, sera from LVS-infected C57-p40 KO mice had no detectable p40 protein (Fig. 3C) or complete IL-12 p70 (<50 pg/ml in all cases; data not shown). However, while sera from LVS-infected C57BL/6J WT mice had large amounts of serum IFN-γ on day 3 that declined below detectable levels by day 22, sera from LVS-infected C57-p40 KO mice had no detectable IFN-γ at either time point (Fig. 3B).
The quantity and isotype of specific anti-LVS antibodies were also measured in all three types of mice. Three weeks after sublethal i.d. LVS infection of C57BL/6J WT mice, sera contained low amounts of IgM (mean end-point titer in sera from five mice of 1:640)- and IgG2a (1:80)-specific anti-LVS antibodies (but not specific antibodies of other isotypes [36]). Sera from LVS-infected C57-p35 KO mice had somewhat higher anti-LVS IgM levels of 1:1,280, IgG2a titers of 1:1,280, and IgG1 titers of 1:20. In the sera of LVS-infected C57-p40 KO mice, anti-LVS IgM titers of 1:2,560, IgG2a titers of 1:1,280, and IgG1 titers of 1:640 (but no titers for IgG2b or IgG3) were found.
Role of T cells, cytokines, and NO in control of intracellular LVS infection in IL-12 KO mice.
IL-12 is considered necessary for appropriate development of Th1 cells and production of IFN-γ (see reference 16). To examine T-cell development and the contribution of IFN-γ in KO mice more directly, we used a recently developed in vitro model system that assesses the ability of immune lymphocytes to control intracellular bacterial growth (4). To date, this in vitro culture system has reflected known immunological parameters of in vivo immunity to LVS, particularly dependence on CD4+ or CD8+ T cells in secondary (memory) responses (4).
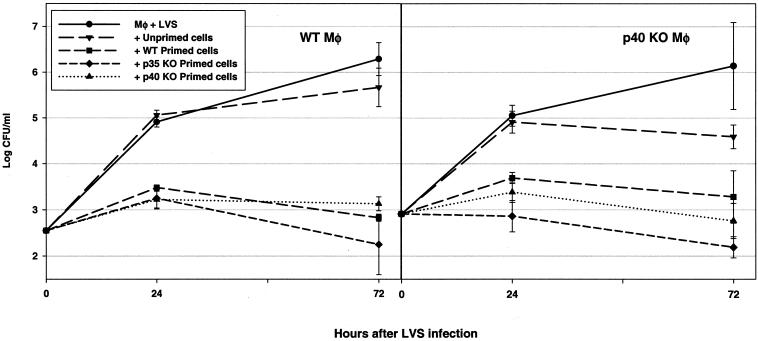
In recognition of the possibility that nonclassical lymphocytes, including γδ+ T cells and even B cells, might participate in control of intracellular bacterial growth, initially all immune lymphocytes were studied. LVS-immune lymphocytes were obtained from spleens of WT and KO mice that survived both primary and secondary LVS infections. All in vitro experiments shown here used cells from C57BL/6J WT and p35 or p40 KO mice in the C57 background, although similar results were obtained using cells from BALB/cByJ WT mice and BALB/c-p40 KO mice (data not shown; BALB/c-p35 KO mice were not available). Macrophages derived from bone marrow (BMMφ) of either WT mice or p40 KO mice were infected in vitro with LVS; macrophages from p35 KO mice were not studied, since the phenotype of LVS infection in p35 KO mice was similar to that of WT mice. Immediately after LVS infection, splenic lymphocytes from nonimmune and LVS-immune mice were added to infected macrophages. Infected macrophages were lysed to determine the extent of bacterial replication and survival over time, and culture supernatants were collected after 72 h. As shown in Fig. 4, LVS replicated exponentially in both C57BL/6J WT and C57-p40 KO BMMφ over 3 days, and bacterial growth was essentially unaffected by the addition of nonimmune WT lymphocytes. However, the addition of immune lymphocytes from either WT, p35 KO, or p40 KO mice to either infected WT or p40 KO macrophages controlled bacterial growth dramatically by 72 h to a comparable degree. Of note, spleens obtained from p40 KO mice themselves contained LVS bacteria, due to chronic infection, at the time of addition to culture; no effort was made to remove this additional bacterial burden before the addition of cells.
FIG. 4.
LVS-immune lymphocytes from WT, p35, or p40 KO mice control intracellular LVS growth in vitro. BMMφ were derived from either WT C57BL/6J mice (left) or from C57-p40 KO mice (right) and infected with LVS. Splenic lymphocytes from the indicated mice (unprimed C57BL/6J mice or LVS-primed mice [mice that were infected with 106 LVS i.d. on day 0 and then challenged on day 35 with 5 × 106 LVS i.p., with spleens removed 4 weeks later]) were added immediately after infection, and growth of bacteria in macrophages was monitored over time. Results shown are the mean CFU/well ± standard deviations (SD) of three triplicate culture wells per condition. Results from one representative experiment of two (using lymphocytes from C57-p35 KO mice) or nine (using lymphocytes from either BALB/c or C57-p40 KO mice) independent experiments of similar design are shown.
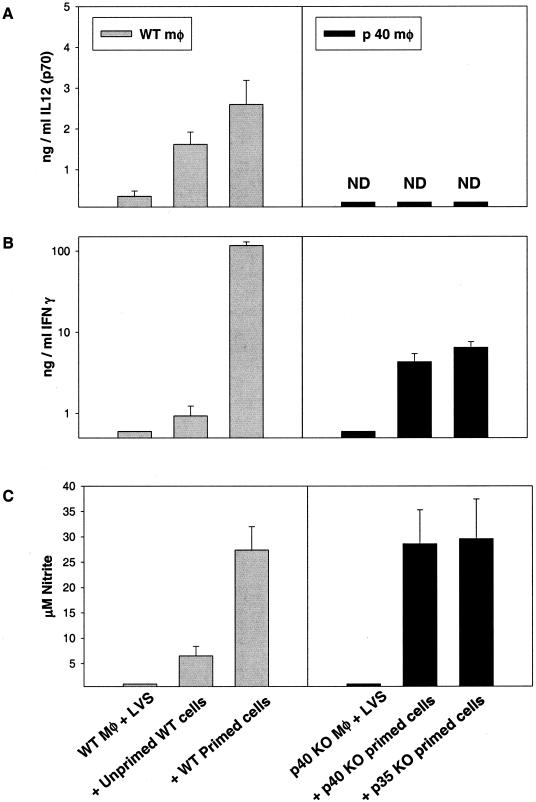
Supernatants obtained after 72 h from these cultures were assayed for the presence of a panel of cytokines as well as for NO. For clarity, only results from cultures containing LVS-infected WT macrophages with WT lymphocytes or LVS-infected p40 KO macrophages with either type of KO lymphocyte are shown (Fig. 5), although other combinations were tested and similar to the homologous cultures (data not shown). As seen previously (4), infected macrophages from WT mice produced small amounts of IL-12 that were increased upon addition of either unprimed or primed cells; as expected, no IL-12 p70 was detected in cultures containing KO macrophages and lymphocytes (Fig. 5A). The addition of primed, but not unprimed, WT cells to infected WT macrophages resulted in production of large amounts of IFN-γ (Fig. 5B) and NO (Fig. 5C). In contrast, much smaller amounts of IFN-γ were produced in cultures containing KO macrophages and either p35 or p40 primed lymphocytes, and the quantities produced were very similar to each other (Fig. 5B; note the logarithmic scale). Interestingly, despite large differences in the levels of IFN-γ produced, levels of NO produced were comparable in cultures containing either WT, p35, or p40 primed lymphocytes (Fig. 5C).
FIG. 5.
Cytokine and NO production during in vitro control of intracellular LVS growth by WT, p35, or p40 LVS-immune lymphocytes. Supernatants were obtained at 72 h from the indicated cultures described for Fig. 4 and assessed by ELISA for the presence of IL-12 p40 protein (A) or IFN-γ (B) or by Greiss reaction for nitrite as an indicator of NO production (C). Results shown are the mean nanograms of cytokine per milliliter or micromolar NO ± SD of three triplicate culture wells per condition. Results for IFN-γ are shown on a log scale because of the large differences in quantities between the levels of C57BL/6J WT and C57-KO mice. Results from one representative experiment of two (using lymphocytes from C57-p35 KO mice) or nine (using lymphocytes from either BALB/c or C57-p40 KO mice) independent experiments of similar design are shown.
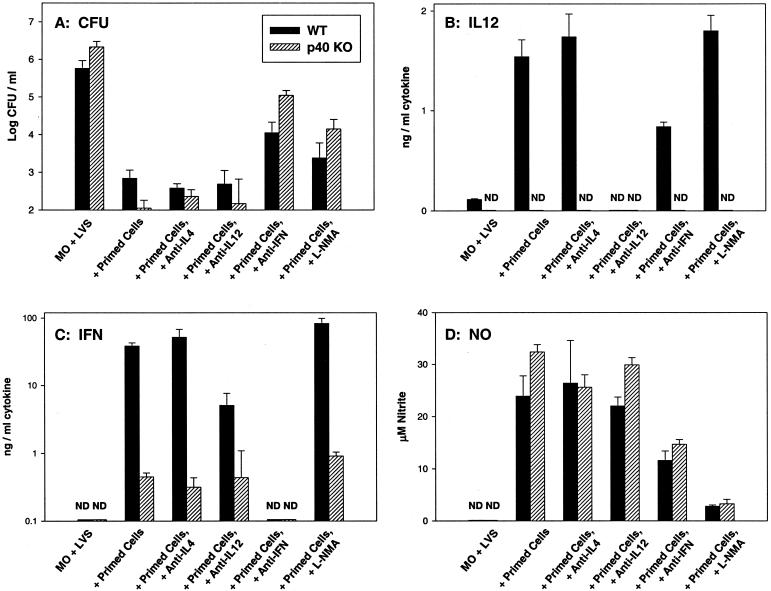
To assess the contribution of IL-12 and IFN-γ to the control of intracellular growth in vitro, neutralizing antibodies to each cytokine were added at the initiation of cultures, and the impact on bacterial growth, cytokine production, and NO production was assessed. In these studies, we focused on comparison of control of intracellular bacterial growth by immune lymphocytes obtained following primary LVS infection of WT and p40 KO mice (since the in vivo phenotype of p40 mice was defective, but the phenotype of p35 KO mice was similar to that of WT mice). For clarity, only results from cultures with LVS-infected C57BL/6J WT macrophages and WT immune lymphocytes or LVS-infected C57-p40 KO macrophages with p40 KO immune lymphocytes at the 72-h time point are shown (Fig. 6). As seen above, addition of both WT and p40 KO primed lymphocytes to either type of infected macrophage effectively and comparably controlled the intracellular growth of LVS bacteria (Fig. 6A). Addition of neutralizing anti-IL-12 antibodies or isotype-matched control anti-IL-4 antibodies had no significant impact (P > 0.05 for all combinations) on the control of intracellular bacterial growth by either type of lymphocytes. In contrast, the addition of anti-IFN-γ partially but not completely reversed the control of intracellular bacterial growth. Control of intracellular bacterial growth in the presence of anti-IFN-γ was significantly less than that seen in the presence of either type of lymphocytes alone (P < 0.05 for all combinations, by Student's t test), but bacterial growth in anti-IFN-γ-treated cultures was still significantly less than that seen in infected macrophages alone (P < 0.05 for all combinations). Importantly, the relative impact of anti-IFN-γ in reversing control of growth was significantly greater in the cultures containing LVS-infected p40 KO macrophages and p40 KO immune lymphocytes than in the corresponding WT cultures (P < 0.05 in five of six repeated experiments).
FIG. 6.
Effect of anticytokine treatment on in vitro control of intracellular LVS growth and production of mediators by WT or p40 LVS-immune lymphocytes. BMMφ were derived from either WT C57BL/6J mice or from C57-p40 KO mice and infected with LVS. Splenic lymphocytes from either C57BL/6J WT or C57-p40 KO immune mice (that had received a primary sublethal i.d. LVS infection of 105 CFU 35 days earlier) were added immediately after infection. Results using only LVS-infected WT macrophages with WT immune lymphocytes (black bars) or LVS-infected p40 KO macrophages with p40 KO immune lymphocytes (hatched bars) are shown. Growth of bacteria in macrophages was assessed at 72 h after infection (A); results shown are the mean CFU/well ± SD of three triplicate culture wells per condition. Supernatants were obtained at 72 h from the same cultures and assessed by ELISA for the presence of IL-12 p40 protein (B) or IFN-γ (C) or by Greiss reaction for nitrite as an indicator of NO production (D). Results shown are the mean nanograms of cytokine per milliliter or micromolar NO ± SD of three triplicate culture wells per condition. Results for IFN-γ are shown on a log scale because of the large differences in quantities between the levels of WT and KO mice. Results from one representative experiment of six independent experiments of similar design are shown.
When the corresponding production of soluble mediators was examined, supernatants from WT cultures treated with anti-IL-12 antibodies contained no detectable IL-12, confirming the effectiveness of neutralization, and all cultures containing p40 KO cells produced no p40 (Fig. 6B) or p70 (data not shown), as expected. Throughout, the addition of isotype-matched control anti-IL-4 antibodies had no significant effect on levels of any of the cytokines tested or on NO levels (Fig. 6; also, see below). In WT cultures, the addition of anti-IL-12 antibodies greatly reduced the amount of IFN-γ produced (Fig. 6C) but not the amount of NO produced (Fig. 6D). However, the addition of anti-IL-12 antibodies to cultures containing p40 KO primed cells, in which the amounts of IFN-γ produced were very low initially (Fig. 6C), had no significant impact on production of either IFN-γ (Fig. 6C) or NO (Fig. 6D).
Supernatants from both WT and p40 cultures treated with anti-IFN-γ antibodies contained no detectable IFN-γ (Fig. 6C), confirming the effectiveness of neutralization (despite the large amounts of IFN-γ produced in WT cultures). Addition of anti-IFN-γ antibodies significantly reduced the amount of IL-12 produced in cultures containing WT primed cells, by about 50% (P < 0.05) (Fig. 6B). Addition of anti-IFN-γ to either type of culture also significantly reduced levels of NO by about 50% (P < 0.05). Of particular note, despite the undetectable levels of IFN-γ in anti-IFN-γ-treated cultures as well as very low levels in cultures containing infected p40 KO macrophages and p40 KO immune lymphocytes, substantial amounts of NO were produced in all cultures containing WT or p40 immune lymphocytes (Fig. 6D).
Supernatants were also assayed for the presence of the following cytokines, with the following results: IL-4, undetectable in all supernatants (<50 pg/ml); IL-18, very low levels (approximately 20 pg/ml) in all supernatants, with no apparent differences in any combination; IL-10, low levels (approximately 100 pg/ml) in all supernatants, with no apparent differences in any combination; and TNF-α, very low levels (approximately 40 pg/ml) in all supernatants, with no apparent differences in any combination.
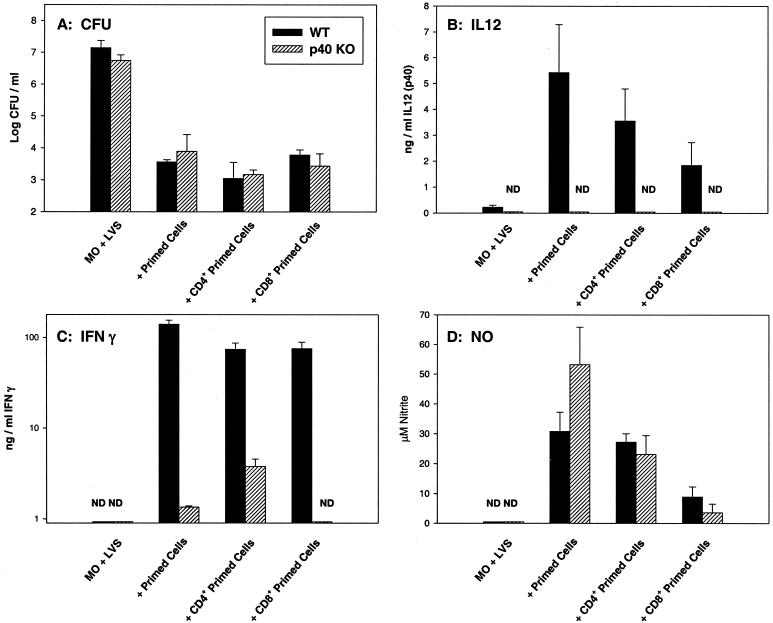
Thus, LVS-immune splenic lymphocytes from WT, p35 KO, and p40 KO mice were comparable in their ability to control intracellular bacterial growth (Fig. 4 to 6), even though p40 KO mice were clearly defective in bacterial clearance in vivo while WT and p35 KO mice were not (Tables 2 and 3; Fig. 2 and 3). Previous data demonstrated that CD4+ and CD8+ T cells, but not B cells, were effector cells in this in vitro system when WT cells were studied (4). Here we compared T cells in particular from LVS-infected (defective) p40 KO mice to those from LVS-immune WT (normal) mice. Purified CD4+ and CD8+ T cells were prepared from both types of LVS-immune primed spleen cells at 4 to 8 weeks after LVS sublethal infection (priming) and assessed using the homologous LVS-infected macrophages (Fig. 7). As seen in Fig. 7A, both WT and p40 KO CD4+ and CD8+ primed T cells were readily able to control intracellular bacterial growth to a degree comparable to that of unseparated primed spleen cells and were not significantly different from each other (P > 0.05 in all cases). Levels of IL-12 were moderate in WT T-cell cultures and undetectable, as expected, in all p40 KO cultures (Fig. 7B). Both CD4+ and CD8+ WT primed T cells produced large amounts of IFN-γ, compared to much lower amounts in p40 KO primed lymphocyte or CD4+-T-cell cultures (note log scale); in repeated experiments, both CD4+ and CD8+ T cells from LVS-immune p40 KO mice produced very low levels of IFN-γ that were near the limit of detection (here, below the limit of detection for CD8+ T cells). IL-4 was undetectable from all cultures, whether they contained WT or p40 KO splenic lymphocytes or separated T-cell subpopulations (<50 pg/ml). Finally, levels of NO produced were high and comparable in cultures containing unseparated spleen cells from both WT and p40 KO mice, as well as those containing both types of CD4+ T cells (Fig. 7D); levels of NO were lower but comparable in cultures containing CD8+ T cells from both types of mice. Overall, levels of NO were not correlated with IFN-γ production from these cultures (Fig. 7C). Although we have been unable to obtain sufficient age-matched WT, p35, and p40 KO mice to simultaneously prepare purified T-cell subpopulations from all three types of mice, more limited studies using purified CD4+ and CD8+ T cells from LVS-immune p35 KO mice with LVS-infected p35 KO macrophages demonstrated that, as expected, purified p35 KO T cells controlled intracellular bacterial growth, comparable to T cells from WT KO mice, but produced reduced levels of IFN-γ compared to cultures from WT mice (data not shown).
FIG. 7.
In vitro control of intracellular LVS growth, cytokine production, and NO production by WT or p40 LVS-immune T-cell subpopulations. BMMφ were derived from either WT C57BL/6J mice or C57-p40 KO mice and infected with LVS. Splenic lymphocytes or purified CD4+ or CD8+ T cells from either C57BL/6J WT or C57-p40 KO immune mice (that had received a primary sublethal i.d. LVS infection of 105 CFU 45 days earlier) were added to the homologous LVS-infected macrophages immediately after infection. By flow cytometry, WT CD4+ T cells were 94.3% CD4+, WT CD8+ T cells were 90.8% CD8+, p40 KO CD4+ T cells were 90.4% CD4+, and p40 KO CD8+ T cells were 87.3% CD8+. Results using only LVS-infected WT macrophages with WT immune lymphocytes (black bars) or LVS-infected p40 KO macrophages with p40 KO immune lymphocytes (hatched bars) are shown. Growth of bacteria in macrophages was assessed at 72 h after infection (A); results shown are the mean CFU/well ± SD of three triplicate culture wells per condition. Supernatants were obtained at 72 h from the same cultures and assessed by ELISA for the presence of IL-12 p40 protein (B) or IFN-γ (C) or by Greiss reaction for nitrite as an indicator of NO production (D). Results shown are the mean nanograms of cytokine per milliliter or micromolar NO ± SD of three triplicate culture wells per condition. Results for IFN-γ are shown on a log scale because of the large differences in quantities between the levels of WT and KO mice. Results from one representative experiment of three independent experiments of similar design using WT and p40 KO cultures are shown.
DISCUSSION
Unlike mice treated with anti-IFN-γ or anti-TNF-α, which die within 5 to 7 days after primary sublethal LVS infection (1, 11, 12, 25), both IL-12 p40 KO mice and mice treated with neutralizing anti-IL-12 antibodies survived and initially controlled primary LVS infection; they then exhibited a chronic infection even following secondary challenge that resulted in tolerable, and nearly homeostatic, numbers of bacteria in organs (Tables 1 and 3; Fig. 1 and 3). In contrast, p35 KO mice, which also lack the ability to produce complete IL-12 p70 protein, readily survived and cleared both primary and secondary LVS infection, with only a slight delay in bacterial clearance (Table 2; Fig. 2). These results clearly lead to the surprising conclusion that resolution of a classic intracellular bacterial infection does not depend on IL-12 p70 but instead is dependent on a separate positive, not antagonistic (29), activity of the p40 protein itself.
Immunity to F. tularensis LVS is considered to be dependent on Th1 T-cell responses and IFN-γ production, similar to many other intracellular pathogens, and IL-12 can clearly be important in directing optimal Th1 T-cell development (16). However, results using the in vitro culture system demonstrated that the inability of p40 KO mice to resolve LVS infection was not due solely to poor T-cell function or memory, as primed T cells from p40 KO mice were functionally comparable to those from WT and p35 KO mice in controlling intracellular bacterial growth (Fig. 5 to 7). Previous results using this culture system demonstrated a close correlation between in vivo and in vitro effector mechanisms, but here no difference in the effectiveness of WT or p40 T cells could be detected, despite obvious differences in the course of in vivo infection (Table 3, Fig. 2 and 3). Of note, not all bacteria are eliminated in this in vitro culture system, even when WT cells are studied and cultures are continued beyond 3 days (see reference 4). To date, we cannot determine whether this is due to inherent limitations in the efficiency of the culture system or to factors that render it impossible for this culture system to detect the final mechanism(s) that is responsible for full in vivo clearance. As might be expected, IFN-γ production from both p40 KO mice or cultures was greatly reduced, but there were minimal or no changes in the magnitude of production of other cytokines (Fig. 3 and 5 to 7) and no obvious Th2 switch in the absence of IL-12. In other infection models, it is also becoming clear that absence of IL-12 does not necessarily result in a switch toward Th2 development. Leishmania infection in mice is indeed associated with increased IL-4 production in IL-12-deficient animals (28, 30). On the other hand, there were no dramatic increases in IL-4, IL-5, and/or IL-13 production in IL-12-deficient animals infected with Mycobacterium tuberculosis (7), Mycobacterium bovis BCG (48), T. gondii (50), Schistosoma mansoni (22), or viruses (34). Our data indicate that development of T cells capable of clearing LVS infection (arguably Th1 cells although they are deficient in IFN-γ production) and maintenance of LVS T-cell memory does not necessarily require IL-12 p70; this is in contrast to results obtained using L. major (44) and T. gondii (50), which suggested that Th1 development and maintenance of memory required IL-12.
The lack of change to a Th2 phenotype may also be reflected in the continued production of larger amounts of IgG2a anti-LVS antibodies in both p35 and p40 LVS-infected KO mice (see text). We previously assumed that IgG2a anti-LVS antibodies were related to the large amounts of IFN-γ produced after LVS infection, but these studies suggest instead that either small amounts of IFN-γ are sufficient or that IgG2a is an inherently dominant isotype of specific antibody produced in response to chronic stimulation by this pathogen. Of note, other studies in viral and parasitic infection models also demonstrate that IgG2a production can be IFN-γ independent (27).
There is substantial literature to date on the outcome of intracellular bacterial and parasitic infections in various models of IL-12 deficiency (see reference 16), with results ranging from dramatic to intermediate to minimal impact. In some cases, infection of any IL-12-deficient mouse resulted in death. These include L. major infection in p35 KO, p40 KO, and anti-IL-12-treated mice (28, 30, 40) and T. gondii infection in p40 and anti-IL-12-treated mice (17, 50). Interestingly, infections with obligate intracellular pathogens (including viruses [34]) have been much less affected by IL-12 deficiency. Anti-IL-12-treated mice survived and cleared both Chlamydia pneumoniae (18) and Chlamydia trachomatis (35), with only minor differences from WT mice in magnitude of organ burdens or length of shedding. Some discrepancies in the phenotype of infection between p35 KO and p40 KO mice have also been reported. Although both types died more rapidly than WT mice, C. neoformans-infected p35 KO mice died more slowly, with fewer obvious pathological differences and lower infection burdens, than p40 KO mice despite comparable deficiencies in IFN-γ production in both types of KO mice (8). Infections with the classic intracellular bacterium Listeria monocytogenes have not been comprehensively characterized in all three types of IL-12 deficiency, but some results suggest that the outcome of Listeria infection may be different between the KO mice and/or may be highly dose dependent. Anti-IL-12 treatment before infection with a large primary sublethal dose of Listeria (approaching the LD50) resulted in death, while secondary lethal Listeria challenge was only minimally affected by anti-IL-12 treatment (46, 47). A recent study demonstrated that p35 KO mice died following large sublethal Listeria doses but survived and cleared lower primary and secondary doses, despite poor IFN-γ production (5). Further, p40 KO mice also survived and cleared, with some delay, a low primary dose of Listeria (34). Of particular note, while intravenous M. tuberculosis infection of p40 KO mice resulted in rapid death (7), very recent results indicate that intravenous M. bovis BCG infection was controlled well by WT and p35 KO mice but was chronic in p35- p40 double KO mice; further, WT and p35 KO mice given a low-dose aerosol infection with M. tuberculosis exhibited greater survival times, stronger delayed-type hypersensitivity responses, and improved granuloma formation compared to M. tuberculosis-infected p35-p40 double KO mice (23). Although p40 single-KO mice were not included in that study, the relative difference in outcome after Mycobacteria infection appears to be quite similar to that seen here with LVS.
While it may appear difficult to generalize about the role of IL-12 p70 in intracellular infections, many studies of facultative intracellular parasites are consistent with the central notion that p40 protein may be necessary for clearance of maximal doses. Direct comparison of LVS infection in all three models of IL-12 deficiency clearly indicates that long-term clearance is dependent on p40 but not on IL-12 p70. In other infections such as Leishmania and Mycobacteria, the role of p40 may be obscured by the absolute dependence on complete IL-12 p70 for control of initial primary infection. There are two obvious possibilities concerning the means by which the IL-12 p40 protein, which is present in mouse serum in excess of the p70 heterodimer (16), may effect its role. As has been previously suggested (19), p40 protein as either a monomer or a homodimer may have positive biological activity in vivo that is not evident from in vitro studies indicating antagonistic activities (29). Alternatively, p40 may be present in complex with other proteins. In particular, recent studies have described the combination of p40 with a 19-kDa protein to form a new cytokine, designated IL-23 (32). IL-23 is apparently produced by macrophages and dendritic cells, and in vitro this molecule supported proliferation of memory T cells; this is therefore an attractive candidate for having a role in T-cell-mediated clearance of bacteria. Thus, future studies testing the ability of the clearance-promoting activities of either p40 alone or the p19-p40 complex are in progress.
These studies also raise new questions about the role and relative importance of IFN-γ in intracellular infection. Since all three types of IL-12-deficient mice restrict initial bacterial organ burdens to the same degree as WT mice but produce little if any systemic IFN-γ (Fig. 1 to 3), this initial control of bacterial replication apparently requires relatively little IFN-γ (perhaps locally produced). Thus, further study is necessary to distinguish between the possibilities that relatively low levels of IFN-γ are sufficient to clear LVS infection or that clearance of late primary or secondary infection is actually independent of IFN-γ. Previous studies clearly demonstrated that initial survival of WT mice was dependent on the availability of IFN-γ within the first 2 days after LVS infection (1, 11, 12, 25), as is initial survival of both p35 and p40 KO mice (S. C. Cowley and K. L. Elkins, manuscript in preparation). However, it is intriguing to note that in LVS-infected WT mice, IFN-γ was readily detected in serum at very early time points only, within 1 to 3 days after LVS infection, but not at later time points (Fig. 1 to 3). Further, mice depleted of IFN-γ immediately before secondary LVS challenge died from higher challenge doses but not from lower doses (42). Other evidence strongly indicates that while IFN-γ may be necessary to control intracellular infection, it is not likely to be sufficient. For example, CD4-deficient mice were clearly defective in their ability to control primary M. tuberculosis infection, despite having only minimal or transient differences in IFN-γ production compared to WT mice (6, 38). Importantly, IFN-γ KO mice vaccinated with an attenuated mutant of L. monocytogenes are nearly normal in their ability to survive secondary lethal Listeria challenge (2, 21). In fact, the relative importance of IFN-γ production in late primary or secondary intracellular infection has not been well studied to date, due in large part to the acute death of IFN-γ-deficient mice following primary intracellular infection.
The relative importance of activated macrophage production of NO in primary or secondary LVS infection also remains to be revealed. In previous in vitro studies of LVS replication in resident peritoneal or inflammatory murine macrophages, inhibition of NO partially but not completely reversed control of intracellular bacterial growth (14). Further, control of LVS growth in IFN-γ-stimulated alveolar macrophages was minimally affected by inhibition of NO (36). Here, there was a correlation between production of NO and in vitro control of intracellular LVS growth but not between IFN-γ and NO production (see Fig. 5 to 7). In particular, large amounts of NO were produced in cultures containing p40 KO immune lymphocytes and LVS-infected p40 KO macrophages, where minimal IFN-γ was produced (Fig. 5 to 7). Thus, results to date suggest the existence of significant IFN-γ- and NO-independent mechanisms in immunity to LVS, but more direct studies using inducible NO synthase KO mice are also planned.
Taken together, these data suggest that one mechanism of control of intracellular LVS growth is due to the well-known IL-12-IFN-γ macrophage activation-NO production cascade but that another equally important mechanism is independent of these elements and dependent on p40. Thus, initial survival of LVS infection is dependent on immediate production (within the first 2 days) of IFN-γ; the cellular source of this early IFN-γ remains to be directly identified, but is clearly not T cells (11, 12), and in other intracellular infections is proposed to be natural killer cells (39) or even CD8α+ lymphoid dendritic cells, which are also an important source of IL-12 (31). Later production of IFN-γ is derived from T cells. We suggest that the activity of IFN-γ is primarily bacteriostatic. A p40-dependent function is responsible for ultimate clearance of in vivo infection, but clearance may be only partially IL-12 p70 and/or IFN-γ dependent. This hypothesis is consistent with the observation (Fig. 5 and 6; also see above) that anti-IFN-γ antibody treatment had a greater impact on control of intracellular growth by LVS-immune p40 KO lymphocytes (which lack the putative p40-dependent mechanism but have the IFN-γ-dependent mechanism) than on that by WT lymphocytes (which have both mechanisms). Thus, future studies will examine the specific role and form of p40 activity and the closely related question of the relative importance of IFN-γ throughout LVS infection.
Acknowledgments
We are most grateful to Suzanne Epstein and Dorothy Scott for thoughtful and critical reviews of the manuscript; to Dorothy Scott for generously providing BALB/c-p40 KO mice; and to Catharine Bosio for continued insightful discussions.
Editor: R. N. Moore
REFERENCES
- 1.Anthony, L. S. D., E. Ghadirian, F. P. Nestel, and P. A. L. Kongshavn. 1989. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb. Pathog. 7:421-428. [DOI] [PubMed] [Google Scholar]
- 2.Badovinac, V. P., and J. T. Harty. 2000. Adaptive immunity and enhanced CD8+ T cell response to Listeria monocytogenes in the absence of perforin and IFN-gamma. J. Immunol. 164:6444-6452. [DOI] [PubMed] [Google Scholar]
- 3.Baker, C. N., D. G. Hollis, and C. Thornsberry. 1985. Antimicrobial susceptibility testing of Francisella tularensis with a modified Mueller-Hinton broth. J. Clin. Microbiol. 22:212-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosio, C. M., and K. L. Elkins. 2001. Susceptibility to secondary Francisella tularensis LVS infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect. Immun. 69:194-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brombacher, F., A. Dorfmuller, J. Magram, W. J. Dai, G. Kohler, A. Wunderlin, K. Palmer-Lehmann, M. K. Gately, and G. Alber. 1999. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int. Immunol. 11:325-332. [DOI] [PubMed] [Google Scholar]
- 6.Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 7.Cooper, A. M., J. Magram, J. Ferrante, and I. M. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decken, K., G. Kohler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M. K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorman, S. E., and S. M. Holland. 2000. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 11:321-333. [DOI] [PubMed] [Google Scholar]
- 10.Elkins, K. L., C. M. Bosio, and T. R. Rhinehart-Jones. 1999. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the model intracellular bacterium Francisella tularensis live vaccine strain. Infect. Immun. 67:6002-6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkins, K. L., T. R. Rhinehart-Jones, S. J. Culkin, D. Yee, and R. K. Winegar. 1996. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 64:3288-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkins, K. L., T. Rhinehart-Jones, C. A. Nacy, R. K. Winegar, and A. H. Fortier. 1993. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect. Immun. 61:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkins, K. L., R. K. Winegar, C. A. Nacy, and A. H. Fortier. 1992. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb. Pathog. 13:417-421. [DOI] [PubMed] [Google Scholar]
- 14.Fortier, A. H., T. Polsinelli, S. J. Green, and C. A. Nacy. 1992. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect. Immun. 60:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortier, A. H., M. V. Slayter, R. Ziemba, M. S. Meltzer, and C. A. Nacy. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 59:2922-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 17.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 18.Geng, Y., K. Berencsi, A. Gyulai, T. Valy-Nagy, E. Gonczol, and G. Trinchieri. 2000. Roles of interleukin-12 and gamma interferon in murine Chlamydia pneumoniae infection. Infect. Immun. 68:2245-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germann, T., and E. Rüde. 1995. The IL-12 p40 homodimer as a specific antagonist of the IL-12 heterodimer. Immunol. Today 16:500-503. [DOI] [PubMed] [Google Scholar]
- 20.Green, L. C., A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 21.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN-γ. Immunity 3:109-117. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann, K. F., S. L. James, A. W. Cheever, and T. A. Wynn. 1999. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J. Immunol. 163:927-938. [PubMed] [Google Scholar]
- 23.Hölscher, C., R. A. Atkinson, B. Arendse, N. Brown, E. Myburgh, G. Alber, and F. Brombacher. 2001. A protective and agonistic function of IL-12 p40 in mycobacterial infection. J. Immunol. 167:6957-6966. [DOI] [PubMed] [Google Scholar]
- 24.Jouanguy, E., R. Doffinger, S. Dupuis, A. Pallier, F. Altare, and J. L. Casanova. 1999. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 11:346-351. [DOI] [PubMed] [Google Scholar]
- 25.Leiby, D. A., A. H. Fortier, R. M. Crawford, R. D. Schreiber, and C. A. Nacy. 1992. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect. Immun. 60:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magram, J., S. E. Connaughton, R. R. Warrier, D. M. Carvajal, C. Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. A. Faherty, and M. K. Gately. 1996. IL-12-deficient mice are defective in IFN-gamma production and type 1 cytokine responses. Immunity 4:471-481. [DOI] [PubMed] [Google Scholar]
- 27.Markine-Goriaynoff, D., J. T. van der Logt, C. Truyens, T. D. Nguyen, F. W. Heessen, G. Bigaignon, Y. Carlier, and J. P. Coutelier. 2000. IFN-gamma-independent IgG2.a production in mice infected with viruses and parasites. Int. Immunol. 12:223-230. [DOI] [PubMed] [Google Scholar]
- 28.Mattner, F., K. Di Padova, and G. Alber. 1997. Interleukin-12 is indispensable for protective immunity against Leishmania major. Infect. Immun. 65:4378-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattner, F., S. Fischer, S. Guckes, S. Jin, H. Kaulen, E. Schmitt, E. Rüde, and T. Germann. 1993. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur. J. Immunol. 23:2202-2208. [DOI] [PubMed] [Google Scholar]
- 30.Mattner, F., J. Magram, J. Ferrante, P. Launois, K. Di Padova, R. Behin, M. K. Gately, J. A. Louis, and G. Alber. 1996. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 26:1553-1559. [DOI] [PubMed] [Google Scholar]
- 31.Ohteki, T., T. Fukao, K. Suzue, C. Maki, M. Ito, M. Nakamura, and S. Koyasu. 1999. Interleukin 12-dependent interferon g production by CD8α+ lymphoid dendritic cells. J. Exp. Med. 189:1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y.-J. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Wall-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12 p40 to form a cytokine, IL-23, with biological activities similar to as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 33.Ottenhoff, T. H., D. Kumararatne, and J. L. Casanova. 1998. Novel immunodeficiencies reveal the essential role of type 1 cytokines in immunity to intracellular bacteria. Immunol. Today 19:491-494. [DOI] [PubMed] [Google Scholar]
- 34.Oxenius, A., U. Karrer, R. M. Zinkernagel, and H. Hengartner. 1999. IL-12 is not required for induction of type 1 cytokine responses in viral infections. Immunology 162:965-973. [PubMed] [Google Scholar]
- 35.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 36.Polsinelli, T., M. S. Meltzer, and A. H. Fortier. 1994. Nitric oxide-independent killing of Francisella tularensis by IFN-γ-stimulated murine alveolar macrophages. J. Immunol. 153:1238-1245. [PubMed] [Google Scholar]
- 37.Rhinehart-Jones, T. R., A. H. Fortier, and K. L. Elkins. 1994. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect. Immun. 62:3129-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scanga, C. A., V. P. Mohan, K. Yu, H. Joseph, K. Tanaka, J. Chan, and J. L. Flynn. 2000. Depletion of CD4+ T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaible, U. E., H. L. Collins, and S. H. Kaufmann. 1999. Confrontation between intracellular bacteria and the immune system. Adv. Immunol. 71:267-377. [DOI] [PubMed] [Google Scholar]
- 40.Scharton-Kersten, T., L. C. Afonso, M. Wysocka, G. Trinchieri, and P. Scott. 1995. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 154:5320-5330. [PubMed] [Google Scholar]
- 41.Sinigaglia, F., D. D'Ambrosio, P. Panina-Bordignon, and L. Rogge. 1999. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol. Rev. 170:65-72. [DOI] [PubMed] [Google Scholar]
- 42.Sjöstedt, A., R. J. North, and J. W. Conlan. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 142:1369-1374. [DOI] [PubMed] [Google Scholar]
- 43.Stenmark, S., D. Sunnemark, A. Bucht, and A. Sjöstedt. 1999. Rapid local expression of interleukin-12, tumor necrosis factor alpha, and gamma interferon after cutaneous Francisella tularensis infection in tularemia-immune mice. Infect. Immun. 67:1789-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stobie, L., S. Gurunathan, C. Prussin, D. L. Sacks, N. Glaichenhaus, C. Y. Wu, and R. A. Seder. 2000. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. USA 97:8427-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tärnvik, A. 1989. Nature of protective immunity to Francisella tularensis. Rev. Infect. Dis. 11:440-451. [PubMed] [Google Scholar]
- 46.Tripp, C. S., M. K. Gately, J. Hakimi, P. Ling, and E. R. Unanue. 1994. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. J. Immunol. 152:1883-1888. [PubMed] [Google Scholar]
- 47.Tripp, C. S., O. Kanagawa, and E. R. Unanue. 1995. Secondary response to Listeria infection requires IFN-γ but is partially independent of IL-12. J. Immunol. 155:3427-3432. [PubMed] [Google Scholar]
- 48.Wakeham, J., J. Wang, J. Magram, K. Croitoru, R. Harkness, P. Dunn, A. Zganiacz, and Z. Xing. 1998. Lack of both type 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis bacille Calmette-Guerin in IL-12-deficient mice. J. Immunol. 160:6101-6111. [PubMed] [Google Scholar]
- 49.Wysocka, M., M. Kubin, L. Q. Vieira, L. Ozmen, G. Garotta, P. Scott, and G. Trinchieri. 1995. Interleukin-12 is required for interferon-gamma production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol. 25:672-676. [DOI] [PubMed] [Google Scholar]
- 50.Yap, G., M. Pesin, and A. Sher. 2000. IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen Toxoplasma gondii. J. Immunol. 165:628-631. [DOI] [PubMed] [Google Scholar]
- 51.Yee, D., T. R. Rhinehart-Jones, and K. L. Elkins. 1996. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J. Immunol. 157:5042-5048. [PubMed] [Google Scholar]